Abstract
BACKGROUND
Superficial hemosiderosis (SS) of the central nervous system is a rare condition that is caused by chronic, repeated hemorrhage into the subarachnoid space. The subsequent deposition of hemosiderin in the brain and spinal cord causes neurological deterioration. In this report, the authors describe a repair procedure for SS associated with a dural defect in the thoracic spine.
OBSERVATIONS
A 75-year-old man presented with tinnitus symptoms that began about 1 year prior. Subsequently, his hearing loss progressed, and he gradually became unsteady on walking. Magnetic resonance imaging (MRI) of the head showed diffuse hemosiderin deposition on the surface of the cerebellum. Thoracic MRI showed ventral cerebrospinal fluid leakage of T2–7, and computed tomography myelography showed leakage of contrast medium that appeared to be a dural defect. Dural closure was successful, and MRI showed decreased fluid collection ventral to the dura. The patient’s symptoms of wobbliness on walking and tinnitus improved dramatically from the postoperative period.
LESSONS
Dural abnormalities of the spine must always be considered as one of the causes of SS. Early dural closure is an effective means of preventing the progression of symptoms.
Keywords: superficial siderosis, dural defect, bleeding source, dural closure, ventral CSF leakage
ABBREVIATIONS : CSF = cerebrospinal fluid, CT = computed tomography, FIESTA = fast imaging employing steady-state acquisition, MRI = magnetic resonance imaging, SS = superficial hemosiderosis
Kumar1 proposed the concept of duropathies for diseases that cause neurological symptoms due to defects or damage to the dura mater in the spinal canal. Duropathies are considered to be a group of diseases mainly caused by defects or damage to the anterior dura mater of the spinal canal and abnormalities in the arachnoid membrane, resulting in leakage of spinal fluid out of the dura mater. The mechanism is spinal fluid leakage occurs at the site of dural defect, intracranial spinal fluid decreases, and abnormalities of the epidural venous plexus in the spinal canal cause hemorrhage from the venous plexus into the subarachnoid space, resulting in hemosiderin deposition on the brain surface. Superficial hemosiderosis (SS) is a relatively rare disease, and the classic type is characterized by hemosiderin deposits in the brainstem and cerebellum, cerebellar ataxia, hearing loss, and sometimes myelopathy. Here, we report a case of SS with a dural defect in the upper thoracic spine in which the dural defect was successfully closed. We also discuss the essential points of preoperative imaging leading to the diagnosis, as well as the details of the actual repair procedure.
Illustrative Case
A 75-year-old man with no history of surgery or trauma had tinnitus and hearing loss that had progressed gradually over the past year. At the same time as the hearing loss, the patient became aware of lightheadedness when walking. Magnetic resonance imaging (MRI) showed diffuse surface hemosiderin deposition mainly on the superior surface of the cerebellum and atrophy of the cerebellum (Fig. 1). Thoracic T2-weighted MRI showed cerebrospinal fluid (CSF) collection ventral to the dura at the T2–7 level, and thoracic spine computed tomography (CT) showed osteophytes at the T2–3 level. CT myelography showed contrast exposure to the extradural ventral side of T2 to the T7 level and contrast continuity on the right ventral side of T2–3, where osteophytes were present (Fig. 2), suggesting a dural defect. Fast imaging employing steady-state acquisition (FIESTA) MRI sequences revealed a ventral dural defect at T2–3 (Fig. 3), which was determined to be the site of the causative dural defect. The patient underwent T2–3 recapping laminoplasty; the site of the dural defect was confirmed by a transdural approach; and a slight posthemorrhagic scar was observed epidurally (Fig. 4). The defect hole was also identified extradurally, and a portion of the dural defect was sutured and covered with DuraGen (Integra LifeSciences), both inside and outside the dura mater, to provide closure reinforcement. Postoperatively, the patient’s tinnitus and wobbling sensation on walking disappeared, but his hearing loss remained unchanged. A follow-up thoracic MRI 6 months later showed the disappearance of CSF collection at the ventral epidural level of the T4–7 vertebral levels.
FIG. 1.

Head T2* MRI shows hemosiderin deposition on the superior surface of the cerebellum and an atrophic cerebellum. A: Midbrain level and under the cerebellar tent. B: Pons level.
FIG. 2.

Sagittal (A) and axial (B, at T2) CT myelography reveals contrast leakage on the ventral side of the right dura mater at T2 on the cranial side, very near the T2–3 osteophyte. Red arrow indicates the location of contrast leakage.
FIG. 3.
FIESTA MRI shows CSF collection ventral to the epidural of the T2–7 vertebrae, with the finding of a dural defect on the right side at T2–3. Sagittal T2-weighted image (A) and axial images from the T2 to the T7 level (B–G), respectively. Red arrows indicate epidural CSF collection.
FIG. 4.
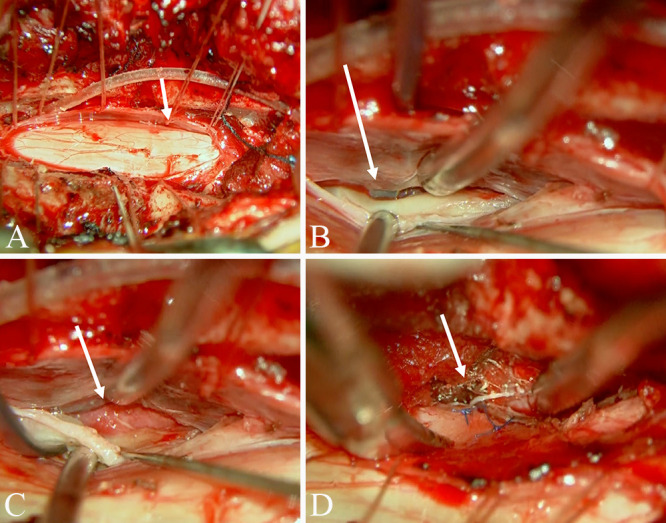
Intraoperative microscopy findings. Dural incision revealed a dural defect on the ventral right side of the spinal cord at the T2–3 level (A). A dissector inserted from the epidural space was observed from within the dura (B). The epidural space with dural defect shows red scar tissue that appears to be posthemorrhagic (C). The defective dura mater was primarily sutured with nylon thread (D). White arrows indicate dural defect.
Discussion
Observations
SS in the central nervous system is a relatively rare, chronic, progressive condition caused by repeated hemorrhages into the subarachnoid space. SS can be divided into brainstem-cerebellar and cortical types. The latter is thought to be associated with cerebral amyloid angiopathy.2 The former brainstem-cerebellar type is mostly due to hemosiderin deposition with dural defects and is characterized by prominent hemosiderin deposition in the cerebellum and brainstem, Wilson et al.3 reported that 83% (40 of 48) had duropathies. The symptoms of the brainstem-cerebellar form are as follows. To summarize previous reports, the common clinical manifestations are hearing loss (95%), progressive cerebellar ataxia (88%), and pyramidal tract signs (76%).4–6 Deposition of hemosiderin under the cerebellar tent gradually leads to cerebellar atrophy, which is easily detected on MRI sagittal images, as has been reported previously.7 Since Kumar et al.8 first reported surgical repair of this defect, the primary focus in the treatment of this disease has been to precisely identify the dural defect. Kumar et al.8 recommended the use of dynamic CT myelography to identify the lesion. With the development of MRI technology for CSF cisternography, diagnostic tools have increased. Egawa et al.9 described two cases of dural defects identified using the constructive interference in steady state sequence, and Takai et al.10 described five of seven SS cases in the high thoracic level (T1–4) that could be identified using a FIESTA (MRI) sequence. In the present study, the patient also had hearing impairment and cerebellar ataxia. In addition to these characteristic symptoms, the presence of hemosiderin deposition, especially in the cerebellum and brainstem, and cerebellar atrophy on head MRI should raise strong suspicions of this disease. Compared with previous reports, the main features of this case were no hemosiderin deposition on the spinal cord surface on MRI and no pyramidal tract signs present preoperatively. The disease concept of duropathies proposed by Kumar1 includes SS with dural defects; when dural defects are evident on radiographic imaging, conservative treatment is considered ineffective, and dural closure is mandatory. Although several studies have reported conservative treatment such as corticosteroids,11 currently the most reliable treatment for duropathy with SS is repair of the dural defect. Takai et al.12 reported that repair of the dura decreases epidural spinal fluid collection, resulting in the cessation of chronic hemorrhage. However, in this condition, successful dural repair often does not lead to symptomatic improvement, and a PubMed search revealed that only 5 of 29 patients treated surgically for SS of the central nervous system had clinical improvement.13 There have been reports of postoperative deterioration of neurological symptoms,9,14 presumably reflecting the irreversible state of the damage to the nervous tissue caused by hemosiderin deposition. Table 1 summarizes previous reports of cases in which dural defects were found in the upper thoracic spine with a diagnosis of SS and repair was performed.8,9,12,13,15–17 In this case, there was no hemosiderin deposition on the surface of the spinal cord on preoperative MRI, and the patient did not present with pyramidal tract signs. The dural closure was completed in a relatively short period of 1 year after the onset of the disease, which was considered to contribute to the favorable prognosis of neurological function. SS is generally characterized by a long period of time from symptom onset to surgical treatment; our case is the shortest in comparison with the past reports. With regard to the dural closure technique, the dura mater was sutured with nylon thread and reinforced with DuraGen (Integra LifeSciences) covering both the outer and inner surfaces of the dura mater to further ensure the closure.
TABLE 1.
Previous reports of repaired dura mater for SS in the upper thoracic spine
| Case No. | Authors & Year | Age (yrs), Sex | Duration (yrs) | Location of Dural Defect | Range of Fluid Collection | Closure Method | Long Tract Sign | Urinary Disturbance | T2-Low on Spinal Cord Surface | Clinical Outcome (FU) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
Kumar et al., 20058 |
42, M |
8 |
T2–3 |
C4–T9 |
Muscle graft |
(+) |
(−) |
(+) |
Unchanged (6 mos) |
| 2 |
Kumar et al., 20058 |
51, F |
4 |
T2 |
T1–3 |
NA |
(+) |
(−) |
(+) |
NA |
| 3 |
Shih et al., 200917 |
70, M |
2 |
T4–5 |
T2–8 |
Dural patch |
(+) |
(−) |
(+) |
Unchanged (15 mos) |
| 4 |
Ikeda et al., 201015 |
71, F |
7 |
T2–3 |
C7–T12 |
NA |
(+) |
(−) |
(+) |
Unchanged (1 yr) |
| 5 |
Kumar et al., 201016 |
54, M |
5 |
T3 |
C2–T7 |
Sutures |
(+) |
(+) |
(+) |
Unchanged |
| 6 |
Egawa et al., 20139 |
61, M |
30 |
T2–3 |
C2–T8 |
Free muscle graft |
(+) |
(+) |
(+) |
Deteriorated |
| 7 |
Egawa et al., 20139 |
54, M |
2 |
T1–2 |
C2–T8 |
Sutures, muscle graft |
(+) |
(−) |
(+) |
Unchanged (18 mos) |
| 8 |
Ryu et al., 201613 |
55, M |
3 |
T5 |
T3–5 |
Sutures, dural patch |
(+) |
(−) |
(+) |
Deteriorated (6 mos) |
| 9 |
Takai et al., 201712 |
58, M |
5 |
T3 |
C4–T7 |
Autologous fascia of neck muscle |
(−) |
(−) |
(−) |
Improved |
| 10 | Present case | 75, M | 1 | T2/3 | T2–7 | Sutures, DuraGen | (−) | (−) | (−) | Improved (6 mos) |
FU = follow-up; NA = not available.
T2 low intensity means hemosiderin deposition on the spinal surface.
Lessons
We report a case of dural repair for SS of the high thoracic level with a dural defect. Rapid treatment based on various imaging techniques led to dramatic symptomatic improvement. Although there are limitations in the results of dural repair at present, prompt diagnosis and reliable dural repair are of utmost significance because early treatment might lead to symptom improvement.
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Author Contributions
Conception and design: Matsuoka, Nogami, Tsuboi. Acquisition of data: Matsuoka, Narikiyo, Ohashi. Analysis and interpretation of data: Matsuoka. Drafting the article: Matsuoka. Critically revising the article: Matsuoka. Reviewed submitted version of manuscript: Matsuoka, Nagasaki. Approved the final version of the manuscript on behalf of all authors: Matsuoka. Statistical analysis: Matsuoka. Administrative/technical/material support: Matsuoka. Study supervision: Matsuoka.
References
- 1. Kumar N. Beyond superficial siderosis: introducing “duropathies.”. Neurology. 2012;78(24):1992–1999. doi: 10.1212/WNL.0b013e318259e272. [DOI] [PubMed] [Google Scholar]
- 2. Pongpitakmetha T, Fotiadis P, Pasi M, et al. Cortical superficial siderosis progression in cerebral amyloid angiopathy: prospective MRI study. Neurology. 2020;94(17):e1853–e1865. doi: 10.1212/WNL.0000000000009321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wilson D, Chatterjee F, Farmer SF, et al. Infratentorial superficial siderosis: classification, diagnostic criteria, and rational investigation pathway. Ann Neurol. 2017;81(3):333–343. doi: 10.1002/ana.24850. [DOI] [PubMed] [Google Scholar]
- 4. Levy M, Turtzo C, Llinas RH. Superficial siderosis: a case report and review of the literature. Nat Clin Pract Neurol. 2007;3(1):54–59. doi: 10.1038/ncpneuro0356. [DOI] [PubMed] [Google Scholar]
- 5. Matsumoto S, Kang Y, Sato S, et al. Spinal meningeal melanocytoma presenting with superficial siderosis of the central nervous system. Case report and review of the literature. J Neurosurg. 1998;88(5):890–894. doi: 10.3171/jns.1998.88.5.0890. [DOI] [PubMed] [Google Scholar]
- 6. Miliaras G, Bostantjopoulou S, Argyropoulou M, Kyritsis A, Polyzoidis K. Superficial siderosis of the CNS: report of three cases and review of the literature. Clin Neurol Neurosurg. 2006;108(5):499–502. doi: 10.1016/j.clineuro.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 7. Bracchi M, Savoiardo M, Triulzi F, et al. Superficial siderosis of the CNS: MR diagnosis and clinical findings. AJNR Am J Neuroradiol. 1993;14(1):227–236. [PMC free article] [PubMed] [Google Scholar]
- 8. Kumar N, Lindell EP, Wilden JA, Davis DH. Role of dynamic CT myelography in identifying the etiology of superficial siderosis. Neurology. 2005;65(3):486–488. doi: 10.1212/01.wnl.0000172345.05810.14. [DOI] [PubMed] [Google Scholar]
- 9. Egawa S, Yoshii T, Sakaki K, et al. Dural closure for the treatment of superficial siderosis. J Neurosurg Spine. 2013;18(4):388–393. doi: 10.3171/2013.1.SPINE12649. [DOI] [PubMed] [Google Scholar]
- 10. Takai K, Taniguchi M. Superficial siderosis of the central nervous system associated with ventral dural defects: bleeding from the epidural venous plexus. J Neurol. 2021;268(4):1491–1494. doi: 10.1007/s00415-020-10319-2. [DOI] [PubMed] [Google Scholar]
- 11. Angstwurm K, Schielke E, Zimmer C, Kivelitz D, Weber JR. Superficial siderosis of the central nervous system: response to steroid therapy. J Neurol. 2002;249(9):1223–1225. doi: 10.1007/s00415-002-0815-0. [DOI] [PubMed] [Google Scholar]
- 12. Takai K, Komori T, Niimura M, Taniguchi M. Superficial siderosis of the central nervous system associated with intraspinal hemorrhage from ventral thoracic epidural veins and a ventral spinal CSF leak: case report. J Neurosurg Spine. 2017;26(6):751–753. doi: 10.3171/2016.11.SPINE16488. [DOI] [PubMed] [Google Scholar]
- 13. Ryu SM, Kim ES, Kim SK, Lee SH, Eoh W. Superficial siderosis of the central nervous system originating from the thoracic spine: a case report. Korean J Spine. 2016;13(2):83–86. doi: 10.14245/kjs.2016.13.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Payer M, Sottas C, Bonvin C. Superficial siderosis of the central nervous system: secondary progression despite successful surgical treatment, mimicking amyotrophic lateral sclerosis. Case report and review. Acta Neurochir (Wien) 2010;152(8):1411–1416. doi: 10.1007/s00701-010-0653-2. [DOI] [PubMed] [Google Scholar]
- 15. Ikeda T, Noto D, Noguchi-Shinohara M, et al. CSF tau protein is a useful marker for effective treatment of superficial siderosis of the central nervous system: two case reports. Clin Neurol Neurosurg. 2010;112(1):62–64. doi: 10.1016/j.clineuro.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 16. Kumar N, Miller GM, Piepgras DG, Mokri B. A unifying hypothesis for a patient with superficial siderosis, low-pressure headache, intraspinal cyst, back pain, and prominent vascularity. J Neurosurg. 2010;113(1):97–101. doi: 10.3171/2009.10.jns091125. [DOI] [PubMed] [Google Scholar]
- 17. Shih P, Yang BP, Batjer HH, Liu JC. Surgical management of superficial siderosis. Spine J. 2009;9(8):e16–e19. doi: 10.1016/j.spinee.2009.03.004. [DOI] [PubMed] [Google Scholar]



