Abstract
Background
While physical inactivity is associated with adverse psychological outcomes, less is known about the psychological outcomes associated with sedentary behaviour, and specifically, its mentally active and passive forms. The COVID-19 pandemic represents a unique opportunity to study associations between these variables in light of widespread stay-at-home mandates and restrictions on outdoor exercise/social activities. Using a cross-sectional dataset acquired during the COVID-19 pandemic in Australia, we examined whether physical activity and sedentary behaviour were associated with subjective quality of life (sQoL) and subjective cognitive dysfunction, and whether these associations were mediated by depressive symptoms.
Methods
658 participants (males = 169, females = 489) self-reported data on physical activity and sedentary behaviour in an online survey during May 2020–May 2021. Data on physical activity and sedentary behaviour (both mentally active and passive types) was compared according to whether it was collected during or out of a lockdown period. Regression models were used to test associations of physical activity and sedentary behaviour with sQoL and subjective cognitive dysfunction, and whether these associations were mediated by depression severity.
Results
Physical activity was beneficially associated with sQoL, whereas sedentary behaviour (both total hours and the reduction of mentally active/increase in mentally passive behaviour) was detrimentally associated with sQoL. These associations were mediated by depression severity. Physical activity and sedentary behaviour were also indirectly associated with subjective cognitive dysfunction by virtue of their associations with depression severity.
Conclusions
There are important differences in the psychological correlates of mentally passive and active sedentary behaviours. Our findings suggest that health promotion strategies should focus on not only increasing physical activity but also reducing passive sedentary behaviours as a means of maintaining good psychological health.
Keywords: Mentally active sedentary behaviour, Mentally passive sedentary behaviour, Subjective cognitive dysfunction, Depression, COVID-19, Subjective quality of life
1. Introduction
Cognitive and psychological health are important public health considerations, with existing literature implicating lifestyle factors as important contributors to adverse outcomes (Zaman, Hankir, & Jemni, 2019). One such factor, physical activity, or its more structured form – exercise – has been linked to better psychological and cognitive functioning in several observational studies (Bangsbo et al., 2019; Kirk-Sanchez & McGough, 2013; Voss, Nagamatsu, Liu-Ambrose, & Kramer, 2011; Zhao et al., 2018). A large body of work demonstrates that physical inactivity (i.e. not meeting physical activity guidelines) correlates with poorer subjective quality of life (sQoL) and psychological health (Nowak, Bożek, & Blukacz, 2019; Pucci, Rech, Fermino, & Reis, 2012; Puciato, Borysiuk, & Rozpara, 2017), and there is evidence that both depression, and to a lesser extent, cognitive performance, can be improved by physical activity interventions (Bangsbo et al., 2019; Josefsson, Lindwall, & Archer, 2014; Kane, Butler, & Fink, 2017; Kirk-Sanchez & McGough, 2013; Krogh, Nordentoft, Sterne, & Lawlor, 2011). Notably, the associations between physical activity and psychological or cognitive functioning appear to be evident irrespective of whether physical activity is measured subjectively or objectively.
Physical inactivity is related to, yet conceptually distinct from, sedentary behaviour, which has commonly been operationalised as periods of sitting or reclining with low energy expenditure, as opposed to the absence of physical activity itself (Tremblay et al., 2017). Associations between psychological health and sedentary behaviour are increasingly evident (Carson et al., 2015; Falck, Davis, & Liu-Ambrose, 2017; Olanrewaju, Stockwell, Stubbs, & Smith, 2020), and can be independent of physical activity levels (Hallgren et al., 2020). This may be the case for mentally passive forms of sedentary behaviour in particular, which can be differentiated from mentally active forms in that the latter is defined by more cognitive effort; and the former much less so (Kikuchi et al., 2014). This differentiation is only just beginning to be recognised (Hallgren et al., 2020; Hallgren et al., 2020; Kikuchi et al., 2014), where on face validity, activities such as tv viewing, passive social media use or commuting from place to place as a passenger index sedentary behaviours that are mentally passive. In contrast, occupational or non-occupational computer use, video games or reading, index sedentary activities that are mentally active.
Preliminary research has associated mentally passive sedentary behaviours with greater levels of psychological distress (Kikuchi et al., 2014) and poorer physical health-related-QoL (Saunders et al., 2020); however, further research is needed to determine whether this extends to QoL more generally, and for sQoL in particular. A small body of work has also linked mentally passive sedentary behaviour to an increased risk of depression, whereas mentally active sedentary behaviour appears to protect against depression onset (Hallgren et al., 2018, 2019) and is associated with better psychological wellbeing in adolescents (A. Kandola, Owen, Dunstan, & Hallgren, 2021). The extent to which this relationship is bi-directional is not clear, as those with greater depressive symptoms may also be more likely to engage in mentally passive sedentary behaviours, while mentally-well individuals may engage in greater levels of mentally active behaviours. Given the infancy of this literature, further work is clearly needed, including in relation to the association of psychological health and different forms of sedentary behaviour. This is particularly pertinent given growing evidence that mentally active sedentary behaviours, such as reading and some forms of computer use (e.g., video gaming), are associated with better cognitive functioning (Olanrewaju et al., 2020; Tun & Lachman, 2010; Wang et al., 2006), whereas the more-passive TV viewing is associated with poorer cognitive functioning (Falck et al., 2017; Hoang et al., 2016; Olanrewaju et al., 2020; Wang et al., 2006).
Notably, physical activity and sedentary behaviour have thus far been studied predominantly in relation to objectively measured cognition, and there is minimal research examining these lifestyle factors in the context of subjective cognitive complaints (Felez-Nobrega, Haro, Erickson, & Koyanagi, 2020; Nemoto et al., 2018; Omura et al., 2020; Zuniga, Mackenzie, Kramer, & McAuley, 2016). This absence is important given that subjective cognitive dysfunction has been shown to predict objective cognitive decline in ageing samples (Lautenschlager, Cox, & Ellis, 2019; Liew, 2019; Mendonça, Alves, & Bugalho, 2016; Reisberg, Shulman, Torossian, Leng, & Zhu, 2010), and is also associated with poor mental health and QoL generally (Hill et al., 2016, 2017). Subjective cognitive dysfunction may even represent a by-product of psychological distress (Hill et al., 2016), since it has been associated with both suicidal ideation and poor psychosocial functioning/QoL in those with mood disorders (Luo, Yinghua, Dali, Kunlun, & Lin, 2020; Sumiyoshi et al., 2019, 2021). Further, a longitudinal study of over 13,000 participants found that objective cognitive impairment was strongly predicted by comorbid depression and subjective cognitive decline together, and by both of these factors individually (Liew, 2019). Accordingly, subjective cognitive dysfunction appears to be important within the holistic profile of health and wellbeing. Thus, further research examining direct and indirect (via psychological distress) associations between physical activity and sedentary behaviours on subjective cognitive functioning is pertinent.
Recently in several countries, a large majority of outdoor exercise and social activities were prohibited or limited as a result of the preventive measures and restrictions put in place to slow or stop transmission of the novel coronavirus disease (COVID-19). As these preventive measures predominantly encompassed physical distancing and isolation, they likely impacted both physical activity and sedentary behaviour. In Australia, for example, there was a documented decrease in self-reported physical exercise during the first wave/lockdown associated with the pandemic (Phillipou et al., 2020). This is consistent with findings from other countries in which COVID-19-related physical distancing restrictions were also adopted (Castañeda-Babarro, Coca, Arbillaga-Etxarri, & Gutiérrez-Santamaría, 2020; Gjaka et al., 2021; Meyer et al., 2020; Woodruff, Coyne, & St-Pierre, 2021). Currently, it is unclear whether levels of physical activity, and in particular sedentary behaviour, have varied as a function of the presence or severity of preventative restrictions in place at a given time. Examining variability in these lifestyle factors during the COVID-19 pandemic, however, presents a unique opportunity to better understand the extent to which physical activity and sedentary behaviour correlate with psychological outcomes.
With this in mind, we leveraged a large Australian dataset acquired during the COVID-19 pandemic for this purpose, where we specifically aimed to evaluate (1) whether physical activity (including moderate/vigorous subtype) and sedentary behaviour (including mentally active and mentally passive subtypes) differed according to whether it was reported during lockdown versus non-lockdown periods during the first year of the pandemic (2) whether self-reported physical activity and sedentary behaviour during the pandemic were associated with sQoL and subjective cognitive dysfunction, and (3) whether these associations were mediated by depression severity. We hypothesised that lower levels of physical activity and higher levels of sedentary behaviour would be associated with lockdown periods. Lower levels of physical activity and mentally active sedentary behaviours, and higher levels of mentally passive sedentary behaviours, were also expected to be associated with worse sQoL and more subjective cognitive dysfunction.
2. Methods
This study received ethics approval from the Swinburne University Human Research Ethics Committee (approval number: 202029174107) and complied with the Declaration of Helsinki. All participants provided informed consent.
2.1. Study design and sample
Data from the COvid-19 and you: mentaL heaLth in AusTralia now survEy (COLLATE) was used to address the aims of this study (Tan et al., 2020; Neill et al., 2020; Phillipou et al., 2020; Rossell et al., 2021; Toh et al., 2021; Van Rheenen et al., 2020, Van Rheenen et al., 2020). The COLLATE project is an ongoing cross-sectional study that involves a series of anonymous online surveys of members of the general Australian public aged 18 years and above. Surveys are active for 72 h on each occasion. Participants are able to complete as many or as few surveys as they would like, to enable the capture of several cross-sectional ‘snapshots’ of mental health and wellbeing at different timepoints of the pandemic (for further detail see Tan et al. (2020). Participants are recruited online through social media, university and community noticeboards, participant registries, and non-discriminative snowball sampling. Participants provide consent online and then complete the survey, which covers three broad topics: a) current concerns, b) current emotional experiences, and c) socio-demographics/risk factors (including presence of mental illness), followed by other topics of relevance that rotate between survey rounds.
The present paper reports data taken from four of the monthly cross-sectional surveys collected during the first year (May 2020–February 2021). Only participants from the state of Victoria were included in the analyses, as the majority of participants resided in Victoria (50–75%) and because the COVID-19 restrictions and rates of infection drastically differed across Australia during this time. Physical activity and sedentary behaviour questions were rotated into the survey design every three months from May 2020 to February 2021. Thus, the data described here were taken from responses to either the May 2020 (round 2), August 2020 (round 5), November 2020 (round 8) and February 2021 (round 11) survey timepoints. There were only a small number of repeat participants across all four of these survey rounds. Given this number of participants was insufficient for longitudinal analysis, we chose to use data from a single cross-sectional survey timepoint for each repeat participant to retain power in the analyses. These data were taken from their last survey completed (i.e., their responses to earlier surveys were not used) and combined with the cross-sectional data of the other single-survey participants that was collected at one of the time points mentioned above.
2.2. Measures
2.2.1. Lockdown status
A dichotomous Lockdown Status variable was created, referring to whether data were acquired during a period of time in which severe physical distancing restrictions were in effect, or when they were not (see Fig. S1 for a detailed description). Data from May 2020 and August 2020 (survey rounds 2 and 5) were considered to have been acquired during a lockdown period, and data from November 2020 and February 2021 (survey rounds 8 and 11) were considered to have been acquired during a non-lockdown period.
2.2.2. Self-reported physical activity and sedentary behaviour
Physical activity was assessed using two distinct questions (see below). Participants were given the option to answer yes or no, and if they answered yes, they were then asked how many days per week, and how many minutes per day, on average.
-
1.
In the past week, did you do any moderate (i.e., able to simultaneously talk but not sing) or vigorous (i.e., unable to simultaneously talk or sing) physical activity for at least 10 min at a time?
-
2.
In the past week, did you do any walking for at least 10 min at time?
Sedentary behaviour was assessed using following three questions (see below).
-
1.
On a typical day, how much time did you spend sitting, lying, or reclining (but not sleeping or napping; please specify the number of hours or minutes per day)?
-
2.
Estimate what percentage of your time sitting, lying, or reclining on a typical day was spent in mentally active activities.
-
3.
Estimate what percentage of your time sitting, lying, or reclining on a typical day was spent in mentally relaxing activities.
Mentally active and mentally passive (relaxing) percentage estimates were required to total 100%. Examples of mentally active and mentally passive activities were given as follows;
-
•
Mentally active: driving to/from a location, for study or employment-related purposes, using social media to read and/or post messages/photos etc., playing video or computer games, writing, or reading, online gambling or shopping, talking with others digitally or in person, knitting and/or sewing, doing a puzzle and/or playing board games and/or problem solving, or similar.
-
•
Mentally passive: commuting to/from a location as a passenger, taking a bath, listening to music (without dancing or physical activity), using a screen (e.g., computer, smartphone, or tablet) to watch TV and/or passively view social media without a specific purpose and/or ‘surfing’ the internet without a specific purpose, or similar.
From these questions, the following scores were derived for analysis:
-
•
Total sedentary behaviour (hours per day) and mentally active sedentary behaviour (percentage of sedentary behaviour spent in mentally active, as opposed to mentally passive, sedentary behaviours on a typical day i.e., sedentary behaviour question 2). Note that only mentally active sedentary behaviour scores were included in the analyses, as the mentally active and mentally passive scores totalled 100, making one of these scores redundant.
-
•
Total physical activity (hours per week) and moderate/vigorous activity (percentage of Total Physical Activity spent in moderate/vigorous activity, as opposed to light activity (walking)).
Further information regarding the calculation of these scores is provided in Table S1.
2.2.3. Psychological outcomes
Subjective cognitive dysfunction was measured using an abbreviated version of the self-report Cognitive Complaints in Bipolar Disorder Rating Assessment (COBRA; (Rosa et al., 2013)). This measure has been validated in non-bipolar disorder populations (Ott et al., 2016; Toyoshima et al., 2019). Responses are rated on a 4-point Likert scale ranging from never (0) to always (3). Higher total scores reflect poorer subjective cognition over the past 4 weeks.
Subjective Quality of life (sQoL) was measured using the 8-item European Health Interview Surveys – Quality of Life index (EUROHIS-QoL; (Schmidt, Muehlan, & Power, 2006)). Four key domains are assessed; psychological, physical, social and environmental. Individual responses are rated on a 5-point Likert scale. Scores are summed to form a total score, where higher scores reflect better sQoL over the past 4 weeks.
Depression severity was measured using depression subscale from the 21-item Depression Anxiety Stress Scale (DASS-21; (Lovibond & Lovibond, 1995)). The DASS is a self-report questionnaire with items rated on a 4-point Likert scale, ranging from never (0) to almost always (3). Scores from the depression subscale are summed and multiplied by two to form the depression sub score, where higher scores reflect higher levels of depression in the past week.
2.3. Statistical analyses
All analyses were completed using the Statistical Package for the Social Sciences (SPSS) version 27 (IBM). COBRA and DASS Depression scores were square root (SQRT) transformed to account for positive skew in the sample, and the SQRT transformed scores were used for all subsequent analyses, consistent with past work (Rossell et al., 2021; Van Rheenen, Meyer, et al., 2020). Differences in demographic data between periods in and out of lockdown were assessed using ꭓ2 tests for categorical variables and one-way ANOVAs for continuous variables as part of the preliminary analyses.
2.3.1. Physical activity and sedentary behaviour levels by lockdown status
A multivariate ANCOVA was used to assess whether there were differences in the reported physical activity or sedentary behaviour levels of participants whose data was collected during periods of lockdown and those whose data was not. Total activity, moderate/vigorous activity, total sedentary behaviour, and mentally active sedentary behaviour were specified as the dependent variables, and lockdown status as the independent variable of interest. Age, sex, and presence of mental illness (yes/no dichotomous variable) were selected as covariates a-priori. A false discovery rate (FDR) of p<.05 was applied to all results to account for multiple comparisons using the Benjamini-Hochberg method.
2.3.2. Associations between key variables
Model 4 of the Preacher and Hayes PROCESS plugin for SPSS (v4.0) was used to determine whether COBRA and EUROHIS-QoL scores were associated with each of the physical activity and sedentary behaviour measures, and whether these associations were mediated by depression. Coefficients with 95% bias-corrected bootstrapped confidence intervals (CIs) were calculated for the indirect path (5000 bootstrap samples used), and significant mediation established if the range of the CI did not span zero. For each of the dependent variables (Y; COBRA or EUROHIS-QoL), separate models were specified with either total activity, moderate/vigorous activity, total sedentary behaviour, or mentally active sedentary behaviour as the independent variable (X) and DASS Depression as the mediator (M) (n = 8 models). Sex, mental illness status, and age were entered as covariates in all models. In the models in which physical activity or sedentary behaviour were not specified as the independent variable, they were entered as covariates to control their respective effects on the dependent variable (e.g., physical activity effect controlling for sedentary behaviour and vice versa). COBRA and EUROHIS-QoL were considered standalone measures, and thus no FDR correction was performed for these outputs.
3. Results
3.1. Sample description
Participant characteristics are displayed in Table 1 . A total of 658 participants completed in at least one of the surveys of the COLLATE project in the four months analysed and provided sufficient data for the specified analyses. This included physical activity and sedentary behaviour data, as well as DASS, COBRA, and EUROHIS-QoL scores.
Table 1.
Characteristics of participants whose data were acquired during a period in or out of lockdown.
| CHARACTERISTIC | Lockdown (n = 386) | No Lockdown (n = 272) | Comparison |
|---|---|---|---|
| Age | 41.2 ± 13.9 | 39.1 ± 15.8 | F = 3.3 p = 0.072 |
| Sex (male/female) | 73/313 | 96/176 | ꭓ2 = 22.4, p < 0.001* |
| Presence of mental illness (yes/no) | 110/276 | 69/203 | ꭓ2 = 0.8, p = 0.374 |
| COBRA | 7.0 ± 4.2 | 6.3 ± 4.2 | F = 4.7 p = 0.030* |
| DASS depression | 12.0 ± 9.8 | 10.3 ± 9.9 | F = 4.6 p = 0.033* |
| EUROHIS-QoL | 29.0 ± 6.3 | 28.7 ± 6.3 | F = 0.5 p = 0.467 |
COBRA = Cognitive Complaints in Bipolar Disorder Rating Assessment, DASS = Depression Anxiety Stress Scale, EURHIS-QoL = European Health Interview.
Survey – Quality of Life Index.
*Significant at p < 0.05.
3.2. Physical activity and sedentary behaviour levels by lockdown status
Table 2 displays the results of the ANCOVAs examining the association of lockdown status with both physical activity and sedentary behaviour. Lockdown status was not significantly associated with total physical activity, moderate/vigorous activity, total sedentary behaviour, or mentally active sedentary behaviour, with responses remaining similar irrespective of whether they were acquired during a period of lockdown or not.2
Table 2.
Differences in self-reported physical activity and sedentary behaviour as a function of lockdown status.
| Comparisonsa | Group | Mb | SD | |
|---|---|---|---|---|
| Total physical activity | ||||
| Lockdown status | F (1,653) = 0.32, p = 0.570 | Lockdown | 4.75 | −0.89 |
| No Lockdown | 4.49 | |||
| Moderate/Vigorous Activity (%) | ||||
| Lockdown status | F (1,653) = 0.95, p = 0.331 | Lockdown | 29.41 | −0.33 |
| No Lockdown | 31.86 | |||
| Total sedentary behaviour | ||||
| Lockdown status | F (1,653) = 0.11, p = 0.741 | Lockdown | 7.93 | −0.82 |
| No Lockdown | 7.83 | |||
| Mentally-active sedentary behaviour (%) | ||||
| Lockdown status | F (1,653) = 0.09, p = 0.768 | Lockdown | 62.51 | −2.79 |
| No Lockdown | 63.00 | |||
Lockdown status: May 2020 and August 2020 = in lockdown, November 2020 and February 2021 = not in lockdown.
Unadjusted for multiple comparisons.
All values are adjusted for age, sex, and presence of mental illness.
3.3. Associations between key variables
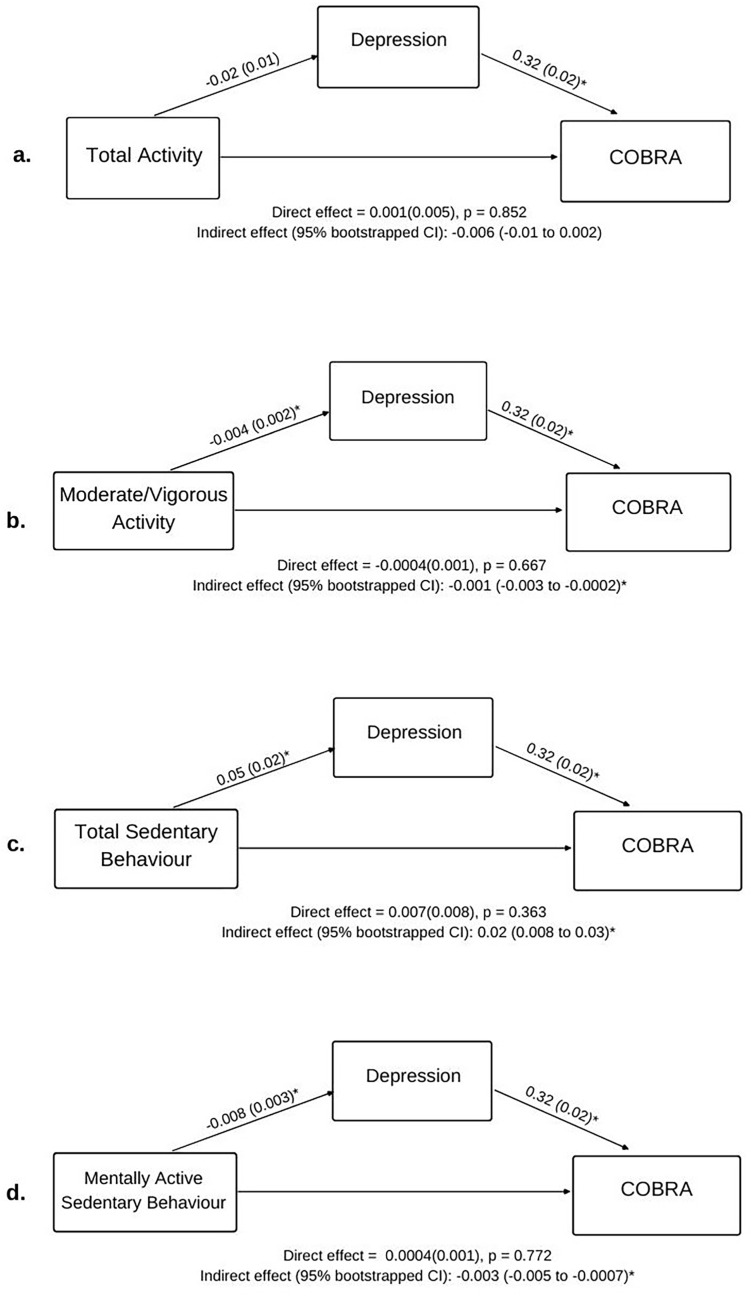
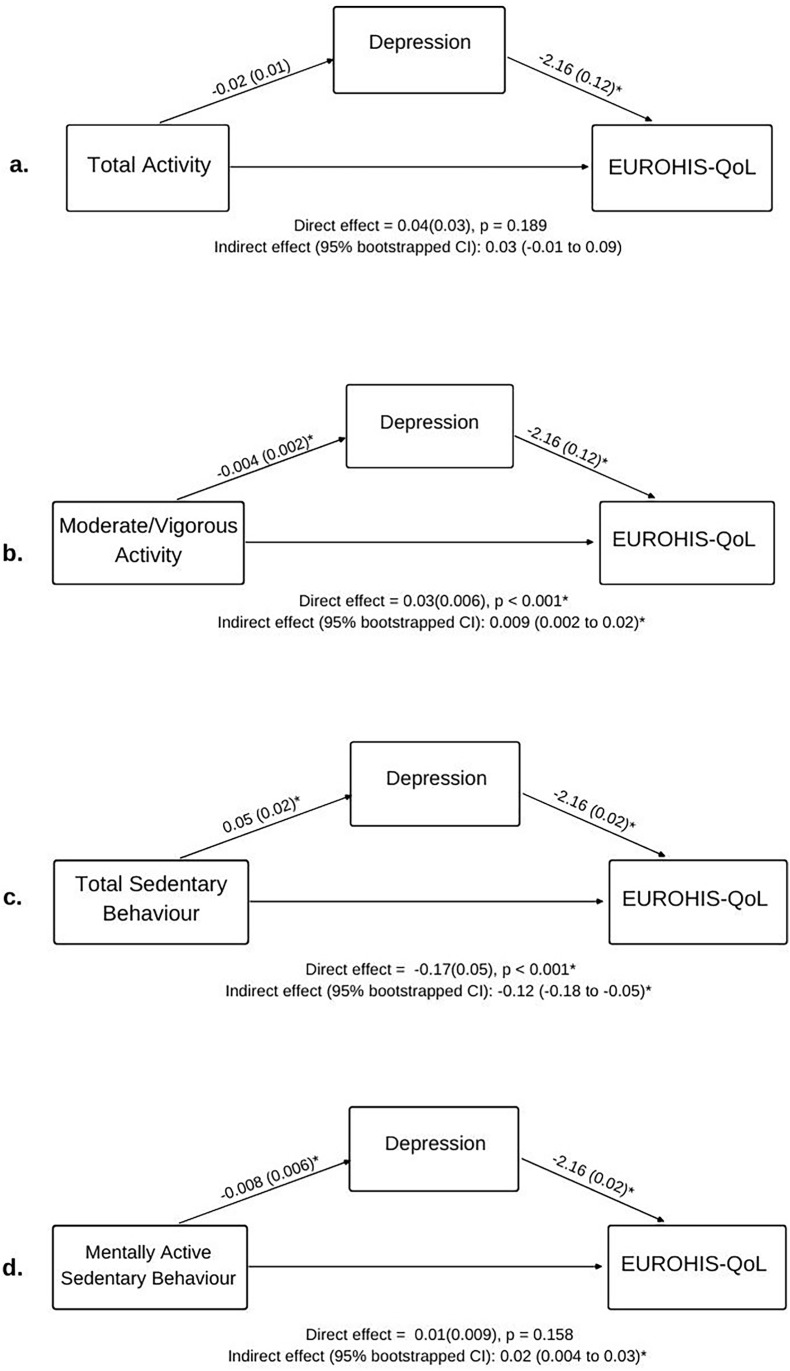
Table 3 and Fig. 1, Fig. 2 detail the direct, indirect, and total effects of all models testing associations between the key variables. The values detailed in brackets below (β) represent the standardised regression coefficient (mean = 0, SD = 1). To our knowledge, a clear heuristic for standardised coefficients does not exist. However, some statisticians have argued that standardised coefficients can be interpreted similarly to correlation coefficients i.e. <0.2 = small, 0.2–0.5 = medium, >0.5 = large (Alcock, 2014).
Table 3.
Total effect of physical activity and sedentary behaviour on subjective cognitive dysfunction and subjective quality of life.
| Effecta | S.E. | p | 95% LLCI | 95% ULCI | |
|---|---|---|---|---|---|
| COBRA | |||||
| Total physical activity | −0.005 | 0.006 | 0.453 | −0.02 | 0.007 |
| Moderate/vigorous activity | −0.002 | 0.001 | 0.117 | −0.004 | 0.0004 |
| Total sedentary behaviour | 0.02 | 0.009 | 0.007* | 0.007 | 0.04 |
| Mentally-active sedentary behaviour | −0.002 | 0.002 | 0.210 | −0.005 | 0.001 |
| EUROHIS-QoL | |||||
| Total physical activity | 0.08 | 0.04 | 0.04* | 0.003 | 0.16 |
| Moderate/vigorous activity | 0.04 | 0.007 | <0.001* | 0.02 | 0.05 |
| Total sedentary behaviour | −0.29 | 0.06 | <0.001* | −0.40 | −0.17 |
| Mentally-active sedentary behaviour | 0.03 | 0.01 | 0.006* | 0.008 | 0.05 |
EUROHIS-QoL = European Health Index Scale - Quality of Life, COBRA = Cognitive Complaints in Bipolar Disorder Rating Assessment.
*Significant at p<.05.
Effect refers to the unstandardised regression coefficients. Note that COBRA scores were SQRT transformed due to positive skew, which may alter the interpretation of the regression coefficient (effect).
Fig. 1.
Effects (bootstrapped standard error in parenthesis) for mediation examining how physical activity, sedentary behaviour, and depression were associated with subjective quality of life after controlling for sex, age, and mental illness status. *p < 0.05. Note DASS depression scores were SQRT transformed due to positive skew, which may alter interpretation of the regression coefficient (effect).
Fig. 2.
Effects (bootstrapped standard error in parenthesis) for mediation examining how physical activity, sedentary behaviour, and depression were associated with subjective cognitive dysfunction after controlling for sex, age, and mental illness status. *p < 0.05. Note COBRA scores and DASS depression scores were SQRT transformed due to positive skew, which may alter interpretation of the regression coefficients (effects).
3.3.1. Physical activity
Total physical activity was significantly and positively associated with EUROHIS-QoL scores (β = 0.07), where participants who spent more time physically active reported better sQoL. This effect was not mediated by DASS Depression score. Moderate/vigorous activity was also significantly positively associated with EUROHIS-QoL scores (β = 0.19), such that participants who spent more time in moderate/vigorous activity reported better sQoL. This association was mediated by DASS Depression score (CI: 0.002–0.02; Fig. 1b). No associations between physical activity and COBRA scores were evident, although depressive severity did mediate the association between COBRA scores and moderate/vigorous activity (CI: 0.003 to −0.0002, Fig. 2b). These significant statistical mediations indicate that those with higher levels of moderate/vigorous physical activity had lower levels of depression, and in turn, better sQoL and subjective cognitive dysfunction.
3.3.2. Sedentary behaviour
Total sedentary behaviour scores were significantly and negatively associated with EUROHIS-QoL (β = −0.17), while mentally active sedentary behaviour scores were significantly and positively associated with EUROHIS-QoL scores (β = 0.10). Thus, poorer sQoL was reported by participants who spent more time sedentary overall, with the effect most pronounced in participants who spent less of this time in mentally active as opposed to mentally passive sedentary behaviours. DASS Depression scores significantly mediated the association between total sedentary behaviour and EUROHIS-QoL scores (partial mediation; CI: 0.18 to −0.05, Fig. 1c) and the association between mentally active sedentary behaviour and EUROHIS-QoL scores (full mediation; CI: 0.004–0.03, Fig. 1d). Total sedentary behaviour was also significantly positively associated with COBRA scores (β = 0.10), such that more time spent in sedentary behaviour was related to more subjective cognitive dysfunction. As seen in Fig. 2, DASS Depression scores significantly and fully mediated this association (CI: 0.008–0.03, Fig. 2c). DASS depression scores also mediated associations between COBRA scores and mentally active sedentary behaviour (CI: 0.005 to −0.0007, Fig. 2d). These significant statistical mediations indicate that those with higher levels of overall sedentary behaviour have higher levels of depression, and in turn, worse sQoL and subjective cognitive dysfunction. In contrast, those with higher levels of mentally active sedentary behaviour had lower levels of depression, and in turn, better sQoL and less subjective cognitive dysfunction.
4. Discussion
In this paper, we found that self-reported physical activity and sedentary behaviour levels did not differ by lockdown status. While somewhat counterintuitive, this finding is nonetheless consistent with prior research in the COLLATE sample indicating that initial pandemic-related reductions in the amount of exercise reported by participants at the outset of the pandemic (Phillipou et al., 2020) remained stable across later stages (Phillipou et al., 2021). While speculative, these data together suggest a possible feedback loop, in which reduced physical activity as a result of the initial lockdown may have reduced motivation to engage in activity going forward. Public health measures that stress the importance of maintaining adequate activity levels early in times of crisis might thus be useful.
We found that higher levels of physical activity generally, and a greater percentage of hours spent in moderate/vigorous activity (as opposed to light physical activity) specifically, were associated with more favourable sQoL, albeit modestly so. Several recent COVID-19 studies have demonstrated associations between higher levels of physical activity and more favourable QoL (Ozdemir et al., 2020; Qi, Li, Moyle, Weeks, & Jones, 2020; Slimani, Paravlic, Mbarek, Bragazzi, & Tod, 2020) and mental health (Jacob et al., 2020; Meyer et al., 2020; Qin et al., 2020; Schuch et al., 2020; Stanton et al., 2020). A substantial evidence base of non-COVID-19 research also shows associations of physical activity with both general and health-related QoL (Alsubaie, Alkathiry, Abdelbasset, & Nambi, 2020; Bize, Johnson, & Plotnikoff, 2007; Penedo & Dahn, 2005; Pucci et al., 2012; Puciato et al., 2017). One prior study found that moderate intensity activity, in comparison to walking or high intensity activity, was most strongly associated with health-related QoL (Alsubaie et al., 2020). When considered alongside the significant association of moderate/vigorous activity with sQoL in the current study, this finding raises the possibility that moderate activity could be the driving factor in the physical activity – QoL relationship. However, given the potential for those who engage only in light or in highly vigorous physical activity to have comorbid conditions that may directly affect sQoL, the relative importance of moderate/vigorous activity versus light physical activity to sQoL remains to be elucidated.
In line with the above findings, poorer sQoL and subjective cognitive dysfunction was reported by those with more hours of sedentary behaviour generally, though a greater percentage of time spent in mentally active sedentary behaviours and less time spent in mentally passive sedentary behaviours was associated with better sQoL. Although small in size, the latter association was notably evident despite physical activity levels being statistically controlled for in the analyses. These findings are consistent with recent meta-analytic data showing an inverse association between health-related QoL and sedentary behaviour (Boberska et al., 2018), as well as with findings showing specific associations of heightened passive sedentary behaviour with worse health related sQoL (Saunders et al., 2020). Although this is the first analysis of sedentary behaviour and subjective cognitive dysfunction, several papers have reported associations of physical inactivity with subjective cognition dysfunction (Felez-Nobrega et al., 2020; Nemoto et al., 2018; Omura et al., 2020; Zuniga et al., 2016). There is also evidence of an association between sedentary behaviour (typically measured by TV viewing) and objective cognitive function (Falck et al., 2017; Hoang et al., 2016; Olanrewaju et al., 2020; Wang et al., 2006), the latter of which is often predicted by level of subjective cognitive function (Lautenschlager et al., 2019; Liew, 2019; Mendonça et al., 2016; Reisberg et al., 2010).
The relationships between total sedentary behaviour and sQoL and between moderate/vigorous activity and sQoL were found to be direct but also partially mediated by depression severity. That is, the association of these variables with sQoL were partially related to their negative association with depression severity. This aligns with evidence linking increased sedentary behaviour and physical inactivity to depression (Aaron Kandola, Ashdown-Franks, Hendrikse, Sabiston, & Stubbs, 2019; Dinas, Koutedakis, & Flouris, 2011; Zhai, Zhang, & Zhang, 2015, pp. 705–709) and depression to low sQoL (Eric Josiah Tan & Rossell, 2016; Van Rheenen & Rossell, 2014). Depression severity also fully mediated the relationship between sedentary behaviour type and sQoL, and indirectly associated sedentary behaviour type with subjective cognitive dysfunction, such that less mentally active sedentary behaviour/more mentally passive sedentary behaviour associated with more severe depression, and this in turn, linked to poorer sQoL/subjective cognitive dysfunction. The latter indirect relationship was also true for moderate/vigorous activity. These findings are consistent with evidence that mentally active sedentary behaviour may have a protective effect, whereas mentally passive behaviour may contribute to increased depression risk (Hallgren et al., 2018, 2019), as well as research implicating subjective cognitive dysfunction as an adverse outcome of psychological distress (Hill et al., 2016).
The association of different types of sedentary behaviour and depression, subjective cognitive dysfunction and sQoL might be explained by the fact that mentally passive sedentary activities typically involve decreased social interaction and isolation. Depression and poor quality of life are widely recognised correlates of social isolation (Hawton et al., 2011; Moreno-Tamayo, Manrique-Espinoza, Ramírez-García, & Sánchez-García, 2020), with social isolation also having been associated with the ruminative behaviours and maladaptive cognitions that commonly characterise people who are depressed (Kovacs & Beck, 1978; Nolen-Hoeksema, 2000). In contrast, mentally active sedentary behaviour usually occurs within the context of educational attainment and employment, the latter of which having been associated with a sense of autonomy, belonging, and achievement as well as providing a platform from which interpersonal relationships can be developed (Modini et al., 2016; Van Dongen, 1996). Relevantly, depressed individuals often experience mental and physical inertia and are less likely to be employed (Amiri, 2021; Koval, Kuppens, Allen, & Sheeber, 2012). Hence, they may be less likely to engage in mentally active sedentary activities by nature of their symptoms/illness. It is possible that the cognitive demands of mentally active sedentary activities contribute to a cognitive reserve that can compensate, cognitively and emotionally, for the known adverse influence of passive sedentary behaviour and physical inactivity on physical health outcomes (Bouchard, Depres, & Tremblay, 1993; Cassidy, Chau, Catt, Bauman, & Trenell, 2017; Keadle et al., 2015). Indeed, poor physical health is commonly comorbid in depression (Glassman, 2007), and has been implicated in brain structural and functional decline (Gunstad et al., 2008), potentially via mechanistic pathways that include abnormal glycaemic regulation and lipid metabolism (Healy, Matthews, Dunstan, Winkler, & Owen, 2011; Henson et al., 2013; Morris, Vidoni, Honea, & Burns, 2014; Wheeler et al., 2017). In some neurological and psychiatric disorders, engagement in long-term mentally stimulating activities has been shown to confer resilience against the adverse effects of brain structural and functional decline on cognition (Sumowski, Wylie, Chiaravalloti, & DeLuca, 2010; Van Rheenen, Cropley, et al., 2020). Such mental engagement has also been shown to protect against adverse physical and psychological outcomes, for example, by reducing migraine chronicity (Gómez-Beldarrain, Anton-Ladislao, Aguirre-Larracoechea, Oroz, & García-Moncó, 2015; Gomez-Beldarrain et al., 2016) and minimising the adverse effect of pain on mental wellbeing (Delgado-Gallén et al., 2021).
There are several factors that should be considered when interpreting our findings. First, participants in the COLLATE surveys were self-selected and the sample used in the current study was restricted to a single Australian state. Thus, the sample is unlikely to be representative of the Australian population. Further, the cross-sectional nature of the findings meant only statistical associations between variables could be assessed, thus precluding inferences regarding causality. This is particularly pertinent in relation to the mediation analyses, as the cross-sectional data means we were unable to assume a temporal ordering of events. Second, physical activity and sedentary behaviour were not the primary focus of the COLLATE project. Although the physical activity questions we reported on were based loosely on the International Physical Activity Questionnaire (Craig et al., 2003), these questions and those relating to sedentary behaviour were not validated as they represented only a small part of the lengthy COLLATE surveys. Given this, they were not entirely comprehensive. For example, the questions did not differentiate between weekday and weekend time, and thus may be confounded by potential differences in activity levels between these times. Third, given all measures of interest in this study relied on self-report, there is potential they are biased by subjective recall. Finally, presence of mental illness (refer to Table S2 for breakdown of diagnoses), which was used as a covariate in several models, was subjectively reported and not clinically verified. Nonetheless, it should be noted that individuals self-identifying with a mental illness usually meet criteria for a psychiatric diagnosis (Kupfer et al., 2002; Sanchez-Villegas et al., 2008). Future research would do well to employ accelerometry data, alongside self-reported activity, in a longitudinal design.
In conclusion, our findings demonstrate that levels of physical activity and sedentary behaviour did not differ as a function of lockdown restrictions throughout the first year of the COVID-19 pandemic in Australia. Nonetheless, we observed a small but direct association of physical activity and sedentary behaviour with sQoL, and an indirect association of sedentary behaviour and moderate/vigorous activity with subjective cognitive dysfunction. Greater levels of mentally active and less mentally passive sedentary behaviours specifically, were modestly associated with increased sQoL and reduced subjective cognitive dysfunction (via a reduction of depression). These findings add to a small but growing literature showing associations of mentally passive sedentary behaviours with greater psychological distress and worse health-related sQoL. Taken together, these findings highlight important differences between mentally passive and active sedentary behaviours and their psychological correlates and provide evidence that public health promotion strategies should focus not only on increasing physical activity but also reducing passive sedentary behaviours as a means of maintaining good psychological health. Future research should aim to formally validate mentally active and passive subtypes of sedentary behaviour, and to explore the effects of variability within these subtypes (i.e., leisure versus occupational activities) on psychological health.
Authors contributions
DM, EN, AP, EJT, WLT, PJS, SLR, and TVR designed the study and collected the data; ER performed the statistical analysis and wrote the first draft of the manuscript; All authors contributed to the interpretation of results and re-drafting of the manuscript; All authors have read and approved the final version of the manuscript and agree with the order of presentation of the authors.
Competing Interests:
Mats Hallgren is currently an associate editor at Mental Health and Physical Activity. The remaining author's declare they have no competing interests.
Role of the funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Contents of this article are the sole responsibility of the authors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank all the participants who took thetime and effort to take part in this study, especially during these challenging and unprecedented times. DD (GNT1078360), NO (GNT1003960), and SLR (GNT1154651) hold a NHMRC Senior Fellowship, and EJT (GNT1142424) and TVR (GNT1088785) hold NHMRC Early Career Fellowships. WLT (GNT1161609) and AP (GNT1159953) are supported by NHMRC New Investigator Project Grants.
Footnotes
Given the absence of association between physical activity or sedentary behaviour and lockdown status, and this variable was not included or controlled for in subsequent analyses.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.mhpa.2022.100481.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Alcock A. Stata Press; 2014. A gentle introduction to stata. [Google Scholar]
- Alsubaie S.F., Alkathiry A.A., Abdelbasset W.K., Nambi G. BioMed Research International; 2020. The physical activity type most related to cognitive function and quality of life. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri S. Unemployment associated with major depression disorder and depressive symptoms: A systematic review and meta-analysis. International Journal of Occupational Safety and Ergonomics. 2021;1 doi: 10.1080/10803548.2021.1954793. –13. [DOI] [PubMed] [Google Scholar]
- Bangsbo J., Blackwell J., Boraxbekk C.J., Caserotti P., Dela F., Evans A.B., et al. Copenhagen Consensus statement 2019: Physical activity and ageing. British Journal of Sports Medicine. 2019;53(14):856–858. doi: 10.1136/bjsports-2018-100451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bize R., Johnson J.A., Plotnikoff R.C. Physical activity level and health-related quality of life in the general adult population: A systematic review. Preventive Medicine. 2007;45(6):401–415. doi: 10.1016/j.ypmed.2007.07.017. [DOI] [PubMed] [Google Scholar]
- Boberska M., Szczuka Z., Kruk M., Knoll N., Keller J., Hohl D.H., et al. Sedentary behaviours and health-related quality of life. A systematic review and meta-analysis. Health Psychology Review. 2018;12(2):195–210. doi: 10.1080/17437199.2017.1396191. [DOI] [PubMed] [Google Scholar]
- Bouchard C., Depres J.-P., Tremblay A. Exercise and obesity. Obesity Research. 1993;1(2):133–147. doi: 10.1002/j.1550-8528.1993.tb00603.x. [DOI] [PubMed] [Google Scholar]
- Carson V., Kuzik N., Hunter S., Wiebe S.A., Spence J.C., Friedman A., et al. Systematic review of sedentary behavior and cognitive development in early childhood. Preventive Medicine. 2015;78:115–122. doi: 10.1016/j.ypmed.2015.07.016. [DOI] [PubMed] [Google Scholar]
- Cassidy S., Chau J.Y., Catt M., Bauman A., Trenell M.I. Low physical activity, high television viewing and poor sleep duration cluster in overweight and obese adults; a cross-sectional study of 398,984 participants from the UK Biobank. International Journal of Behavioral Nutrition and Physical Activity. 2017;14(1):57. doi: 10.1186/s12966-017-0514-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castañeda-Babarro A., Coca A., Arbillaga-Etxarri A., Gutiérrez-Santamaría B. Physical activity change during COVID-19 confinement. International Journal of Environmental Research and Public Health. 2020;17(18):1–10. doi: 10.3390/ijerph17186878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig C.L., Marshall A.L., Sjöström M., Bauman A.E., Booth M.L., Ainsworth B.E., et al. International physical activity questionnaire: 12-Country reliability and validity. Medicine & Science in Sports & Exercise. 2003 doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- Delgado-Gallén S., Soler M.D., Albu S., Pachón-García C., Alviárez-Schulze V., Solana-Sánchez J., et al. Cognitive reserve as a protective factor of mental health in middle-aged adults affected by chronic pain. Frontiers in Psychology. 2021;12 doi: 10.3389/fpsyg.2021.752623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinas P.C., Koutedakis Y., Flouris A.D. Effects of exercise and physical activity on depression. Irish Journal of Medical Science. 2011;180(2):319–325. doi: 10.1007/s11845-010-0633-9. [DOI] [PubMed] [Google Scholar]
- Falck R.S., Davis J.C., Liu-Ambrose T. What is the association between sedentary behaviour and cognitive function? A systematic review. British Journal of Sports Medicine. 2017;51(10):800–811. doi: 10.1136/bjsports-2015-095551. [DOI] [PubMed] [Google Scholar]
- Felez-Nobrega M., Haro J.M., Erickson K.I., Koyanagi A. Physical activity is associated with fewer subjective cognitive complaints in 47 low- and middle-income countries. Journal of the American Medical Directors Association. 2020;21(10):1423–1429. doi: 10.1016/j.jamda.2020.02.014. e2. [DOI] [PubMed] [Google Scholar]
- Gjaka M., Feka K., Bianco A., Tishukaj F., Giustino V., Parroco A.M., et al. The effect of COVID-19 lockdown measures on physical activity levels and sedentary behaviour in a relatively young population living in Kosovo. Journal of Clinical Medicine. 2021;10(4) doi: 10.3390/jcm10040763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glassman A.H. Depression and cardiovascular comorbidity. Dialogues in Clinical Neuroscience. 2007;9(1):9–17. doi: 10.31887/DCNS.2007.9.1/ahglassman. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Beldarrain M., Anton-Ladislao A., Aguirre-Larracoechea U., Oroz I., García-Moncó J.C. Low cognitive reserve is associated with chronic migraine with medication overuse and poor quality of life. Cephalalgia. 2015;35(8):683–691. doi: 10.1177/0333102414553822. [DOI] [PubMed] [Google Scholar]
- Gomez-Beldarrain M., Oroz I., Zapirain B.G., Ruanova B.F., Fernandez Y.G., Cabrera A., et al. Right fronto-insular white matter tracts link cognitive reserve and pain in migraine patients. The Journal of Headache and Pain. 2016;17(1):4. doi: 10.1186/s10194-016-0593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunstad J., Paul R.H., Cohen R.A., Tate D.F., Spitznagel M.B., Grieve S., et al. Relationship between body mass index and brain volume in healthy adults. International Journal of Neuroscience. 2008;118(11):1582–1593. doi: 10.1080/00207450701392282. [DOI] [PubMed] [Google Scholar]
- Hallgren M., Dunstan D.W., Owen N. Passive versus mentally active sedentary behaviors and depression. Exercise and Sport Sciences Reviews. 2020;48(1):20–27. doi: 10.1249/JES.0000000000000211. [DOI] [PubMed] [Google Scholar]
- Hallgren M., Nguyen T., Owen N., Stubbs B., Vancampfort D., Lundin A., et al. Cross-sectional and prospective relationships of passive and mentally active sedentary behaviours and physical activity with depression. The British Journal of Psychiatry. 2019:1–7. doi: 10.1192/bjp.2019.60. [DOI] [PubMed] [Google Scholar]
- Hallgren M., Nguyen T.-T.-D., Owen N., Stubbs B., Vancampfort D., Lundin A., et al. Cross-sectional and prospective relationships of passive and mentally active sedentary behaviours and physical activity with depression. The British Journal of Psychiatry. 2020;217(2):413–419. doi: 10.1192/bjp.2019.60. [DOI] [PubMed] [Google Scholar]
- Hallgren M., Nguyen T., Owen N., Vancampfort D., Dunstan D.W., Wallin P., et al. Associations of sedentary behavior in leisure and occupational contexts with symptoms of depression and anxiety. Preventive Medicine. 2020;133(September 2019) doi: 10.1016/j.ypmed.2020.106021. [DOI] [PubMed] [Google Scholar]
- Hallgren M., Owen N., Stubbs B., Zeebari Z., Vancampfort D., Schuch F., et al. Passive and mentally-active sedentary behaviors and incident major depressive disorder: A 13-year cohort study. Journal of Affective Disorders. 2018;241(July):579–585. doi: 10.1016/j.jad.2018.08.020. [DOI] [PubMed] [Google Scholar]
- Hawton A., Green C., Dickens A.P., Richards S.H., Taylor R.S., Edwards R., et al. The impact of social isolation on the health status and health-related quality of life of older people. Quality of Life Research. 2011;20(1):57–67. doi: 10.1007/s11136-010-9717-2. [DOI] [PubMed] [Google Scholar]
- Healy G.N., Matthews C.E., Dunstan D.W., Winkler E.A.H., Owen N. Sedentary time and cardio-metabolic biomarkers in US adults: Nhanes 200306. European Heart Journal. 2011;32(5):590–597. doi: 10.1093/eurheartj/ehq451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson J., Yates T., Biddle S.J.H., Edwardson C.L., Khunti K., Wilmot E.G., et al. Associations of objectively measured sedentary behaviour and physical activity with markers of cardiometabolic health. Diabetologia. 2013;56(5):1012–1020. doi: 10.1007/s00125-013-2845-9. [DOI] [PubMed] [Google Scholar]
- Hill N.L., Mcdermott C., Mogle J., Munoz E., Depasquale N., Wion R., et al. Subjective cognitive impairment and quality of life: A systematic review. International Psychogeriatrics. 2017;29(12):1965–1977. doi: 10.1017/S1041610217001636. [DOI] [PubMed] [Google Scholar]
- Hill N.L., Mogle J., Wion R., Munoz E., Depasquale N., Yevchak A.M., et al. Subjective cognitive impairment and affective symptoms : A systematic review. The Gerontologist. 2016;56(6):109–127. doi: 10.1093/geront/gnw091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoang T.D., Reis J., Zhu N., Jacobs D.R., Launer L.J., Whitmer R.A., et al. Effect of early adult patterns of physical activity and television viewing on midlife cognitive function. JAMA Psychiatry. 2016;73(1):73. doi: 10.1001/jamapsychiatry.2015.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob L., Tully M.A., Barnett Y., Lopez-Sanchez G.F., Butler L., Schuch F., et al. The relationship between physical activity and mental health in a sample of the UK public: A cross-sectional study during the implementation of COVID-19 social distancing measures. Mental Health and Physical Activity. 2020;19:1–5. doi: 10.1016/j.mhpa.2020.100345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josefsson T., Lindwall M., Archer T. Physical exercise intervention in depressive disorders: Meta-analysis and systematic review. Scandinavian Journal of Medicine & Science in Sports. 2014;24(2):259–272. doi: 10.1111/sms.12050. [DOI] [PubMed] [Google Scholar]
- Kandola A., Ashdown-Franks G., Hendrikse J., Sabiston C.M., Stubbs B. Physical activity and depression: Towards understanding the antidepressant mechanisms of physical activity. Neuroscience & Biobehavioral Reviews. 2019;107:525–539. doi: 10.1016/j.neubiorev.2019.09.040. August. [DOI] [PubMed] [Google Scholar]
- Kandola A., Owen N., Dunstan D.W., Hallgren M. Prospective relationships of adolescents' screen-based sedentary behaviour with depressive symptoms: The millennium cohort study. Psychological Medicine. 2021:1–9. doi: 10.1017/S0033291721000258. [DOI] [PubMed] [Google Scholar]
- Kane R.L., Butler M., Fink H.A. Interventions to prevent age-related cognitive decline, mild cognitive impairment, and clinical Alzheimer’s-type dementia. Comparative Effectiveness Reviews. 2017;188(188):86–91. www.effectivehealthcare.ahrq.gov/reports/final.cfm.%0Ahttp://www.effectivehealthcare.ahrq.gov/index.cfm/search-for-guides-reviews-and-reports/?pageaction=displayproduct&productid=2417 Retrieved from. [PubMed] [Google Scholar]
- Keadle S.K., Moore S.C., Sampson J.N., Xiao Q., Albanes D., Matthews C.E. Causes of death associated with prolonged TV viewing. American Journal of Preventive Medicine. 2015;49(6):811–821. doi: 10.1016/j.amepre.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi H., Inoue S., Sugiyama T., Owen N., Oka K., Nakaya T., et al. Distinct associations of different sedentary behaviors with health-related attributes among older adults. Preventive Medicine. 2014;67:335–339. doi: 10.1016/j.ypmed.2014.08.011. [DOI] [PubMed] [Google Scholar]
- Kirk-Sanchez N.J., McGough E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clinical Interventions in Aging. 2013;9:51–62. doi: 10.2147/CIA.S39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs M., Beck A.T. Maladaptive cognitive structures in depression. American Journal of Psychiatry. 1978;135(5):525–533. doi: 10.1176/ajp.135.5.525. [DOI] [PubMed] [Google Scholar]
- Koval P., Kuppens P., Allen N.B., Sheeber L. Getting stuck in depression: The roles of rumination and emotional inertia. Cognition & Emotion. 2012;26(8):1412–1427. doi: 10.1080/02699931.2012.667392. [DOI] [PubMed] [Google Scholar]
- Krogh J., Nordentoft M., Sterne J.A.C., Lawlor D.A. The effect of exercise in clinically depressed adults. Journal of Clinical Psychiatry. 2011;72:529–538. doi: 10.4088/JCP.08r04913blu. 04. [DOI] [PubMed] [Google Scholar]
- Kupfer D.J., Frank E., Grochocinski V.J., Cluss P.A., Houck P.R., Stapf D.A. Demographic and clinical characteristics of individuals in a bipolar disorder case registry. Journal of Clinical Psychiatry. 2002;63(2):120–125. doi: 10.4088/jcp.v63n0206. [DOI] [PubMed] [Google Scholar]
- Lautenschlager N.T., Cox K.L., Ellis K.A. Physical activity for cognitive health: What advice can we give to older adults with subjective cognitive decline and mild cognitive impairment. Dialogues in Clinical Neuroscience. 2019;21(1):61–68. doi: 10.31887/DCNS.2019.21.1/nlautenschlager. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew T.M. Depression, subjective cognitive decline, and the risk of neurocognitive disorders. Alzheimer's and Dementia. 2019;11(70) doi: 10.1186/s13195-019-0527-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovibond P., Lovibond S. The structure of negative emotional states: Comparison of the depression anxiety stress scales (DASS) with the Beck depression and anxiety inventories. Behaviour Research and Therapy. 1995;33(3):335–343. doi: 10.1016/0005-7967(94)00075-U. [DOI] [PubMed] [Google Scholar]
- Luo X., Yinghua Z., Dali L., Kunlun Z., Lin X. Subjective cognitive dysfunction in patients with bipolar disorder: The prevalence, related factors and eff ects on predicting psychosocial functioning and suicidal ideation. Psychiatry Research. 2020;284(74) doi: 10.1016/j.psychres.2019.112669. [DOI] [PubMed] [Google Scholar]
- Mendonça M.D., Alves L., Bugalho P. From subjective cognitive complaints to dementia. American Journal of Alzheimer's Disease and Other Dementias. 2016;31(2):105–114. doi: 10.1177/1533317515592331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J., McDowell C., Lansing J., Brower C., Smith L., Tully M., et al. Changes in physical activity and sedentary behavior in response to covid-19 and their associations with mental health in 3052 us adults. International Journal of Environmental Research and Public Health. 2020;17(18):1–13. doi: 10.3390/ijerph17186469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modini M., Joyce S., Mykletun A., Christensen H., Bryant R.A., Mitchell P.B., et al. The mental health benefits of employment: Results of a systematic meta-review. Australasian Psychiatry. 2016;24(4):331–336. doi: 10.1177/1039856215618523. [DOI] [PubMed] [Google Scholar]
- Moreno-Tamayo K., Manrique-Espinoza B., Ramírez-García E., Sánchez-García S. Social isolation undermines quality of life in older adults. International Psychogeriatrics. 2020;32(11):1283–1292. doi: 10.1017/S1041610219000310. [DOI] [PubMed] [Google Scholar]
- Morris J.K., Vidoni E.D., Honea R.A., Burns J.M. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiology of Aging. 2014;35(3):585–589. doi: 10.1016/j.neurobiolaging.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill E., Meyer D., Toh W.L., van Rheenen T.E., Phillipou A., Tan E.J., et al. Alcohol use in Australia during the early days of the COVID-19 pandemic: Initial results from the COLLATE project. Psychiatry and Clinical Neurosciences. 2020;74(10):542–549. doi: 10.1111/pcn.13099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto Y., Sato S., Takahashi M., Takeda N., Matsushita M., Kitabatake Y., et al. The association of single and combined factors of sedentary behavior and physical activity with subjective cognitive complaints among community-dwelling older adults: Cross-sectional study. PLoS One. 2018;13(4) doi: 10.1371/journal.pone.0195384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S. The role of rumination in depressive disorders and mixed anxiety/depressive symptoms. Journal of Abnormal Psychology. 2000;109(3):504–511. doi: 10.1037/0021-843X.109.3.504. [DOI] [PubMed] [Google Scholar]
- Nowak P.F., Bożek A., Blukacz M. Physical activity, sedentary behavior, and quality of life among university students. BioMed Research International. 2019;1–10 doi: 10.1155/2019/9791281. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanrewaju O., Stockwell S., Stubbs B., Smith L. Sedentary behaviours, cognitive function, and possible mechanisms in older adults: A systematic review. Aging Clinical and Experimental Research. 2020;32(6):969–984. doi: 10.1007/s40520-019-01457-3. [DOI] [PubMed] [Google Scholar]
- Omura J.D., Brown D.R., McGuire L.C., Taylor C.A., Fulton J.E., Carlson S.A. Cross-sectional association between physical activity level and subjective cognitive decline among US adults aged ≥45 years. Preventive Medicine. 2020;141(October) doi: 10.1016/j.ypmed.2020.106279. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott C.V., Bjertrup A.J., Jensen J.H., Ullum H., Sjælland R., Purdon S.E., et al. Screening for cognitive dysfunction in unipolar depression: Validation and evaluation of objective and subjective tools. Journal of Affective Disorders. 2016;190:607–615. doi: 10.1016/j.jad.2015.10.059. [DOI] [PubMed] [Google Scholar]
- Ozdemir F., Cansel N., Kizilay F., Guldogan E., Ucuz I., Sinanoglu B., et al. The role of physical activity on mental health and quality of life during COVID-19 outbreak: A cross-sectional study. European Journal of Integrative Medicine. 2020;40 doi: 10.1016/j.eujim.2020.101248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penedo F.J., Dahn J.R. Exercise and well-being: A review of mental and physical health benefits associated with physical activity. Current Opinion in Psychiatry. 2005;18(2):189–193. doi: 10.1097/00001504-200503000-00013. [DOI] [PubMed] [Google Scholar]
- Phillipou A., Meyer D., Neill E., Tan E.J., Toh W.L., Van Rheenen T.E., et al. Eating and exercise behaviors in eating disorders and the general population during the COVID-19 pandemic in Australia: Initial results from the COLLATE project. International Journal of Eating Disorders. 2020;53(7):1158–1165. doi: 10.1002/eat.23317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillipou A., Tan E.J., Toh W.L., Van Rheenen T.E., Meyer D., Neill E., et al. Mental health of individuals with and without eating disorders across six months and two waves of COVID-19. Eating Behaviors. 2021;43(August) doi: 10.1016/j.eatbeh.2021.101564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pucci G.C.M.F., Rech C.R., Fermino R.C., Reis R.S. Association between physical activity and quality of life in adults. Revista de Saúde Pública. 2012;46(1):166–179. doi: 10.1590/S0034-89102012000100021. [DOI] [PubMed] [Google Scholar]
- Puciato D., Borysiuk Z., Rozpara M. Quality of life and physical activity in an older working-age population. Clinical Interventions in Aging. 2017;12:1627–1634. doi: 10.2147/CIA.S144045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M., Li P., Moyle W., Weeks B., Jones C. Physical activity, health-related quality of life, and stress among the Chinese adult population during the COVID-19 pandemic. International Journal of Environmental Research and Public Health. 2020;17(18):6494. doi: 10.3390/ijerph17186494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin F., Song Y., Nassis G.P., Zhao L., Cui S., Lai L., et al. Prevalence of insufficient physical activity, sedentary screen time and emotional well-being during the early days of the 2019 novel coronavirus (COVID-19) outbreak in China: A national cross-sectional study. SSRN Electronic Journal. 2020 doi: 10.2139/ssrn.3566176. [DOI] [Google Scholar]
- Reisberg B., Shulman M.B., Torossian C., Leng L., Zhu W. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimer's and Dementia. 2010;6(1) doi: 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa A., Mercade C., Sanchez-Moreno J., Sole B., del Mar Bonnin C., Torrent C., et al. Validity and reliability of a rating scale on subjective cognitive deficits in bipolar disorder (COBRA) Journal of Affective Disorders. 2013;150(1):29–36. doi: 10.1016/j.jad.2013.02.022. [DOI] [PubMed] [Google Scholar]
- Rossell S.L., Neill E., Phillipou A., Tan E.J., Toh W.L., Van Rheenen T.E., et al. An overview of current mental health in the general population of Australia during the COVID-19 pandemic: Results from the COLLATE project. Psychiatry Research. 2021;296 doi: 10.1016/j.psychres.2020.113660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Villegas A., Schlatter J., Ortuno F., Lahortiga F., Pla J., Benito S., et al. Validity of a self-reported diagnosis of depression among participants in a cohort study using the Structured Clinical Interview for DSM-IV (SCID-I) BMC Psychiatry. 2008;8:1–8. doi: 10.1186/1471-244X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders T.J., McIsaac T., Douillette K., Gaulton N., Hunter S., Rhodes R.E., et al. Sedentary behaviour and health in adults: An overview of systematic reviews. Applied Physiology Nutrition and Metabolism. 2020;45(10):S197–S217. doi: 10.1139/apnm-2020-0272. [DOI] [PubMed] [Google Scholar]
- Schmidt S., Muehlan H., Power M. The EUROHIS-QOL 8-item index: Psychometric results of a cross-cultural field study. The European Journal of Public Health. 2006;16(4):420–428. doi: 10.1093/eurpub/cki155. [DOI] [PubMed] [Google Scholar]
- Schuch F.B., Bulzing R.A., Meyer J., Vancampfort D., Firth J., Stubbs B., et al. Associations of moderate to vigorous physical activity and sedentary behavior with depressive and anxiety symptoms in self-isolating people during the COVID-19 pandemic: A cross-sectional survey in Brazil. Psychiatry Research. 2020;292 doi: 10.1016/j.psychres.2020.113339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slimani M., Paravlic A., Mbarek F., Bragazzi N.L., Tod D. The relationship between physical activity and quality of life during the confinement induced by COVID-19 outbreak: A pilot study in Tunisia. Frontiers in Psychology. 2020;11 doi: 10.3389/fpsyg.2020.01882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanton R., To Q.G., Khalesi S., Williams S.L., Alley S.J., Thwaite T.L., et al. Depression, anxiety and stress during COVID-19: Associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. International Journal of Environmental Research and Public Health. 2020;17(11):1–13. doi: 10.3390/ijerph17114065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi T., Watanabe K., Noto S., Sakamoto S., Moriguchi Y., Hammer-Helmich L., et al. Relationship of subjective cognitive impairment with psychosocial function and relapse of depressive symptoms in patients with major depressive disorder: Analysis of longitudinal data from perform-j. Neuropsychiatric Disease and Treatment. 2021;17:945–955. doi: 10.2147/NDT.S288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumiyoshi T., Watanabe K., Noto S., Sakamoto S., Moriguchi Y., Tan K.H.X., et al. Relationship of cognitive impairment with depressive symptoms and psychosocial function in patients with major depressive disorder: Cross–sectional analysis of baseline data from PERFORM-J. Journal of Affective Disorders. 2019;258(July):172–178. doi: 10.1016/j.jad.2019.07.064. [DOI] [PubMed] [Google Scholar]
- Sumowski J.F., Wylie G.R., Chiaravalloti N., DeLuca J. Intellectual enrichment lessens the effect of brain atrophy on learning and memory in multiple sclerosis. Neurology. 2010;74(24):1942–1945. doi: 10.1212/WNL.0b013e3181e396be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E.J., Meyer D., Neill E., Phillipou A., Toh W.L., Van Rheenen T.E., et al. Considerations for assessing the impact of the COVID-19 pandemic on mental health in Australia. Australian and New Zealand Journal of Psychiatry. 2020;54(11) doi: 10.1177/0004867420947815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan E.J., Rossell S.L. Comparing how co-morbid depression affects individual domains of functioning and life satisfaction in schizophrenia. Comprehensive Psychiatry. 2016;66:53–58. doi: 10.1016/j.comppsych.2015.12.007. [DOI] [PubMed] [Google Scholar]
- Toh W.L., Meyer D., Phillipou A., Tan E.J., Van Rheenen T.E., Neill E., et al. Mental health status of healthcare versus other essential workers in Australia amidst the COVID-19 pandemic: Initial results from the collate project. Psychiatry Research. 2021;298(July 2020) doi: 10.1016/j.psychres.2021.113822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyoshima K., Inoue T., Masuya J., Ichiki M., Fujimura Y., Kusumi I. Evaluation of subjective cognitive function using the cognitive complaints in bipolar disorder rating Assessment (COBRA) in Japanese adults. Neuropsychiatric Disease and Treatment. 2019;15:2981–2990. doi: 10.2147/NDT.S218382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M.S., Aubert S., Barnes J.D., Saunders T.J., Carson V., Latimer-Cheung A.E., et al. Sedentary behavior research network (SBRN) - terminology consensus project process and outcome. International Journal of Behavioral Nutrition and Physical Activity. 2017 doi: 10.1186/s12966-017-0525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tun P.A., Lachman M.E. The association between computer use and cognition across adulthood: Use it so you won't lose it? Psychology and Aging. 2010;25(3):560–568. doi: 10.1037/a0019543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dongen C.J. Quality of life and self-esteem in working and nonworking persons with mental illness. Community Mental Health Journal. 1996;32(6):535–548. doi: 10.1007/BF02251064. [DOI] [PubMed] [Google Scholar]
- Van Rheenen T.E., Cropley V., Fagerlund B., Wannan C., Bruggemann J., Lenroot R.K., et al. Cognitive reserve attenuates age-related cognitive decline in the context of putatively accelerated brain ageing in schizophrenia-spectrum disorders. Psychological Medicine. 2020;50(9):1475–1489. doi: 10.1017/S0033291719001417. [DOI] [PubMed] [Google Scholar]
- Van Rheenen T.E., Meyer D., Neill E., Phillipou A., Tan E.J., Toh W.L., et al. Mental health status of individuals with a mood-disorder during the COVID-19 pandemic in Australia: Initial results from the COLLATE project. Journal of Affective Disorders. 2020;275(June):69–77. doi: 10.1016/j.jad.2020.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rheenen T.E., Rossell S.L. Objective and subjective psychosocial functioning in bipolar disorder: An investigation of the relative importance of neurocognition, social cognition and emotion regulation. Journal of Affective Disorders. 2014;162:134–141. doi: 10.1016/j.jad.2014.03.043. [DOI] [PubMed] [Google Scholar]
- Voss M.W., Nagamatsu L.S., Liu-Ambrose T., Kramer A.F. Exercise, brain, and cognition across the life span. Journal of Applied Physiology. 2011;111(5):1505–1513. doi: 10.1152/japplphysiol.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J.Y.J., Zhou D.H.D., Li J., Zhang M., Deng J., Tang M., et al. Leisure activity and risk of cognitive impairment: The Chongqing aging study. Neurology. 2006;66(6):911–913. doi: 10.1212/01.wnl.0000192165.99963.2a. [DOI] [PubMed] [Google Scholar]
- Wheeler M.J., Dempsey P.C., Grace M.S., Ellis K.A., Gardiner P.A., Green D.J., et al. Sedentary behavior as a risk factor for cognitive decline? A focus on the influence of glycemic control in brain health. Alzheimer's and Dementia: Translational Research & Clinical Interventions. 2017;3(3):291–300. doi: 10.1016/j.trci.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff S.J., Coyne P., St-Pierre E. Stress, physical activity, and screen-related sedentary behaviour within the first month of the COVID-19 pandemic. Applied Psychology: Health and Well-Being. 2021;13(2):454–468. doi: 10.1111/aphw.12261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman R., Hankir A., Jemni M. Lifestyle factors and mental health. Psychiatria Danubina. 2019;31:217–220. [PubMed] [Google Scholar]
- Zhai L., Zhang Y., Zhang D. 2015. Sedentary behaviour and the risk of depression : A meta-analysis; pp. 705–709. [DOI] [PubMed] [Google Scholar]
- Zhao C., Noble J.M., Marder K., Hartman J.S., Gu Y., Scarmeas N. Dietary patterns, physical activity, sleep, and risk for dementia and cognitive decline. Current Nutrition Reports. 2018;7(4):335–345. doi: 10.1007/s13668-018-0247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga K.E., Mackenzie M.J., Kramer A., McAuley E. Subjective memory impairment and well-being in community-dwelling older adults. Psychogeriatrics. 2016;16(1):20–26. doi: 10.1111/psyg.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.