Abstract
Objectives
To assess the efficacy and safety of olokizumab (OKZ), a monoclonal antibody against the interleukin-6 (IL-6) cytokine, versus placebo (PBO) in patients with prior inadequate response to tumour necrosis factor inhibitors (TNFi-IRs).
Methods
In this 24-week multicentre, placebo-controlled, double-blind study, the patients were randomised in a 2:2:1 ratio to receive subcutaneously administered OKZ 64 mg once every 2 weeks (q2w), OKZ 64 mg once every 4 weeks (q4w) or PBO plus methotrexate. At week 16, the patients on PBO were randomised to receive either OKZ regime. The primary endpoint was the proportion of patients achieving an American College of Rheumatology 20% (ACR20) response at week 12. Disease Activity Score 28-joint count C-reactive protein (DAS28 (CRP))<3.2 at week 12 was the major secondary efficacy endpoint. Safety and immunogenicity were assessed.
Results
In 368 patients randomised, ACR20 response rates were 60.9% in OKZ q2w, 59.6% in OKZ q4w and 40.6% in PBO (p<0.01 for both comparisons). Achievement of DAS28 (CRP) <3.2 was significantly different, favouring the OKZ arms. Improvements in efficacy and patient-reported outcomes were maintained throughout 24 weeks and were noted after week 16 in patients who switched from PBO.
Dose-related treatment-emergent serious adverse events were 7% in OKZ q2w, 3.2% in OKZ q4w and none in the PBO group.
Conclusions
Direct inhibition of IL-6 with OKZ resulted in significant improvements in the signs and symptoms of rheumatoid arthritis compared with PBO in TNF-IR patients with a similar safety profile as observed for monoclonal antibodies to the IL-6 receptor.
Trial registration number
Keywords: antirheumatic agents; arthritis, rheumatoid; autoimmune diseases
WHAT IS ALREADY KNOWN ON THIS TOPIC
Olokizumab (OKZ) is a new humanised monoclonal antibody targeting the interleukin-6 (IL-6) ligand in development for the treatment of rheumatoid arthritis (RA).
OKZ was previously shown to be safe and effective in two-dose ranging placebo controlled phase II studies conducted in patients with RA who had failed prior treatment with anti-tumour necrosis factor (TNF) biologics, and two phase III trials in those who were methotrexate inadequate responders.
WHAT THIS STUDY ADDS
This is a placebo-controlled randomised phase III trial conducted in patients with active RA despite prior treatment with anti-TNF agents.
In fact, an increasing medical need in patients with RA after failure of anti-TNF agents requires further adequately designed phase III trials to delineate their specific clinical outcomes.
The current CREDO 3 study met its predefined key efficacy endpoints and provided meaningful safety and efficacy data for two dose regimens of olokizumab.
It adds to accumulating knowledge about targeting the IL-6 axis in general, and IL-6 ligand specifically.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
The CREDO programme includes three phase III randomised controlled trials (RCTs) each with its specific features to provide relevant clinical data for physicians in different clinical settings.
This study provides further evidence that OKZ, a direct inhibitor of IL-6, is safe and highly effective and thus represents a new treatment approach in the management of refractory RA.
Introduction
Rheumatoid arthritis (RA) is a chronic progressive autoimmune disease that primarily affects the joints and is associated with significant morbidity, mortality and reduced quality of life, when insufficiently treated.1–3 Early treatment of RA with conventional synthetic disease modifying drugs (csDMARDs) such as methotrexate (MTX) in a treat-to-target setting is recommended. Although tumour necrosis factor inhibitors (TNFis) are frequently used in patients with active RA who fail to achieve their treatment goal with MTX,4 5 both American College of Rheumatology (ACR) and European Alliance of Associations for Rheumatology (EULAR) suggest that after MTX, a biological DMARD (bDMARD) or targeted synthetic DMARD (tsDMARD) may be used especially in patients with poor prognosis.3 6 There are several approved bDMARDs and tsDMARDs which target molecules beside TNF that have been shown to be effective in patients who fail to respond to TNFi. Interleukin-6 (IL-6) is a proinflammatory cytokine that has been shown to play a key role in the pathogenesis of RA.7 Currently, there are two approved bDMARDs for RA that target IL-6 by blocking the IL-6 receptor.8 9 While other agents have been studied that also target the IL-6 cytokine directly, none has been approved.10 As a potential relevant difference with respect to the mode of action, these anti-IL-6 monoclonal antibodies all target site 1 of the cytokine, whereas olokizumab (OKZ) binds to site 3.11 OKZ was previously shown to be generally safe and effective in reducing signs and symptoms of active RA in patients with an incomplete response to TNFi in two relatively small and short-term phase II randomised controlled trials (RCTs).12 13 Two phase III study of OKZ in MTX-IR was previously reported with positive results.14 15 In the present global phase III study, we evaluated the efficacy and safety of OKZ 64 mg every 2 weeks (q2w) and every 4 weeks (q4w) in patients with active RA and inadequate response to TNFi.
Methods
Study design
This study was a 24-week phase III, randomised, double-blind, placebo-controlled, multicentre trial (ClinicalTrials.gov Identifier NCT02760433, CREDO 3), conducted at 123 centres in 11 countries across Asia, EU, Latin America, Russia and the USA from January 2017 to October 2019. After week 24, the patients were offered the opportunity to participate in an open-label extension study (OLE) or stop the drug and enter the safety follow-up period (SFU) of 20 weeks duration.
Patient inclusion and exclusion criteria
Adult patients with active RA (swollen joint count ≥6 (66-joint count), tender joint count ≥6 (68-joint count) and CRP >6 mg/L) meeting the ACR/EULAR 2010 revised classification criteria8 for at least 24 weeks prior to screening, and who received treatment with MTX for at least 12 weeks prior to screening at a dose of 15 to 25 mg/week (or ≥10 mg/week if intolerant to higher doses) were enrolled. The patients had failed to achieve an adequate response to >1 anti-TNF agent after at least 12 weeks of treatment. Prior use of other bDMARDs, with the exception of other anti-IL-6 or anti-IL-6R products, and cell depleting agents other than rituximab, was allowed if the drugs were discontinued at least for a specified period of time before randomisation. Non-steroidal anti-inflammatory drugs and glucocorticoids in doses<10 mg/day prednisone or equivalent were allowed in stable doses. Patients with latent tuberculosis infection were allowed to participate if they had started appropriate anti-TB therapy at least 30 days prior to randomisation (online supplementary materials, online supplemental table S1; exclusion criteria in the online supplemental materials).
ard-2022-222630supp001.pdf (462.1KB, pdf)
Randomisation and blinding
Patients were randomised 2:2:1 to receive subcutaneous injections of OKZ 64 mg q2w, OKZ 64 mg q4w or placebo (PBO) for 24 weeks using an automated randomisation system. At week 16, all subjects in the PBO group were randomised in a 1:1 ratio in a blinded fashion to receive either OKZ SC 64 mg q2w or 64 mg q4w. Subjects who discontinued the randomised treatment prior to week 24 were requested to continue the study without study treatment.
All patients, investigators, clinical site staff, contract research organisation’s staff and the sponsor’s staff involved in the study were blinded. Joint assessments were performed by independent assessors, blinded to study drug assignment and all other study assessments (online supplemental materials).
Rescue medication
At week 14, non-responders, defined as subjects who did not improve by at least 20% in both swollen and tender joint counts, in all study arms were prescribed rescue medication (sulfasalazine and/or hydroxychloroquine) in addition to the study treatment (online supplemental materials).
Endpoints
The primary endpoint was the proportion of patients achieving the American College of Rheumatology 20 (ACR20) response at week 12.
Ranked secondary endpoints were percentage of subjects achieving Disease Activity Score 28-joint count C-reactive protein (DAS28 (CRP))<3.2, improvement in the Health Assessment Questionnaire-Disability Index (HAQ-DI), ACR50 response and percentage of subjects with Clinical Disease Activity Index (CDAI) ≤2.8 (remission), all at week 12 (online supplemental materials).
Other patient-reported outcomes (PROs) were Short Form-36 Health Survey (SF-36), Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-Fatigue) and European Quality of Life Questionnaire 5-Dimensions (EQ-5D).
Safety monitoring, including assessment of adverse events (AEs), serious adverse events (SAEs) and laboratory tests via central laboratory were performed at multiple time points.
Determination of anti-drug antibodies (ADAs) in plasma samples was accomplished using electrochemiluminescense assay (Covance Laboratories, Otley Road, Harrogate, North Yorkshire, HG3 1PY, UK). For the detection of neutralising ADAs, a cell-based assay was used (Eurofins BioPharma Product Testing Munich GmbH, Robert-Koch-Str. 3a-Haus 2, 82152 Planegg/Munich, Germany).
An independent external Data and Safety Monitoring Board reviewed the safety data throughout the study. Major adverse cardiovascular events (MACEs) were adjudicated by a Cardiovascular Adjudication Committee. MACE included cardiovascular death or death from undetermined cause, non-fatal myocardial infarction, non-fatal stroke, transient ischaemic attack, hospitalisation for unstable angina requiring unplanned revascularisation and coronary revascularisation procedures.
Statistical analyses
To detect a difference between at least one OKZ dose regimen and placebo, a sample size of 320 patients randomised in a 2:2:1 ratio was estimated to ensure sufficient discriminatory power (99% for testing the primary hypothesis (ACR20 at week 12) and 82% for the primary secondary endpoint of DAS28 (CRP) <3.2 rate at week 12).
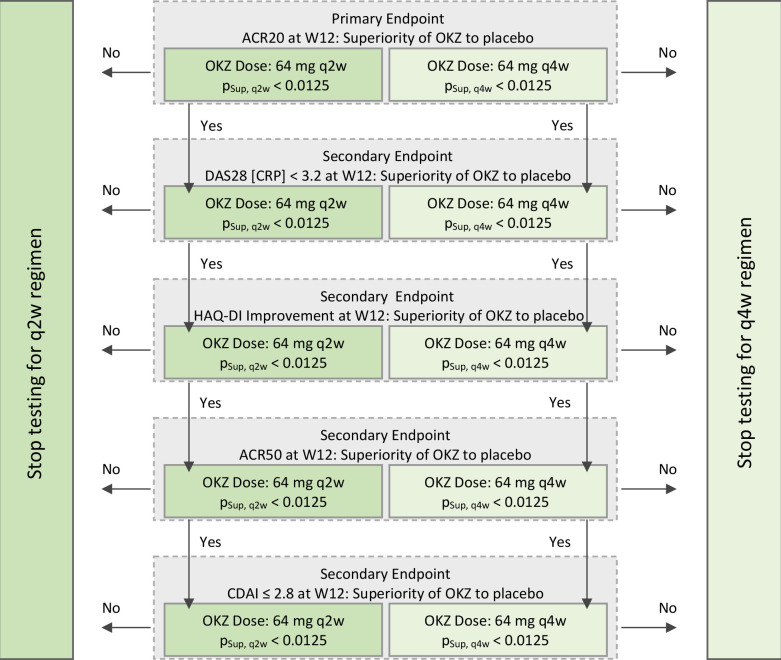
The ACR20 response at week 12 for each of the active treatment groups was compared with placebo using a 2×2 χ2 test for equality of proportions. To control the overall type I error rate at a one-sided α=0.025, the Bonferroni adjustment was used for the tests related to each of the OKZ dose regimens versus placebo (ie, one-sided α=0.0125 for each dose group for primary and secondary endpoints). The secondary endpoints that were binary in nature were analysed as per primary endpoint. Efficacy endpoints that were continuous in nature were analysed using an analysis of covariance model adjusted for the baseline value of the corresponding parameter. A gatekeeping strategy with a fixed order of hypotheses was used for the primary and secondary endpoints within each OKZ dose regimen independently (figure 1).
Figure 1.
Gatekeeping strategy. pSup, q2w and pSup, q4w represent p values from a one-sided test of superiority vs placebo for OKZ dose regimens 64 mg q2w and q4w, respectively. ACR, American College of Rheumatology; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; DAS28 (CRP), Disease Activity Score 28 based on CRP; HAQ-DI, Health Assessment Questionnaire Disability Index; OKZ, olokizumab; q2w, every 2 weeks; q4w, every 4 weeks; Wk, week.
For analyses of binary variables, inability to remain on randomised treatment through the time point of interest was defined as non-response with respect to the corresponding endpoint. In case of missing visits or assessments not performed for the reason other than treatment or study discontinuation, intermediate missing data were imputed using surrounding visits. For the analyses of continuous endpoints, subjects who discontinued randomised treatment prematurely but remained in the study through the time point of interest were included using all collected measurements, including those from assessments post-treatment discontinuation; in case of missing values, return to baseline values was assumed and was implemented using multiple imputation methodology allowing to account for the uncertainty of missing data according to the methodology of Rubin.16
The primary analysis was performed for the intent-to-treat (ITT) population defined as all randomised patients. The safety population included all subjects who received at least one dose of the study treatment (see online supplemental materials for additional details).
Protocol-specified statistical analyses were performed using Statistical Analysis System V.9.4 or higher (SAS Institute).
ResultsT
Disposition
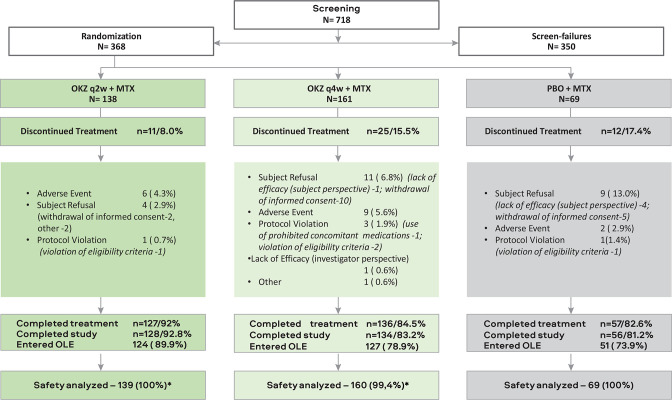
A total of 368 patients were randomised to OKZ 64 mg q2w (n=138), OKZ 64 mg q4w (n=161) or placebo (n=69) (figure 2). The three treatment groups were well balanced for baseline demographic and disease characteristics (table 1, online supplemental tables S2 and S3). The majority of patients had a previous exposure to TNF blockers of more than 6 months (online supplemental table S4).
Figure 2.
Patient disposition. *One patient was randomised to OKZ 64 mg q4w but actually received OKZ 64 mg q2w. Patients who discontinued treatment early and entered safety follow-up period were considered completers for the whole study if they performed all three follow-up visits. Therefore, the number of those who completed study can be higher than the number of treatment completers. AE, adverse event; IC, informed consent; ITT, intention-to-treat; MTX, methotrexate; N, number patient in the arm; N (%), number (%) patients; %, the percentage of subjects is calculated relative to the total number of subjects in the population; OKZ, olokizumab; OLE, open-label extension; PBO, placebo; q2w, every 2 weeks; q4w, every 4 weeks.
Table 1.
Demographic and other baseline characteristics (ITT population)*
| Characteristics, n (%) unless otherwise specified | OKZ q2w, n=138 | OKZ q4w, n=161 | PBO, n=69 |
| Age, years; mean (SD) | 53.4 (12.7) | 53.9 (11.7) | 53.0 (13.7) |
| Female | 122 (88.4) | 130 (80.7) | 55 (79.7) |
| Race | |||
| Asian | 6 (4.3) | 3 (1.9) | 2 (2.9) |
| Black or African American | 11 (8.0) | 11 (6.8) | 1 (1.4) |
| White | 110 (79.7) | 139 (86.3) | 53 (76.8) |
| Other/mixed | 11 (8.0) | 8 (5.0) | 13 (18.8) |
| Ethnicity | |||
| Hispanic or Latino ethnicity | 64 (46.4) | 77 (47.8) | 42 (60.9) |
| Not Hispanic or Latino | 74 (53.6) | 84 (52.2) | 27 (39.1) |
| Duration of RA, years; mean (SD) | 11.8 (9.2) | 12.7 (8.8) | 9.8 (7.0) |
| MTX dose, mg*; mean (SD) | 16.3 (3.7) | 16.7 (3.8) | 16.5 (3.8) |
| Duration of prior MTX use, months; mean (SD) | 74.7 (68.2) | 71.3 (56.7) | 66.3 (56.7) |
| Systemic corticosteroids use | 78 (56.5) | 94 (58.4) | 46 (66.7) |
| Prednisone dose or equivalent, mg; mean (SD) | 5.9 (2.3) | 6.0 (2.3) | 5.9 (2.1) |
| Prior exposure to ≥2 bDMARD | 26 (18.8) | 36 (22.4) | 16 (23.2) |
| Prior exposure to ≥3 bDMARD | 4 (2.9) | 10 (6.2) | 6 (8.7) |
| BMI, kg/m; mean (SD) | 28.8 (7.0) | 29.2 (6.0) | 28.4 (5.6) |
| RF+ (≥20 IU/mL) | 105 (76.1) | 128 (79.5) | 55 (79.7) |
| Anti-CCP+ (>10 U/mL) | 96 (69.6) | 124 (77.0) | 58 (84.1) |
| CRP (mg/L)†; mean (SD) | 20.7 (21.7) | 21.4 (24.3) | 19.4 (20.2) |
| TJC‡; mean (SD) | 26.0 (13.7) | 25.6 (12.8) | 28.2 (13.7) |
| SJC‡; mean (SD) | 16.8 (8.2) | 17.0 (7.8) | 19.3 (9.5) |
| DAS28 (CRP); mean (SD) | 5.9 (0.9) | 6.0 (0.8) | 6.2 (0.9) |
| CDAI (0–76); mean (SD) | 40.7 (12.5) | 41.7 (10.6) | 44.4 (11.7) |
| HAQ-DI; mean (SD) | 1.8 (0.6) | 1.8 (0.6) | 1.8 (0.6) |
| HAQ-DI <0.5, n (%) | 2 (1.4) | 3 (1.9) | 5 (7.2) |
| PtGA (VAS) (mm); mean (SD) | 64.8 (20.5) | 68.1 (19.1) | 72.1 (18.5) |
| Pain (VAS) (mm); mean (SD) | 67.2 (19.5) | 69.3 (19.1) | 69.6 (21.9) |
| PGA (VAS) (mm); mean (SD) | 64.6 (17.8) | 65.9 (17.5) | 69.5 (14.9) |
*100% patients were on MTX.
†Upper limit of normal=6 mg/L.
‡Joints were assessed based on 66–68 joint counts.
Anti-CCP, anti-cyclic citrullinated peptide positivity; BMI, body mass index; CDAI, Clinical Disease Activity Index; DAS28 (CRP), Disease Activity Score 28 based on C-reactive protein; HAQ-DI, Health Assessment Questionnaire Disability Index; IIT, intention-to-treat; MTX, methotrexate; N, number of subjects; OKZ, olokizumab; Pain, patient assessment of pain; PBO, placebo; PGA, Physician Global Assessment of Disease Activity; PtGA, Patient Global Assessment of Disease Activity; q2w, every 2 weeks; q4w, every 4 weeks; RA, rheumatoid arthritis; RF+, rheumatoid factor positivity; SJC, swollen joint count; TJS, tender joint count; VAS, visual analogue scale.
A total of 326 patients completed week 16 of the study: 129 (93.5%) in OKZ q2w, 139 (86.3%) in OKZ q4w and 58 (84.1%) in the placebo treatment group. Of patients randomised to placebo, 32 and 26 were re-randomised to OKZ q2w and OKZ q4w groups, respectively.
A total of 87.0% (320) of randomised subjects completed the treatment period of 24 weeks: 127 (92.0%) in OKZ q2w, 136 (84.5%) in OKZ q4w, 31 (96.9%) in placebo to OKZ q2w and 26 (100%) in placebo to OKZ q4w group. Most of the patients in the study rolled over to OLE; a minority continued to SFU (9 (6.5%) in OKZ q2w, 14 (8.7%) in OKZ q4w, 2 (6.3%) in placebo to OKZ q2w and 3 (11.5%) in placebo to OKZ q4w group) (figure 2).
Efficacy
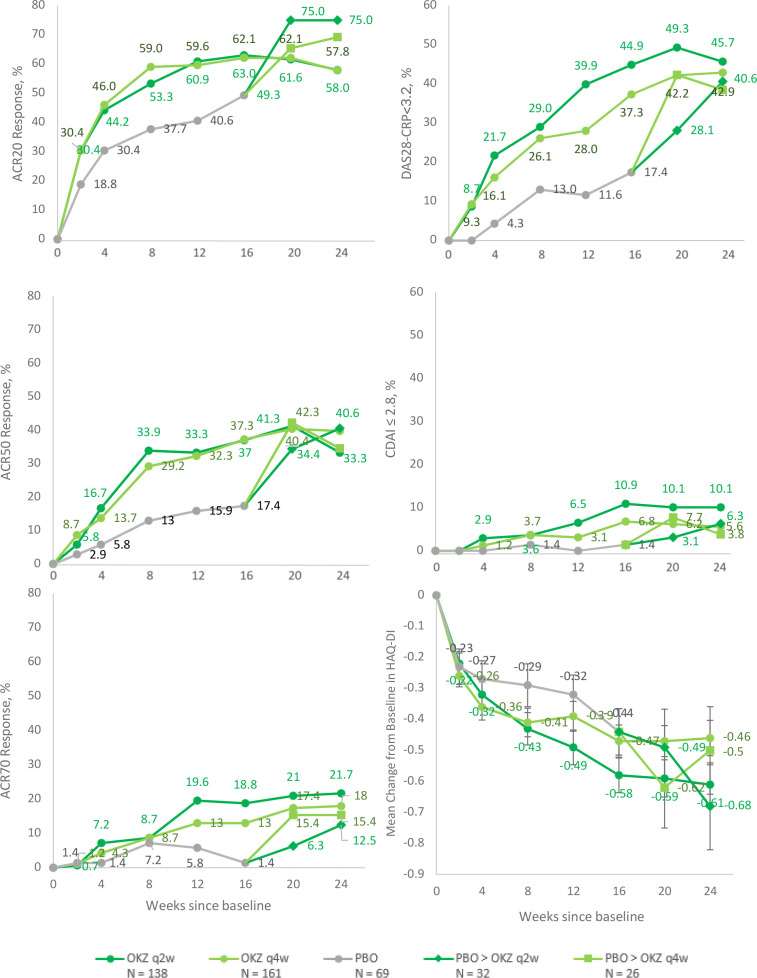
The primary efficacy endpoint, ACR20 response rate at week 12, was 60.9% in the OKZ q2w group and 59.6% in the OKZ q4w group compared with 40.6% in the placebo group (p<0.01 for both comparisons) (table 2, figure 3). Achievement of ACR20 response in the OKZ treatment groups separated from the placebo group as early as week 2 and persisted throughout the 24-week treatment period (figure 3, online supplemental figure S1). Statistically significant difference in the first secondary endpoint in the hierarchy (DAS28 (CRP) <3.2 at week 12) was observed in patients receiving either dose of OKZ compared with PBO (p<0.0001 for OKZ q2w and 0.0035 for OKZ q4w) (table 2).
Table 2.
Main efficacy results at week 12 in the intent‐to‐treat population
| Outcomes, n (%) unless otherwise specified | OKZ q2w, n=138 | OKZ q4w, n=161 | PBO, n=69 |
| Primary endpoint | |||
| ACR20 response (NRI) | 84 (60.9) | 96 (59.6) | 28 (40.6) |
| Comparison vs PBO risk difference | 0.203 (0.038 to 0.353)** | 0.190 (0.030 to 0.337)** | |
| Secondary endpoints | |||
| DAS28 (CRP) <3.2 | 55 (39.9) | 45 (28.0) | 8 (11.6) |
| Comparison vs PBO risk difference* | 0.283 (0.139 to 0.396)*** | 0.164 (0.029 to 0.268)** | |
| HAQ-DI LSM (SE), mean difference from baseline | −0.49 (0.05) | −0.39 (0.04) | −0.32 (0.07) |
| Comparison vs PBO risk difference* | −0.17 (−0.35 to 0.02)* | −0.07 (−0.26 to 0.11) | |
| ACR50 response (NRI) | 46 (33.3) | 52 (32.3) | 11 (15.9) |
| Comparison vs PBO risk difference* | 0.174 (0.027 to 0.294)** | 0.164 (0.020 to 0.278)** | |
| CDAI≤2.8 (NRI) | 9 (6.5) | 5 (3.1) | 0 |
| Comparison vs PBO risk difference* | 0.065 (−0.023 to 0.134)* | 0.031 (−0.052 to 0.083) | |
| Other endpoints | |||
| DAS28 (CRP) <2.6† | 30 (21.7) | 25 (15.5) | 3 (4.3) |
| Comparison vs PBO risk difference* | 0.174 (0.059 to 0.267)** | 0.112 (0.005 to 0.192)* | |
| CDAI <10† | 43 (31.2) | 41 (25.5) | 9 (13.0) |
| Comparison vs PBO risk difference* | 0.181 (0.040 to 0.296)** | 0.124 (−0.011 to 0.231)* | |
| ACR70 response (NRI) | 27 (19.6) | 21 (13.0) | 4 (5.8) |
| Comparison vs PBO risk difference* | 0.138 (0.021 to 0.232)** | 0.072 (−0.037 to 0.153) | |
| HAQ-DI improvement of ≥0.22 (NRI) | 75 (54.3) | 89 (55.3) | 33 (47.8) |
| Comparison vs PBO risk difference* | 0.08 (−0.086 to 0.236) | 0.074 (−0.084 to 0.229) | |
*p≤0.025; **p<0.01; ***p<0.001 compared with placebo.
*97.5% CI was calculated for comparison of OKZ vs PBO
†Not predefined by protocol (post hoc).
ACR, American College of Rheumatology response; CDAI, Clinical Disease Activity Index; CRP, C-reactive protein; DAS28 (CRP), Disease activity Score 28 based on CRP; HAQ-DI, Health Assessment qQuestionnaire Disability Index; LSM, least squares mean; n (%), number and percentage of responders; N, number of subjects; NRI, non-responder imputation; OKZ, olokizumab; PBO, placebo; q2w, every 2 weeks; q4w, every 4 weeks.
Figure 3.
Efficacy results during the double‐blind treatment period (ITT population). ACR, American College of Rheumatology; CDAI, Clinical Disease Activity Index; DAS28 (CRP), Disease Activity Score 28 based on C-reactive protein; HAQ-DI, Health Assessment Questionnaire Disability Index; ITT, intention-to-treat; OKZ, olokizumab; PBO, placebo; q2w, every 2 weeks; q4w, every 4 weeks.
While numerically higher improvements from baseline in HAQ-DI were observed at week 12 for subjects in OKZ q2w and OKZ q4w treatment groups in comparison to patients on PBO, the differences were not statistically significant at the prespecified level of p<0.0125 (p=0.0227 for OKZ q2w, p=0.1814 for OKZ q4w).
Due to the gatekeeping strategy of statistical testing, differences from placebo for the ranked outcomes of ACR50 and disease remission defined as CDAI <2.8 could not be assessed for statistical significance and should be considered nominal. Achievement of ACR70 was an exploratory endpoint and therefore not ranked in the hierarchy of statistical testing.
Subgroup analyses of the ACR20 response showed no influence of country, gender, age, weight, body mass index (BMI), baseline disease severity, time since diagnosis, duration of prior MTX use, or anti-CCP and RF status on the efficacy of OKZ (online supplemental figure S2 (region), other data available on request).
Re-randomisation from placebo to OKZ at week 16 resulted in prompt improvements in all assessed efficacy parameters (figure 3).
In parallel with the main efficacy endpoints, there were marked improvements in several PRO measurements such as SF-36 mental and physical component scores (table 3, online supplemental figure S1).
Table 3.
Mean baseline values and LSM changes from baseline to week 12 for PROs
| Baseline, mean (SD) | 12 weeks LSM changes (SE) | |||||
| OKZ q2w, n=138 | OKZ q4w, n=161 | PBO, n=69 | OKZ q2w, n=138 | OKZ q4w, n=161 | PBO, n=69 | |
| PtGA-VAS (mm) | 64.8 (20.5) | 68.1 (19.1) | 72.1 (18.5) | −24.9 (2.1) | −25.0 (1.9) | −16.9 (2.9) |
| Pain-VAS (mm) | 67.2 (19.5) | 69.3 (19. 1) | 69.6 (21.9) | −28.2 (2.2)** | −27.5 (2.0)** | −15.0 (3.0) |
| HAQ-DI | 1.79 (0.53) | 1.78 (0.56) | 1.78 (0.64) | −0.49 (0.05)* | −0.39 (0.04) | −0.32 (0.07) |
| SF-36 PCS score | 31.4 (6.8) | 30.6 (7.2) | 30.6 (5.9) | 6.9 (0.7)** | 5.7 (0.6) | 3.9 (0.9) |
| SF-36 MCS score | 44.3 (12.6) | 44.5 (11.1) | 45.1 (10.2) | 4.1 (0.8)* | 3.4 (0.8) | 0.5 (1.1) |
| FACIT-Fatigue | 27.0 (10.2) | 26.6 (10.6) | 27.3 (9.9) | 7.8 (0.9) | 6.8 (0.8) | 4.6 (1.2) |
| EQ-5D Health Today Score | 45.0 (23.35) | 43.7 (22.42) | 50.4 (28.31) | 17.8 (2.06) | 18.0 (1.92) | 12.6 (2.92) |
Missing data resulted from study withdrawal imputed based on the return to baseline assumption.
*p≤0.025; **p<0.01; ***p<0.001 compared with placebo.
*Secondary endpoint: OKZ q2w p=0.0227 and OKZ q4w p=0.1814 compared with placebo.
EQ-5D, EuroQol 5-Dimensions; FACIT, Functional Assessment of Chronic Illness Therapy-Fatigue; HAQ-DI, Health Assessment Questionnaire Disability Index; LSM, least squares mean; MCS, Mental Component Summary; OKZ, olokizumab; PBO, placebo; PCS, Physical Component Summary; PRO, patient-reported outcome; PtGA, Patient Global Assessment of Disease Activity; q2w, every 2 weeks; q4w, every 4 weeks; SF-36, Short Form-36 Health Survey; VAS, visual analogue scale.
Safety
A total of 238 patients (64.7%) reported treatment emergent adverse events (TEAE) up to week 44: 110 (64.3%) in any OKZ q2w group (those on OKZ q2w from randomisation and those who were re-randomised to this group from placebo at week 16), 111 (59.7%) in any OKZ q4w group and 35 (50.7%) on placebo (up to week 16) (online supplemental table S7). Most TEAEs were mild to moderate in severity and non-serious and infections were the most common TEAEs. TEAEs leading to study treatment discontinuation were more commonly observed in OKZ q2w (7 (4.1%) and OKZ q4w (10 (5.4%)) than in the PBO-treated patients (1 (1.4%)) for 16 weeks prior to re-randomisation (online supplemental table S7).
In total, 197 patients reported TEAEs up to week 16 (table 4). TESAEs were reported in 9 (6.5%) subjects in OKZ q2w group and in 3 (1.9%) in OKZ q4w group, no serious events were reported in the placebo group (table 4). An anaphylaxis reaction with lip oedema and decreased blood pressure was reported in a patient from the OKZ q4w treatment group. This adverse drug reaction resolved with prednisone 10 mg orally two times per day and loratadine 10 mg orally two times per day for 2 days. No TEAEs leading to death, MACE, active TB, or gastrointestinal perforations were reported during the study. TESAEs up to week 44 were numerically higher for the any OKZ 64 mg q2w group (online supplemental table S6). One opportunistic infection (non-serious Herpes zoster infection) was reported in the study in any OKZ q2w group (online supplemental table S5).
Table 4.
Incidence of treatment-emergent adverse events by system organ class in >than 3% of patients and serious adverse events up to week 16 (safety population)
| System organ class, n (%) | OKZ q2w n=139 |
OKZ q4w n=160 |
PBO n=69 |
| Subjects with ≥1 TEAE | 74 (53.2) | 88 (55.0) | 35 (50.7) |
| Blood and lymphatic system disorders | 7 (5.0) | 8 (5.0) | 5 (7.2) |
| Gastrointestinal disorders | 12 (8.6) | 10 (6.2) | 6 (8.7) |
| General disorders and administration site conditions | 7 (5.0) | 12 (7.5) | 3 (4.3) |
| Hepatobiliary disorders | 6 (4.3) | 5 (3.1) | 1 (1.4) |
| Infections and infestations | 28 (20.1) | 36 (22.5) | 18 (26.1) |
| Injury, poisoning and procedural complications | 3 (2.2) | 10 (6.2) | 1 (1.4) |
| Investigations | 21 (15.1) | 21 (13.1) | 4 (5.8) |
| Metabolism and nutrition disorders | 9 (6.5) | 11 (6.9) | 1 (1.4) |
| Musculoskeletal and connective tissue disorders | 9 (6.5) | 8 (5.0) | 5 (7.2) |
| Nervous system disorders | 3 (2.2) | 5 (3.1) | 2 (2.9) |
| Skin and subcutaneous tissue disorders | 9 (6.5) | 12 (7.5) | 1 (1.4) |
| Vascular disorders | 4 (2.9) | 3 (1.9) | 3 (4.3) |
| TEAE, leading to death | 0 | 0 | 0 |
| Subjects with ≥1 TESAE* | 9 (6.5) | 3 (1.9) | 0 |
n, number of subjects; %, percentage of subjects calculated relative to the total number of subjects in the treatment arm. MedDRA (Medical Dictionary for Regulatory Activities) V.21.1 was used to code AEs. A TEAE is defined as an AE that first occurred or worsened in severity after the first dose of the study treatment.
*TEASE by organ class/preferred term were: 1 pt with hepatobiliary disorders/cholecystitis; 1 pt with immune system disorders/anaphylactic reaction; 3 pts with infections and infestations/cellulitis (1pt), pilonidal cyst (1pt), sepsis (1pt); 3 pts with investigations/alanine aminotransferase increased (1pt), aspartate aminotransferase increased (1pt), transaminases increased (1pt); 2 pts with musculoskeletal and connective tissue disorders/intervertebral disc protrusion (1pt), musculoskeletal chest pain (1pt); 1pt with psychiatric disorders/anxiety and 1 pt with vascular disorders/hypertensive crisis.
pt, patient; TEAE, treatment-emergent adverse event; TESAE, treatment-emergent serious adverse event.
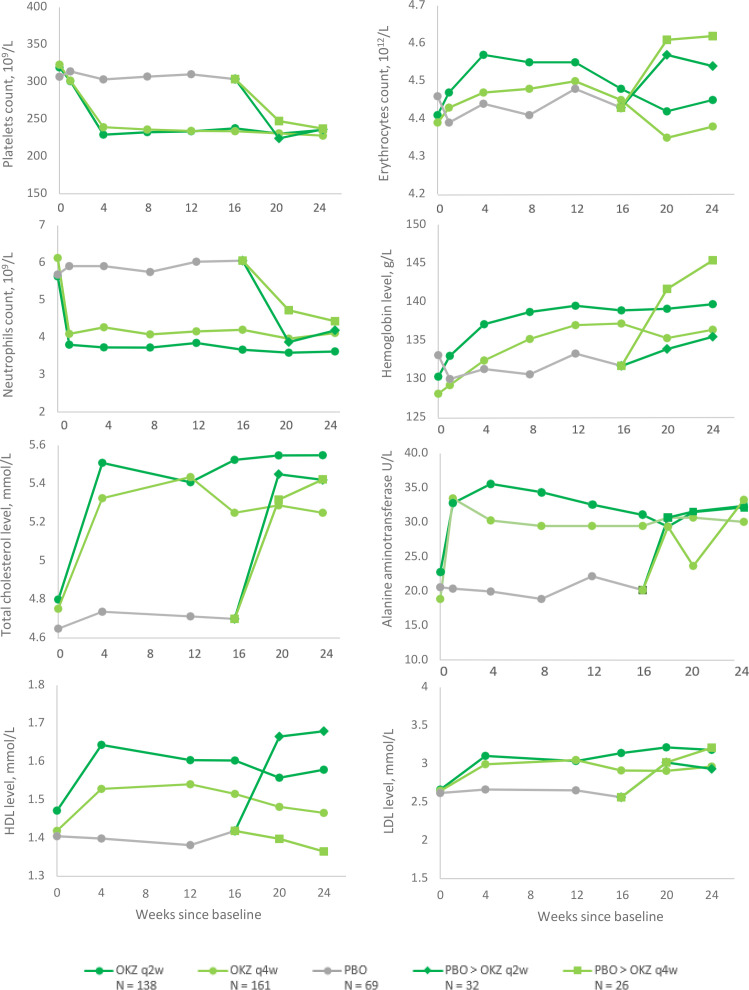
Elevations in serum ALT value from 1x ULN to ≤3x ULN at any time during the study were seen in 17 (12.2%) patients in any OKZ q2w, in 12 (7.5%) subjects in any OKZ q4w and in 6 (8.7%) in the PBO group; and elevations above 3x ULN ALT were seen in OKZ arms only: 12 subjects (8.7%) and 16 subjects (10%), respectively, none with concomitant elevation of bilirubin >2x ULN (online supplemental table S9). Other selected abnormal results of haematology and chemistry assessments are presented (online supplemental tables S8 and S9), as well as mean changes in laboratory values dynamic are shown (figure 4).
Figure 4.
Mean changes in laboratory values during the double‐blind treatment period (Safety population). HDL, high-density lipoproteins; LDL, low-density lipoproteins; OKZ, olokizumab; PBO, placebo; q2w, every 2 weeks; q4w, every 4 weeks. Elements of these data were presented at the annual meeting of the American College of Rheumatology 202129 and the British Society of Rheumatology Conference 2021.30
Overall, 23 subjects (6.9%) had positive confirmatory ADA results at any time post-baseline among patients who received OKZ with no neutralising antibodies detected. Although the clinical significance of this is not clear for the general RA population, there was no difference in clinical responses or safety outcomes in the patients who developed ADA compared with those who did not in this study.
Discussion
This phase III study was conducted to assess efficacy and safety of OKZ in TNFi-IR patients with active RA, a population of patients in high need of additional therapies. The study met the primary endpoint and the first secondary endpoint of DAS28 (CRP) <3.2: it was shown that both dose regimens of OKZ were statistically superior to placebo for these two key endpoints. Moreover, there were numerically higher clinical responses observed in most clinical and some PRO domains with OKZ every 2 week compared with the OKZ every 4 week, but the study was neither designed nor powered to detect differences between doses.
Several clinical outcomes did not show significant improvement by week 12 including HAQ-DI and evidence of deep response determined by CDAI remission. However, more stringent endpoints generally do not plateau by 12 weeks (which was chosen as the time for assessment of the primary endpoint for ethical reasons); they usually plateau by week 20 to week 24 and achieve significance compared with placebo.17 18 Indeed, increased levels of improvement were also observed in this study between week 12 and week 24, as seen in figure 3. Regarding the HAQ-DI, it is well established that with increasing disease duration the difference between active treatment and placebo decreases until it disappears, presumably due to an increasing irreversibility of functional impairment with increasing damage, related to RA duration.19 20
Because of the failure of statistical significance for HAQ-DI, subsequent secondary endpoints could only be statistically evaluated with nominal p values. Using nominal p values, the ranked secondary endpoints of ACR50 and CDAI <2.8 were supportive of the primary endpoint. Clinical efficacy of OKZ was sustained throughout the entire 24-week treatment period. Importantly, re-randomisation from placebo to OKZ at week 16 resulted in prompt improvements in all disease activity parameters to the degree that these patients approached the same level of disease control by week 24 as those who received OKZ for the entire 24-week period.
Reductions in disease activity were paralleled by improvements in most PROs including SF-36 (both physical and mental), pain, EQ-5D and fatigue.
OKZ was generally safe and well tolerated with few subjects discontinuing treatment. However, a dose-dependent increase of SAEs was observed with more SAE in the q2w regimen; this had not been observed in other studies with OKZ in RA.14 21
There were no deaths, few serious infections and no unexpected safety findings. The safety profile of OKZ, including its effect on serum lipids and hepatic transaminases, was consistent with that seen in other studies of OKZ as well as the approved anti-IL-6 drugs tocilizumab and sarilumab.8 9 The findings suggest that there may be a numerical advantage with respect to some clinical outcomes with the q2w regimen versus the q4w regimen counterbalanced by better safety with the q4w regimen; however, this trial may be too small to draw any definitive conclusions with respect to the optimal dose of OKZ in an individual patient. Post-marketing surveillance and registry data are required to capture further information on rare safety issues, as has been done with other agents.
It has been shown that proinflammatory cytokines such as IL-6 play an essential role in the pathogenesis of RA and the inhibition of the signal cascade at the IL-6 receptor is an established and highly effective approach in the treatment of RA. The IL-6 ligand itself has the potential to be a particularly attractive therapeutic target due to the presumable different levels of the circulating pluripotent cytokine and expression of its soluble as well as cell-associated receptors. It is thus important to fully explore this mode of action, especially in patients who have failed an anti-TNF agent.
With respect to the potential antigenic sites of IL-6,22 sirukumab and clazakizumab target site 1; interfering with the binding of IL-6 to the cognate IL-6R (IL-6Rα) in the trimolecular IL-6–IL-6R–gp130 complex. Of note, olokizumab binds to site 3 and inhibits the interaction of IL-6 and the IL-6–IL6-R dimer with the signal-transducing β-receptor subunit gp130 of the receptor complex.12–14 21 As a result, OKZ blocks the final hexamer formation on the molecular level, while the other anti-IL-6 inhibitors prevent dimer formation. This has the advantage that dimers of IL-6 and the soluble IL-6R cannot continue to bind to the signalling moiety of the receptor on the cell membrane with continued cell activation.
The mode of action is also different from the two approved IL-6 pathway inhibitors, which are monoclonal antibodies to the IL-6 receptor. In theory, sIL-6R levels far exceeds those of the IL-6 cytokine in patients with RA and therefore neutralisation of the ligand requires less monoclonal antibody than targeting the IL-6R. This could represent a significant pharmacokinetic and pharmacodynamic difference compared with the IL-6R blockers.23 24
The advantages of OKZ are, that as a direct inhibitor of IL-6, less protein needs to be injected to obtain a therapeutic response, and every 4-week dosing may be advantageous to the patient rather than the weekly or every 2-week dosing required with the two approved anti-IL-6R antibodies.
Two other IL-6 ligand blockers, sirukumab and clazakizumab, have been evaluated in RA. Although both drugs have demonstrated clinical efficacy, sirukumab was not approved by the United States Food and Drug Administration for RA due to an observed increased mortality with prolonged treatment. (NCT01604343).10 Although clazakizumab showed efficacy in phase 2 (NCT02015520), the company stopped further development in RA in favour of an ongoing investigation in chronic kidney transplant rejection (NCT03744910).
Major limitations of this study are its relatively small size, although comparable to studies of other molecules in this patient group, which limits the generalisability of our findings, and the short placebo-controlled portion (for ethical reasons).
The high placebo response rate is another limitation. This phenomenon has been observed in the more recent trials in RA.25 26 Proposed reasons for this are better adherence to MTX due to the scrutiny of the investigators in current clinical trials.27 28 Similar to other studies in patients with RA who are TNF-IR, an active comparator arm was not used.
In summary, this study confirms and extends the results of the two previous phase III trials demonstrating significant efficacy with acceptable toxicity for this novel IL-6 inhibitor.
Conclusion
In this phase III trial in patients with active RA inadequately controlled by TNF-α inhibitor therapy, treatment with OKZ 64 mg q2w and 64 mg q4w plus MTX was associated with significant improvements in the signs and symptoms of RA compared with PBO plus MTX over a 24-week period with a safety profile similar to approved IL-6 inhibitors.
Footnotes
Handling editor: Désirée van der Heijde
Contributors: CJSC R-Pharm was involved in the study design, collection, and analysis, interpretation of data and checking of information provided in the manuscript. MS, SF and EK were involved with study conceptualisation and conducted the data analysis. All authors had unrestricted access to study data and contributed to the interpretation of the results. The authors received no honoraria related to the development of this publication. All authors were responsible for all content and editorial decisions. Medical writing assistance, under the direction of the authors, and editorial support was provided by Sofia Kuzkina, MD (CJSC R-Pharm, RF) according to CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials (https://www.bmj.com/content/340/bmj.c332) and Good Publication Practice guidelines (http://annals.org/aim/article/2424869). EF is responsible for the overall content as guarantor.
Funding: NCT02760433 RCT was funded by CJSC R-Pharm.
Competing interests: The analysis was funded by JSC R-Pharm. EF: Research grants from BMS, Eli Lilly, Novartis, Roche; consulting fees from Abbvie, BMS, Eli Lilly, Gilead Sciences, Galapagos, Novartis, Roche, Sanofi, Sobi; speakers’ bureau for Abbvie, BMS, Eli Lilly, Gilead Sciences, Galapagos, Medac, Novartis, Roche, Sanofi, Sobi. SF: Consulting fees from ICON and PPD contract research organisations, shareholder of Pfizer, INC stocks, consulting fees from R-Pharm. EK: Employee of R-Pharm, with no R-Pharm stock; ML: Research grants from Amgen, Biogen, UCB, Sun Pharmaceuticals, Abbvie, Pfizer, Novartis, Lilly, GSK, R-Pharm. EN: Speakers’ bureau for AbbVie, Eli Lilly, Janssen, Novartis, Pfizer. SG and MS: Employees of R-Pharm, with no R-Pharm stock. JS: Research grants from Abbvie, Astra-Zeneca, Lilly; consulting fees from Abbvie, Galapagos/Gilead, Novartis-Sandoz, BMS, Samsung, Sanofi, Chugai, R-Pharma, Lilly; speakers’ bureau for Samsung, Lilly, R-Pharm, Chugai, MSD, Janssen, Novartis-Sandoz; Editor, ARD. RF: Consulting fees from AbbVie, BMS, Gilead, Galvani, GSK, Janssen, Eli Lilly, Novartis, Pfizer, R-Pharm, UCB; speakers bureaus for AbbVie; Pfizer; R-Pharm. Disclosure forms provided by the authors are available in the full text of this article.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available in a public, open access repository. Data are available upon reasonable request. Data generated by the present research available via https://clinicaltrials.gov and additional data are available on reasonable request to Sergey Grishin: sa.grishin@rpharm.ru.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
The study protocol was approved by all individual centres’ ethics committees and regulatory authorities and written informed consent was obtained from each patient. The study was conducted in accordance with the ICH GCP and the Declaration of Helsinki requirements.
References
- 1. Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet 2016;388:2023–38. 10.1016/S0140-6736(16)30173-8 [DOI] [PubMed] [Google Scholar]
- 2. Singh JA, Saag KG, Bridges SL, et al. 2015 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2016;68:1–26. 10.1002/art.39480 [DOI] [PubMed] [Google Scholar]
- 3. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis 2020;79:685–99. 10.1136/annrheumdis-2019-216655 [DOI] [PubMed] [Google Scholar]
- 4. Aletaha D, Smolen JS. Diagnosis and management of rheumatoid arthritis: a review. JAMA 2018;320:1360–72. 10.1001/jama.2018.13103 [DOI] [PubMed] [Google Scholar]
- 5. Johnson KJ, Sanchez HN, Schoenbrunner N. Defining response to TNF-inhibitors in rheumatoid arthritis: the negative impact of anti-TNF cycling and the need for a personalized medicine approach to identify primary non-responders. Clin Rheumatol 2019;38:2967–76. 10.1007/s10067-019-04684-1 [DOI] [PubMed] [Google Scholar]
- 6. Fraenkel L, Bathon JM, England BR, et al. 2021 American College of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol 2021;73:1108–23. 10.1002/art.41752 [DOI] [PubMed] [Google Scholar]
- 7. Hunter CA, Jones SA. Il-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 8. Genovese MC, van der Heijde D, Lin Y, et al. Long-Term safety and efficacy of sarilumab plus methotrexate on disease activity, physical function and radiographic progression: 5 years of sarilumab plus methotrexate treatment. RMD Open 2019;5:e000887. 10.1136/rmdopen-2018-000887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hushaw LL, Sawaqed R, Sweis G, et al. Critical appraisal of tocilizumab in the treatment of moderate to severe rheumatoid arthritis. Ther Clin Risk Manag 2010;6:143–52. 10.2147/tcrm.s5582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kenney B. Janssen receives complete response letter from U.S. FDA for sirukumab biologics license application, 2017. Available: https://www.jnj.com/media-center/press-releases/janssen-receives-complete-response-letter-from-us-fda-for-sirukumab-biologics-license-application [Accessed 1 Jul 2022].
- 11. Shaw S, Bourne T, Meier C, et al. Discovery and characterization of olokizumab: a humanized antibody targeting interleukin-6 and neutralizing gp130-signaling. MAbs 2014;6:774–82. 10.4161/mabs.28612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Genovese MC, Fleischmann R, Furst D, et al. Efficacy and safety of olokizumab in patients with rheumatoid arthritis with an inadequate response to TNF inhibitor therapy: outcomes of a randomised phase IIb study. Ann Rheum Dis 2014;73:1607–15. 10.1136/annrheumdis-2013-204760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takeuchi T, Tanaka Y, Yamanaka H, et al. Efficacy and safety of olokizumab in Asian patients with moderate-to-severe rheumatoid arthritis, previously exposed to anti-TNF therapy: results from a randomized phase II trial. Mod Rheumatol 2016;26:15–23. 10.3109/14397595.2015.1074648 [DOI] [PubMed] [Google Scholar]
- 14. Smolen JS, Feist E, Fatenejad S, et al. Olokizumab versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2022;387:715–26. 10.1056/NEJMoa2201302 [DOI] [PubMed] [Google Scholar]
- 15. Nasonov E, Fatenejad S, Feist E, et al. Olokizumab, a monoclonal antibody against interleukin 6, in combination with methotrexate in patients with rheumatoid arthritis inadequately controlled by methotrexate: efficacy and safety results of a randomised controlled phase III study. Ann Rheum Dis 2022;81:469–79. 10.1136/annrheumdis-2021-219876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rubin DB. Multiple imputation for nonresponse in surveys. Hoboken, NJ, USA: John Wiley & Sons, Inc, 1987. [Google Scholar]
- 17. Fleischmann R, van Adelsberg J, Lin Y, et al. Sarilumab and Nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol 2017;69:277–90. 10.1002/art.39944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fleischmann R, Pangan AL, Song I-H, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019;71:1788–800. 10.1002/art.41032 [DOI] [PubMed] [Google Scholar]
- 19. Aletaha D, Strand V, Smolen JS, et al. Treatment-Related improvement in physical function varies with duration of rheumatoid arthritis: a pooled analysis of clinical trial results. Ann Rheum Dis 2008;67:238–43. 10.1136/ard.2007.071415 [DOI] [PubMed] [Google Scholar]
- 20. Smolen JS, Aletaha D, Grisar JC, et al. Estimation of a numerical value for joint damage-related physical disability in rheumatoid arthritis clinical trials. Ann Rheum Dis 2010;69:1058–64. 10.1136/ard.2009.114652 [DOI] [PubMed] [Google Scholar]
- 21. Feist E, Chohan S, Fatenejad S, et al. P131 Efficacy and safety of olokizumab in a phase III trial of patients with moderately to severely active RA inadequately controlled by methotrexate: placebo and active controlled study. Rheumatology 2021;60:keab247.126. 10.1093/rheumatology/keab247.126 [DOI] [Google Scholar]
- 22. Hunter CA, Jones SA. Il-6 as a keystone cytokine in health and disease. Nat Immunol 2015;16:448–57. 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 23. Robak T, Gladalska A, Stepień H, et al. Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators Inflamm 1998;7:347–53. 10.1080/09629359890875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scheller J, Garbers C, Rose-John S. Interleukin-6: from basic biology to selective blockade of pro-inflammatory activities. Semin Immunol 2014;26:2–12. 10.1016/j.smim.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 25. Dougados M, van der Heijde D, Chen Y-C, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. 10.1136/annrheumdis-2016-210094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Combe B, Kivitz A, Tanaka Y, et al. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis 2021;80:848–58. 10.1136/annrheumdis-2020-219214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerschbaumer A, Rivai ZI, Smolen JS, et al. OP0127 CONFOUNDING EFFECTS OF CONTINUED METHOTREXATE IN PLACEBO ARMS (PLC) OF RHEUMATOID ARTHRITIS (RA) CLINICAL TRIALS – A POST-HOC ANALYSIS OF TWO RANDOMIZED CONTROLLED TRIALS (RCTS). Ann Rheum Dis 2021;80:71–2. 10.1136/annrheumdis-2021-eular.1532 33158881 [DOI] [Google Scholar]
- 28. Kerschbaumer A, Rivai ZI, Smolen JS, et al. Impact of pre-existing background therapy on placebo responses in randomised controlled clinical trials of rheumatoid arthritis. Ann Rheum Dis 2022;81:1374–8. 10.1136/annrheumdis-2021-221807 [DOI] [PubMed] [Google Scholar]
- 29. Fleischmann RM, Feist E, Fatenejad S. Efficacy and safety of Olokizumab in a phase III trial of patients with moderately to severely active rheumatoid arthritis inadequately controlled by TNF-α inhibitor therapy. Arthritis Rheumatol 2021;73 https://acrabstracts.org/abstract/efficacy-and-safety-of-olokizumab-in-a-phase-iii-trial-of-patients-with-moderately-to-severely-active-rheumatoid-arthritis-inadequately-controlled-by-tnf-%ce%b1-inhibitor-therapy/ [Google Scholar]
- 30. Feist E, Fatenejad S, Grishin S, et al. P136 Efficacy and safety of olokizumab in a phase III trial in patients with moderately to severely active RA inadequately controlled by TNF-α inhibitor therapy. Rheumatology 2021;60:keab247.132. 10.1093/rheumatology/keab247.132 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ard-2022-222630supp001.pdf (462.1KB, pdf)
Data Availability Statement
Data are available in a public, open access repository. Data are available upon reasonable request. Data generated by the present research available via https://clinicaltrials.gov and additional data are available on reasonable request to Sergey Grishin: sa.grishin@rpharm.ru.