Abstract
Significance
Electronic cigarettes (e-cigarettes) aerosolise liquids that contain nicotine, propylene glycol, glycerol and appealing flavours. In the USA, regulations have limited the availability of flavoured e-cigarettes in pod-based systems, and further tightening is expected. In response, some e-cigarette users may attempt to make their e-liquids (do-it-yourself, DIY). This study examined toxicant emissions from several aerosolised DIY e-liquids.
Methods
DIY additives were identified by reviewing users’ responses to a hypothetical flavour ban, e-cigarette internet forums and DIY mixing internet websites. They include essential oils, cannabidiol, sucralose and ethyl maltol. E-liquids with varying concentrations and combinations of additives and tobacco and menthol flavours were prepared and were used to assess reactive oxygen species (ROS), carbonyl and phenol emissions in machine-generated aerosols.
Results
Data showed that adding DIY additives to unflavoured, menthol-flavoured or tobacco-flavoured e-liquids increases toxicant emissions to levels comparable with those from commercial flavoured e-liquids. Varying additive concentrations in e-liquids did not have a consistently significant effect on the tested emissions, yet increasing power yielded significantly higher ROS, carbonyl and phenol emissions for the same additive concentration. Adding nicotine to DIY e-liquids with sucralose yielded increase in some emissions and decrease in others, with freebase nicotine-containing e-liquid giving higher ROS emissions than that with nicotine salt.
Conclusion
This study showed that DIY additives can impact aerosol toxicant emissions from e-cigarettes and should be considered by policymakers when restricting commercially available flavoured e-liquids.
Keywords: Addiction, Carcinogens, Electronic nicotine delivery devices, Harm Reduction, Toxicology
WHAT IS ALREADY KNOWN ON THIS TOPIC
Electronic cigarette (e-cigarette) liquids are available in many flavours; however, policies have been proposed or implemented that prohibit many flavoured e-liquids.
E-cigarette users often add unregulated additives to their e-liquids to obtain desired flavours.
Previous research has demonstrated that liquid composition can impact e-cigarette aerosol toxicant profile.
WHAT THIS STUDY ADDS
This study identified ingredients that e-cigarette users may add to e-liquids in response to flavour-limiting policies and systematically assessed the impact of do-it-yourself (DIY) additives on e-cigarette toxicant emissions.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Because DIY e-liquids may result in similar or increased toxicant emissions to commercially available flavoured e-liquids, regulations that limit e-cigarette flavours may have unintended consequences if the use of DIY e-liquids increases.
Policies that prevent the use of DIY liquids, such as prohibiting ‘open-system’ e-cigarettes, may strengthen the effects of policies that limit e-cigarette flavours.
Introduction
Electronic cigarette (e-cigarette) use has increased globally in the last decade, especially among youth.1–3 Epidemiological data have linked e-cigarette use trends to efficient nicotine delivery, flavour availability and harm reduction claims.4–7 The availability of flavours marketed heavily with vivid descriptions of taste and sensory experiences has contributed to e-cigarette experimentation and use among youth.8 9 Importantly, using multiple flavours is associated with increased frequency of e-cigarette use,10 11 and adolescents who use flavoured e-cigarettes are more likely to become regular users.12
Recently, the US Food and Drug Administration implemented an enforcement policy removing all flavoured (except tobacco or menthol) ‘cartridge-based e-cigarettes’ from the market.13 However, many flavoured e-liquids are still available and can be used in refillable ‘open-system e-cigarettes’, until the enforcement of the Premarket Tobacco Product Authorization which denied the marketing of ~1 million flavoured e-cigarettes.14 Some users of open systems prepare their e-liquids,15 a practice known as do-it-yourself (DIY).16 DIY liquids can be prepared from propylene glycol and glycerol (PG/G), ready-to-use DIY flavour concentrates with unknown ingredients, and/or other DIY additives that may or may not be intended for use in e-cigarettes. There are limited data regarding the prevalence of DIY behaviours (and subsequent health risks), although previous research has reported less than 5% of current users make DIY liquids.17 However, flavour concentrates used in DIY preparations constitute a large share of the e-cigarette market.18 Additionally, research examining the potential impact of a hypothetical e-cigarette flavour ban suggests some users may consider making their e-liquids in response.19 20 Hence, this study aimed to assess the impact of DIY behaviours on toxicant emissions from e-cigarettes.
Methods
DIY flavour additive identification
A concept mapping study was conducted to identify and describe reactions that current e-cigarette users (n=71) may take in response to a hypothetical policy that only allows the sale of tobacco, menthol or unflavoured e-liquids.19 A primary response was making DIY e-liquids and adding compounds such as cannabidiol (CBD) and essential oils. To identify DIY recipes that contained the identified ingredients, a search of 42 e-cigarette forums21 and a Google search for ‘DIY e-liquid recipes’ were conducted.
Study design
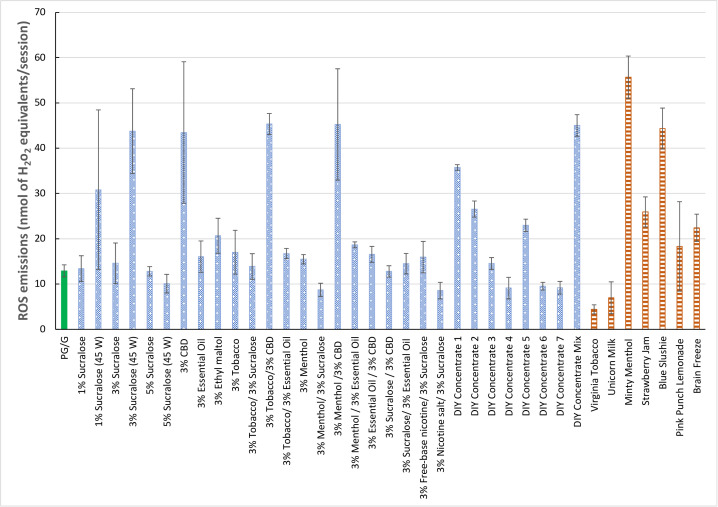
We differentiate between DIY additives that are based on chemical compounds (eg, sucralose, CBD, ethyl maltol and essential oil), DIY concentrates that could be added to e-liquids as flavourings (eg, concentrates 1–7 in figure 1) and ready-to-use flavoured e-liquids (eg, menthol, tobacco and flavoured e-liquids mentioned in figure 1). We prepared e-liquids with one additive (3% concentration) in PG/G (30/70) or a mixture of two additives (each at 3% concentration). A constant mass of CBD powder (m=0.25 g) was used for all CBD-containing liquids. Tobacco-flavoured or menthol-flavoured e-liquids were prepared at 3% level with or without additives. To assess the impact of nicotine on toxicant emissions, two e-liquids were prepared with sucralose (3%) and either freebase nicotine (3%) or nicotine salt (3%). Also, e-liquids with different sucralose levels (1%, 3% and 5%) were tested at two different powers (30 W and 45 W). Seven DIY concentrates were tested individually or as a mixture. Seven commercially available flavoured e-liquids selected from top flavours in the market were tested for comparison. We measured reactive oxygen species (ROS), carbonyl and phenol emissions in machine-generated aerosols. Statistical significance was evaluated using t-test on head-to-head comparisons (p<0.05).
Figure 1.
ROS emissions from 27 laboratory-prepared DIY e-cigarette liquids (dotted fill) in comparison with unflavoured PG/G liquid (solid fill) and seven commercially available flavoured e-liquids (horizontal stripes), all generated at 30 W. Emissions from e-liquids with different levels of sucralose at 45 W were added for comparison. CBD, cannabidiol; DIY, do it yourself; e-cigarette, electronic cigarette; G, glycerol; PG, propylene glycol; ROS, reactive oxygen species.
Aerosol generation and sampling
The AUB Aerosol Lab Vaping Instrument22 was used to generate aerosols from a Kangertech Subox Mini e-cigarette. The puffing regimen consisted of 10 puffs of 4 s puff duration, 10 s interpuff interval and 8 L/min flow rate. The flow was split into three branches: a 1 L/min branch was used for carbonyl quantification using a 2,4-dinitrophenylhydrazine gas sampling cartridge placed downstream a filter pad,23 a 3.5 L/min branch was used for quantification of phenols in the particle phase trapped on the filter pads,24 and the filter of the other 3.5 L/min branch was directly submerged in 2′,7′-dichlorofluorescein probe solution for ROS analysis. Each condition was tested in triplicate.25
Results
Data showed that ROS emissions from DIY concentrates or from menthol and tobacco flavours mixed with DIY additives were often significantly higher than PG/G base e-liquid and like those emitted from commercially available flavoured e-liquids (figure 1). Mixing menthol flavour with CBD or essential oil yielded significantly higher (192% and 20% increase, respectively) while sucralose yielded significantly lower (44% decrease) ROS emissions than menthol with no additives. Only CBD additive significantly increased ROS emissions from tobacco flavour (166%). Although the nature of the additive was impactful on ROS emissions, different concentrations of sucralose did not yield higher emissions than unflavoured PG/G liquid. Also, the effect of power increase (from 30 W to 45 W) was dependent on sucralose concentration (significant at 3% only). Interestingly, nicotine form affected ROS emissions from a liquid containing sucralose, with nicotine salt yielding lower ROS emissions than freebase nicotine.
Carbonyl and phenol emissions from DIY concentrates or from menthol and tobacco flavours mixed with DIY additives were also comparable with commercial flavoured e-liquids (online supplemental table S1 and figures S1–S4). The addition of 1% and 5% of sucralose to PG/G led to a significant decrease in acetone (Ac) (~50%), crotonaldehyde (CA) (~30%), methacrolein (MAcr) (~40%) and catechol (not detected, ND), yet a significant increase in propionaldehyde (PA) (255% and 123%, respectively). The addition of 3% sucralose and 3% CBD had no significant effect on emissions and 3% essential oil increased CA and MAcr (19% and 6%, respectively). Adding 3% ethyl maltol resulted in increasing acetaldehyde (AA) (9%) and methyl glyoxal (MGA) (38%), while decreasing Ac (47%), CA (27%), MAcr (41%) and catechol (ND). Higher power (45 W vs 30 W) led to higher emissions of AA (458%), Ac (288%), MAcr (300%) and phenol (290%) for e-liquids with 1% sucralose, higher CA (1661%), glyoxal (GA) (2347%), MGA (4094%) and phenol (237%) for 3% sucralose, and higher AA (346%), Ac (267%), MAcr (269%) and GA (359%) for 5% sucralose. The influence of sucralose percentage on emissions at 30 W was mixed, as was the influence of mixing more than one additive with PG/G base.
tc-2022-057505supp001.pdf (2.9MB, pdf)
The addition of freebase nicotine or nicotine salt to PG/G with sucralose led to a significant increase in AA (50% for freebase) and PA (203% and 300%, respectively) and a significant decrease in Ac (~40%), MAcr (~34%), GA (~25%) and catechol (ND with nicotine). DIY additives increased certain emissions from tobacco-flavoured e-liquids like Ac (54%), CA (32%), MAcr (46%) and phenol (144%) with sucralose, formaldehyde (FA) (534%), PA (102%) and CA (33%) with CBD, and Ac (79%), CA (52%), MAcr (57%) and MGA (114%) with essential oil. However, for menthol-flavoured e-liquids, CBD showed no significant effects (due to the presence of an outlier), while sucralose and essential oil increased Ac (62% and 74%), CA (37% and 69%) and MAcr (46% and 62%). It should be noted that additives decreased some emissions, like the case of sucralose added to menthol-flavoured e-liquids (FA: 47%; AA: 47%; Acr: ND; and PA: 86%).
Discussion
The results highlight that if flavoured e-liquids were removed from the market, as well as DIY concentrates, the addition of DIY additives to unflavoured PG/G or tobacco and menthol-flavoured e-liquids may yield similar toxicant exposure. Also, mixing concentrates and/or additives, which is a common practice among e-cigarette users,10 may lead to significantly higher ROS emissions compared with individual concentrates (figure 1).
The data on carbonyl and phenol emissions showed mixed observations with increase in some emissions and decrease in others for the same e-liquid condition. This could be attributed to the different mechanisms of formation during e-cigarette operation,26 27 or to coil variability in triplicate measurements that can mask the impact of liquid ingredients on emissions.28 Also, focusing on toxicants that are mainly formed from PG/G degradation23 24 29 may lead to overlooking other degradation pathways, as in the case of sucralose leading to chloropropanols.28 30 Moreover, toxicant assessments should consider secondary interactions between e-liquid constituents.31 This could explain the lower ROS emissions from nicotine salt that could be attributed to ROS trapping on the benzene ring of the benzoate counter anion.32 33 Nevertheless, the current work shows that DIY additives can contribute significantly to e-cigarette toxicant emissions and questions the need for ‘open-system’ e-cigarettes that allow for such exposure to unregulated additives.34
Limitations of the current work include using ready-to-use DIY additives dissolved in PG (eg, sucralose) or containing unidentified chemicals (eg, essential oil). The data only represent 10 puffs, and more puffs or chronic use may reveal further toxicant emissions. Additionally, after a wider flavour ban is implemented, more ‘novice’ DIY users may start making liquids with additive concentrations that exceed those assessed in this study. It may be worth considering more ‘extreme’ scenarios in the future.
Conclusion
E-cigarette users vaping e-liquids with DIY concentrates or menthol/tobacco flavours mixed with DIY additives may be exposed to the same or greater levels of toxicants as from the ‘to-be banned’ commercial flavoured e-liquids. If ‘open-system’ devices remain on the market allowing users to add DIY e-liquids to their e-cigarettes, regulators should consider how DIY behaviours could undermine the impact of any potential flavour ban.
Footnotes
Twitter: @aal_najat
Contributors: AE-H, ES, RS, REH and ST conceived of the study idea and study design. RS, REH, MD, ME-K, OA and AY conducted the experimental work. AE-H and ES wrote the first and final version of the manuscript. All authors revised the manuscript and approved its final version.
Funding: This research was supported by a pilot project award (grant number U54CA229974) from the National Cancer Institute of the National Institutes of Health (NIH) and the Center for Tobacco Products (CTP) of the US Food and Drug Administration (FDA) and by a grant (U54DA036105) from the National Institute on Drug Abuse of the NIH and the CTP of the FDA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Competing interests: AS is a paid consultant in litigation against the tobacco and e-cigarette industry and is named on one patent for a device that measures the puffing behaviour of e-cigarette users and another patent application for a smoking cessation intervention.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data related to the concept mapping and carbonyl and phenol emissions are available upon request.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Jerzyński T, Stimson GV, Shapiro H, et al. Estimation of the global number of e-cigarette users in 2020. Harm Reduct J 2021;18:109. 10.1186/s12954-021-00556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tarasenko Y, Ciobanu A, Fayokun R, et al. Electronic cigarette use among adolescents in 17 European study sites: findings from the global youth tobacco survey. Eur J Public Health 2022;32:126–32. 10.1093/eurpub/ckab180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Park-Lee E, Ren C, Sawdey MD, et al. Notes from the field: E-cigarette use among middle and high school students - national youth tobacco survey, United States, 2021. MMWR Morb Mortal Wkly Rep 2021;70:1387–9. 10.15585/mmwr.mm7039a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Berg CJ. Preferred flavors and reasons for e-cigarette use and discontinued use among never, current, and former smokers. Int J Public Health 2016;61:225–36. 10.1007/s00038-015-0764-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kong G, Morean ME, Cavallo DA, et al. Reasons for electronic cigarette experimentation and discontinuation among adolescents and young adults. Nicotine Tob Res 2015;17:847–54. 10.1093/ntr/ntu257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sapru S, Vardhan M, Li Q, et al. E-cigarettes use in the United States: reasons for use, perceptions, and effects on health. BMC Public Health 2020;20:1518. 10.1186/s12889-020-09572-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Baker AN, Wilson SJ, Hayes JE. Flavor and product messaging are the two most important drivers of electronic cigarette selection in a choice-based task. Sci Rep 2021;11:4689. 10.1038/s41598-021-84332-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen-Sankey JC, Kong G, Choi K. Perceived ease of flavored e-cigarette use and e-cigarette use progression among youth never tobacco users. PLoS One 2019;14:e0212353. 10.1371/journal.pone.0212353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Soule EK, Sakuma K-LK, Palafox S, et al. Content analysis of Internet marketing strategies used to promote flavored electronic cigarettes. Addict Behav 2019;91:128–35. 10.1016/j.addbeh.2018.11.012 [DOI] [PubMed] [Google Scholar]
- 10. Morean ME, Butler ER, Bold KW, et al. Preferring more e-cigarette flavors is associated with e-cigarette use frequency among adolescents but not adults. PLoS One 2018;13:e0189015. 10.1371/journal.pone.0189015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Rao PD, Nanding H, Strasser AA, et al. Pilot experiment: the effect of added flavorants on the taste and pleasantness of mixtures of glycerol and propylene glycol. Chemosens Percept 2018;11:1–9. 10.1007/s12078-017-9231-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Leventhal AM, Goldenson NI, Cho J, et al. Flavored e-cigarette use and progression of vaping in adolescents. Pediatrics 2019;144. 10.1542/peds.2019-0789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. FDA . FDA finalizes enforcement policy on unauthorized flavored cartridge-based e-cigarettes that appeal to children, including fruit and mint, 2020. Available: https://www.fda.gov/news-events/press-announcements/fda-finalizes-enforcement-policy-unauthorized-flavored-cartridge-based-e-cigarettes-appeal-children
- 14. FDA . FDA Makes Significant Progress in Science-Based Public Health Application Review, Taking Action on Over 90% of More Than 6.5 Million ‘Deemed’ New Tobacco Products Submitted, 2021. Available: https://www.fda.gov/news-events/press-announcements/fda-makes-significant-progress-science-based-public-health-application-review-taking-action-over-90
- 15. Breland A, Soule E, Lopez A, et al. Electronic cigarettes: what are they and what do they do? Ann N Y Acad Sci 2017;1394:5–30. 10.1111/nyas.12977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cox S, Leigh NJ, Vanderbush TS, et al. An exploration into “do-it-yourself” (DIY) e-liquid mixing: usUsers' motivations, practices and product laboratory analysis. Addict Behav Rep 2019;9:100151. 10.1016/j.abrep.2018.100151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soule EK, Mayne S, Snipes W, et al. Impacts of COVID-19 on electronic cigarette purchasing, use and related behaviors. Int J Environ Res Public Health 2020;17. doi: 10.3390/ijerph17186762. [Epub ahead of print: 16 09 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. MordorIntelligence . E-cigarette market - growth, trends and forecast (2019 - 2024), 2018. Available: https://www.mordorintelligence.com/industry-reports/global-e-cigarettes-market-industry
- 19. Soule E, Mayne S, Snipes W. Electronic cigarette users’ reactions and responses to a hypothetical ban on flavored electronic cigarette liquids. Tob Control In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gravely S, Smith DM, Liber AC, et al. Responses to potential nicotine vaping product flavor restrictions among regular vapers using non-tobacco flavors: findings from the 2020 ITC smoking and vaping survey in Canada, England and the United States. Addict Behav 2022;125:107152. 10.1016/j.addbeh.2021.107152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maloney SF, Soule EK, Palafox S, et al. A longitudinal analysis of electronic cigarette forum participation. Addict Behav 2019;91:75–81. 10.1016/j.addbeh.2018.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Talih S, Balhas Z, Eissenberg T, et al. Effects of user puff topography, device voltage, and liquid nicotine concentration on electronic cigarette nicotine yield: measurements and model predictions. Nicotine Tob Res 2015;17:150–7. 10.1093/ntr/ntu174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. El-Hellani A, Salman R, El-Hage R, et al. Nicotine and carbonyl emissions from popular electronic cigarette products: correlation to liquid composition and design characteristics. Nicotine Tob Res 2018;20:215–23. 10.1093/ntr/ntw280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El-Hage R, El-Hellani A, Salman R, et al. Vaped humectants in e-cigarettes are a source of phenols. Chem Res Toxicol 2020;33:2374–80. 10.1021/acs.chemrestox.0c00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Haddad C, Salman R, El-Hellani A, et al. Reactive oxygen species emissions from supra- and sub-ohm electronic cigarettes. J Anal Toxicol 2019;43:45–50. 10.1093/jat/bky065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jensen RP, Strongin RM, Peyton DH. Solvent chemistry in the electronic cigarette reaction vessel. Sci Rep 2017;7:42549. 10.1038/srep42549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kerber PJ, Duell AK, Powers M, et al. Effects of common e-liquid flavorants and added nicotine on toxicant formation during vaping analyzed by 1H NMR spectroscopy. Chem Res Toxicol 2022. 10.1021/acs.chemrestox.2c00110. [Epub ahead of print: 23 Jun 2022]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duell AK, McWhirter KJ, Korzun T, et al. Sucralose-enhanced degradation of electronic cigarette liquids during vaping. Chem Res Toxicol 2019;32:1241–9. 10.1021/acs.chemrestox.9b00047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Uchiyama S, Ohta K, Inaba Y, et al. Determination of carbonyl compounds generated from the e-cigarette using coupled silica cartridges impregnated with hydroquinone and 2,4-dinitrophenylhydrazine, followed by high-performance liquid chromatography. Anal Sci 2013;29:1219–22. 10.2116/analsci.29.1219 [DOI] [PubMed] [Google Scholar]
- 30. El-Hage R, El-Hellani A, Haddad C, et al. Toxic emissions resulting from sucralose added to electronic cigarette liquids. Aerosol Science and Technology 2019;53:1197–203. 10.1080/02786826.2019.1645294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Erythropel HC, Jabba SV, DeWinter TM, et al. Formation of flavorant–propylene glycol adducts with novel toxicological properties in chemically unstable e-cigarette liquids. Nicotine Tobacco Research 2019;21:1248–58. 10.1093/ntr/nty192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Herrera-Vásquez A, Salinas P, Holuigue L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. Front Plant Sci 2015;6:171. 10.3389/fpls.2015.00171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Son Y, Mishin V, Laskin JD, et al. Hydroxyl radicals in e-cigarette vapor and E-vapor oxidative potentials under different vaping patterns. Chem Res Toxicol 2019;32:1087–95. 10.1021/acs.chemrestox.8b00400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Eissenberg T, Soule E, Shihadeh A, et al. 'Open-system' electronic cigarettes cannot be regulated effectively. Tob Control 2021;30:234–5. 10.1136/tobaccocontrol-2019-055499 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tc-2022-057505supp001.pdf (2.9MB, pdf)
Data Availability Statement
Data related to the concept mapping and carbonyl and phenol emissions are available upon request.