Abstract
Background
There is increasing interest in better understanding the biology and clinical presentation of invasive lobular cancer (ILC), which is the most common special histological subtype of breast cancer. Limited large contemporary data sets are available allowing comparison of clinicopathologic features between ILC and invasive ductal cancer (IDC).
Methods
The Great Lakes Breast Cancer Consortium was formed to compare clinical behavior of ILC (n = 3617) and IDC (n = 30 045) from 33 662 patients treated between 1990 and 2017 at 3 large clinical centers. We used Kaplan-Meier analysis, Cox proportional hazards modeling, and propensity score matching to evaluate treatment differences and outcomes. All statistical testing used 2-sided P values.
Results
Compared with IDC, patients with ILC were more frequently diagnosed at later stages and with more lymph node involvement (corrected P < .001). Estrogen receptor–positive ILCs were of lower grade (grade 1 and 2: 90% in ILC vs 72% in IDC) but larger in size (T3 and 4: 14.3% in ILC vs 3.4% in IDC) (corrected P < .001), and since 1990, the mean ILC size detected at diagnosis increased yearly. Patients with estrogen receptor (ER)–positive ILC underwent statistically significantly more mastectomies compared with ER-positive IDC (57% vs 46%). Using Kaplan-Meier analysis, patients with ER-positive ILC had statistically significantly worse disease-free survival and overall survival than ER-positive IDC although 6 times more IDCs were classified as high risk by OncotypeDx Breast Recurrence Score assay.
Conclusions
This large, retrospective, collaborative analysis with 3 clinical centers identified meaningful differences in clinicopathological features between ILC and IDC, providing further evidence that these are 2 different entities requiring different clinical management.
Invasive lobular carcinoma (ILC) is the most common special subtype of breast cancer, accounting for 10%-15% of all breast cancers, which represents approximately 26 000-40 000 cases annually in the United States (1). There is an increasing recognition that ILC has distinct clinical, histologic, molecular, and biological characteristics compared with invasive ductal cancer (IDC) [reviewed in (1-6)].
The hallmark of ILC is the loss of E-cadherin, resulting in a more linear growth pattern of cells as compared with round masses usually seen in IDC. This unique growth pattern is associated with challenges in detection of ILC and, hence, larger, lobulated, and more spiculated tumors at the time of detection. This, together with increased rates of multifocality, is associated with an increased rate of mastectomy (7).
ILC is more often estrogen receptor (ER) positive compared with IDC (8-11), and hence, hormonal therapy is given in the majority of cases. The use of chemotherapy remains an open question, with limited data on efficacy in ILC. Further complicating use of chemotherapy is the limited data on the utility of multigene prognostic tests in ILC. Some assays have been tested in ILC with mixed results (12-20), and there are efforts to develop assays specifically based on information from ILC (21). These studies are necessary given the large number of ILC variants (22), which may harbor unique mutations that change clinical phenotype.
ILC outcome is similar to ER-positive IDC in that the majority of patients experience a favorable outcome. Recurrence sites are shared with IDC such as bone, brain, lung, and liver, but lobular metastases can also be found in less well-characterized sites such in the gastrointestinal and urogenital tracts (23,24). There is evidence that patients with ILC have worse long-term outcome compared with IDC because of late recurrences (25-27).
It is promising to see overall progress in the understanding of ILC; nevertheless, many open questions remain. Many previous studies comparing clinicopathological features and outcomes have been based on relatively small numbers of patients with ILC or larger numbers of patients but seen at a large number of hospitals (8,11,23,27-29), thereby limiting granular detail. We set out to investigate the clinicopathologic features, treatment trends, and outcomes for ILC and IDC across a large cohort of patients using cancer registries from 3 high-volume cancer centers.
Methods
Data Sources and Study Population
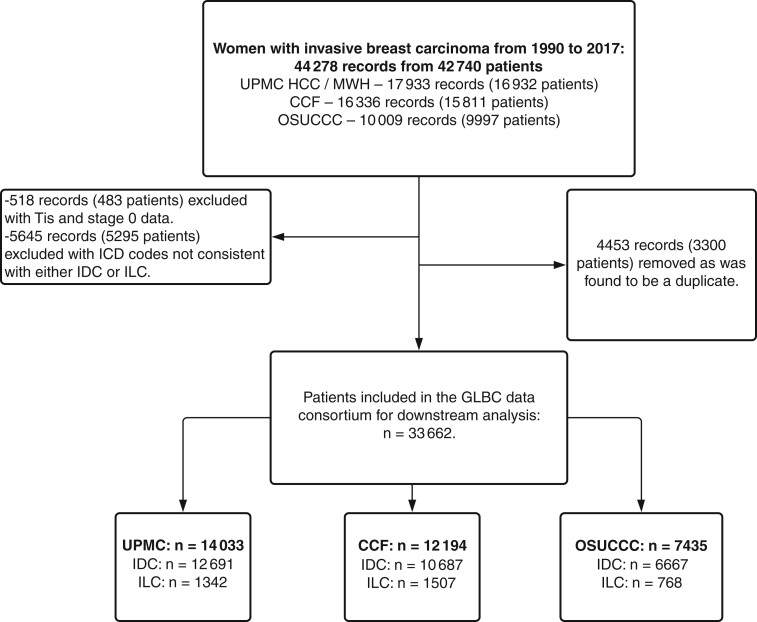
This retrospective cohort study included female adults (aged older than 18 years) who were diagnosed with breast cancer from 1990 to 2017 treated at the University of Pittsburgh Medical Center (Magee Women’s Hospital and Hillman Cancer Center), Cleveland Clinic, and the Ohio State University Comprehensive Cancer Center. In the raw datasets, there were 44 278 records from 42 740 patients (see Figure 1). Institutional review boards at each institution approved this study and waived informed consent because all of the data used were deidentified.
Figure 1.
CONSORT diagram. CONSORT diagram of the patients included in the Great Lakes Breast Cancer (GLBC) study showing the filtering steps used to arrive at the final cohort and breakdown of participants by originating institution. CCF = Cleveland Clinic Foundation; HCC = Hillman Cancer Center; ICD = International Classification of Diseases; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma; MWH = Magee Womens Hospital; OSUCCC = Ohio State University Comprehensive Cancer Center; UPMC = University of Pittsburgh Medical Center.
Oncotype Dx Breast Recurrence Score (RS) result data were received from Exact Sciences (Redwood City, CA, USA) for a subset of patients. Some data were missing, mostly because of lack of collection of specific variables in earlier years as summarized in Supplementary Figure 1 (available online). There was no statistically significant association between the missing data and histology.
Additional details are provided in the Supplementary Methods (available online).
Statistical Analysis
All continuous data are expressed as median (interquartile range), and categorial or ordinal variables are expressed as count (frequencies [%]). Mann-Whitney U test was used for the continuous variables, Pearson χ2 test with no continuity correction was used for categorical variables, and Cochran-Armitage test was used for ordinal variables in the comparative analyses. Kaplan-Meier curves are used for visualizing survival, and the corresponding P values are calculated by log-rank test to test for difference between the groups. Cox proportional hazard regression models are fitted when covariates adjustment was needed, and the estimated hazard ratios (HR) are reported with the 95% confidence interval (CI). All statistical testing used 2-sided P values.
To determine whether treatments were more beneficial for either IDC or ILC, we selected comparable cohorts using propensity score matching (PSM) approaches. We used nearest neighbor matching strategy in R package MatchIt (30) to match over age, stage, grade, nodal status, and institution, and the treatment information was adjusted in the subsequent survival analysis.
Additional details are provided in the Supplementary Methods (available online).
Results
Patient and Tumor Characteristics
From 42 740 patients seen at 3 institutions, we identified 33 662 patients to be included into the Great Lakes Breast Cancer (GLBC) ILC data consortium, consisting of 3617 (10.7%) patients with ILC and 30 045 (89.3%) patients with IDC (Figure 1). The overall cohort is similar to other cohorts in IDC and ILC, such as studies by Arpino et al. (23) and Chen et al. (8), as well as the cohorts used for The Cancer Genome Atlas Plan (9), METABRIC (31), and the Sweden Cancerome Analysis Network (SCAN-B) (32) (Supplementary Table 1, available online). Furthermore, our cohort is representative of populations in the states of Ohio and Pennsylvania (covering the GLBC cohort) and the overall United States using data from National Program of Cancer Registries (33) within the Surveillance, Epidemiology, and End Results (SEER) Program data set (Supplementary Table 2, available online).
Patient characteristics separated by institutions reveal some differences, but overall trends are similar (Supplementary Table 3, available online). The median follow-up time for the entire cohort was 66 (range = 0-345) months.
Table 1 shows differences in baseline characteristics between IDC and ILC in the entire cohort: patients with ILC are older (aged 61 vs 57 years; corrected P < .001) and slightly more likely to be White women (91% vs 89%; corrected P = .046), but this small effect is lost when limiting the analysis to patients with ER-positive disease. There was no statistically significant difference in body mass index comparing patients with IDC and ILC (Supplementary Table 4, available online). In the entire cohort, 88% of ILCs were grade 1 and 2, whereas only 60% of IDC fell into these categories (corrected P < .001). ILCs were more frequently higher stage (stage III and IV: 20.7% vs 10.4% in IDC), and more patients were diagnosed with de novo metastatic disease (stage IV: 3.7% ILC vs 2.4% IDC) (corrected P < .001). ILCs were larger in size (T3 and 4: 14.7% in ILC vs 4.0% in IDC), and there was more nodal involvement at time of diagnosis (N2 and 3: 9.9% in IDC vs 5.5% in IDC) (corrected P < .001).
Table 1.
Patients characteristics for the entire cohort
| Characteristic | Entire cohort |
Cohort with ER-positive disease |
||||||
|---|---|---|---|---|---|---|---|---|
| ILC | IDC | P a | Q b | ILC | IDC | P a | Q b | |
| Total No. | 3617 | 30 045 | 2564 | 17 224 | ||||
| Median age (IQR), y | 61 (52- 70) | 57 (48- 67) | <.001 | <.001 | 62 (52-70) | 59 (49-68) | <.001 | <.001 |
| Unknown, No. | 0 | 1 | ||||||
| Median BMI (IQR), kg/m2 | 27 (24-32) | 27 (24-32) | .15 | >.99 | 27 (24-32) | 27 (24-32) | .30 | >.99 |
| Unknown, No. | 1102 | 8759 | 345 | 2291 | ||||
| Laterality, No. (%) | .60 | >.99 | .40 | >.99 | ||||
| Left | 1083 (52) | 9841 (51) | 902 (52) | 6347 (51) | ||||
| Right | 1017 (48) | 9482 (49) | 838 (48) | 6167 (49) | ||||
| Unknown | 1517 | 10 722 | 824 | 4710 | ||||
| Race, No. (%) | .004 | .046 | .30 | >.99 | ||||
| Black | 276 (7.6) | 2746 (9.2) | 195 (7.6) | 1220 (7.1) | ||||
| White | 3275 (91) | 26 633 (89) | 2321 (91) | 15 612 (91) | ||||
| Otherc | 60 (1.7) | 592 (2.0) | 43 (1.7) | 360 (2.1) | ||||
| Unknown | 6 | 74 | 5 | 32 | ||||
| Stage, No. (%) | <.001 | <.001 | <.001 | <.001 | ||||
| I | 1406 (46) | 13 377 (54) | 1007 (45) | 8873 (59) | ||||
| II | 1008 (33) | 8615 (35) | 791 (35) | 4642 (31) | ||||
| III | 502 (17) | 1968 (8.0) | 380 (17) | 1154 (7.7) | ||||
| IV | 112 (3.7) | 593 (2.4) | 61 (2.7) | 292 (2.0) | ||||
| Unknown | 589 | 5492 | 325 | 2263 | ||||
| Grade, No. (%) | <.001 | <.001 | <.001 | <.001 | ||||
| 1 | 631 (24) | 4048 (16) | 553 (25) | 3394 (21) | ||||
| 2 | 1656 (64) | 10 984 (44) | 1420 (65) | 8047 (51) | ||||
| 3 | 294 (11) | 10 147 (40) | 213 (9.7) | 4393 (28) | ||||
| Unknown | 1036 | 4866 | 378 | 1390 | ||||
| ER, No. (%) | <.001 | <.001 | ||||||
| Positive | 2564 (96) | 17 224 (77) | ||||||
| Negative | 104 (3.9) | 5266 (23) | ||||||
| Unknown | 949 | 7555 | ||||||
| PR, No. (%) | <.001 | <.001 | .12 | >.99 | ||||
| Positive | 2144 (81) | 14 930 (67) | 2107 (83) | 14 374 (84) | ||||
| Negative | 508 (19) | 7437 (33) | 425 (17) | 2650 (16) | ||||
| Unknown | 965 | 7678 | 32 | 200 | ||||
| HER2, No. (%) | <.001 | <.001 | <.001 | <.001 | ||||
| Positive | 169 (9.4) | 2144 (18) | 145 (8.4) | 1375 (15) | ||||
| Negative | 1607 (90) | 9412 (80) | 1558 (91) | 7650 (83) | ||||
| Equivocal | 19 (1.1) | 250 (2.1) | 17 (1.0) | 212 (2.3) | ||||
| Unknown | 1822 | 18 239 | 844 | 7987 | ||||
| Lymph nodes, No. (%) | <.001 | <.001 | <.001 | <.001 | ||||
| N0 | 1892 (57) | 17 374 (63) | 1444 (59) | 10 785 (64) | ||||
| N1 | 805 (24) | 6416 (23) | 543 (22) | 3581 (21) | ||||
| N2 | 173 (5.3) | 1112 (4.0) | 155 (6.3) | 766 (4.6) | ||||
| N3 | 150 (4.6) | 417 (1.5) | 132 (5.4) | 281 (1.7) | ||||
| NX | 273 (8.3) | 2415 (8.7) | 187 (7.6) | 1310 (7.8) | ||||
| Unknown | 324 | 2311 | 103 | 501 | ||||
| Size, No. (%) | <.001 | <.001 | <.001 | <.001 | ||||
| T0 | 13 (0.6) | 927 (3.3) | 9 (0.5) | 344 (2.0) | ||||
| T1 | 1107 (49) | 17 525 (63) | 939 (51) | 11 557 (69) | ||||
| T2 | 695 (31) | 6534 (23) | 583 (31) | 3565 (21) | ||||
| T3 | 307 (14) | 776 (2.8) | 263 (14) | 397 (2.4) | ||||
| T4 | 15 (0.7) | 334 (1.2) | 6 (0.3) | 161 (1.0) | ||||
| TX | 100 (4.5) | 1761 (6.3) | 59 (3.2) | 811 (4.8) | ||||
| Unknown | 1380 | 2188 | 705 | 389 | ||||
| Oncotype, No. (%) | <.001 | <.001 | <.001 | <.001 | ||||
| Low risk | 407 (65%) | 2026 (58%) | 396 (65%) | 2002 (58%) | ||||
| Intermediate risk | 204 (33%) | 1105 (31%) | 201 (33%) | 1091 (32%) | ||||
| High risk | 12 (1.9%) | 383 (11%) | 12 (2.0%) | 364 (11%) | ||||
| Unknown | 2994 | 26 531 | 1955 | 13 767 | ||||
Wilcoxon rank sum test; Pearson χ2 test; Cochran-Armitage test for trend. All statistical tests were 2-sided. BMI = body mass index; ER = estrogen receptor; IDC = invasive ductal cancer; ILD = invasive lobular carcinoma; IQR = interquartile range; PR = progesterone receptor.
Bonferroni correction for multiple testing.
Other includes Hispanic, Asian, and American Indian women.
ILCs were more likely ER positive (96% vs 77%) and progesterone receptor (PR) positive (81% vs 67%) (all corrected P < .001). When limiting the analyses to ER positive only, the associations with age, stage, grade, tumor size, and nodal status remained statistically significant, whereas there were no statistically significant differences in body mass index, race, left or right laterality, and PR (Table 1). Information on HER2 was missing for the majority of the GLBC cohort because of age of the cohort (1990-2017), and thus numbers of patients in this cohort are smaller, but limiting the analysis to ER positive/HER2 negative only (Supplementary Table 5, available online), the conclusions remain the same with the exception of PR negativity, which is statistically significantly higher in ER-positive/HER2-negative ILC (16%) vs ER-positive/HER2-negative IDC (12%) (corrected P = .002).
For a subset of the patient cohort (n = 4138), information on RS result was available. The majority of ILCs were low risk (65%), and only 1.9% were classified as high risk, in contrast to 11% high-risk IDCs (corrected P < .001) (Table 1). This difference remained when limiting the analysis to patients with ER-positive/HER2-negative disease (9.3% vs 1.4%; corrected P < .001)
Treatment of Patients With ILC vs IDC
Patients with ILC were less likely to receive radiation (52% vs 57%; corrected P < .001) and chemotherapy (44% vs 47%; corrected P = .014) and more likely to receive hormonal therapy (78% vs 61%; corrected P < .001) (Table 2) compared with patients with IDC. When limiting the analysis to patients with ER-positive breast cancer, the increase in the use of hormonal therapy for patients with ILC remained statistically significant (90% vs 87%; corrected P < .001), but the use of chemotherapy was similar (41% in both groups).
Table 2.
Treatment differences in ILC and IDC
| Characteristic | Entire cohort |
Cohort with ER-positive disease |
||||||
|---|---|---|---|---|---|---|---|---|
| ILC | IDC | P a | Q b | ILC | IDC | P a | Q b | |
| No. (%) | No. (%) | No. (%) | No. (%) | |||||
| Total No. | 3617 | 30 045 | 2564 | 17 224 | ||||
| Radiation | <.001 | <.001 | <.001 | <.001 | ||||
| Yes | 1876 (52) | 16 883 (57) | 1467 (58) | 10 925 (64) | ||||
| No | 1700 (48) | 12 744 (43) | 1060 (42) | 6028 (36) | ||||
| Unknown | 41 | 418 | 37 | 271 | ||||
| Hormone | <.001 | <.001 | <.001 | <.001 | ||||
| Yes | 2771 (78) | 18 046 (61) | 2269 (90) | 14 729 (87) | ||||
| No | 791 (22) | 11 567 (39) | 244 (9.7) | 2164 (13) | ||||
| Unknown | 55 | 432 | 51 | 331 | ||||
| Chemotherapy | .004 | .014 | .70 | >.99 | ||||
| Yes | 972 (44) | 14 060 (47) | 761 (41) | 6989 (41) | ||||
| No | 1236 (56) | 15 710 (53) | 1074 (59) | 10 052 (59) | ||||
| Unknown | 1409 | 275 | 729 | 183 | ||||
| Surgery | <.001 | <.001 | <.001 | <.001 | ||||
| Lumpectomy | 1084 (31) | 12 238 (41) | 925 (37) | 8555 (50) | ||||
| Mastectomy | 2116 (60) | 14 899 (50) | 1435 (57) | 7769 (46) | ||||
| None | 333 (9.4) | 2380 (8.1) | 139 (5.6) | 639 (3.8) | ||||
| Unknown | 84 | 528 | 65 | 261 | ||||
Pearson χ2 test. All statistical tests were 2-sided. ER = estrogen receptor; IDC = invasive ductal cancer; ILD = invasive lobular carcinoma.
Bonferroni correction for multiple testing.
Analysis of local therapy revealed a statistically significant increase in rates of mastectomy in patients with ILC compared with IDC (60% vs 50%; corrected P < .001). The reduced rates of radiation in patients with ILC remained when excluding patients who underwent mastectomies (68% vs 77%; corrected P < .001).
Outcome Analysis
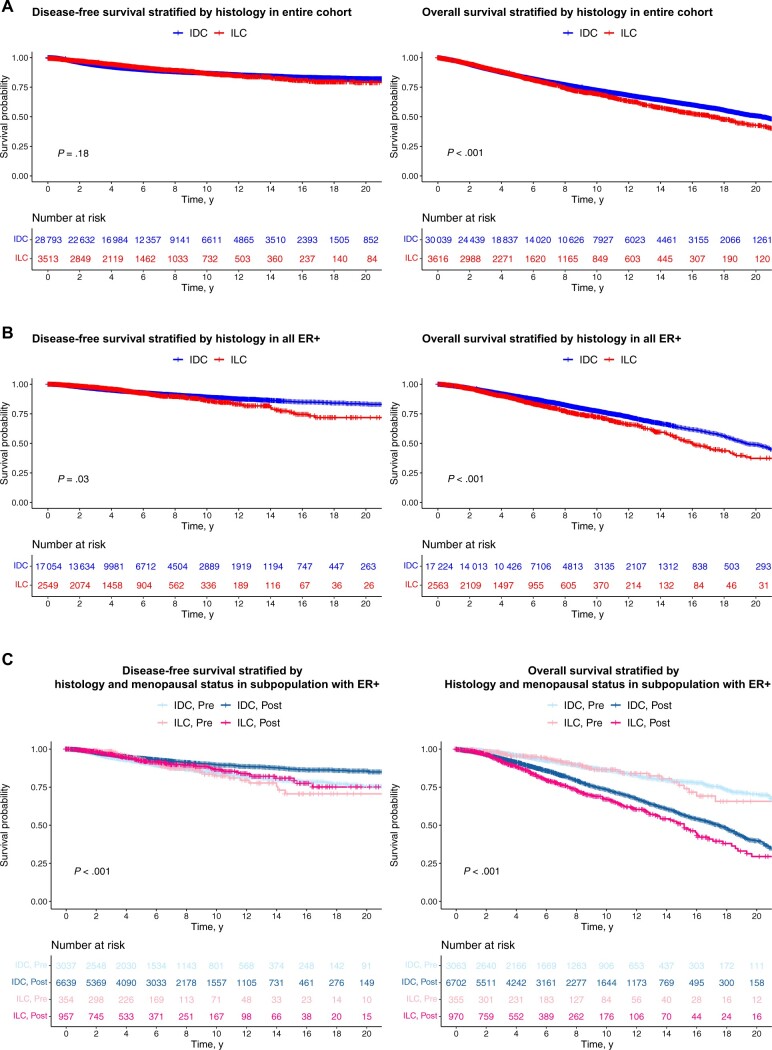
There was no difference in disease-free survival (DFS) (HR = 0.92, 95% CI = 0.82 to 1.04; P = .18) comparing patients with IDC and ILC in the entire cohort, although OS (HR = 1.17, 95% CI = 1.09 to 1.25; P < .001) was statistically significantly worse in patients with ILC (Figure 2, A). In patients with ER-positive and HER2-negative breast cancer, we observed statistically significantly worse DFS (HR = 1.18, 95% CI = 1.01 to 1.38; P = .03) and OS (HR = 1.32, 95% CI = 1.19 to 1.45; P < .001) in patients with ILC (Figure 2, B). Specifically, the 5-, 10-, 15-, and 20-year DFS probabilities for women diagnosed with ER-positive ILC and ER-positive IDC were 94%, 86%, 77%, and 72%, and 94%, 89%, 86%, and 83%, respectively, reflecting an increase in late recurrences in patients with ILC.
Figure 2.
DFS and OS of patients with IDC and ILC. A) A total of 32 306 records in the cohort (28 793 patients with IDC and 3513 patients with ILC) are included for DFS analysis with the median follow-up time of 5.03 years. Log-rank tests were used. Specifically, the 5-, 10-, 15-, and 20-year DFS probabilities for women diagnosed with ER-positive ILC and ER-positive IDC were 94%, 86%, 77%, and 72%, and 94%, 89%, 86%, and 83%, respectively. A total of 33 655 records in the whole cohort (30 039 patients with IDC and 3616 patients with ILC) are included for OS analysis with the median follow-up time of 5.46 years. The estimated 10-year OS probabilities are 0.733 (95% CI = 0.726 to 0.739) for patients with IDC and 0.696 (95% CI = 0.676 to 0.717) for patients with ILC (P < .0001). B) A total of 19 603 records in the ER-positive cohort (17 054 patients with IDC and 2549 patients with ILC) are included for DFS analysis with the median follow-up time of 4.75 years. Log-rank tests were used. A total of 19 787 records in the ER-positive cohort (17 224 patients with IDC and 2563 patients with ILC) are included for OS analysis with the median follow-up time of 5 years. The estimated 10-year OS probabilities are 0.771 (95% CI = 0.762 to 0.781) for patients with IDC and 0.720 (95% CI = 0.694 to 0.748) for patients with ILC (P < .0001). C) DFS and OS comparing patients with IDC and ILC stratified by menopausal status (unadjusted for other covariates using the Kaplan-Meier method). CI = confidence interval; DFS = disease-free survival; ER+ = estrogen receptor positive; IDC = invasive ductal cancer; ILC = invasive lobular carcinoma; OS = overall survival; pre = premenopausal; post = postmenopausal.
We observed a statistically significant difference (partial log-rank, P = .02) in DFS after 10 years in patients with ER-positive and PR-negative IDC (n = 2650) vs ER-positive and PR-negative ILC (n = 425). However, we noticed a similar worse survival in the ER-positive and PR-positive ILC (n = 2107) cohort compared with the ER-positive and PR-positive IDC (n = 14 374) cohort (partial log-rank, P < .001), suggesting that differences in late DFS were independent of PR status.
The shortest DFS after 10 years was in premenopausal women with ILC, whereas OS was shortest for postmenopausal women with ILC (Figure 2, C). However, there was no statistically significant interaction between histology and menopause for OS (HR = 1.30, 95% CI = 0.91 to 1.87; P = .153) and DFS (HR = 1.29, 95% CI = 0.86 to 1.94; P = .215).
To control for confounding factors associated with retrospective, multi-institutional analyses, we conducted PSM analysis (see Supplementary Methods, available online). We created PSM cohorts (Supplementary Table 6, available online) for each of the 5 main treatments (surgery [lumpectomy or mastectomy], radiation, hormone therapy, and chemotherapy). The DFS hazard ratio of 0.80 (95% CI = 0.48 to 1.32; HR = 0.85, 95% CI = 0.63 to 1.14; HR = 0.80, 95% CI = 0.57 to 1.12) for lumpectomy, hormonal therapy, and radiation, respectively, suggests increased efficacies in patients with ILC, however, none of these differences reached statistical significance (Table 3).
Table 3.
Disease-free survival and overall survival resulting from each propensity score matched cohort to evaluate differences between treatment benefit in IDC vs ILC (IDC is reference) in the cohort with ER-positive disease
| Treatment | HR (95% CI) | P a | Sample size (for both ILC and IDC) |
|---|---|---|---|
| Disease-free survival | |||
| Lumpectomy | 0.80 (0.48 to 1.32) | .38 | 630 |
| Mastectomy | 0.92 (0.65 to 1.28) | .61 | 733 |
| Radiation therapy | 0.80 (0.57 to 1.12) | .19 | 907 |
| Chemotherapy | 0.90 (0.64 to 1.27) | .56 | 547 |
| Hormone therapy | 0.85 (0.63 to 1.14) | .27 | 1286 |
| Overall survival | |||
| Lumpectomy | 1.07 (0.78 to 1.47) | .67 | 630 |
| Mastectomy | 1.32 (1.01 to 1.73) | .04 | 733 |
| Radiation therapy | 1.08 (0.84 to 1.40) | .55 | 907 |
| Chemotherapy | 1.14 (0.84 to 1.55) | .41 | 547 |
| Hormone therapy | 1.12 (0.91 to 1.39) | .30 | 1286 |
Cox proportional hazards regression were used; all statistical testing is 2-sided. CI = confidence interval; ER = estrogen receptor; HR = hazard ratio; IDC = invasive ductal carcinoma; ILC = invasive lobular carcinoma.
Our large cohort allowed us to address outcomes in HER2-positive ILC (n = 168; 9.4%) and triple-negative (TN) ILC (n = 29; 0.8%). As expected, we observed statistically significantly worse DFS (P < .001) and OS (P < .001) in patients with TN IDC and HER2-positive IDC compared with ER-positive IDC (Supplementary Figure 2, A, available online). Whereas there was no difference in DFS (P = .52) comparing patients with TN ILC (or HER2-positive ILC) vs ER-positive ILC (Supplementary Figure 2, B, available online), the trends were consistent with IDC, and there was statistically significantly worse OS for patients with TN ILC compared with ER-positive ILC (P = .043). There was no statistically significant difference in outcome (P = .97 for DFS and P = 1 for OS) within all patients with HER2-positive (Supplementary Figure 2, C, available online) or TN breast cancer disease (Supplementary Figure 2, D, available online) between IDC and ILC.
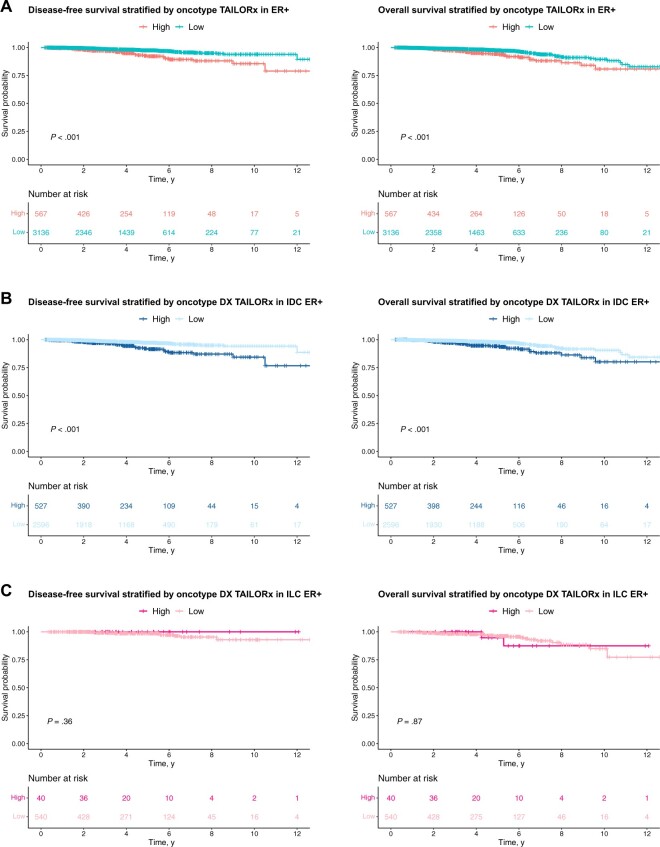
Using the TAILORx cutoff for high (>25) or low and intermediate (≤25) RS, we identified a statistically significant difference in DFS (HR = 0.36, 95% CI = 0.24 to 0.55; P < .0001, high RS as the reference) and OS (HR = 0.49, 95% CI = 0.33 to 0.74; P = .00052) in patients with ER-positive disease (Figure 3, A) and in the ER-positive IDC cohort (HR = 0.34, 95% CI = 0.22 to 0.53; P < .0001 for DFS, and HR = 0.44, 95% CI = 0.28 to 0.68; P = .00014 for OS) (Figure 3, B). In contrast, there was no statistically significant difference in outcome (P = .36 for DFS; HR = 0.88, 95% CI = 0.21 to 3.77; P = .87 for OS) between patients with high and low RS result ILC (Figure 3, C). Similar conclusions were made when repeating the analyses separately for node-negative (Supplementary Figure 3, A, available online) and node-positive disease (Supplementary Figure 3, B, available online). Although limited by small sample size, it is worth noting that there was not a recurrence in the cohort of patients (n = 40) with high RS ILC (median follow-up time is 48.2 months) (DFS, left panel, Figure 3, C), and 2 patients had not recurred 10 years post original diagnosis. We also evaluated the performance of RS result as a continuous variable, or using the original OncotypeDx cutoffs in the cohort of patients with ER-positive disease by fitting the Cox proportional hazards model for OS and DFS (Supplementary Table 7, available online). Regardless of the cutoff, the results remain consistent, that is, using TAILORx, Oncotype DX, or a continuous score, we detected statistically significant associations between RS and outcome in IDC but not in ILC.
Figure 3.
Survival curves stratified by Oncotype Dx TAILORx RS result. Oncotype DX test status is identified as low and intermediate if the RS result is ≤25 and identified as high if the RS result is >25 according to the TAILORx trial groups. DFS and OS analysis (A) in patients with ER-positive disease, (B) in patients with ER-positive IDC, and (C) in patients with ER-positive ILC. Log-rank tests were used to compare the curves (unadjusted for other covariates using the Kaplan-Meier method). DFS = disease-free survival; ER+ = estrogen receptor positive; IDC = invasive ductal cancer; ILC = invasive lobular carcinoma; OS = overall survival; RS = recurrence score.
Metastases and Metastatic Sites Comparing Patients With IDC and ILC
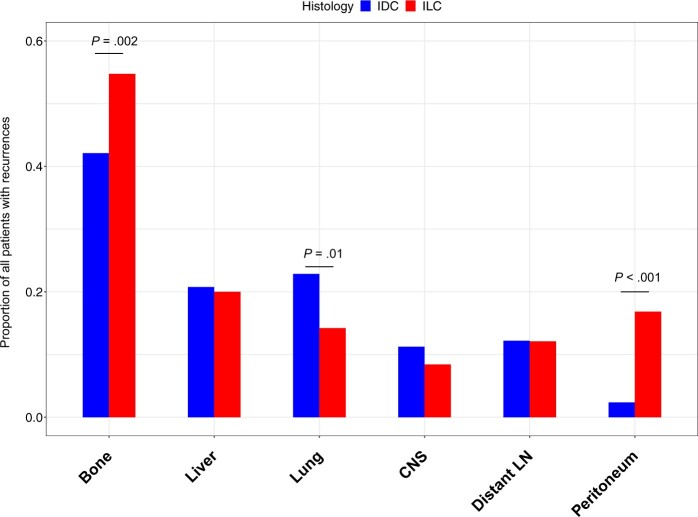
More patients with ILC presented with stage IV de novo metastases compared with patients with IDC (3.7% vs 2.4%; P < .001) (Table 1). The cohort had 2883 recurrences, and among those with information on sites of metastases, we noted an increased propensity to metastasize to the bone and peritoneum and decreased propensity to metastasize to the lung in patients with ILC (Figure 4; Supplementary Figure 4, A, available online).
Figure 4.
Site of recurrence in patients with ER-positive metastatic breast cancer. The bars represent proportion of patients with ER-positive IDC or ER-positive ILC who have recorded information on metastases with recurrence to specific sites as indicated. A 2-sided χ2 test was used. CNS = central nervous system; ER = estrogen receptor; IDC = invasive ductal cancer; ILC = invasive lobular carcinoma; LN = lymph node.
Limiting the analysis to patients diagnosed between 1990 and 2000, to increase follow-up time, the results are similar although only the difference in metastases to peritoneum remains statistically significant (Supplementary Figure 4, B, available online). When comparing median time with recurrence for different metastatic sites, we observed statistically significantly longer timer to metastases in bone in patients with ILC and borderline significance for metastases to the peritoneum (Supplementary Table 8, available online).
Treatment and Tumor Feature Changes Over Time
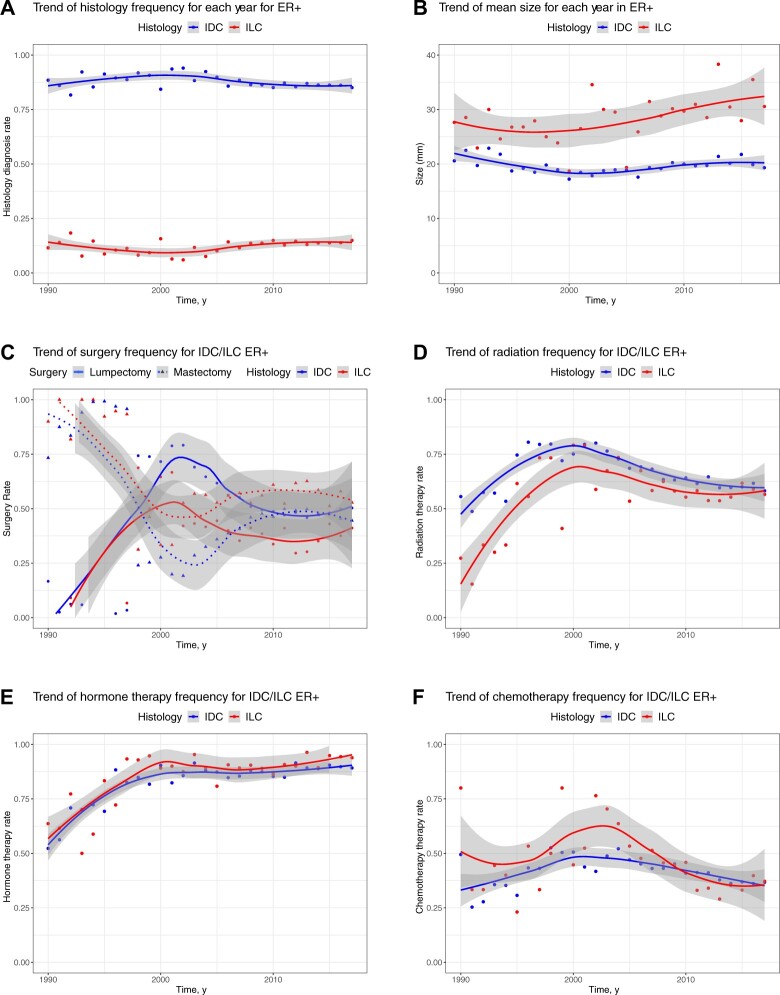
Rates of ILC diagnosis (ie, the distribution of IDC and ILC histology) stayed constant over time (Figure 5, A; Supplementary Figure 5, available online). There is a trend for an increase in ILC size over time, which is not observed in IDC (Figure 5, B).
Figure 5.
Change over time in histology type, tumor size, and treatment types in patients with ER-positive disease from 1990 to 2017. A) Shows the frequency of histology types in 19 788 patients with ER-positive from 1990 to 2017. B) Shows the trend of tumor size (averaged for each year) in UPMC cohort for 10 495 patients with ER-positive from 1990 to 2017. The mean tumor sizes are 20.58 mm for IDC and 27.64 mm for ILC in 1990 and 19.32 mm for IDC and 30.56 mm for ILC in 2017. C) Shows trends of surgery rates across years for 19 462 patients with ER-positive IDC and ER-positive ILC in 3 sites from 1990 to 2017. There were 9480 patients who had lumpectomy and 9204 patients had mastectomy. D) Shows trends of radiation therapy rates across years for 19 480 patients with ER-positive IDC and ER-positive ILC in 3 sites from 1990 to 2017. In total, 12 392 patients received radiation therapy. E) Shows the trend of hormone therapy rates across years for 19 406 patients with ER-positive IDC and ER-positive ILC in 3 sites from 1990 to 2017. In total, 16 998 patients received hormone therapy. F) Shows the trend of chemotherapy rates across years for 18 876 patients with ER-positive IDC and ER-positive ILC in 3 sites from 1990 to 2017. In total, 7750 patients received chemotherapy therapy. ER+ = estrogen receptor positive; IDC = invasive ductal cancer; ILC = invasive lobular carcinoma; UPMC = University of Pittsburgh Medical Center.
Rates of mastectomies increased over the last decade in patients with IDC, getting closer to the higher rates seen in ILC (Figure 5, C). The use of radiation decreased in patients with IDC over the last decade, and a similar trend was seen for patients with ILC (Figure 5, D). There was a slight increase in the use of hormonal therapy over the last decade for both patients with ILC and IDC (Figure 5, E). Finally, there was an increase in the use of chemotherapy in patients with ILC in the early 2000s, but this has sharply declined over the last decade, and a constant decrease in the use of chemotherapy has also been observed for patients with IDC (Figure 5, F). Chemotherapy use was largely driven by nodal status over this period of time.
Discussion
In this retrospective cohort analysis of 33 662 patients, lobular histology represented 10.7% of the cases. The cohort was established at a limited number of institutions, which is a strength and difference to other previously performed retrospective studies (8,11,23,27-29). Comparative analyses showed that the GLBC cohort is representative of the larger IDC and ILC population in the United States. In our study, diagnosis of ILC was associated with larger tumors, older age, lower grade, ER and PR positivity, and lower expression of HER2, and our findings are in agreement with data from these prior studies. The design and main conclusions of the study are depicted in Supplementary Figure 6 (available online).
We observed twice as many diagnoses of stage III (18% vs 7.7%) and stage IV (3% vs 1.6%) ER-positive ILC compared with ER-positive IDC. In addition, there was more lymph node involvement (N2 and N3) in patients with ILC. This has been seen in other prior studies including the analysis of the SEER database (8) and is likely because of later detection as a result of limitations with imaging of ILC.
In our study, we also address changes in diagnosis over time. There was no increase in rate of detection of ILC, which is in contrast to a recent analysis in the Ontario Cancer Registry (28). Analysis of the SEER database also revealed an increase in ILC incidence until 2000 (34), but subsequently such trend attenuated. The lack of a consistent change in incidence might result from challenges in the diagnosis of ILC, as such changes might not reflect true changes in the number of cases but rather result from varying diagnostic criteria.
Patients with ER-positive ILC had statistically significantly worse DFS and OS than ER-positive IDC, a result that is in agreement with a number of prior studies (8,25-27,29). The increased late recurrences in ILC may reflect the overall slower growth or point toward an increased role for cancer cell dormancy in ILC. The shortest 10-year DFS was seen in premenopausal women with ILC, and a recent SEER analysis showed that patients with premenopausal ILC had worse breast cancer–specific outcome compared with premenopausal IDC (35). The aforementioned SEER study (35) did reveal a statistically significant time-dependent effect of histology for outcome, indicating late recurrences in patients with ILC. Curiously, this increase was seen even though 6 times more IDC cases were classified as high risk by RS. Patients with IDC with high RS had statisticallysignificantly worse prognosis compared with those with low RS, but that was not seen in ILC. Indeed, within our cohort, we didn’t identify a single recurrence in high RS ILC cases. Of note, this study was conducted independently from ExactScience. These findings emphasize the need for larger studies for patients with ILC as well as the need for specific molecular tests for ILC (12-14,19-21,36,37).
In general, patients with ILC benefited more from lumpectomy, radiation, and hormonal therapies as indicated by smaller hazard ratio for DFS (0.59, 0.67, and 0.65, respectively), however, this analysis is limited by small sample sizes. Nevertheless, our study shows that clinical management of the 2 diseases are somewhat different. Higher rates of mastectomies are seen in patients with ILC, and after an increase in the use of chemotherapies in the early 2000s, there has been a sharp decline of its use for patients with ILC. Given neoadjuvant chemotherapy has been associated with lower pathological complete response (38) and relative ineffectiveness in adjuvant therapy [(39), reviewed in 40)], it is somewhat surprising that the recent use of chemotherapy is similar in patients with IDC and ILC. Of note, lack of pathological complete response did not remain statistically significant in a multivariable analysis in a study at MD Anderson Cancer Center (41). This topic deserves further attention including specific genomic studies in ILC that can predict responses to different modalities of therapies.
We focused our main analyses and data interpretation to ER-positive disease, which represents 96% of ILC cases in our cohort. There were few patients with HER2-positive disease (<5%) (n = 168; n = 21 were HER2 positive/ER negative and n = 145 were HER2 positive/ER positive; 3 had unknown ER status); however, the topic of HER2 signaling is of increasing interest because of enrichment of HER2 mutations in ILC in the absence of HER2 amplification (42-47). The trend in survival for TN ILC mirrored that seen in TN IDC. Poor survival in TN ILC was also seen in 2 other recent studies with n = 38 (48) and n = 74 TN ILC cases (15). These rare ILC variants with poor prognosis should be studied further, preferentially in larger consortia, because of potential clinical relevance of such findings. For example, preliminary data in the GELATO trial show an efficacy signal of programmed death ligand 1 (PD-L1) blockade in combination with carboplatin in patients with metastatic TN ILC (49), and further molecular studies showed enhanced ErbB-signaling, androgen receptor expression, and activation of DNA damage response pathways (15,48).
Limitations of our paper are the retrospective nature of the study and incomplete information on site of recurrence. Other variables were limited and not included, such as comorbidities. In some subanalyses in the study, small sample sizes were observed (ie, those with ILC with high RS result). With respect to RS result, we note that a selection bias may have occurred for the patients who actually underwent Oncotype Dx testing and that the assay was not routinely run for patients with nodal positivity during the study period. Finally, we lack information on ILC variants, which will be critical to consider in future analyses.
In summary, our study contributes to the growing literature of the unique clinicopathological features of ILC but also highlights those shared with IDC. These findings highlight the need for more ILC research on imaging to improve early detection and staging, on predictive markers to prevent over- and undertreatment, and on dormancy and late recurrence. Rare ILC subtypes such as TN and the more aggressive pleomorphic variant require formation of larger consortia to obtain sufficient numbers.
Funding
The work was supported by the Breast Cancer Research Foundation (BCRF) (SO), the National Cancer Institute (NCI) of the National Institutes of Health (NCI P30CA047904 to UPMC HCC, and NCI P30CA016058 to OSUCCC; NCI 1F30CA264963-01 [NC]). AVL and SO are Komen Scholars and Hillman Foundation Fellows, and AN was supported by a Gianni Bonadonna Breast Cancer Research Fellowship provided by the Conquer Cancer Foundation of the American Society of Clinical Oncology. The work was also supported in part by the MacDonald Fund at Cleveland Clinic Taussig Cancer Center and the Anderson Breast Cancer Fund at OSUCCC.
Notes
Role of the funder: The funders of this study did not participate and gave no input with respect to the design of the study; the collection, analysis, and interpretation of the data; the writing of the manuscript; and the decision to submit the manuscript for publication.
Disclosures: The authors have no conflicts of interest to report.
Author contributions: SO: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Supervision, Project Administration, Funding Acquisition; AN: Conceptualization, Investigation, Data Curation, Writing—Original Draft, Funding Acquisition; JZ: Methodology, Software, Formal Analysis, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization; NC: Methodology, Formal Analysis, Investigation, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Funding Acquisition; TO: Investigation, Resources, Data Curation, Writing—Review & Editing; MDW: Investigation, Resources, Data Curation, Writing—Review & Editing; YL: Methodology, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization; KD: Methodology, Data Curation, Formal Analysis, Investigation, Writing—Original Draft, Writing—Review & Editing; BR: Conceptualization, Data Curation, Funding Acquisition, Supervision, Writing—Review & Editing, Project Administration; GT: Methodology, Software, Formal Analysis, Data Curation, Writing—Original Draft, Writing—Review & Editing, Visualization, Project Administration; AVL: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Supervision, Project Administration, Funding Acquisition; AN: Conceptualization, Investigation, Data Curation, Writing—Original Draft, Funding Acquisition; NW: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Supervision, Project Administration, Funding Acquisition; AN: Conceptualization, Investigation, Data Curation, Writing—Original Draft, Funding Acquisition; MK: Conceptualization, Methodology, Investigation, Resources, Data Curation, Writing—Original Draft, Writing—Review & Editing, Supervision, Project Administration, Funding Acquisition; AN: Conceptualization, Investigation, Data Curation, Writing—Original Draft, Funding Acquisition;.
Acknowledgments: We would like to acknowledge the valuable contribution of the cancer registries at the participating institutions and the support by many physicians at the participating institutions. We would also like to thank Ms Susan MacDonald for her longstanding encouragement of collaborative registry studies aimed at improving understanding of ILC.
Supplementary Material
Contributor Information
Steffi Oesterreich, UPMC Hillman Cancer Center, Pittsburgh, PA, USA; Magee-Women’s Research Institute and Women’s Cancer Research Center, Pittsburgh, PA, USA; Department of Pharmacology and Chemical Biology, University of Pittsburgh, Pittsburgh, PA, USA.
Azadeh Nasrazadani, UPMC Hillman Cancer Center, Pittsburgh, PA, USA; Magee-Women’s Research Institute and Women’s Cancer Research Center, Pittsburgh, PA, USA; Division of Hematology/Oncology, Department of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
Jian Zou, Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, USA.
Neil Carleton, UPMC Hillman Cancer Center, Pittsburgh, PA, USA; Magee-Women’s Research Institute and Women’s Cancer Research Center, Pittsburgh, PA, USA; Medical Scientist Training Program, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Tiffany Onger, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA.
Matthew D Wright, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA.
Yujia Li, Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, USA.
Kathryn Demanelis, UPMC Hillman Cancer Center, Pittsburgh, PA, USA.
Bhuvaneswari Ramaswamy, James Cancer Hospital, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
George Tseng, Department of Biostatistics, University of Pittsburgh, Pittsburgh, PA, USA.
Adrian V Lee, UPMC Hillman Cancer Center, Pittsburgh, PA, USA; Magee-Women’s Research Institute and Women’s Cancer Research Center, Pittsburgh, PA, USA; Department of Pharmacology and Chemical Biology, University of Pittsburgh, Pittsburgh, PA, USA.
Nicole Williams, James Cancer Hospital, The Ohio State University Wexner Medical Center, Columbus, OH, USA.
Megan Kruse, Cleveland Clinic Taussig Cancer Institute, Cleveland, OH, USA; Case Western Comprehensive Cancer Center, Cleveland, OH, USA.
Data Availability
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available. A data manual describing the variables included in the study is available upon request. Code used for data analysis in this manuscript is available upon reasonable request to the corresponding author.
References
- 1. Van Baelen K, Geukens T, Maetens M, et al. Current and future diagnostic and treatment strategies for patients with invasive lobular breast cancer. Ann Oncol. 2022;33(8):769-785. doi: 10.1016/j.annonc.2022.05.006. [DOI] [PubMed] [Google Scholar]
- 2. Christgen M, Derksen P.. Lobular breast cancer: molecular basis, mouse and cellular models. Breast Cancer Res. 2015;17(1):16. doi: 10.1186/s13058-015-0517-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Desmedt C, Zoppoli G, Sotiriou C, Salgado R.. Transcriptomic and genomic features of invasive lobular breast cancer. Semin Cancer Biol. 2017;44:98-105. doi: 10.1016/j.semcancer.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 4. McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR.. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res. 2021;23(1):6. doi: 10.1186/s13058-020-01384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pramod N, Nigam A, Basree M, et al. Comprehensive review of molecular mechanisms and clinical features of invasive lobular cancer. Oncologist. 2021;26(6):e943-e953. doi: 10.1002/onco.13734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thomas M, Kelly ED, Abraham J, Kruse M.. Invasive lobular breast cancer: a review of pathogenesis, diagnosis, management, and future directions of early stage disease. Semin Oncol. 2019;46(2):121-132. doi:10.1053/j.seminoncol.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 7. Mamtani A, King TA.. Lobular breast cancer: different disease, different algorithms? Surg Oncol Clin N Am. 2018;27(1):81-94. doi: 10.1016/j.soc.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 8. Chen Z, Yang J, Li S, et al. Invasive lobular carcinoma of the breast: a special histological type compared with invasive ductal carcinoma. PLoS One. 2017;12(9):e0182397.doi: 10.1371/journal.pone.0182397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ciriello G, Gatza ML, Beck AH, et al. ; for TCGA Research Network. Comprehensive molecular portraits of invasive lobular breast cancer. Cell. 2015;163(2):506-519. doi: 10.1016/j.cell.2015.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li CI, Uribe DJ, Daling JR.. Clinical characteristics of different histologic types of breast cancer. Br J Cancer. 2005;93(9):1046-1052. doi: 10.1038/sj.bjc.6602787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yang C, Lei C, Zhang Y, et al. Comparison of overall survival between invasive lobular breast carcinoma and invasive ductal breast carcinoma: a propensity score matching study based on SEER database. Front Oncol. 2020;10:590643.doi: 10.3389/fonc.2020.590643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Abel MK, Shui AM, Melisko M, et al. The incidence of discordant clinical and genomic risk in patients with invasive lobular or ductal carcinoma of the breast: a National Cancer Database Study. NPJ Breast Cancer. 2021;7(1):156.21 doi:10.1038/s41523-021-00366-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Beumer IJ, Persoon M, Witteveen A, et al. Prognostic value of MammaPrint((R)) in invasive lobular breast cancer. Biomark Insights. 2016;11:139-146. doi: 10.4137/BMI.S38435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Christgen M, Gluz O, Harbeck N, et al. ; for the West German Study Group Plan B Investigators. Differential impact of prognostic parameters in hormone receptor-positive lobular breast cancer. Cancer. 2020;126(22):4847-4858. doi: 10.1002/cncr.33104. [DOI] [PubMed] [Google Scholar]
- 15. Conforti F, Pala L, Pagan E, et al. Biological and clinical features of triple negative invasive lobular carcinomas of the breast. Clinical outcome and actionable molecular alterations. Breast. 2021;59:94-101. doi:10.1016/j.breast.2021.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kizy S, Huang JL, Marmor S, Tuttle TM, Hui JYC.. Impact of the 21-gene recurrence score on outcome in patients with invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2017;165(3):757-763. doi:10.1007/s10549-017-4355-9. [DOI] [PubMed] [Google Scholar]
- 17. Lænkholm A-V, Jensen M-B, Eriksen JO, et al. Population-based study of prosigna-PAM50 and outcome among postmenopausal women with estrogen receptor-positive and HER2-negative operable invasive lobular or ductal breast cancer. Clin Breast Cancer. 2020;20(4):e423-e432. doi:10.1016/j.clbc.2020.01.013. [DOI] [PubMed] [Google Scholar]
- 18. Metzger-Filho O, Michiels S, Bertucci F, et al. Genomic grade adds prognostic value in invasive lobular carcinoma. Ann Oncol. 2013;24(2):377-384. doi: 10.1093/annonc/mds280. [DOI] [PubMed] [Google Scholar]
- 19. Nunes R, Sella T, Treuner K, et al. Prognostic utility of breast cancer index to stratify distant recurrence risk in invasive lobular carcinoma. Clin Cancer Res. 2021;27(20):5688-5696. doi: 10.1158/1078-0432.CCR-21-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sestak I, Filipits M, Buus R, et al. Prognostic value of endopredict in women with hormone receptor-positive, HER2-negative invasive lobular breast cancer. Clin Cancer Res. 2020;26(17):4682-4687. doi:10.1158/1078-0432.CCR-20-0260. [DOI] [PubMed] [Google Scholar]
- 21. McCart Reed AE, Lal S, Kutasovic JR, et al. LobSig is a multigene predictor of outcome in invasive lobular carcinoma. NPJ Breast Cancer. 2019;5:18.doi: 10.1038/s41523-019-0113-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Christgen M, Cserni G, Floris G, et al. Lobular breast cancer: histomorphology and different concepts of a special spectrum of tumors. Cancers (Basel). 2021;13(15):3695. doi: 10.3390/cancers1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Arpino G, Bardou VJ, Clark GM, Elledge RM.. Infiltrating lobular carcinoma of the breast: tumor characteristics and clinical outcome. Breast Cancer Res. 2004;6(3):R149-56. doi: 10.1186/bcr767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mathew A, Rajagopal PS, Villgran V, et al. Distinct pattern of metastases in patients with invasive lobular carcinoma of the breast. Geburtshilfe Frauenheilkd. 2017;77(6):660-666. doi: 10.1055/s-0043-109374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adachi Y, Ishiguro J, Kotani H, et al. Comparison of clinical outcomes between luminal invasive ductal carcinoma and luminal invasive lobular carcinoma. BMC Cancer. 2016;16:248. doi: 10.1186/s12885-016-2275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engstrom MJ, Opdahl S, Vatten LJ, Haugen OA, Bofin AM.. Invasive lobular breast cancer: the prognostic impact of histopathological grade, E-cadherin and molecular subtypes. Histopathology. 2015;66(3):409-419. doi:10.1111/his.12572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pestalozzi BC, Zahrieh D, Mallon E, et al. Distinct clinical and prognostic features of infiltrating lobular carcinoma of the breast: combined results of 15 International Breast Cancer Study Group clinical trials. J Clin Oncol. 2008;26(18):3006-3014. doi:10.1200/J Clin Oncol.2007.14.9336. [DOI] [PubMed] [Google Scholar]
- 28. Findlay-Shirras LJ, Lima I, Smith G, Clemons M, Arnaout A.. Population trends in lobular carcinoma of the breast: the Ontario experience. Ann Surg Oncol. 2020;27(12):4711-4719. doi: 10.1245/s10434-020-08895-8. [DOI] [PubMed] [Google Scholar]
- 29. Yang LY, Yang LP, Zhu B.. Clinicopathological characteristics and survival outcomes of invasive lobular carcinoma in different races. Oncotarget. 2017;8(43):74287-74298. doi: 10.18632/oncotarget.19396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ho DE, Imai K, King G, Stuart EA.. MatchIt: nonparametric preprocessing for parametric causal inference. J Stat Softw. 2011;42(8):1-28. doi: 10.18637/jss.v042.i08. [DOI] [Google Scholar]
- 31. Curtis C, Shah SP, Chin SF, et al. ; the METABRIC Group. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346-352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Saal LH, Vallon-Christersson J, Hakkinen J, et al. The Sweden Cancerome Analysis Network - Breast (SCAN-B) Initiative: a large-scale multicenter infrastructure towards implementation of breast cancer genomic analyses in the clinical routine. Genome Med. 2015;7(1):20.doi: 10.1186/s13073-015-0131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results SEERStat Database. NPCR and SEER Incidence - U.S. Cancer Statistics 2001-2017 Public Use Research Database, 2019 Submission (2001-2017), United States Department of Health and Human Services, Centers for Disease Control and Prevention and National Cancer Institute. Released June 2020. www.cdc.gov/cancer/uscs/public-use. Accessed June 6, 2022.
- 34. Li CI, Anderson BO, Daling JR, Moe RE.. Trends in incidence rates of invasive lobular and ductal breast carcinoma. JAMA. 2003;289(11):1421-1424. doi: 10.1001/jama.289.11.1421. [DOI] [PubMed] [Google Scholar]
- 35. Kim HJ, Kim S-O, Freedman RA, Partridge AH, Metzger-Filho O.. Survival outcomes of premenopausal patients diagnosed with invasive lobular carcinoma. J Clin Oncol. 2019;37(suppl 15):1093-1093. doi:10.1200/J Clin Oncol.2019.37.15_suppl.1093. [Google Scholar]
- 36. Jenkins JA, Marmor S, Hui JYC, et al. The 70-gene signature test as a prognostic and predictive biomarker in patients with invasive lobular breast cancer. Breast Cancer Res Treat. 2022;191(2):401-407. doi: 10.1007/s10549-021-06429-8. [DOI] [PubMed] [Google Scholar]
- 37. Makower D, Qin J, Lin J, Xue X, Sparano JA.. The 21-gene recurrence score in early non-ductal breast cancer: a National Cancer Database analysis. NPJ Breast Cancer. 2022;8(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cristofanilli M, Gonzalez-Angulo A, Sneige N, et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23(1):41-48. doi:10.1200/J Clin Oncol.2005.03.111 [DOI] [PubMed] [Google Scholar]
- 39. Marmor S, Hui JYC, Huang JL, et al. Relative effectiveness of adjuvant chemotherapy for invasive lobular compared with invasive ductal carcinoma of the breast. Cancer. 2017;123(16):3015-3021. doi: 10.1002/cncr.30699. [DOI] [PubMed] [Google Scholar]
- 40. Colleoni M, Russo L, Dellapasqua S.. Adjuvant therapies for special types of breast cancer. Breast. 2011;20(suppl 3):S153-S157. doi:10.1016/S0960-9776(11)70315-0. [DOI] [PubMed] [Google Scholar]
- 41. Delpech Y, Coutant C, Hsu L, et al. Clinical benefit from neoadjuvant chemotherapy in oestrogen receptor-positive invasive ductal and lobular carcinomas. Br J Cancer. 2013;108(2):285-291. doi: 10.1038/bjc.2012.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Desmedt C, Zoppoli G, Gundem G, et al. Genomic characterization of primary invasive lobular breast cancer. J Clin Oncol. 2016;34(16):1872-1881. doi:10.1200/J Clin Oncol.2015.64.0334 [DOI] [PubMed] [Google Scholar]
- 43. Kurozumi S, Alsaleem M, Monteiro CJ, et al. Targetable ERBB2 mutation status is an independent marker of adverse prognosis in estrogen receptor positive, ERBB2 non-amplified primary lobular breast carcinoma: a retrospective in silico analysis of public datasets. Breast Cancer Res. 2020;22(1):85. doi: 10.1186/s13058-020-01324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lien HC, Chen YL, Juang YL, Jeng YM.. Frequent alterations of HER2 through mutation, amplification, or overexpression in pleomorphic lobular carcinoma of the breast. Breast Cancer Res Treat. 2015;150(2):447-455. doi: 10.1007/s10549-015-3336-0. [DOI] [PubMed] [Google Scholar]
- 45. Rosa-Rosa JM, Caniego-Casas T, Leskela S, et al. High frequency of ERBB2 activating mutations in invasive lobular breast carcinoma with pleomorphic features. Cancers (Basel). 2019;11(1):74. doi: 10.3390/cancers11010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ross JS, Wang K, Sheehan CE, et al. Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin Cancer Res. 2013;19(10):2668-2676. doi: 10.1158/1078-0432.CCR-13-0295 [DOI] [PubMed] [Google Scholar]
- 47. Sokol ES, Feng YX, Jin DX, et al. Loss of function of NF1 is a mechanism of acquired resistance to endocrine therapy in lobular breast cancer. Ann Oncol. 2019;30(1):115-123. doi: 10.1093/annonc/mdy497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bergeron A, MacGrogan G, Bertaut A, et al. Triple-negative breast lobular carcinoma: a luminal androgen receptor carcinoma with specific ESRRA mutations. Mod Pathol. 2021;34(7):1282-1296. doi: 10.1038/s41379-021-00742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Voorwerk L, Horlings H, Van Dongen M, et al. Atezolizumab with carboplatin as immune induction in metastatic lobular breast cancer: first results of the GELATO-trial. Ann Oncol. 2021;32(suppl 2):S58-S59. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the nature of this research, participants of this study did not agree for their data to be shared publicly, so supporting data is not available. A data manual describing the variables included in the study is available upon request. Code used for data analysis in this manuscript is available upon reasonable request to the corresponding author.