This randomized clinical trial investigates whether an intervention to provide health coaching, training, and tools to dyads consisting of 1 adult patient with diabetes and 1 adult supporter improves patient activation and diabetes management compared with standard diabetes care.
Key Points
Question
Does the Caring Others Increasing Engagement in Patient Aligned Care Teams (CO-IMPACT) intervention, which provides health coaching and automated monitoring telephone calls to dyads of adults with diabetes and their family supporters, improve patient activation, diabetes management, and outcomes compared with standard care?
Findings
In this randomized clinical trial with 239 patient-supporter dyads, CO-IMPACT improved patient activation, diabetes self-efficacy, and healthy eating. There were no between-group differences in diabetes-specific cardiac risk or glycemic control.
Meaning
Engaging family supporters in a low-intensity coaching and monitoring program improved patients’ capacity to manage diabetes, but a higher-intensity program may be necessary to improve outcomes.
Abstract
Importance
More than 75% of US adults with diabetes do not meet treatment goals. More effective support from family and friends (“supporters”) may improve diabetes management and outcomes.
Objective
To determine if the Caring Others Increasing Engagement in Patient Aligned Care Teams (CO-IMPACT) intervention improves patient activation, diabetes management, and outcomes compared with standard care.
Design, Setting, and Participants
This randomized clinical trial was conducted from November 2016 to August 2019 among participants recruited from 2 Veterans Health Administration primary care sites. All patient participants were adults aged 30 to 70 years with diabetes who had hemoglobin A1c (HbA1c) levels greater than 8% of total hemoglobin (to convert to proportion of total hemoglobin, multiply by 0.01) or systolic blood pressure (SBP) higher than 150 mm Hg; each participating patient had an adult supporter. Of 1119 recruited, 239 patient-supporter dyads were enrolled between November 2016 and May 2018, randomized 1:1 to receive the CO-IMPACT intervention or standard care, and followed up for 12 to 15 months. Investigators and analysts were blinded to group assignment.
Interventions
Patient-supporter dyads received a health coaching session focused on dyadic information sharing and positive support techniques, then 12 months of biweekly automated monitoring telephone calls to prompt dyadic actions to meet diabetes goals, coaching calls to help dyads prepare for primary care visits, and after-visit summaries. Standard-care dyads received general diabetes education materials only.
Main Outcomes and Measures
Intent-to-treat analyses were conducted according to baseline dyad assignment. Primary prespecified outcomes were 12-month changes in Patient Activation Measure–13 (PAM-13) and UK Prospective Diabetes Study (UKPDS) 5-year diabetes-specific cardiac event risk scores. Secondary outcomes included 12-month changes in HbA1c levels, SBP, diabetes self-management behaviors, diabetes distress, diabetes management self-efficacy, and satisfaction with health system support for the involvement of family supporters. Changes in outcome measures between baseline and 12 months were analyzed using linear regression models.
Results
A total of 239 dyads enrolled; among patient participants, the mean (SD) age was 60 (8.9) years, and 231 (96.7%) were male. The mean (SD) baseline HbA1c level was 8.5% (1.6%) and SBP was 140.2 mm Hg (18.4 mm Hg). A total of 168 patients (70.3%) lived with their enrolled supporter; 229 patients (95.8%) had complete 12-month outcome data. In intention-to-treat analyses vs standard care, CO-IMPACT patients had greater 12-month improvements in PAM-13 scores (intervention effect, 2.60 points; 95% CI, 0.02-5.18 points; P = .048) but nonsignificant differences in UKPDS 5-year cardiac risk (intervention effect, 1.01 points; 95% CI, −0.74 to 2.77 points; P = .26). Patients in the CO-IMPACT arm also had greater 12-month improvements in healthy eating (intervention effect, 0.71 d/wk; 95% CI, 0.20-1.22 d/wk; P = .007), diabetes self-efficacy (intervention effect, 0.40 points; 95% CI, 0.09-0.71 points; P = .01), and satisfaction with health system support for the family supporter participants’ involvement (intervention effect, 0.28 points; 95% CI, 0.07-0.49 points; P = .009); however, the 2 arms had similar improvements in HbA1c levels and in other measures.
Conclusions and Relevance
In this randomized clinical trial, the CO-IMPACT intervention successfully engaged patient-supporter dyads and led to improved patient activation and self-efficacy. Physiological outcomes improved similarly in both arms. More intensive direct coaching of supporters, or targeting patients with less preexisting support or fewer diabetes management resources, may have greater impact.
Trial Registration
ClinicalTrials.gov Identifier: NCT02328326
Introduction
More than 75% of US adults with diabetes do not meet all glycemic, blood pressure, and lipid treatment targets.1 These patients often need more intensive monitoring and support than health systems can provide. One potentially powerful source of support is patients’ family and friends (family supporters). Among adults with diabetes and without significant functional limitations, 50% to 75% have a family supporter who is regularly involved in their diabetes management.2,3 Support for diabetes care from family and friends is associated with better self-management behaviors and risk factor control and is associated with decreased risk of hospitalization and death.4,5,6,7,8 Recent Diabetes Self-management Education and Support (DSMES) guidelines call for health care professionals to actively include family supporters in diabetes education and support programs.9
Interventions that provide general diabetes information to family supporters have not improved patient outcomes.10,11 Studies on specific aspects of dyadic diabetes management have identified more promising approaches to increasing effective family support,12 including training family supporters in techniques to facilitate behavior change (eg, goal-setting,13 action planning,14,15 and use of autonomy-supportive communication styles15,16,17,18), enhancing supporter access to information about the patient’s diabetes regimen and test results,19 prompting supporters to routinely discuss diabetes management with the patient, and training supporters in techniques to support patient engagement in health care visits.20 However, health care teams do not have structured programs or tools to deliver these promising approaches to patient-supporter pairs.
To address this gap, we designed the Caring Others Increasing Engagement in Patient Aligned Care Teams (CO-IMPACT) intervention as a structured approach to providing training and tools to adult patient-supporter dyads. In this article, we report the main patient health, behavioral, and satisfaction outcomes of a 12-month randomized clinical trial of the CO-IMPACT intervention vs standard, comprehensive diabetes care. Our primary hypotheses were that patients’ activation, defined as “having the knowledge, skills, and confidence to manage one’s health,”21 would increase and that diabetes-specific cardiac risk would decrease more among patients receiving the CO-IMPACT intervention than among those receiving standard care.
Methods
Study Design and Setting
This randomized clinical trial was conducted from November 2016 to August 2019. Dyads consisting of 1 adult with diabetes plus 1 family supporter were recruited from 1 hospital-based and 1 community-based US Veterans Health Administration primary care clinic. Both clinics offered comprehensive diabetes care, including assignment to a patient-centered medical home team22; access to nurse care management, a clinical pharmacist, and dietitian services; and diabetes education and weight management classes. A detailed study protocol and rationale were previously published.23 The full initial and final study protocol and statistical analysis plans were approved by the Veterans Affairs (VA) Ann Arbor Institutional Review Board and are included as Supplement 1. All patients provided written informed consent, and supporters provided oral informed consent. Analyses and reporting followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.24
Participants and Randomization
Potentially eligible patients were identified via administrative databases. Patient inclusion criteria included an age of 30 to 70 years; type 2 diabetes, based on the encounter diagnoses or a chronic diabetes medication prescription other than metformin; poor glycemic control (most recent hemoglobin A1c [HbA1c] level in the past 9 months >8% of total hemoglobin) (to convert to proportion of total hemoglobin, multiply by 0.01) or poor blood pressure (BP) control (most recent systolic BP [SBP] >150 mm Hg and mean SBP during the past 9 months >150 mm Hg); 2 or more visits to the VA primary care clinic in the previous 12 months; English speaker; and able to communicate via touch-tone telephone. Exclusion criteria included diabetes managed by a specialist or non-VA physician, living in a nursing home or assisted living facility, requiring help with 2 or more basic activities of daily living, life-limiting severe illness, being pregnant or planning pregnancy, serious mental illness, moderate to severe cognitive impairment, or active substance use disorder.
Supporters were nominated by the patient participant. Supporter eligibility criteria included age 21 years or older, talking with the patient about their health at least twice per month, helping the patient regularly with 1 or more aspects of their health care (eg, filling medications, conducting home testing), not receiving pay to care for the patient, and no severe mental illness, dementia, life-limiting severe illness, or need for help with basic activities of daily living.
Participants were enrolled between November 2016 and May 2018 and were observed for up to 15 months after enrollment (until August 2019). Dyads were randomized using sequential treatment assignment with minimization in a 1:1 ratio between arms, initially balanced across strata of supporter-patient cohabitation (yes or no), using a level of determinism of 5 on a scale of 1 to 10.25 The randomization protocol was amended to add a second stratification criterion (Patient Activation Measure–13 [PAM-1326] score of ≥40 vs <40 [score range, 0-100, with higher scores indicating greater activation]) after interim analyses revealed that the mean baseline PAM-13 scores differed significantly between arms. Investigators and analysts were blinded to group assignment.
Interventions
Dyads assigned to the CO-IMPACT arm received 4 program components over 12 months: an initial coaching session, biweekly automated interactive voice response (IVR) telephone calls, primary care visit preparation calls, and primary care visit summaries. Details on the intervention-component delivery mode and topics are included in Table 1 and the eAppendix in Supplement 2. Each coach-delivered component was designed to be usable within typical physician, diabetes educator, or nurse care management patient encounters. Study coaches had social work or public health degrees and received training in diabetes care, health behavior change principles, and dyadic communication. Coaches delivered the intervention using manualized scripts. Specific IVR call topics and information on accessing intervention materials are included in the eFigure in Supplement 2. Dyads assigned to either CO-IMPACT or standard care (termed enhanced usual care in initial protocols) were offered home glucose and BP monitoring equipment and were provided general diabetes management information via a study handbook and website. Patients in both arms could receive standard clinic diabetes care, which followed national VA clinical practice guidelines.27 Supporters in both arms were not limited from participating in any patient clinic visits or programs. Fidelity to intervention delivery was assessed via investigator (A.R., S.S., M.H.) review of all coach call and mailing records and of fidelity rating forms for recordings of 10% of the initial sessions and visit preparation calls.
Table 1. Intervention Component Details of the CO-IMPACT and Standard Care Armsa.
| Timeline | Component | Participants | Deliverer, mode | Content and topics addressed |
|---|---|---|---|---|
| Enrollmentb | Self-monitoring equipment and general diabetes information | P, S | RA, in person |
|
| Within 1 mo of enrollment | Initial 1-1.5 h coaching session | P, S | Dyad coach, synchronous in person |
|
| CO-IMPACT handbook and website | P, S | NA |
|
|
| From the date of the initial coaching session to a date 12 mo after the patient baseline survey (approximately 11 mo) | IVR calls every 2 wk | P | Automated phone call |
|
| IVR summary with follow-up tips | S | Automated email |
|
|
| Visit reminder 2-5 d before each scheduled clinic visit | S | Dyad coach, email |
|
|
| Visit preparation calls 2-5 d before each scheduled clinic visit | P and/or S | Dyad coach, synchronous telephone discussion |
|
|
| Visit summaries | P, S | Dyad coach, mailed or posted to website automatically after a completed visit |
|
Abbreviations: IVR, interactive voice response; NA, not applicable; P, patient participant; RA, research assistant; S, supporter participant.
Termed enhanced usual care in initial protocols.
Received by participants in both arms (CO-IMPACT and standard care).
Data Sources and Measures
Patients and supporters were surveyed in person or via telephone at baseline and 12 months. Patient BP and laboratory measurements were collected by study staff at baseline and 12 months. Patient medical record data were obtained from central VA databases. Structured qualitative interviews were conducted with participants in the CO-IMPACT arm at the end of the 12-month intervention. Interviews were transcribed, then analyzed for thematic content using editing analysis style.28
Outcomes
Prespecified primary outcomes were changes from baseline to 12 months in patients’ (1) activation, measured by the PAM-13,26 and (2) diabetes-specific cardiac event risk, measured by the UK Prospective Diabetes Study (UKPDS) 5-year risk engine.29 The 13 items on the PAM-13 include “I am confident that I can follow through on medical treatments I may need to do at home” and “I am confident I can figure out solutions when new problems arise with my health,” with item response options ranging from “strongly disagree” (1) to “strongly agree” (4). The item response total is transformed into an activation score ranging from 0 to 100 by using the creators’ established algorithm.26 Diabetes-specific cardiac risk was chosen as the main physiological outcome to provide a summary measure of multiple risk factors for diabetes complications that may have been modified by improved self-management, including HbA1c levels, SBP, lipid levels, and smoking status. Other components included age, sex, race and ethnicity (ascertained by self-report and collected because the official calculation of the UKPDS score includes a variable for race and ethnicity), and years since diabetes diagnosis.
Prespecified secondary patient outcomes included 12-month changes in glycemic, BP, and cholesterol control; diabetes self-management behaviors (assessed by the Summary of Diabetes Self-care Activities30 as the number of days of self-management adherence in the past week; range 0-7 days); diabetes distress (measured by the Problem Areas in Diabetes Scale [PAID]31; score range 0-20, with higher scores indicating greater distress); diabetes management self-efficacy (measured by the Stanford Self-efficacy for Diabetes Scale32; score range 0-10, with higher scores indicating greater self-efficacy); efficacy in health care participation (measured by the Perceived Efficacy in Patient-Physician Interactions Questionnaire33; score range 5-25, with higher scores indicating greater participation in interactions between patients and health care professionals); and satisfaction with health care system support for the supporter participants (assessed by a survey item with response options ranging from 1, “strongly disagree,” to 5, “strongly agree”). eTable 1 in Supplement 2 contains details for the measures used.
Statistical Analysis
Given 80% power and a 2-tailed α of .05, we estimated we needed data from 204 dyads to detect a 4-point difference in PAM-13 score changes between groups and 198 dyads to detect a 2% difference in UKPDS score changes. These estimates assumed a baseline mean (SD) PAM-13 score of 70 (13), with correlation of 0.70 in 2 measurements in 1 year, and a baseline mean (SD) UKPDS score of 18% (12%).
Intent-to-treat analyses were conducted according to baseline dyad assignment. If a supporter withdrew from the study, the patient could continue participating in their assigned arm. If a patient withdrew, their supporter was also withdrawn. Descriptive patient and supporter data at baseline and changes between baseline and 12 months were analyzed for each group. Analyses of outcomes were initially conducted using hierarchical linear models; however, identical results were obtained using linear regression models with continuous change from baseline to 12 months as the dependent variable, adjusted for the baseline value of the outcome, and these are reported for ease of interpretation. Outcomes models were adjusted for variables determining randomization strata and baseline patient use of insulin, known to be independently correlated with patient diabetes outcomes.34 Models included only patients with complete data for the relevant outcome. No data from variables included in the outcome regression models was missing from among patients with complete outcome data. Statistical analyses were performed using Stata, version 17 (StataCorp LLC). Two-sided P < .05 was considered significant.
Results
Among the 239 enrolled patients, the mean (SD) age was 60 (8.9) years; 8 (3.3%) were female and 231 (96.7%) were male. Thirty patients (12.6%) were Black or African American, 7 (2.9%) were Native American, 182 (76.2%) were White, 6 (2.5%) identified as more than 1 race, 14 (5.9%) identified as other race (specific races within this category were not provided) or did not answer, and 14 (5.9%) were Latino or Latina ethnicity. Fifty-six patients (23.4%) had completed college (Table 2). The mean (SD) time since diabetes diagnosis was 12.5 (9.0) years, and 142 patients (59.4%) used insulin at baseline. The mean (SD) baseline HbA1c level was 8.5% (1.6%), and the mean (SD) SBP was 140.2 mm Hg (18.4 mm Hg). At baseline, patient activation (mean [SD] PAM-13 score, 61.3 [23.0]) and diabetes self-efficacy (mean [SD] Stanford Self-efficacy for Diabetes Scale score, 8.2 [1.7]) were comparatively high, and diabetes distress was comparatively low (mean [SD] PAID score, 5.9 [5.2]).
Table 2. Participant Baseline Characteristics.
| Characteristic | Participants, No. (%) | ||
|---|---|---|---|
| CO-IMPACT intervention (n = 123) | Standard care (n = 116) | Overall (n = 239) | |
| Patient | |||
| Age at baseline, mean (SD), y | 60 (8.4) | 60 (9.4) | 60 (8.9) |
| Sex | |||
| Female | 6 (4.9) | 2 (1.7) | 8 (3.3) |
| Male | 117 (95.1) | 114 (98.3) | 231 (96.7) |
| Race | |||
| Black or African American | 12 (9.8) | 18 (15.5) | 30 (12.6) |
| Native Americana | 6 (4.9) | 1 (0.9) | 7 (2.9) |
| White | 90 (73.2) | 92 (79.3) | 182 (76.2) |
| More than 1 race | 6 (4.9) | 0 | 6 (2.5) |
| Other or not reportedb | 9 (7.3) | 5 (4.3) | 14 (5.9) |
| Latino or Latina ethnicity | 10 (8.1) | 4 (3.4) | 14 (5.9) |
| Completed college | 29 (23.6) | 27 (23.3) | 56 (23.4) |
| Annual income, $c | |||
| <30 000 | 35 (29.2) | 39 (34.2) | 74 (31.6) |
| 30 000 to <50 000 | 31 (25.8) | 34 (29.8) | 65 (27.8) |
| 50 000 to <75 000 | 31 (25.8) | 17 (14.9) | 48 (20.5) |
| ≥75 000 | 23 (19.2) | 24 (21.1) | 47 (20.1) |
| Low health literacyd | 57 (46.3) | 52 (44.8) | 109 (45.6) |
| Used insulin at baseline | 78 (63.4) | 64 (55.2) | 142 (59.4) |
| Time since diabetes diagnosis, mean (SD), y | 13.7 (10.0) | 11.2 (7.7) | 12.5 (9.0) |
| Study site was hospital-based, vs community clinic | 78 (63.4) | 87 (75.0) | 165 (69.0) |
| PAM-13 score, mean (SD)e | 62.8 (11.2) | 59.8 (12.6) | 61.3 (12.0) |
| UKPDS 5-y cardiac event risk, mean (SD), % | 14.7 (10.0) | 13.6 (9.9) | 14.2 (9.9) |
| HbA1c level, mean (SD), % | 8.4 (1.5) | 8.6 (1.8) | 8.5 (1.6) |
| Systolic blood pressure, mean (SD), mm Hg | 141.0 (18.3) | 139.3 (18.5) | 140.2 (18.4) |
| Total cholesterol to HDL ratio, mean (SD) | 4.7 (1.6) | 4.5 (1.5) | 4.6 (1.5) |
| Current smoker | 20 (16.3) | 16 (13.8) | 36 (15.1) |
| Diabetes distress (PAID), mean (SD)f | 5.6 (5.2) | 6.2 (5.2) | 5.9 (5.2) |
| Diabetes self-efficacy, mean (SD)g | 8.5 (1.4) | 7.9 (1.9) | 8.2 (1.7) |
| Healthcare participation (PEPPI), mean (SD)h | 21.7 (4.1) | 21.2 (4.3) | 21.5 (4.2) |
| Self-management adherence in the past week (SDSCA), mean (SD), di | |||
| Healthy eating | 4.1 (2.4) | 4.1 (2.3) | 4.1 (2.4) |
| Physical activity | 2.9 (2.2) | 2.3 (2.0) | 2.6 (2.2) |
| Blood glucose home testingj | 5.2 (2.2) | 4.6 (2.3) | 4.9 (2.3) |
| Blood pressure home testingk | 2.7 (2.7) | 2.1 (2.4) | 2.4 (2.5) |
| Check feet | 3.7 (2.3) | 3.2 (2.3) | 3.5 (2.3) |
| Take oral medications as prescribedl | 6.3 (1.3) | 6.3 (1.3) | 6.3 (1.3) |
| Take insulin as prescribedm | 6.4 (1.1) | 6.0 (2.0) | 6.2 (1.6) |
| Satisfaction with health care system supporter engagement, mean (SD)n | 3.6 (1.0) | 3.5 (1.0) | 3.6 (1.0) |
| Supporter | |||
| Relationship to patient | |||
| Spouse or partner | 75 (61.0) | 70 (60.3) | 145 (60.7) |
| Friend | 25 (20.3) | 16 (13.8) | 41 (17.2) |
| Adult child | 9 (7.3) | 18 (15.5) | 27 (11.3) |
| Other relative | 14 (11.4) | 12 (10.3) | 26 (10.9) |
| Supporter lives in patient household | 86 (69.9) | 82 (70.7) | 168 (70.3) |
| Sex | |||
| Female | 109 (88.6) | 106 (91.4) | 215 (90.0) |
| Male | 14 (11.4) | 10 (8.6) | 24 (10.0) |
| Race | |||
| Asian | 0 | 1 (0.9) | 1 (0.4) |
| Black or African American | 10 (8.1) | 16 (13.8) | 26 (10.9) |
| Native Americana | 1 (0.8) | 0 | 1 (0.4) |
| White | 103 (83.7) | 92 (79.3) | 195 (81.6) |
| More than 1 race | 5 (4.1) | 2 (1.7) | 7 (2.9) |
| Other or not reportedb | 4 (3.3) | 5 (4.3) | 9 (3.8) |
| Latino or Latina ethnicity | 6 (4.9) | 0 | 6 (2.5) |
| Completed college | 25 (20.3) | 30 (25.9) | 55 (23.0) |
| Annual income, $o | |||
| <30 000 | 33 (28.7) | 34 (31.5) | 67 (30.0) |
| 30 000 to <50 000 | 31 (27.0) | 31 (28.7) | 62 (27.8) |
| 50 000 to <75 000 | 29 (25.2) | 21 (19.4) | 50 (22.4) |
| ≥75 000 | 22 (19.1) | 22 (20.4) | 44 (19.7) |
| Supporter has diabetesp | 22 (18.2) | 24 (20.7) | 46 (19.4) |
Abbreviations: HbA1c, hemoglobin A1c; HDL, high-density lipoprotein; PAID, Problem Areas in Diabetes Scale; PAM-13, Patient Activation Measure–13; PEPPI, Perceived Efficacy in Patient-Physician Interactions Questionnaire; SDSCA, Summary of Diabetes Self-care Activities measure; UKPDS, UK Prospective Diabetes Study.
SI conversion factor: To convert percentage of total hemoglobin to proportion of total hemoglobin, multiply by 0.01.
The Native American category includes participants who identified as Native American, Alaskan Native, Native Hawaiian, or Pacific Islander.
Participant chose the “other” category (races included in this category were not provided) or did not answer. No patient participants indicated Asian race.
All but 3 patients in the intervention arm and 2 in the standard-care arm reported income data.
Assessment of health literacy is explained in eTable 1 in Supplement 2.
Score range 0-100, with higher scores indicating greater activation.
Score range 0-20, with higher scores indicating greater distress.
Stanford Self-efficacy for Diabetes Scale; score range 0-10, with higher scores indicating greater self-efficacy.
Score range 5-25, with higher scores indicating greater participation in interactions between patients and health care professionals.
Range, 0-7 days, with higher scores indicating more frequent adherence.
All but 4 participants in the intervention arm and 6 in the standard-care arm.
All but 14 participants in the intervention arm and 22 in the standard-care arm (patients who reported not being advised by their clinicians to test glucose or blood pressures at home were not asked self-testing questions).
All but 14 participants in the intervention arm and 16 in the standard-care arm.
All but 45 participants in the intervention arm and 51 in the standard-care arm (patients who did not take oral diabetes medications or insulin were not asked these questions).
All but 3 participants in the intervention arm and 1 in the standard-care arm; score range 1-5, with 1 indicating “strongly disagree” and 5, “strongly agree.”
All but 8 supporter participants in the intervention arm and 8 in the standard-care arm reported income data.
All but 2 supporter participants in the intervention arm and 0 in the standard-care arm.
Among the 239 enrolled supporters, 215 were (90.0%) were female and 24 (10.0%) were male. One supporter (0.4%) was Asian, 26 (10.9%) were Black or African American, 1 (0.4%) was Native American, 195 (81.6%) were White, 7 (2.9%) identified as more than 1 race, 9 (3.8%) identified as other race or did not answer, and 6 (2.5%) were Latino or Latina ethnicity. A total of 168 supporters (70.3%) resided with the patient, and 145 (60.7%) were spouses or partners. Forty-six supporters (19.4%) had diabetes.
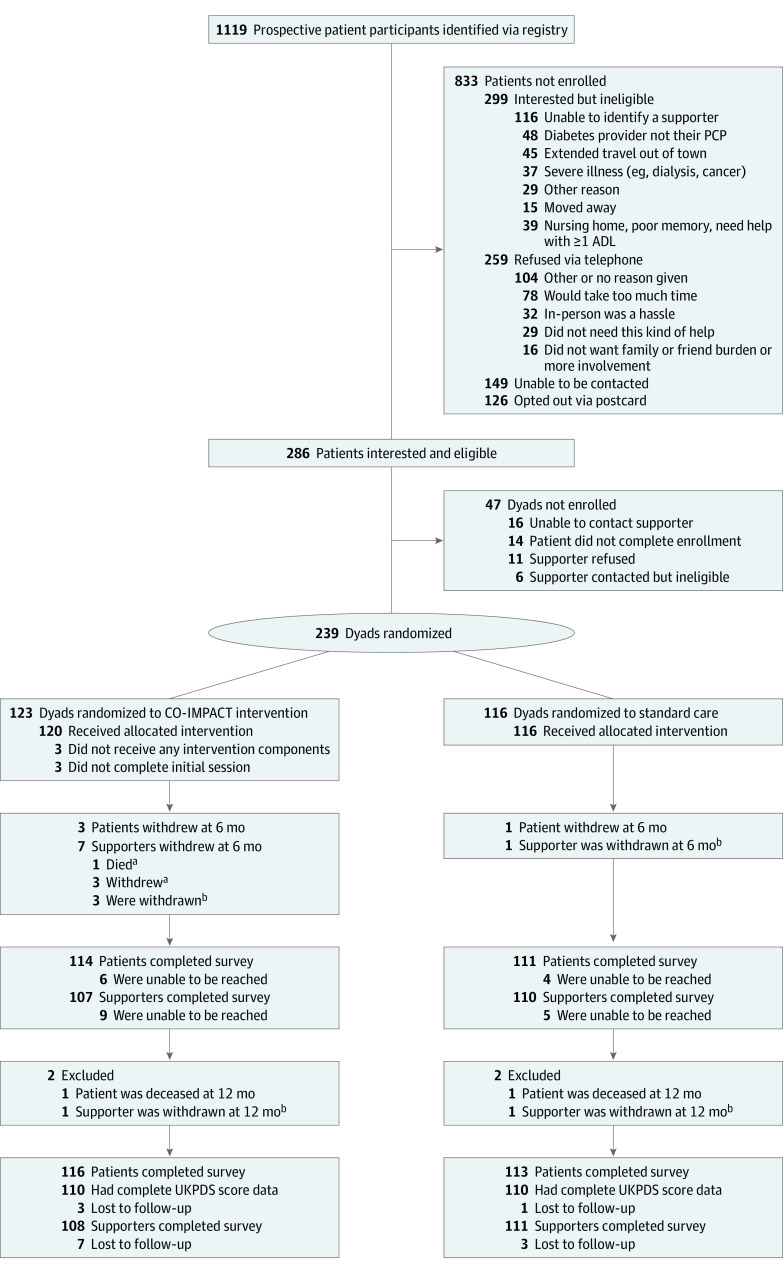
Figure 1 shows the study flow diagram, with 1119 recruitment letters resulting in 239 enrolled dyads. Among the 259 recruited individuals who declined to participate and provided reasons, the most common refusal reason was too much time or hassle (110 [42.5%]); only 16 (6.2%) refused due to not wanting increased supporter involvement or burden. However, among the 299 individuals who were interested but ineligible, inability to identify a supporter to participate with them was the most common reason for ineligibility (116 [38.8%]). Among those identified via the registry (eTable 2 in Supplement 2), enrolled patients were 3 years younger on average than those who did not enroll (mean [SD] age, 60 [9.0] vs 63 [7.4] years). Those qualifying via both a high HbA1c level and SBP were more likely to enroll than were those meeting just 1 of these criteria. Four patients (1.7%) withdrew and 2 (0.8%) died during the study period. A total of 229 patients (95.8%) completed 12-month survey assessments, and 220 (92.1%) had complete vital sign and laboratory data.
Figure 1. Study Participant Flow Diagram.
ADL indicates activities of daily living; CO-IMPACT, Caring Others Increasing Engagement in Patient Aligned Care Teams; PCP, primary care physician; and UKPDS, UK Prospective Diabetes Study.
aIf a supporter died or withdrew, the patient was allowed to continue in the patient-focused parts of their originally assigned intervention.
bThe supporter was automatically withdrawn if the patient died or withdrew from the study.
With regard to CO-IMPACT intervention participation (Figure 2), 120 of 123 CO-IMPACT arm dyads (97.6%) completed the initial session and were enrolled in the IVR system. Of those, 114 (95.0%) completed at least 1 IVR call, with a mean (SD) of 19 (7) completed calls per patient. Ninety-eight dyads (79.7%) completed 1 or more visit preparation calls (mean [SD], 2.4 [1.9] calls per dyad), and 113 (91.9%) received 1 or more visit summaries (mean [SD], 4.2 [2.6] summaries per dyad).
Figure 2. Participation in CO-IMPACT Intervention Components.
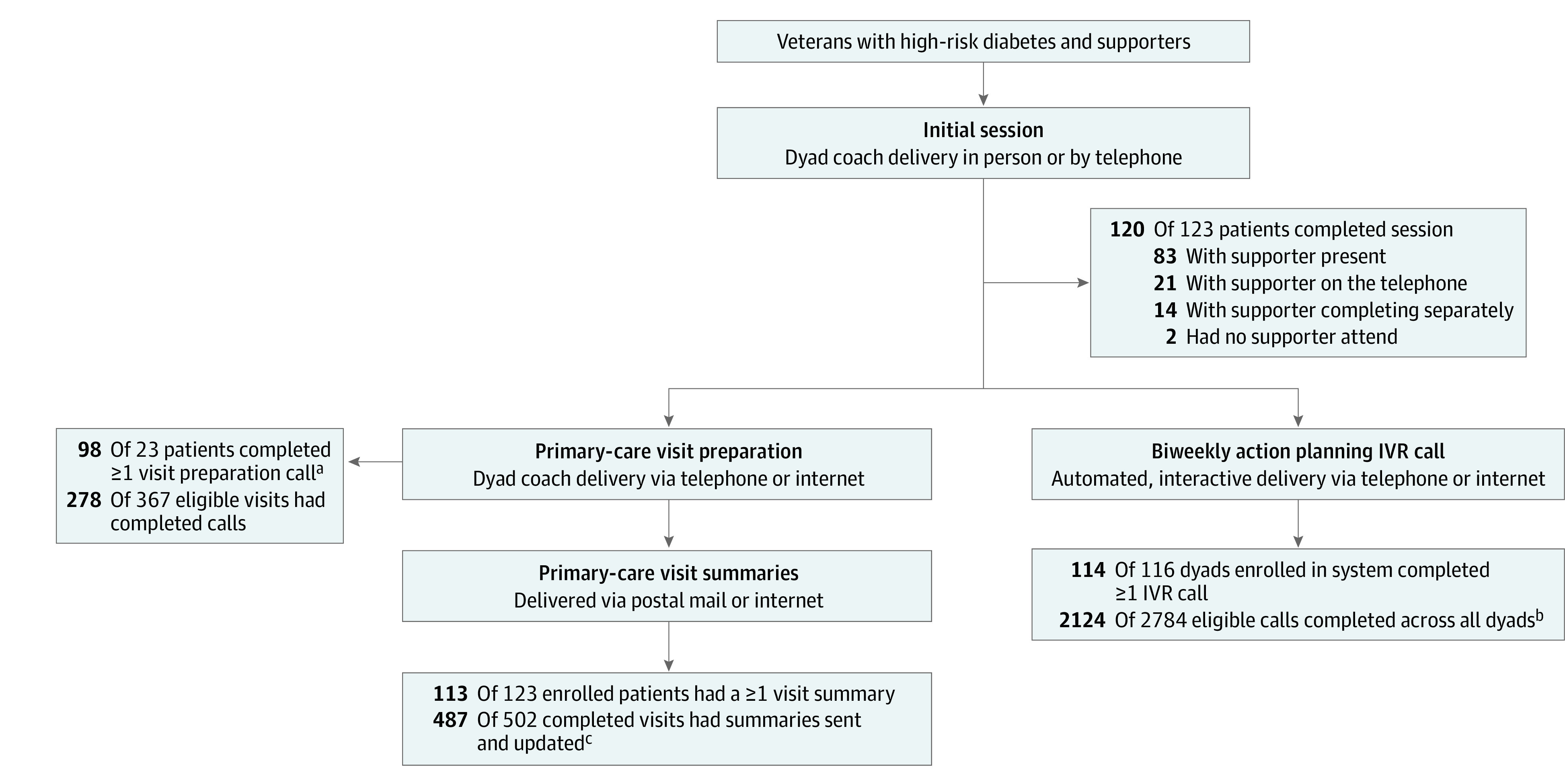
IVR indicates interactive voice response.
aMean (SD) calls per patient over 12 months was 2.4 (1.9).
bMean (SD) calls per dyad over 12 months was 18.6 (6.9).
cMean (SD) summaries per patient over 12 months was 4.2 (2.6).
Patient Outcomes
Unadjusted mean (SD) 12-month PAM-13 score changes (eTable 3 in Supplement 2) were greater for the CO-IMPACT arm than for the standard-care arm (2.9 [9.9] points vs 1.8 [10.8] points). The UKPDS 5-year cardiac event risk score increased slightly on average in the CO-IMPACT group (mean [SD], 0.1% [6.8%]) and decreased slightly in the standard-care group (mean [SD], −0.7% [6.5%]). The HbA1c level and SBP decreased on average in both arms, but HbA1c levels decreased more in the standard-care group, and SBP decreased more in the CO-IMPACT group.
Fully adjusted models (Table 3; eTable 4 in Supplement 2) showed that PAM-13 scores improved more in the CO-IMPACT arm than the standard-care arm (intervention effect, 2.60 points; 95% CI, 0.02-5.18 points; P = .048). The UKPDS risk score changes were not statistically significantly different by arm (intervention effect, 1.01 points; 95% CI, −0.74 to 2.77 points; P = .26). Self-reported healthy eating (intervention effect, 0.71 d/wk; 95% CI, 0.20-1.22 d/wk; P = .007) and diabetes self-efficacy (intervention effect, 0.40 points; 95% CI, 0.09-0.71; P = .01) improved more over 12 months in those receiving the intervention compared with standard care. Hemoglobin A1c levels, SBP, diabetes distress, efficacy in health care participation, and other self-management behavior changes did not differ significantly by study arm.
Table 3. Adjusted Intervention Effect on 12-Month Changes in Patient Primary and Secondary Outcomes From Multivariable Linear Regression Modelsa.
| Adjusted coefficient (95% CI) for intervention from baseline to 12-mo change, vs standard care | Patients included in the model, No. | P value | |
|---|---|---|---|
| Prespecified primary outcomes | |||
| PAM-13 score | 2.60 (0.02 to 5.18) | 229 | .048 |
| UKPDS 5-y cardiac event risk score | 1.01 (−0.74 to 2.77) | 220 | .26 |
| Prespecified secondary outcomes | |||
| HbA1c level, % | 0.17 (−0.17 to 0.51) | 227 | .33 |
| Systolic blood pressure, mm Hg | −2.82 (−7.00 to 1.35) | 220 | .18 |
| Total cholesterol to HDL-C ratio | 0.15 (−0.09 to 0.40) | 227 | .29 |
| Diabetes distress (PAID) | 0.12 (−0.95 to 1.19) | 225 | .83 |
| Diabetes self-efficacyb | 0.40 (0.09 to 0.71) | 228 | .01 |
| Efficacy in health care participation (PEPPI) | 0.11 (−0.71 to 0.93) | 228 | .79 |
| Self-management adherence (SDSCA) | |||
| Healthy eating | 0.71 (0.20 to 1.22) | 229 | .007 |
| Physical activity | −0.04 (−0.56 to 0.47) | 228 | .87 |
| Blood glucose home testing | 0.23 (−0.22 to 0.68) | 195 | .31 |
| Blood pressure home testing | 0.47 (−0.25 to 1.19) | 137 | .20 |
| Check feet | 0.26 (−0.22 to 0.75) | 228 | .29 |
| Medication adherence | |||
| Oral medications | −0.10 (−0.42 to 0.23) | 195 | .56 |
| Insulin | 0.07 (−0.26 to 0.41) | 134 | .67 |
| Satisfaction with health care system supporter engagement | 0.28 (0.07 to 0.49) | 218 | .009 |
Abbreviations: HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; PAID, Problem Areas in Diabetes Scale; PAM-13, Patient Activation Measure–13; PEPPI, Perceived Efficacy in Patient-Physician Interactions Questionnaire; SDSCA, Summary of Diabetes Self-care Activities Measure; UKPDS, UK Prospective Diabetes Study.
Each model adjusted for whether the patient and supporter lived together (yes or no), a baseline PAM-13 score higher than 40 vs lower than 40 (total score range, 0-100, with higher scores indicating greater activation), whether the patient used insulin at baseline (yes or no), and the baseline value of the outcome measure. Full model results are included in eTable 4 in Supplement 2.
Stanford Self-efficacy for Diabetes Scale.
Satisfaction
Patient satisfaction with health system support for the involvement of supporters improved more in the CO-IMPACT arm (intervention effect, 0.28 points; 95% CI, 0.07-0.49; P = .009). Most patients and supporters in the CO-IMPACT arm rated each component as helping a great deal or somewhat (eTable 5 in Supplement 2); the initial coaching session received the highest rating (189 of 215 patients and supporters [87.9%] said it helped a great deal or somewhat) and visit preparation calls received the lowest (only patients were asked this question; 76 of 109 [69.7%] said the calls helped a great deal or somewhat). Eighteen of 105 supporters (17.1%) did not recall receiving email summaries of patient IVR entries. Most participants (108 of 111 patients [97.3%]; 98 of 105 supporters [93.3%]) said they would definitely or probably recommend CO-IMPACT to others like them, and 87 of 105 supporters (82.9%) felt they and the patient improved how they worked together to manage diabetes.
Qualitative Analyses
Thematic analysis of CO-IMPACT participant interview transcripts indicated ways that patients and supporters worked together to increase goal setting and other activated patient behaviors (eTable 6 in Supplement 2). Participants gave multiple examples of supporters learning better ways to help patients, of the dyad increasing how often they discussed the patient’s diabetes, and of ways the dyad used team-based approaches, sometimes identified via “we-talk.”35,36 Although there were many positive comments about the IVR system, there were also reasons for dissatisfaction, including repetitiveness, patients not knowing that supporters received call summaries, and wariness to reveal “bad” results. Some patient and supporter participants desired more interaction with human coaches.
Discussion
In this randomized clinical trial of a dyadic intervention to engage adults with diabetes along with a family supporter in diabetes care, patients receiving the CO-IMPACT intervention had a greater improvement in activation (PAM-13 score), 1 of 2 prespecified primary outcomes. The clinical significance of the PAM-13 score change of the magnitude we observed (a 2.6-point improvement) is supported by multiple studies showing associations between this level of PAM-13 score change and significant, positive changes in patient behaviors and health outcomes.37,38,39 The CO-IMPACT patients’ diabetes self-efficacy and healthy eating behavior also improved compared with those receiving standard care. Improvements in self-efficacy of the magnitude we observed have been associated with mediating improvements in diabetes clinical outcomes in patient-only interventions.40,41 However, in this trial, improvements in cardiac risk score and individual physiological outcomes were similar in both arms.
Increases in patient activation and the related concept of self-efficacy are key patient-centered diabetes outcomes. Moreover, each provides an essential foundation for patients to make healthy behavior changes and engage in medical care. In other studies, these measures have been associated with improvements in health behaviors, glycemic control, and hospitalization rates among adults with diabetes.38,42,43 The difference in PAM-13 score changes we observed between study arms had a P value slightly less than .05 and are unlikely to be spurious given the supporting finding of significant differences in self-efficacy change. These improvements were achieved despite using no in-person clinician time and only brief phone or digital interactions with participants. In separate analyses from this trial, supporters in the CO-IMPACT arm experienced significantly increased involvement in several patient diabetes-management tasks and increased use of positive, autonomy-supportive communication compared with those receiving standard care.44
Several factors may explain the lack of significant impact of CO-IMPACT on physiological measures beyond improvements observed in the standard care group. Our intervention was primarily delivered via automated telephone calls, and supporters received less direct interaction than did patients after the initial coaching session. Some supporters indicated in qualitative interviews that they did not receive automated messages and desired more direct contact with coaches. Future studies should test more intensive and direct follow-up with supporters. Our qualitative data indicate that studies using automated systems with patients and supporters may be more engaging by varying message content and intensity, providing periodic notifications that the other member of the dyad is receiving summary messages, and reminders reminding participants of the benefits of discussing supportive follow-up plans, particularly when diabetes care is not going well. In addition, patients received health care in VA settings with high documented quality of primary and diabetes care45,46 and extensive resources to support diabetes management. Interventions to increase family support may benefit patients more in settings with fewer diabetes support resources.
Strengths and Limitations
This study has strengths. It was highly successful in recruiting, engaging, and retaining adult patient-supporter dyads, which has been challenging in prior research.47,48 A key strategy was accommodating supporters who lived with or apart from the patient and those who were related to the patient. Many health care systems are trying to increase their engagement of patients’ caregivers, as promoted by recent legislation.49,50 Concurrent with the time frame of this study, the VA has prioritized expanding programs to engage and support patients’ caregivers.51,52 Among CO-IMPACT patients, satisfaction with health care system support for their supporter’s involvement in care increased significantly more than among patients in standard care, and most supporters felt CO-IMPACT improved their ability to help patients. Possible factors in this success, supported by our qualitative findings, include the intervention’s focus on a positive, patient-supporter teamwork approach, increased sharing of health care information with supporters, and supporter encouragement of patients’ engagement in health care.
This study also has limitations. The generalizability of findings is limited because most participants were non-Latino White men. We only enrolled patients who could identify a family or friend supporter who was willing and able to be involved in patient care. However, this mirrors the selection process for existing clinical programs that engage patients’ existing informal supporters or caregivers. For patients without family supporters, peer support or more intensive nurse care management may be more appropriate. The study was conducted in the VA health care system, with integrated payment and widespread implementation of care based on patient-centered medical home teams. However, patient-centered medical home teams are being increasingly implemented, and our intervention models how supporters could be included in team-based care.53 Interactive voice response systems are not yet commonly available in health care systems. However, automated monitoring programs, by text, app, or telephone, are increasingly common and provide unique opportunities for family supporters to monitor and respond to patient health information asynchronously or from a distance. Patients in our trial started with high baseline activation and self-efficacy scores; patients with lower levels of activation and efficacy may experience different outcomes. We evaluated 2 primary outcomes and several prespecified secondary outcomes without adjusting P value thresholds for multiple testing, as per our prespecified statistical analysis plan. The clinical significance of results with confidence intervals should be interpreted with this in mind. In addition, using both HbA1c levels and SBP for eligibility may have limited our ability to detect change in either separate outcome. However, our intervention sessions and choice of complication risk as the primary physiological outcome reflect that both glycemic and BP control are associated with lower risk of diabetes complications.54,55 Our success in recruiting patients who met both criteria suggests that diabetes programs may be more appealing to participants if multiple risks for complications are addressed.
Conclusions
The CO-IMPACT dyadic intervention successfully engaged family supporters in adults’ diabetes care in ways participants found valuable and led to improved patient activation and self-efficacy in managing diabetes. Our findings from this randomized clinical trial indicate that increasing family supporters’ engagement in the care of adults with diabetes is feasible and may improve key behavioral determinants. Future studies should investigate whether interacting more directly with patients’ supporters and targeting patients with higher needs for support would help translate the observed benefits into physiological improvements.
Trial Protocol
eTable 1. Key Study Measure Details
eReferences
eTable 2. Predictors of Enrollment Among the 1119 Patients With Diabetes Sent a Recruitment Letter
eTable 3. Unadjusted Changes in Patient Outcomes by Study Arm, Baseline to 12 Months
eTable 4. Intervention Effect on Baseline–to–12-Month Changes in Patient Outcomes From Multivariable Linear Regression, Full Model Results
eTable 5. Patient and Supporter Ratings of Experience With CO-IMPACT Intervention Components
eTable 6. Qualitative Themes Among CO-IMPACT Intervention Arm Patient and Supporter Participant Comments
eFigure. CO-IMPACT IVR Topic Flow Chart
eAppendix. CO-IMPACT Intervention Materials Toolkit Location
Data Sharing Statement
References
- 1.Fang M, Wang D, Coresh J, Selvin E. Trends in diabetes treatment and control in US adults, 1999-2018. N Engl J Med. 2021;384(23):2219-2228. doi: 10.1056/NEJMsa2032271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosland AM, Heisler M, Choi HJ, Silveira MJ, Piette JD. Family influences on self-management among functionally independent adults with diabetes or heart failure: do family members hinder as much as they help? Chronic Illn. 2010;6(1):22-33. doi: 10.1177/1742395309354608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silliman RA, Bhatti S, Khan A, Dukes KA, Sullivan LM. The care of older persons with diabetes mellitus: families and primary care physicians. J Am Geriatr Soc. 1996;44(11):1314-1321. doi: 10.1111/j.1532-5415.1996.tb01401.x [DOI] [PubMed] [Google Scholar]
- 4.Woods SB, Bridges K, Carpenter EN. The critical need to recognize that families matter for adult health: a systematic review of the literature. Fam Process. 2020;59(4):1608-1626. doi: 10.1111/famp.12505 [DOI] [PubMed] [Google Scholar]
- 5.Strom JL, Egede LE. The impact of social support on outcomes in adult patients with type 2 diabetes: a systematic review. Curr Diab Rep. 2012;12(6):769-781. doi: 10.1007/s11892-012-0317-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luttik ML, Jaarsma T, Moser D, Sanderman R, van Veldhuisen DJ. The importance and impact of social support on outcomes in patients with heart failure: an overview of the literature. J Cardiovasc Nurs. 2005;20(3):162-169. doi: 10.1097/00005082-200505000-00007 [DOI] [PubMed] [Google Scholar]
- 7.Lett HS, Blumenthal JA, Babyak MA, Strauman TJ, Robins C, Sherwood A. Social support and coronary heart disease: epidemiologic evidence and implications for treatment. Psychosom Med. 2005;67(6):869-878. doi: 10.1097/01.psy.0000188393.73571.0a [DOI] [PubMed] [Google Scholar]
- 8.Nicklett EJ, Heisler MEM, Spencer MS, Rosland AM. Direct social support and long-term health among middle-aged and older adults with type 2 diabetes mellitus. J Gerontol B Psychol Sci Soc Sci. 2013;68(6):933-943. doi: 10.1093/geronb/gbt100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Powers MA, Bardsley JK, Cypress M, et al. Diabetes self-management education and support in adults with type 2 diabetes: a consensus report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Sci Diabetes Self Manag Care. 2021;47(1):54-73. doi: 10.1177/0145721720987936 [DOI] [PubMed] [Google Scholar]
- 10.Hogan BE, Linden W, Najarian B. Social support interventions: do they work? Clin Psychol Rev. 2002;22(3):383-442. doi: 10.1016/S0272-7358(01)00102-7 [DOI] [PubMed] [Google Scholar]
- 11.Thirsk LM, Schick-Makaroff K. Family interventions for adults living with type 2 diabetes mellitus: a qualitative meta-synthesis. Patient Educ Couns. 2021;104(12):2890-2899. doi: 10.1016/j.pec.2021.04.037 [DOI] [PubMed] [Google Scholar]
- 12.Rosland AM, Piette JD. Emerging models for mobilizing family support for chronic disease management: a structured review. Chronic Illn. 2010;6(1):7-21. doi: 10.1177/1742395309352254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whitehead L, Glass C, Coppell K. The effectiveness of goal setting on glycaemic control for people with type 2 diabetes and prediabetes: a systematic review and meta-analysis. J Adv Nurs. 2022;78(5):1212-1227. doi: 10.1111/jan.15084 [DOI] [PubMed] [Google Scholar]
- 14.Roddy MK, Nelson LA, Greevy RA, Mayberry LS. Changes in family involvement occasioned by FAMS mobile health intervention mediate changes in glycemic control over 12 months. J Behav Med. 2022;45(1):28-37. doi: 10.1007/s10865-021-00250-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosland AM, Heisler M, Piette JD. The impact of family behaviors and communication patterns on chronic illness outcomes: a systematic review. J Behav Med. 2012;35(2):221-239. doi: 10.1007/s10865-011-9354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yun Q, Ji Y, Liu S, et al. Can autonomy support have an effect on type 2 diabetes glycemic control? results of a cluster randomized controlled trial. BMJ Open Diabetes Res Care. 2020;8(1):e001018. doi: 10.1136/bmjdrc-2019-001018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee AA, Piette JD, Heisler M, Janevic MR, Rosland AM. Diabetes self-management and glycemic control: the role of autonomy support from informal health supporters. Health Psychol. 2019;38(2):122-132. doi: 10.1037/hea0000710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stawnychy MA, Teitelman AM, Riegel B. Caregiver autonomy support: a systematic review of interventions for adults with chronic illness and their caregivers with narrative synthesis. J Adv Nurs. 2021;77(4):1667-1682. doi: 10.1111/jan.14696 [DOI] [PubMed] [Google Scholar]
- 19.Lee AA, Piette JD, Heisler M, Janevic MR, Langa KM, Rosland AM. Family members’ experiences supporting adults with chronic illness: a national survey. Fam Syst Health. 2017;35(4):463-473. doi: 10.1037/fsh0000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janevic MR, Piette JD, Ratz DP, Kim HM, Rosland AM. Correlates of family involvement before and during medical visits among older adults with high-risk diabetes. Diabet Med. 2016;33(8):1140-1148. doi: 10.1111/dme.13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greene J, Hibbard JH. Why does patient activation matter? an examination of the relationships between patient activation and health-related outcomes. J Gen Intern Med. 2012;27(5):520-526. doi: 10.1007/s11606-011-1931-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosland AM, Nelson K, Sun H, et al. The patient-centered medical home in the Veterans Health Administration. Am J Manag Care. 2013;19(7):e263-e272. [PubMed] [Google Scholar]
- 23.Rosland AM, Piette JD, Trivedi R, et al. Engaging family supporters of adult patients with diabetes to improve clinical and patient-centered outcomes: study protocol for a randomized controlled trial. Trials. 2018;19(1):394. doi: 10.1186/s13063-018-2785-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.EQUATOR Network. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Accessed August 3, 2022. https://www.equator-network.org/reporting-guidelines/consort/
- 25.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 26.Hibbard JH, Stockard J, Mahoney ER, Tusler M. Development of the Patient Activation Measure (PAM): conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39(4, pt 1):1005-1026. doi: 10.1111/j.1475-6773.2004.00269.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conlin PR, Colburn J, Aron D, Pries RM, Tschanz MP, Pogach L. Synopsis of the 2017 US Department of Veterans Affairs/US Department of Defense Clinical Practice Guideline: management of type 2 diabetes mellitus. Ann Intern Med. 2017;167(9):655-663. doi: 10.7326/M17-1362 [DOI] [PubMed] [Google Scholar]
- 28.Miller WL, Crabtree BF. Clinical research: a multimethod typology and qualitative roadmap. In: Crabtree BF, Miller WF, eds. Doing Qualitative Research. 2nd ed. Sage Publications;1999:3-32. [Google Scholar]
- 29.Stevens RJ, Kothari V, Adler AI, Stratton IM; United Kingdom Prospective Diabetes Study (UKPDS) Group . The UKPDS risk engine: a model for the risk of coronary heart disease in type II diabetes (UKPDS 56). Clin Sci (Lond). 2001;101(6):671-679. doi: 10.1042/CS20000335 [DOI] [PubMed] [Google Scholar]
- 30.Toobert DJ, Hampson SE, Glasgow RE. The Summary of Diabetes Self-Care Activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943-950. doi: 10.2337/diacare.23.7.943 [DOI] [PubMed] [Google Scholar]
- 31.McGuire BE, Morrison TG, Hermanns N, et al. Short-form measures of diabetes-related emotional distress: the Problem Areas in Diabetes Scale (PAID)-5 and PAID-1. Diabetologia. 2010;53(1):66-69. doi: 10.1007/s00125-009-1559-5 [DOI] [PubMed] [Google Scholar]
- 32.Lorig K, Stewart A, Ritter P, Gonazalez V, Laurent D, Lynch J. Outcomes Measures for Health Education and Other Health Care Interventions. Sage Publications; 1996. [Google Scholar]
- 33.Maly RC, Frank JC, Marshall GN, Robin M, Reuben DB. Perceived Efficacy in Patient–Physician Interactions (PEPPI): validation of an instrument in older persons. J Am Geriatr Soc. 1998;46(7):889-894. doi: 10.1111/j.1532-5415.1998.tb02725.x [DOI] [PubMed] [Google Scholar]
- 34.Lingsma H, Roozenbeek B, Steyerberg E; IMPACT Investigators . Covariate adjustment increases statistical power in randomized controlled trials. J Clin Epidemiol. 2010;63(12):1391. doi: 10.1016/j.jclinepi.2010.05.003 [DOI] [PubMed] [Google Scholar]
- 35.Lee J, Helgeson VS, Van Vleet M, et al. Implications of we-talk for relationships and health among patients with type 1 diabetes and their spouses. J Soc Pers Relat. 2020;37(1):345-354. doi: 10.1177/0265407519865613 [DOI] [Google Scholar]
- 36.Rohrbaugh MJ, Mehl MR, Shoham V, Reilly ES, Ewy GA. Prognostic significance of spouse we talk in couples coping with heart failure. J Consult Clin Psychol. 2008;76(5):781-789. doi: 10.1037/a0013238 [DOI] [PubMed] [Google Scholar]
- 37.Struwe LA, Schmaderer MS, Zimmerman L. Changes in patient activation in a self-management intervention. West J Nurs Res. 2020;42(3):194-200. doi: 10.1177/0193945919848091 [DOI] [PubMed] [Google Scholar]
- 38.Hibbard JH, Greene J, Tusler M. Improving the outcomes of disease management by tailoring care to the patient’s level of activation. Am J Manag Care. 2009;15(6):353-360. [PubMed] [Google Scholar]
- 39.Hibbard JH, Greene J. The impact of an incentive on the use of an online self-directed wellness and self-management program. J Med internet Res. 2014;16(10):e217. doi: 10.2196/jmir.3239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang J, Ding S, Xiong S, Liu Z. Medication adherence and associated factors in patients with type 2 diabetes: a structural equation model. Front Public Health. 2021;9:730845. doi: 10.3389/fpubh.2021.730845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Juarez LD, Presley CA, Howell CR, Agne AA, Cherrington AL. The mediating role of self-efficacy in the association between diabetes education and support and self-care management. Health Educ Behav. Published online April 24, 2021. doi: 10.1177/10901981211008819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood). 2013;32(2):207-214. doi: 10.1377/hlthaff.2012.1061 [DOI] [PubMed] [Google Scholar]
- 43.Marks R, Allegrante JP, Lorig K. A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part I). Health Promot Pract. 2005;6(1):37-43. doi: 10.1177/1524839904266790 [DOI] [PubMed] [Google Scholar]
- 44.Zupa MF, Lee A, Piette JD, et al. Impact of a dyadic intervention on family supporter involvement in helping adults manage type 2 diabetes. J Gen Intern Med. Published online July 8, 2021. doi: 10.1007/s11606-021-06946-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Woodard LD, Adepoju OE, Amspoker AB, et al. Impact of patient-centered medical home implementation on diabetes control in the Veterans Health Administration. J Gen Intern Med. Published online April 2, 2018. doi: 10.1007/s11606-018-4386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosland AM, Wong E, Maciejewski M, et al. Patient-centered medical home implementation and improved chronic disease quality: a longitudinal observational study. Health Serv Res. 2018;53(4):2503-2522. doi: 10.1111/1475-6773.12805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trivedi RB, Szarka JG, Beaver K, et al. Recruitment and retention rates in behavioral trials involving patients and a support person: a systematic review. Contemp Clin Trials. 2013;36(1):307-318. doi: 10.1016/j.cct.2013.07.009 [DOI] [PubMed] [Google Scholar]
- 48.Regan T, Lambert SD, Kelly B. Uptake and attrition in couple-based interventions for cancer: perspectives from the literature. Psychooncology. 2013;22(12):2639-2647. doi: 10.1002/pon.3342 [DOI] [PubMed] [Google Scholar]
- 49.Cacchione PZ. The Recognize, Assist, Include, Support and Engage (RAISE) Family Caregivers Act. Clin Nurs Res. 2019;28(8):907-910. doi: 10.1177/1054773819876130 [DOI] [PubMed] [Google Scholar]
- 50.Reinhard SC, Young HM, Ryan E, Choula RB. The CARE Act Implementation: Progress and Promise. AARP Public Policy Institute; March 2019. Accessed November 29, 2021. https://www.hahusersgroup.org/wp-content/uploads/2021/06/The-Care-Act-Implementation-and-Progress.pdf [Google Scholar]
- 51.VA MISSION Act of 2018, S 2372, 115th Cong (2018). [Google Scholar]
- 52.Caregivers and Veterans Omnibus Health Services Act of 2010, S 1963, 111th Cong (2010).
- 53.Nelson KM, Helfrich C, Sun H, et al. Implementation of the patient-centered medical home in the Veterans Health Administration: associations with patient satisfaction, quality of care, staff burnout, and hospital and emergency department use. JAMA Intern Med. 2014;174(8):1350-1358. doi: 10.1001/jamainternmed.2014.2488 [DOI] [PubMed] [Google Scholar]
- 54.Clarke PM, Gray AM, Briggs A, Stevens RJ, Matthews DR, Holman RR; UKPDS 72 United Kingdom Prospective Diabetes Study . Cost-utility analyses of intensive blood glucose and tight blood pressure control in type 2 diabetes (UKPDS 72). Diabetologia. 2005;48(5):868-877. doi: 10.1007/s00125-005-1717-3 [DOI] [PubMed] [Google Scholar]
- 55.de Boer IH, Bangalore S, Benetos A, et al. Diabetes and hypertension: a position statement by the American Diabetes Association. Diabetes Care. 2017;40(9):1273-1284. doi: 10.2337/dci17-0026 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Key Study Measure Details
eReferences
eTable 2. Predictors of Enrollment Among the 1119 Patients With Diabetes Sent a Recruitment Letter
eTable 3. Unadjusted Changes in Patient Outcomes by Study Arm, Baseline to 12 Months
eTable 4. Intervention Effect on Baseline–to–12-Month Changes in Patient Outcomes From Multivariable Linear Regression, Full Model Results
eTable 5. Patient and Supporter Ratings of Experience With CO-IMPACT Intervention Components
eTable 6. Qualitative Themes Among CO-IMPACT Intervention Arm Patient and Supporter Participant Comments
eFigure. CO-IMPACT IVR Topic Flow Chart
eAppendix. CO-IMPACT Intervention Materials Toolkit Location
Data Sharing Statement