Abstract
Colchicine is commonly used as part of the treatment of acute and recurrent pericarditis. Neuromyopathy is a well-known, but probably underreported, side effect of colchicine. Here we present a unique case of a 56-year-old woman with recurrent episodes of colchicine-induced neuromyopathy over many years. (Level of Difficulty: Beginner.)
Key Words: colchicine, neuromyopathy, pericarditis, vacuolar myopathy
Abbreviations and Acronyms: CPK, creatinine phosphokinase; EMG, electromyography; GBS, Guillain-Barré syndrome
Central Illustration
History of Presentation
A 56-year-old woman presented to the emergency department with a 4-week history of watery diarrhea and gradual proximal weakness, with no fever or usage of any new medication. Physical examination was remarkable for severe proximal weakness, mainly of the lower limbs, with inability to rise from a wheelchair, areflexia, and mild glove and stocking paresthesia.
Learning Objectives
-
•
To recognize the clinical manifestations and differential diagnosis of colchicine toxicity.
-
•
To identify the potential precipitants of colchicine toxicity.
-
•
To discuss the proper use of colchicine in the long-term treatment of recurrent pericarditis.
Table 1 presents the main laboratory abnormalities, which included mild pancytopenia, moderate mixed elevation of liver enzymes, acute kidney injury, and mild elevation of creatinine phosphokinase (CPK) and C-reactive protein. Antinuclear antibody, rheumatoid factor, and antineutrophil cytoplasmic antibodies were negative. Complement C3 was low (79.7 mg/dL [90-180 mg/dL]), whereas C4 was normal. Haptoglobin and hematinics were within normal limits.
Table 1.
Main Laboratory Abnormalities
| At Presentation | Reference Range | |
|---|---|---|
| White blood count (× 109/L) | 1.8 | 4.05-11.84 |
| Platelet count (× 109/L) | 104 | 203-445 |
| Hemoglobin (g/dL) | 11.2 | 12.4-16.1 |
| Creatinine (μmol/L) (estimated glomerular filtration rate, mL/min) |
93 (48) | 49-90 (>60)a |
| Albumin (g/L) | 41 | 32-48 |
| International normalized ratio | 3.4 | 0.9-1.2b |
| Alanine aminotransferase (U/L) | 344 | 10-49 |
| Aspartate transaminase (U/L) | 157 | 0-34 |
| Alkaline phosphatase (U/L) | 299 | 46-116 |
| Creatine kinase (U/L) | 283 | 34-145 |
| C-reactive protein (mg/dL) | 2.06 | 0-0.5 |
| Erythrocyte sedimentation rate (mm/h) | 26 | 0-20 |
The patient’s personal creatinine baseline was 55 μmol/L. Because of her low weight (50 kg) estimated glomerular filtration rate was calculated based on the Cockcroft-Gault equation, taking into account that such estimation during acute kidney injury is imperfect.
Personal international normalized ratio goal while taking warfarin, 2.5-3.5.
Past Medical History
The patient had a history of mitral stenosis secondary to rheumatic heart disease, paroxysmal atrial fibrillation, and recurrent pericarditis over a decade before the current presentation that was attributed to systemic lupus erythematosus based on a positive antinuclear antibody in the serum and pericardial effusion, along with a positive direct Coombs and thrombocytopenia. She also experienced a presumed episode of gastroenteritis-related transient Guillain-Barré syndrome (GBS) 5 years before the current presentation. That diagnosis was based on acute limb weakness, areflexia, and a positive response to plasma exchange; lumbar puncture was not performed because she was using warfarin.
Her list of medications included amiodarone, 300 mg once daily; prednisone, 5 mg once daily; colchicine, 0.5 mg twice daily (taken regularly for at least 15 years); hydroxychloroquine, 200 mg once daily (taken regularly for at least 15 years); and warfarin, 1 mg once daily.
Differential Diagnosis
This patient presented with proximal weakness and areflexia accompanied with mild elevation of CPK and inflammatory markers. In addition, she had diarrhea, liver and kidney injuries, and pancytopenia. At this point, the main differential diagnosis included inflammatory myositis, recurrent episode of post viral GBS, and colchicine toxicity.
Investigations
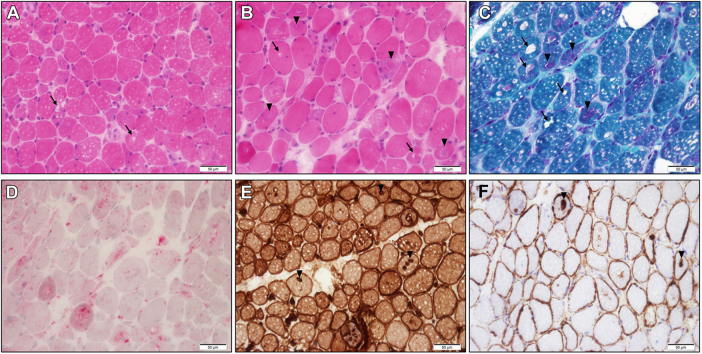
Abdominal ultrasound, cerebrospinal fluid, and urine analysis were unremarkable. Blood smear showed no signs of schistocytes or blasts. Stool samples were negative for pathogens. Lower limb magnetic resonance imaging was notable for bilateral myositis of the thigh muscles (mainly the adductors). Electromyography (EMG) and nerve conduction velocity of the thighs demonstrated a nonspecific myopathic pattern with axonal damage. Muscle biopsy of the vastus medialis was remarkable for vacuolar myopathy without significant inflammation, compatible with colchicine toxicity (Figure 1).
Figure 1.
Vacuolar Myopathy
Frozen sections stained with hematoxylin and eosin (A, B, original magnification ×40), Gömöri trichrome (C, original magnification ×40), acid phosphatase (D, original magnification ×40), and immunohistochemical stains for major histocompatibility complex (MHC) class I (E, original magnification ×40) and membrane attach complex (MAC) C5b-9 (F, original magnification ×40). Note display vacuoles (arrows) and basophilic inclusions (arrowheads) of variable size. Additional findings include variation in myofiber size with scattered atrophic myofibers, mildly increased endomysial connective tissue, and MHC class I and MAC C5b-9 overexpression. There was no significant inflammation or reduced COX staining (not shown). These findings are compatible with colchicine toxicity.
Review of the patient medical records was notable for many clinical and laboratory similarities between the current presentation and the previous episode of presumed GBS: diarrhea, pancytopenia, CPK elevation, and liver and kidney injuries. In retrospect, objective evidence of previous episode of GBS, such as supportive lumbar puncture or nerve conduction velocity/EMG findings, was not found. The earlier diagnosis was based on clinical presentation and it is possible that improvement in her condition was related to temporary colchicine withdrawal due to diarrhea. In addition, objective findings of recurrent pericarditis in the past decade were not found. In accordance, we cautiously concluded that she suffered from recurrent colchicine-induced neuromyopathy, and that her pericardial disease was probably stable over the years.
Management
In the face of objective harm and probably no clinical benefit, colchicine was discontinued, and the patient was given supportive treatment, including intravenous fluids and physical rehabilitation. No corticosteroids were given.
Discussion
The described patient was treated for more than 15 years with colchicine for recurrent pericarditis. Colchicine is the standard of care in many cases for the treatment of pericarditis, familial Mediterranean fever, and gout. It was also recently found to lower the risk of ischemic cardiovascular events among patients with chronic coronary disease or after myocardial infarction.1
In acute pericarditis, colchicine is commonly used as part of the initial treatment, along with nonsteroidal anti-inflammatory drugs. It improves the response to therapy and reduces the recurrence rate and the need for glucocorticoids. Colchicine is also a key part of the treatment of recurrent pericarditis, with a well-proven efficacy in reducing recurrence on randomized trials.2 The guidelines advise duration of treatment with colchicine for at least 3 months for acute pericarditis, and at least 6 months for recurrent pericarditis, with a gradual discontinuation after obtaining a complete response. In case of contraindication, intolerance, or refractoriness to colchicine, low-dose corticosteroids can be used as a temporalizing measure. In these cases, azathioprine or anakinra, a recombinant interleukin-1b receptor antagonist, can be used as a steroid-sparing agent.3
Although serious adverse events were uncommon in the short-term (3-6 months) use of colchicine in pericarditis, a Cochrane review found inconsistent serious adverse events reporting and uncertainty about its safety with long-term use in general, and in the "cardiovascular patient" with comorbidity and potential drug interactions, in particular.4
Colchicine is excreted and undergoes extensive metabolism mainly by the liver, and approximately 20% of the dose administered is excreted unchanged in the urine (probably higher in the setting of hepatic dysfunction). Colchicine has a long half-life, large volume of distribution, and a narrow therapeutic index, with potential multisystemic toxicity, especially in patients with hepatic or renal dysfunction and in the setting of drug interactions, the most cited being cytochrome (CYP) 3A4 and P-glycoprotein inhibitors.5 Some of these potential interactions are detailed in Table 2. The spectrum of clinical presentation in colchicine toxicity includes renal and hepatic injuries, diarrhea and vomiting, cytopenia, azoospermia, and neuromyopathy. Notably, our patient had no clinical, laboratory, or radiological signs of chronic liver dysfunction as a potential predisposing factor for her presentation, and the international normalized ratio was easily normalized with vitamin K as part of the periprocedural management before the muscle biopsy.
Table 2.
Major Drug Interactions That Might Increase the Serum Concentration of Colchicine
| Cytochrome 3A4 inhibitorsa | Clarithromycin, erythromycin, itraconazole, ketoconazole, voriconazole, cyclosporine, ritonavir, indinavir, verapamil, imatinib |
| P-glycoprotein inhibitorsa | Cyclosporine, amiodarone, ritonavir, carvedilol |
Partial lists.
Colchicine neuromyopathy usually has a subacute presentation and tends to develop in patients using a relatively small colchicine dosage over a long period. It presents mainly as proximal lower limb weakness with areflexia and distal sensory abnormality. The elevated CPK, myopathic pattern in EMG, and vacuolar myopathy in biopsy distinguish it from other diagnoses, which include GBS and polymyositis, among others.6 The diagnosis is usually made on clinical grounds, and the treatment is mainly supportive. Although there were a few reports of benefit with the use of Fab fragments, plasma exchange, and red blood cell exchange transfusion in acute severe toxicity, no treatment modality has proven to be effective to date.7,8 The prognosis depends on the clinical setting and the dose, with acute and high-dose (>0.8 mg/kg) toxicity resulting in a dismal outcome (∼100% death), and subacute or chronic toxicity, usually associated with a low dose, being mostly reversible within a few weeks,6,7 as in our case.
Notably, long-term use of hydroxychloroquine and amiodarone have also been described as a cause of vacuolar myopathy and axonal damage.9 In addition, amiodarone was reported to elevate the colchicine level.10 These factors, together with the fact that our patient was prescribed a relatively high dose of colchicine for her weight (0.5 mg twice a day for a 50-kg patient) and suffered from kidney injury, probably contributed to the clinical presentation.
Follow-Up
The patient had gradual but full clinical and laboratory recovery, with no signs of weakness, recurrence of pericarditis, or any laboratory anomaly 2 months after discharge and at 1-year follow-up.
Conclusions
This case describes the classical neuromuscular manifestations of colchicine toxicity, and hypothesizes that hydroxychloroquine and amiodarone may have contributed to toxicity and the clinical presentation. It highlights some of the potential participating factors for toxicity even while using low-dose colchicine for recurrent pericarditis, and stresses the importance of clinical follow-up, including consideration of supervised colchicine withdrawal after obtaining a complete response.
Funding Support and Author Disclosures
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Nidorf S.M., Fiolet A.T.L., Mosterd A., et al. Colchicine in patients with chronic coronary disease. N Engl J Med. 2020;383(19):1838–1847. doi: 10.1056/NEJMoa2021372. [DOI] [PubMed] [Google Scholar]
- 2.Imazio M., Belli R., Brucato A., et al. Efficacy and safety of colchicine for treatment of multiple recurrences of pericarditis (CORP-2): a multicentre, double-blind, placebo-controlled, randomised trial. Lancet. 2014;383(9936):2232–2237. doi: 10.1016/S0140-6736(13)62709-9. [DOI] [PubMed] [Google Scholar]
- 3.Adler Y., Charron P., Imazio M., et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hemkens L.G., Ewald H., Gloy V.L., et al. Cardiovascular effects and safety of long-term colchicine treatment: Cochrane review and meta-analysis. Heart. 2016;102(8):590–596. doi: 10.1136/heartjnl-2015-308542. [DOI] [PubMed] [Google Scholar]
- 5.Putterman C., Ben-Chetrit E., Caraco Y., Levy M. Colchicine intoxication: clinical pharmacology, risk factors, features, and management. Semin Arthritis Rheum. 1991;21(3):143–155. doi: 10.1016/0049-0172(91)90003-i. [DOI] [PubMed] [Google Scholar]
- 6.Kuncl R.W., Duncan G., Watson D., Alderson K., Rogawski M.A., Peper M. Colchicine myopathy and neuropathy. N Engl J Med. 1987;316(25):1562–1568. doi: 10.1056/NEJM198706183162502. [DOI] [PubMed] [Google Scholar]
- 7.Baud F.J., Sabouraud A., Vicaut E., et al. Brief report: treatment of severe colchicine overdose with colchicine-specific Fab fragments. N Engl J Med. 1995;332(10):642–645. doi: 10.1056/NEJM199503093321004. [DOI] [PubMed] [Google Scholar]
- 8.Mulkareddy V., Sokach C., Bucklew E., et al. Colchicine toxicity: the fatal masquerader. J Am Coll Cardiol Case Rep. 2020;2(4):678–680. doi: 10.1016/j.jaccas.2020.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le Quintrec J.S., Le Quintrec J.L. Drug-induced myopathies. Baillieres Clin Rheumatol. 1991;5(1):21–38. doi: 10.1016/s0950-3579(05)80294-8. [DOI] [PubMed] [Google Scholar]
- 10.Salem C.B., Sakhri J., Fathallah N., Trimech B., Hmouda H., Kamel B. Colchicine-induced rhabdomyolysis and possible amiodarone interaction. Pharmacology & Pharmacy. 2010;1:39–41. [Google Scholar]