Abstract
Background
Risk of nonrelapse mortality (NRM) after hematopoietic cell transplantation (HCT) is high. Patient-level clinical prediction models such as the HCT–comorbidity index (HCT-CI) help identify those at increased risk for NRM, but the independent contribution of social determinants of health on HCT outcomes is not well characterized.
Methods
This study included 1602 patients who underwent allogeneic HCT between 2013 and 2019 at City of Hope. Census tract–level social vulnerability was measured using the social vulnerability index (SVI). Fine-Gray multivariable regression evaluated the association between SVI and 1-year NRM. Subgroup analysis examined risk of NRM across combined SVI and HCT-CI categories and by race and ethnicity.
Results
Cumulative incidence of 1-year NRM after HCT was 15.3% (95% confidence interval [CI] = 13.6% to 17.1%). In multivariable analysis, patients in the highest SVI tertile (highest social vulnerability) had a 1.4-fold risk (subdistribution hazard ratio [sHR] = 1.36, 95% CI = 1.04 to 1.78) of NRM compared with individuals in the lower tertiles; patients in the highest SVI tertile who also had elevated (≥3) HCT-CI scores had the highest risk (sHR = 1.81, 95% CI = 1.26 to 2.58) of 1-year NRM (reference: lower SVI tertiles and HCT-CI < 3). High social vulnerability was associated with risk of 1-year NRM in Asian (sHR = 2.03, 95% CI = 1.09 to 3.78) and Hispanic (sHR = 1.63, 95% CI = 1.04 to 2.55) but not non-Hispanic White patients.
Conclusions
High social vulnerability independently associated with 1-year NRM after HCT, specifically among minority populations and those with a high comorbidity burden at HCT. These findings may inform targeted approaches for needs assessment during and after HCT, allowing for timely interventions to improve health outcomes in at-risk patients.
Allogeneic hematopoietic cell transplantation (HCT) is an established treatment for malignant and nonmalignant hematologic diseases. Advances in treatment strategies have contributed to a growing number of patients undergoing HCT, with an estimated 10 000 patients who undergo allogeneic HCT annually in the United States alone (1). However, the benefit of HCT should be balanced by the risk of nonrelapse mortality (NRM) during the first year after transplantation attributed, in part, to complications associated with prolonged cytopenia and the development of graft-versus-host disease (GVHD) after HCT. Established patient-related risk factors for NRM after HCT include older age, male sex, and the burden of comorbidities at the time of HCT (2-4). To this end, clinical prediction models such as the HCT–comorbidity index (HCT-CI) have been developed to identify those at increased risk for NRM after HCT (2,5), allowing for appropriate risk-stratification and real-time decision making regarding the intensity of HCT conditioning and supportive care during HCT. That said, it is increasingly recognized that these patient-level variables alone do not explain the variability in outcomes after HCT. Studies conducted mostly in non-oncology populations suggest that social determinants of health, which include individual factors (eg, education, employment) as well as the neighborhood and environment in which people work and live, can substantially impact health outcomes (6-8).
The Centers for Disease Control (CDC) social vulnerability index (SVI) was initially developed as a tool to evaluate communities’ resilience when confronted by disaster (9). More recently, SVI has been used to determine the impact of neighborhood-level social vulnerability on health outcomes in oncology and non-oncology populations (9-11). The SVI provides an overall composite vulnerability score as well as scores for each component of the index, allowing for delineation of specific neighborhood-level components (eg, socioeconomic status [SES], education, housing, transportation) that constitute social determinants of health (12). Specifically, studies have found that higher SVI scores, which indicate higher social vulnerability, are associated with poorer surgical (eg, adverse postoperative outcomes) and oncologic (eg, mortality) outcomes, with the impact particularly notable among minority populations (10,13,14). The SVI differs from other indices such as the area deprivation index and community risk score, because of its ability to provide additional information on important environment and neighborhood-level determinants of health.
To date, there have been no studies examining the association between a patient’s neighborhood-level socioeconomic environment and NRM after HCT. Importantly, little is known about the interaction between established risk factors (eg, HCT-CI) for NRM and neighborhood-level socioeconomic factors. To fill this gap, we used the CDC SVI to evaluate the relationship between census tract–level social vulnerability and NRM after HCT in a demographically diverse population of patients who underwent HCT and further explored the interaction of baseline comorbidity at the time of HCT and race and ethnicity on social vulnerability.
Methods
Study Population and Definitions
This was a retrospective cohort study of patients living in California who underwent a first allogeneic HCT for hematologic disease at City of Hope (COH) from 2013 to 2019. Patients were identified from the COH Long-term Follow-up program, which supports follow-up of all patients receiving HCT at COH. The Long-term Follow-up protocol was approved by the COH institutional review board, and all patients or their legal representatives provided informed consent. This protocol collects information on patients’ demographics (age, sex, race and ethnicity [Alaska Native, American Indian, Asian, Black, Hispanic, multiracial, Native Hawaiian, non-Hispanic White, White with ethnicity not specified, missing or unknown], and address), insurance, diagnosis, HCT conditioning, GVHD prophylaxis, leukocyte antigen [HLA] match, cytomegalovirus (CMV) status, comorbidities to derive the HCT-CI, and performance score (Lansky, Karnofsky). Euclidean distance to COH was calculated for all patients, given the recognized importance of timely access to subspeciality care in populations with high comorbidity burden (15,16). Vital status and cause of death were obtained from the National Death Index. Causes of deaths were classified as primary disease, subsequent malignant neoplasms, GVHD, infection, cardiopulmonary, organ failure, external, or other. NRM included causes other than primary disease.
Myeloablative conditioning was defined as receipt of at least 8 Gy of fractionated body and/or marrow irradiation or busulfan (>8mg/kg) with cyclophosphamide (120 mg/kg) or with clofarabine (40 mg/m2) (17). HLA match was categorized as matched (8 of 8), mismatched, or haploidentical, and CMV status was positive if the donor and/or recipient was CMV positive. High HCT-CI was defined as having a score of at least 3. Low performance status was defined as a performance score of no more than 80. Established criteria were used to define severity of acute GVHD (18) and relapse risk (high, standard) (19) at HCT.
Social Vulnerability
The CDC SVI was used to measure social vulnerability. The SVI comprises 4 themes constructed using 15 social and environmental variables from the US census (theme 1: SES [poverty, unemployment, per capita income, no high school diploma]; theme 2: household composition and disability [persons aged 65 years and older, persons aged 17 years and younger, single parent households, civilian with a disability]; theme 3: minority status and language [minority status, limited English]; and theme 4: housing and transportation [multi-unit housing, mobile homes, crowded housing, no vehicle access, group quarters]) (12). Overall, SVI and individual theme scores are ranked across the California census tracts as percentiles ranging from 0 (least vulnerable) to 1 (most vulnerable). Patient addresses at the time of HCT were geocoded to determine census tract–level Federal Information Processing Standards codes, which were used to determine the SVI scores for each individual. Census 2014, 2016, and 2018 data were used for patients who underwent HCT between 2013-2015, 2016-2017, and 2018-2019, respectively.
Statistical Analysis
Descriptive statistics of patient demographics, insurance status, diagnosis, HCT-related variables, HCT-CI scores, performance scores, and risk of relapse were generated for the cohort and by vital status (alive, NRM). The primary outcome measure was NRM at 1-year post-HCT. Patients were at risk for NRM from the date of HCT until date of death, date last known alive, or 1 year after HCT, whichever came first. Death due to non-NRM and receipt of a second HCT was treated as competing risks. Patients alive at beyond 1 year after HCT were censored at 1 year.
Cumulative incidence of NRM was calculated taking into consideration competing risk of relapse-related mortality and second HCT. All univariable and multivariable analyses were conducted using the Fine-Gray subdistribution hazard model (20). Subdistribution hazard ratios (sHRs) and their 95% confidence intervals (CIs) were determined to quantify magnitude of risk. We first estimated a clinical model by examining the association between baseline variables at time of HCT (ie, age, HCT-CI, distance to COH, performance status, sex, race and ethnicity [Asian, Hispanic, non-Hispanic White (NHW), other]), diagnosis, conditioning, risk of relapse, CMV status, and HLA match individually with 1-year NRM in univariable analysis. Multivariable regression was conducted by including all the variables in the univariable analyses with a P value of no more than .1. Acute lymphoblastic leukemia, acute myeloid leukemia, lymphoma, and other diagnoses were combined because there was no significant difference in their subdistribution hazard ratios. Because of high collinearity between GVHD prophylaxis and HLA match, GVHD prophylaxis was not included in the multivariable model. After establishing the clinical model, the interaction between SVI and race and ethnicity was found to be significant (P = .048). Hence, race and ethnicity–specific analyses were performed to evaluate the association between SVI and 1-year NRM. Univariable and multivariable analyses were conducted as described above to develop a clinical model for each group. SVI as a binary variable (ie, highest tertile [most vulnerable] vs lower 2 tertiles [less vulnerable]), defined by the population as a whole, was added to the model. This was done for the overall SVI ranking as well as for each of the 4 themes.
To assess the risk of NRM among the most vulnerable and at-risk patients, we created an ordinal variable combining overall SVI tertile and HCT-CI into 1) lower 2 SVI tertiles (low social vulnerability) and low (<3) HCT-CI score (reference); 2) low social vulnerability and high (≥3) HCT-CI score; 3) high social vulnerability (upper SVI tertile) and low HCT-CI score; and 4) high social vulnerability and high HCT-CI score. The variables included in the multivariable model for risk of 1-year NRM by SVI and HCT-CI categories were the same as those included in the clinical model described above (excluding HCT-CI score).
All statistical analyses were 2-sided, and a P value less than .05 was considered statistically significant. All analyses were conducted using SAS 9.4 (SAS Institute Inc, Cary, NC).
Results
Patient Characteristics
Between 2013 and 2019, 1602 patients underwent a first allogeneic HCT at COH. Patient characteristics are detailed in Table 1. The median age at HCT was 52.0 (range = 1.0-78.0) years. The race and ethnicity composition of the cohort was 13.2% Asian, 35.1% Hispanic, 44.6% NHW, and 7.1% other (4.6% Black, 2.5% other or missing). The majority was male (55.8% vs 44.2% female), had private insurance (59.1%), had an underlying diagnosis of acute myeloid leukemia (40.6%), received reduced intensity conditioning (54.1%), had low risk of relapse (60.3%), and had an HLA-matched donor (69.7%).
Table 1.
Cohort characteristics
| Characteristic | Overall cohort | Alive at 1 year | 1-year NRM |
|---|---|---|---|
| (n = 1602) | (n = 1245) | (n = 245) | |
| No (%) | No (%) | No (%) | |
| Age at HCT, median (range) | 52.00 (1-78) | 51.00 (1-76) | 57.00 (2-78) |
| Sex | |||
| Female | 708 (44.2) | 548 (44.0) | 104 (42.4) |
| Male | 894 (55.8) | 697 (56.0) | 141 (57.6) |
| Race and ethnicity | |||
| Asian | 211 (13.2) | 156 (12.5) | 40 (16.3) |
| Hispanic | 563 (35.1) | 432 (34.7) | 89 (36.3) |
| Non-Hispanic White | 715 (44.6) | 565 (45.4) | 98 (40.0) |
| Other or unknowna | 113 (7.1) | 92 (7.4) | 18 (7.4) |
| Insurance status | |||
| Public | 565 (35.3) | 428 (34.4) | 97 (39.6) |
| Private | 947 (59.1) | 739 (59.4) | 138 (56.3) |
| Uninsured or unknown | 90 (5.6) | 78 (6.3) | 10 (4.1) |
| Diagnosis | |||
| AML | 651 (40.6) | 503 (40.4) | 93 (38.0) |
| ALL | 369 (23.0) | 293 (23.5) | 56 (22.9) |
| Lymphoma | 116 (7.2) | 92 (7.4) | 14 (5.7) |
| MDS | 291 (18.2) | 208 (16.7) | 65 (26.5) |
| Other | 175 (10.9) | 149 (12.0) | 17 (6.9) |
| Conditioning | |||
| Myeloablative | 736 (45.9) | 587 (47.1) | 97 (39.6) |
| Reduced intensity | 866 (54.1) | 658 (52.9) | 148 (60.4) |
| Risk relapse | |||
| Low | 966 (60.3) | 798 (64.1) | 121 (49.4) |
| High | 636 (39.7) | 447 (35.9) | 124 (50.6) |
| GVHD prophylaxis | |||
| Tacrolimus/Sirolimus | 1021 (63.7) | 800 (64.3) | 140 (57.1) |
| Calcineurin inhibitor with MMF or MTX | 17 (16.3) | 209 (16.8) | 45 (18.4) |
| Post-HCT cyclophosphamide | 292 (18.2) | 220 (17.7) | 59 (24.1) |
| Other | 18 (1.1) | 16 (1.3) | 1 (0.4) |
| Performance status, median (range) | 90 (50-100) | 90 (50-100) | 80 (60-100) |
| HCT-CI score, median (range) | 2.00 (0-11) | 2 (0-11) | 3 (0-11) |
| CMV status | |||
| Negative | 214 (13.4) | 172 (13.8) | 27 (11.0) |
| Positive | 1388 (86.6) | 1073 (86.2) | 218 (89.0) |
| HLA match | |||
| Matched | 1116 (69.7) | 888 (71.3) | 151 (61.6) |
| Mismatched | 275 (17.2) | 203 (16.3) | 47 (19.2) |
| Haplo | 211 (13.2) | 154 (12.4) | 47 (19.2) |
| Distance to City of Hope, median (range), miles | 28.0 (0.5-369.6) | 28.0 (0.5-369.6) | 27.2 (3.0-359.9) |
| Overall SVI ranking, median (range) | 0.46 (0-1.00) | 0.45 (0-1.00) | 0.49 (0.01-1.00) |
| SVI socioeconomic status, median (range) | 0.45 (0-1.00) | 0.45 (0-1.00) | 0.51 (0-1.00) |
| SVI household composition and disability, median (range) | 0.45 (0.01-1.00) | 0.45 (0.01-1.00) | 0.49 (0.02-1.00) |
| SVI minority status and language, median (range) | 0.51 (0-1.00) | 0.51 (0-1.00) | 0.58 (0.01-1.00) |
| SVI housing and transportation, median (range) | 0.42 (0-1.00) | 0.42 (0-1.00) | 0.42 (0.01-1.00) |
Includes 74 (4.6%) Black, 39 (2.5%) other or missing. ALL = acute lymphoblastic leukemia; AML = acute myeloid leukemia; CMV = cytomegalovirus; GVHD = graft-versus-host disease; HCT = hematopoietic cell transplantation; HCT-CI = hematopoietic cell transplantation–comorbidity index; HLA = human leukocyte antigen; MDS = myelodysplastic syndrome; MMF = mycophenolate mofetil; MTX = methotrexate; NRM = nonrelapse mortality; SVI = social vulnerability index.
The cumulative incidence of NRM in the first year after HCT was 15.3% (95% CI = 13.6% to 17.1%). The most common causes of NRM for the overall cohort were infection (48.2%), GVHD (18.8%), and respiratory failure (13.1%), and these proportions were similar across SVI tertiles (data not shown).
Risk Factors for 1-Year NRM
In univariable analysis, risk factors for 1-year NRM were older age (sHR = 1.02, 95% CI = 1.01 to 1.02; P < .001), lower performance status (≤80) at HCT (sHR = 1.73, 95% CI = 1.34 to 2.22), diagnosis of myelodysplastic syndrome (sHR = 1.56, 95% CI = 1.09 to 2.23; reference = ALL), high relapse risk (sHR = 1.63, 95% CI = 1.27 to 2.09), and HCT-CI score (sHR = 1.10, 95% CI = 1.04 to 1.17; per integer increase; Table 2). The multivariable clinical model included age at HCT, relapse risk, performance status, HCT-CI, and HLA match. Added to this model, patients in the highest tertile SVI had a 1.3-fold risk (sHR = 1.36, 95% CI = 1.04 to 1.78) of NRM compared with individuals in the lower 2 tertiles. Highest tertiles of SVI themes independently associated with NRM included SES (sHR = 1.39, 95% CI = 1.06 to 1.81), household composition and disability (sHR = 1.31, 95% CI = 1.01 to 1.69), and minority status and language (sHR = 1.33, 95% CI = 1.02 to 1.75; Table 3). Of note, SVI was associated with all-cause but not relapse-related mortality (Supplementary Tables 1 and 2, available online).
Table 2.
Univariable analysis for risk of 1-year nonrelapse mortality
| Variable | sHR (95% CI) | P |
|---|---|---|
| Age at HCT | 1.02 (1.01 to 1.02) | <.001 |
| Sex | ||
| Female | 1.00 (Referent) | —b |
| Male | 1.07 (0.83 to 1.38) | .61 |
| Race and ethnicity | .29 | |
| Asian | 1.43 (0.99 to 2.07) | .06 |
| Hispanic | 1.15 (0.86 to 1.53) | .35 |
| Non-Hispanic White | 1.00 (Referent) | —b |
| Other | 1.17 (0.72 to 1.93) | .53 |
| Insurance status at time of HCT | .24 | |
| Private | 1.00 (Referent) | —b |
| Public | 1.19 (0.92 to 1.54) | .19 |
| Uninsured or unknown | 0.76 (0.40 to 1.45) | .41 |
| Diagnosis | .002 | |
| Acute lymphoblastic leukemia | 1.00 (Referent) | —b |
| Acute myeloid leukemia | 0.94 (0.67 to 1.31) | .71 |
| Lymphoma | 0.79 (0.44 to 1.42) | .43 |
| Myelodysplastic syndrome | 1.56 (1.09 to 2.23) | .01 |
| Other | 0.63 (0.36 to 1.07) | .09 |
| Conditioning | ||
| Myeloablative | 1.00 (Referent) | —b |
| Reduced intensity | 1.34 (1.04 to 1.73) | .02 |
| Risk relapse | ||
| Low | 1.00 (Referent) | —b |
| High | 1.63 (1.27 to 2.09) | <.001 |
| GVHD prophylaxis | .01 | |
| Tacrolimus/Sirolimus | 1.00 (Referent) | —b |
| Calcineurin inhibitor with MMF or MTX | 1.25 (0.89 to 1.75) | .19 |
| Post-HCT cyclophosphamide | 1.45 (1.07 to 1.96) | .02 |
| Other | 0.38 (0.05 to 2.82) | .34 |
| Performance status ≤80 | 1.73 (1.34 to 2.22) | <.001 |
| HCT-CI score | 1.10 (1.04 to 1.17) | <.001 |
| CMV status | ||
| Negative | 1.00 (Referent) | —b |
| Positive | 1.27 (0.85 to 1.89) | .24 |
| HLA match | .003 | |
| Matched | 1.00 (Referent) | —b |
| Haploidentical | 1.74 (1.25 to 2.41) | <.001 |
| Mismatched | 1.28 (0.93 to 1.78) | .13 |
| Distance to City of Hope | 1.00 (0.99 to 1.00) | .10 |
| Overall SVIa | 1.26 (0.98 to 1.63) | .07 |
| SVI socioeconomic statusa | 1.26 (0.98 to 1.62) | .07 |
| SVI household composition and disabilitya | 1.24 (0.96 to 1.60) | .10 |
| SVI minority status and languagea | 1.24 (0.96 to 1.60) | .09 |
| SVI housing and transportationa | 1.18 (0.92 to 1.52) | .20 |
Analysis conducted for highest tertile of SVI compared with lower 2 tertiles as referent groups. CI = confidence interval; CMV = cytomegalovirus; GVHD = graft-versus-host disease; HCT-CI = hematopoietic cell transplantation–comorbidity index; HLA = human leukocyte antigen; MMF = mycophenolate mofetil; MTX = methotrexate; sHR = subdistribution hazard ratio; SVI = social vulnerability index.
Not applicable.
Table 3.
Multivariable analysis for risk of 1-year nonrelapse mortalitya
| Covariable | sHR (95% CI) | P |
|---|---|---|
| Age at HCT | 1.02 (1.01 to 1.02) | <.001 |
| Relapse risk | ||
| Low | 1.00 (Referent) | —d |
| High | 1.50 (1.17 to 1.92) | .002 |
| Performance status | 0.97 (0.96 to 0.99) | <.001 |
| HCT-CI score | 1.09 (1.03 to 1.15) | .006 |
| HLA match | ||
| Matched | 1.00 (Referent) | —d |
| Haploidentical | 1.92 (1.38 to 2.67) | <.001 |
| Mismatched | 1.48 (1.06 to 2.07) | .02 |
| SVI overall (highest tertile)b,c | 1.36 (1.04 to 1.78) | .02 |
| SVI socioeconomic status (highest tertile)b,c | 1.39 (1.06 to 1.81) | .02 |
| SVI household composition and disability (highest tertile)b,c | 1.31 (1.01 to 1.69) | .04 |
| SVI minority status and language (highest tertile)b,c | 1.33 (1.02 to 1.75) | .04 |
| SVI housing and transportation (highest tertile)b,c | 1.26 (0.97 to 1.63) | .09 |
SVI overall and SVI themes were each adjusted for the preceding non-SVI variables but not for each other. CI = confidence interval; CMV = cytomegalovirus; HCT-CI = hematopoietic cell transplantation–comorbidity index; HLA = human leukocyte antigen; sHR = subdistribution hazard ratio; SVI = social vulnerability index.
Each SVI component was added independently to the baseline clinical model.
Reference: lower 2 SVI tertiles.
Not applicable.
The cumulative incidence of 1-year NRM increased incrementally in the following SVI-HCT-CI categories: low social vulnerability and low HCT-CI (12.8%, 95% CI = 10.3% to 15.6%); high social vulnerability and low HCT-CI (14.4%, 95% CI = 10.8% to 18.6%); low social vulnerability and high HCT-CI (15.9%, 95% CI = 12.7% to 19.4%); high social vulnerability and high HCT-CI (21.8%, 95% CI = 16.8% to 27.2%) (Table 4 and Figure 1). In multivariable analysis, patients with high social vulnerability and high HCT-CI had the greatest risk of 1-year NRM (sHR = 1.81, 95% CI = 1.26 to 2.58; Table 4).
Table 4.
Cumulative incidence and risk of 1-year nonrelapse mortality per SVI and HCT-CI category
| SVI or HCT-CI category | No. of events/No. at risk | Cumulative incidence, % (95% CI) |
Unadjusted |
Adjusted |
||||
|---|---|---|---|---|---|---|---|---|
| Day 100 | Day 180 | Day 365 | sHR (95% CI) | P a | sHR (95% CI) | P a | ||
| Model 1 | ||||||||
| Lower SVI tertiles | 149/1058 | 6.5 (5.1 to 8.1) | 9.9 (8.2 to 11.8) | 14.1 (12.1 to 16.3) | 1.00 (Referent) | —b | 1.00 (Referent) | —b |
| Highest SVI tertile | 96/544 | 6.8 (4.9 to 9.1) | 10.3 (7.9 to 13.1) | 17.7 (14.6 to 20.1) | 1.26 (0.98 to 1.63) | .07 | 1.36 (1.04 to 1.78) | .02 |
| Model 2 | ||||||||
| Lower SVI tertiles | ||||||||
| Low HCT-CI (<3) | 78/611 | 5.6 (3.9 to 7.6) | 9.3 (7.2 to 11.8) | 12.8 (10.3 to 15.6) | 1.00 (Referent) | —b | 1.00 (Referent) | —b |
| High HCT-CI (≥3) | 71/447 | 7.8 (5.6 to 10.6) | 10.7 (8.1 to 13.8) | 15.9 (12.7 to 19.4) | 1.26 (0.92 to 1.74) | .007 | 1.20 (0.87 to 1.66) | .003 |
| Highest SVI tertile | ||||||||
| Low HCT-CI (<3) | 44/305 | 6.2 (3.9 to 9.3) | 9.5 (6.6 to 13.1) | 14.4 (10.8 to 18.6) | 1.13 (0.78 to 1.64) | 1.23 (0.85 to 1.79) | ||
| High HCT-CI (≥3) | 52/239 | 7.5 (4.6 to 11.3) | 11.3 (7.7 to 15.7) | 21.8 (16.8 to 27.2) | 1.75 (1.24 to 2.48) | 1.81 (1.26 to 2.58) | ||
Model 1 adjusted for age at HCT, risk of relapse, performance status, HLA match, and HCT-CI; model 2 adjusted for age at HCT, risk of relapse, performance status, HLA match. CI = confidence interval; HCT-CI = hematopoietic cell transplantation–comorbidity index; sHR = subdistribution hazard ratio; SVI = social vulnerability index.
Not applicable.
Figure 1.
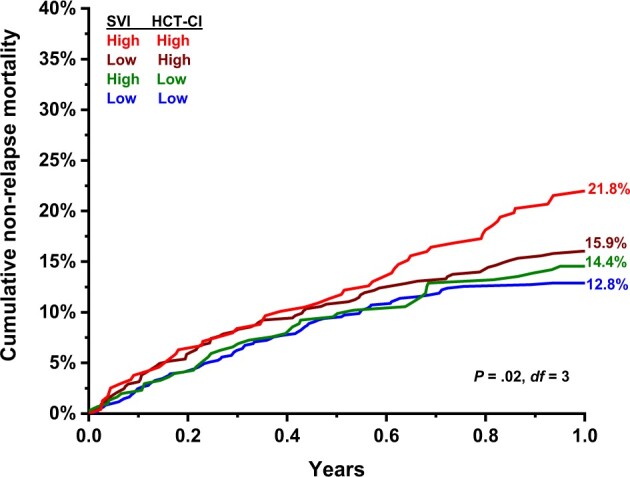
One-year NRM by SVI and HCT-CI. Cumulative incidence of 1-year NRM by highest or lower 2 SVI tertiles and high (≥3) or low (<3) HCT-CI. HCT-CI = hematopoietic cell transplantation–comorbidity index; NRM = nonrelapse mortality; SVI = social vulnerability index.
Impact of SVI on NRM by Race and Ethnicity
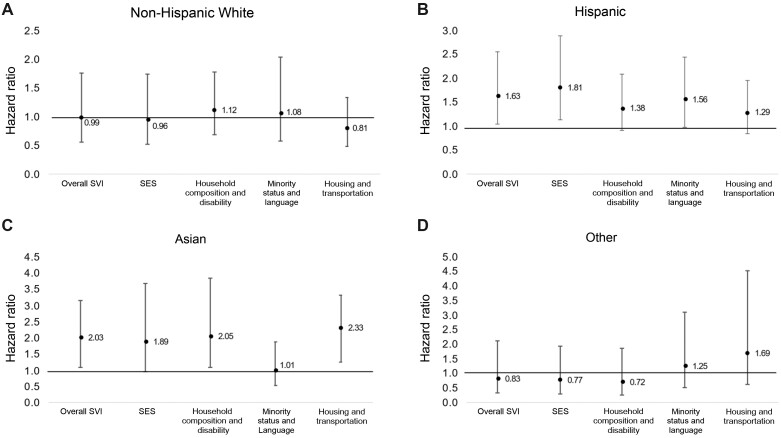
In NHW patients, neither high tertile of overall SVI nor any of the individual subthemes were associated with risk of NRM. High overall SVI tertile was associated with increased risk of NRM in Hispanic (sHR = 1.63, 95% CI = 1.04 to 2.55) and Asian (sHR = 2.03, 95% CI = 1.09 to 3.79) patients, and the risk was increased for high tertile of SES (sHR = 1.81, 95% CI = 1.14 to 2.90) among Hispanic patients and high tertiles of household composition and disability (sHR = 2.05, 95% CI = 1.09 to 3.85) and housing and transportation (sHR = 2.33, 95% CI = 1.25 to 4.34) among Asian patients (Figure 2).
Figure 2.
Impact of SVI by race and ethnicity. Risk of 1-year nonrelapse mortality for those in the highest SVI tertile (reference: lower 2 tertiles) stratified by race and ethnicity: (A) non-Hispanic White, adjusted for age and performance status; (B) Hispanic, adjusted for age and risk of relapse; (C) Asian, adjusted for performance status and diagnosis; (D) other, adjusted for hematopoietic cell transplantation–comorbidity index, performance status, and diagnosis. Adjusted covariables varied by race and ethnicity because multivariable models were fitted separately for each race and ethnicity group resulting in differently adjusted models. Error bars represent 95% confidence interval. SES = socioeconomic status; SVI = social vulnerability index.
Discussion
In this large, contemporary cohort of adults who underwent allogeneic HCT, we found significant associations between neighborhood-level social vulnerability, measured by the SVI, and risk of 1-year NRM. The dyad of living in neighborhoods with higher social vulnerability and having a high comorbidity burden posed a substantial risk of 1-year NRM. The components of SVI that increased risk of 1-year NRM differed by race and ethnicity. In Hispanic patients, overall SVI and SES were associated with 1-year NRM, and in Asian patients, overall SVI, household composition and disability, and housing and transportation were associated with 1-year NRM. We found no association between SVI and 1-year NRM in NHW patients undergoing HCT. These findings highlight the important role local social-environmental factors play in health outcomes following HCT, specifically among different racial and ethnic groups.
Previous studies have highlighted how low SES and Medicaid insurance status can be associated with inferior outcomes after cancer treatment, including HCT (21-30). However, these studies have largely relied on SES derived from zip codes or individual-level insurance status, which do not provide a complete picture of social determinants of health outcomes. A previous study did find that worse county-level community health status was associated with poorer survival and increased risk of NRM following HCT (31), however county-level data are not reflective of individuals’ immediate surroundings, especially in large, diverse, densely populated areas. Indices that incorporate additional aspects of social determinants of health such as community resources and housing conditions at the neighborhood level are needed to comprehensively evaluate how social and environmental factors influence health outcomes. In this study, we found that higher vulnerability in each individual component of SES, household composition and disability, and minority status and language was associated with increased risk of 1-year NRM by more than 30%. There are several potential explanations related to health behaviors and maintenance for the associations seen between individual SVI themes and NRM. For example, lower SES has been associated with neighborhood safety concerns and decreased physical activity (32), which are in turn related to overall health status and comorbidities. Household composition has been associated with increased caregiver burden and decreased medication adherence (19), which can be compounded by lack of access to transportation (33). Furthermore, multiple studies have found that health-related outcomes are better for patients receiving language-concordant care (34). Translating measures such as SVI into clinical care may identify which patients would benefit from approaches such as evaluating neighborhood safety and resources, implementing home physical activity and health maintenance programs, providing home health assistance, and allocating additional resources to ensure equitable language-concordant care.
The HCT-CI has been a useful clinical tool to determine the burden of comorbidities prior to HCT and the risk of NRM after HCT. To our knowledge, our study is the first to examine the impact of living in socially vulnerable areas in patients with high comorbidity burden at the time of HCT. The cumulative incidence of NRM in patients who had a high comorbidity burden and also lived in areas with high social vulnerability at the time of HCT approached 25% at 1-year post-HCT, and this translated into a nearly twofold increased risk of NRM compared with patients who had a low comorbidity burden and also lived in areas with lower social vulnerability at the time of HCT. Tools such as the SVI can be complementary to the HCT-CI to identify which would derive particular benefit from modified intervention and monitoring approaches. For example, if patients live in areas with higher vulnerability within the themes of household composition or housing and transportation, additional support for local housing, medical transportation, medication delivery, or remote health monitoring to decrease the burden associated with frequent in-person visits may help improve health behaviors including clinical follow-up and medication adherence. Additionally, with continued advancements in health-care delivery, incorporation of composite indices into clinical decision-making algorithms may help identify which patients would be candidates for approaches such as outpatient HCT and cellular immunotherapy and which patients may need closer monitoring and additional resource allocation.
Interestingly, although living in a neighborhood with higher social vulnerability was not associated with 1-year NRM in NHW patients, it was in Asian and Hispanic patients, who constituted 48% of our patient population. Racial disparities in health outcomes have been well reported, including in HCT recipients, and have largely been attributed to associations between race and ethnicity, SES, and access to health care, as well as unmeasured comorbidities and inherent differences in disease biology (8,21,24,30). In an effort to better elucidate the mechanistic links between social determinants of health and HCT outcomes, this study included measures of all of these variables. Access to health care, as assessed by insurance status and distance to the treating center, was not associated with 1-year NRM. On the other hand, worse neighborhood-level SES, as measured by the SVI, was independently associated with 1-year NRM in the overall cohort and was especially pronounced in certain racial and ethnic populations. This suggests that attributing inferior outcomes among racial and ethnic minorities to more traditional measures of SES (eg, household income) alone may underestimate the complex social determinants of health that impact outcomes after HCT. Of note, although SVI-based minority status and language was associated with increased risk of 1-year NRM in the overall cohort, the association was not significant in race and ethnicity–specific subanalyses, potentially because of the lack of racial and ethnic diversity by census tract.
A strength of our study is that we used census tract–level data, which are more reflective of a person’s immediate neighborhood and environment than use of zip code or county-level data. Additionally, applying the SVI to our cohort allowed us to evaluate overall social vulnerability as well as specific themes to identify which social and environmental factors had the greatest impact on outcomes. Integration of individual-level SES information in future SVI-based analyses may further elucidate neighborhood vs individual determinants of health. We acknowledge that this was a single-center study and that differences in supportive care practices could have influenced outcomes, which may speak to the generalizability of our findings to other HCT populations. That said, our cohort comprised a diverse patient population, which enabled more specific analyses by race and ethnicity than previous studies. The relatively small proportion of Black patients included in the current study is reflective of the broader demographics of southern California, with its high Hispanic and Asian minority populations. Additional studies may be needed to determine whether the associations seen in our study persist on a geographically broader scale. Lastly, whereas the outcome of interest in this study was 1-year NRM, future studies may also consider evaluating the relationship between social vulnerability and longer-term (>1 year) health outcomes post-HCT.
In conclusion, we found that higher social vulnerability, especially among minorities, is associated with increased risk of 1-year NRM following allogeneic HCT, and the combination of SVI and HCT-CI may identify which patients are at highest risk for 1-year NRM. As HCT is increasingly used to treat hematologic diseases, it is critical to continue identifying and mitigating risk factors for adverse HCT outcomes. For the clinical team, use of an index such as the SVI can aid in risk stratification, needs assessment, and resource allocation, such as language, caregiver, transportation, and housing support pre- and post-HCT. An improved understanding of the dynamic interplay between disease, host, and social-environmental factors may also inform health policy to improve community-level resources and equitable access to care to ultimately improve outcomes in particularly at-risk patients.
Funding
This work was supported, in part, by a Lymphoma & Leukemia Society Scholar Award: 2315-17 (Armenian).
Notes
Role of the funder: The funding source had no role in the study design, the conduct or management of the study; in the collection, analysis, or the interpretation of the data; in the writing of the manuscript; nor in the decision to submit the manuscript for publication.
Disclosures: The authors report no relevant conflicts to disclose.
Author contributions: RB: conceptualization, methodology, analysis, writing—original draft, writing—review & editing; JBT: conceptualization, methodology, data curation, analysis, writing—review & editing; TH: analysis, writing—original draft, writing—review & editing; RN: methodology, writing—review & editing; ASA: methodology, writing—review & editing; MMJ: methodology, writing—review & editing; SJF: supervision, writing—review & editing; FLW: conceptualization, methodology, writing—original draft, writing—review & editing, analysis; SHA: conceptualization, methodology, analysis, writing—original draft, writing—review & editing, supervision.
Prior presentation: This work was presented, in part, as an oral abstract at the American Society of Hematology meeting, December 2021, Atlanta, Georgia.
Supplementary Material
Contributor Information
Rusha Bhandari, Department of Pediatrics, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Jennifer Berano Teh, Department of Population Sciences, City of Hope Comprehensive Cancer Center, Duarte, CA, USA; Current affiliation: The Heart Institute, Children’s Hospital Los Angeles, Los Angeles, CA, USA.
Tianhui He, Department of Population Sciences, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Ryotaro Nakamura, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Andrew S Artz, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Marta M Jankowska, Department of Population Sciences, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Stephen J Forman, Department of Hematology & Hematopoietic Cell Transplantation, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
F Lennie Wong, Department of Population Sciences, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Saro H Armenian, Department of Population Sciences, City of Hope Comprehensive Cancer Center, Duarte, CA, USA.
Data Availability
The protocol, the statistical code, and the anonymized dataset are available upon request from the corresponding author: sarmenian@coh.org.
References
- 1. Phelan R, Arora M, Chen M. Current Use and Outcome of Hematopoietic Stem Cell Transplantation: CIBMTR US Summary Slides; 2020. https://www.cibmtr.org/ReferenceCenter/SlidesReports/SummarySlides/pages/index.aspx.
- 2. Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106(8):2912-2919. DOI: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong FL, Teh JB, Atencio L, et al. Conditional survival, cause-specific mortality, and risk factors of late mortality after allogeneic hematopoietic cell transplantation. J Natl Cancer Inst. 2020;112(11):1153-1161. DOI: 10.1093/jnci/djaa022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yanada M, Konuma T, Mizuno S, et al. Predicting non-relapse mortality following allogeneic hematopoietic cell transplantation during first remission of acute myeloid leukemia. Bone Marrow Transplant. 2021;56(2):387-394. DOI: 10.1038/s41409-020-01032-9. [DOI] [PubMed] [Google Scholar]
- 5. Sorror ML, Storb RF, Sandmaier BM, et al. Comorbidity-age index: a clinical measure of biologic age before allogeneic hematopoietic cell transplantation. J Clin Oncol. 2014;32(29):3249-3256. DOI: 10.1200/JCO.2013.53.8157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Namburi N, Timsina L, Ninad N, Ceppa D, Birdas T.. The impact of social determinants of health on management of stage I non-small cell lung cancer. Am J Surg. 2022;223(6):1063-1066. DOI: 10.1016/j.amjsurg.2021.10.022. [DOI] [PubMed] [Google Scholar]
- 7. Khan S, Bajwa S, Brahmbhatt D, et al. Multi-level socioenvironmental contributors to childhood asthma in New York City: a cluster analysis. J Urban Health. 2021;98(6):700-710. DOI: 10.1007/s11524-021-00582-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abraham IE, Rauscher GH, Patel AA, et al. Structural racism is a mediator of disparities in acute myeloid leukemia outcomes. Blood. 2022;139(14):2212-2226. DOI: 10.1182/blood.2021012830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Flanagan BE, Gregory EW, Hallisey EJ, Heitgerd JL, Lewis B.. A social vulnerability index for disaster management. J Homeland Secur Emerg Manage. 2011;8(1):Article 3. [Google Scholar]
- 10. Hyer JM, Tsilimigras DI, Diaz A, et al. High social vulnerability and “textbook outcomes” after cancer operation. J Am Coll Surg. 2021;232(4):351-359. DOI: 10.1016/j.jamcollsurg.2020.11.024. [DOI] [PubMed] [Google Scholar]
- 11. Diaz A, Hyer JM, Barmash E, Azap R, Paredes AZ, Pawlik TM.. County-level social vulnerability is associated with worse surgical outcomes especially among minority patients. Ann Surg. 2021;274(6):881-891. DOI: 10.1097/sla.0000000000004691. [DOI] [PubMed] [Google Scholar]
- 12.CDC SVI Documentation 2018. Agency for Toxic Substances and Disease Registry. https://www.atsdr.cdc.gov/placeandhealth/svi/documentation/SVI_documentation_2018.html. Published 2020. Accessed March 1, 2021.
- 13. Azap RA, Paredes AZ, Diaz A, Hyer JM, Pawlik TM.. The association of neighborhood social vulnerability with surgical textbook outcomes among patients undergoing hepatopancreatic surgery. Surgery. 2020;168(5):868-875. DOI: 10.1016/j.surg.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 14. Diaz A, Dalmacy D, Hyer JM, Tsilimigras D, Pawlik TM.. Intersection of social vulnerability and residential diversity: postoperative outcomes following resection of lung and colon cancer. J Surg Oncol. 2021;124(5):886-893. DOI: 10.1002/jso.26588. [DOI] [PubMed] [Google Scholar]
- 15. Diaz A, Cloyd JM, Manilchuk A, et al. Travel patterns among patients undergoing hepatic resection in California: does driving further for care improve outcomes? J Gastrointest Surg. 2021;25(6):1471-1478. DOI: 10.1007/s11605-019-04501-9. [DOI] [PubMed] [Google Scholar]
- 16. Diaz A, Burns S, Paredes AZ, Pawlik TM.. Accessing surgical care for pancreaticoduodenectomy: patient variation in travel distance and choice to bypass hospitals to reach higher volume centers. J Surg Oncol. 2019;120(8):1318-1326. DOI: 10.1002/jso.25750. [DOI] [PubMed] [Google Scholar]
- 17. Bacigalupo A, Ballen K, Rizzo D, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transplant. 2009;15(12):1628-1633. DOI: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Przepiorka D, Weisdorf D, Martin P, et al. 1994 consensus conference on acute GVHD grading. Bone Marrow Transplant. 1995;15(6):825-828. [PubMed] [Google Scholar]
- 19. Merenstein D, Schneider MF, Cox C, et al. Association of child care burden and household composition with adherence to highly active antiretroviral therapy in the Women’s Interagency HIV Study. AIDS Patient Care Stds. 2009;23(4):289-296. DOI: 10.1089/apc.2008.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. [Google Scholar]
- 21. Baker KS, Davies SM, Majhail NS, et al. Race and socioeconomic status influence outcomes of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15(12):1543-1554. DOI: 10.1016/j.bbmt.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu S, Rybicki L, Abounader D, et al. Association of socioeconomic status with long-term outcomes in 1-year survivors of allogeneic hematopoietic cell transplantation. Bone Marrow Transplant. 2015;50(10):1326-1330. DOI: 10.1038/bmt.2015.166. [DOI] [PubMed] [Google Scholar]
- 23. Gupta S, Wilejto M, Pole JD, Guttmann A, Sung L.. Low socioeconomic status is associated with worse survival in children with cancer: a systematic review. PLoS One. 2014;9(2):e89482. DOI: 10.1371/journal.pone.0089482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kehm RD, Spector LG, Poynter JN, Vock DM, Altekruse SF, Osypuk TL.. Does socioeconomic status account for racial and ethnic disparities in childhood cancer survival? Cancer. 2018;124(20):4090-4097. DOI: 10.1002/cncr.31560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Woods LM, Rachet B, Coleman MP.. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5-19. DOI: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 26. Bradley CJ, Gardiner J, Given CW, Roberts C.. Cancer, Medicaid enrollment, and survival disparities. Cancer. 2005;103(8):1712-1718. DOI: 10.1002/cncr.20954. [DOI] [PubMed] [Google Scholar]
- 27. Khera N, Chang YH, Slack J, et al. Impact of race and ethnicity on outcomes and health care utilization after allogeneic hematopoietic cell transplantation. Leuk Lymphoma. 2015;56(4):987-992. DOI: 10.3109/10428194.2014.941834. [DOI] [PubMed] [Google Scholar]
- 28. Koroukian SM, Bakaki PM, Raghavan D.. Survival disparities by Medicaid status: an analysis of 8 cancers. Cancer. 2012;118(17):4271-4279. DOI: 10.1002/cncr.27380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Niu X, Roche LM, Pawlish KS, Henry KA.. Cancer survival disparities by health insurance status. Cancer Med. 2013;2(3):403-411. DOI: 10.1002/cam4.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bona K, Brazauskas R, He N, et al. Neighborhood poverty and pediatric allogeneic hematopoietic cell transplantation outcomes: a CIBMTR analysis. Blood. 2021;137(4):556-568. DOI: 10.1182/blood.2020006252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hong S, Brazauskas R, Hebert KM, et al. Community health status and outcomes after allogeneic hematopoietic cell transplantation in the United States. Cancer. 2020;127(4):609-618. DOI: 10.1002/cncr.33232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Meyer OL, Castro-Schilo L, Aguilar-Gaxiola S.. Determinants of mental health and self-rated health: a model of socioeconomic status, neighborhood safety, and physical activity. Am J Public Health. 2014;104(9):1734-1741. DOI: 10.2105/AJPH.2014.302003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cornelius T, Jones M, Merly C, Welles B, Kalichman MO, Kalichman SC.. Impact of food, housing, and transportation insecurity on ART adherence: a hierarchical resources approach. AIDS Care. 2017;29(4):449-457. DOI: 10.1080/09540121.2016.1258451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Diamond L, Izquierdo K, Canfield D, Matsoukas K, Gany F.. A systematic review of the impact of patient-physician non-English language concordance on quality of care and outcomes. J Gen Intern Med. 2019;34(8):1591-1606. DOI: 10.1007/s11606-019-04847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The protocol, the statistical code, and the anonymized dataset are available upon request from the corresponding author: sarmenian@coh.org.