Abstract
Chronic Helicobacter pylori infection is associated with mucosal inflammation. The aim of the present study was to assess human neutrophil and monocyte activation by H. pylori strains obtained from patients with different clinical presentations. Bacterial sonicates from 12 strains were used to stimulate phagocyte upregulation of CD11b/CD18 adherence molecules assessed by fluorescence flow cytometry and oxidative burst responses assessed by chemiluminescence. A dose-dependent activation of CD11b/CD18 adherence molecules was observed with all strains on both neutrophils and monocytes. The activities were similar for strains from patients with duodenal ulceration and for strains from asymptomatic volunteers irrespective of histopathologic grades of the biopsy specimens from the antral mucosa. The neutrophil chemiluminescence response correlated with histopathologic severity. We conclude that upregulation of neutrophil and monocyte adherence molecules by H. pylori sonicates is not associated with clinical presentation of the infection.
Infection with Helicobacter pylori is a major risk factor for gastroduodenal disease. Initial changes after gastric infection include an acute neutrophilic inflammatory response, which in the majority of individuals progresses to chronic antrum-predominant gastritis. A prominent feature of H. pylori infection of the antral mucosa is the abundant neutrophil inflammation together with mononuclear phagocytes and lymphoid follicles. Several observations support the close interrelationship between bacterial load and neutrophil influx: (i) a patchy distribution of H. pylori is associated with a similar variation in mucosal neutrophil inflammation (5); (ii) histopathologic scores for intensity of inflammation correlate with the density of H. pylori infection (1, 4, 8, 14); and (iii) following antibacterial therapy of the infection, neutrophil inflammation scores return to normal values in those subjects who are cured of the infection (16, 24). The relevance of neutrophils in the pathogenesis of gastroduodenal disorders is supported by the observations of increased concentrations of toxic oxygen radicals in the mucosa (8) and enhanced activity of nitric oxide synthase (19), both of which could contribute to tissue injury. Proinflammatory activity of H. pylori with neutrophils and monocytes is well recognized by demonstration of bacterial components exhibiting chemotactic activity (15, 17) and induction of oxidative burst responses (18, 20).
A crucial initial event in mucosal inflammation is the intravascular upregulation of phagocyte membrane adherence molecules preceding transendothelial migration (3). Nonstimulated neutrophils and monocytes constitutively express l-selectin (defined by CD62L) on their surface, whereas Mac-1 (defined by CD11b/CD18, complement receptor type 3) is present in very low numbers on the surface membranes. Upon activation, a proteolytic shedding of l-selectin takes place, whereby neutrophils and monocytes change behavior and begin rolling on the inner surface of the endothelial layer (21). Upon further activation, the neutrophils and monocytes upregulate CD11b/CD18 by fusion of intracellular vesicles (6), rich in CD11b/CD18, with the surface membrane and the cells adhere firmly to the endothelial cells. An activation of neutrophil adherent properties has been demonstrated with H. pylori stimulation. The active component has been suggested to be a 50-kDa protein (10) or a 150-kDa protein composed of 10 identical 15-kDa polypeptides (11). The proadhesive activity has been examined in very few clinical isolates so far (11), and its relationship with clinical presentation of the infection is not known. The interactions of H. pylori components with monocyte adhesion molecules have not been studied.
The aim of the present study was to further examine the relationship between clinical presentation of the infected patient and phagocyte inflammatory activation, with special reference to initial upregulation of adherence molecules. We chose to assess strains from subjects with the two extremes of clinical presentation of the H. pylori infection: (i) patients with endoscopically defined duodenal ulceration and clear clinical symptoms, and (ii) healthy volunteers with asymptomatic gastritis without any history of dyspepsia. Further, in the upregulation of adherence molecules, the variation among the donors and the effect of H. pylori seropositivity were addressed.
The subject population included seven patients presenting with endoscopically documented duodenal ulcers and five healthy volunteers with H. pylori gastritis who had no current or prior history of gastroduodenal disease or symptoms. All subjects lived in the Houston, Tex., area. No subject had received treatment for H. pylori infection or was taking antibiotics or acid-suppressing agents at the time of the study. All patients provided written informed consent. This study was performed in compliance with the Institutional Review Board of the Baylor College of Medicine. Gastric biopsy specimens were obtained at the time of endoscopic evaluation. Slides from each specimen were stained with a triple stain, including hematoxylin and eosin, silver stain, and alcian blue, at pH 2.5 (12). Each specimen was graded for the presence of H. pylori infection, active and chronic inflammation, atrophy, intestinal metaplasia, and lymphoid follicles in accordance with the updated Sydney System classification (9). The inflammation of the specimens was then scored as mild, moderate, or severe. Of the patients presenting with duodenal ulcer (n = 7), specimens from four were graded mild to moderate and those from three were graded severe. Of the healthy volunteers presenting with gastritis (n = 5), specimens from three were graded mild to moderate and those from two were graded as severe. Clinical isolates were cultured on blood agar plates and incubated under microaerobic conditions and high humidity for up to 7 days. The organisms were identified as H. pylori by gram-negative staining, typical colony morphology, and positive oxidase, catalase, and urease reactions. Pure cultures of H. pylori were grown on chocolate agar plates under microaerobic conditions. After 24 to 48 h of incubation, the confluent cultures were harvested in sterile distilled water. Bacteria were washed twice in distilled water (resuspended in phosphate-buffered saline [pH 7.4] at 0.5 g/ml or approximately 109/ml) and sonicated three times for 45 s at 20,000 Hz and 400 W. The crude sonicate was centrifuged at 14,000 × g for 1 h at 4°C. The supernatants were filtered through a 0.22-μm-pore-size Millipore filter. The protein concentration was determined by use of solid-phase dye binding assays as described previously (24). Concanavalin A was used as a standard, and binding was measured by spectrophotometry at 590 nm. The sonicate was stored in small aliquots at −20°C. The effects of the H. pylori sonicates on surface expression of the adhesion molecules were examined in peripheral heparinized blood from healthy volunteers from the laboratory staff. A whole-blood system was used for the assessment of upregulation of CD11b/CD18 adherence molecules since even careful isolation procedures will interfere with degranulation processes of neutrophils (23) and monocytes. A 100-μl bacterial sonicate at a final concentration of 0.5, 5, or 50 μg/ml was added to 100 μl of whole blood for 30 min at 37°C. Sonicates from all 12 strains examined were assessed in parallel with cells from the same volunteer. Preliminary kinetic experiments confirmed that an incubation period of 30 min in this system was optimal. Following incubation, the samples were cooled immediately in an ice bath, and phycoerythrin-conjugated mouse anti-human CD11b/CD18 monoclonal antibody (DAKO, Glostrup, Denmark) was added for 30 min at 4°C. Subsequently, the erythrocytes were lysed for 10 min at room temperature (Becton Dickinson lysing solution with formalin) and the phagocytes were washed in phosphate-buffered saline and resuspended in sheath fluid (Becton Dickinson) with 2.7% formalin. The analysis was performed in a flow cytometer (FACscan; Becton Dickinson). Mean fluorescence intensity was corrected by subtracting the mean value from unstimulated controls. In each analysis, a stimulated and unstimulated sample were used and fluorescein-conjugated mouse-anti-human CD14 monoclonal antibodies (DAKO) were added to determine the monocyte distribution in the analysis. The level of immunoglobulin G antibodies against H. pylori in the donors was assessed by an enzyme-linked immunosorbent assay (2); the presence of H. pylori was not confirmed among the phagocyte donors. Peripheral venous blood was separated by dextran sedimentation followed by density gradient centrifugation on metrizoate-polysucrose (Lymphoprep; Nyegaard, Oslo, Norway). Mononuclear cells were washed twice in Eagle’s minimal essential medium (Difco) and adjusted to 5 × 105 monocytes per ml in Eagle’s minimal essential medium. The percentage of monocytes on cytocentrifuge preparations was in the range of 20 to 28% as assessed by morphology with Wright’s stain and cytochemical identification of nonspecific esterase (25). Neutrophils were washed twice in Gey’s solution, the remaining erythrocytes were removed by hypotonic lysis, and cells were adjusted to 5 × 105 per ml in Gey’s solution. The purity of the neutrophils was always more than 95%. The viability of cells was tested by trypan blue and nigrosin exclusion assays and was always more than 95%. A chemiluminescence system was used for the examination of the oxidative burst response of neutrophils and monocytes, as previously described (18). In brief, the assay was performed in duplicate in glass scintillation vials containing 2.5 × 105 neutrophils or monocytes, and the responses after stimulation with fMLP (N-formyl-methionyl-leucyl-phenylalanine; Sigma Chemical Co., St. Louis, Mo.) were enhanced with 2 × 10−4 mol of N,N′-dimethyl-9,9′-biacridinium dinitrate (Lucigenin; Sigma). Neutrophils and monocytes were preincubated for 30 min with H. pylori sonicate at 10 and 100 μg/ml before stimulation. Control vials with Krebs Ringer solution were included in parallel. A Beckman L 8000 scintillation counter in an air-conditioned and thermostat-controlled room at 21 ± 1°C was used in the out-of-coincidence mode. All reagents were dark adapted before use, and the experiments were performed under a red light. Sequential 0.5-min counts were made on each vial over a period of 60 min. Results are presented as the peak response of stimulated cells after subtracting the response of unstimulated cells. The S-plus program was used for data processing. The Mann-Whitney test was used for nonparametric data, and analysis of variance was done by the random-effect model.
The sonicate preparations from all strains tested showed a dose-dependent upregulation of CD11b/CD18 adhesion molecules. The maximal intensity of fluorescence was obtained at a protein concentration of 50 μg/ml for both neutrophils and monocytes. The donor variance was 10.8 (95% confidence limits, 5.2 to 36.0), when analysis of 10 healthy volunteers with all 12 strains was performed. The data showed a normal distribution. The reactivity of phagocytes was unrelated to the anti-H. pylori serological status of cell donors, although seropositive donors had a slightly higher response than seronegative donors (data not shown).
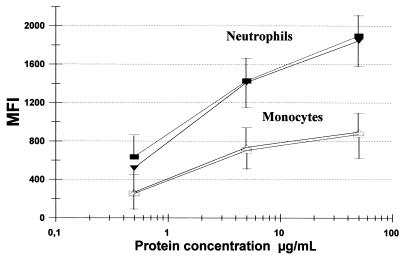
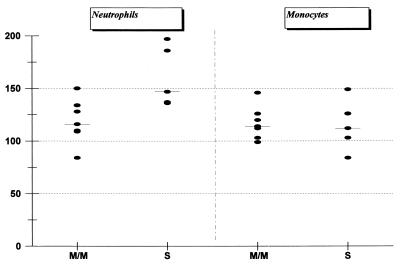
The potencies of bacterial sonicates were similar for monocytes and neutrophils, but the increase in CD11b/CD18 intensity was highest in neutrophils (Fig. 1). The ability to induce adhesion molecules upregulated on neutrophils and monocytes was no different for strains from individuals with duodenal ulcers or asymptomatic gastritis (Fig. 1), and it was unrelated to the severity of the mucosal inflammation as assessed by histopathology (data not shown). For all strains examined, an enhanced chemiluminescence response was obtained following bacterial sonicate preincubation (priming). The highest activity was found in two of the strains with an inflammation grade of severe, and the group of strains with severe inflammation, based upon the histopathological examination, was statistically associated with increased oxidative burst induction in neutrophils (P < 0.05) (Fig. 2). Monocyte responsiveness to bacterial sonicate priming was not related to the histological grade (Fig. 2). There was no correlation between activity of sonicates for upregulation of adherence molecules and stimulation of oxidative burst responses.
FIG. 1.
Upregulation of CD11b/CD18 on neutrophils (solid symbols) and monocytes (open symbols) activated for 30 min by H. pylori sonicate proteins. The results are expressed as the mean fluorescence intensities (MFI) from seven strains isolated from duodenal ulcer patients (squares) and from five strains from volunteers with asymptomatic gastritis (triangles) defined by endoscopy. The results represent mean values from 10 experiments ± standard deviations.
FIG. 2.
Neutrophil and monocyte oxidative burst response upon fMLP stimulation after preincubation with sonicates from H. pylori strains isolated from seven subjects with mild to moderate gastritis (M/M) and from five subjects with severe gastritis (S). Results are expressed as neutrophil or monocyte response with fMLP treatment or without (i.e., after preincubation in sonicate or after preincubation in medium). Each strain was examined by using cells from four to six different healthy volunteers, and the results are expressed as the mean value from the results for each strain (horizontal bars indicate median values).
Chronic active gastritis with H. pylori will progress to clinical disease in only a minority of cases, but the relative role of bacterial factors and host responses in the pathogenesis is unknown. Previous reports have suggested that H. pylori strains from ulcer patients have stronger potential for activation of neutrophil oxidative burst than strains from patients with gastritis only (17, 18). These studies, however, have compared H. pylori strains from patients referred for upper gastrointestinal dyspepsia, and the role of H. pylori infection in nonulcer dyspepsia is controversial. In the present study, we chose to compare patients with definitive duodenal ulcer disease to healthy volunteers with asymptomatic H. pylori gastritis, hoping to better define any difference in bacterial factors responsible for inflammatory reactions.
Although H. pylori is a noninvasive pathogen, it has been proposed that protein components and secreted products of the bacterium transversing the epithelial barrier may, by direct or indirect action on leukocytes, lead to their activation and enhanced local migration into mucosal tissue. A central prerequisite for transendothelial migration of leukocytes into the lamina propria of gastric mucosa is the primary adhesion of these cells to activated endothelium. This is regulated by the alteration of the density and avidity of different adhesion molecules present on the surface of the leukocytes and endothelial cells. Previous experiments suggest that H. pylori water-soluble extracts can promote neutrophil-endothelial cell-adhesive interactions by upregulating the β2-integrin CD11b/CD18 on the neutrophil surface (10, 11, 26) as well as the counterreceptor ICAM-1 (defined by CD54) on vascular endothelial tissue in the gastric mucosa (13) and on cultured gastric epithelial cell lines (7). Enders et al. found upregulation of CD11b/CD18 on neutrophils associated with a water-soluble protein destroyed by pronase and heat treatment (10). Preliminary experiments suggested a molecular mass between 30 and 100 kDa (10), whereas Evans et al. reported a protein of 150 kDa as the neutrophil-activating factor (11). Of 21 clinical isolates, all had proadhesive activity although with obvious variation in activity (11), but no analysis of an association of activity with clinical or histopathological diagnosis was provided (10, 11).
We found no difference in the β2-integrin upregulation on neutrophils or monocytes between the H. pylori strains from duodenal ulcer patients and those from asymptomatic volunteers. Likewise, histopathological assessment of the severity of gastritis was not correlated with in vitro activation properties of adherence molecule upregulation. Confirming previous observations, however, we found that induction of oxidative burst responsiveness in neutrophils correlates with severity of inflammation assessed in mucosal biopsy specimens.
Acknowledgments
The expert technical assistance of Susan Small, Birgitte Sander Nielsen, and Anne Elbaek is appreciated.
The study was supported by a grant from the medical association of the county of Northern Jutland, Denmark, from Aalborg Stifts Julelotteri, from the U.S. Department of Veterans Affairs, and NIH/NCI grant R01 CA67469.
REFERENCES
- 1.Alam K, Schubert T T, Bologna S D, Ma C K. Increased density of Helicobacter pylori on antral biopsy is associated with severity of acute and chronic inflammation and likelihood of duodenal ulceration. Am J Gastroenterol. 1992;87:424–428. [PubMed] [Google Scholar]
- 2.Andersen L P, Espersen F, Souckova A, Sedlackova M, Soucek A. Isolation and primary evaluation of a low-molecular-mass antigen preparation for improved detection of Helicobacter pylori immunoglobulin G antibodies. Clin Diagn Lab Immunol. 1995;2:156–159. doi: 10.1128/cdli.2.2.156-159.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arnaout M A. Structure and function of the leucocyte adhesion molecules CD11/CD18. Blood. 1990;75:1037–1050. [PubMed] [Google Scholar]
- 4.Atherton J C, Tham K T, Peek R M, Cover T L, Blaser M J. Density of Helicobacter pylori infection in vivo as assessed by quantitative culture and histology. J Infect Dis. 1996;174:552–556. doi: 10.1093/infdis/174.3.552. [DOI] [PubMed] [Google Scholar]
- 5.Bayerdörffer E, Oertel H, Lehn N, Kasper G, Mannes G A, Sauerbach T, Stolte M. Topographic association between active gastritis and Campylobacter pylori colonisation. J Clin Pathol. 1989;42:834–839. doi: 10.1136/jcp.42.8.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borregaard N, Kjeldsen L, Sengeloev H, Diamond M S, Springer T A, Anderson H C, Kishimoto T K, Bainton D F. Changes in subcellular location and surface expression of l-selectin, alkaline phosphatase and Mac-1 in human neutrophils during stimulation with inflammatory mediators. J Leucoc Biol. 1994;56:80–87. doi: 10.1002/jlb.56.1.80. [DOI] [PubMed] [Google Scholar]
- 7.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Muller M, Shermab P, Patel J, Jin J, Ernst P B. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 8.Davies G R, Banatvala N, Collins C E, Sheaff M T, Abdi Y, Clements L, Rampton D S. Relationship between infective load of Helicobacter pylori and reactive oxygen metabolite production in antral mucosa. Scand J Gastroenterol. 1994;29:419–424. doi: 10.3109/00365529409096832. [DOI] [PubMed] [Google Scholar]
- 9.Dixon M F, Genta R M, Yardley J H, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–81. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Enders G, Brooks W, Bayerdörffer E, Hatz R. Expression of adhesion molecules on human granulocytes after stimulation with Helicobacter pylori membrane proteins: comparison with membrane proteins from other bacteria. Infect Immun. 1995;63:2473–2477. doi: 10.1128/iai.63.7.2473-2477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Evans D J, Jr, Evans D G, Takemura T, Nakano H, Lampert H C, Graham D Y, Granger D N, Kvietys P R. Characterization of Helicobacter pylori neutrophil-activating protein. Infect Immun. 1995;63:2213–2220. doi: 10.1128/iai.63.6.2213-2220.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Genta R M, Robason G O, Graham D Y. Simultaneous visualization of Helicobacter pylori and gastric morphology: a new stain. Hum Pathol. 1994;25:221–226. doi: 10.1016/0046-8177(94)90191-0. [DOI] [PubMed] [Google Scholar]
- 13.Hatz R A, Rieder G, Stolte M, Bayerdorffer E, Meimarakis G, Schildberg F W, Enders G. Pattern of adhesion molecule expression on vascular endothelium in Helicobacter pylori-associated antral gastritis. Gastroenterology. 1997;112:1908–1919. doi: 10.1053/gast.1997.v112.pm9178683. [DOI] [PubMed] [Google Scholar]
- 14.Khulusi S, Mendall M A, Patel P, Levy J, Badve S, Northfield T C. Helicobacter pylori infection density and gastric inflammation in duodenal ulcer and non-ulcer subjects. Gut. 1995;37:319–324. doi: 10.1136/gut.37.3.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mai U E H, Perez-Perez G I, Allen J B, Wahl L M, Wahl S M, Blaser M J. Surface proteins from Helicobacter pylori exhibit chemotactic activity for human leucocytes and are present in gastric mucosa. J Exp Med. 1992;175:517–525. doi: 10.1084/jem.175.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moss S F, Legons S, Davies J, Calam J. Cytokine gene expression in Helicobacter pylori associated antral gastritis. Gut. 1994;35:1567–1570. doi: 10.1136/gut.35.11.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nielsen N, Andersen L P. Chemotactic activity of Helicobacter pylori sonicate for human polymorphonuclear leucocytes and monocytes. Gut. 1992;33:738–742. doi: 10.1136/gut.33.6.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen H, Andersen L P. Activation of human phagocyte oxidative metabolism by Helicobacter pylori. Gastroenterology. 1992;103:1747–1753. doi: 10.1016/0016-5085(92)91430-c. [DOI] [PubMed] [Google Scholar]
- 19.Rachmilewitz D, Karmeli F, Eliakim R, Stalnikowicz R, Ackerman Z, Amir G, Stamler J S. Enhanced gastric nitric oxide synthase activity in duodenal ulcer patients. Gut. 1994;35:1393–1397. doi: 10.1136/gut.35.10.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rautelin H, Blomberg B, Fredlund H, Järnerot G, Danielsson D. Incidence of Helicobacter pylori strains activating neutrophils in patients with peptic ulcer disease. Gut. 1993;34:599–603. doi: 10.1136/gut.34.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sengeloev H, Kjeldsen L, Diamond M S, Springer T A, Borregaard N. Subcellular location and dynamics of Mac-1 in human neutrophils. J Clin Investig. 1993;92:1467–1476. doi: 10.1172/JCI116724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stolte, M., C. Bätz, S. Eidt, and E. Bayerdörffer. Hypertrophic gastritis in H. pylori infection, p. 362–371. In R. H. Hunt and G. N. J. Tytgat (ed.), Helicobacter pylori—basic mechanisms to clinical cure—1994. Kluwer Academic Publications, Lancaster, United Kingdom.
- 23.Watson F, Robinson J J, Edwards S W. Neutrophil function in whole blood and after purification: changes in receptor expression, oxidase activity and responsiveness to cytokines. Biosci Rep. 1992;2:123–133. doi: 10.1007/BF02351217. [DOI] [PubMed] [Google Scholar]
- 24.Winterbourne D J. Cell growth determined by a dye binding assay. Biochem Soc Trans. 1986;14:1179. [Google Scholar]
- 25.Yam L T, Li C Y, Crosby W H. Cytochemical identification of monocytes and granulocytes. Am J Pathol. 1971;55:283–290. doi: 10.1093/ajcp/55.3.283. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida N, Granger D N, Evans D J, Jr, Evans D G, Graham D Y, Anderson D C, Wolf R E, Kvietys P R. Mechanisms involved in Helicobacter pylori-induced inflammation. Gastroenterology. 1993;105:1431–1440. doi: 10.1016/0016-5085(93)90148-6. [DOI] [PubMed] [Google Scholar]