Abstract
Previous investigations have demonstrated that immunization with Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase significantly protects rabbits from subsequent treponeme challenge. In this report, we show that the glycerophosphodiester phosphodiesterase amino acid sequence is conserved among 12 strains from a total of five pathogenic treponemes. The invariant nature of this immunoprotective antigen makes it an attractive candidate for inclusion in a universal subunit vaccine against T. pallidum infection. In addition, these studies show a silent nucleotide substitution at position 579 of the gpd open reading frame which is consistently observed in the non-T. pallidum subsp. pallidum strains. This sequence alteration introduces a PleI restriction site in the nonsyphilis strains and thus allows genetic differentiation from T. pallidum subsp. pallidum strains.
The human-infective Treponema pallidum subsp. pallidum, pertenue, and endemicum are the causative agents of syphilis, yaws, and bejel, respectively. Two highly related treponemes that are naturally infectious for animals have also been identified; these are the rabbit-infective species T. paraluiscuniculi and the Simian isolate obtained from skin lesions of a monkey (12). Although the human-infective pathogenic treponemes cause clinically distinct diseases, differentiation between T. pallidum subsp. pallidum and the other T. pallidum subspecies on the genetic level has only recently been described (6).
Syphilis remains a public health concern worldwide, with an estimated 3.5 million cases occurring annually (16). Successful control of syphilis depends on the development of an effective vaccine that demonstrates cross-protection between T. pallidum subsp. pallidum strains. To date, complete protection has been demonstrated only in experimental animals using impractical immunization protocols involving gamma-irradiated treponemes (15). Partial protection has been achieved in experimental animals by immunizing with treponemes that were antiformin treated (21) or aged at 4°C (14), as well as with several recombinant or native T. pallidum subsp. pallidum proteins, including protein 4D (1), purified endoflagella (8), and TmpB (23). Recent investigations conducted in our laboratory have identified three additional recombinant antigens that provide significant protection against experimental syphilis infections; these are TprK (5), Tp92 (3), and glycerophosphodiester phosphodiesterase (Gpd) (2, 20). Antigens that confer complete protection against infection have yet to be discovered, and successful vaccination regimens against syphilis may involve concurrent vaccination with promising immunoprotective antigens as part of a vaccine cocktail.
In this study, we further extend our investigations into the suitability of Gpd as a potential vaccine candidate by determining the degree of Gpd sequence conservation among pathogenic treponemes.
Bacterial species.
The Gpd coding sequence was PCR amplified from genomic DNAs isolated from a variety of treponemal strains. All strains were propagated in New Zealand White rabbits as previously described (13). T. pallidum subsp. pallidum Nichols was originally sent to the University of Washington by James N. Miller (University of California, Los Angeles) in 1979, and T. pallidum subsp. pertenue Gauthier was supplied by Peter Perine (Centers for Disease Control and Prevention, Atlanta, Ga.) in 1981. T. pallidum subsp. pallidum Bal-3, Bal-7, and Bal 73-1; T. paraluiscuniculi Cuniculi A; T. pallidum subsp. pertenue Haiti B; T. pallidum subsp. endemicum Iraq B; and the Simian isolate were supplied by Paul Hardy (Johns Hopkins University, Baltimore, Md.). T. pallidum subsp. pallidum Sea 81-3 and Sea 83-1 were isolated by Sheila A. Lukehart from the cerebrospinal fluid of untreated syphilis patients.
PCR amplifications.
To obtain the entire gpd open reading frame, primers were designed from the 5′ (5′-TGCACGGTGACGATCTGTGC-3′) and 3′ (5′-GGTACCAGGCGACACTGAAC-3′) noncoding regions flanking the gpd gene (11). These primers are located 48 bp upstream and 51 bp downstream, respectively, of the gpd open reading frame. PCR amplification of the gpd gene was performed by using a 100-μl reaction mixture containing 200 μM deoxynucleoside triphosphates, each primer at 0.25 μM, 1× Taq polymerase buffer (50 mM Tris-HCl [pH 9.0] at 20°C, 1.5 mM MgCl2, 20 mM NH4SO4), and 1 μl of genomic DNA containing 5,000 to 10,000 treponeme equivalents for each strain. The PCR conditions were 30 cycles of 1 min of denaturation at 94°C, 1 min of annealing at 60°C, and 2 min of extension at 74°C. For each reaction, a hot-start PCR (9) was performed by adding 2.5 U of Taq polymerase after the initial denaturation step. Following the PCR, the amplification products were cloned into the pGEM-T vector (Promega, Madison, Wis.) and each insert was sequenced in its entirety in both directions. To reduce the possibility of PCR- or sequencing-induced errors, two clones derived from independent PCR amplifications were sequenced for each strain.
Sequence analysis.
Double-stranded plasmid DNA was extracted by using the Qiagen Plasmid Mini Kit (Qiagen, Chatsworth, Calif.), and both strands of insert DNA were sequenced by using the Applied Biosystems dye terminator sequencing kit (PE Applied Biosystems, Foster City, Calif.) and the ABI 373A DNA sequencer in accordance with the manufacturer’s instructions. In all cases, both universal sequencing primers and internal primers designed from the insert sequence were used. Nucleotide sequences were translated and analyzed by using the Sequencher Version 3.1RC4 sequence analysis software (Gene Codes Corporation, Ann Arbor, Mich.). Alignment of protein and DNA sequences was performed by using the Clustal W general-purpose multiple-alignment program (22).
RFLP analysis.
RFLP analysis was performed on the gpd open reading frame amplified from each treponeme strain. One microgram of each of the amplified templates was digested with PleI (New England Biolabs, Beverly, Mass.) for 4 h at 37°C prior to electrophoresis on a 1.5% NuSieve (FMC BioProducts, Rockland, Maine) agarose gel.
As shown in Table 1, all six strains of T. pallidum subsp. pallidum have identical gpd gene sequences, while the other human subspecies (pertenue and endemicum) and the animal pathogens (Simian strain and T. paraluiscuniculi) have a silent A-to-G change at bp 579. Interestingly, T. paraluiscuniculi (the only different species represented) has five additional base pair changes, one of which (bp 263) results in a conservative amino acid substitution at residue 88. This demonstrates genetic divergence of the nonvenereal treponemal strains and the rabbit pathogen away from the syphilis strains, consistent with their different clinical diseases and host ranges. The Simian strain has been thought to be very closely related (or identical) to the human pertenue subspecies (10, 18), and this study supports this hypothesis.
TABLE 1.
Summary of Gpd sequence conservation between T. pallidum subsp. pallidum Nichols and various pathogenic treponeme strains
| T. pallidum subspecies | Strain | Sequence divergence from strain Nichols
|
|
|---|---|---|---|
| Nucleotides | Amino acids | ||
| pallidum | Bal-3 | None | None |
| pallidum | Bal-7 | None | None |
| pallidum | Bal-73-1 | None | None |
| pallidum | Sea 81-3 | None | None |
| pallidum | Sea 83-1 | None | None |
| pallidum | Mexico A | None | None |
| pertenue (?) | Haiti B | None | None |
| pertenue | Gauthier | Bp 579, A to G | None |
| endemicum | Iraq B | Bp 579, A to G | None |
| ? | Simian | Bp 579, A to G | None |
| paraluiscuniculi | Cuniculi A | Bp 263, G to A | Residue 88, R to H |
| Bp 459, A to G | None | ||
| Bp 579, A to G | None | ||
| Bp 711, A to G | None | ||
| Bp 960, C to T | None | ||
| Bp 999, G to C | None | ||
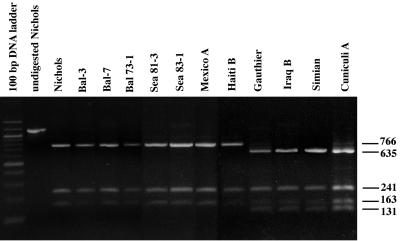
The base pair change at position 579 in the nonsyphilis strains introduces a PleI restriction site that creates different restriction fragment length polymorphism (RFLP) patterns between the T. pallidum subsp. pallidum strains and the other human and animal pathogens. As shown in Fig. 1, PleI digestion of the T. pallidum subsp. pallidum strains generates three restriction fragments with sizes of 766, 241, and 163 bp. The presence of the additional PleI site in the nonsyphilis strains generates four restriction fragments with sizes of 635, 241, 163, and 131 bp. These characteristic RFLP patterns provide a means of genetically differentiating between infections caused by the pallidum subspecies and those caused by the various other pathogenic treponemes.
FIG. 1.
RFLP analysis of the gpd amplicons from various pathogenic treponeme strains. The gpd open reading frame was amplified from each of the specified strains, digested with PleI, and subjected to agarose gel electrophoresis and ethidium bromide staining. The left lane shows the 100-bp DNA ladder (New England Biolabs). Shown also is the undigested gpd amplicon from the Nichols strain. The sizes, in base pairs, of the DNA fragments generated by PleI digestion of the gpd amplicons from the various strains are shown on the right.
The finding that the Haiti B strain, which is reportedly a T. pallidum subsp. pertenue strain, shows sequence identity with the pallidum subspecies and not with the nonsyphilis strains supports the proposal by Centurion-Lara et al. (6) that this strain was misidentified and should be classified as a T. pallidum subsp. pallidum strain. Similar sequence analyses performed on the tprK (7) and tp92 (4) sequences from the Haiti B strain further support its identification as a T. pallidum subsp. pallidum strain.
Homologues of Gpd from other bacterial species also demonstrate remarkable conservation of the amino acid sequence. The enzyme from Haemophilus influenzae, designated protein D, is 98% conserved among eight strains (19). The corresponding molecule from the relapsing-fever spirochete Borrelia hermsii, GlpQ, exhibits a range of 96.5 to 100% amino acid sequence similarity among 26 B. hermsii isolates (17). Similarly, results reported here show that Gpd is highly conserved among 12 strains that encompass a total of five pathogenic treponemes. The invariant nature of Gpd, combined with the immunoprotective capability previously described for this molecule in the experimental syphilis model (2), makes it an attractive candidate for inclusion in a universal subunit vaccine against T. pallidum infection.
Nucleotide sequence accession numbers.
The nucleotide sequences of the gpd genes from the Nichols, Bal-3, Bal-7, Bal 73-1, Sea 81-3, Sea 83-1, Mexico A, Haiti B, Gauthier, Iraq B, Simian, and Cuniculi A strains have been assigned GenBank accession no. AF004286 and AF127415 to AF127425, respectively.
Acknowledgments
This work was supported by grants AI 34616, AI 18988, and AI 42143 from the National Institutes of Health (W.C.V.V. and S.A.L.) and postdoctoral fellowships from the Natural Sciences and Engineering Research Council of Canada and the Medical Research Council of Canada (C.E.C.).
REFERENCES
- 1.Borenstein L A, Radolf J D, Fehniger T E, Blanco D R, Miller J N, Lovett M A. Immunization of rabbits with recombinant Treponema pallidum surface antigen 4D alters the course of experimental syphilis. J Immunol. 1988;140:2415–2421. [PubMed] [Google Scholar]
- 2.Cameron C E, Castro C, Lukehart S A, Van Voorhis W C. Function and protective capacity of Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase. Infect Immun. 1998;66:5763–5770. doi: 10.1128/iai.66.12.5763-5770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. Unpublished data.
- 4.Cameron, C. E., C. Castro, S. A. Lukehart, and W. C. Van Voorhis. Unpublished data.
- 5.Centurion-Lara A, Castro C, Barrett L, Cameron C, Mostowfi M, Van Voorhis W C, Lukehart S A. Treponema pallidum major sheath protein homologue TprK is a target of opsonic antibody and the protective immune response. J Exp Med. 1999;189:647–656. doi: 10.1084/jem.189.4.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centurion-Lara A, Castro C, Castillo R, Shaffer J M, Van Voorhis W C, Lukehart S A. The flanking region sequences of the 15-kDa lipoprotein gene differentiate pathogenic treponemes. J Infect Dis. 1998;177:1036–1040. doi: 10.1086/515247. [DOI] [PubMed] [Google Scholar]
- 7.Centurion-Lara, A., C. Castro, W. C. Van Voorhis, and S. A. Lukehart. Unpublished data.
- 8.Champion C I, Miller J N, Borenstein L A, Lovett M A, Blanco D R. Immunization with Treponema pallidum endoflagella alters the course of experimental rabbit syphilis. Infect Immun. 1990;58:3158–3161. doi: 10.1128/iai.58.9.3158-3161.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chou Q, Russell M, Birch D E, Raymond J, Bloch W. Prevention of pre-PCR mis-priming and primer dimerization improves low-copy-number amplifications. Nucleic Acids Res. 1992;20:1717–1723. doi: 10.1093/nar/20.7.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Felsenfeld O, Wolf R H. Serological reactions with treponemal antigens in nonhuman primates and the natural history of treponematosis in man. Folia Primatol. 1971;16:294–305. doi: 10.1159/000155411. [DOI] [PubMed] [Google Scholar]
- 11.Fraser C M, Norris S J, Weinstock G M, White O, Sutton G G, Dodson R, Gwinn M, Hickey E K, Clayton R, Ketchum K A, Sodergren E, Hardham J M, McLeod M P, Salzberg S, Peterson J, Khalak H, Richardson D, Howell J K, Chidambaram M, Utterback T, McDonald L, Artiach P, Bowman C, Cotton M D, Fujii C, Garland S, Hatch B, Horst K, Watthey L, Weidman J, Smith H O, Venter J C. Complete genome sequence of Treponema pallidum, the syphilis spirochete. Science. 1998;281:375–388. doi: 10.1126/science.281.5375.375. [DOI] [PubMed] [Google Scholar]
- 12.Fribourg-Blanc A, Niel G, Mollaret H H. Note sur quelques aspects immunologiques du cynocephale africain. Bull Soc Pathol Exot. 1963;56:474–485. [PubMed] [Google Scholar]
- 13.Lukehart S A, Baker-Zander S A, Sell S. Characterization of lymphocyte responsiveness in early experimental syphilis. I. In vitro response to mitogens and Treponema pallidum antigens. J Immunol. 1980;124:454–460. [PubMed] [Google Scholar]
- 14.Metzger M, Michalska E, Podwinska J, Smogór W. Immunogenic properties of the protein component of Treponema pallidum. Br J Vener Dis. 1969;45:299–303. doi: 10.1136/sti.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miller J N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by gamma-irradiation. J Immunol. 1973;110:1206–1215. [PubMed] [Google Scholar]
- 16.Rolfs T T. Surveillance for primary and secondary syphilis—United States, 1991. Morbid Mortal Weekly Rep. 1993;42:13–19. [PubMed] [Google Scholar]
- 17.Schwan, T. G., and S. F. Porcella. Personal communication.
- 18.Sepetjian M, Guerraz F T, Salussola D, Thivolet J, Monier J C. Contribution a l’etude du treponeme isole du dinge par A. Fribourg-Blanc Bull WHO. 1969;40:141–151. [PMC free article] [PubMed] [Google Scholar]
- 19.Song X-M, Forsgren A, Janson H. The gene encoding protein D (hpd) is highly conserved among Haemophilus influenzae type b and nontypeable strains. Infect Immun. 1995;63:696–699. doi: 10.1128/iai.63.2.696-699.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stebeck C E, Shaffer J M, Arroll T W, Lukehart S A, Van Voorhis W C. Identification of the Treponema pallidum subsp. pallidum glycerophosphodiester phosphodiesterase homologue. FEMS Microbiol Lett. 1997;154:303–310. doi: 10.1111/j.1574-6968.1997.tb12660.x. [DOI] [PubMed] [Google Scholar]
- 21.Tani T, Inoue R, Asano O. Studies on the preventive inoculation against syphilis. Jpn Med J. 1951;4:71–86. doi: 10.7883/yoken1948.4.71. [DOI] [PubMed] [Google Scholar]
- 22.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wicher K, Schouls L M, Wicher V, Van Embden J D A, Nakeeb S S. Immunization of guinea pigs with recombinant TmpB antigen induces protection against challenge infection with Treponema pallidum Nichols. Infect Immun. 1991;59:4343–4348. doi: 10.1128/iai.59.12.4343-4348.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]