Abstract
OBJECTIVE
Despite its worldwide use, reviews of oxytocin for labor augmentation include mainly studies from high-income countries. Meanwhile, oxytocin is a potentially harmful medication and risks may be higher in low-resource settings. We conducted a systematic review and meta-analysis of practices, benefits, and risks of oxytocin for labor augmentation in low- and lower-middle-income countries.
DATA SOURCES
PubMed, Embase, PsycINFO, Index Medicus, Cochrane, and Google Scholar were searched for publications until January 1, 2022.
STUDY ELIGIBILITY CRITERIA
All studies evaluating oxytocin augmentation rates were included. To investigate benefits and risks, randomized and quasi-randomized trials comparing oxytocin augmentation with placebo or no oxytocin were included. To explore risks more broadly, cohort and case–control studies were also included.
METHODS
Data were extracted and quality-assessed by 2 researchers using a modified Newcastle–Ottawa scale. Generic inverse variance outcome and a random-effects model were used. Adjusted or crude effect measures with 95% confidence intervals were used.
RESULTS
In total, 42 studies were included, presenting data from 885 health facilities in 25 low- and lower-middle-income countries (124,643 women). Rates of oxytocin for labor augmentation varied from 0.7% to 97.0%, exceeding 30% in 14 countries. Four studies investigated timing of oxytocin for augmentation and found that 89.5% (2745) of labors augmented with oxytocin did not cross the partograph's action line. Four cohort and 7 case–control studies assessed perinatal outcomes. Meta-analysis revealed that oxytocin was associated with: stillbirth and day-1 neonatal mortality (relative risk, 1.45; 95% confidence interval, 1.02–2.06; N=84,077; 6 studies); low Apgar score (relative risk, 1.54; 95% confidence interval, 1.21–1.96; N=80,157; 4 studies); neonatal resuscitation (relative risk, 2.69; 95% confidence interval, 1.87–3.88; N=86,750; 3 studies); and neonatal encephalopathy (relative risk, 2.90; 95% confidence interval, 1.87–4.49; N=1383; 2 studies). No studies assessed effects on cesarean birth rate and uterine rupture.
CONCLUSION
This review discloses a concerning level of oxytocin use, including in labors that often did not fulfill criteria for dystocia. Although this finding is limited by confounding by indication, oxytocin seems associated with increased perinatal risks, which are likely mediated by inadequate fetal monitoring. We call for cautious use on clear indications and robust implementation research to support evidence-based guidelines for labor augmentation, particularly in low-resource settings.
Key words: Apgar score, birth asphyxia, childbirth, clinical guidelines, low- and lower-middle-income countries, low-resource setting, neonatal encephalopathy, neonatal mortality, neonatal resuscitation, oxytocin augmentation, partograph, perinatal mortality, prolonged labor, stillbirths
AJOG Global Reports at a Glance.
Why was this study conducted?
Reviews of oxytocin for labor augmentation include mainly studies from high-income countries. We hypothesize that risks are more pronounced in low- and lower-middle-income countries (LLMIC).
Key findings
In studies from LLMIC, rates of oxytocin augmentation exceeded 30% in 14 countries (56%). In many cases, criteria for dystocia were not fulfilled. Although limited by confounding, this meta-analysis indicates the association between oxytocin for labor augmentation and stillbirth, day-1 mortality, neonatal resuscitation, neonatal encephalopathy, and low Apgar score.
What does this add to what is known?
This review suggests that suboptimal intrapartum care in LLMIC drives risks mediated by oxytocin augmentation. Robust implementation research is warranted to understand overuse and guide realistic, safe, and effective use based on clear indications.
Introduction
Oxytocin has been used to stimulate uterine contractions since the 1950s and is the most commonly used drug during labor around the world. Although oxytocin augmentation is proven to reduce labor duration by 2 hours, evidence that it reduces cesarean birth for prolonged labor is missing.1,2
Oxytocin remains on the list of 12 specific high-alert medications that require “special safeguards to reduce the risk of errors” (Institute for Safe Medication Practices).3 Oxytocin has a variable individual therapeutic index, whereby the effect of 1 dose may result in no effect in some women and hypertonic uterine contractions in others. Hypertonic contractions can cause decreased placental blood perfusion and oxygen flow to the fetus, which may lead to brain damage or intrauterine death.4,5 Randomized controlled trials from high-income countries suggest that oxytocin for labor augmentation is associated with fetal heart rate (FHR) abnormalities. These randomized trials are, however, underpowered to assess perinatal death and Apgar score.1 Observational studies, from high-income countries mainly, suggest an association with acidemia, low Apgar score, and neonatal encephalopathy (NE).6, 7, 8
We hypothesize that adverse effects caused by oxytocin are likely to be much larger in low-resource settings because of absence of 1-to-1 care, electronic infusion pumps, continuous fetal and uterine monitoring, and delays in access to cesarean birth if fetal distress occurs.9 Use of oxytocin for labor augmentation seems to follow the trend of increasing medicalization of childbirth seen in many parts of the world.10 For instance, the World Health Organization's (WHO) Better Outcomes in Labour Difficulty (BOLD) study showed that 35% of Nigerian and Ugandan women had labor augmented with oxytocin.11
This study aimed to perform a systematic review investigating clinical practices, benefits, and risks of oxytocin for labor augmentation in low- and lower-middle-income countries (LLMIC). In addition, by using an exploratory approach, we unfold gaps for future research.
Materials and Methods
Search strategy
As registered in the International Prospective Register of Systematic Reviews (PROSPERO) and in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) and MOOSE (Meta-analyses of Observational Studies in Epidemiology) guidelines, a systematic literature search and meta-analysis was carried out. PubMed, Embase, PsycINFO, Cochrane, Index Medicus, and Google Scholar Citations were searched for publications until January 1, 2022 (Supplementary table 1). Search terms included 3 themes for search strings: oxytocin for labor augmentation, birth outcomes (“perinatal mortality,” “neonatal resuscitation,” “Apgar score,” “neonatal encephalopathy,” “uterine rupture,” “labor duration,” and “cesarean section”), and LLMICs (World Bank 2020 country classification). Medical Subject Headings terms were used whenever available. References of included studies were screened to identify additional studies. Language was restricted to papers in English and French (spoken in 60 of 79 LLMICs). Studies were published in both peer-reviewed and non–peer-reviewed journals.
Selection criteria
Exposure to oxytocin for labor augmentation was defined as oxytocin given after onset of labor and before the third stage of labor. Outcomes were intrapartum stillbirth, day-1 neonatal mortality, neonatal resuscitation, NE, low Apgar score, cesarean birth for prolonged labor, labor duration, and uterine rupture. All studies providing oxytocin augmentation rates were included. For assessing timing of oxytocin, only women in spontaneous labor were included. To investigate benefits and risks, randomized and quasi-randomized trials, comparing oxytocin augmentation with placebo or no oxytocin augmentation, were included. To explore possible oxytocin-mediated risks more broadly, cohort and case–control studies were also included. Studies that investigated only subgroups of women (ie, with a previous cesarean birth), did not include oxytocin for labor augmentation, did not differentiate between oxytocin used for induction vs augmentation, or did not distinguish oxytocin from other methods of augmentation were excluded. Likewise, conference abstracts and studies without a reference group, such as case series or case reports, were excluded.
Data extraction and risk of bias assessment
Titles and abstracts were screened for eligibility according to predefined criteria. If immediate exclusion was impossible, the full text was assessed for eligibility. Data were extracted into a structured, pilot-tested sheet, which included a risk of bias table. Assessment of risk of bias was based on the Newcastle–Ottawa scale and Cochrane Handbook for Systematic Reviews of Interventions (Supplementary table 2 and 3). Literature search, inclusion of studies, data extraction, and quality assessment were conducted independently by 2 researchers. In case of discrepancies, a third researcher was consulted.
Data synthesis
Data on oxytocin administration practices, monitoring of FHR and contractions, partograph use, ratio of birth attendants to women, and hospital volume were collected for narrative analysis of the context. Rates of oxytocin for labor augmentation were analyzed as proportion of women augmented among all women in the study. For pre-post studies, only preintervention data were included to represent baseline care. For case–control studies, only data from controls were included to reflect exposure in the study population. A definition was needed to assess appropriate timing of oxytocin initiation because no definition is uniformly applied.12 WHO's partograph is the most prevalent labor monitoring tool in LLMICs, and crossing its action line was defined as the appropriate time to apply oxytocin. This correlates well with WHO's recommendation that “a slower than 1 cm/hour cervical dilatation rate alone (ie, the partograph's alert line) should not be a routine indication for obstetric intervention” and to WHO's recent labor progression study from Nigeria and Uganda, where few women would have received oxytocin augmentation unnecessarily if an action-line-based indication had been used.11,13,14
Cochrane Collaboration Review Manager software (RevMan, version 5.4.1; Cochrane, London, England) was used for meta-analysis.15 Adjusted effect measures were applied when available. If unavailable, crude risk ratios (RRs) or odds ratios (ORs) were included with 95% confidence intervals (CIs). Effect measures were entered into RevMan using the “generic inverse variance” outcome. Because of rare outcomes, ORs and RRs were combined in the meta-analysis. Low Apgar score was included as 1 composite outcome regardless of the definition used by the author. A random-effects model was used for analysis because we expected heterogeneity among studies.
Results
Study selection
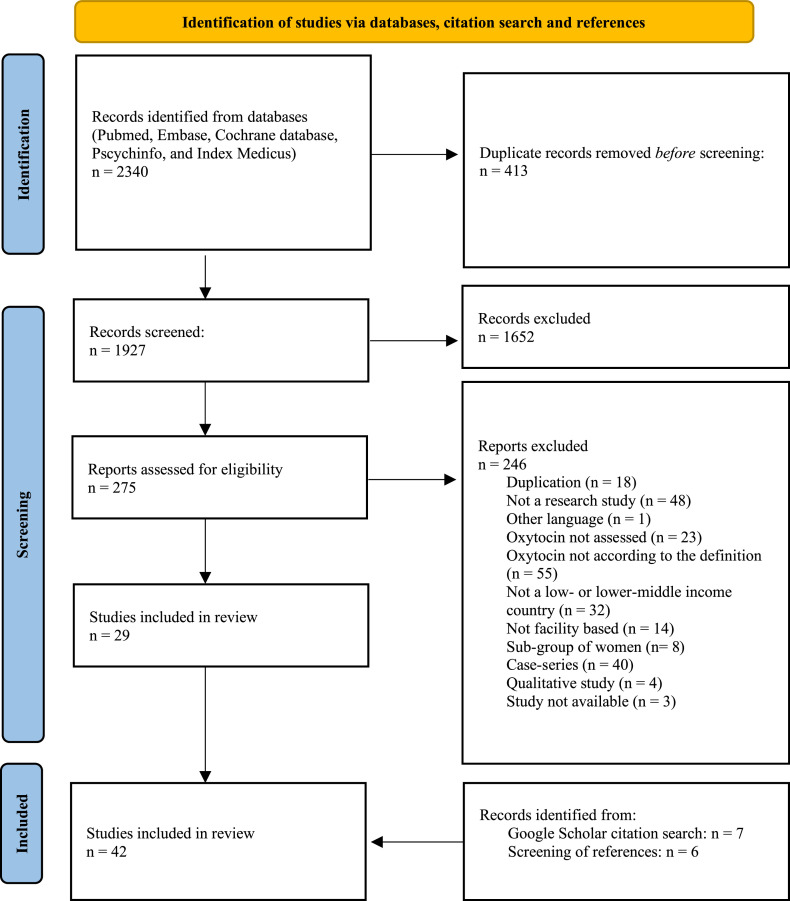
A total of 2340 studies were identified, of which 413 were duplicates and 1652 were excluded on the basis of titles or abstracts (Figure 1). Of 275 full-text articles, 246 did not meet eligibility criteria (Supplementary table 4), leaving 29 studies for inclusion. By screening references of included articles and through Google Scholar Citation search, 13 additional studies were identified. Finally, 42 studies were included: 27 studies simply provided rates of oxytocin augmentation (Figure 2), 4 studies reported oxytocin use according to labor progress (Figure 3), and 4 cohort and 7 case–control studies reported associations between oxytocin and perinatal outcomes (Table; Figure 4). No studies assessed effects of oxytocin augmentation on cesarean birth rates, labor duration, or uterine rupture. No randomized trials met the inclusion criteria.
Figure 1.
PRISMA flow diagram
PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Lauridsen Kujabi. A systematic review of oxytocin augmentation in low- and lower-middle-income country. Am J Obstet Gynecol Glob Rep 2022.
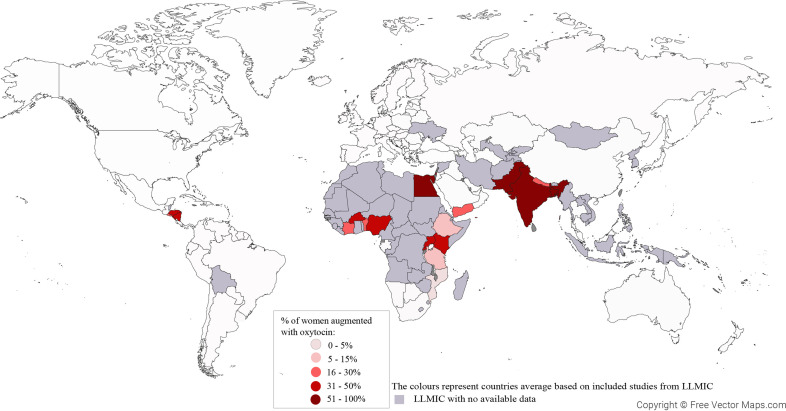
Figure 2.
Average percentage of labors augmented with oxytocin in LLMICs
Average facility-based use of oxytocin for labor augmentation in LLMICs (World Bank 2020 classification) after year 2000. Based on 41 studies reporting from 885 health facilities in 24 countries.
LLMIC, low- and lower-middle-income country.
Lauridsen Kujabi. A systematic review of oxytocin augmentation in low- and lower-middle-income country. Am J Obstet Gynecol Glob Rep 2022.
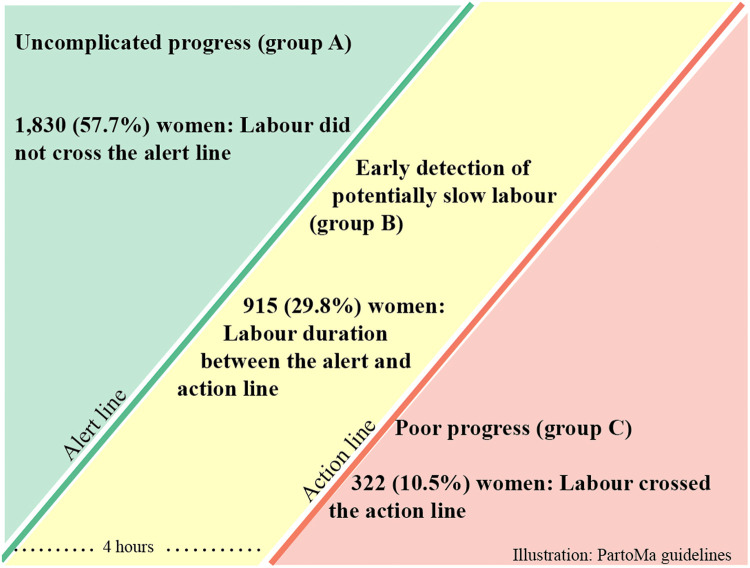
Figure 3.
Assessment of 3067 women augmented with oxytocin
Including studies from Benin, India, and Rwanda (9000 women in spontaneous labor).16, 17, 18, 19 Of these, 359 (3.9%) crossed the action line and 3067 (34.0%) received oxytocin for labor augmentation.
Lauridsen Kujabi. A systematic review of oxytocin augmentation in low- and lower-middle-income country. Am J Obstet Gynecol Glob Rep 2022.
Table.
Study characteristics of case–control and cohort studies
| Case-control studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, y; study year, country, data source | Facility | Study population | Confounders | Oxytocin | Outcome | Exposed cases/all cases | Exposed controls/all controls | Effect estimate |
| Delaney et al,20 2021a; 2014–2017, India. DO (oxytocin and bag-mask). MR (stillbirths and oxytocin). IN (perinatal mortality). |
30 facilities: 8 primary health centers; 18 community health centers; 4 first referral units. | All women admitted to a study facility for childbirth (stillbirths and bag-mask). All women with known health outcomes (perinatal mortality). | Not adjusted | 32%–78%. Intramuscular injections |
Perinatal mortality, not defined | 1597/87 | 1265/47 | OR, 1.47 (0.99–2.16) |
| Bag-and-mask ventilation | 3291/247 | 2193/44 | OR, 3.74 (2.37–5.90) | |||||
| Stillbirths | Numbers not available | Numbers not available | No difference (numbers not available) | |||||
| Litorp et al,21 2021a; 2017–2018, Nepal. MR. | 12 public referral hospitals. | All women excluding women with elective CD (5.4%), missing data on augmentation of labor (15%), and absent or no recording of FHR on admission (3.7%). | Multivariate logistic regression adjusted for parity, induction, maternal age, GA, complications during pregnancy or labor, BW, suboptimal partograph use, suboptimal FHR monitoring, ethnicity, educational level, and mode of delivery. |
37%. Gravity-fed infusion or electronic infusion pumps. |
All | All exposed: 28,915 (applies to all outcomes) | All unexposed: 50,016 (applies to all outcomes) | aRR |
| Stillbirths and day-1 neonatal mortality | 64 | 130 | 1.24 (0.65–2.40) | |||||
| Neonatal death at discharge | 234 | 422 | 1.93 (1.46–2.56) | |||||
| 5-min Apgar <7 | 1136 | 1553 | 1.65 (1.49–1.86) | |||||
| Bag-and-mask ventilation | 439 | 346 | 2.10 (1.80–2.50) | |||||
| ECD | 356 | 968 | 0.62 (0.59–0.66) | |||||
| Postpartum hemorrhage | 67 | 155 | 0.80 (0.55–1.20) | |||||
| Dujardin et al,22 1995a; 1990–1991, Benin, Democratic Republic of the Congo, and Senegal. DO. | 8 peripheral maternity clinics and 2 reference hospitals. | All women, <10 cm dilated, singleton, vertex, BW >1000 g. | Multivariate logistic regression adjusted for primiparity, previous complicated delivery, presence of meconium during labor, ruptured membranes, education. | Benin: 21%; Senegal: 11%; Congo: 6%. Gravity-fed infusion. |
Stillbirths (analysis restricted to oxytocin applied in normally progressing labor) | 279/16 | 2131/53 | RR, 1.9 (1.06–3.40) |
| Manual respiratory assistance | 266/76 | 2069/206 | aOR, 2.88 (1.84–4.50)b | |||||
| Mola and Rageau,23 1990a; 1989, Papua New Guinea. DO. | General hospital. | All women in spontaneous labor, singleton, vertex. Cases: oxytocin augmentation. Controls: next delivery with same parity. |
Not adjusted, but matching was done on parity, and only women in spontaneous labor were included. | 10.3%. Gravity-fed infusion, no infusion pumps available. | Stillbirths, intrapartum or neonatal death not defined | 329/3 | 329/2 | RR, 1.50 (0.25–8.92) |
| 5-min Apgar £6 | 329/1 | 329/1 | RR, 1 (0.06–16.6) | |||||
| Case–control studies | ||||||||
| Author, y; study year, country, data source (variable) | Facility | Study population | Confounders | Oxytocin | Outcome | Exposed cases/all cases | Exposed controls/all controls | Effect estimate |
| Mohan et al,24 2020a; 2008–2010b, India. IN. | All facility births in India. | Cases: neonatal day-1 mortality. Controls: death between day 8 and 28 (late neonatal deaths). |
Adjusted for the presence of skilled birth attendant. Stratified by sex and parity. The following were included in a supplementary adjusted analysis with 2% difference in point estimate: age, multiple pregnancy, APH, prolonged laborc, foul smelling amniotic fluid, PROM, cord prolapse, preterm, assisted deliveries, malpresentation, fever on the day delivery began, received ANC. | Cases: 74%. Controls: 62%. Intramuscular injections. |
Neonatal day-1 mortality | Government hospitals: 212/28 Private hospitals: 672/792 |
51/67 127/166 |
aOR, 0.96 (0.59–1.6) aOR, 1.8 (1.2–2.5) |
| Ellis et al,25 2000; 1995–1996, Nepal. DO. | Principal maternity hospital. | GA >37. Cases: NE. Controls: unmatched, every 25th infant. Excluding congenital malformations, hepatosplenomegaly, cataracts, signs of infection, infants who normalized after hypoglycemia was corrected. |
Adjusted for maternal age, parity, education, height, previous neonatal death, antenatal care, preeclampsia, BW, sex of infant, and plurality. No infants were >4 kg. Balance between groups for prolonged labor. | Cases: 39%. Controls: 22%. Administration not described. |
NE within 24 h,Amiel–Tison score assessed by trained Junior doctors | 50/131 | 139/635 | aOR, 3.51 (2.04–6.07) |
| Tann et al,26 2018; 2011–2012, Uganda. MR. | Referral hospital. | GA >27. Cases: NE. Controls: unmatched, Thompson score <3, recruited in a ratio of 79:21 from high-risk and low-risk wards, respectively. Excluding antibiotics given, mothers living 20 km away, out-born infants. |
Adjusted for primiparity, socioeconomic group, age >20 y, weight <50 kg, height <150 cm, >4 ANC visits, sex, previous birth asphyxia, previous perinatal death, severe anemia, hypertension, HIV, sex, BW, twins, noncephalic, no IAS of FHR during labor, prolonged rupture of membranes >24 h, obstructed labor. Balance between the groups for prolonged labor.c | Controls: 10.5%. Cases: 20.1%. Administration not described. |
NE: Thompson score >5 within 12 h assessed by the author or other study doctors |
42/209 | 43/408 | aOR, 2.23 (1.17–4.23) |
| Maaløe et al,27 2016; 2014–2015, Tanzania. MR. | Tertiary referral hospital. | Singleton, BW ³2 kg, positive FHR on admission. Cases: stillbirths. Controls: unmatched, Apgar ≥7, every 10th delivery, ratio 1:4. |
Not adjusted. Balance between the 2 groups for induction and parity. More cases crossed the partograph alert and action line than controls. | Cases: 36%. Controls: 23%. Infusion, not further specified. |
Stillbirths with positive FHR on admission | 26/72 | 58/249 | OR, 1.86 (1.06–3.27) |
| Onyearugha and Ugboma,28 2010; 2004, Nigeria. DO (Apgar score), MR (oxytocin). | Tertiary hospital, serving both as a secondary healthcare center and referral center for peripheral hospitals. | Cases: severe birth asphyxia. Controls: same weight bracket, Apgar 8–10, consecutively recruited. Excluding severe congenital malformation. |
Not adjusted. Prolonged labor was more common in cases than in controls. | Cases: 7%. Controls: 5%. Administration not described. |
Apgar 1–3 at 1 min and <5 at 5 min, assessed by the author or a resident | 7/98 | 5/98 | OR, 1.43 (0.44–4.67) |
| Hailu et al,29 2018; 2018 Ethiopia. MR. | 5 hospitals (2 governmental and 3 private). | Cases: infants with asphyxia. Controls: unmatched, ratio 1:4. |
Not adjusted. Balance between the 2 groups for parity. Labor duration >12 h was more common in cases than in controls. | Cases: 10.5%. Controls: 12.5%. Administration not described. |
Asphyxia: inability to sustain adequate respiration with an Apgar <7 at 5 min, assessed by trained midwives | 8/76 | 38/296 | OR, 0.80 (0.36–1.79) |
| Geelhoed et al,30 2015; 2009–2011, Mozambique. MR. | 2 urban health centers (providing basic emergency obstetrical care) and 1 provincial hospital (providing comprehensive emergency obstetrical care). | Cases: stillbirths with GA >28 wk and BW >1.5 kg; 33% had positive FHR on arrival. Controls: live births matched on health facility attended, maternal age, and parity. First subsequent delivery. |
Not adjusted. Active first stage of labor >6 h was more common in cases than in controls. | Cases: 2%. Controls: 2.7% Intravenous infusion, not further specified. |
Stillbirths (including prefacility stillbirths) | 3/150 | 8/300 | OR, 0.81 (0.31–2.16) |
ANC, antenatal care; aOR, adjusted odds ratio; APH, antepartum hemorrhage; aRR, adjusted risk ratio; BW, birthweight; CD, cesarean delivery; DO, direct observations; ECD, emergency cesarean delivery; FHR, fetal heart rate; GA, gestational age; IAS, intermittent auscultation; IN, interviews; MR, medical records; NE, neonatal encephalopathy; OR, odds ratio; PROM, prelabor rupture of membranes; RR, risk ratio.
Studies with an objective of assessing the association between oxytocin augmentation and perinatal outcomes
Combined OR including OR from the 4 countries is calculated using RevMan 5.3, inverse variance outcome
Defined as labor duration >12 hours for multiparous women and >24 hours for nulliparous women.
Lauridsen Kujabi. A systematic review of oxytocin augmentation in low- and lower-middle-income country. Am J Obstet Gynecol Glob Rep 2022.
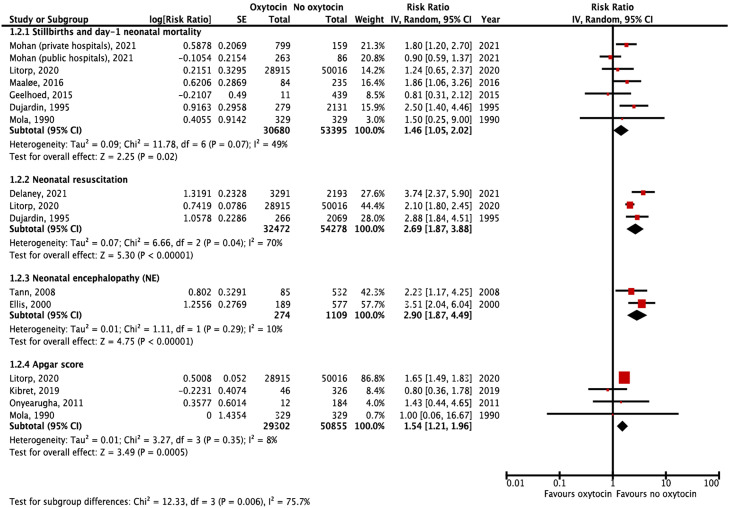
Figure 4.
Association between oxytocin for labor augmentation and perinatal outcomes
Forest plots include studies from Tanzania, India, Uganda, Benin, Democratic Republic of the Congo, Senegal, Papua New Guinea, Nepal, Mozambique, Ethiopia, and Nigeria.
CI, confidence interval; IV, inverse variance; SE, standard error.
Lauridsen Kujabi. A systematic review of oxytocin augmentation in low- and lower-middle-income country. Am J Obstet Gynecol Glob Rep 2022.
Monitoring of labor in low- and lower-middle-income countries
The 42 studies presented data from 885 health facilities in 25 countries (Table; Supplementary table 5). Of these, 32 (76.2%) included exclusively hospitals and 10 (23.8%) included both hospitals and lower-level health facilities. Inclusion periods of participants spanned from 1989 to 2021, with 35 of 42 (83.3%) conducted after 2000. Substandard use of the WHO partograph was described in facilities in India, Nigeria, Uganda, Zimbabwe, and Côte d'Ivoire.11,16,21,27,31, 32, 33 Intermittent auscultation with fetoscope or Doppler was reported in 20 of 42 (47.6%) studies as the FHR monitoring method. In the remaining studies, no information was given about FHR monitoring devices. Information on actual monitoring frequencies was scarce in most studies; 10 studies reported substandard FHR recordings in >40% of laboring women or substandard monitoring of contractions.21,26,27,31,34, 35, 36 In an Egyptian hospital, drip count in gravity-fed oxytocin infusions was only checked in 62 of 171 (36.3%) women receiving oxytocin augmentation.35 Only 1 study from Nepal reported that a motor-driven infusion pump was sometimes used.21 Studies from India, Côte d'Ivoire, and Nepal reported intramuscular oxytocin injections during labor.24,31,35,37, 38, 39 No studies reported on titration practices and maximum doses of oxytocin.
Rates and timing of oxytocin augmentation
To assess the rate of oxytocin for labor augmentation, 41 studies were eligible, including either all women in labor,16,22,33,34,40, 41, 42, 43 vaginal births only,9,31,37,39,44, 45, 46 women with uncomplicated singleton cephalic pregnancies at term,11,17, 18, 19,32,35,38,47, 48, 49, 50, 51 or other criteria.36,52, 53, 54 Data collection methods were by nonvalidated medical records,11,16,17,19,28,32,33,40, 41, 42, 43,45,46,51,54 clinical observations,9,18,28,31,34, 35, 36, 37, 38,48, 49, 50,53 questionnaires,44 or interviews with women,39,47 whereas 4 studies had no methods described.18,23,29,52 Studies reported up to 24% missing data.30,33,45,46 Figure 2 presents average rates of oxytocin for labor augmentation in each country in studies after the year 2000. Studies from Bangladesh, Pakistan, India, and Egypt (totally 3698 women) reported >50% of women receiving oxytocin labor augmentation, 10 countries (101,954 women) reported 30% to 49%, 5 countries (3586 women) reported 15% to 29%, and 3 countries (2245 women) reported <14% (Supplementary table 6). Notably, no study before 2000 (17,819 women) had oxytocin augmentation rates of >21%.
To assess timing of oxytocin for labor augmentation, 4 studies from Benin, Rwanda, and India (9000 women) assessed oxytocin augmentation in relation to progress on the partograph and divided women into 3 groups: (A) at or to the left of the partograph's alert line (ie, progress of cervical dilatation ≥1 cm/h); (B) between the alert and action lines (the action line is located parallel to the alert line, but 4 hours later); and (C) crossing the action line (Figure 3).16, 17, 18, 19 In these studies, a total of 3067 women were augmented with oxytocin (augmentation rate, 34.1%). Among these, 1830 (59.7%) were still in group A when giving birth, 915 (29.8%) in group B, and 322 (10.5%) in group C. In other words, 59.7% of women augmented with oxytocin during active labor had labor progress of ≥1 cm per hour, whereas only 10.5% actually had prolonged labor.
Association with adverse birth outcomes
To assess risks of oxytocin for labor augmentation, 4 cohort20, 21, 22, 23 and 7 case–control studies were identified in Tanzania, Nepal, Benin, Democratic Republic of the Congo, Senegal, Papua New Guinea, Uganda, Nigeria, and Ethiopia (Table).24, 25, 26, 27, 28, 29, 30 The studies had varying quality (Supplementary table 3); all but 2 studies20,22 used nonvalidated records21,23,25, 26, 27, 28, 29, 30 or verbal autopsies24 to assess oxytocin exposure; 4 studies assessed used clinical observations to assess outcomes,22,25,26,28 whereas the remaining studies used nonvalidated records. All studies had high risk of confounding because they did not adequately adjust for labor duration. Finally, 6 studies did not adjust for any confounders.20,23,27, 28, 29, 30 Results of the meta-analysis unanimously suggest that oxytocin used for labor augmentation may be associated with adverse perinatal outcomes (Figure 4), including: stillbirth and day-1 neonatal mortality (RR, 1.45; 95% CI, 1.02–2.06; N=84,077; 6 studies)21, 22, 23, 24,27,30; low Apgar score (RR, 1.54; 95% CI, 1.21–1.96; N=80,157; 4 studies)21,23,28,29; NE (RR, 2.90; 95% CI, 1.87–4.49; N=1383; 2 studies)25,26; and neonatal resuscitation (RR, 2.69; 95% CI, 1.87–3.88; N=86,750; 3 studies).20, 21, 22 No studies assessed association with cesarean birth rate, labor duration, or uterine rupture.
Comments
Principal findings
This review discloses major practice variations and high frequencies of oxytocin augmentation in many LLMICs. In many cases, the criteria for dystocia were not fulfilled. Although compromised by confounding by indication, our meta-analysis amplifies these concerns by indicating associations between oxytocin augmentation and stillbirth, day-1 neonatal mortality, neonatal resuscitation, NE, and low Apgar score. For decades, potential risks of unsafe use of oxytocin for labor augmentation have been a concern, and this review confirms that risks are most pronounced in the context of busy low-resource settings with poor means to monitor FHR and contractions—possibly compromising the desired effects of its use.9,55
Oxytocin augmentation: too much, too soon
In only 10.5% of women who received oxytocin for labor augmentation, the drug was administered in women with prolonged labor, defined as crossing the partograph's action line. Similar findings have been reported in studies from high-income countries. For instance, in Norway and Sweden, approximately half of all women in labor were augmented with oxytocin, with more than a third augmented without being diagnosed with prolonged labor.56,57 Notably, this contradicts the growing evidence that spontaneous labor progression is slower than previously anticipated, which is further reflected in the recently adopted WHO Labour Care Guide.58,59
The recommended rate of oxytocin for labor augmentation has not been defined, but rates above 6% to 12% do not seem to result in lower cesarean birth rates.33,34 Likewise, only 3.9% of 9000 women in our studies and only 15% of 8489 women in the WHO multicenter BOLD study crossed the partograph's action line, which has been proposed as a relevant indication for when to consider initiating oxytocin for labor augmentation.60 Therefore, rates of oxytocin for labor augmentation >15%, which was the case in most studies, cause worry for inappropriate use. However, heterogeneity of studies on oxytocin rates makes it difficult to provide generalizable recommendations for rates, which depend on the characteristics of women giving birth in the facilities. Importantly, other parts of prolonged labor management (mental support, ambulation, pain relief, etc.) and decision-making around cesarean births are likely to be just as influential on mode of birth as oxytocin itself.
With the currently limited evidence available,20,56 3 drivers seem central for such overuse in LLMICs. Firstly, overburdened maternity units: as illustrated in an Egyptian hospital with 8 laboring women per health provider, a high caseload contributing to massive bed shortages was an important reason that 91% of laboring women were given oxytocin to enhance labor and free up beds and hands.35 Secondly, increasing availability of obstetrical care in LLMICs has led to overmedicalization, whereas other aspects of maternity care are still absent. This may cause a dangerous coexistence of “too little, too late” and “too much, too soon” care where oxytocin is overused, whereas labor monitoring remains limited.10 Finally, vague and ambiguous clinical guidelines for diagnosis and management of prolonged labor and oxytocin seem crucial12,61; that is, although WHO meticulously describes how to up-titrate oxytocin, recommendations about when to prescribe, reduce, stop, and possibly restart oxytocin are absent, even in the recent WHO Labour Care Guide.58,61 Furthermore, guidance regarding safe maximum rates does not account for clinical realities where lack of 1-to-1 care, controlled infusion pumps, and delays in monitoring and treatment inevitably result in higher risks of unsafe oxytocin use, particularly if many women are treated simultaneously.
In contrast, unambiguous clinical guidelines for restricted oxytocin augmentation seem effective in promoting timely and safe use.33,60 For example, WHO's multicenter trial among 35,484 women in Indonesia, Malaysia, and Thailand in the 1990s introduced the partograph with clear guidelines recommending that oxytocin be first administered after crossing the action line.60 After implementation, a decline in oxytocin for labor augmentation from 20.7% to 9.1% was reported, together with an insignificant reduction in emergency cesarean birth rate (9.9% to 8.7%; P=.68). Following this strategy seems promising in reducing rates of oxytocin augmentation. In India, reduction of the use of intrapartum oxytocin through a coaching-based intervention led to a decrease from 77.8% to 32.1%; however, 1 year after implementation, the rate increased to 48.2%.20 This indicates that understanding the multiple factors influencing oxytocin use is highly needed to sustainably reduce current overuse.
Possible risks and no studies on benefits
Our meta-analysis revealed associations between oxytocin for labor augmentation and adverse perinatal outcomes. This is in line with observational studies from high-income countries, but with more severe consequences.6, 7, 8,62,63 Suboptimal monitoring of FHR and contractions, as described in the studies, is likely mediating the severity of risks. The influence of substandard care was, however, not assessed in the studies.
Supporting the association between oxytocin for labor augmentation and adverse perinatal outcomes is a high level of consistency between studies. A recent study in India, furthermore, supports a causal link between oxytocin augmentation and adverse effects given that the association between oxytocin augmentation and day-1 neonatal mortality seemed to be mediated entirely by birth asphyxia.24 The association was strongest for stillbirths, waned during the first 24 hours, and was negligible in the subsequent 6 days of life.24 Yet, a few inconsistent results related to stillbirths require further discussion. A multisite cohort study of 78,931 births in Nepal found associations between oxytocin augmentation and low Apgar score, neonatal resuscitation, and neonatal mortality before discharge, but no association with stillbirth (Table).21 Given that these outcomes are all part of the spectrum of morbidity caused by intrauterine hypoxia, such inconsistency warrants further exploration. The study excluded 3828 (3.7%) women because of absent or no recording of FHR on admission, which probably concealed underreported intrahospital stillbirths, of which the study reported only 194 (0.3%). Distinguishing between prehospital, intrahospital, macerated, and fresh stillbirths in medical records is a well-known challenge.64 A similar situation was observed in a recent study from India, in which oxytocin augmentation was associated with bag-and-mask ventilation and perinatal mortality, but not with stillbirths.20 Likewise, a multicountry study from Benin, Democratic Republic of the Congo, and Senegal found stronger associations between oxytocin augmentation and stillbirth when macerated stillbirths were excluded, indicating that including these may underestimate harmful effects.22 The remaining studies included only women with positive FHR on admission.
One of the studies from India found increased risks of intrapartum stillbirth and day-1 neonatal mortality only in private hospitals and home births, but not in public hospitals with similar augmentation rates.24 This supports the notion that the risks mediated by oxytocin for labor augmentation are influenced by factors related to care, such as fetal monitoring and administration practices; therefore, inconsistent findings are not surprising. In fact, it is promising that this study did not find such an association in public hospitals, which suggests that oxytocin augmentation may be safely used in a low-resource setting. What precisely constitutes such safe use, when advanced equipment is not available, is yet to be explored.
Unfortunately, no studies met the inclusion criteria for assessing the influence of oxytocin augmentation on cesarean birth and labor duration. Absence of studies supporting the effect of oxytocin on reducing cesarean birth rates is especially worrying because prolonged labor is the most common indication for first cesarean birth.65 The high frequency of use together with other factors affecting decision to perform cesarean birth possibly explain why studies globally fail to document any effect of oxytocin on cesarean birth rates.1,2,66 Because of the scarcity of evidence, it remains unknown whether reducing the use of oxytocin finally increases or reduces cesarean birth rates.
Strengths and limitations
The main strength of this review is its comprehensive inclusion of studies from LLMICs targeting vulnerable populations, which is currently overlooked in reviews of oxytocin augmentation.1,2,66 Through searching in international and regional databases to ensure that all available data were included, we found studies from 25 out of 79 LLMICs. Although the explorative approach resulted in heterogeneous studies, which hampered generalizability, unfolding the complexity enabled us to identify important gaps in research and practice. An important limitation is confounding by indication, which may bias the results of the meta-analysis. Distinguishing between risks of prolonged labor and risks of oxytocin augmentation is challenging. The studies did not elaborate sufficiently on this. It is important to notice that for women who actually had prolonged labor when oxytocin was administered, it is not possible to distinguish whether the harm was a marker of oxytocin or prolonged labor, or a combination. Some studies, however, reported no differences in prolonged labor between groups.21,24, 25, 26 This may be explained by high levels of use without clear indication or routine use suspected in studies with high oxytocin rates. New studies including women with documented prolonged labor are highly warranted to provide stronger conclusions and guidance for practice.
Some studies did not have a primary objective to investigate oxytocin, thereby increasing the risk of type 1 error because of random findings and publication bias.25, 26, 27 Another limitation is the use of nonvalidated hand-written medical records in some studies, which may have been of poor quality. Lack of quality restrictions in the studies is a central limitation. However, stratifying by quality levels did not change conclusions. Finally, use of oxytocin involves aspects related to timing, titration and duration, manual administration of gravity-fed infusion, frequency of fetal monitoring, administration forms (intravenous/intramuscular bolus injections), and human resources. These factors may be important mediators of increased risks. Many of these factors are particularly pertinent to the context of busy low-resource settings and were often not included, suggesting an important area for future research.
Research implications
Although new medications must pass through multiple testing phases before approval, use of oxytocin for labor augmentation was approved before strong trials were the standard. Postapproval monitoring of medications is now standard; however, oxytocin has not been evaluated in this way, and we continue to use oxytocin for labor augmentation with scarce evidence of effect and data suggesting risks. Although this review provides a starting point, more research is needed to provide insight into such use of oxytocin in low-resource settings. In response, we recommend 3 simultaneous areas of action. Firstly, clear and unambiguous clinical guidelines, adjusted to the context, must be established to assist frontline health providers in LLMICs. As called for by the WHO-INTEGRATE framework, aspects of safety, benefits, health system feasibility, and women's and health providers’ views should inform such guidelines.67 Secondly, because physiological labor involves multiple receptors and biomarkers in addition to oxytocin, such as lactate, embedded studies of the pathophysiology of prolonged labor may foster novel, effective, and safer approaches to diagnosing and treating prolonged labor.68 Last, but not least, the unconducive low-resource clinical realities that women and health providers work in both compound increased harm and seem to drive overuse of oxytocin augmentation in LLMICs. Therefore, broader efforts remain essential to tackle the human resource crisis in health, the increasingly overloaded urban maternity units, suboptimal routine monitoring during labor, and delays in accessing emergency cesarean birth.
Conclusion
Our review discloses great practice variation and high frequency of oxytocin use for labor augmentation. In half of the studies, rates of oxytocin for labor augmentation exceeded 30%. Meanwhile, a recent WHO multicenter study presented that only approximately 15% of labors crossed the partograph's action line. This indicates high levels of use in normally progressing labors and is in line with studies where data on labor progression were available: 89.5% of women augmented with oxytocin did not cross the partograph's action line.
Alarmingly high rates in settings with the poorest resources for childbirth amplify concern for safety. Evidence from these studies suggests that labor augmentation with oxytocin may result in severe adverse outcomes. Importantly, however, the studies had methodological limitations that hamper quantification of confounding by indication. Harmful effects are likely mediated through suboptimal quality of care in the busy low-resource context, including lack of electronic drip count, intermittent rather than continuous FHR monitoring, and lack of electronic contraction monitoring. Robust implementation research in real-world low-resourced labor wards is warranted to bridge the gap between universal guidelines and clinical realities.69 Finally, we urge judicious use of oxytocin on clear indications (such as crossing the partograph's action line) while calling for prioritization of safe childbirth care, particularly where most of the world's preventable deaths occur.
Footnotes
Received June 30, 2022; revised September 29, 2022; accepted October 13, 2022.The authors report no conflict of interest.
Funding was provided by Laerdal Global Health, Danida Fellowship Centre, and Bodil Pedersen Fonden. The funding sources had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Patient consent was not required because no personal information or details were included.
Registered in the International Prospective Register of Systematic Reviews (PROSPERO) on April 7, 2021. Registration number: CRD42020219821.
An abstract with preliminary findings was presented online at the 12th European Congress on Tropical Medicine and International Health, Bergen, Norway, September 27, 2021.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.xagr.2022.100123.
Appendix. Supplementary materials
References
- 1.Bugg GJ, Siddiqui F, Thornton JG. Oxytocin versus no treatment or delayed treatment for slow progress in the first stage of spontaneous labour. Cochrane Database Syst Rev. 2013;6 doi: 10.1002/14651858.CD007123.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei S, Wo BL, Qi H, et al. Early amniotomy and early oxytocin for prevention of, or therapy for, delay in first stage spontaneous labour compared with routine care. Cochrane Database Syst Rev. 2013;8 doi: 10.1002/14651858.CD006794.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simpson KR, Knox GE. Oxytocin as a high-alert medication: implications for perinatal patient safety. MCN Am J Matern Child Nurs. 2009;34:8–15. doi: 10.1097/01.NMC.0000343859.62828.ee. [DOI] [PubMed] [Google Scholar]
- 4.Bakker PC, Kurver PH, Kuik DJ, van Geijn HP. Elevated uterine activity increases the risk of fetal acidosis at birth. Am J Obstet Gynecol. 2007;196 doi: 10.1016/j.ajog.2006.11.035. 313.e1–6. [DOI] [PubMed] [Google Scholar]
- 5.Hirayama T, Hiraoka Y, Kitamura E, et al. Oxytocin induced labor causes region and sex-specific transient oligodendrocyte cell death in neonatal mouse brain. J Obstet Gynaecol Res. 2020;46:66–78. doi: 10.1111/jog.14149. [DOI] [PubMed] [Google Scholar]
- 6.Jonsson M, Nordén-Lindeberg S, Ostlund I, Hanson U. Acidemia at birth, related to obstetric characteristics and to oxytocin use, during the last two hours of labor. Acta Obstet Gynecol Scand. 2008;87:745–750. doi: 10.1080/00016340802220352. [DOI] [PubMed] [Google Scholar]
- 7.Milsom I, Ladfors L, Thiringer K, Niklasson A, Odeback A, Thornberg E. Influence of maternal, obstetric and fetal risk factors on the prevalence of birth asphyxia at term in a Swedish urban population. Acta Obstet Gynecol Scand. 2002;81:909–917. doi: 10.1034/j.1600-0412.2002.811003.x. [DOI] [PubMed] [Google Scholar]
- 8.Burgod C, Pant S, Morales MM, et al. Effect of intra-partum oxytocin on neonatal encephalopathy: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2021;21:736. doi: 10.1186/s12884-021-04216-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovold A, Stanton C, Armbruster D. How to avoid iatrogenic morbidity and mortality while increasing availability of oxytocin and misoprostol for PPH prevention? Int J Gynaecol Obstet. 2008;103:276–282. doi: 10.1016/j.ijgo.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Miller S, Abalos E, Chamillard M, et al. Beyond too little, too late and too much, too soon: a pathway towards evidence-based, respectful maternity care worldwide. Lancet. 2016;388:2176–2192. doi: 10.1016/S0140-6736(16)31472-6. [DOI] [PubMed] [Google Scholar]
- 11.Souza JP, Oladapo OT, Fawole B, et al. Cervical dilatation over time is a poor predictor of severe adverse birth outcomes: a diagnostic accuracy study. BJOG. 2018;125:991–1000. doi: 10.1111/1471-0528.15205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daly D, Minnie KCS, Blignaut A, et al. How much synthetic oxytocin is infused during labour? A review and analysis of regimens used in 12 countries. PLoS One. 2020;15 doi: 10.1371/journal.pone.0227941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oladapo OT, Souza JP, Fawole B, et al. Progression of the first stage of spontaneous labour: a prospective cohort study in two sub-Saharan African countries. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. WHO recommendations. Intrapartum care for a positive childbirth experience. 2018. Available at: https://apps.who.int/iris/bitstream/handle/10665/260178/9789241550215-eng.pdf. Accessed November, 05, 2022. [PubMed]
- 15.Review Manager (RevMan) (computer program). The Cochrane Collaboration 2020. Version 5.4.1.
- 16.Azandegbé N, Testa J, Makoutodé M. Assessment of partogram utilisation in Benin. Sante. 2004;14:251–255. [PubMed] [Google Scholar]
- 17.Kalisa R, Rulisa S, van den Akker T, van Roosmalen J. Is prolonged labor managed adequately in rural Rwandan hospitals? Afr J Reprod Health. 2019;23:27–34. doi: 10.29063/ajrh2019/v23i2.3. [DOI] [PubMed] [Google Scholar]
- 18.Penumadu K, Hariharan C. Role of partogram in the management of spontaneous labour in primigravida and multigravida. Int J Reprod Contracept Obstet Gynecol. 2014;3:1043–1049. [Google Scholar]
- 19.Shah N, Maitra N, Pagi SL. Evaluating role of parity in progress of labour and its outcome using modified WHO partograph. Int J Reprod Contracept Obstet Gynecol. 2017;5:860–863. [Google Scholar]
- 20.Marx Delaney M, Kalita T, Hawrusik B, et al. Modification of oxytocin use through a coaching-based intervention based on the WHO Safe Childbirth Checklist in Uttar Pradesh, India: a secondary analysis of a cluster randomised controlled trial. BJOG. 2021;128:2013–2021. doi: 10.1111/1471-0528.16856. [DOI] [PubMed] [Google Scholar]
- 21.Litorp H, Sunny AK, Kc A. Augmentation of labor with oxytocin and its association with delivery outcomes: A large-scale cohort study in 12 public hospitals in Nepal. Acta Obstet Gynecol Scand. 2021;100:684–693. doi: 10.1111/aogs.13919. [DOI] [PubMed] [Google Scholar]
- 22.Dujardin B, Boutsen M, de Schampheleire I, et al. Oxytocics in developing countries. Int J Gynaecol Obstet. 1995;50:243–251. doi: 10.1016/0020-7292(95)02389-t. [DOI] [PubMed] [Google Scholar]
- 23.Mola G, Rageau O. Augmentation of labour by a standard protocol in Papua New Guinea. Asia Oceania J Obstet Gynaecol. 1990;16:219–224. doi: 10.1111/j.1447-0756.1990.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 24.Brahmawar Mohan SB, Sommerfelt H, Frøen JF, et al. Antenatal uterotonics as a risk factor for intrapartum stillbirth and first-day death in Haryana, India: a nested case-control study. Epidemiology. 2020;31:668–676. doi: 10.1097/EDE.0000000000001224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis M, Manandhar N, Manandhar DS, Costello AM. Risk factors for neonatal encephalopathy in Kathmandu, Nepal, a developing country: unmatched case-control study. BMJ. 2000;320:1229–1236. doi: 10.1136/bmj.320.7244.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tann CJ, Nakakeeto M, Willey BA, et al. Perinatal risk factors for neonatal encephalopathy: an unmatched case-control study. Arch Dis Child Fetal Neonatal Ed. 2018;103:F250–F256. doi: 10.1136/archdischild-2017-312744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maaløe N, Housseine N, Bygbjerg IC, et al. Stillbirths and quality of care during labour at the low resource referral hospital of Zanzibar: a case-control study. BMC Pregnancy Childbirth. 2016;16:351. doi: 10.1186/s12884-016-1142-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Onyearugha C, Ugboma H. Severe birth asphyxia: risk factors as seen in a tertiary institution in the Niger Delta area of Nigeria. Cont J Trop Med. 2010;4:11–19. [Google Scholar]
- 29.Hailu G, Kibret Y, Angaw K. Determinants of birth-asphyxia among newborns in Dessie Town hospitals, north-central Ethiopia. Int J Sex Heal Reprod Health Care. 2018;1:1–12. [Google Scholar]
- 30.Geelhoed D, Stokx J, Mariano X, Mosse Lázaro CM, Roelens K. Risk factors for stillbirths in Tete, Mozambique. Int J Gynecol Obstet. 2015;130:148–152. doi: 10.1016/j.ijgo.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Delvaux T, Aké-Tano O, Va Gohou-Kouassi, Bosso P, Collin S, Ronsmans C. Quality of normal delivery care in Côte d'Ivoire. Afr J Reprod Health. 2007;11:22–32. [PubMed] [Google Scholar]
- 32.Mukamurigo J, Dencker A, Nyirazinyoye L, Ntaganira J, Berg M. Quality of intrapartum care for healthy women with spontaneous onset of labour in Rwanda: a health facility-based, cross-sectional study. Sex Reprod Healthc. 2019;19:78–83. doi: 10.1016/j.srhc.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 33.Maaløe N, Housseine N, Meguid T, et al. Effect of locally tailored labour management guidelines on intrahospital stillbirths and birth asphyxia at the referral hospital of Zanzibar: a quasi-experimental pre-post study (The PartoMa study) BJOG. 2018;125:235–245. doi: 10.1111/1471-0528.14933. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen BL, Rasch V, Massawe S, Nyakina J, Elsass P, Nielsen BB. Impact of ALSO training on the management of prolonged labor and neonatal care at Kagera Regional Hospital, Tanzania. Int J Gynaecol Obstet. 2010;111:8–12. doi: 10.1016/j.ijgo.2010.04.031. [DOI] [PubMed] [Google Scholar]
- 35.Khalil K, Cherine M, Elnoury A, Sholkamy H, Breebaart M, Hassanein N. Labor augmentation in an Egyptian teaching hospital. Int J Gynaecol Obstet. 2004;85:74–80. doi: 10.1016/S0020-7292(03)00311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agha S, Fitzgerald L, Fareed A, et al. Quality of labor and birth care in Sindh Province, Pakistan: findings from direct observations at health facilities. PLoS One. 2019;14 doi: 10.1371/journal.pone.0223701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stanton CK, Deepak NN, Mallapur AA, et al. Direct observation of uterotonic drug use at public health facility-based deliveries in four districts in India. Int J Gynecol Obstet. 2014;127:25–30. doi: 10.1016/j.ijgo.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Singh S, Kashyap JA, Chandhiok N, et al. Labour & delivery monitoring patterns in facility births across five districts of India: a cross-sectional observational study. Indian J Med Res. 2018;148:309–316. doi: 10.4103/ijmr.IJMR_103_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iyengar SD, Iyengar K, Suhalka V, Agarwal K. Comparison of domiciliary and institutional delivery-care practices in rural Rajasthan. India. J Health Popul Nutr. 2009;27:303–312. doi: 10.3329/jhpn.v27i2.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spitzer RF, Steele SJ, Caloia D, et al. One-year evaluation of the impact of an emergency obstetric and neonatal care training program in Western Kenya. Int J Gynaecol Obstet. 2014;127:189–193. doi: 10.1016/j.ijgo.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 41.Muylder X de, Thiery M. The cesarean delivery rate can be safely reduced in a developing country. Obstet Gynecol. 1990;75:360–364. [PubMed] [Google Scholar]
- 42.van Roosmalen J. Perinatal mortality in rural Tanzania. Br J Obstet Gynaecol. 1989;96:827–834. doi: 10.1111/j.1471-0528.1989.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 43.van Roosmalen J, Brand R. Maternal height and the outcome of labor in rural Tanzania. Int J Gynaecol Obstet. 1992;37:169–177. doi: 10.1016/0020-7292(92)90377-u. [DOI] [PubMed] [Google Scholar]
- 44.Onah HE, Obi SN, Oguanuo TC, Ezike HA, Ogbuokiri CM, Ezugworie JO. Pain perception among parturients in Enugu, South-Eastern Nigeria. J Obstet Gynaecol. 2007;27:585–588. doi: 10.1080/01443610701467937. [DOI] [PubMed] [Google Scholar]
- 45.Frega A, Puzio G, Maniglio P, et al. Obstetric and neonatal outcomes of women with FGM I and II in San Camillo Hospital, Burkina Faso. Arch Gynecol Obstet. 2013;288:513–519. doi: 10.1007/s00404-013-2779-y. [DOI] [PubMed] [Google Scholar]
- 46.Obel J, Martin AIC, Mullahzada AW, Kremer R, Maaløe N. Resilience to maintain quality of intrapartum care in war torn Yemen: a retrospective pre-post study evaluating effects of changing birth volumes in a congested frontline hospital. BMC Pregnancy Childbirth. 2021;21:36. doi: 10.1186/s12884-020-03507-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hassan SJ, Sundby J, Husseini A, Bjertness E. Translating evidence into practice in childbirth: a case from the Occupied Palestinian Territory. Women Birth. 2013;26:e82–e89. doi: 10.1016/j.wombi.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 48.Sharma R, Bhojwani P, Meena P, Mathur S. Does admission in labour room during latent phase of labour versus active phase really matters in low risk women presenting at term? A cross-sectional observational study. 2016. Available at: https://aimdrjournal.com/wp-content/uploads/2021/08/OG1_OA_Raksha.pdf. Accessed November, 05, 2022.
- 49.Maimbolwa MC, Ransjö-Arvidson AB, Ng'andu N, Sikazwe N, Diwan VK. Routine care of women experiencing normal deliveries in Zambian maternity wards: a pilot study. Midwifery. 1997;13:125–131. doi: 10.1016/s0266-6138(97)90002-4. [DOI] [PubMed] [Google Scholar]
- 50.Janna JR, Chowdhury SB. Impact of timing of admission in labour on subsequent outcome. Comm Based Med J. 2013;2:21–28. [Google Scholar]
- 51.Rana TG, Rajopadhyaya R, Bajracharya B, Karmacharya M, Osrin D. Comparison of midwifery-led and consultant-led maternity care for low risk deliveries in Nepal. Health Policy Plan. 2003;18:330–337. doi: 10.1093/heapol/czg039. [DOI] [PubMed] [Google Scholar]
- 52.Ijaiya MA, Adesina KT, Raji HO, et al. Duration of labor with spontaneous onset at the University of Ilorin Teaching Hospital, Ilorin, Nigeria. Ann Afr Med. 2011;10:115–119. doi: 10.4103/1596-3519.82074. [DOI] [PubMed] [Google Scholar]
- 53.Bood T. Experience with an active labour management protocol and reduction of caesarean section rate in Nicaragua. Trop Doct. 1990;20:115–118. doi: 10.1177/004947559002000309. [DOI] [PubMed] [Google Scholar]
- 54.Munan R, Kakudji Y, Nsambi J, et al. Childbirth among primiparous women in Lubumbashi: maternal and perinatal prognosis. Pan Afr Med J. 2017;28:77. doi: 10.11604/pamj.2017.28.77.13712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Clark SL, Simpson KR, Knox GE, Garite TJ. Oxytocin: new perspectives on an old drug. Am J Obstet Gynecol. 2009;200 doi: 10.1016/j.ajog.2008.06.010. 35.e1–6. [DOI] [PubMed] [Google Scholar]
- 56.Selin L, Almström E, Wallin G, Berg M. Use and abuse of oxytocin for augmentation of labor. Acta Obstet Gynecol Scand. 2009;88:1352–1357. doi: 10.3109/00016340903358812. [DOI] [PubMed] [Google Scholar]
- 57.Dalbye R, Bernitz S, Olsen IC, et al. The Labor Progression Study: the use of oxytocin augmentation during labor following Zhang's guideline and the WHO partograph in a cluster randomized trial. Acta Obstet Gynecol Scand. 2019;98:1187–1194. doi: 10.1111/aogs.13629. [DOI] [PubMed] [Google Scholar]
- 58.World Health Organization. WHO labour Care Guide: User's manual. 2020. Available at: https://apps.who.int/iris/bitstream/handle/10665/337693/9789240017566-eng.pdf. Accessed November, 05, 2022.
- 59.Oladapo OT, Diaz V, Bonet M, et al. Cervical dilatation patterns of “low-risk” women with spontaneous labour and normal perinatal outcomes: a systematic review. BJOG. 2018;125:944–954. doi: 10.1111/1471-0528.14930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.World health organization partograph in management of labour World Health Organization Maternal Health and Safe Motherhood Programme. Lancet. 1994;343:1399–1404. [PubMed] [Google Scholar]
- 61.World Health Organization. WHO recommendations for augmentation of labour. 2014. Available at: https://apps.who.int/iris/bitstream/handle/10665/112825/9789241507363_eng.pdf?sequence=1. Accessed November, 05, 2022. [PubMed]
- 62.Oscarsson ME, Amer-Wåhlin I, Rydhstroem H, Källén K. Outcome in obstetric care related to oxytocin use. A population-based study. Acta Obstet Gynecol Scand. 2006;85:1094–1098. doi: 10.1080/00016340600804530. [DOI] [PubMed] [Google Scholar]
- 63.Hayes BC, McGarvey C, Mulvany S, et al. A case-control study of hypoxic-ischemic encephalopathy in newborn infants at >36 weeks gestation. Am J Obstet Gynecol. 2013;209 doi: 10.1016/j.ajog.2013.03.023. 29.e1–19. [DOI] [PubMed] [Google Scholar]
- 64.Peven K, Day LT, Ruysen H, et al. Stillbirths including intrapartum timing: EN-BIRTH multi-country validation study. BMC Pregnancy Childbirth. 2021;21(Suppl1):226. doi: 10.1186/s12884-020-03238-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sobhy S, Arroyo-Manzano D, Murugesu N, et al. Maternal and perinatal mortality and complications associated with caesarean section in low-income and middle-income countries: a systematic review and meta-analysis. Lancet. 2019;393:1973–1982. doi: 10.1016/S0140-6736(18)32386-9. [DOI] [PubMed] [Google Scholar]
- 66.Budden A, Chen LJY, Henry A. High-dose versus low-dose oxytocin infusion regimens for induction of labour at term. Cochrane Database Syst Rev. 2014;10 doi: 10.1002/14651858.CD009701.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rehfuess EA, Stratil JM, Scheel IB, Portela A, Norris SL, Baltussen R. The WHO-INTEGRATE evidence to decision framework version 1.0: integrating WHO norms and values and a complexity perspective. BMJ Glob Health. 2019;4(Suppl1) doi: 10.1136/bmjgh-2018-000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wray S, Alruwaili M, Prendergast C. Hypoxia and reproductive health: hypoxia and labour. Reproduction. 2021;161:F67–F80. doi: 10.1530/REP-20-0327. [DOI] [PubMed] [Google Scholar]
- 69.Maaløe N, Ørtved AMR, Sørensen JB, et al. The injustice of unfit clinical practice guidelines in low-resource realities. The Lancet Global Health. 2021;9:e875–e879. doi: 10.1016/S2214-109X(21)00059-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.