Abstract
Background
Exercise parameters are not routinely incorporated in decision making for cardiac resynchronisation therapy (CRT). Submaximal exercise parameters better reflect daily functional capacity of heart failure patients than parameters measured at maximal exertion, and may therefore better predict response to CRT. We compared various exercise parameters, and sought to establish which best predict CRT response.
Methods
In 31 patients with chronic heart failure (61% male; age 68±7 years), submaximal and maximal cycling testing was performed before and 3 months after CRT. Submaximal oxygen onset (τVO2 onset) and recovery kinetics (τVO2 recovery), peak oxygen uptake (VO2 peak) and oxygen uptake efficiency slope (OUES) where measured. Response was defined as ≥15% relative reduction in end-systolic volume.
Results
After controlling for age, New York Heart Association and VO2 peak, fast submaximal VO2 kinetics were significantly associated with response to CRT, measured either during onset or recovery of submaximal exercise (area under the curve, AUC=0.719 for both; p<0.05). By contrast, VO2 peak (AUC=0.632; p=0.199) and OUES (AUC=0.577; p=0.469) were not associated with response. Among patients with fast onset and recovery kinetics, below 60 s, a significantly higher percentage of responders was observed (91% and 92% vs 43% and 40%, respectively).
Conclusions
Impaired VO2 kinetics may serve as an objective marker of submaximal exercise capacity that is age-independently associated with non-response following CRT, whereas maximal exercise parameters are not. Assessment of VO2 kinetics is feasible and easy to perform, but larger studies should confirm their clinical utility.
Keywords: HEART FAILURE; Cardiomyopathy, Dilated; Pacemaker, Artificial; Outcome Assessment, Health Care; Heart Failure, Systolic
WHAT IS ALREADY KNOWN ON THIS TOPIC
Novel ECG and imaging markers are widely investigated to improve patient selection criteria for cardiac resynchronisation therapy. Exercise parameters may add to prediction models, but whether maximal or submaximal parameters are better remain unclear.
WHAT THIS STUDY ADDS
Not maximal exercise parameters, but impaired submaximal VO2 kinetics are associated with non-response, independently of age, New York Heart Association or VO2 peak.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Incorporation of VO2 kinetics in clinical practice omits the need for strenuous exertion, while assessment is easy and better reflects daily functional capacity.
Introduction
Cardiac resynchronisation therapy (CRT) is an effective treatment for patients with chronic heart failure (HF) and a reduced left ventricular (LV) ejection fraction (EF). Although patient eligibility is currently primarily based on the presence of left bundle branch block (LBBB) on the ECG, approximately 30%–40% of patients are non-responders.1 2 Research has focused on deriving new and more advanced markers from the ECG or echocardiogram to better predict response after CRT.3 However, sensitivity and specificity should be further improved in order to truly affect clinical decision making.4 5
Incorporation of exercise parameters may further improve patient selection, but these are not yet part of routine clinical decision making for CRT.5 However, several studies showed a positive relationship between pre-implantation maximal exercise capacity (peak oxygen uptake; VO2 peak) and CRT response,6 7 suggesting that more severely impaired HF patients are less likely to achieve an echocardiographic response. This may be because their exercise capacity is more severely limited by peripheral derangements. Yet, studies investigating the influence of baseline ‘exercise’ characteristics on echocardiographic response are relatively scarce, as opposed to trials investigating electrical and mechanical substrates.3 5
A barrier for the widespread implementation of assessment of maximal exercise capacity is that symptom limiting exercise testing is burdensome, and often not truly achievable for patients with severe HF.5 8 Therefore, ‘submaximal’ exercise testing could be a valuable alternative. One of the submaximal exercise parameters that reflect exercise capacity are oxygen kinetics.9–12 Oxygen kinetics describe the rate of increase of oxygen uptake during a short constant-load submaximal exercise bout (VO2 onset kinetics) and the rate of decline during recovery (VO2 recovery kinetics). Assessment of VO2 kinetics is safe, feasible and not physically burdensome for severely impaired patients, as it only requires an exercise effort of several minutes below the anaerobic threshold. In HF patients, oxygen kinetics were shown to be reproducible and well correlated with exercise capacity and prognosis.12–15 In addition, VO2 recovery kinetics were shown to be useful as a predictor of the effect of an exercise training programme.16
The main goal of this study was to investigate whether submaximal exercise testing, and in particular oxygen uptake kinetics, can be used to predict the response to CRT. In addition, use of submaximal exercise parameters was compared with conventional parameters that require maximal exertion.
Methods
Patient population and study protocol
We performed a prospective multicentre trial in an open cohort of patients. A total of 34 patients with HF eligible for CRT were enrolled. Inclusion criteria were based on ESC guidelines implemented at the time of inclusion: (1) New York Heart Association (NYHA) II or III HF despite optimal medical treatment; (2) EF ≤35% and (3) QRS-duration ≥120 ms. Participation in an exercise training programme in the last year and during the study were excluded.
Resting echocardiography and two cardiopulmonary exercise tests (CPET) were performed at baseline and 3 months after CRT implantation. After echocardiography, patients performed a submaximal constant load CPET, followed by a symptom limited maximal CPET, both with respiratory gas analysis. In order to familiarise the patient with the test procedure, and to determine the workload for the constant load CPET, an additional symptom limited CPET was performed at baseline. Implantation of CRT devices was performed according to standard techniques and local protocols in the Catharina Hospital, Eindhoven and University Medical Center, Utrecht, the Netherlands. Algorithms based on the intracardiac electrogram were used for device optimisation.
Echocardiographic response was used as a well-accepted surrogate marker of long-term prognosis after CRT, and was defined as a decline in end-systolic volume (ESV)≥15%, 3 months after implantation.17 A clinical response was defined as an increase in VO2 peak ≥1 mL/kg/min.18 19
Echocardiography
Standard two-dimensional, colour and spectral Doppler measurements were performed using a Phillips Epic 7C echo machine and an X5-1 transducer. LV dimensions were measured in the parasternal long axis view. EF was determined using the Simpson’s rule algorithm by tracing the LV 2D-area in both standard apical two-chamber and four-chamber view at end-systole and end-diastole. Images were analysed independently by two physicians.
Exercise testing
All exercise tests were performed in an upright seated position on an electromagnetically braked cycle ergometer (Lode Corrival; Lode BV, Groningen, The Netherlands). A 12-lead ECG was registered continuously. During the test, ventilatory parameters were measured breath-by-breath (ZAN 680 USB; ZAN Messgeräte, Oberthulba, Germany) and were averaged over 10 s intervals after the removal of outliers (values >3 SD from the local mean). Volume and gas analysers were calibrated before each test.
Maximal CPET consisted of a symptom-limited test using an individualised ramp protocol for a total test duration of 8–12 min. The test was preceded by 4 min of unloaded pedalling and ended when the patient was unable to maintain the required pedalling frequency. The results of this baseline test were used in order to determine the workload for the submaximal CPET. Exercise performance was determined as the maximally achieved resistance at the end of the exercise. VO2 peak was defined as the final 20 s averaged value of the maximal CPET. Ventilatory aerobic threshold (VAT) was assessed by the V-slope method, using the mean of the value calculated by two blinded physicians. The efficiency of CO2 exchange was measured by assessing the required minute ventilation for CO2 elimination (ie, VE/VCO2). Finally, the oxygen uptake efficiency slope (OUES) was measured, as it is a reliable and reproducible measure of effort-independent cardiopulmonary reserve. The OUES was derived from the logarithmic relation between VO2 and VE during incremental exercise.20
Submaximal constant load exercise testing commenced with a 2 min resting period, followed by a 6 min bout at 80% of the workload corresponding to the VAT achieved during the preceding maximal exercise test. Patients were instructed to maintain a pedalling frequency of 70 rotations per minute. After the load phase, there was a 5 min recovery phase with no movement of the leg in order to assess VO2 kinetics.
Analysis of oxygen uptake kinetics
Analysis of VO2 kinetics during onset and recovery of the constant load tests was reported previously.11 All data were resampled into 10 s intervals and the first 20 s of the VO2 data (eg, exercise onset) were omitted. This was done because during this period (cardiodynamic phase), increases in VO2 merely reflect an increase in pulmonary blood flow, rather than actual changes in tissue gas exchange. A non-linear least squares regression procedure (Matlab V.8.6 R2015b, MathWorks, USA) was applied to the onset phase in order to calculate the time constant of VO2 onset (formula 1).
Formula 1. VO2 kinetics for onset phase.
where A=amplitude during exercise onset; Td=time delay (seconds) and τ=time constant (seconds). Recovery kinetics of VO2 were calculated as well (formula 2); Y= VO2.
Formula 2. VO2 kinetics for recovery phase.
where A=amplitude during recovery from steady state; Td=time delay (seconds) and τ=time constant (seconds); Y= VO2
Statistical analysis
Data were analysed using SPSS statistics (V.25.0, SPSS). Continuous variables were tested for normality using a Shapiro-Wilk test and were expressed as the mean±SD. Data with a normal distribution were evaluated using the paired samples t-test for within group differences and by independent samples t-test concerning between-group differences. Categorical data were presented as absolute count and were compared with a Fisher’s exact test.
Receiver operator characteristic curves were created for exercise-related predictors of CRT outcome. Optimal cut-off values were based on the level that resulted in the highest (sensitivity – [1 – specificity]), and subsequently an integer within this numeric range was selected in order to prevent unjustified overfitting of data. The relationship between these predictors and echocardiographic response was established using analysis of covariance with age, NYHA class and peak VO2 as covariates. Agreement between exercise kinetics and NYHA class was assessed using Cohen’s kappa coefficient. All statistical tests performed were two tailed and a p<0.05 was considered statistically significant.
Results
A total of 34 patients were included. One patient did not complete the CPET on the visit after CRT implantation due to dizziness when sitting on the bike, two other patients did not show up for the examinations post-CRT without giving reason. A total of 31 patients completed all tests; all patients underwent CRT implantation, without complications. Paired respiratory gas analysis data during (sub)maximal exercise testing could be successfully retrieved. The majority of the population were male (61%) with non-ischaemic cardiomyopathy (58%) and a mean age of 68±7 years (table 1). An overview of changes in echocardiographic and exercise parameters before and after CRT of the total study population is presented in online supplemental table 1.
Table 1.
Characteristics at baseline of study population
| Characteristic | Total (n=31) |
Non-responder (n=11) |
Responder (n=20) |
P value |
| Age (years) | 68.4±7.1 | 72.2±4.0 | 66.3±7.6 | 0.026 |
| Male (%) | 19 (61) | 10 (91) | 9 (45) | 0.020 |
| Ischaemic aetiology—n (%) | 13 (42) | 7 (64) | 6 (30) | 0.128 |
| NYHA III—n (%) | 21 (69) | 10 (91) | 11 (55) | 0.055 |
| HF duration (months) | 16 (6–66) | 39 (14–66) | 13 (6–60) | 0.215 |
| Sinus rhythm—n (%) | 23 (82) | 8 (73) | 18 (90) | 0.224 |
| LBBB—n (%) | 26 (84) | 8 (80) | 18 (95) | 0.267 |
| QRS width (ms) | 154±17 | 157±21 | 153±15 | 0.556 |
| LVEF (%) | 26.6±8.1 | 24.5±8.6 | 27.7±7.7 | 0.298 |
| ACE/ARB—n (%) | 31 (100) | 11 (100) | 20 (100) | 1.000 |
| Beta blocker—n (%) | 28 (90) | 10 (91) | 18 (90) | 1.000 |
| VO2 peak (mL/kg/min) | 16.4±5.4 | 14.8±3.9 | 17.3±6.1 | 0.215 |
Values are presented as mean±SD, median with IQR, number or percentage.
ARB, angiotensin II receptor blocker; LBBB, left bundle branch block; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; VO2 peak, peak oxygen uptake capacity.
openhrt-2022-002047supp001.pdf (109.6KB, pdf)
Echocardiographic responders versus non-responders
Relative changes in exercise-related variables between the group of echocardiographic non-responders versus responders are presented in table 2. No significant between-group differences in exercise capacity were observed at baseline. Yet, in contrast with non-responders, only responders showed a significant improvement in peak power output (P peak), VO2 peak, OUES and VE/VCO2 slope following CRT. Although τVO2 recovery was significantly reduced after CRT on group level (77±39 vs 61±18; p=0.030), no significant changes were seen when stratified according to response groups. In both responders and non-responders, the extent of LVESV-reduction and clinical improvement (∆VO2 peak) after CRT were not correlated significantly (R=0.284; p=0.135).
Table 2.
Relative changes in echocardiographic and exercise-related variables in patients with or without echocardiographic response, before and 3 months after CRT implantation
| Variable | Non-responder (n=11) | Responder (n=20) | ||
| Baseline | 3 months | Baseline | 3 months | |
| Rest | ||||
| EF (%) | 24.5±8.6 | 24.7±8.0 | 27.7±7.7 | 40.5±8.8* |
| EDV (mL) | 289±105 | 287±101 | 221±72 | 171±52* |
| ESV (mL) | 223±102 | 218±92 | 161±59† | 106±45* |
| Maximal | ||||
| P peak (W) | 97±35 | 102±39 | 107±47 | 118±47* |
| VO2 peak (mL/kg/min) | 14.8±3.9 | 16.0±3.2 | 17.3±6.1 | 19.7±6.6* |
| OUES | 1445±479 | 1610±520 | 1610±521 | 1943±586* |
| VE/VCO2 slope | 38.9±9.9 | 37±6.1 | 34.9±8.4 | 31.7±7.0* |
| Submaximal | ||||
| τVO2 on (s) | 87.2±39.1 | 90.1±48.8 | 58.6±34.2 | 55.4±32.2 |
| τVO2 rec (s) | 79.5±21.4 | 67.0±19.4 | 75.0±47.3 | 57.6±17.3 |
Values presented as mean±SD.
*p<0.05 for within-group difference.
†p<0.05 for between group difference at baseline.
CRT, cardiac resynchronisation therapy; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; OUES, oxygen uptake efficiency slope; P, performance; VE/VCO2 slope, minute ventilation for CO2 elimination; τVO2, time constant of oxygen uptake.
Exercise-related predictors for echocardiographic response
The optimal cut-off values of various exercise parameters, measured at baseline, to predict echocardiographic response are shown in table 3. For τVO2 onset and τVO2 recovery, the highest area under the curve (AUC) was found at a cut-off of 60 s (AUC 0.719; p<0.05 for both). τVO2 onset ≤60 s predicted echocardiographic response with a specificity of 90% and a sensitivity of 61%, whereas τVO2 recovery predicted echocardiographic response with a specificity of 90% and a sensitivity of 67%. Maximal exercise parameters (VO2 peak, VE/VCO2 slope, OUES) showed lower AUC’s (0.632, 0.600, 0.577, respectively), which were all not significant (online supplemental table 2). None of the exercise parameters were significantly associated with a clinical response (p>0.05 for all).
Table 3.
Baseline test characteristics of exercise-related predictors for echocardiographic response 3 months after CRT implantation
| Predictor | AUC | P value | Cut-off value | Met? | Total | Sens (%) | Spec (%) |
| τVO2 onset | 0.719 | 0.029 | ≤60 s | Yes | 11 | 61 | 90 |
| No | 16 | ||||||
| τVO2 recovery | 0.719 | 0.025 | ≤60 s | Yes | 12 | 67 | 90 |
| No | 15 | ||||||
| VO2 peak | 0.632 | 0.199 | ≥18 mL/kg/min | Yes | 12 | 50 | 82 |
| No | 18 | ||||||
| VE/VCO2 slope | 0.600 | 0.371 | ≤40 | Yes | 22 | 80 | 45 |
| No | 8 | ||||||
| OUES | 0.577 | 0.469 | ≥1500 | Yes | 14 | 55 | 73 |
AUC, area under the curve; CRT, cardiac resynchronisation therapy; OUES, oxygen uptake efficiency slope; sens, sensitivity; spec, specificity; VE/VCO2 slope, minute ventilation for CO2 elimination; τVO2, time constant of oxygen uptake.
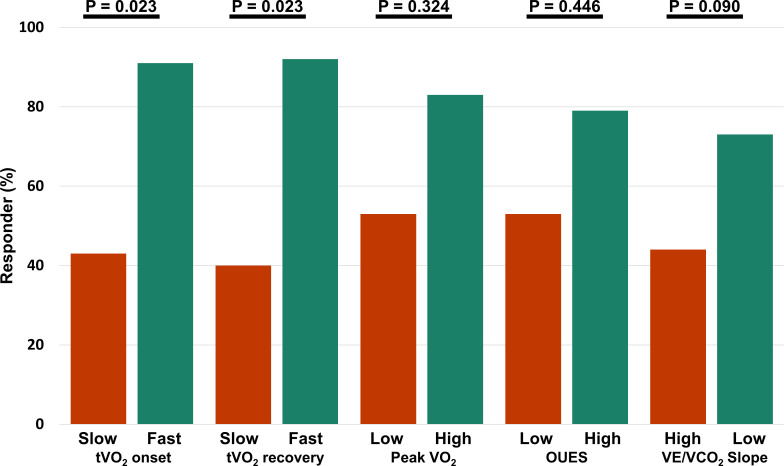
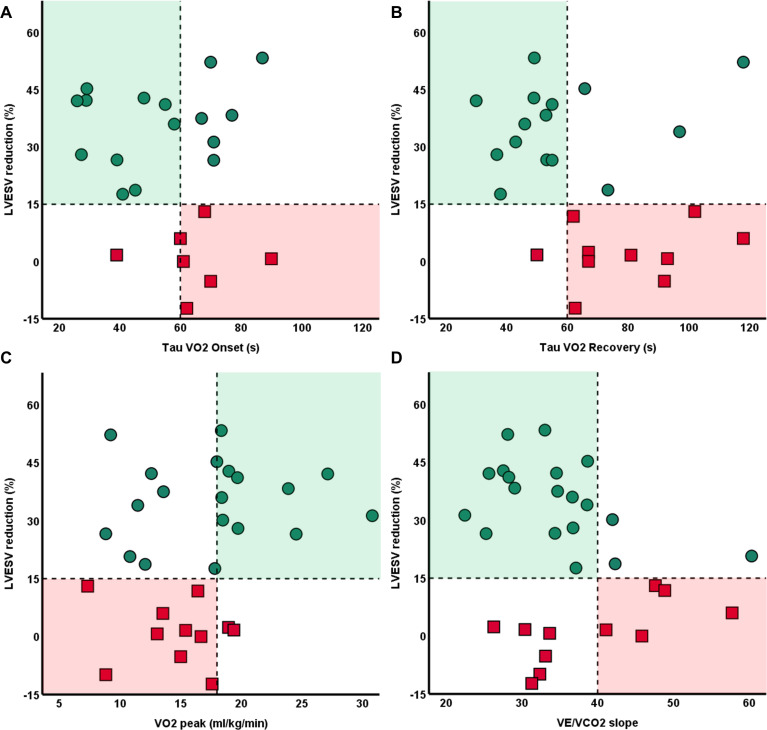
Only among patients with fast onset and recovery kinetics (τVO2 onset and τVO2 recovery ≤60 s) at baseline, a significantly higher percentage of responders were observed (92% and 92% vs 44% and 40%, respectively). By contrast, maximal parameters (VO2 peak, OUES, VE/VCO2 slope) could not differentiate response after CRT. Controlling for age, NYHA class and VO2 peak as covariates did not significantly influence these results (figure 1). Patients with fast τVO2 recovery demonstrated significantly more ESV-reduction than patients with delayed τVO2 recovery (32±14 vs 17±21; p=0.033), and had a twofold larger increase in LVEF (online supplemental table 3). A scatterplot with individual values of submaximal VO2 kinetics at baseline and their association with reverse remodelling is shown in figure 2.
Figure 1.
Difference in echocardiographic response based on various exercise-related parameters at baseline, with significance corrected for age, NYHA and VO2 peak. Patients with poor (red) and good (green) testing results during (sub)maximal exercise. NYHA, New York Heart Association; OUES, oxygen uptake efficiency slope; VE/VCO2, minute ventilation for carbon dioxide elimination; VO2, oxygen uptake.
Figure 2.
Distribution of patients with (green dots) and without (red squares) echocardiographic response relative to their exercise performance at baseline. Responders, defined as ≥15% decrease in LVESV, are characterised by faster baseline oxygen VO2 during onset (A) and recovery (B) of exercise. By contrast, no significant association were observed when evaluating VO2 peak (C) or VE/VCO2 slope (D). LVESV, left ventricular end-systolic-volume; VE/VCO2, minute ventilation for carbon dioxide elimination; VO2, oxygen uptake.
Baseline onset and recovery VO2 kinetics were not correlated to each other (R=0.092; p=0.640), which suggests their interaction with echocardiographic response is due to independent mechanisms. Patients that demonstrated an echocardiographic response despite poor VO2 recovery kinetics were more frequently female (p=0.002) and tended to have a smaller LVESV and LVEDV (p<0.01, for both). For tVO2 onset, these patients had a higher LVEF (p=0.008).
Comparison with NYHA functional class
Baseline NYHA functional class was not significantly associated with response (AUC=0.680; p=0.103). When comparing NYHA II and NYHA III, similar reductions in ESV were observed (28±15 vs 20±21; p=0.289). In patients classified as NYHA III functional impairment, fast τVO2 onset and recovery kinetics <60 s were seen in 35% of patients regardless. There was no agreement between NYHA class and VO2 kinetics dichotomised at 60 s (Cohen’s kappa=0.000), or NYHA class and VO2 peak dichotomised at 18 mL/kg/min (Cohen’s kappa=0.000).
Factors associated with oxygen kinetics
Patients were stratified according to fast and slow baseline τVO2 recovery, that is, below or above 60 s, respectively. Age was significantly higher in patients with prolonged VO2 recovery, as compared with fast recovery time (72±4 vs 64±8; p=0.001). Importantly, in addition to peak VO2, no other associations were found with other well-known patient characterises associated with response, including sex, aetiology, NYHA, LBBB morphology or QRS duration (online supplemental table 4). Age correlated with τVO2 recovery (R=0.453; p=0.016), but not with τVO2 onset (R=0.164; p=0.403). Lastly, no significant differences were found when comparing patients with fast and slow oxygen kinetics during ‘onset’ of submaximal exercise.
Discussion
This study suggests that oxygen uptake kinetics during onset and recovery from submaximal exercise may add to the prediction of the effect of CRT. An association with volumetric response was seen independently of age, VO2 peak and NYHA class. Conversely, more conventional exercise parameters such as VO2 peak, OUES and VE/VCO2 slope, measured during peak exercise intensity, were not significantly associated with CRT response. Moreover, both submaximal VO2 kinetics and VO2 peak were not significantly associated with NYHA functional class, suggesting limited clinical representability of this assessment.
Exercise-related predictors of response to CRT
To our knowledge, this is one of the first studies that show that submaximal exercise parameters can help in predicting outcome of CRT. Previously, Berger et al showed that the OUES at baseline was related to the CRT response.21 However, we were unable to reproduce the high sensitivity and specificity of OUES that was reported. VO2 peak and VE/VCO2 slope were shown to be related to CRT response in other studies, possibly because of their higher sample size.6 7 Regardless, our results point towards a superior predictive value of VO2 kinetics, also when directly compared with maximal exercise parameters. In addition, our findings were consistent, both during onset and recovery of submaximal exercise. Moreover, determining a valid OUES in patients with severe HF requires exercise testing above anaerobic threshold,22 whereas this is not required for determining VO2 kinetics.
Lastly, although very easy to use and therefore widely adopted into clinical practice, assessment of NYHA functional class is subjective and non-specific. Indeed, interobserver agreement for NYHA is only 54%,23 and NYHA poorly reflects exercise capacity.24 This in line with our results, which showed no agreement with objective assessments of (sub)maximal exercise capacity, and no significant association with response to CRT.
Pathophysiology of impaired submaximal exercise performance
The association between low VO2 peak at baseline and better CRT-induced improvements in VO2 peak has been established previously.25 It should, however, be noted that increasingly worse VO2 peak may be associated with improved clinical response of the same parameter. As such, and in line with our findings, actual prognostic benefit from CRT has been linked to higher levels of VO2 peak at baseline in 181 CRT recipients before.26 Conversely, τVO2 recovery kinetics can be used to objectively quantify one’s (in)ability to physiologically recover from submaximal exercise. Hence, opposed to VO2 peak and NYHA, submaximal testing may be more representative of the functional capacity of patients with HF during ordinary daily activity.
A possible explanation for the finding that patients with slow VO2 kinetics are less likely to respond to CRT lies in the pathophysiological background of delayed VO2 kinetics in HF. In fact, submaximal VO2 kinetics reflect the ability of the heart to increase cardiac output and the capacity of skeletal muscles to increase oxygen utilisation (online supplemental graphical abstract). This, in turn, is determined by peripheral vascular function and the metabolic capacity of skeletal muscles.27 In fact, previous studies showed that peripheral vascular function is impaired28 29 and related to prolonged recovery after submaximal exercise in HF patients.30 Peripheral vascular function is, among others, influenced by neurohumoral and systemic inflammatory processes. Impaired peripheral haemodynamics, reflected in impaired VO2 kinetics, could therefore point towards a proinflammatory state and an unbalanced neurohumoral system, which also impairs myocardial adaptation to CRT.
openhrt-2022-002047supp002.pdf (242.5KB, pdf)
In turn, significant improvements in tVO2 recovery were seen during the follow-up. Because CRT primarily alters cardiac function, actual changes in tVO2 recovery ‘following CRT’ are most likely predominantly owed to improved local oxygen delivery at the skeletal muscle level. Further research is warranted to assess whether CRT can alter local oxygen utilisation as well.
Submaximal exercise-capacity and age
Age is a well-documented independent cause of CRT non-response, but the actual reason for this has remained largely unexplained.3 31 In our study, older age was associated with both echocardiographic non-response and prolonged peripheral oxygen uptake recovery kinetics, irrespective of sex or HF aetiology. This link is not surprising, since decreased skeletal muscle performance has been shown to be age-dependent before.32 33 However, VO2 recovery kinetics were more strongly associated with non-response, also when corrected for differences in age, NYHA class and peak exercise capacity. Submaximal exercise capacity may, therefore, be a more specific marker of response to CRT than age, while also being more objective than assessment of NYHA functional class. Moreover, submaximal VO2 recovery kinetics can be assessed as a continuum, as opposed to NYHA functional class.
Although current prediction models of CRT-response incorporate age and indices of electrical and mechanical dyssynchrony,31 clinical information of exercise capacity is currently lacking and perhaps insufficiently explored in this context. To date, no literature has related VO2 kinetics to CRT response. Our results, therefore, point for the first time towards the potential role of impaired oxygen uptake kinetics as a mechanism of non-response. Clearly, future studies are warranted to investigate whether performing submaximal exercise tests in patients with dyssynchronous HF can be of added value in the refinement of patient selection criteria.
Clinical implications
To our knowledge, this is the first study that shows that submaximal exercise parameters can be used to predict CRT response. Although we acknowledge that larger prospective studies are warranted to establish definite cut-off values, the utility of submaximal VO2 kinetics for predicting echocardiographic response after CRT was superior to VO2 peak. Previous research34 has suggested VO2 peak to be more predictive than submaximal prognostic markers such as the 6 min walking test.35 36 However, submaximal VO2 kinetics provides physicians with reliable prognostic and objective information on the functional status of the individual patient, that is, the ability to adapt to and recover from daily activities. Assessment of VO2 kinetics may, therefore, be a more accurate and potentially better reproducible method to determine one’s submaximal exercise capacity as opposed to better known and more widely implemented indices such as the 6 min walking test.15 37 It also does not require achieving peak exertion, which is unobtainable in as many as half of all patients with HF.8 In addition, assessment of VO2 kinetics is non-invasive, objective, affordable and can easily be performed repetitively over time as an indicator of clinical status.
Limitations
First, as a relatively small number of patients were included, statistical power was limited. As such, a type 2 error should not be ruled out for conventional exercise parameters, and no adequately powered subgroup analyses could be performed. Therefore, especially results concerning exercise-related predictors should be interpreted with caution, and larger prospective trials are warranted before finite conclusions can be drawn whether potential predictors should be applied in clinical practice. Nonetheless, to our best knowledge, incorporation of submaximal exercise-related predictors has not been performed previously, and therefore, extends our current knowledge of eligible CRT patients. Second, a relatively short follow-up duration was carried out, possibly resulting in overestimation of the percentage of non-responders as a consequence of a potential delayed effect. Alternatively, some patients illustrate temporary effects after CRT that diminish over time, and our response percentages were similar to those observed in larger trials.8 9 Third, day-to-day reproducibility of recovery kinetics are superior relative to onset kinetics, rendering them most clinically suitable.15
Conclusion
This study showed that delayed onset and recovery of peripheral oxygen uptake kinetics at baseline are associated with non-response in CRT, whereas parameters measured during maximal exertion were not. Although this association was independent of age, prolonged recovery from submaximal exercise may also—in part—explain the relative lack of reverse remodelling as more frequently seen in older individuals. Since assessment of VO2 kinetics is feasible, objective and easy to perform, requiring only a short bout of submaximal exercise, this method is promising for use in clinical practice. However, additional research in larger populations, allowing for multivariate analysis, is needed to establish definite cut-off values, and show whether submaximal exercise testing can be of added value in the clinical decision process of CRT as a whole.
Footnotes
PW and TS contributed equally.
Contributors: PW was involved in data analysis and representation, statistical analysis, drafting the manuscript, and accepts full responsibility for the finished work as guarantor. TS was involved in acquisition, data collection, data analysis and drafting the manuscript. VN and RFS were involved in acquisition, data collection, data analysis and revision of the manuscript. HK was involved in study design and revision of the manuscript.
Funding: This study was supported by a research grant from Medtronic Trading NL B.V., Eindhoven, the Netherlands.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data will be shared on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was approved by local Medical Ethics Committee at Máxima MC. NL33115.015.10. Participants gave informed consent to participate in the study before taking part.
References
- 1.Yu C-M, Abraham WT, Bax J, et al. Predictors of response to cardiac resynchronization therapy (PROSPECT)--study design. Am Heart J 2005;149:600–5. 10.1016/j.ahj.2004.12.013 [DOI] [PubMed] [Google Scholar]
- 2.Auricchio A, Prinzen FW. Non-responders to cardiac resynchronization therapy: the magnitude of the problem and the issues. Circ J 2011;75:521–7. 10.1253/circj.CJ-10-1268 [DOI] [PubMed] [Google Scholar]
- 3.Wouters PC, van Everdingen WM, Vernooy K, et al. Does mechanical dyssynchrony in addition to QRS area ensure sustained response to cardiac resynchronization therapy? Eur Heart J Cardiovasc Imaging 2021. doi: 10.1093/ehjci/jeab264. [Epub ahead of print: 06 Dec 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung ES, Leon AR, Tavazzi L, et al. Results of the predictors of response to crt (prospect) trial. Circulation 2008;117:2608–16. 10.1161/CIRCULATIONAHA.107.743120 [DOI] [PubMed] [Google Scholar]
- 5.Fixsen LS, Wouters PC, Lopata RGP, et al. Strain-based discoordination imaging during exercise in heart failure with reduced ejection fraction: feasibility and reproducibility. BMC Cardiovasc Disord 2022;22:127. 10.1186/s12872-022-02578-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mastenbroek MH, Van't Sant J, Versteeg H, et al. Relationship between reverse remodeling and cardiopulmonary exercise capacity in heart failure patients undergoing cardiac resynchronization therapy. J Card Fail 2016;22:385–94. 10.1016/j.cardfail.2015.08.342 [DOI] [PubMed] [Google Scholar]
- 7.Piepoli MF, Villani GQ, Corrà U, et al. Time course of effects of cardiac resynchronization therapy in chronic heart failure: benefits in patients with preserved exercise capacity. Pacing Clin Electrophysiol 2008;31:701–8. 10.1111/j.1540-8159.2008.01073.x [DOI] [PubMed] [Google Scholar]
- 8.Chase PJ, Kenjale A, Cahalin LP, et al. Effects of respiratory exchange ratio on the prognostic value of peak oxygen consumption and ventilatory efficiency in patients with systolic heart failure. JACC Heart Fail 2013;1:427–32. 10.1016/j.jchf.2013.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niemeijer VM, Snijders T, Verdijk LB, et al. Skeletal muscle fiber characteristics in patients with chronic heart failure: impact of disease severity and relation with muscle oxygenation during exercise. J Appl Physiol 2018;125:1266–76. 10.1152/japplphysiol.00057.2018 [DOI] [PubMed] [Google Scholar]
- 10.Sietsema KE, Ben-Dov I, Zhang YY, et al. Dynamics of oxygen uptake for submaximal exercise and recovery in patients with chronic heart failure. Chest 1994;105:1693–700. 10.1378/chest.105.6.1693 [DOI] [PubMed] [Google Scholar]
- 11.Kemps HM, Schep G, Zonderland ML, et al. Are oxygen uptake kinetics in chronic heart failure limited by oxygen delivery or oxygen utilization? Int J Cardiol 2010;142:138–44. 10.1016/j.ijcard.2008.12.088 [DOI] [PubMed] [Google Scholar]
- 12.Belardinelli R, Barstow TJ, Nguyen P, et al. Skeletal muscle oxygenation and oxygen uptake kinetics following constant work rate exercise in chronic congestive heart failure. Am J Cardiol 1997;80:1319–24. 10.1016/S0002-9149(97)00672-3 [DOI] [PubMed] [Google Scholar]
- 13.Bailey CS, Wooster LT, Buswell M, et al. Post-Exercise Oxygen Uptake Recovery Delay: A Novel Index of Impaired Cardiac Reserve Capacity in Heart Failure. JACC Heart Fail 2018;6:329–39. 10.1016/j.jchf.2018.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fortin M, Turgeon P-Y, Nadreau Éric, et al. Prognostic value of oxygen kinetics during recovery from cardiopulmonary exercise testing in patients with chronic heart failure. Can J Cardiol 2015;31:1259–65. 10.1016/j.cjca.2015.02.015 [DOI] [PubMed] [Google Scholar]
- 15.Kemps HMC, De Vries WR, Hoogeveen AR, et al. Reproducibility of onset and recovery oxygen uptake kinetics in moderately impaired patients with chronic heart failure. Eur J Appl Physiol 2007;100:45–52. 10.1007/s00421-007-0398-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kemps HM, Schep G, de Vries WR, et al. Predicting effects of exercise training in patients with heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 2008;102:1073–8. 10.1016/j.amjcard.2008.05.054 [DOI] [PubMed] [Google Scholar]
- 17.Yu C-M, Bleeker GB, Fung JW-H, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation 2005;112:1580–6. 10.1161/CIRCULATIONAHA.105.538272 [DOI] [PubMed] [Google Scholar]
- 18.Swank AM, Horton J, Fleg JL, et al. Modest increase in peak VO2 is related to better clinical outcomes in chronic heart failure patients: results from heart failure and a controlled trial to investigate outcomes of exercise training. Circ Heart Fail 2012;5:579–85. 10.1161/CIRCHEARTFAILURE.111.965186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Neill JO, Young JB, Pothier CE, et al. Peak oxygen consumption as a predictor of death in patients with heart failure receiving beta-blockers. Circulation 2005;111:2313–8. 10.1161/01.CIR.0000164270.72123.18 [DOI] [PubMed] [Google Scholar]
- 20.Baba R, Nagashima M, Goto M, et al. Oxygen uptake efficiency slope: a new index of cardiorespiratory functional reserve derived from the relation between oxygen uptake and minute ventilation during incremental exercise. J Am Coll Cardiol 1996;28:1567–72. 10.1016/S0735-1097(96)00412-3 [DOI] [PubMed] [Google Scholar]
- 21.Berger T, Zwick RH, Stuehlinger M, et al. Impact of oxygen uptake efficiency slope as a marker of cardiorespiratory reserve on response to cardiac resynchronization therapy. Clin Res Cardiol 2011;100:159–66. 10.1007/s00392-010-0226-7 [DOI] [PubMed] [Google Scholar]
- 22.Niemeijer VM, van 't Veer M, Schep G, et al. Causes of nonlinearity of the oxygen uptake efficiency slope: a prospective study in patients with chronic heart failure. Eur J Prev Cardiol 2014;21:347–53. 10.1177/2047487312472075 [DOI] [PubMed] [Google Scholar]
- 23.Raphael C, Briscoe C, Davies J, et al. Limitations of the New York heart association functional classification system and self-reported walking distances in chronic heart failure. Heart 2007;93:476–82. 10.1136/hrt.2006.089656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim FY, Yap J, Gao F, et al. Correlation of the New York heart association classification and the cardiopulmonary exercise test: a systematic review. Int J Cardiol 2018;263:88–93. 10.1016/j.ijcard.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 25.Arora S, Aarones M, Aakhus S, et al. Peak oxygen uptake during cardiopulmonary exercise testing determines response to cardiac resynchronization therapy. J Cardiol 2012;60:228–35. 10.1016/j.jjcc.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 26.Stelken AM, Younis LT, Jennison SH, et al. Prognostic value of cardiopulmonary exercise testing using percent achieved of predicted peak oxygen uptake for patients with ischemic and dilated cardiomyopathy. J Am Coll Cardiol 1996;27:345–52. 10.1016/0735-1097(95)00464-5 [DOI] [PubMed] [Google Scholar]
- 27.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol 2012;2:933–96. 10.1002/cphy.c100072 [DOI] [PubMed] [Google Scholar]
- 28.Van Craenenbroeck EM, Conraads VM. Mending injured endothelium in chronic heart failure: a new target for exercise training. Int J Cardiol 2013;166:310–4. 10.1016/j.ijcard.2012.04.106 [DOI] [PubMed] [Google Scholar]
- 29.Conraads VM, Van Craenenbroeck EM, De Maeyer C, et al. Unraveling new mechanisms of exercise intolerance in chronic heart failure: role of exercise training. Heart Fail Rev 2013;18:65–77. 10.1007/s10741-012-9324-0 [DOI] [PubMed] [Google Scholar]
- 30.Kemps HMC, Prompers JJ, Wessels B, et al. Skeletal muscle metabolic recovery following submaximal exercise in chronic heart failure is limited more by O2 delivery than O2 utilization. Clin Sci 2010;118:203–10. 10.1042/CS20090220 [DOI] [PubMed] [Google Scholar]
- 31.Maass AH, Vernooy K, Wijers SC, et al. Refining success of cardiac resynchronization therapy using a simple score predicting the amount of reverse ventricular remodelling: results from the markers and response to crt (mARC) study. Europace 2018;20:e1–10. 10.1093/europace/euw445 [DOI] [PubMed] [Google Scholar]
- 32.Tieland M, Trouwborst I, Clark BC. Skeletal muscle performance and ageing. J Cachexia Sarcopenia Muscle 2018;9:3–19. 10.1002/jcsm.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLorey DS, Paterson DH, Kowalchuk JM. Effects of ageing on muscle O2 utilization and muscle oxygenation during the transition to moderate-intensity exercise. Appl Physiol Nutr Metab 2007;32:1251–62. 10.1139/H07-121 [DOI] [PubMed] [Google Scholar]
- 34.Lamp B, Vogt J, Schmidt H. Impact of cardiopulmonary exercise testing on patient selection for cardiac resynchronisation therapy. Eur Heart J Suppl 2004;6:D5–9. 10.1016/j.ehjsup.2004.05.020 [DOI] [Google Scholar]
- 35.Guyatt GH, Sullivan MJ, Thompson PJ. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure sur sa capacite dans les activites de la vie quotidienne. colleagues’0 introduced the 12-minute walking test, in. Can Med Assoc J 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 36.Lucas C, Stevenson LW, Johnson W, et al. The 6-min walk and peak oxygen consumption in advanced heart failure: aerobic capacity and survival. Am Heart J 1999;138:618–24. 10.1016/S0002-8703(99)70174-2 [DOI] [PubMed] [Google Scholar]
- 37.Guimarães GV, Carvalho VO, Bocchi EA. Reproducibility of the self-controlled six-minute walking test in heart failure patients. Clinics 2008;63:201–6. 10.1590/S1807-59322008000200008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2022-002047supp001.pdf (109.6KB, pdf)
openhrt-2022-002047supp002.pdf (242.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. Data will be shared on reasonable request.