Abstract
A man in his 20s presented with headache and acute deterioration in visual acuity. He was found to have panuveitis and raised intracranial pressure with papilloedema. MRI and F-fluorodeoxyglucose positron emission tomography confirmed a subclinical, but active, inflammatory mastoid process. Histology of the mastoid showed immunoglobulin G4 (IgG4) cells, plasma cells and storiform fibrosis.
This presentation of IgG4 disease has not been previously described.
Treatment with high-dose steroids was initiated, followed by long-term immunosuppressive therapy. The patient’s symptoms improved, although he remains dependent on azathioprine and low dose oral steroids for symptom control. To date, there has been no progression of the disease.
Keywords: Ear, nose and throat/otolaryngology; Neurology; Ophthalmology; Pathology
Background
Immunoglobulin G4 (IgG4) disease, a condition first described in 2001 as autoimmune pancreatitis,1 is a multisystem inflammatory disorder, which typically presents as pancreatitis, retroperitoneal fibrosis, sclerosing cholangitis or interstitial lung disease. The central nervous system (CNS) is rarely affected, with manifestations limited to pachymeningitis, hypophysitis and cranial nerve involvement. Infrequent cranial presentations include orbital disease, sinusitis, pharyngitis and bone lesions.2 3
This is the first description of IgG4 disease presenting as panuveitis with associated mastoiditis and raised intracranial pressure. Ophthalmologic involvement has been limited to orbital disease and inflammatory pseudotumour, with associated involvement of dura, pituitary gland, ventricles and cranial nerves.4–6 Two cases of IgG4 disease presenting with mastoiditis have been described, and both were associated with CNS involvement.7 8
Case presentation
The patient is a man in his 20s who presented with sudden onset right sided frontotemporal headache. This progressed to acute bilateral loss of vision and associated tinnitus after a few days. He was found to have a non-granulomatous panuveitis (1+cells were identified in the anterior and posterior chambers) and papilloedema with retinal haemorrhages. Visual acuity was 6/12 in the left eye and 6/18 in the right eye. The remainder of his neurological exam was normal.
Investigations
Brain CT demonstrated thickened optic nerves bilaterally and hyperostosis of the right mastoid (figure 1). A right mastoid effusion in keeping with chronic mastoiditis was found on MRI (figure 2). A lumbar puncture (LP) showed an initial opening pressure exceeding 50 cm water, with normal chemistry and cell count.
Figure 1.
Empty sella turcica on CT brain.
Figure 2.
Hyperintensity compatible with inflammation in the right mastoid on T2 sequence MRI brain.
LPs were repeated, and with the fourth procedure, the opening pressure normalised to 14 cm water. Microscopy and culture on the samples were negative for bacterial infections, including cryptococcus and Mycobacterium tuberculosis. PCR testing and 42-day cultures for M.tuberculosis from two cerebrospinal fluid (CSF) samples, taken 12 days apart, were also negative. Blood workup was negative for HIV, syphilis and mycoplasma infection. Similarly, autoimmune tests (anti-Sjogren’s syndrome related antigen A and B, rheumatoid factor and anti-neutrophil cytoplasmic antibody (ANCA)) were negative. Serum angiotensin-converting enzyme (ACE) was raised at 115 U/L (reference 8–52 U/L). CT chest imaging was negative for features of sarcoidosis.
Otorhinolaryngology review demonstrated an injected, dull, tender and hypomobile tympanic membrane. Facial nerve function was normal. Right-sided severe mixed hearing loss and left-sided mild conductive hearing loss were found on audiometric testing. F-fluorodeoxyglucose positron emission tomography (FDG PET) CT demonstrated high uptake in the right mastoid, and an exploratory cortical mastoidectomy found the mastoid antrum to be filled with thick, pink tumour-like tissue which extended into the aditus ad antrum medially.
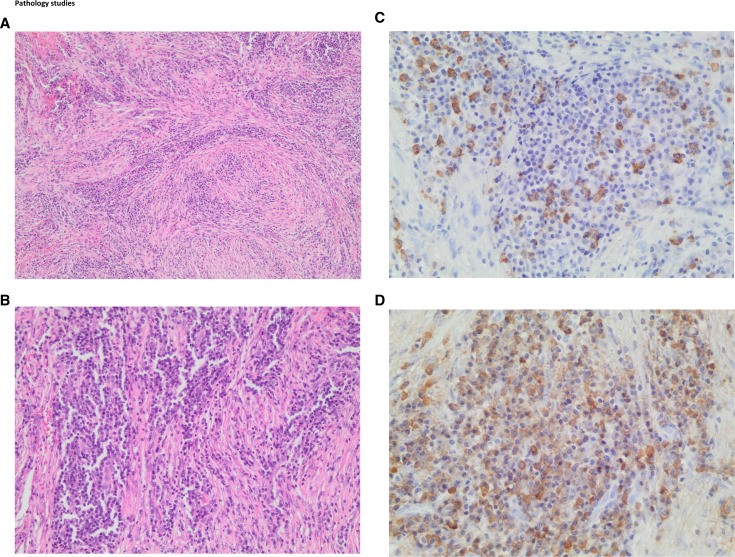
Tissue from the mastoid antrum (figure 3A–D) demonstrated a dense lymphoplasmacytic infiltrate, with an increased number of plasma cells. There were greater than 30 IgG4 positive plasma cells per high power field with a patchy distribution. The IgG4+/IgG+ plasma cell ratio was found to be greater than 40%. A prominent storiform pattern of fibrosis with wirelike bands of fibrotic collagen deposition radiating outward from a central point was observed. Obliterative vasculitis was not a feature. The serum IgG4 level was raised at 2.66 g/L (reference 0.03–2.01).
Figure 3.
IgG-4 related mastoiditis: (A) 100× low power magnification showing large areas of storiform fibrosis with a dense lymphoplasmacytic infiltrate. (B) 200× closer microscopic examination reveals storiform fibrosis with an abundance of plasma cells. (C) 400× immunohistochemistry showing more than 10 IgG-4 positive cells per high power field (approximately 30 cells). (D) 400× immunohistochemistry showing an increasing number of IgG positive staining cells, with an IgG-4/IgG ratio of more than 40%.
Differential diagnosis
The constellation of headache with reduced vision, raised intracranial pressure and imaging findings of thickened optic nerves was concerning for idiopathic intracranial hypertension, although atypical in a mesomorphic male. Repeated LPs provided some improvement in headache, however, vision remained poor. The presence of panuveitis and additional findings of mastoiditis on imaging, broadened the differential to include infective, inflammatory, and non-benign causes of a systemic disorder with a diffuse meningeal process. Tuberculosis is highly endemic in our setting and was excluded. Similarly, HIV, syphilis and sarcoidosis were also investigated for and a raised serum ACE was found. Subsequent CT chest imaging was negative for features of sarcoid.
Other less common causes for a diffuse meningitis that were excluded were histiocytosis × and Mycoplasma pneumoniae. Pachymeningitis secondary to autoimmune conditions such as Sjogren’s syndrome, rheumatoid arthritis, and ANCA-related disease were excluded by the relevant blood tests. Localised areas of infiltration and tissue destruction, such as in mastoiditis with resultant transverse sinus thrombosis (otitic hydrocephalus), were excluded by the normal MR venogram.9 10
Whole body FDG-PET confirmed mastoiditis. There was no evidence of an underlying malignancy elsewhere, furthermore CSF cytology was normal. Biopsy of the mastoid followed the FDG PET findings and confirmed IgG4 disease. Since this is both an unusual, as well as relatively recently described disorder, the pathological diagnosis of IgG4 disease was due to the skill of the anatomical pathologist and their ability to identify the important pathognomonic features.11 Histopathology is the gold standard for diagnosis and the prototypic findings are highly specific.12 13 Serum IgG4 levels were performed as a supportive test after the biopsy results became available and were elevated.
The constellation of chronic mastoiditis, panuveitis and raised intracranial pressure has not been described in IgG4 disease, and the more commonly described systemic manifestations were not present in this patient (pancreatitis, sclerosing cholangitis, retroperitoneal fibrosis and interstitial lung disease).2 3 As such, histopathological diagnosis in this case was pivotal.
Treatment
Raised intracranial pressure was treated with a combination of therapeutic LPs and acetazolamide. Although the headache responded, the visual loss did not improve, and as there was a concern of an underlying autoimmune or inflammatory process, intravenous steroid therapy was initiated at one gram daily for 3 days, followed by high-dose oral prednisone at 1 mg per kilogram daily. Topical steroid eye drops were prescribed for the panuveitis. Once the diagnosis of IgG4 disease was established, azathioprine 75 mg daily was initiated, with a view to escalating the dose. The patient only took treatment for 1 month and was then lost to follow-up for 8 months. Unfortunately, rituximab is not readily available at our facility.
Outcome and follow-up
When the patient represented, he had reports of headache and right eye pain. Visual acuity in both eyes remained unchanged. High-dose oral steroids, followed by a gradual taper, as well as azathioprine were commenced. Two months later, he again reported defaulting on his medication for 1 month and had ongoing reports of pain. Ophthalmology review was that visual acuity was unchanged, and papilloedema and retinal haemorrhages had improved. Azathioprine and a tapering dose of oral steroids, as well as acetazolamide were given.
Investigations for other common sites of involvement in IgG4 disease were performed. Abdominal and pelvic ultrasound was negative for evidence of retroperitoneal fibrosis, and echocardiogram did not reveal pericarditis. Serum IgG4 levels were raised at 2.66 g/L (reference 0.03–2.01), serum ACE was normal.
Discussion
IgG4 disease is a multisystem disorder characterised by reactive T cells. The causative antigen is yet to be identified. However, while usually present, raised levels of IgG4 are not considered pathognomonic, nor pathogenic.2
IgG4 is a subclass of IgG antibodies, produced by B cells. The pathogenesis of IgG4 disease is presumed to follow the presentation of an unidentified antigen by B cells (CD20 B lymphocytes and CD 19/27/38 plasmablasts) to T cells (CD4 T helper cells). The activated T helper cells release interleukin 1-beta, interferon gamma and transforming growth factor beta, which lead to the formation of storiform fibrosis. A subset of T cells, T follicular cells, are responsible for driving a class switch whereby B cells produce more IgG4 relative to other IgG subclasses. IgG4 cell levels, however, can be normal even in the presence of disease, and plasmablasts can be raised independently of IgG4 levels. In some cases, IgG4 levels are markedly raised and may not normalise despite treatment due to continuous production of IgG4 from long-lived plasma cells. Despite the disease nomenclature, the pathogenicity of IgG4 antibodies in IgG4 disease remains unclear. The antibodies are known to have low affinity for Fc receptors on haematopoietic cells, thereby minimising the degree of antibody-dependent phagocytic activation, cellular toxicity, and complement-mediated damage.14 15 The presentation is usually indolent and subacute, with a relapsing-remitting course and organs affected characteristically have fibrotic, mass-like lesions rather than diffusely infiltrating lesions.2 16
IgG4 disease only rarely affects the nervous system. When the nervous system is affected, it is usually in the form of pachymeningitis involving the dura or cavernous sinus, hypophysitis or meningeal inflammation, in conjunction with other organ involvement. Papilloedema, in the absence of inflammatory pseudotumour or a secondary obstructive hydrocephalus following pachymeningitis, has not been previously described as a manifestation of IgG4 disease. Similarly, there are no reported cases of panuveitis as a presentation of this disease and there are only a limited number of reports of IgG4 chronic mastoiditis.
Levels of IgG4 >1.35 g/L in serum are regarded as high (sensitivity 87.2%, specificity 82.6%).17
However, raised IgG4 is a non-specific finding and can also be a feature of sarcoidosis, eosinophilic granulomatosis with polyangiitis, and allergic disorders. CSF analysis shows a lymphocytic pleocytosis with oligoclonal bands, and FDG PET/CT imaging can be used to identify active lesions.18
Histopathology of affected tissue is the gold standard for diagnosis and is characterised by lymphoplasmacytic infiltration, eosinophils, secondary obliterative phlebitis and ultimately fibrosis, described as storiform with a cartwheel pattern due to the arrangement of fibroblasts and inflammatory cells. Immunohistochemistry can also be used to stain IgG4 and the presence of >10 IgG4 cells/high power field, or >40% IgG4 to IgG, is typical.12 13
Of particular interest is the potential overlap between IgG4 disease and sarcoidosis. The chest radiograph features may have similar characteristics in both conditions. A large series of patients with pulmonary sarcoidosis found that the two conditions may coexist, and some patients with clinicoradiological and bronchoalveolar lavage-confirmed sarcoidosis had raised IgG4 levels which returned to normal following treatment. Histopathological features of both sarcoidosis and IgG4 disease were noted in the lacrimal gland of a 59-year-old woman with raised serum ACE, but normal IgG4 levels. There is also an overlap in sarcoidosis between raised serum ACE and immunoglobulin levels, although across all classes (IgA, IgG, IgM).19 There is a single case of IgG4 disease-related pancreatitis presenting with pulmonary and hilar lymphadenopathy with non-caseating granulomas in the accessory salivary glands and coeliac lymph nodes.20
IgG4 disease remains a highly treatable condition with a good prognosis if diagnosed early. There are no randomised controlled trials investigating therapeutic options and current guidelines therefore stem from systematic reviews and expert opinion. Glucocorticoids are regarded as first-line therapy but relapses after withdrawal or tapering are frequently seen in up to a third of patients. Long-term immunosuppressive therapy is necessary, and rituximab is reported to have shown promising results.2 Poor prognostic factors include higher serum IgG4 levels, multiorgan involvement, eosinophil elevation at baseline and re-elevation of serum IgG4.21
Patient’s perspective.
When I was not feeling well, my head felt like it was heavy, as if there was something on my head. My eyes were painful, and my vision was poor. When I arrived at the hospital and was given treatment, I began feeling much better. I was worried because many tests were being performed and there was difficulty in trying to establish my diagnosis. What was wrong with me? Now that I have been home for nearly a year, and stable on treatment, I can appreciate in hindsight the improvement in my symptoms.
Learning points.
Panuveitis, papilloedema and mastoiditis are atypical presentations of IgG4 disease, a newly described disease entity.
The confirmatory tissue diagnosis adds to expanding the potential scope of IgG4 disease.
The combination of F-fluorodeoxyglucose positron emission tomography/CT, along with biopsy may be prudent in atypical presentations, particularly as the spectrum of the disease is being defined.
Footnotes
Contributors: HNJ: original layout of the study and composition of the manuscript. NB: critical revision of manuscript. JW: provided a perspective on the clinical and intra-operative findings. AA: head and neck pathologist who provided a perspective on the histology findings. FvdC: initial ophthalmological assessment and management.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Consent obtained directly from patient.
References
- 1.de las Heras Flórez S, Pérez MC, Sanz Díaz CT, et al. Igg4-Related disease: a case report. Advances in Laboratory Medicine / Avances en Medicina de Laboratorio 2020;1. 10.1515/almed-2019-0045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baptista B, Casian A, Gunawardena H, et al. Neurological manifestations of IgG4-related disease. Curr Treat Options Neurol 2017;19:14. 10.1007/s11940-017-0450-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bittencourt AG, Pereira LV, Cabral F, et al. Igg4-Related sclerosing disease of the temporal bone. Otol Neurotol 2013;34:e20–1. 10.1097/MAO.0b013e31827f1948 [DOI] [PubMed] [Google Scholar]
- 4.Lui PCW, Fan YS, Wong SS, et al. Inflammatory pseudotumors of the central nervous system. Hum Pathol 2009;40:1611–7. 10.1016/j.humpath.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 5.Katsura M, Morita A, Horiuchi H, et al. Igg4-Related inflammatory pseudotumor of the trigeminal nerve: another component of IgG4-related sclerosing disease? AJNR Am J Neuroradiol 2011;32:E150–2. 10.3174/ajnr.A2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong S, Lam WY, Wong WK, et al. Hypophysitis presented as inflammatory pseudotumor in immunoglobulin G4-related systemic disease. Hum Pathol 2007;38:1720–3. 10.1016/j.humpath.2007.06.011 [DOI] [PubMed] [Google Scholar]
- 7.Schiffenbauer AI, Wahl C, Pittaluga S, et al. Igg4-Related disease presenting as recurrent mastoiditis. Laryngoscope 2012;122:681–4. 10.1002/lary.22486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barnado AL, Cunningham MA. Igg4-Related disease presenting as recurrent mastoiditis with central nervous system involvement. J Investig Med High Impact Case Rep 2014;2:232470961455367. 10.1177/2324709614553670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viswanatha B. Otitic hydrocephalus: a report of 2 cases. Ear Nose Throat J 2010;89:E34–7. 10.1177/014556131008900708 [DOI] [PubMed] [Google Scholar]
- 10.Foley J. Benign forms of intracranial hypertension; toxic and otitic hydrocephalus. Brain 1955;78:1–41. 10.1093/brain/78.1.1 [DOI] [PubMed] [Google Scholar]
- 11.Deshpande V. Igg4 related disease of the head and neck. Head Neck Pathol 2015;9:24–31. 10.1007/s12105-015-0620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deshpande V, Zen Y, Chan JK, et al. Consensus statement on the pathology of IgG4-related disease. Mod Pathol 2012;25:1181–92. 10.1038/modpathol.2012.72 [DOI] [PubMed] [Google Scholar]
- 13.Masaki Y, Kurose N, Yamamoto M, et al. Cutoff values of serum IgG4 and histopathological IgG4+ plasma cells for diagnosis of patients with IgG4-related disease. Int J Rheumatol 2012;2012:1–5. 10.1155/2012/580814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.AbdelRazek MA, Venna N, Stone JH. Igg4-Related disease of the central and peripheral nervous systems. Lancet Neurol 2018;17:183–92. 10.1016/S1474-4422(17)30471-4 [DOI] [PubMed] [Google Scholar]
- 15.Mahajan VS, Mattoo H, Deshpande V, et al. Igg4-Related disease. Annu Rev Pathol 2014;9:315–47. 10.1146/annurev-pathol-012513-104708 [DOI] [PubMed] [Google Scholar]
- 16.Salzman OA. Demyelinating and inflammatory diseases. In: Osborne’s Brain. 489. Elsevier, 2017. [Google Scholar]
- 17.Hao M, Liu M, Fan G, et al. Diagnostic value of serum IgG4 for IgG4-related disease: a PRISMA-compliant systematic review and meta-analysis. Medicine 2016;95:e3785. 10.1097/MD.0000000000003785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.AbdelRazek M, Stone JH. Neurologic features of immunoglobulin G4-related disease. Rheum Dis Clin North Am 2017;43:621–31. 10.1016/j.rdc.2017.06.010 [DOI] [PubMed] [Google Scholar]
- 19.Schoenfeld N, Schmolke B, Schmitt M, et al. Specification and quantitation of circulating immune complexes in the serum of patients with active pulmonary sarcoidosis. Thorax 1994;49:688–91. 10.1136/thx.49.7.688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Boshier PR, Priest OH, Hanna GB. Association of IgG4-related disease and sarcoidosis. Thorax 2011;66:919–20. 10.1136/thx.2011.161208 [DOI] [PubMed] [Google Scholar]
- 21.Peng Y, Li JQ, Zhang PP, et al. Clinical outcomes and predictive relapse factors of IgG4-related disease following treatment: a long-term cohort study. J Intern Med 2019;286:542–52. 10.1111/joim.12942 [DOI] [PubMed] [Google Scholar]