Abstract
Endoscopy remains the reference standard for the diagnosis and assessment of patients with inflammatory bowel disease (IBD), but it has several important limitations. Cross-sectional imaging techniques such as magnetic resonance enterography (MRE) and intestinal ultrasound (IUS) are better tolerated and safer. Moreover, they can examine the entire bowel, even in patients with stenoses and/or severe inflammation. A variety of cross-sectional imaging activity scores strongly correlate with endoscopic measures of mucosal inflammation in the colon and terminal ileum. Unlike endoscopy, cross-sectional techniques allow complete visualisation of the small-bowel and assess for extraintestinal disease, which occurs in nearly half of patients with IBD. Extramural findings may predict outcomes better than endoscopic mucosal assessment, so cross-sectional techniques might help identify more relevant therapeutic targets. Coupled with their high sensitivity, these advantages have made MRE and IUS the primary non-invasive options for diagnosing and monitoring Crohn’s disease; they are appropriate first-line investigations, and have become viable alternatives to colonoscopy. This review discusses cross-sectional imaging in IBD in current clinical practice as well as research lines that will define the future role of these techniques.
Keywords: CROHN'S DISEASE, MAGNETIC RESONANCE IMAGING, GASTROINTESTINAL ULTRASOUND, INFLAMMATORY BOWEL DISEASE
Key messages.
Ileocolonoscopy remains the standard reference for the diagnosis and monitoring of patients with inflammatory bowel disease.
Cross-sectional imaging techniques such as magnetic resonance enterography (MRE) and intestinal ultrasound (IUS) are alternatives to ileocolonoscopy for assessing disease activity in patients with Crohn’s disease and for monitoring the short-term and long-term response to therapeutic interventions.
Cross-sectional imaging techniques are less invasive and better tolerated than ileocolonoscopy, and they visualise the entire small-bowel. They assess the full thickness of the bowel wall and can detect extra-enteric complications.
Several MRE and bowel ultrasound disease activity scores have been developed against a range of reference standards. Optimal operational characteristics including validity, reliability and responsiveness have been reported, facilitating their use in clinical trials.
Transmural healing measured by IUS or MRE is associated with better long-term patient outcomes than endoscopic mucosal healing, and has been proposed as a desirable treatment target. Optimal criteria for quantifying transmural healing on cross-sectional imaging are not yet defined, and prospective studies are needed.
Introduction
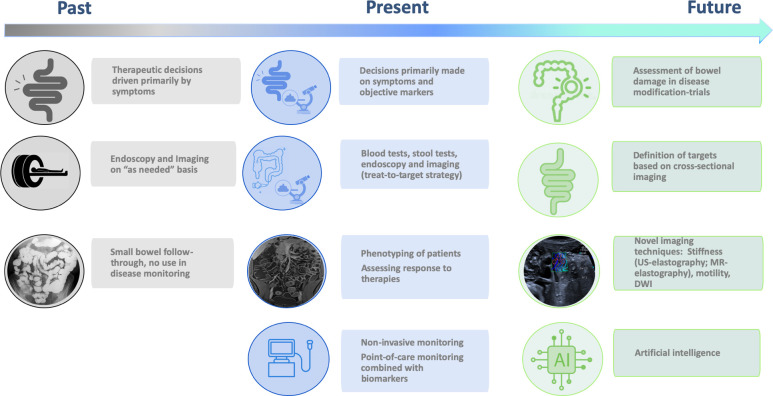
Inflammatory bowel disease (IBD), including Crohn’s disease (CD) and ulcerative colitis (UC), is a chronic, progressive, life-long disease that often requires surgery and results in bowel damage and disability.1 2 In recent decades, the management of IBD has changed considerably. Treatment goals have shifted from controlling symptoms to achieving sustained deep remission. Accordingly, management strategies have evolved toward early introduction of effective therapy with frequent assessments to monitor disease activity (tight-monitoring), and adjustment of therapy based on these assessments (treat-to-target strategy).3
Cross-sectional imaging techniques such as CT enterography (CTE), magnetic resonance enterography (MRE) and intestinal ultrasound (IUS) provide complementary information in the diagnosis of IBD, in the assessment of its complications, and in monitoring disease activity and therapeutic response. Although endoscopic remission is the currently accepted treatment target,3 patients often prefer to forgo repeated endoscopic assessments of disease activity.4 Furthermore, endoscopy often cannot examine segments proximal to the terminal ileum necessary for the complete phenotyping and evaluation of the extent of CD.
Cross-sectional imaging is useful in assessing therapeutic response. Multiple features improve or normalise on successful treatment, and cross-sectional imaging findings have good overall correlation with endoscopic scores, suggesting that these techniques should be incorporated in tight-monitoring and treat-to-target strategies.5–7 Point-of-care IUS to monitor disease activity promises to enable earlier treatment optimisation. Long-term patient outcomes are better when transmural healing is achieved compared with endoscopic mucosal healing only, although inconsistencies between cross-sectional and endoscopic assessment need to be clarified.8 9 While both approaches measure disease activity, cross-sectional techniques can also monitor disease progression, demonstrating the progression of cumulative bowel damage,10 11 an important endpoint in disease modification trials. However, more prospective studies are needed to determine the best use of cross-sectional imaging techniques during treatment follow-up and how to optimise their role in conjunction with non-invasive biomarkers and/or endoscopy. This review, based on current recent evidence published in the literature (see online supplemental material), will discuss cross-sectional imaging in IBD in current clinical practice and priority research lines that will define the future role of cross-sectional imaging in IBD (table 1).
Table 1.
Priority areas for future research
| Topic of interest | Comments |
| Multicentre validation of ultrasound disease activity scores and responsiveness | Reproducibility of scoring criteria. Definitions and performance of treatment response. |
| Integration of cross-sectional imaging in tight monitoring of treatment response, and treat-to-target strategies | Optimised use of endoscopy, blood and stool markers, point-of-care ultrasound and magnetic resonance enterography (MRE) in treat to target strategies. |
| Utility of cross-sectional imaging in stricture diagnosis and treatment follow-up | Optimised criteria to diagnose stricturing disease and best imaging parameters to monitor therapeutic response. |
| Development and validation of novel Imaging biomarkers for active and fibrotic disease | Development of novel cross-sectional imaging biomarkers of activity and fibrosis. Multicentre validation of ultrasound (and MRE) elastography, contrast enhanced ultrasound and bowel motility. |
| Cross-sectional imaging definition, and clinical utility of transmural healing as treatment target | Optimised and reproducible cross-sectional imaging definitions of transmural healing. Clinical impact of implementing transmural healing as a treatment target. |
| Utility of artificial intelligence in cross-sectional imaging | Automated segmentation of diseased bowel and extraction of activity/ fibrosis scores. Extraction of novel imaging biomarkers (eg, radiomics). Artificial intelligence driven individualised patient monitoring strategies. |
gutjnl-2021-326562supp001.pdf (106KB, pdf)
Reporting cross-sectional imaging assessments of activity and severity
Recent consensus guidelines provide detailed standardised nomenclature to increase reporting consistency for cross-sectional imaging at diagnosis, staging, activity assessment and evaluation of stricturing and penetrating complications.12 13
Because cross-sectional techniques assess the full thickness of the bowel wall and surrounding tissues, they can detect features not visible to endoscopy.14 Several imaging signs of activity have been validated against different reference standards, including biochemical markers, endoscopic scores and both mucosal and full thickness histopathology.13 15–17 Bowel-wall thickness (BWT) is probably the most robust parameter for determining disease activity, although nearly all pathological processes of the gut can cause thickening.12 18–20 A recent expert consensus proposed a threshold of BWT>3 mm for active IBD in both the ileum and colon.13 However, as both acute inflammatory and chronic fibrotic changes are nearly ubiquitous in CD-affected bowel, other activity parameters should also be assessed. In particular, neoangiogenesis and increased vascularisation contribute to chronic intestinal inflammation in CD and UC21–23 (figure 1). Several semi-quantitative colour Doppler IUS scores have been developed to assess vascularisation, although their use is mainly restricted to clinical trials. These scores correlate well with histological and endoscopic measures of inflammation.24–26 Using ultrasound contrast agents may improve IUS’s correlation with endoscopic activity,27 but the technique is not yet standardised and its contribution to the categorisation of inflammation remains controversial.28 29 SICUS (small intestinal contrast enhanced ultrasound) using orally administered contrast medium has been suggested to improve distension and visualisation of the small-bowel. Even though SICUS has shown advantages in evaluating the small-bowel30 31 and to determine postoperative recurrence32 it is more time consuming and therefore not widely used. Recently, a transmural damage score called sonographic lesion index for Crohn's disease (SLIC) was developed for SICUS to monitor transmural bowel damage.33
Figure 1.
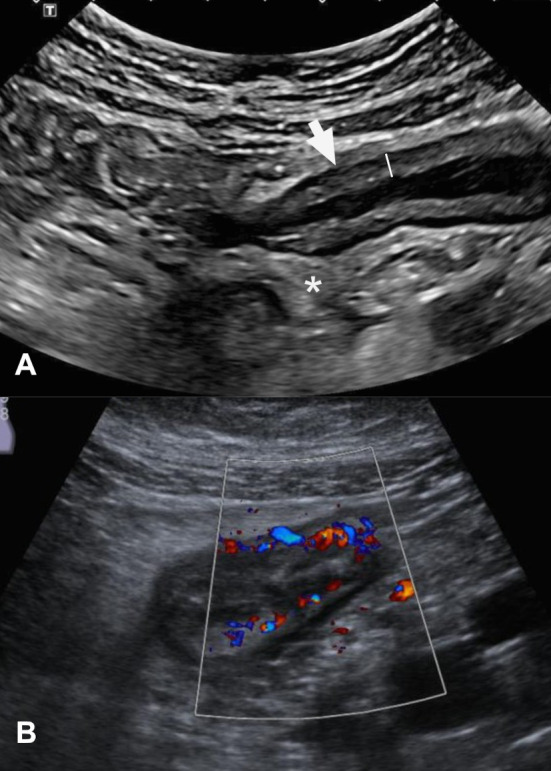
Small-bowel inflammation in Crohn’s disease detected using intestinal ultrasound. Diffusely increased bowel wall thickness (measured using the line that appears in the image, resulting in a distance of 5.3 mm) in the terminal ileum (arrow) is associated with partial loss of echostratification. The surrounding mesenteric fat appears (asterisk) is hyperechogenic due to perienteric inflammatory changes (A). Intestinal ultrasound in a different patient shows hypervascularisation of the small-bowel measured by colour Doppler (B). The wall of the terminal ileum is thickened and has a high colour Doppler signal that extends outside the bowel wall. These findings are consistent with active inflammatory small-bowel Crohn’s disease.
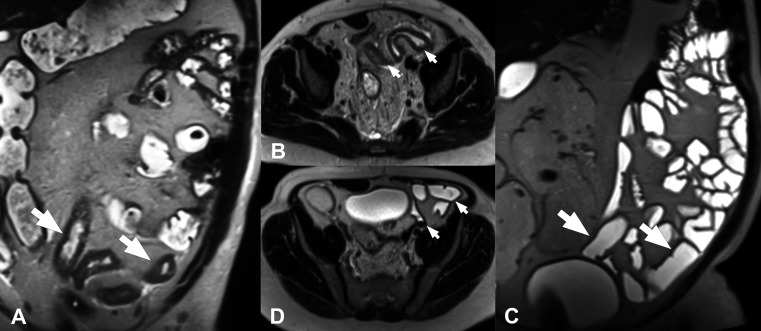
On MRE, increased mural enhancement following intravenous gadolinium injection can be asymmetric, stratified or homogenous, and both subjective and quantitative assessments generally correlate modestly with inflammatory activity.12 13 Like BWT, however, increased enhancement is non-specific and can reflect mural fibrosis, for example.34 On IUS, mural oedema may cause disruption of the bowel wall echostratification. On MRE, increased mural T2 signal, typically submucosal, is a highly specific sign of disease activity that generally suggests severe inflammation; at least in part, this finding likely reflects mural oedema.12 17 Similarly, increased T2 signal and stranding of the mesenteric fat due to transmural oedema and inflammation are generally specific signs of activity in the context of diseased bowel in CD16 (figure 2). The term fibrofatty proliferation (or fat wrapping) describes hypertrophy and expansion of the mesenteric fat side, which produces a mass-effect on adjacent bowel loops. It is difficult to quantify mesenteric fat by IUS, so assessments are mostly limited to ‘present’ or ‘absent’. Another marker of disease activity, ulceration, is seen on MRE (provided adequate luminal distension is achieved) as thin high signal intensity lines within thickened bowel wall12 and on IUS as breaks in the mucosal layer.
Figure 2.
Small-bowel inflammation in Crohn’s disease detected using magnetic resonance enterography. Axial post-contrast fat-saturated T1-weighted images (A) show moderate bowel-wall thickening and hyperenhancement (arrow); compare with the normal thickness in the uninvolved segments (arrowhead). The same segment seen on axial fat-saturated T2-weighted images (B). Note the increased signal intensity within the wall due to oedema (arrows) and the thin perienteric rim, due to the presence of fluid around the ileum. In the proximal sections of the same segment (C), ulcerations are seen as small disruptions of the inner surface of the thickened bowel wall (arrowhead).
In UC, the role of IUS is less defined, and until recently few studies had addressed its role in measuring disease activity.35–37 However, growing evidence suggests that IUS could also be used to determine disease activity38 39 and to follow-up the trajectory of patients with active UC.40 The most relevant measures of disease activity in UC include BWT and vascularisation on colour Doppler. Loss of haustration is less specific because it is associated with chronic changes and fibrosis as well as with endoscopic activity.39
Activity scores
Various MRE activity scores have been developed and validated (table 2).15 Despite differences in their details, the scores include similar components, and growing evidence supports their satisfactory inter-reader and intra-reader reproducibility, and high sensitivity and specificity for active disease against a range of reference standards (online supplemental table 1).41 These scoring systems make the assessment of MRE findings more objective and systematic, increasing the usefulness of these techniques in therapeutic clinical trials for selecting patients and potentially as therapeutic endpoints.42 43 As scoring systems become simpler, they may also prove useful in routine clinical practice.44
Table 2.
Summary of magnetic resonance enterography activity scoring index
|
Scoring index |
Formula | Strengths | Limitations | Further research questions |
| sMaRIA | *(1 × WT>3 mm) + (1 × wall oedema) + (1 × fat stranding) + (2 × ulcers) | Can be applied to small-bowel and large-bowel segments, can grade by severity, good responsiveness to treatment change, quick to derive, does not require gadolinium. | Reproducibility needs evaluating. | Reproducibility and specificity in multicentre and multireader settings with a range of reference standards. |
| London | †1.79 + 1.34 × mural thickness + 0.94 × mural T2 score | Quick to derive, does not require gadolinium, can assess treatment response. | Only applicable to small-bowel segments, does not allow grading by severity | Specificity in multicentre, multireader settings with a range of reference standards |
| ‘Extended’ London | ‡Mural thickness +mural T2 score +perimural T2 signal +contrast | Quick to derive, can assess treatment response. | Requires gadolinium, only applicable to small-bowel segments, does not allow grading by severity. | Ability to assess responsiveness to treatment; specificity in multicentre, multireader settings with a range of reference standards. |
| MaRIA | §1.5 × WT + 0.02 × RCE + 5 × oedema + 10 × ulcer | Accurate for small and large-bowel segments, allows grading by severity, can assess treatment response, most investigated. | Time consuming to calculate, requires gadolinium. | Practicality in clinical practice. |
| Clermont | −1.321 × ADC (mm2/s) + 1.646 × WT + 8.306 × ulcers + 5.613 × oedema + 5.039 | Accurate for small and large-bowel segments, allows grading by severity, can assess treatment response. | Time consuming to calculate, requires diffusion-weighted sequences. | Practicality in clinical practice. |
*WT=Wall thickness >3 mm scores 1 point, presence of oedema scores 1 point, presence of fat stranding scores 1 point, presence of ulcers scores 2 points. Thus, the score for each segment ranges from 0 (completely normal) to 5 (markedly inflamed).
†Where mural thickness and mural T2 score are classified in four categories according to severity (0=absent, 1=mild, 2=moderate, 3=marked).
‡Where mural thickness, mural T2 score and contrast enhancement are classified in four categories according to severity (0=absent, 1=mild, 2=moderate, 3=marked).
§Where WT is measured in millimeters and RCE in arbitrary units, and evidence of oedema or ulcer is assigned a value of 1 if present and a value of 0 if absent.
ADC, apparent diffusion coefficients; MaRIA, Magnetic Resonance Index of Activity; RCE, Relative Contrast Enhancement; sMaRIA, simplified MaRIA.
The first and best validated is the Magnetic Resonance Index of Activity (MaRIA), calculated from four independent predictors of the presence of endoscopic inflammation (wall thickening, mural contrast enhancement) and presence of endoscopic ulcerations (mural oedema, and ulceration detected by MRE).17 45 For each individual intestinal segment, an MaRIA score ≥7 indicates active disease and those ≥11 indicate severe (ulcerative) disease. However, calculating the MaRIA score is time-consuming because it requires measurement of bowel wall signal intensity by manually placing multiple regions of interest in the bowel wall on pre and post contrast enhanced images; moreover normal segments contribute to the global MaRIA score (which is computed as the sum of each subscore obtained in each individual intestinal segment) and in patients with resected segments an underestimation of the global score occurs.46 These drawbacks led to the development of the simplified MaRIA (sMaRIA), which requires much less time to calculate, and normal segments do not account in the global score. The sMaRIA has also been validated against endoscopic standards of reference (Crohn's disease endoscopic index of activity [CDEIS] and the simple endoscopic score for Crohn's disease (SES-CD)).47–49
The London and ‘extended’ scores were derived and validated using a histological standard of reference50 and were subsequently validated against the CDEIS.51 The components of the sMaRIA and extended London score are very similar; however, whereas the sMaRIA and London scores can be applied regardless of whether contrast material administration, the extended London score cannot. The Clermont score was derived from the MaRIA and uses the same descriptors, but it includes apparent diffusion coefficients (ADC) derived from diffusion-weighted imaging (DWI) (discussed in detail below) instead of contrast enhancement (table 2). Because of the need for relatively time-consuming region of interest placement, and because they offer more granularity, Clermont and MaRIA scores are usually employed in the setting of clinical trials of new therapeutics. Both the London (and extended London if intravenous gadolinium administered) and sMaRIA are relatively quick to calculate and although infrequently applied in routine clinical reporting, their constituent parameters are used as part of radiologists’ review of disease activity and treatment response.
Several IUS activity scores have been developed for CD and for UC (table 3). While few have been rigorously validated and methodological concerns remain,22 there is now growing consensus on the most appropriate parameters to include, and how these should be measured.20 52 Scores for UC have more recently been proposed, but most are yet to be validated.
Table 3.
Intestinal ultrasound scoring systems for disease activity in Crohn’s disease and ulcerative colitis
| Scoring index | Disease | Formula | Strengths | Limitations | Further research questions |
| Simple IUS score. Novak et al 103 |
CD | (0.0563 × BWT1) + (2.0047 × BWT2) + (3.0881 × BWT3) + (1.0204 × Doppler1) + (1.5460 × Doppler2) | Validated | Ultrasonographers and endoscopists were not blinded. Needs to be validated in external cohorts. |
Future studies should use MRE as a reference. Future research should focus on establishing scores that are validated against endoscopy and/or MRE in multiple centres through central reading. Responsiveness should be assessed with clear definitions for response and remission. |
| Simple IUS score. Saevik et al 2021104 |
CD | BWT × 1.053 + (colour Doppler × 1.934) + (fatty wrapping × 1.275) +(stratification × 1.225) + 0.242 or simply summarising the numerical values of the variables assessed | Validated. Good inter-rater reliability. |
Needs to be evaluated in external cohorts. Sensitivity to change not assessed. |
|
| Bowel US Score (BUSS) Allocca et al 26 |
CD | 0.75 × BWT + 1.65 × BWF; where BWF=1 if present, or BWF=0 if absent | Validated. Correlates well with endoscopic activity BUSS determined endoscopic response with high accuracy. |
Small validation cohort. Needs to be validated in external cohorts. |
|
| Milan US Criteria (MUC). Allocca et al 38 105 |
UC | 1.4 × BWT + 2 × BWF | Validated. Accurate discrimination between active and non-active UC (MUC score >6.2). |
Validity to assess treatment response has not been assessed. Does not discriminate different degrees of inflammation. |
Further investigation is needed to determine these criteria’s sensitivity to change and their validity to assess treatment response and outcomes in clinical practice as well as in clinical trials. |
| UC IUS Index. Bots et al 39 |
UC | Point-based index with four parameters graded on a 7-point scale: BWT, Doppler signal, abnormal haustration, fat wrapping. | Not validated. Strong correlation with the Mayo endoscopic subscore (p=0.830; p<0.001). Moderate-to-substantial inter-rater agreement. |
The coefficient from the multivariable analysis have not been used for the score. Instead, an arbitrary grading system (a 7-point scale) was used. Needs to be validated in external cohorts. |
Validity to assess treatment response needs to be assessed. Assessment of rectum needs to be included. |
BWF, bowel wall flow; BWT, bowel wall thickness; CD, Crohn’s disease; IUS, intestinal ultrasound; MRE, magnetic resonance enterography; UC, ulcerative colitis; US, ultrasound.
There is a current need for multicentre studies to validate IUS scores against endoscopy and/or MRE, employing precise definitions of response and remission to assess the scores’ responsiveness. Akin to MRE activity scores, reliable, standardised IUS activity scores for CD and UC would be helpful in monitoring treatment outcomes in clinical practice and could also be applied as endpoints in clinical trials.
Using cross-sectional imaging to assess therapeutic response
The treatment paradigm in CD has shifted from targeting symptoms toward reducing inflammatory activity and achieving endoscopic healing (figure 3). This approach requires frequent monitoring of disease activity, to allow timely adjustment of therapy in the event endoscopic healing has not been achieved despite symptom improvement (treat-to-target strategy). However, endoscopy-based monitoring is limited by its invasiveness and poor patient tolerability; moreover, endoscopic assessments of mucosal healing inadequately reflect transmural (eg, oedema) and perienteric changes or complications. In clinical practice, transmural response and remission are usually subjectively assessed by the changes in parameters known to reflect activity such as BWT.13 In clinical research these endpoints are usually quantified using activity scores. Recent consensus statements12 13 classify therapeutic response according to observable changes in individual imaging features of disease activity (such as BWT, T2 signal) into four categories: transmural remission (normalisation of all features), response (unequivocal decrease in the severity or extent of imaging findings within an inflamed segment), stable (no clear change in severity or extent) or progression (worsening in parameters of inflammation and/or new disease sites, and/or development of CD-related complications). Furthermore, it is plausible that disease duration and accumulated damage over time may have an impact on the chances of achieving transmural healing; in that case, the possibility of achieving transmural healing might be limited to a window early in the course of the disease.
Figure 3.
Evolving role of cross-sectional imaging in the field of inflammatory bowel disease. DWI, diffusion-weighted imaging; US, ultrasound.
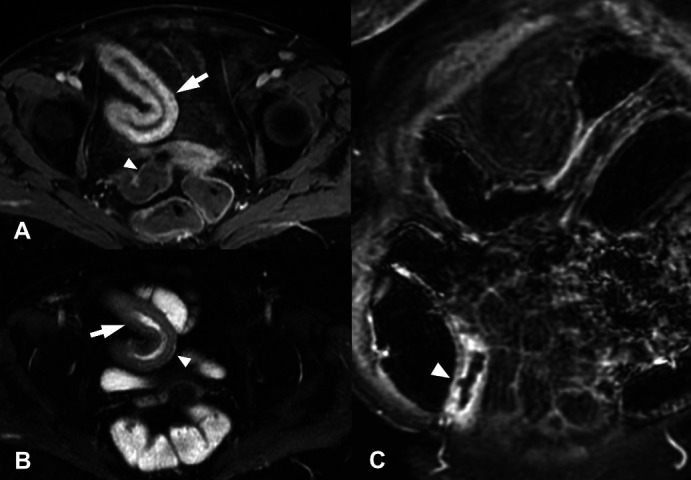
In patients with CD, the stringent definition of transmural remission requires complete resolution of inflammatory mural and perimural findings so that the bowel wall recovers its normal appearance: BWT<3 mm and no signs of hyperperfusion, oedema (on MRE), disrupted echostratification (on IUS), ulcers or fat stranding13 (figure 4). A less stringent and perhaps more realistic definition would also allow the resolution of inflammation with residual findings or sequelae such as mild mural thickening or mild hyperperfusion and/or asymmetric fat deposits within the bowel wall resulting from multiple episodes of activity and healing. In other words, the definition of transmural healing may be dependent on disease duration and accumulated bowel damage.
Figure 4.
Coronal (A, C) and axial (B, D) T2 non-fat saturated weighted images from an MRE enterography examination before (A, B) and after (C, D) anti TNF-α therapy. Before treatment there is active small-bowel Crohn’s disease with mural thickening and increased mural T2 signal (arrows). Following 6 months of anti-TNF-α therapy the bowel has returned to near normal with subtle low T2 signal thickening only (arrows). MRE, magnetic resonance enterography; TNF, tumour necrosis factor.
One of the advantages of IUS is its easy use and the rapid evaluation of the treatment response in patients with IBD53 (figure 5). In CD, transmural response could already be determined as soon as 4 weeks after treatment initiation. In UC, normalisation of BWT could be observed 2 weeks after treatment initiation in the majority of patients.40
Figure 5.
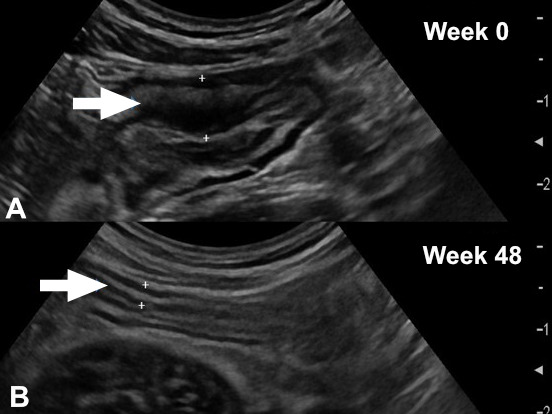
Monitoring disease activity using intestinal ultrasound. Composite image of small-bowel inflammation due to Crohn’s disease in the terminal ileum showing a mural wall thickness of 7 mm and partially abrogated echostratification at week 0 (A). The same part of terminal ileum at week 48 after treatment with ustekinumab showing normalisation of mural thickness, normal echostratification (B). Overall, findings are consistent with achievement of transmural remission.
Further studies are needed to establish clear definitions of therapeutic response based on individual activity parameters in IBD. To best define transmural response criteria and the optimal time for reassessment, it is critical to characterise changes in individual imaging activity parameters over time, because some parameters (eg, BWT, in particular ileal) are slower to change than others.47 54 55
The discrepancy between active ileal inflammation on cross-sectional imaging and normal mucosa at endoscopy likely reflects active inflammation confined to intramural portions of the terminal ileum, inaccessible at endoscopy.56–58 Indeed, as discussed in more detail below (targets in Crohn’s disease), when assessing the response of inflamed bowel segments to medical therapy, cross-sectional imaging findings better predict clinically relevant clinical outcomes57 59 60 and progression of bowel damage61 than endoscopy.
To use MRE and IUS in clinical trials, it essential to develop validated indices to measure changes after medical treatment. Studies using different methodological approaches have demonstrated that various scores correlate well with endoscopic indices and endpoints47 55 58 62–65 (online supplemental table 2). Hanzel et al 42 recently compared the responsiveness of MRE scores. All scores were substantially responsive with the standardised effect size of sMaRIA (1.17; 95% CI: 0.56 to 1.77) similar to that of the full MaRIA (0.98; 95% CI: 0.42 to 1.55) and higher than that of the London score (0.85; 95% CI: 0.31 to 1.39). Moreover, the correlation between the change in the MRE index score and the change in endoscopic disease activity was numerically largest for the sMaRIA (r=0.72), suggesting that it is potentially the preferred score for use in clinical trials due to its practicality and responsiveness.
By contrast, the operating characteristics of IUS indices are poorly defined, with insufficient data available regarding their efficacy in assessing the response-to-treatment compared with endoscopy.21
Choice of cross-sectional imaging techniques in clinical practice
No imaging modality is ideal in all situations. The choice of cross-sectional technique depends on the clinical question, the patient’s characteristics and preferences, local interpretative expertise and availability of imaging platforms. The aim is to use the best test for a particular patient at a given time point in the course of their disease. All modalities have strengths and limitations. Compared with MRE and IUS, CTE is faster, less dependent on body habitus and less prone to artefacts; however, CTE uses ionising radiation, making it unsuitable for the serial examinations required in the management of IBD and thus relegating its role largely to the acute setting.
Regarding patients’ experience and preferences, a recent prospective multicentre study comparing MRE and IUS in patients undergoing both tests found that although the burden of MRE was low, it was significantly greater than that of IUS; furthermore, 99% of patients were willing to undergo IUS again, with 91% willing to undergo MRE again.66 67 Nonetheless, patients preferred MRE to colonoscopy, and patients considered diagnostic accuracy the most important test attribute.
Meta-analyses suggest that both IUS and MRE are accurate for diagnosis and staging CD, although prospective multicentre head-to-head comparison suggests MRE is more accurate, particularly for defining the extent of small-bowel CD, and likely preferable for the initial diagnostic workup to define the disease distribution and phenotype.13 66 68 69 Moreover, as discussed above, validated MRE disease activity scores support its use in follow-up and assessing treatment response in both clinical practice and research.62 However, IUS can be performed at the point-of-care, enabling real-time clinical decisions. This is a distinct advantage should robust data demonstrate the benefit of activity assessment using cross-sectional imaging during tight-monitoring strategies, particularly in established non-complex disease phenotypes.
Usefulness of cross-sectional imaging in guiding clinical decisions
Several studies have analysed the impact of cross-sectional imaging findings on clinical decisions. In a prospective study, Novak et al 70 found that IUS assessment changed clinical decisions in about 60% of cases. In a study comparing colonoscopy followed by MRE versus MRE followed by colonoscopy in patients with CD, Garcia-Bosch et al 71 found that information from MRE alone was sufficient for management in 80% of cases, whereas information from colonoscopy alone was sufficient in only 34%. Adding information from MRE to that from colonoscopy led to changes in therapy in 28% of patients. Moreover, adding information from MRE to that obtained from colonoscopy changed clinicians’ grade of confidence in a higher proportion of patients than adding colonoscopy to information from MRE for diagnosing disease activity, stenosis, fistula, and internal abscesses. Anti-tumour necrosis factor (TNF) therapy and surgery were indicated more often when MRE was done first. Another study comparing point-of-care IUS with MRE in patients with CD found that both modalities had a high impact on clinical decision-making, and the changes to management resulting from IUS and MRE were highly concordant.72
Cost-effectiveness
Across the majority of healthcare systems, IUS is cheaper than MRE and CTE. Relatively few data are available on the cost-effectiveness of MRE and IUS; however, a detailed modelling exercise based on prospective data from the METRIC trial showed similar cost-effectiveness for the two techniques in both newly diagnosed patients and those with clinically suspected relapse.73
Near-future perspectives
Avoiding contrast media
Current MRE protocols typically include rapid sequences with gadolinium-chelate-enhanced sequences that provide morphological and functional information. Although gadolinium-based contrast agents are generally considered safe, recent confirmation of gadolinium deposits in brain tissue has raised concerns about its repeated use, especially in younger patients. Moreover, using contrast agents increases costs. Therefore, alternative or complementary image strategies are being investigated.
Diffusion-weighted imaging
DWI is an MR sequence deriving its image from the Brownian motion of free water molecules in tissues, and reflects the underlying tissue architecture and histological properties. The diffusion of water molecules can be restricted by interactions with cell membranes and macromolecules. In addition to providing images, DWI intrinsically provides a quantitative parameter, that is, the ADC. On DWI, restricted diffusion is typically seen in active inflammation, where it manifests as high signal with corresponding low ADC values on ADC maps. To date, research into the use of DWI and ADC in IBD has focused mainly on identifying activity in the bowel wall. A critical review of 15 studies using diverse methodologies concluded that although DWI offered high sensitivity (80%‒100%) for detecting enteric inflammation, high rates of false-positives (up to 40%) suggest that restricted diffusion is most useful as a supportive feature when other findings of mural inflammation are present on conventional sequences.74 Further studies are necessary to define the role of DWI in monitoring the response-to-treatment.
Motility
The mechanisms underlying reduced motility in CD-affected bowel are multifactorial, but inflammatory and fibrotic infiltration, neuritis within the bowel wall, and systemic effects of the inflammatory burden mediated via hormonal and neuronal pathways likely play a role. One of the main advantages of IUS is the direct bedside evaluation of motility and of bowel wall stiffness although clear parameters for quantification are still lacking. Technological advances have enabled MR scanners to capture small-bowel motility in a single breath hold and postprocessing software can quantify this motility (figure 6). Reductions in segmental bowel motility are inversely correlated with histopathological and endoscopic activity grades, and recovery of motility may better capture early treatment response than morphological measurement; however, this promising approach requires further validation.75
Figure 6.
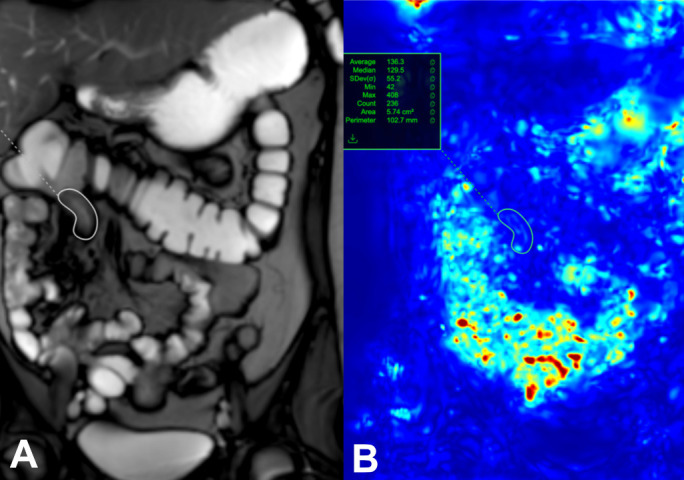
A single image from a dynamic set with a region of interest (white circle) placed on a diseased terminal Ileum (A). Motion is assessed by assigning each pixel in the image with an associated displacement value, and expressed as quantitative value (green circle) (B).
Elastography
Ultrasound-based elastography is increasingly used to quantifying tissue stiffness as a biomarker for fibrosis. Elastography measures tissue stiffness in response to a mechanical force, applied by the operator in strain elastography or generated by the transducer in shear-wave elastography. Strain elastography findings are expressed through semi-quantitative colour maps and the ratio of strains between the area of interest (eg, stricture) and normal tissue, whereas shear-wave elastography provides quantitative measures of the propagation of shear waves in kPa or metres per second as well as colour maps. Therefore, shear-wave elastography is less operator dependent and reproducible.
There are promising emerging data showing an association between elastography measurement and the degree of underlying fibrosis in CD-related strictures. A recent systematic review found moderate-to-good accuracy for ultrasound elastography in identifying histological fibrosis, with point-shear wave elastography performing best.76
The use of elastography as a biomarker to predict the response to TNF-inhibitors has also been explored. Orlando et al 77 found that strain ratios were lower in patients who achieved transmural healing (BWT≤3 mm) during treatment, and patients with higher ratios required surgery more frequently.
Elastography can also be performed with MRI. A recent study using MR elastography in a prospective cohort of 69 patients found that bowel wall stiffness >3.57 kPa was associated with an increased risk of adverse clinical events (p<0.0001).78 Elastography is a promising tool for guiding clinical decisions, but the wide heterogeneity of modalities and reported values currently hampers its implementation in daily practice.
Combining imaging findings and other biomarkers
Given the limited ability of cross-sectional imaging techniques’ to identify early mucosal inflammation, it seems reasonable to supplement cross-sectional imaging with alternative biomarkers of mucosal inflammation such as faecal calprotectin (fCalpro) to compose a more complete picture of transmural and mucosal disease activity. In patients with IBD, fCalpro correlates well with colonic inflammation,79 although fCalpro is less sensitive in patients with proctitis alone and in patients with CD with small-bowel inflammation.79 80 Nevertheless, a meta-analysis of studies comparing fCalpro with video capsule endoscopy indicated that fCalpro <50 µg/g suggests a very limited likelihood of small-bowel inflammation.81
In clinical practice, combining IUS and fCalpro at different time points might be helpful in monitoring disease activity and the response-to-treatment in both UC and CD, but more data are needed to determine the value of this approach.
Artificial intelligence
Conventional imaging evaluation relies to a great extent on subjective assessment by radiologists, risking interpretative variability. Advancements in intestinal segmentation methodologies for cross-sectional imaging using artificial intelligence (AI) may potentially power disease activity measurements while reducing variability. Improvements in semi-automated bowel segmentation are key to automated extraction of standardised, reproducible CD activity measures using MRE or CTE.82 83
In the last decade, radiomics (software-based extraction of multiple imaging features from radiographic images) has been explored as a way of harnessing hidden biological signatures to aid diagnosis and prognostication, forming part of more general strategies aiming towards more personalised medicine. One area where radiomics research can expand is correlating models obtained from the imaging features extracted with histological findings. In a pilot study in 16 patients, Makanyanga et al 84 found that MRI texture features were associated with histological measures of CD activity and MRI CD activity scores. Other recent works have focused on detecting intestinal fibrosis. One retrospective multicentre study found that a radiomic model based on data extracted from CTE and MRE identified moderate-to-severe fibrosis in the bowel with diagnostic accuracy comparable with radiologists’ perception.42 The same group went on to develop a deep-learning model that outperformed radiologists in diagnosing intestinal fibrosis on CTE in patients with CD and that was not inferior to the radiomics model.85 Unlike radiomics models, deep-learning models are capable of automatically learning features without predefined characteristics or human intervention.
There is no doubt that AI systems will eventually be integrated into medical imaging work-flow schemes, improving diagnostic accuracy, lowering reporting times and reducing radiologists’ workloads. However, various challenges must be overcome before these systems can be widely implemented in clinical practice or research. Among these, efforts to develop and train AI systems must take patient and data diversity and non-uniform image acquisition protocols into account, ensuring the most comprehensive data sets for ground truthing to minimise biases. These efforts would benefit greatly from standardised reporting as well as from increased data sharing, which is currently hindered by regulatory restrictions.
At this very early stage in this promising era, it is difficult to predict how AI advances will be implemented and how they will impact the interpretation of medical imaging and patient outcomes.
Targets in Crohn’s disease
Since CD is a transmural disease, it seems logical to use MRE or IUS to assess the transmural response-to-treatment and remission or healing. The recent Selecting Therapuetic Targets in Inflammatory Bowel Disease (STRIDE II) consensus proposed transmural healing as a desirable treatment target.3 Several studies have demonstrated that transmural healing is associated with better long-term outcomes. For example, a recent study using MRI to determine transmural healing found lower rates of hospital admission, therapy escalation and surgery in patients with transmural healing compared with patients with endoscopic mucosal healing alone or no healing.57 Another study showed that transmural healing on MRI is associated with a lower risk of bowel damage progression than endoscopic healing in CD.61 In patients with CD receiving anti-TNF therapy, those achieving a complete response on IUS had better long-term outcomes, including less hospitalisation, less surgery and less steroid use compared with patients with incomplete responses.86 Similarly, in patients with CD, after 1-year treatment with biologics, clinical outcomes were better in those with transmural healing on IUS than in those with no healing or only mucosal healing.59
One of the inherent challenges in adopting transmural healing as a treatment target is the relatively low rate of patients who achieve it with currently available drugs. In the STARDUST IUS study, where transmural healing was defined as normalisation of all pathological IUS parameters 1 year after treatment with ustekinumab, only 24% of patients had achieved this endpoint.54 Similarly, in the VERSIFY trial, where transmural healing was defined as MaRIA <7 in all segments, only 22% of patients had achieved this endpoint after treatment with vedolizumab for 26 weeks, and only 38% after treatment for 52 weeks.87 The rate of response to medical therapy observed in the small-bowel and large bowel are consistently different, possibly due to differences in the pathophysiology of CD in the two locations42 88; however, these differences are still poorly understood, so attention is currently focused on developing specific studies for small-bowel CD.
Very little is known about the comparative impact of drugs with different mechanisms of action in inducing transmural healing, whether treatment optimisation can increase the rates of transmural healing, or the length of time necessary to induce transmural healing in patients with CD. Furthermore, most of the available information comes from retrospective studies with few patients.
Heterogeneity in the definitions of transmural healing and the techniques used to measure it hamper the identification of relevant therapeutic targets based on cross-sectional imaging.7 Furthermore, the paucity of data showing the relationship between cross-sectional activity scores and clinical outcomes further limits using these scores as an endpoint in clinical trials. Transmural healing can be the new treatment target; however, the optimal set of therapeutic targets to predict clinically relevant long-term outcomes in CD can only be settled through large-scale long-term prospective studies to provide physicians with some guidance in clinical practice and therapeutic goals in clinical research.
Development of targets in fibrostenosing CD
Current therapeutic strategies for CD target inflammation. Although administering corticosteroids, immunosuppressive therapies or biological agents can bring about disease remission in about 30% of patients, recurrent flares alternating with periods of remission will still result in cumulative bowel wall remodelling. Indeed, despite these therapeutic strategies to control inflammation, the incidence of fibrostenosis in patients with IBD is not declining.89 Thus, to avoid the irreversible progression to stenosis in patients with IBD, it is essential to detect fibrosis early and treat it adequately. However, since no drug therapy for fibrostenosis is available, this condition evolves irreversibly and eventually requires surgical resection. Clinical trials for potential anti-fibrotic therapies are difficult to set up and carry out for three main reasons: they require a very long time, predictive models for the development of strictures are lacking and no surrogate endpoints are available.
In this context, it is crucial to develop and validate novel targets specific for fibrosis and to define how to diagnose, prevent and cure fibrosis in patients with CD. With the aim of establishing a standard, reliable and objective definition for strictures based on MRE, a panel of experts proposed the following diagnostic criteria for fibrostenosis (1) BWT>3 mm, (2) luminal narrowing >50% and (3) pre-stenotic dilation >3 cm.12 13 90
Although the definition of strictures containing some degree of fibrosis is in the process of validation, various single-centre studies have reported other MR quantitative metrics. For instance, the dynamic pattern of gadolinium enhancement (DGE) of the bowel wall differs depending on the amount of dense, compact fibrotic matrix in the submucosa. Whereas predominantly inflammatory lesions become saturated with contrast material early, bowel wall areas with moderate and high degrees of fibrosis are delineated through the delayed, progressive enhancement of deep intestinal layers over a 7 min period, regardless of the degree of inflammation present.91 ADC values have also shown promise for correlating fibrosis in small-bowel CD. Lower ADC values correlate significantly with Chiorean scores for histological fibrosis,92 likely because fibrosis increases the density of the extracellular space, restricting the diffusion of water molecules. Another parameter apparently associated with fibrosis is the magnetisation transfer ratio (MTR). Derived from the magnetisation transfer technique, the MTR reflects the burden of macromolecules (eg, collagen) that accumulate in the bowel wall and correlates with fibrotic deposition in resected bowel segments.93 However, a recent prospective multicentre study that aimed to validate these imaging biomarkers in 61 surgical samples from 51 patients with CD with strictures found that ADC and MaRIA correlated strongly with fibrosis (R=−0.71, p<0.0001 and 0.59, p<0.001), but correlations of fibrosis with DGE and MTR were weaker or non-existent.94 An additional composite quantitative score obtained using regression analysis including MaRIA, ADC and DGE was a good predictor of histological fibrosis (area under receiver operating characteristic curve=0.910) and promises to be useful in the development of fibrosis-targeted therapies.
Monitoring disease progression
The ultimate goal of therapy in IBD is to bring about deep and sustained remission, prevent the progression of disease and restore patients’ quality of life over the long-term. The progressive nature of CD is well established, and CD studies have used changes from inflammatory behaviour to stricturing or penetrating behaviour as hallmarks of disease progression.95 To better assess the effects of therapeutic interventions on disease progression, it is important to separate the long-term outcomes related to the modification of disease from the short-term effects of treatment on symptoms and disease activity.
The Lémann Index (LI) was developed and validated to provide an objective and reproducible measure of CD progression.10 96 It is based on a comprehensive assessment of structural bowel damage, including stricturing lesions, penetrating lesions and history of surgery, and increases with disease duration.96 Its main applicability will likely be as an outcome measure in disease modification trials, and applied over a long period of follow-up time.97 Importantly, the LI has also been shown to also to decrease over time. In a population of patients with CD responding to anti-TNF drugs, the LI decreased in 60% 1 year after starting anti-TNF therapy,98 suggesting that this index also includes inflammatory components in the penetrating domain (eg, fistula) and in its stricturing domain. Nonetheless, a retrospective study in 221 patients with CD assessing the LI’s sensitivity to changes in bowel damage found that more than 50% had substantial bowel damage (LI>2.0) 2–10 years after the initial diagnosis. In addition to the duration of clinical activity and intestinal resection, an elevated (>2.0) LI at first evaluation was associated with bowel damage over time. In another cohort of 30 subjects who achieved clinical remission with anti-TNF and were followed prospectively for a median time of 32.5 months, the authors proposed a cut-off of the LI>4.8 for significant bowel damage, and found that an increase >0.3 in the LI was associated with bowel damage progression and.99 Besides, bowel damage progression was found to be predictive for major surgery in the follow-up period (HR 0.19, p=0.005).99 In two additional retrospective cohorts LI stabilisation or decrease was observed in patients receiving anti-TNF, as opposed to patients receiving azathioprine,100 101 or mesalamine.100 It is important to highlight that all these studies have employed the non-validated and updated version of the LI,10 and over a relatively short period of time.
Currently ongoing, prospective CD Cohort Study aims to prospectively measure the validated LI in a population of recently diagnosed CD, and to assess predictors of bowel damage (measured by the LI) progression over time (CROCO trial; NCT05420233). Although the concept of bowel damage is not as well accepted in UC as in CD, some evidence suggests that inflammation in UC can lead to damage, manifesting as reduced rectal compliance, altered motility and overlapping functional symptoms.102 Thus, it seems plausible that the cross-sectional imaging could provide new insights into the effects of UC on the bowel wall beyond the mucosa.
Conclusion
Cross-sectional imaging plays a vital role in the management of patients with IBD and overcomes many of the limitations of endoscopy. Many studies demonstrate the utility of cross-sectional imaging for diagnosis, staging, activity assessment and severity grading. Furthermore, as treatment goals shift to achieving sustained deep remission, MRE and IUS are vital adjuncts to endoscopy as objective tools for monitoring disease activity. New techniques such as motility assessment and elastography hold promise as potential biomarkers for activity and fibrosis, respectively, with relatively few barriers to clinical implementation once appropriately validated. Cross-sectional imaging also allows quantification of cumulative progressive bowel damage, and the ability to assess transmural response and healing opens intriguing possibilities for new treatment targets and prognostication. The criteria for, and utility of, transmural healing on cross-sectional imaging is a current research priority.
Footnotes
Twitter: @JordiRimola
Contributors: All authors equally contributed to discuss the outline of the manuscript, drafting the manuscript and critically reviewed the manuscript. All authors contributed to providing figures. JR coordinated the execution of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: JR has received grants from AbbVie and consulting fees from Boehringer Ingelheim, Janssen, Origo, Lument, Takeda and Alimentiv. JT has received grants from AbbVie and Janssen, advisory board fees and/or speaker fees from Janssen, Galapagos and Pfizer. SK has no declarations. SAT is a previous research consultant to Alimentiv. Shareholder in Motilent. TK has received grants from AbbVie, Janssen and Takeda, advisory board fees and/or speaker fees from AbbVie, Amgen, Arena Pharmaceuticals, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Celltrion, Dr Falk Pharma, Ferring Arzneimittel, Galapagos, Gilead, Janssen, MSD Sharp & amp; Dome GmbH, Pfizer, Roche, Takeda Pharma and Vifor Pharma.
Provenance and peer review: Commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
Not applicable.
References
- 1. Torres J, Mehandru S, Colombel J-F, et al. Crohn’s disease. The Lancet 2017;389:1741–55. 10.1016/S0140-6736(16)31711-1 [DOI] [PubMed] [Google Scholar]
- 2. Ungaro R, Mehandru S, Allen PB, et al. Ulcerative colitis. Lancet 2017;389:1756–70. 10.1016/S0140-6736(16)32126-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Turner D, Ricciuto A, Lewis A, et al. STRIDE-II: an update on the selecting therapeutic targets in inflammatory bowel disease (STRIDE) initiative of the International organization for the study of IBD (IOIBD): determining therapeutic goals for Treat-to-Target strategies in IBD. Gastroenterology 2021;160:1570–83. 10.1053/j.gastro.2020.12.031 [DOI] [PubMed] [Google Scholar]
- 4. Goodsall TM, Noy R, Nguyen TM, et al. Systematic review: patient perceptions of monitoring tools in inflammatory bowel disease. J Can Assoc Gastroenterol 2021;4:e31–41. 10.1093/jcag/gwaa001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wu Y-W, Tang Y-H, Hao N-X, et al. Crohn's disease: CT enterography manifestations before and after treatment. Eur J Radiol 2012;81:52–9. 10.1016/j.ejrad.2010.11.010 [DOI] [PubMed] [Google Scholar]
- 6. Paredes JM, Ripollés T, Cortés X, et al. Abdominal sonographic changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn's disease. Dig Dis Sci 2010;55:404–10. 10.1007/s10620-009-0759-7 [DOI] [PubMed] [Google Scholar]
- 7. Geyl S, Guillo L, Laurent V, et al. Transmural healing as a therapeutic goal in Crohn's disease: a systematic review. Lancet Gastroenterol Hepatol 2021;6:659–67. 10.1016/S2468-1253(21)00096-0 [DOI] [PubMed] [Google Scholar]
- 8. Ma L, Li W, Zhuang N, et al. Comparison of transmural healing and mucosal healing as predictors of positive long-term outcomes in Crohn's disease. Therap Adv Gastroenterol 2021;14:17562848211016259. 10.1177/17562848211016259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weinstein-Nakar I, Focht G, Church P, et al. Associations among mucosal and transmural healing and fecal level of calprotectin in children with Crohn's disease. Clin Gastroenterol Hepatol 2018;16:1089–97. 10.1016/j.cgh.2018.01.024 [DOI] [PubMed] [Google Scholar]
- 10. Pariente B, Torres J, Burisch J, et al. Validation and update of the Lémann index to measure cumulative structural bowel damage in Crohn's disease. Gastroenterology 2021;161:853–64. 10.1053/j.gastro.2021.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Le Berre C, Peyrin-Biroulet L. Selecting end points for disease-modification trials in inflammatory bowel disease: the spirit consensus from the IOIBD. Gastroenterology 2021;160:1452–60. [DOI] [PubMed] [Google Scholar]
- 12. Bruining DH, Zimmermann EM, Loftus EV, et al. Consensus recommendations for evaluation, interpretation, and utilization of computed tomography and magnetic resonance Enterography in patients with small bowel Crohn's disease. Radiology 2018;286:776–99. 10.1148/radiol.2018171737 [DOI] [PubMed] [Google Scholar]
- 13. Kucharzik T, Tielbeek J, Carter D, et al. ECCO-ESGAR topical review on optimizing reporting for cross-sectional imaging in IBD. J Crohns Colitis 2021. [DOI] [PubMed] [Google Scholar]
- 14. Wilkens R, Novak KL, Maaser C, et al. Relevance of monitoring transmural disease activity in patients with Crohn's disease: current status and future perspectives. Therap Adv Gastroenterol 2021;14:17562848211006672. 10.1177/17562848211006672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Grand DJ, Deepak P, Rimola J. Mre evaluation of intestinal inflammation: qualitative and quantitative assessment. Top Magn Reson Imaging 2021;30:13–22. 10.1097/RMR.0000000000000270 [DOI] [PubMed] [Google Scholar]
- 16. Rao N, Kumar S, Taylor S, et al. Diagnostic pathways in Crohn's disease. Clin Radiol 2019;74:578–91. 10.1016/j.crad.2019.03.013 [DOI] [PubMed] [Google Scholar]
- 17. Rimola J, Rodriguez S, García-Bosch O, et al. Magnetic resonance for assessment of disease activity and severity in ileocolonic Crohn's disease. Gut 2009;58:1113–20. 10.1136/gut.2008.167957 [DOI] [PubMed] [Google Scholar]
- 18. Zappa M, Stefanescu C, Cazals-Hatem D, et al. Which magnetic resonance imaging findings accurately evaluate inflammation in small bowel Crohn's disease? A retrospective comparison with surgical pathologic analysis. Inflamm Bowel Dis 2011;17:984–93. 10.1002/ibd.21414 [DOI] [PubMed] [Google Scholar]
- 19. Maconi G, Nylund K, Ripolles T, et al. EFSUMB recommendations and clinical guidelines for intestinal ultrasound (GIUS) in inflammatory bowel diseases. Ultraschall Med 2018;39:304–17. 10.1055/s-0043-125329 [DOI] [PubMed] [Google Scholar]
- 20. Goodsall TM, Jairath V, Feagan BG, et al. Standardisation of intestinal ultrasound scoring in clinical trials for luminal Crohn's disease. Aliment Pharmacol Ther 2021;53:873–86. 10.1111/apt.16288 [DOI] [PubMed] [Google Scholar]
- 21. Goodsall TM, Nguyen TM, Parker CE, et al. Systematic review: gastrointestinal ultrasound scoring indices for inflammatory bowel disease. J Crohns Colitis 2021;15:125–42. 10.1093/ecco-jcc/jjaa129 [DOI] [PubMed] [Google Scholar]
- 22. Bots S, Nylund K, Löwenberg M, et al. Ultrasound for assessing disease activity in IBD patients: a systematic review of activity scores. J Crohns Colitis 2018;12:920–9. 10.1093/ecco-jcc/jjy048 [DOI] [PubMed] [Google Scholar]
- 23. Ripollés T, Martínez MJ, Paredes JM, et al. Crohn disease: correlation of findings at contrast-enhanced us with severity at endoscopy. Radiology 2009;253:241–8. [DOI] [PubMed] [Google Scholar]
- 24. Paredes JM, Ripollés T, Cortés X, et al. Non-invasive diagnosis and grading of postsurgical endoscopic recurrence in Crohn's disease: usefulness of abdominal ultrasonography and (99m)Tc-hexamethylpropylene amineoxime-labelled leucocyte scintigraphy. J Crohns Colitis 2010;4:537–45. 10.1016/j.crohns.2010.03.002 [DOI] [PubMed] [Google Scholar]
- 25. Sasaki T, Kunisaki R, Kinoshita H, et al. Use of color Doppler ultrasonography for evaluating vascularity of small intestinal lesions in Crohn's disease: correlation with endoscopic and surgical macroscopic findings. Scand J Gastroenterol 2014;49:295–301. 10.3109/00365521.2013.871744 [DOI] [PubMed] [Google Scholar]
- 26. Allocca M, Craviotto V, Dell'Avalle C, et al. Bowel ultrasound score is accurate in assessing response to therapy in patients with Crohn's disease. Aliment Pharmacol Ther 2022;55:446–54. 10.1111/apt.16700 [DOI] [PubMed] [Google Scholar]
- 27. Ripollés T, Martínez MJ, Paredes JM, et al. Crohn disease: correlation of findings at contrast-enhanced us with severity at endoscopy. Radiology 2009;253:241–8. 10.1148/radiol.2531082269 [DOI] [PubMed] [Google Scholar]
- 28. Serafin Z, Białecki M, Białecka A, et al. Contrast-Enhanced ultrasound for detection of Crohn's disease activity: systematic review and meta-analysis. J Crohns Colitis 2016;10:354–62. 10.1093/ecco-jcc/jjv196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ripollés T, Martínez-Pérez MJ, Paredes JM, et al. The role of intravenous contrast agent in the sonographic assessment of Crohn's disease activity: is contrast agent injection necessary? J Crohns Colitis 2019;13:585–92. 10.1093/ecco-jcc/jjy204 [DOI] [PubMed] [Google Scholar]
- 30. Calabrese E, Zorzi F, Onali S, et al. Accuracy of small-intestine contrast ultrasonography, compared with computed tomography enteroclysis, in characterizing lesions in patients with Crohn's disease. Clin Gastroenterol Hepatol 2013;11:950–5. 10.1016/j.cgh.2013.01.015 [DOI] [PubMed] [Google Scholar]
- 31. Kumar S, Hakim A, Alexakis C, et al. Small intestinal contrast ultrasonography for the detection of small bowel complications in Crohn's disease: correlation with intraoperative findings and magnetic resonance enterography. J Gastroenterol Hepatol 2015;30:86–91. 10.1111/jgh.12724 [DOI] [PubMed] [Google Scholar]
- 32. Calabrese E, Petruzziello C, Onali S, et al. Severity of postoperative recurrence in Crohn's disease: correlation between endoscopic and sonographic findings. Inflamm Bowel Dis 2009;15:1635–42. 10.1002/ibd.20948 [DOI] [PubMed] [Google Scholar]
- 33. Zorzi F, Stasi E, Bevivino G, et al. A sonographic lesion index for Crohn's disease helps monitor changes in transmural bowel damage during therapy. Clin Gastroenterol Hepatol 2014;12:2071–7. 10.1016/j.cgh.2014.04.036 [DOI] [PubMed] [Google Scholar]
- 34. Mazza S, Conforti FS, Forzenigo LV, et al. Agreement between real-time elastography and delayed enhancement magnetic resonance enterography on quantifying bowel wall fibrosis in Crohn's disease. Dig Liver Dis 2022;54:69–75. 10.1016/j.dld.2021.05.018 [DOI] [PubMed] [Google Scholar]
- 35. Maconi G, Ardizzone S, Parente F, et al. Ultrasonography in the evaluation of extension, activity, and follow-up of ulcerative colitis. Scand J Gastroenterol 1999;34:1103–7. 10.1080/003655299750024904 [DOI] [PubMed] [Google Scholar]
- 36. Parente F, Molteni M, Marino B, et al. Are colonoscopy and bowel ultrasound useful for assessing response to short-term therapy and predicting disease outcome of moderate-to-severe forms of ulcerative colitis?: a prospective study. Am J Gastroenterol 2010;105:1150–7. 10.1038/ajg.2009.672 [DOI] [PubMed] [Google Scholar]
- 37. Parente F, Molteni M, Marino B, et al. Bowel ultrasound and mucosal healing in ulcerative colitis. Dig Dis 2009;27:285–90. 10.1159/000228562 [DOI] [PubMed] [Google Scholar]
- 38. Allocca M, Fiorino G, Bonovas S, et al. Accuracy of Humanitas ultrasound criteria in assessing disease activity and severity in ulcerative colitis: a prospective study. J Crohns Colitis 2018;12:1385–91. 10.1093/ecco-jcc/jjy107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bots S, Nylund K, Löwenberg M, et al. Intestinal ultrasound to assess disease activity in ulcerative colitis: development of a novel UC-Ultrasound index. J Crohns Colitis 2021;15:1264–71. 10.1093/ecco-jcc/jjab002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Maaser C, Petersen F, Helwig U, et al. Intestinal ultrasound for monitoring therapeutic response in patients with ulcerative colitis: results from the TRUST&UC study. Gut 2020;69:1629–36. 10.1136/gutjnl-2019-319451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jairath V, Ordas I, Zou G, et al. Reliability of measuring Ileo-Colonic disease activity in Crohn's disease by magnetic resonance Enterography. Inflamm Bowel Dis 2018;24:440–9. 10.1093/ibd/izx040 [DOI] [PubMed] [Google Scholar]
- 42. Hanzel J, Jairath V, Ma C. Responsiveness of Magnetic Resonance Enterography Indices for Evaluation of Luminal Disease Activity in Crohn’s Disease. Clin Gastroenterol Hepatol 2022. [DOI] [PubMed] [Google Scholar]
- 43. Coimbra AJF, Rimola J, O'Byrne S, et al. Magnetic resonance enterography is feasible and reliable in multicenter clinical trials in patients with Crohn's disease, and may help select subjects with active inflammation. Aliment Pharmacol Ther 2016;43:61–72. 10.1111/apt.13453 [DOI] [PubMed] [Google Scholar]
- 44. Kumar S, Parry T, Mallett S, et al. Diagnostic performance of magnetic resonance enterography disease activity indices compared with a histological reference standard for adult terminal ileal Crohn’s disease: experience from the METRIC trial. Journal of Crohn's and Colitis 2022;389. 10.1093/ecco-jcc/jjac062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Rimola J, Ordás I, Rodriguez S, et al. Magnetic resonance imaging for evaluation of Crohn's disease: validation of parameters of severity and quantitative index of activity. Inflamm Bowel Dis 2011;17:1759–68. 10.1002/ibd.21551 [DOI] [PubMed] [Google Scholar]
- 46. Rimola J, Alvarez-Cofiño A, Pérez-Jeldres T, et al. Comparison of three magnetic resonance enterography indices for grading activity in Crohn's disease. J Gastroenterol 2017;52:585–93. 10.1007/s00535-016-1253-6 [DOI] [PubMed] [Google Scholar]
- 47. Capozzi N, Ordás I, Fernandez-Clotet A, et al. Validation of the Simplified Magnetic Resonance Index of Activity [sMARIA] Without Gadolinium-enhanced Sequences for Crohn's Disease. J Crohns Colitis 2020;14:1074–81. 10.1093/ecco-jcc/jjaa030 [DOI] [PubMed] [Google Scholar]
- 48. Roseira J, Ventosa AR, de Sousa HT, et al. The new simplified MARIA score applies beyond clinical trials: a suitable clinical practice tool for Crohn's disease that parallels a simple endoscopic index and fecal calprotectin. United European Gastroenterol J 2020;8:1208–16. 10.1177/2050640620943089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tao Y, Li H, Xu H, et al. Can the simplified magnetic resonance index of activity be used to evaluate the degree of activity in Crohn's disease? BMC Gastroenterol 2021;21:409. 10.1186/s12876-021-01987-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Steward MJ, Punwani S, Proctor I, et al. Non-perforating small bowel Crohn's disease assessed by MRI enterography: derivation and histopathological validation of an MR-based activity index. Eur J Radiol 2012;81:2080–8. 10.1016/j.ejrad.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 51. Puylaert CAJ, Nolthenius CJT, Tielbeek JAW, et al. Comparison of MRI activity scoring systems and features for the terminal ileum in patients with Crohn disease. AJR Am J Roentgenol 2019;212:W25–31. 10.2214/AJR.18.19876 [DOI] [PubMed] [Google Scholar]
- 52. Ilvemark JFKF, Hansen T, Goodsall TM, et al. Defining transabdominal intestinal ultrasound treatment response and remission in inflammatory bowel disease: systematic review and expert consensus statement. J Crohns Colitis 2022;16:554-580. 10.1093/ecco-jcc/jjab173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kucharzik T, Wittig BM, Helwig U, et al. Use of intestinal ultrasound to monitor Crohn's disease activity. Clin Gastroenterol Hepatol 2017;15:535–42. 10.1016/j.cgh.2016.10.040 [DOI] [PubMed] [Google Scholar]
- 54. Kucharzik T, Wilkens R, Maconi G, et al. Intestinal ultrasound response and transmural healing after 48 weeks of treatment with ustekinumab in Crohn’s disease: STARDUST trial substudy. UEGW 2020. [Google Scholar]
- 55. Ordás I, Rimola J, Rodríguez S, et al. Accuracy of magnetic resonance enterography in assessing response to therapy and mucosal healing in patients with Crohn's disease. Gastroenterology 2014;146:374–82. 10.1053/j.gastro.2013.10.055 [DOI] [PubMed] [Google Scholar]
- 56. Deepak P, Fletcher JG, Fidler JL, et al. Radiological response is associated with better long-term outcomes and is a potential treatment target in patients with small bowel Crohn's disease. Am J Gastroenterol 2016;111:997–1006. 10.1038/ajg.2016.177 [DOI] [PubMed] [Google Scholar]
- 57. Fernandes SR, Rodrigues RV, Bernardo S, et al. Transmural healing is associated with improved long-term outcomes of patients with Crohn's disease. Inflamm Bowel Dis 2017;23:1403–9. 10.1097/MIB.0000000000001143 [DOI] [PubMed] [Google Scholar]
- 58. Fernandez-Clotet A, Sapena V, Capozzi N. Avoiding contrast-enhanced sequences does not compromise the precision of the simplified MaRIA for the assessment of non-penetrating Crohn’s disease activity. Eur Radiol 2022;33:3334–45. [DOI] [PubMed] [Google Scholar]
- 59. Castiglione F, Imperatore N, Testa A, et al. One-year clinical outcomes with biologics in Crohn's disease: transmural healing compared with mucosal or no healing. Aliment Pharmacol Ther 2019;49:1026–39. 10.1111/apt.15190 [DOI] [PubMed] [Google Scholar]
- 60. Jauregui-Amezaga A, Rimola J, Ordás I, et al. Value of endoscopy and MRI for predicting intestinal surgery in patients with Crohn's disease in the era of biologics. Gut 2015;64:1397–402. 10.1136/gutjnl-2014-308101 [DOI] [PubMed] [Google Scholar]
- 61. Lafeuille P, Hordonneau C, Vignette J, et al. Transmural healing and MRI healing are associated with lower risk of bowel damage progression than endoscopic mucosal healing in Crohn's disease. Aliment Pharmacol Ther 2021;53:577–86. 10.1111/apt.16232 [DOI] [PubMed] [Google Scholar]
- 62. Buisson A, Pereira B, Goutte M, et al. Magnetic resonance index of activity (MaRIA) and Clermont score are highly and equally effective MRI indices in detecting mucosal healing in Crohn's disease. Dig Liver Dis 2017;49:1211–7. 10.1016/j.dld.2017.08.033 [DOI] [PubMed] [Google Scholar]
- 63. Ordás I, Rimola J, Alfaro I, et al. Development and validation of a simplified magnetic resonance index of activity for Crohn's disease. Gastroenterology 2019;157:432–9. 10.1053/j.gastro.2019.03.051 [DOI] [PubMed] [Google Scholar]
- 64. Stoppino LP, Della Valle N, Rizzi S, et al. Magnetic resonance enterography changes after antibody to tumor necrosis factor (anti-TNF) alpha therapy in Crohn's disease: correlation with SES-CD and clinical-biological markers. BMC Med Imaging 2016;16:37. 10.1186/s12880-016-0139-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Takenaka K, Ohtsuka K, Kitazume Y, et al. Utility of magnetic resonance Enterography for small bowel endoscopic healing in patients with Crohn's disease. Am J Gastroenterol 2018;113:283–94. 10.1038/ajg.2017.464 [DOI] [PubMed] [Google Scholar]
- 66. Taylor SA, Mallett S, Bhatnagar G, et al. Diagnostic accuracy of magnetic resonance enterography and small bowel ultrasound for the extent and activity of newly diagnosed and relapsed Crohn's disease (metric): a multicentre trial. Lancet Gastroenterol Hepatol 2018;3:548–58. 10.1016/S2468-1253(18)30161-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miles A, Bhatnagar G, Halligan S, et al. Magnetic resonance enterography, small bowel ultrasound and colonoscopy to diagnose and stage Crohn's disease: patient acceptability and perceived burden. Eur Radiol 2019;29:1083–93. 10.1007/s00330-018-5661-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Puylaert CAJ, Tielbeek JAW, Bipat S, et al. Grading of Crohn's disease activity using CT, MRI, US and scintigraphy: a meta-analysis. Eur Radiol 2015;25:3295–313. 10.1007/s00330-015-3737-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Horsthuis K, Bipat S, Bennink RJ, et al. Inflammatory bowel disease diagnosed with US, MR, scintigraphy, and CT: meta-analysis of prospective studies. Radiology 2008;247:64–79. 10.1148/radiol.2471070611 [DOI] [PubMed] [Google Scholar]
- 70. Novak K, Tanyingoh D, Petersen F, et al. Clinic-based point of care transabdominal ultrasound for monitoring Crohn's disease: impact on clinical decision making. J Crohns Colitis 2015;9:795–801. 10.1093/ecco-jcc/jjv105 [DOI] [PubMed] [Google Scholar]
- 71. García-Bosch O, Ordás I, Aceituno M, et al. Comparison of diagnostic accuracy and impact of magnetic resonance imaging and colonoscopy for the management of Crohn's disease. J Crohns Colitis 2016;10:663–9. 10.1093/ecco-jcc/jjw015 [DOI] [PubMed] [Google Scholar]
- 72. Allocca M, Fiorino G, Bonifacio C, et al. Comparative accuracy of bowel ultrasound versus magnetic resonance Enterography in combination with colonoscopy in assessing Crohn's disease and guiding clinical decision-making. J Crohns Colitis 2018;12:1280–7. 10.1093/ecco-jcc/jjy093 [DOI] [PubMed] [Google Scholar]
- 73. Taylor SA, Mallett S, Bhatnagar G. Magnetic resonance enterography compared with ultrasonography in newly diagnosed and relapsing Crohn’s disease patients: the METRIC diagnostic accuracy study. Health Technol Assess 2019;23:1–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dohan A, Taylor S, Hoeffel C, et al. Diffusion-weighted MRI in Crohn's disease: current status and recommendations. J Magn Reson Imaging 2016;44:1381–96. 10.1002/jmri.25325 [DOI] [PubMed] [Google Scholar]
- 75. Menys A, Puylaert C, Tutein Nolthenius CE, et al. Quantified terminal ileal motility during Mr Enterography as a biomarker of Crohn disease activity: prospective Multi-Institution study. Radiology 2018;289:428–35. 10.1148/radiol.2018180100 [DOI] [PubMed] [Google Scholar]
- 76. Dal Buono A, Faita F, Peyrin-Biroulet L, et al. Ultrasound elastography in inflammatory bowel diseases a systematic review of accuracy compared to histopathological assessment. J Crohns Colitis 2022. 10.1093/ecco-jcc/jjac082. [Epub ahead of print: 13 Jun 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Orlando S, Fraquelli M, Coletta M, et al. Ultrasound elasticity imaging predicts therapeutic outcomes of patients with Crohn's disease treated with anti-tumour necrosis factor antibodies. J Crohns Colitis 2018;12:63–70. 10.1093/ecco-jcc/jjx116 [DOI] [PubMed] [Google Scholar]
- 78. Avila F, Caron B, Hossu G, et al. Magnetic resonance elastography for assessing fibrosis in patients with Crohn's disease: a pilot study. Dig Dis Sci 2021. doi: 10.1007/s10620-021-07311-9. [Epub ahead of print: 21 Nov 2021]. [DOI] [PubMed] [Google Scholar]
- 79. Sakuraba A, Nemoto N, Hibi N, et al. Extent of disease affects the usefulness of fecal biomarkers in ulcerative colitis. BMC Gastroenterol 2021;21:197. 10.1186/s12876-021-01788-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Zittan E, Kelly OB, Gralnek IM, et al. Fecal calprotectin correlates with active colonic inflammatory bowel disease but not with small intestinal Crohn's disease activity. JGH Open 2018;2:201–6. 10.1002/jgh3.12068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kopylov U, Yung DE, Engel T, et al. Fecal calprotectin for the prediction of small-bowel Crohn's disease by capsule endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2016;28:1137–44. 10.1097/MEG.0000000000000692 [DOI] [PubMed] [Google Scholar]
- 82. Naziroglu RE, Puylaert CAJ, Tielbeek JAW, et al. Semi-automatic bowel wall thickness measurements on MR enterography in patients with Crohn's disease. Br J Radiol 2017;90:20160654. 10.1259/bjr.20160654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Stidham RW, Enchakalody B, Waljee AK, et al. Assessing small bowel Stricturing and morphology in Crohn's disease using semi-automated image analysis. Inflamm Bowel Dis 2020;26:734–42. 10.1093/ibd/izz196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Makanyanga J, Ganeshan B, Rodriguez-Justo M, et al. MRI texture analysis (MRTA) of T2-weighted images in Crohn's disease may provide information on histological and MRI disease activity in patients undergoing ileal resection. Eur Radiol 2017;27:589–97. 10.1007/s00330-016-4324-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Meng J, Luo Z, Chen Z, et al. Intestinal fibrosis classification in patients with Crohn’s disease using CT enterography–based deep learning: comparisons with radiomics and radiologists. Eur Radiol 2022;50. 10.1007/s00330-022-08842-z [DOI] [PubMed] [Google Scholar]
- 86. Zorzi F, Ghosh S, Chiaramonte C, et al. Response assessed by ultrasonography as target of biological treatment for Crohn's disease. Clin Gastroenterol Hepatol 2020;18:2030–7. 10.1016/j.cgh.2019.10.042 [DOI] [PubMed] [Google Scholar]
- 87. Danese S, Sandborn WJ, Colombel J-F, et al. Endoscopic, radiologic, and histologic healing with Vedolizumab in patients with active Crohn's disease. Gastroenterology 2019;157:1007–18. 10.1053/j.gastro.2019.06.038 [DOI] [PubMed] [Google Scholar]
- 88. Atreya R, Siegmund B. Location is important: differentiation between ileal and colonic Crohn's disease. Nat Rev Gastroenterol Hepatol 2021;18:544–58. 10.1038/s41575-021-00424-6 [DOI] [PubMed] [Google Scholar]
- 89. Rieder F, Latella G, Magro F, et al. European Crohn's and colitis organisation topical review on prediction, diagnosis and management of Fibrostenosing Crohn's disease. J Crohns Colitis 2016;10:873–85. 10.1093/ecco-jcc/jjw055 [DOI] [PubMed] [Google Scholar]
- 90. Rieder F, Bettenworth D, Ma C, et al. An expert consensus to standardise definitions, diagnosis and treatment targets for anti-fibrotic stricture therapies in Crohn's disease. Aliment Pharmacol Ther 2018;48:347–57. 10.1111/apt.14853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rimola J, Planell N, Rodríguez S, et al. Characterization of inflammation and fibrosis in Crohn's disease lesions by magnetic resonance imaging. Am J Gastroenterol 2015;110:432–40. 10.1038/ajg.2014.424 [DOI] [PubMed] [Google Scholar]
- 92. Tielbeek JAW, Ziech MLW, Li Z, et al. Evaluation of conventional, dynamic contrast enhanced and diffusion weighted MRI for quantitative Crohn's disease assessment with histopathology of surgical specimens. Eur Radiol 2014;24:619–29. 10.1007/s00330-013-3015-7 [DOI] [PubMed] [Google Scholar]
- 93. Li X-H, Mao R, Huang S-Y, et al. Characterization of degree of intestinal fibrosis in patients with Crohn disease by using magnetization transfer MR imaging. Radiology 2018;287:494–503. 10.1148/radiol.2017171221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Coimbra A, Rimola J, Cuatrecasas M, et al. Magnetic resonance Enterography and histology in patients with Fibrostenotic Crohn's disease: a multicenter study. Clin Transl Gastroenterol 2022;13:e00505. 10.14309/ctg.0000000000000505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Louis E, Collard A, Oger AF, et al. Behaviour of Crohn's disease according to the Vienna classification: changing pattern over the course of the disease. Gut 2001;49:777–82. 10.1136/gut.49.6.777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Pariente B, Mary J-Y, Danese S, et al. Development of the Lémann index to assess digestive tract damage in patients with Crohn's disease. Gastroenterology 2015;148:52–63. 10.1053/j.gastro.2014.09.015 [DOI] [PubMed] [Google Scholar]
- 97. Le Berre C, Peyrin-Biroulet L, SPIRIT-IOIBD study group . Selecting end points for disease-modification trials in inflammatory bowel disease: the spirit consensus from the IOIBD. Gastroenterology 2021;160:1452–60. 10.1053/j.gastro.2020.10.065 [DOI] [PubMed] [Google Scholar]
- 98. Gilletta C, Lewin M, Bourrier A, et al. Changes in the Lémann index values during the first years of Crohn's disease. Clin Gastroenterol Hepatol 2015;13:1633–40. 10.1016/j.cgh.2015.02.041 [DOI] [PubMed] [Google Scholar]
- 99. Fiorino G, Bonifacio C, Allocca M, et al. Bowel damage as assessed by the Lémann index is reversible on anti-TNF therapy for Crohn's disease. J Crohns Colitis 2015;9:633–9. 10.1093/ecco-jcc/jjv080 [DOI] [PubMed] [Google Scholar]
- 100. Bodini G, Giannini EG, De Maria C, et al. Anti-TNF therapy is able to stabilize bowel damage progression in patients with Crohn's disease. A study performed using the Lémann index. Dig Liver Dis 2017;49:175–80. 10.1016/j.dld.2016.10.014 [DOI] [PubMed] [Google Scholar]
- 101. Ribaldone DG, Caviglia GP, Pellicano R, et al. Adalimumab versus azathioprine to halt the progression of bowel damage in Crohn's disease: application of Lémann index. Scand J Gastroenterol 2019;54:1339–45. 10.1080/00365521.2019.1686057 [DOI] [PubMed] [Google Scholar]
- 102. Krugliak Cleveland N, Torres J, Rubin DT. What does disease progression look like in ulcerative colitis. and How Might It Be Prevented? Gastroenterology 2022;162:1396–408. [DOI] [PubMed] [Google Scholar]
- 103. Novak KL, Kaplan GG, Panaccione R, et al. A simple ultrasound score for the accurate detection of inflammatory activity in Crohn's disease. Inflamm Bowel Dis 2017;23:2001–10. 10.1097/MIB.0000000000001174 [DOI] [PubMed] [Google Scholar]
- 104. Sævik F, Eriksen R, Eide GE, et al. Development and validation of a simple ultrasound activity score for Crohn's disease. J Crohns Colitis 2021;15:115–24. 10.1093/ecco-jcc/jjaa112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Allocca M, Filippi E, Costantino A. Milan ultrasound criteria are accurate in assessing disease activity in ulcerative colitis: external validation. United European Gastroenterol J 2020;2050640620980203. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2021-326562supp001.pdf (106KB, pdf)