Abstract
Main conclusion
Cytochorme P450s (CYPs) play a critical role in the catalysis of secondary metabolite biosynthetic pathways. For their commercial use, various strategies for metabolic pathway engineering using CYP as a potential target have been explored.
Abstract
Plants produce a vast diversity of secondary metabolites which are being used to treat various ailments and diseases. Some of these metabolites are difficult to obtain in large quantities limiting their industrial use. Cytochrome P450 enzymes (CYPs) are important catalysts in the biosynthesis of highly valued secondary metabolites, and are found in all domains of life. With the development of high-throughput sequencing and high-resolution mass spectrometry, new biosynthetic pathways and associated CYPs are being identified. In this review, we present CYPs identified from medicinal plants as a potential game changer in the metabolic engineering of secondary metabolic pathways. We present the achievements made so far in enhancing the production of important bioactivities through pathway engineering, giving some popular examples. At last, current challenges and possible strategies to overcome the limitations associated with CYP engineering to enhance the biosynthesis of target secondary metabolites are also highlighted.
Keywords: Cytochrome P450, CYPs, Secondary metabolites, Medicinal plants, Metabolic pathway engineering
Introduction
Plants synthesize a rich diversity of heterogeneous secondary metabolites that affect their development and naturally provide protection against a variety of pests, pathogens, and unfavorable environment (Dhar et al. 2015). Medicinal plants produce a variety of secondary metabolites such as phenolics, terpenes, steroids, alkaloids, and flavonoids, and have been proved useful in Unani, Ayurveda, Siddha, and Chinese medicine since time immemorial (Malhotra et al. 2001). Even today, these plants and their extracts are being used as an alternative treatment for many ailments, including diabetes, fibrosis, oxidative stress, inflammation, cardiovascular disorders, COVID-19, Alzheimer’s disease, and cancer (Crozier et al. 2008; Shree et al. 2022). Along with this, secondary metabolites are known for their rejuvenating, health-promoting, and immune system boosting properties. As per WHO reports, over 80% of the world’s population dwells upon herbs and derived products for primary healthcare needs. The major benefit of herbal drugs is attributed to their multiple benefits with minimal side effects. Moreover, the application of secondary metabolites for developing semi-synthetic and synthetic drugs for treating inflammatory bowel diseases, malaria, cardiovascular disorders, cancer, and others is well evident (Seca and Pinto 2019). However, season dependence, low yield, and high extraction cost of therapeutic compounds pose the biggest challenge for the utilization of medicinal plants in herbal industries (Renault et al. 2014). Additionally, reckless harvesting and habitat destruction of medicinally important species have made several of them endangered. Strategies such as breeding to develop high yielding varieties take years to get a stable phenotype. Furthermore, due to their complex biosynthetic pathways, synthetic production of secondary metabolites is technically challenging and also costly.
Metabolic pathway engineering utilizes various strategies such as the introduction of a biosynthetic gene, transcription factor or precursor feeding, overexpression of rate-limiting enzymes, or silencing or deletion of alternate branch pathway genes to shift the metabolic flux toward the desired pathway to enhance the production of rare metabolites for commercial use (Kulkarni 2016). A Nobel prize-winning research on Artemisia annua is a great example of using metabolic engineering for the production of a pharmaceutically important compound, Artemisinin as an antimalarial drug. Several strategies such as overexpression, suppression, and global regulation of genes were used to get the desired Artemisinin yield (Lu et al. 2016).
CYPs belong to the monooxygenase superfamily of proteins found in all domains of life including plants, and are critical players in the production of some of the highly valued secondary metabolites. They contain a heme prosthetic group (Fe-protoporphyrin IX) with anionic, thiolate sulfur of a cysteine residue ligating to the heme iron (Fig. 1) (Lamb and Waterman 2013; Xu et al. 2015). The cofactor heme plays an important role in catalysis, and when bound to carbon monoxide produces a spectrum of wavelength at 450 nm; hence, CYPs are also called P450s. The thiolate bond associated with the formation of Fe (IV) oxo species (compound I), a highly reactive intermediate which is involved in oxidizing inactivated C–H bonds, is indispensable in the CYP catalytic cycle (Rittle and Green 2010). All CYPs share a common mechanism of reduction and oxidation, wherein these reductases are involved in the transfer of two electrons from NADPH to the substrate, and then the addition of one oxygen to the substrate and the other one yields water (Xu et al. 2015). Other than this, CYPs are also involved in hydroxylation, dealkylation, dimerization, and isomerization reactions. Due to their ability to catalyze diverse reactions, they are found to be involved in a variety of biological pathways, including antioxidant biosynthesis, fatty acid metabolism, defence, secondary metabolite biosynthesis, hormonal regulations, and metabolism of xenobiotic substances (Pandian et al. 2020). Next-generation sequencing has facilitated identification of over 30,000 CYPs in various domains of life and their functional role (Pandian et al. 2020). Due to their diverse catalytic functions (mentioned above), they have been used as a prime candidate for biotechnological interventions and metabolite engineering of secondary metabolite biosynthesis (Villa-Ruano et al. 2015; Rasool and Mohamed 2016). In this review, a detailed account on the emergence of CYPs as a game changer in metabolic engineering in medicinal plants along with the challenges and forthcoming possibilities are being discussed.
Fig. 1.
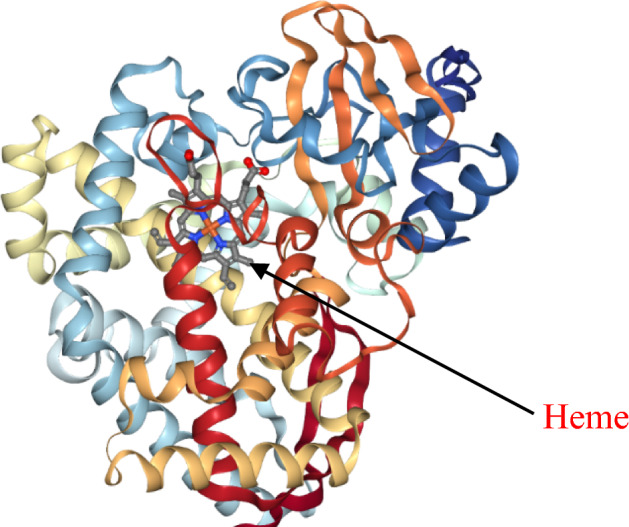
A representative ribbon structure of Arabidopsis thaliana allene oxide synthase (CYP74A) protein along with heme cofactor (PDB entry: 3CLI; Lee et al. 2008)
General mechanism of CYP catalysis
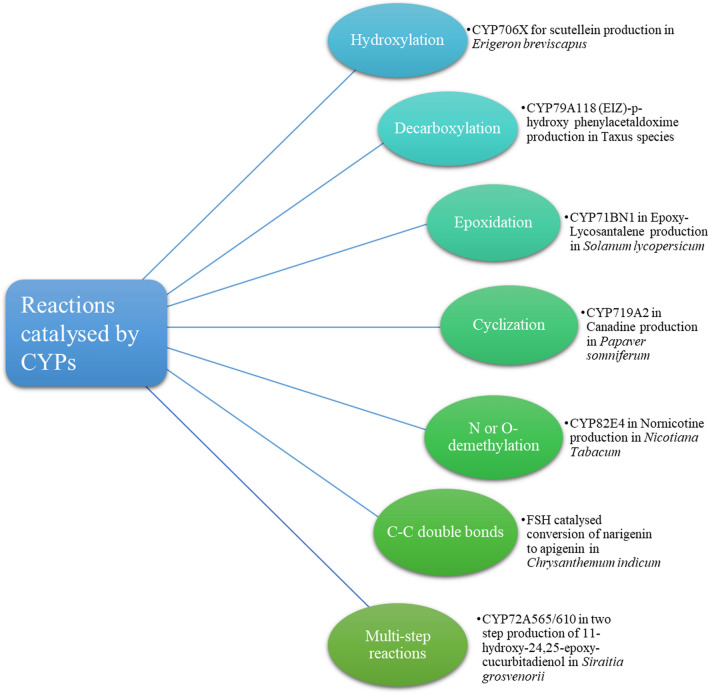
CYPs are known to catalyze a wide variety of complex biochemical reactions such as aryl–aryl coupling, ring contractions and expansions, S-dealkylations, N-dealkylations, and O-dealkylations, decarboxylation, oxidative cyclization, alcohol and aldehyde oxidation, desaturation, sulfoxidation, nitrogen oxidation, epoxidation, C–C bond scission, decarbonylation, and nitration (Fig. 2) (McIntosh et al. 2014; Rasool and Mohamed 2016).
Fig. 2.
Some of the important reactions catalyzed by CYPs in medicinal plants.
Adapted from Rasool and Mohamed (2016)
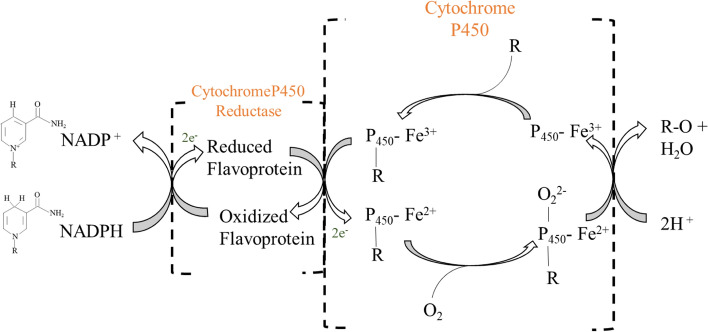
Mostly CYPs are found to be localized at the cytoplasmic surface of the endoplasmic reticulum along with the presence of cytochrome P450 reductase (CPR) for their proper functioning (Li et al. 2019a). They show high regio- and stereo-selectivity during catalysation steps in the biosynthesis of bioactive compounds (Rasool and Mohamed 2016). The presence of a nonpolar active site and high conformational flexibility, makes them a perfect catalyst in the biosynthesis of pharmaceutically important natural products (Jensen and Møller 2010). Reduced pyridine nucleotides (NAD(P)H) act as an electron source for its catalytic function which is assisted by auxiliary redox partner proteins. Generally, these redox partners can be majorly divided into three classes: Fe-containing ferredoxin (Fdx) and a flavin adenine dinucleotide (FAD)-containing ferredoxin reductase (FDR) involved in prokaryotic CYPs reduction; a membrane-bound flavin mononucleotide (FMN)/FAD with NADPH CYP oxidoreductase involved in eukaryotic CYPs; and adrenodoxin (Adx) and adrenodoxin reductase (ADR) involved in mitochondrial CYPs (Lamb and Waterman 2013). The basic mechanism of catalysis of CYP involves firstly binding of organic substrate (R) to the heme group of the enzyme (Fig. 3). This induces electron transfer from NADPH through FAD and FMN domains which leads to the reduction of then heme ferric iron (Fe3+) to the ferrous state (Fe2+). Then, ferrous CYPs get converted to ferrous CYP–dioxygen complex as a result of molecular oxygen binding which leads to the transfer of a second electron from CPR or any other associated reductase. This results in the formation of a short-lived peroxo complex that rapidly gets protonated twice, leading to the formation of one molecule of water and an iron–oxo complex. Lastly, the oxygen atom in the complex binds to the organic substrate (R) and forms the oxidized reaction product (RO) (Pandian et al. 2020).
Fig. 3.
A general mechanism of CYP catalysis.
Adapted from Kalra (2007)
The most important interacting partners for efficient catalysis by CYPs are reductases. Along with this, a conserved active site and axial cysteine ligating the heme iron are involved in the production of other key intermediates. The threonine (T268) promotes the scission of dioxygen bond by protonating the iron-peroxo and iron-hydroperoxy intermediates with the help of water. The thiol bond is also involved in the promotion of dioxygen bond scission of iron-hydroperoxy intermediates. It also makes ferric ion a poorer electron acceptor which prevents the catalytic cycle to start without the presence of a substrate (McIntosh et al. 2014).
CYPs in the production of medicinally important secondary metabolites
A large variety of natural products derived from medicinal plants have been used as traditional medicine since time immemorial (Dhar et al. 2015). For instance, vinblastine, vincristine, vindesine, and vinorelbine isolated from Catharanthus roseus, have been used in chemotherapy. Similarly, the role of other medicinal plants such as Artemisia annua (artemisinin) in malaria, Papaver somniferum (morphine, codeine, papaverine, noscapine) in pain, cough, and cramps, Physostigma venenosum (physostigmine) in Alzheimer’s disease, has been reported (Bhattacharjee 2000). Ayurveda, Siddha, Homeopathy, Unani, and Traditional Chinese Medicine (TCM) are the alternative system of medicine that make use of natural products to treat different ailments (Malhotra et al. 2001). Today, procedures like chromatography have made it possible to analyze and understand the secondary metabolite composition of herbal extracts (Singh 2003). These secondary metabolites comprises of majorly flavonoids, terpenoids, phenylpropanoids, alkaloids, steroids, cyanogenic glycosides, and glucosinolates are known to have a variety of biological and medicinal properties (Villa-Ruano et al. 2015; Xu et al. 2015). Due to their functionally diverse roles, CYPs are considered as the most versatile biological catalysts in nature (Rana et al. 2014). The role of various CYPs in plants used in medicines is given in Table 1.
Table 1.
A few examples of CYPs involved in the secondary metabolic pathway of plants used in medicine
| CYP | Role | Plant species | References |
|---|---|---|---|
| CYP88D6 | Licorice β-amyrin 11-oxidase involved in the biosynthesis of the triterpene sweetener glycyrrhizin | Glycyrrhiza spp. | Seki et al. (2008) |
| CYP71AU87 | Marrubiin biosynthesis | Marrubium vulgare | Karunanithi et al. (2019) |
| CYP76B6 | Iridoid monoterpenoids biosynthesis | Catharanthus roseus | Collu et al. (2001) |
| CYP72A1 | Converts loganin into secologanin | Catharanthus roseus | Irmler et al. (2000) |
| CYP706X | Hydroxylation of Apigenin for the production of Scutellein | Erigeron breviscapus | Liu et al. (2018) |
| CYP76AD1/6 | Hydroxylation of Tyrosin for the production of L-DOPA, the precursor of dopamine | Beta vulgaris and Mirabilis jalapa | Polturak et al. (2016) |
| CYP73A | Encodes Cinnamic acid 4-hydroxylase (CA4H) which is involved in phenylpropanoid biosynthesis | Populus kitakamiensis | Kawai et al. (1996) |
| CYP75 | A hydroxylating enzyme involved in flavonoid biosynthesis | Solanum melongena | Toguri et al. (1993) |
| CYP82D | Flavone-6-hydroxylase and 7-demethylase involved in flavonoid metabolism | Ocimum basilicum | Berim and Gang (2013) |
| CYP93B1 | Involved in hydroxylation of flavones at C-2 position | Glycyrrhiza echinata | Akashi et al. (1998) |
| CYP71D | Encodes flavonoid 6-hydroxylase that catalyzes the conversion of flavanones | Glycine max | Latunde-Dada et al. (2001) |
| CYP719 | Biosynthesis of berberine | Coptis japonica | Ikezawa et al. (2003) |
| CYP80F1 | Rearrangement of (R)-littorine to (S)-hyoscyamine, | Hyoscyamus niger | Li et al., (2006) |
| CYP716A | Hydroxylation of steroidal saponin precursor cycloartenol | Aquilegia coerulea | Miettinen et al. (2017) |
| CYP706C55 | Conversion of phenyl acetaldoxime into phenyl acetonitrile in prunasin biosynthesis | Eucalyptus cladocalyx | Hansen et al. (2018) |
| CYP71E1 | Catalyze the conversion of p-hydroxyphenylacetaldoxime to p-hydroxymandelonitrile | Sorghum bicolor | Kahn et al. (1999) |
| CYP71D18 | Encodes spearmint limonene 6-hydroxylase that hydroxylates (4S)-limonene to (-)-trans-carveol | Mentha spicata | Wust et al. (2001) |
| CYP725A3 | Encodes for taxoid 14 beta-hydroxylase involved in the biosynthesis of taxoid | Taxus cuspidata | Jennewein et al. (2003) |
| CYP76AH1 | Catalyzes the conversion of miltiradiene to tanshinones | Salvia miltiorrhiza | Guo et al. (2013) |
| CYP84A1 | Involved in the biosynthesis of phenylpropanoid by transforms coniferaldehyde and coniferyl alcohol into 5-hydroxylated derivatives | Dendrobium officinale | Humphreys et al. (1999) |
| CYP716A244 | Involved in oleanane-type saponin biosynthesis from 2,3-oxidosqualene | Eleutherococcus senticosus | Jo et al. (2017) |
| CYP72A154 | Involved in C-30 oxidation in the glycyrrhizin biosynthesis pathway | Glycyrrhiza glabra | Seki et al. (2011) |
| CYP722C | Catalyzes the conversion of carlactonoic acid to 5-deoxystrigol | Gossypium arboreum | Wakabayashi et al. (2020) |
| CYP87D18 | Involved in C-11 oxidation of cucurbitadienol | Siraitia grosvenorii | Zhang et al., (2016) |
CYP: a promising target for metabolic engineering
Previous studies imply that CYPs are highly evolvable and adaptable to the mutations in their active site that makes them accept novel substrates readily (Jung et al. 2011). New specificities of CYPs are exhibited by the forceful molding of protein sequences which in turn are filtered by natural selection to favor the formation of certain intermediates (McIntosh et al. 2014). This enables the CYPs to generate valuable natural and non-natural products, and the synthesize the precursors of important metabolites from medicinal plants (Jung et al. 2011; Li et al. 2020). The application of CYPs in metabolic engineering has greatly facilitated the field of synthetic biology.
CYPs are considered to be the prime candidate for silencing of competitive pathways, optimizing the metabolic fluxes toward desired pathway, heterologous expression of desired metabolite, increasing enzyme–host compatibility and others (Li et al. 2020; Liu et al. 2020). There are several approaches used for CYP engineering such as gene overexpression, suppression, functional and structural manipulation by integration of computational designing of protein, evolutionary information related to that protein followed by optimization of the experimental data for alteration of the specificity of the substrate, and also integrated protein, substrate, and cofactor engineering (Li et al. 2019a). Some traditional methods of metabolic engineering using CYPs in plants for the discovery and regulation of pharmaceutically important metabolites and genes involved in their production (Lu et al. 2016) are discussed below:
(i) Overexpression of pathway genes: Overexpression of a single gene or co-expression of multiple genes of the target pathway is the most commonly used technique for enhancing the production of the desired metabolite. For instance, overexpression of CYP716A52v2, a key gene in the ginsenoside biosynthesis pathway showed enhanced production of oleanane-type ginsenoside in Panax ginseng plants (Han et al. 2013). Combined overexpression of genes involved in artemisinin biosynthesis ADS (Amorpha-4,11-diene synthase gene), CYP71AV1 and CPR promoted the accumulation of artemisinin in A. annua up to 2.4-fold higher than the controlled plant (Lu et al. 2013, 2016).
(ii) Downregulating competing metabolic pathways: Cassava (Manihot esculenta) is one of the main staple foods in some countries but it contains cyanogenic glucosides in its tuber which makes it toxic. CYP79D1 and CYP79D2 are two genes which are responsible for catalyzing the first few committed reactions in the biosynthesis of lotaustralin and linamarin. In the transgenic cassava, the downregulation of these enzymes leads to a significant decrease (by up to 92%) in cyanogenic glucoside production in its tuber (Jørgensen et al. 2005).
(iii) Global pathway regulation by regulating transcription factors, endogenous phytohormones, and primary metabolism: Overexpression of a transcription factor, i.e., AabZIP1 which belongs to the basic leucine zipper family leads to the upregulation of ADS and CYP71AV1. It also increases the artemisinin content by 1.5-fold in transgenic A. annua plants (Zhang et al. 2015). Jasmonic acid is also important in artemisinin biosynthesis. Overexpression of the allene oxide cyclase gene from A. annua leads to increase levels of endogenous jasmonate which further leads to upregulation of FDS, CYP71AV1 and DBR2, which resulted in a significantly increased production of artemisinin, DHAA and artemisinic acid (Lu et al. 2014, 2016).
(iv) Heterologous expression of genes: Heterologous expression of a gene can be observed in plant or a microbial host. CYP76B1 from Helianthus tuberosus catalyzes rapid oxidative dealkylation of various phenyl urea herbicides. Ectopic constitutive expression of CYP76B1 in tobacco (Nicotiana tabacum) and Arabidopsis showed a high tolerance against herbicides (Didierjean et al. 2002). Microbial systems can also be considered as a good heterologous host due to several factors such as their fast-doubling time, easy scalability, easy extraction of product, and cost-effectiveness. For example, the expression of CYP716A12 from M. truncatula, together with the bAS and CPR from Lotus japonicus, resulted in the production of oleanolic acid in yeast (Fukushima et al. 2011; Moses et al. 2013). When expressed in a heterologous host, for the enhancement of CYPs catalytic activity, the membrane-bound domains can also be abridged (Xiao et al. 2019).
Successful metabolic engineering of CYP utilizes one or more of these techniques for obtaining high productivity of desired metabolites. For instance, two opioid drugs were completely biosynthesized in a heterologous host (S. cerevisiae) using CYP SalSyn from Papaver somniferum. This engineered SalSyn showing overactivity of salutaridine, when co-expressed with 21/23 heterologous enzymes and two native enzymes with one native yeast gene deleted, resulted in the production of thebaine/hydrocodone (Galanie et al. 2015). A CYP protopanaxadiol synthase from Panax ginseng was designed in an engineered S. cerevisiae strain. When it was co-expressed with AtCPR, it resulted in the production of 1400 mg/liter of protopanaxadiol (Li et al. 2020; Wei et al. 2018; Zhao et al. 2016).
The newer methodologies are focusing more on protein engineering for enhanced metabolite flux which includes techniques such as directed evolution, rational structurally guided mutation, and random approaches like error-prone PCR, terminal modification, integrated approach combining computational protein design, evolutionary information, and experimental data-driven optimization (Caswell et al. 2013; Li et al. 2019a; Xiao et al. 2019). Directed evolution is a method which is used for the optimization of enzyme properties as per application demands (Xiao et al. 2019). It involves error-prone PCR, saturation mutagenesis, and gene recognition. This technique prerequisites a deep knowledge and understanding of the relationship of sequence, structure, and function of a protein. An integrated database of CYPs, using extensive sequence analysis within members of protein family, serves as a tool for the identification of functionally related and amino acid residues determining the selectivity of the proteins (Moses et al. 2013). In this method, a catalytically improved and completely functional CYP CinA-ADD-CinC fusion protein variant (KB8) is generated for bio-electrocatalysis (an alternative for the replacement of NADPH in cell-free CYP-catalyzed reactions). This variant is found to be more suitable in several synthetic and electrochemical approaches by replacing the reconstituted CYP in the system. It will be a step further to use multi-component CYP systems in different bioelectrochemical applications (Belsare et al. 2017). Random design involves the engineering of enzyme by improving CYP performance with a recognized crystal structure or an existing homology model (Xiao et al. 2019). In a study, a combination of random site-directed and saturation mutagenesis techniques were used which led to a significant change in the regioselectivity of progesterone hydroxylation from the 15β- to the 11α-position which is dependent on CYP106A2 (Nguyen et al. 2012). Terminal modification involves modification of the membrane anchor region of CYP as they are bound to ER membrane which can make it insoluble, unstable, or lead to total loss of activity in heterologous CYP expression (Xiao et al. 2019). For instance, a gene from Jerusalem artichoke (Helianthus tuberosus), i.e., CYP73A1 is known for hydroxylation of cinnamic acid for the synthesis of lignin monomers and many phenolic compounds in higher plants. For the purpose of simple purification from recombinant yeast, improved solubility and stability of this gene in the absence of detergent, it was engineered. The hydrophobic N-terminal was replaced by the peptitergent amphipathic sequence PD1 (Schoch et al. 2003a).
Directed evolution, random mutagenesis and structure-guided protein designing are time consuming and complex. This might be due to the absence of efficient high-throughput screening and crystal structure of the protein. De novo computational protein design is a method which is used for the introduction of new structures and functions to the enzymes, guided by the physical principles that underlie protein folding (Huang et al. 2016; Li et al. 2019a). There are several online databases and sites available which are related to the discovery and engineering of CYP (Box 1). Rosetta is a popularly used method for macromolecular modeling, docking, and designing of proteins (Leman et al. 2020). Along with Rosetta, GERMLIN is used to compute co-evolving amino acids which assists in generating high-resolution protein structure and ligands docking. This integrated approach can be followed where information related to protein structure, its folding ability, binding and assembly is used with computational all-energy atom functions (Kellogg et al. 2011; Shang and Huang 2020). For instance, in 2019, Li and his team members have successfully engineered CYP87D20 which is involved in the cucurbitacin C biosynthetic pathway and was transformed into single specific hydroxylation performing CYP at the C-11 position of cucurbitadienol. Then, this approach was used to fashion a de novo pathway for the production of mogrol, a natural sweetener mogroside’s precursor (Li et al. 2019a). Different approaches for CYP engineering and factors limiting them have been summarized in Fig. 4. Successful usage of these techniques is explained in the later sections of the review.
Fig. 4.
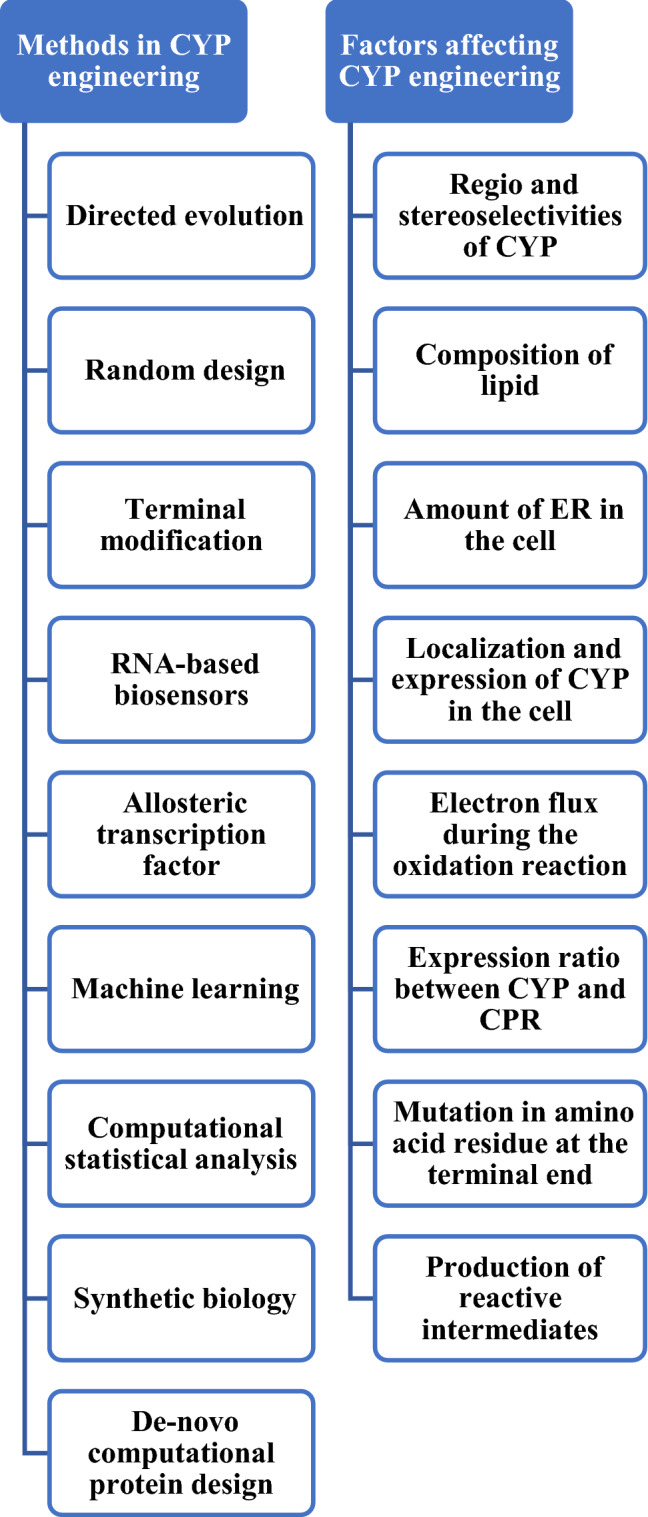
A summary of methods in CYP engineering and factors affecting it (Shang and Huang 2020)
Box 1: Popular tools and databases related to CYP discovery and engineering
CYPED: A collection of tools for classification and analysis of CYPs (Sirim et al. 2009).
Cytochrome P450 (https://drnelson.uthsc.edu/): A database with organized and systematic collection of CYP genes (Nelson et al. 2009).
The Plant Cytochrome P450 Database (erda.dk/public/vgrid/PlantP450/): An online compiled data of functionally characterized Plant CYPs (Hansen et al. 2021).
LICRED: A Versatile Drop-In Vector for Rapid Generation of Redox-Self-Sufficient Cytochrome P450s (Sabbadin et al. 2010).
PCPD: Plant cytochrome P450 database and web-based tools for structural construction and ligand docking (Wang et al. 2021).
CYPminer: An automated cytochrome P450 identification, classification, and data analysis tool for genome data sets across kingdoms (Kweon et al. 2020).
CYPSI: A structure-based interface for CYPs and ligands in Arabidopsis thaliana (Zhang et al. 2012).
Arabidopsis Cytochrome P450 and TAIR(http://www.p450.kvl.dk/p450.shtml and https://www.arabidopsis.org/): CYP genes annotated from the genome database of Arabidopsis (Rhee et al. 2003).
Phytozome (http://www.phytozome.net/)—Database for accurate and insightful comparative genomics studies having large datasets of plant genomes (Goodstein et al. 2012).
Phytometasyn project (https://www.bioinformatics.tugraz.at/phytometasyn/)—Assembled transcriptome databases of non-model plants (Xiao et al. 2013).
Achievements in metabolic engineering of CYPs
Several cytochromes involved in the production of important secondary metabolites have been identified in medicinal plants. A few of them have been engineered in heterologous host or utilized for (semi) synthetic production. A classic example of important metabolite synthesis from a medicinal plant is the biosynthesis of taxol, an anti-cancer drug produced by engineering of a CYP (CYP725A4) involved in selective oxygenation. Optimization of the expression of CYP, interaction of reductase partner, and N-terminus modifications lead to the highest yield of oxygenated taxanes in E. coli (Biggs et al. 2016). Cellular redox balance and availability of cofactors are playing a significant role in influencing the yield of metabolites (Liu et al. 2020). Another major achievement with CYP engineering was semi-synthetic production of Artemisinin which is a sesquiterpene lactone having antimalarial properties, produced by the plant Artemisia annua (Paddon et al. 2013). It was difficult to produce it in its natural form in bulk because of its unstable nature, which often resulted in drug shortage and price fluctuation. CYP71AV1 was found to be involved in catalyzing three successive oxidations steps converting amorpha-4,11-diene to artemisinic acid (Renault et al. 2014). Overexpression of CYP71AV1 in plants or heterologous system provides an opportunity to enhance artemisinin through semi-synthesis (Teoh et al. 2006). Other examples of CYP engineering include the co-expression of Glycyrrhiza uralensis CYP88 and CYP72A154 in Saccharomyces cerevisiae in combination with Arabidopsis thaliana beta-amyrin synthase (β-AS) and AtNADPH-CPR leads to the accumulation of glycyrrhetinic acid (GA) and a small amount of β-amyrin. GA production is further enhanced by Glycyrrhiza uralensis cytochrome b5 (GuCYB5) (Wang et al. 2019). For the biosynthesis of morphine in Papaver somnifera, a critical bioconversion of (S)-reticuline into (R)-reticuline was required. It was achieved by fusion of CYP82Y2-like P450 from an aldo–keto reductase (AKR) leading to this epimerization of reticuline via 1,2-dehydroreticuline (Farrow et al. 2015; Galanie et al. 2015). A comprehensive list of CYPs identified in medicinal plants and engineered in heterologous host is compiled in Table 2.
Table 2.
CYPs involved in secondary metabolite biosynthesis in medicinal plants, and their application in metabolic engineering in heterologous system
| CYP | Organism | Enzyme/reaction | Engineering method | Objective/modulations after engineering | References |
|---|---|---|---|---|---|
| CYP88D | Glycyrrhiza uralensis | β-amyrin 11-oxidase | Codon optimisation of CYP88D6 and CYP72A154 with co-expression of β-amyrin synthase encoding gene and AtNADPH + GuCYB5 expression in S. cerevisiae | Glycyrrhizin accumulation | Seki et al. (2008), Wang et al. (2019) |
| CYP71AV1 | Artemisia annua | Amorpha-4,11-diene C-12 oxidase | Expressed in conjunction with CPR and N -terminus modification, high copy number plasmid and a strong inducible promoter in S. cerevisiae | Anti-malaria-drug precursor artemisinic acid, artemisinic alcohol, artemisinic aldehyde and dihydroartemisinic aldehyde production | Chen et al. (2017), Teoh et al. (2006) |
| CYP76B1 | Helianthus tuberosus | 7-ethoxycoumarin O-de-ethylase involved in double N-dealkylation of phenylurea | Ectopic constitutive expression in Nicotiana tabacum and Arabidopsis | Increase in tolerance for several phenylurea herbicides | Didierjean et al. (2002) |
| CYP87D20 | Cucurbitaceae family | C11 carbonylase and C20 hydroxylase | Structural and data-driven approach using Rosetta software and GREMLIN | Creating a de novo pathway to produce mogrol | Li et al. (2019a) |
| CYP706B1 | Gossypium arboreum | Cadinene-8-hydroxylase | N-terminal modification in E. coli | 8-Hydroxycadinene production | Chang et al. (2007) |
| CYP75A | Camellia sinensis | Flavonoid 3,5-hydroxylase | Gene overexpression in conjunction with CPR in tobacco | Accumulation of catechin | Wang et al. (2014) |
| CYP73A1 | Helianthus tuberosus | Trans-cinnamate 4-hydoxylase | N-terminus modification in S. cerevisiae | Trans-cinnamic acid hydroxylation product | Schoch, et al. (2003b) |
| P450 isoflavone synthase 1 | Glycine max | Isoflavone synthase | Electron flux optimisation by fusion of CPR in E. coli | Genistein accumulation | Leonard & Koffas (2007) |
| CYP82D1.1 | Scutellaria baicalensis | Flavone hydroxylases | N-terminal modification and truncation in E. coli | Improved yield of baicalein and scutellarein | Li et al. (2019b) |
| CYP716a47 | Panax ginseng | Encodes protopanaxadiol synthase | Fusion with AtCPR in S. cerevisiae | Increase in protopanaxadiol production | Zhao et al. (2016) |
| CYP76AH15 | Coleus forskohlii | hydroformylation (converts 13R‑manoyl oxide to 11‑oxo‑13R‑manoyl oxide) | SRSs (Substrate Recognition Sites) engineering in S. cerevisiae | Increased production of forskolin | Forman et al. (2018) |
| CYP76AH1 | Salvia miltiorrhiza | Ferruginol synthase | In-vivo optimization of redox partners in S. cerevisiae | Ferruginol production | Guo et al. (2013) |
| CYP725A4 | Taxus cuspidata | Taxadiene-5α-Hydroxylase | Optimizing expression of CYP, interaction with reduction partner and N-terminal modificaiton in E. coli | Increased oxygenated taxanes production | Biggs et al. (2016) |
| AmI2′H/CYP81E42 | Astragalus membranaceus | Isoflavone 2′-hydroxylase | N-terminal and ORF modification in E. coli | Medicarpin malonyl glucoside accumulation | Chen et al. (2015) |
| CYP82Y2-like | Papaver bracteatum | 1,2-dehydroreticuline synthase | Gene mining, protein mutagenesis, codon optimization, and heterologous expression in yeast | Bioconversion of (S)-reticuline to (R)-reticuline for morphine biosynthesis | Farrow et al. (2015), Galanie et al. (2015) |
| P450 SalSyn | Papaver somniferum | Salutaridine synthase | Co-expression of heterologous proteins and deletion of one host gene in yeast | Production of thebaine/ hydrocodone | Galanie et al. (2015) |
| CYP716a155 | Rosmarinus officinalis | BA-specific lupeol C-28 oxidase | Cognate expression of CYP/CPR in S. cerevisiae | A potent anti-cancer agent, betulinic acid accumulation | An et al. (2020), Huang et al. (2019) |
Challenges in CYP engineering and biotechnological intervention
For engineering any metabolic pathway, it is a prerequisite to have complete information of its biosynthetic pathway. But sequencing and characterization of genes remain broadly limited to crop and horticulturally important plants. Lack of sequence and secondary metabolic pathway information of medicinal plants has been the biggest challenge in the identification and characterization of CYPs which show huge sequence and functional diversities. Expression of these enzymes varies with tissue, developmental stage, and environmental conditions, which makes their regulation very complex.
As CYPs are the largest family of enzymes, they are shown to be involved in the regulation of multiple biosynthetic pathways. They are flexible enough to recognize multiple substrates and produce a spectrum of products. Metabolic engineering of CYPs to produce desired product may lead to silent metabolism and/or hidden crosstalk among various pathways, giving rise to unintended metabolic outcomes (Lynch et al. 2021). For example, overexpression of CYP71BE79 during partial reconstitution of gossypol biosynthetic pathway in tobacco leaves leads to unintended production of glycosylated product 8,11-dihydroxy-7-keto-d-cadinene instead of 11-dihydroxy-7-keto-δ-cadinene itself (Tian et al. 2018). Great versatility for multiple substrates confers low catalytic efficiencies to the CYPs (Bar-Even and Salah Tawfik 2013; Bernhardt and Urlacher 2014; Shang and Huang 2020). Missense mutation in AtCYP83B1 is also a great example of this. The mutation shows reduced levels of indole glucosinolate, and accumulation of indole-3-acetaldoxime, while surprisingly limiting the phenylpropanoid metabolism (Kim et al. 2015; Lynch et al. 2021). Further, the catalytic activity of CYPs relies on transfer of electrons from reductases (CPR). An extrication between the formation of product and availability of NAD(P)H to provide electrons may significantly affect the catalytic activity and stability of CYP (Lundemo and Woodley 2015).
CYP shows varied expression in heterologous systems which makes the determination of CYP function difficult. Renault and his team concluded that almost 40% of plant CYPs are poorly expressed in yeast. This makes it difficult to predict the protein expression and its application in industrial production (Nørholm et al. 2013; Renault et al. 2014). Heterologous production of bioactive compounds can also be toxic for the system. For instance, a CYP71D51v2 from tobacco expressed in recombinant yeast and a CYP reductase from Arabidopsis were used for the optimization of valencene conversion into nootkatol and nootkatone. But because of the toxicity of the bioproduct, i.e., β-nootkatol in yeast system, it did not match the requirements for implementation of an industrial process (Gavira et al. 2013).
Plants provide an outstanding platform for core metabolism to produce desired products by grafting specialized pathways but alongside this, due to the activity of host plant enzymes, they tend to convert those desired products further into conjugates or other derivatives. According to a study, it was shown that CYP76B6, a multifunctional enzyme from Catharanthus roseus is involved in sequential oxidation steps leading to the formation of 8-oxogeraniol from geraniol. In planta, it was observed that the first step of geraniol hydroxylation was very efficient and fast. However, when expressed in leaf tissues, in the absence of the next enzyme of the secoiridoid pathway, 8-oxogeraniol was converted into further oxidized and/or reduced compounds (Höfer et al. 2013). Therefore, it is very important to channel the desired metabolic intermediate to downstream product rapidly (Renault et al. 2014). In summary, instability, insolubility, low activity, poor specificities, incompatibility with non-native hosts, protein designing difficulties due to inadequate amount of substrate bound 3D structure, cofactor requirement, inefficiency of electron transfer as a result of either the lack of sufficient NAD(P)H levels, or poor interactions between CYPs and CPRs are among the major challenges to successful metabolic engineering (Armstrong et al. 2013; Bernhardt & Urlacher 2014; Shang & Huang 2020).
Circumventing the limitations of CYP for pathway engineering
Researchers have been trying to find ways to overcome the challenges in using this versatile multifunctional enzyme for the production of high-value metabolites at large scale through biotechnological interventions. The main challenge of lack of knowledge of genome sequence and mechanisms of silent metabolism was because of the limited ability of a systematic generation of large metabolites datasets and analyzing them computationally. But, recent advances in technology lead to the usage of omics approaches which include transcriptomics, proteomics, genomics and metabolomics that can alleviate this problem. Synthetic biology in combination with computational tools can also be used to overcome these challenges (Jacobowitz and Weng 2020; Lynch et al. 2021). It also gives a deep insight into the plant metabolic networks and potential hidden silent constrictions (Erb and Kliebenstein 2020). Metabolic flux can be controlled by overexpressing the upstream enzymes or by silencing the side-branch pathways. It can also be done by a more appropriate genetic background. Co-expression of optimal protein sets or reconstitution of modular pathways can also be used to control metabolic flux (Renault et al. 2014). Optimization of codon partially or reduction of RNA secondary structure at the N-terminal can be used to solve the problem of heterologous expression of plant CYPs in yeast (Goodman et al. 2013). Toxicity of substrate/intermediates/product can be controlled by a biphasic bioreactor, trapping the products by anion exchange, conjugation of products, compartmentation of products, or boosting the flux to final non-toxic products (Renault et al. 2014). Directed evolution is used to improve the tolerability of CYPs toward organic solvent which is required for substrate molecules’ solubility. Immobilization and directed evolution have shown to increase the shelf life of these multifunctional enzymes (Armstrong et al. 2013). Implying all these techniques with appropriate analysis tools will help to resolve many problems such as the distribution of carbon flux within the networks of metabolic pathways. This will help in unveiling the channeling of metabolites and inactivated metabolic pools (Lynch et al. 2021; Shih & Morgan 2020). Further to solve the problem of cofactor requirement in CYP, scientists have used—(1) Regenerating NADPH cofactor by coupling of oxidation reaction with reductase generating NADPH; (2) Electrochemical reduction of CYP via either by mediators’ actions (indirect approach) or enzyme’s direct attachment to an electrode. Cofactor can also be reduced electrochemically; (3) Photoinduction methods can also be used for electron transfer; (4) Using cheaper cofactors for the reduction of CYP, for instance directed evolution can be used to produce efficient cofactors; (5) Peroxide shunt can also be used, but many CYPs are not efficient in the usage of this pathway, so researchers are attempting to increase their efficiency and lastly, by fusing the reductase with the CYP (Armstrong et al. 2013).
Conclusion and future perspectives
Medicinal plants are critical bioresources, synthesizing a wide array of secondary metabolites that have been found to play important role in treating different ailments in various system of medicines. However, low bioaccumulation of some of the high-valued secondary metabolites is the major bottleneck for their utilization for commercial application. Metabolic pathway engineering is a promising approach to alter the genes and their expression in a way to enhance the accumulation of desired product. CYPs are known to catalyze the synthesis of a wide variety of useful metabolites, and often are the target of choice for metabolic engineering. In a short span of time, a lot of progress has been made in the discovery and modification of plant CYPs to produce natural, semi-synthetic, and synthetic products. However, incomplete pathway information of secondary metabolites, multiple reactions catalyzed by versatile CYPs producing undesired products, and lack of structural information for substrate and cofactor binding limit their application at industrial level. With the evolution of more affordable high-throughput sequencing, computational modeling for structure prediction and possible substrate binding, and development of superior bioreactors, a lot of unexplored possibilities for the production of valuable medicinal products through precise metabolic engineering of CYPs are underway.
Author contribution statement
AS and AB: contributed equally. PK: Conceptualized the idea. AS and AB: wrote the original draft. PK: Edited the manuscript. All the authors have read and approved the final version of manuscript.
Acknowledgements
This work was supported by the Department of Biotechnology (DBT-NER/AGRI/33/2016), Government of India, New Delhi, and Centre for Agricultural Research and Innovation (CARI) under the Rashtriya Uchchattar Shiksha Abhiyan (RUSA-II), GNDU, Amritsar. AB is thankful to CSIR for research associateship (60(0115)/17/EMR-II).
Data availability statement
Data sharing does not apply to this article as no datasets were generated during the current study.
Declarations
Conflict of interest
The authors declare no known conflict of interests or personal relationships.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Anshika Sethi and Abhishek Bhandawat have contributed equally to this work.
References
- Akashi T, Aoki T, Ayabe SI. Identification of a cytochrome P450 cDNA encoding (2S)-flavanone 2-hydroxylase of licorice (Glycyrrhiza echinata L.; Fabaceae) which represents licodione synthase and flavone synthase II. FEBS Lett. 1998;431:287–290. doi: 10.1016/S0014-5793(98)00781-9. [DOI] [PubMed] [Google Scholar]
- An T, Zha W, Zi J. Biotechnological production of betulinic acid and derivatives and their applications. Appl Microbiol Biotechnol. 2020;104:3339–3348. doi: 10.1007/s00253-020-10495-1. [DOI] [PubMed] [Google Scholar]
- Armstrong CT, Watkins DW, Anderson JLR. Constructing manmade enzymes for oxygen activation. Dalt Trans. 2013;42:3136–3150. doi: 10.1039/c2dt32010j. [DOI] [PubMed] [Google Scholar]
- Bar-Even A, Salah Tawfik D. Engineering specialized metabolic pathways-is there a room for enzyme improvements? Curr Opin Biotechnol. 2013;24:310–319. doi: 10.1016/j.copbio.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Belsare KD, Horn T, Ruff AJ, et al. Directed evolution of P450cin for mediated electron transfer. Protein Eng Des Sel. 2017;30:119–127. doi: 10.1093/protein/gzw072. [DOI] [PubMed] [Google Scholar]
- Berim A, Gang DR. The roles of a flavone-6-hydroxylase and 7-o-demethylation in the flavone biosynthetic network of sweet basil. J Biol Chem. 2013;288:1795–1805. doi: 10.1074/jbc.M112.420448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R, Urlacher VB. Cytochromes P450 as promising catalysts for biotechnological application: chances and limitations. Appl Microbiol Biotechnol. 2014;98:6185–6203. doi: 10.1007/s00253-014-5767-7. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee SK (2000) Handbook of medicinal plants. Aavishkar Publishers
- Biggs BW, Lim CG, Sagliani K, et al. Overcoming heterologous protein interdependency to optimize P450-mediated Taxol precursor synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 2016;113:3209–3214. doi: 10.1073/pnas.1515826113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caswell JM, O’Neill M, Taylor SJC, Moody TS. Engineering and application of P450 monooxygenases in pharmaceutical and metabolite synthesis. Curr Opin Chem Biol. 2013;17:271–275. doi: 10.1016/j.cbpa.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Chang MCY, Eachus RA, Trieu W, et al. Engineering Escherichia coli for production of functionalized terpenoids using plant P450s. Nat Chem Biol. 2007;3:274–277. doi: 10.1038/nchembio875. [DOI] [PubMed] [Google Scholar]
- Chen J, Yuan H, Zhang L, et al. Cloning, expression and purification of isoflavone-2′-hydroxylase from Astragalus membranaceus Bge. Var. mongolicus (Bge.) Hsiao. Protein Expr Purif. 2015;107:83–89. doi: 10.1016/j.pep.2014.11.010. [DOI] [PubMed] [Google Scholar]
- Chen X, Zhang C, Too HP. Multienzyme biosynthesis of dihydroartemisinic acid. Molecules. 2017;22:1–12. doi: 10.3390/molecules22091422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collu G, Unver N, Peltenburg-Looman AMG, et al. Geraniol 10-hydroxylase, a cytochrome P450 enzyme involved in terpenoid indole alkaloid biosynthesis. FEBS Lett. 2001;508:215–220. doi: 10.1016/S0014-5793(01)03045-9. [DOI] [PubMed] [Google Scholar]
- Crozier A, Clifford MN, Michael N, Ashihara H. Plant secondary metabolites: occurrence, structure and role in the human diet. Blackwell Pub; 2008. [Google Scholar]
- Dhar N, Razdan S, Rana S, et al. A decade of molecular understanding of withanolide biosynthesis and in vitro studies in Withania somnifera (L) Dunal: prospects and perspectives for pathway engineering. Front Plant Sci. 2015;6:1031. doi: 10.3389/fpls.2015.01031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierjean L, Gondet L, Perkins R, et al. Engineering herbicide metabolism in tobacco and Arabidopsis with CYP76B1, a cytochrome P450 enzyme from Jerusalem artichoke. Plant Physiol. 2002;130:179–189. doi: 10.1104/pp.005801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M, Kliebenstein DJ. Plant secondary metabolites as defenses, regulators, and primary metabolites: the blurred functional trichotomy1 [OPEN] Plant Physiol. 2020;184:39–52. doi: 10.1104/PP.20.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow SC, Hagel JM, Beaudoin GAW, et al. Stereochemical inversion of (S)-reticuline by a cytochrome P450 fusion in opium poppy. Nat Chem Biol. 2015;11:728–732. doi: 10.1038/nchembio.1879. [DOI] [PubMed] [Google Scholar]
- Forman V, Bjerg-Jensen N, Dyekjær JD, et al. Engineering of CYP76AH15 can improve activity and specificity towards forskolin biosynthesis in yeast. Microb Cell Fact. 2018;17:1–17. doi: 10.1186/s12934-018-1027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima EO, Seki H, Ohyama K, et al. CYP716A subfamily members are multifunctional oxidases in triterpenoid biosynthesis. Plant Cell Physiol. 2011;52:2050–2061. doi: 10.1093/pcp/pcr146. [DOI] [PubMed] [Google Scholar]
- Galanie S, Thodey K, Trenchard IJ, et al. Complete biosynthesis of opioids in yeast. Science. 2015;349:1095–1100. doi: 10.1126/science.aac9373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavira C, Höfer R, Lesot A, et al. Challenges and pitfalls of P450-dependent (+)-valencene bioconversion by Saccharomyces cerevisiae. Metab Eng. 2013;18:25–35. doi: 10.1016/j.ymben.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Goodman DB, Church GM, Kosuri S. Causes and effects of N-terminal codon bias in bacterial genes. Science. 2013;342:475–479. doi: 10.1126/science.1241934. [DOI] [PubMed] [Google Scholar]
- Goodstein DM, Shu S, Howson R, et al. Phytozome: a comparative platform for green plant genomics. Nucleic Acids Res. 2012;40:1178–1186. doi: 10.1093/nar/gkr944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo J, Zhou YJ, Hillwig ML, et al. CYP76AH1 catalyzes turnover of miltiradiene in tanshinones biosynthesis and enables heterologous production of ferruginol in yeasts. Proc Natl Acad Sci U S A. 2013;110:12108–12113. doi: 10.1073/pnas.1218061110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Kim MJ, Ban YW, et al. The involvement of β-amyrin 28-oxidase (cyp716a52v2) in oleanane-type ginsenoside biosynthesis in panax ginseng. Plant Cell Physiol. 2013;54:2034–2046. doi: 10.1093/pcp/pct141. [DOI] [PubMed] [Google Scholar]
- Hansen CC, Sørensen M, Veiga TAM, et al. Reconfigured cyanogenic glucoside biosynthesis in eucalyptus cladocalyx involves a cytochrome P450 CYP706C55. Plant Physiol. 2018;178:1081–1095. doi: 10.1104/pp.18.00998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen CC, Nelson DR, Møller BL, Werck-Reichhart D. Plant cytochrome P450 plasticity and evolution. Mol Plant. 2021;14:1244–1265. doi: 10.1016/j.molp.2021.06.028. [DOI] [PubMed] [Google Scholar]
- Höfer R, Dong L, André F, et al. Geraniol hydroxylase and hydroxygeraniol oxidase activities of the CYP76 family of cytochrome P450 enzymes and potential for engineering the early steps of the (seco)iridoid pathway. Metab Eng. 2013;20:221–232. doi: 10.1016/j.ymben.2013.08.001. [DOI] [PubMed] [Google Scholar]
- Huang PS, Boyken SE, Baker D. The coming of age of de novo protein design. Nature. 2016;537:320–327. doi: 10.1038/nature19946. [DOI] [PubMed] [Google Scholar]
- Huang J, Zha W, An T, et al. Identification of RoCYP01 (CYP716A155) enables construction of engineered yeast for high-yield production of betulinic acid. Appl Microbiol Biotechnol. 2019;103:7029–7039. doi: 10.1007/s00253-019-10004-z. [DOI] [PubMed] [Google Scholar]
- Humphreys JM, Hemm MR, Chapple C. New routes for lignin biosynthesis defined by biochemical characterization of recombinant ferulate 5-hydroxylase, a multifunctional cytochrome P450-dependent monooxygenase. Proc Natl Acad Sci. 1999;96:10045–10050. doi: 10.1073/pnas.96.18.10045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezawa N, Tanaka M, Nagayoshi M, et al. Molecular cloning and characterization of CYP719, a methylenedioxy bridge-forming enzyme that belongs to a novel P450 family, from cultured Coptis japonica cells. J Biol Chem. 2003;278:38557–38565. doi: 10.1074/jbc.M302470200. [DOI] [PubMed] [Google Scholar]
- Irmler S, Schröder G, St-Pierre B, et al. Indole alkaloid biosynthesis in Catharanthus roseus: new enzyme activities and identification of cytochrome P450 CYP72A1 as secologanin synthase. Plant J. 2000;24:797–804. doi: 10.1046/j.1365-313X.2000.00922.x. [DOI] [PubMed] [Google Scholar]
- Jacobowitz JR, Weng JK. Exploring uncharted territories of plant specialized metabolism in the postgenomic era. Annu Rev Plant Biol. 2020;71:631–658. doi: 10.1146/annurev-arplant-081519-035634. [DOI] [PubMed] [Google Scholar]
- Jennewein S, Rithner CD, Williams RM, Croteau R. Taxoid metabolism: taxoid 14β-hydroxylase is a cytochrome P450-dependent monooxygenase. Arch Biochem Biophys. 2003;413:262–270. doi: 10.1016/S0003-9861(03)00090-0. [DOI] [PubMed] [Google Scholar]
- Jensen K, Møller BL. Plant NADPH-cytochrome P450 oxidoreductases. Phytochemistry. 2010;71:132–141. doi: 10.1016/j.phytochem.2009.10.017. [DOI] [PubMed] [Google Scholar]
- Jo HJ, Han JY, Hwang HS, Choi YE. β-Amyrin synthase (EsBAS) and β-amyrin 28-oxidase (CYP716A244) in oleanane-type triterpene saponin biosynthesis in Eleutherococcus senticosus. Phytochemistry. 2017;135:53–63. doi: 10.1016/j.phytochem.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Jørgensen K, Bak S, Busk PK, et al. Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Plant Physiol. 2005;139:363–374. doi: 10.1104/pp.105.065904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung ST, Lauchli R, Arnold FH. Cytochrome P450: taming a wild type enzyme. Curr Opin Biotechnol. 2011;22:809–817. doi: 10.1016/j.copbio.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn RA, Fahrendorf T, Halkier BA, Møller BL (1999) ABB,1999,363,9.pdf. 363:9–18 [DOI] [PubMed]
- Kalra B. Cytochrome P450 enzyme isoforms and their therapeutic implications: an update. Indian J Med Sci. 2007;61:102–116. doi: 10.4103/0019-5359.30351. [DOI] [PubMed] [Google Scholar]
- Karunanithi PS, Dhanota P, Addison JB, et al. Functional characterization of the cytochrome P450 monooxygenase CYP71AU87 indicates a role in marrubiin biosynthesis in the medicinal plant Marrubium vulgare. BMC Plant Biol. 2019 doi: 10.1186/s12870-019-1702-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai S, Mori A, Shiokawa T, et al. Isolation and analysis of cinnamic acid 4-hydroxylase homologous genes from a hybrid aspen, populus kitakamiensis. Biosci Biotechnol Biochem. 1996;60:1586–1597. doi: 10.1271/bbb.60.1586. [DOI] [PubMed] [Google Scholar]
- Kellogg EH, Leaver-Fay A, Baker D. Role of conformational sampling in computing mutation-induced changes in protein structure and stability. Proteins Struct Funct Bioinforma. 2011;79:830–838. doi: 10.1002/prot.22921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JI, Dolan WL, Anderson NA, Chapple C. Indole glucosinolate biosynthesis limits phenylpropanoid accumulation in Arabidopsis thaliana. Plant Cell. 2015;27:1529–1546. doi: 10.1105/tpc.15.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni R. Metabolic engineering. Tissue Eng Artif Organs. 2016 doi: 10.1201/b16050-18. [DOI] [Google Scholar]
- Kweon O, Kim SJ, Kim JH, et al. CYPminer: an automated cytochrome P450 identification, classification, and data analysis tool for genome data sets across kingdoms. BMC Bioinform. 2020;21:1–11. doi: 10.1186/s12859-020-3473-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb DC, Waterman MR. Unusual properties of the cytochrome P450 superfamily. Philos Trans R Soc B Biol Sci. 2013 doi: 10.1098/rstb.2012.0434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latunde-Dada AO, Cabello-Hurtado F, Czittrich N, et al. Flavonoid 6-hydroxylase from soybean (Glycine max L.), a novel plant P-450 monooxygenase. J Biol Chem. 2001;276:1688–1695. doi: 10.1074/jbc.M006277200. [DOI] [PubMed] [Google Scholar]
- Lee DS, Nioche P, Hamberg M, Raman CS. Structural insights into the evolutionary paths of oxylipin biosynthetic enzymes. Nature. 2008;455:363–368. doi: 10.1038/nature07307. [DOI] [PubMed] [Google Scholar]
- Leman JK, Weitzner BD, Lewis SM, et al. Macromolecular modeling and design in Rosetta: recent methods and frameworks. Nat Methods. 2020;17:665–680. doi: 10.1038/s41592-020-0848-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard E, Koffas MAG. Engineering of artificial plant cytochrome P450 enzymes for synthesis of isoflavones by Escherichia coli. Appl Environ Microbiol. 2007;73:7246–7251. doi: 10.1128/AEM.01411-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Reed DW, Liu E, et al. Functional genomic analysis of alkaloid biosynthesis in Hyoscyamus niger reveals a cytochrome P450 involved in littorine rearrangement. Chem Biol. 2006;13:513–520. doi: 10.1016/j.chembiol.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Li D, Ma Y, Zhou Y, et al. A structural and data-driven approach to engineering a plant cytochrome P450 enzyme. Sci China Life Sci. 2019;62:873–882. doi: 10.1007/s11427-019-9538-3. [DOI] [PubMed] [Google Scholar]
- Li J, Tian C, Xia Y, et al. Production of plant-specific flavones baicalein and scutellarein in an engineered E. coli from available phenylalanine and tyrosine. Metab Eng. 2019;52:124–133. doi: 10.1016/j.ymben.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Li Z, Jiang Y, Guengerich FP, et al. Engineering cytochrome P450 enzyme systems for biomedical and biotechnological applications. J Biol Chem. 2020;295:833–849. doi: 10.1016/s0021-9258(17)49939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Cheng J, Zhang G, et al. Engineering yeast for the production of breviscapine by genomic analysis and synthetic biology approaches. Nat Commun. 2018 doi: 10.1038/s41467-018-02883-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu X, Wang H, et al. Discovery and modification of cytochrome P450 for plant natural products biosynthesis. Synth Syst Biotechnol. 2020;5:187–199. doi: 10.1016/j.synbio.2020.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Shen Q, Zhang L, et al. Promotion of artemisinin biosynthesis in transgenic Artemisia annua by overexpressing ADS, CYP71AV1 and CPR genes. Ind Crops Prod. 2013;49:380–385. doi: 10.1016/j.indcrop.2013.04.045. [DOI] [Google Scholar]
- Lu X, Zhang F, Shen Q, et al. Overexpression of allene oxide cyclase improves the biosynthesis of artemisinin in Artemisia annua L. PLoS ONE. 2014;9:1–8. doi: 10.1371/journal.pone.0091741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Tang K, Li P. Plant metabolic engineering strategies for the production of pharmaceutical terpenoids. Front Plant Sci. 2016;7:1–11. doi: 10.3389/fpls.2016.01647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundemo MT, Woodley JM. Guidelines for development and implementation of biocatalytic P450 processes. Appl Microbiol Biotechnol. 2015;99:2465–2483. doi: 10.1007/s00253-015-6403-x. [DOI] [PubMed] [Google Scholar]
- Lynch JH, Huang XQ, Dudareva N. Silent constraints: the hidden challenges faced in plant metabolic engineering. Curr Opin Biotechnol. 2021;69:112–117. doi: 10.1016/j.copbio.2020.12.014. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Bhatia GS, Pandhi P. Patterns of use of unconventional therapies in the medical outpatient department of a tertiary care hospital in India. J Ethnopharmacol. 2001;75:71–75. doi: 10.1016/S0378-8741(00)00380-9. [DOI] [PubMed] [Google Scholar]
- McIntosh JA, Farwell CC, Arnold FH. Expanding P450 catalytic reaction space through evolution and engineering. Curr Opin Chem Biol. 2014;19:126–134. doi: 10.1016/j.cbpa.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miettinen K, Pollier J, Buyst D, et al. The ancient CYP716 family is a major contributor to the diversification of eudicot triterpenoid biosynthesis. Nat Commun. 2017 doi: 10.1038/ncomms14153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses T, Pollier J, Thevelein JM, Goossens A. Bioengineering of plant (tri)terpenoids: from metabolic engineering of plants to synthetic biology in vivo and in vitro. New Phytol. 2013;200:27–43. doi: 10.1111/nph.12325. [DOI] [PubMed] [Google Scholar]
- Nelson DR. The cytochrome P450 homepage. Hum Genomics. 2009;4:59–65. doi: 10.1186/1479-7364-4-1-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen KT, Virus C, Günnewich N, et al. Changing the regioselectivity of a P450 from C15 to C11 hydroxylation of progesterone. ChemBioChem. 2012;13:1161–1166. doi: 10.1002/cbic.201100811. [DOI] [PubMed] [Google Scholar]
- Nørholm MHH, Toddo S, Virkki MTI, et al. Improved production of membrane proteins in Escherichia coli by selective codon substitutions. FEBS Lett. 2013;587:2352–2358. doi: 10.1016/j.febslet.2013.05.063. [DOI] [PubMed] [Google Scholar]
- Paddon CJ, Westfall PJ, Pitera DJ, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- Pandian BA, Sathishraj R, Djanaguiraman M, et al. Role of cytochrome P450 enzymes in plant stress response. Antioxidants. 2020;9:454. doi: 10.3390/antiox9050454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polturak G, Breitel D, Grossman N, et al. Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. New Phytol. 2016;210:269–283. doi: 10.1111/nph.13796. [DOI] [PubMed] [Google Scholar]
- Rana S, Waheed Bhat W, Dhar N, et al (2014) Molecular characterization of two A-type P450s, WsCYP98A and WsCYP76A from Withania somnifera (L.) Dunal: expression analysis and withanolide accumulation in response to exogenous elicitations [DOI] [PMC free article] [PubMed]
- Rasool S, Mohamed R. Plant cytochrome P450s: nomenclature and involvement in natural product biosynthesis. Protoplasma. 2016;253:1197–1209. doi: 10.1007/s00709-015-0884-4. [DOI] [PubMed] [Google Scholar]
- Renault H, Bassard JE, Hamberger B, Werck-Reichhart D. Cytochrome P450-mediated metabolic engineering: current progress and future challenges. Curr Opin Plant Biol. 2014;19:27–34. doi: 10.1016/j.pbi.2014.03.004. [DOI] [PubMed] [Google Scholar]
- Rhee SY, Beavis W, Berardini TZ, et al. The Arabidopsis Information Resource (TAIR): A model organism database providing a centralized, curated gateway to Arabidopsis biology, research materials and community. Nucleic Acids Res. 2003;31:224–228. doi: 10.1093/nar/gkg076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittle J, Green MT. Cytochrome P450 compound I: capture, Ch. Science. 2010;330:933–937. doi: 10.1126/science.1193478. [DOI] [PubMed] [Google Scholar]
- Sabbadin F, Hyde R, Robin A, et al. LICRED: a versatile drop-in vector for rapid generation of redox-self-sufficient cytochrome P450s. ChemBioChem. 2010;11:987–994. doi: 10.1002/cbic.201000104. [DOI] [PubMed] [Google Scholar]
- Schoch GA, Attias R, Belghazi M, et al. Engineering of a water-soluble plant cytochrome P450, CYP73A1, and NMR-based orientation of natural and alternate substrates in the active site. Plant Physiol. 2003;133:1198–1208. doi: 10.1104/pp.103.020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoch GA, Attias R, Le Ret M, Werck-Reichhart D. Key substrate recognition residues in the active site of a plant cytochrome P450, CYP73A1: homology model guided site-directed mutagenesis. Eur J Biochem. 2003;270:3684–3695. doi: 10.1046/j.1432-1033.2003.03739.x. [DOI] [PubMed] [Google Scholar]
- Seca AML, Pinto DCGA. Biological potential and medical use of secondary metabolites. Medicines. 2019;6:66. doi: 10.3390/medicines6020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki H, Ohyama K, Sawai S, et al. Licorice β-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci U S A. 2008;105:14204–14209. doi: 10.1073/pnas.0803876105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki H, Sawai S, Ohyama K, et al. Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell. 2011;23:4112–4123. doi: 10.1105/tpc.110.082685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Huang S. Engineering plant cytochrome P450s for enhanced synthesis of natural products: past achievements and future perspectives. Plant Commun. 2020;1:100012. doi: 10.1016/j.xplc.2019.100012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih ML, Morgan JA. Metabolic flux analysis of secondary metabolism in plants. Metab Eng Commun. 2020;10:e00123. doi: 10.1016/j.mec.2020.e00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shree P, Mishra P, Selvaraj C, et al. Targeting COVID-19 (SARS-CoV-2) main protease through active phytochemicals of ayurvedic medicinal plants–Withania somnifera (Ashwagandha), Tinospora cordifolia (Giloy) and Ocimum sanctum (Tulsi)–a molecular docking study. J Biomol Struct Dyn. 2022;40:190–203. doi: 10.1080/07391102.2020.1810778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AP. The role of natural products in pharmacotherapy of Alzheimer’s disease. Ethnobot Leafl. 2003;2005:46. [Google Scholar]
- Sirim D, Wagner F, Lisitsa A, Pleiss J. The cytochrome P450 engineering database: integration of biochemical properties. BMC Biochem. 2009;10:1–4. doi: 10.1186/1471-2091-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teoh KH, Polichuk DR, Reed DW, et al. Artemisia annua L. (Asteraceae) trichome-specific cDNAs reveal CYP71AV1, a cytochrome P450 with a key role in the biosynthesis of the antimalarial sesquiterpene lactone artemisinin. FEBS Lett. 2006;580:1411–1416. doi: 10.1016/j.febslet.2006.01.065. [DOI] [PubMed] [Google Scholar]
- Tian X, Ruan JX, Huang JQ, et al. Characterization of gossypol biosynthetic pathway. Proc Natl Acad Sci U S A. 2018;115:E5410–E5418. doi: 10.1073/pnas.1805085115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toguri T, Umemoto N, Kobayashi O, Ohtani T. Activation of anthocyanin synthesis genes by white light in eggplant hypocotyl tissues, and identification of an inducible P-450 cDNA. Plant Mol Biol. 1993;23:933–946. doi: 10.1007/BF00021810. [DOI] [PubMed] [Google Scholar]
- Villa-Ruano N, Pacheco-Hernández Y, Lozoya-Gloria E, et al. Cytochrome P450 from plants: platforms for valuable phytopharmaceuticals. Trop J Pharm Res. 2015;14:731–742. doi: 10.4314/tjpr.v14i4.24. [DOI] [Google Scholar]
- Wakabayashi T, Shida K, Kitano Y, et al. CYP722C from Gossypium arboreum catalyzes the conversion of carlactonoic acid to 5-deoxystrigol. Planta. 2020;251:1–6. doi: 10.1007/s00425-020-03390-6. [DOI] [PubMed] [Google Scholar]
- Wang YS, Xu YJ, Gao LP, et al. Functional analysis of Flavonoid 3′,5′-hydroxylase from Tea plant (Camellia sinensis): critical role in the accumulation of catechins. BMC Plant Biol. 2014;14:1–14. doi: 10.1186/s12870-014-0347-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Su X, Sun M, et al. Efficient production of glycyrrhetinic acid in metabolically engineered Saccharomyces cerevisiae via an integrated strategy. Microb Cell Fact. 2019;18:1–15. doi: 10.1186/s12934-019-1138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Wang Q, Liu Y, et al. PCPD: plant cytochrome P450 database and web-based tools for structural construction and ligand docking. Synth Syst Biotechnol. 2021;6:102–109. doi: 10.1016/j.synbio.2021.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei Y, Ang EL, Zhao H. Recent developments in the application of P450 based biocatalysts. Curr Opin Chem Biol. 2018;43:1–7. doi: 10.1016/j.cbpa.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Wust M, Little DB, Schalk M, Croteau R. Hydroxylation of limonene enantiomers and analogs by recombinant (-)-limonene 3- and 6-hydroxylases from mint (Mentha) species: evidence for catalysis within sterically constrained active sites. Arch Biochem Biophys. 2001;387:125–136. doi: 10.1006/abbi.2000.2248. [DOI] [PubMed] [Google Scholar]
- Xiao M, Zhang Y, Chen X, et al. Transcriptome analysis based on next-generation sequencing of non-model plants producing specialized metabolites of biotechnological interest. J Biotechnol. 2013;166:122–134. doi: 10.1016/j.jbiotec.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Xiao H, Zhang Y, Wang M. Discovery and engineering of cytochrome P450s for terpenoid biosynthesis. Trends Biotechnol. 2019;37:618–631. doi: 10.1016/j.tibtech.2018.11.008. [DOI] [PubMed] [Google Scholar]
- Xu J, Wang XY, Guo WZ. The cytochrome P450 superfamily: key players in plant development and defense. J Integr Agric. 2015;14:1673–1686. doi: 10.1016/S2095-3119(14)60980-1. [DOI] [Google Scholar]
- Zhang F, Fu X, Lv Z, et al. A basic leucine zipper transcription factor, aabzip1, connects abscisic acid signaling with artemisinin biosynthesis in Artemisia annua. Mol Plant. 2015;8:163–175. doi: 10.1016/j.molp.2014.12.004. [DOI] [PubMed] [Google Scholar]
- Zhang J, Dai L, Yang J, et al. Oxidation of cucurbitadienol catalyzed by CYP87D18 in the biosynthesis of Mogrosides from Siraitia grosvenorii. Plant Cell Physiol. 2016;57:1000–1007. doi: 10.1093/pcp/pcw038. [DOI] [PubMed] [Google Scholar]
- Zhang G, Zhang Y, Su Z. CYPSI: a structure-based interface for cytochrome P450s and ligands in Arabidopsis thaliana. BMC Bioinform. 2012;13(1):332. doi: 10.1186/1471-2105-13-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao F, Bai P, Liu T, et al. Optimization of a cytochrome P450 oxidation system for enhancing protopanaxadiol production in Saccharomyces cerevisiae. Biotechnol Bioeng. 2016;113:1787–1795. doi: 10.1002/bit.25934. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated during the current study.