While preparing to teach the Microbial Pathogenesis graduate course at our institution, we found ourselves struggling to find basic definitions of virulence and pathogenicity that incorporated the contributions of both the host and the pathogen. The generally used definition of a pathogen as a microbe that causes disease in a host (Table 1) seemed inadequate, because some microbes do not cause clinically evident disease in all hosts. As we investigated the origins of the modern concepts of microbial pathogenesis, we found that while the importance of a host’s susceptibility for a microbe’s virulence was often recognized, the existing definitions did not account for the contributions of both pathogen and host. Historical definitions of pathogens were based on their ability to cause disease as an invariant trait. An integrated view of microbial pathogenesis accounting for the contributions of both host and pathogen has not been developed. In this article, we review historical concepts of microbial pathogenicity and virulence, propose new definitions, and suggest a classification system for microbial pathogens based on their ability to cause damage as a function of the host’s immune response.
TABLE 1.
Definitions and proposed revisions
| Term | Definition(s) from the literature | Proposed definition |
|---|---|---|
| Pathogen | A microbe capable of causing disease (17, 34) | A microbe capable of causing host damage; the definition can encompass classical pathogens and opportunistic pathogens; host damage can result from either direct microbial action or the host immune response |
| A microorganism that can increase in living tissue and produce disease (12) | ||
| Any microorganism whose survival is dependent upon its capacity to replicate and persist on or within another species by actively breaching or destroying a cellular or humoral host barrier that ordinarily restricts or inhibits other microorganisms (10) | ||
| A parasite capable of causing or producing some disturbance in the host (29) | ||
| Pathogenicity | The capacity of a microbe to produce disease (27, 32) | The capacity of a microbe to cause damage in a host |
| Virulence | Degree of pathogenicity (33, 34) | The relative capacity of a microbe to cause damage in a host |
| Virulence ∝ 1/resistance (8) | ||
| Strength of the pathogenic activity (12) | ||
| Relative capacity to overcome available defenses (31) | ||
| Disease severity as assessed by reductions in host fitness following infection (24) | ||
| Percent of death per infection (7) | ||
| A synonym for pathogenicity (34) | ||
| Property of invasive power (35) | ||
| Measure of the capacity of a microorganism to infect or damage a host (21) | ||
| Relative capacity to enter and multiply in a given host (29) | ||
| Virulence factor (or determinant) | A component of a pathogen that when deleted specifically impairs virulence but not viability (33) | A component of a pathogen that damages the host; can include components essential for viability including modulins (16) |
| Microbial products that permit a pathogen to cause disease (27) |
HISTORICAL VIEWS OF MICROBIAL PATHOGENICITY AND VIRULENCE
Early views of pathogenicity and virulence were primarily pathogen centered and were based on the assumption that these characteristics were intrinsic properties of microorganisms, although it was recognized that pathogenicity was neither invariant nor absolute. Two influential early 20th century investigators, Bail and Rosenow, independently proposed aggressins and virulins, respectively, as microbial products that allowed pathogens to establish themselves in the host (for a review, see reference 35). Rosenow’s virulin was a substance extracted from virulent pneumococci that conferred virulence when it was mixed with avirulent pneumococci (26). In retrospect, virulin was probably capsular polysaccharide (5). In 1913, Smith recognized the importance of the host but continued to emphasize microbial characteristics as primarily responsible for microbial virulence (28). In his view, pathogenic microbes were endowed with “offensive” and “defensive” functions that separated them from nonpathogenic microbes and determined the type and outcome of the host-pathogen interaction (28). Diphtheria was viewed as a pathogen with primarily offensive functions that allowed it to injure the mucosa with toxin and establish itself, whereas the tubercle bacillus had primarily defensive functions that allowed persistence in tissue (28). Zinsser, in his 1914 treatise on infectious diseases, grouped microorganisms into three classes: pure saprophytes, which were unable to establish themselves in living tissue; pure parasites, which could establish themselves easily in normal hosts; and half parasites, which had low invasive power and caused infection only in certain circumstances (35). However, he noted that the terms “do not cover each other absolutely” (35).
Although Zinsser interpreted the term pathogenic to mean capable of producing disease, he suggested that virulence had two attributes: a passive one that consisted of microbial characteristics, such as capsules, which allowed persistence in the host, and an aggressive one that included toxins, etc. (35). He defined infectious disease as “parasitism in which no such mutual adaptation has taken place, and in which the invasion of the host by the microorganism is marked by a struggle, the local and systemic manifestations of which constitute the disease” and virulence as invasive power (35). Other authorities modified the definitions of virulence and pathogenicity in an attempt to differentiate between pathogens and their characteristics. Ford defined virulence as infectiousness, or the ability of the microbe to reproduce in the body, and differentiated it from toxicity resulting from toxigenic organisms (12). Watson and Brandly noted that the term virulence was often used in the context of qualitative and quantitative properties associated with the capacity of a microbe to cause disease and that the term pathogenicity was used for defining the degree of involvement for microbes that did not cause rapidly fatal infections (32). Adding to the complexity of the terminology, virulence was thought to depend on various independent variables that included the qualities of microbial aggressiveness, invasiveness, infectivity, toxigenicity, and communicability (14, 21, 32, 35). Hoeprich identified three attributes of virulence that varied depending on the pathogen: invasiveness, intoxication, and hypersensitivity (17). In his view, toxin-producing organisms such as Clostridium tetani had high intoxication properties, Staphylococcus aureus was highly invasive, and Mycobacterium tuberculosis had both invasive and hypersensitivity-eliciting properties (17). The association of virulence with hypersensitivity for some pathogens implied that virulence was linked to the host response. However, others have considered distinctions between the terms “pathogenic” and “virulence” to be confusing and proposed that they should be used as synonyms (34).
The prominence of diseases due to toxigenic bacteria in the late 19th and early 20th centuries, such as diphtheria, promoted microbe-centered views of pathogenesis, because toxins produce disease irrespective of the immune status of the host (see below). Koch’s postulate, which followed the dawn of the germ theory of disease, placed the entire responsibility for pathogenesis on the microbe (reviewed in reference 19). However, even at the time of its introduction, it was clear that Koch’s postulate could not account for microbes that could not be cultured (e.g., viruses) (6). Defining pathogenicity as a microbial characteristic was almost inevitable at the time, when the overwhelming majority of infections occurred in individuals who had relatively constant immune function throughout their lifetimes unless they suffered trauma or starvation. It is not known if it was recognized that some microbes caused disease only in certain hosts before immunodeficiency was understood. However, it was recognized that some microbes did not cause disease in hosts with prior exposure (e.g., cow maids who came into contact with cow pox lesions did not get smallpox). Nevertheless, the fact that Koch’s postulate could not account for microbes that caused disease only in some hosts was not fully accepted until later in the 20th century with the advent of vaccines and the subsequent introduction of immunosuppressive therapies (19). In 1928, Falk defined virulence as the inverse of resistance (or immunity), after noting that discussions on the relative contributions of microbial virulence and host resistance were “futile” since the variables could not be separated (8). By the mid-20th century, the idea that virulence was solely a microbial property had been largely abandoned, and most authorities defined virulence in the context of the host-pathogen relationship (32). Dubos considered microbial virulence an “immensely complex property”, such that the “infectious agent must be able to penetrate the protective barriers which shelter the host from the environment, it must be able to survive the many defense mechanisms, cellular and humoral, which attack it soon as it reaches the tissues; it must find an environment favorable for multiplication; and finally it must be able to produce disease, i.e., to produce substances or conditions which cause physiological and pathological disturbances” (5).
More recent views of microbial pathogenesis emanating primarily from studies of bacterial virulence have continued to focus on the ability of a pathogen to cause disease. Smith cited microbial surface characteristics as critical determinants of the virulence of microorganisms (27). In this view, the chemistry of the microbial surface was the major distinction between pathogenic and nonpathogenic microorganisms (27). Falkow and colleagues also viewed bacterial pathogenicity as a microbial characteristic (9–11). Falkow noted that “a key distinction is that a pathogen has an inherent capacity to breach host cell barriers, whereas commensal and opportunistic pathogens do not” (10). This view is supported by molecular distinctions between pathogenic and nonpathogenic bacteria which reveal that the former have unique virulence factors that allow them to establish themselves in the host (11). The pathogen-centered view is reinforced by the fact that many genes required for virulence in bacteria are in discrete DNA segments, e.g., pathogenicity islands (13), which implies that their acquisition is sufficient for a bacterium to become virulent. By considering pathogen-related variables, Falkow proposed “Molecular Koch’s Postulates,” a set of conceptual tools for dissection of bacterial pathogenesis based on the identification of the genes responsible for causing disease (9). The observation that genetic variation in a tissue-specific adhesion factor of Escherichia coli can result in transition from commensal to pathogen supports a central role for the microorganism in the pathogenic process (30). Another pathogen-based perspective was provided by Deitsch et al., who suggested that a unifying theme in microbial pathogenesis is the capacity for antigenic variation, because it permits selection of traits that allow escape from the host immune defenses (4). Brubaker viewed the distinction between saprophytes and pathogens as the loss of functions necessary for saprophytic existence and the gain of virulence factors required for overwhelming host defenses (3).
In areas of the world with poor sanitation, overcrowding, poverty, and little or no access to medical care, infectious diseases continue to cause significant morbidity and mortality in individuals with apparently normal immune function. In contrast, in developed countries, mortality from infectious diseases has been reduced significantly by improvements in sanitation, widespread vaccination, and access to medical care. Unfortunately, some medical advances have also given rise to new problems. Organ transplantation, invasive surgery, implantation of prosthetic devices, and the use of immunosuppressive therapies have prolonged survival for some diseases but also result in compromised immunity and render previously normal individuals susceptible to microbes formerly considered to be pure saprophytes (2). Medical progress and the human immunodeficiency virus epidemic have each resulted in a marked increase in infections by organisms that rarely cause disease in healthy hosts. At present, commensals such as Candida albicans and coagulase-negative Staphylococcus spp. are frequent causes of morbidity and mortality in individuals with a wide spectrum of immune response abnormalities ranging from impaired host defense to alterations in the microbial flora resulting from antimicrobial therapy. Infections by saprophytes are difficult to reconcile with pathogen-centered views of microbial pathogenesis.
THE NEED FOR A UNIFIED CONCEPT TO DEFINE THE INTERACTION BETWEEN HOST AND PATHOGEN
The many definitions proposed for pathogen, pathogenicity, and virulence (Table 1) illustrate the difficulty and complexity involved in formulating precise terms and the evolution of thought in this field. Existing concepts of pathogenesis are becoming cumbersome. Multiple qualifiers are often required to account for the status of the host as well as the pathogen, and this has led to a proliferation of adjectives to describe pathogens, including primary, opportunistic, commensal, emergent, and nosocomial. These terms are often used in a vague, imprecise, and sometimes confusing manner. The innate capacity of some microbes to cause disease in normal hosts and the ability of commensals and opportunistic pathogens to cause disease only in hosts with impaired immunity cannot be inferred from the definitions of “pathogen” and “pathogenicity” in Table 1. The definitions of pathogens listed in Table 1 place the responsibility for causing disease primarily on the pathogen. These definitions and those listed for pathogenicity and virulence are imprecise because they are unable to define an entity (e.g., a pathogen), or a quality (e.g., disease, pathogenicity, or virulence) as the product of an interaction (e.g., between the host and the microbe) (24). Hence, neither microbe- nor host-related qualities can independently characterize the disease-causing potential of many microbes.
A significant problem with historical concepts is that they are often dependent on the type of pathogen. For example, the virulence and pathogenicity concepts derived from the study of viral diseases are not necessarily applicable to bacterial diseases and vice versa. This was evident from the early studies of viral diseases, for which Koch’s postulate was not applicable, and new criteria for establishing that viruses were responsible for disease were developed, e.g. immunological proof of causation (reviewed in reference 6). In addition, for fungal pathogens, the immunological status of the host is a central determinant of the outcome of infection, and as a consequence, neither virus-derived nor bacterium-derived concepts of virulence or pathogenicity are easily applicable. Furthermore, existing definitions (Table 1) do not account for pathogens that cause disease only in the presence of certain other pathogens (e.g., synergistic infections). For example, Meleney’s gangrene and acute necrotizing ulcerative gingivitis are processes that require more than one organism to cause disease (18). The definition of a virulence factor is also problematic. Standard definitions do not work for low-virulence organisms, e.g., commensals and opportunistic pathogens, for which it is difficult to distinguish virulence determinants from common traits. Defining a virulence factor as a microbial component which specifically affects virulence but not viability excludes some microbial products that produce tissue pathology by inducing cytokine synthesis, such as the modulins (16).
PROPOSAL: A DAMAGE-RESPONSE CONTINUUM TO DEFINE MICROBIAL PATHOGENESIS
From the perspective of disease pathogenesis, the host-pathogen interaction is reducible to two outcomes: those that result in damage to the host and those that result in no damage. Disease occurs when the host sustains sufficient damage to perturb homeostasis. In this respect, damage is an inclusive term that encompasses cell, tissue, and organ damage. Damage at the cellular level includes necrosis, apoptosis, and malignant transformation. Damage at the organ and tissue levels includes granulomatous inflammation, fibrosis resulting from chronic inflammation, and tumor. The recognition that damage is a central feature of infectious disease is evident from previous discussions of microbial pathogenesis. Sparling included tissue damage in the requirement for the occurrence of a clinically significant infection (31) and Lipsitch and Moxon cited cytotoxicity, or damage to host tissues, as a component of microbial virulence (21).
Damage can be mediated by either the pathogen or the host. For most infectious diseases, the nature and extent of host damage depend on the immune status of the host. Damage in hosts that mount weak immune responses is primarily pathogen mediated. Damage in hosts that mount very strong immune responses is primarily host mediated. However, in many interactions between pathogens and normal hosts, there is a continuum between pathogen-mediated and host-mediated damage which results in disease only when the nature of the damage impairs the normal function of the host. Hence, disease itself is a complex outcome which can arise because of pathogen-mediated damage (e.g., pathogens that induce cell necrosis), host-mediated damage (e.g., aberrant immune responses such as those associated with rheumatic fever or mediastinal fibrosis), or both. For example, Streptococcus pneumoniae replicates in lung tissue and does not induce tissue necrosis, but it can elicit an intense inflammatory response. Damage due to the latter is the basis of the clinical and histopathologic manifestations of pneumococcal pneumonia. Similarly, Staphylococcus aureus does induce necrosis, but it also stimulates host inflammatory responses, whereas M. tuberculosis elicits strong immune responses in hosts which produce severe inflammation that damages host tissue. Defining microbial pathogenesis in terms of host damage permits the inclusion of many variables which affect the host-pathogen relationship. In this view, virulence is a property of the pathogen, but it is modulated by host susceptibility and resistance.
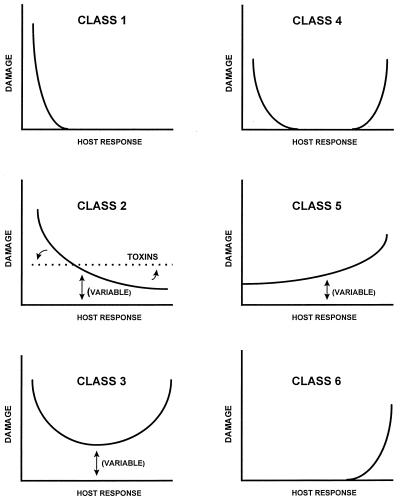
By considering damage as a reflection of either host immune responses or intrinsic pathogen characteristics, or both, it is possible to categorize most, if not all, pathogenic microorganisms into one of six groups (Fig. 1). The curves in Fig. 1 depict host damage as a function of the immune response, which includes both innate and adaptive immunity. We have chosen to characterize host responses by the magnitude and type of damage that occur with microbial infections. These responses include quantitative and qualitative factors that modify the magnitude of a given response. Quantitative factors are defined as the amount of any entity required for protection. Qualitative factors are independent of quantity and are defined as a specific feature of a response. Some examples of quantitatively and qualitatively weak and strong immune responses are listed in Table 2. Any response that avoids or minimizes host damage without allowing pathogen-induced damage is considered appropriate. The specific nature of responses that avoid host damage is a function of the individual host and the particular pathogen. Either weak or strong responses may be appropriate depending on the specific host-pathogen interaction. Each damage-response curve shown in Fig. 1 should be viewed as a possible outcome in a given host, with the actual outcome of the host-pathogen interaction for an individual dependent on the genetic makeup of the pathogen and the host. Although each individual host-pathogen interaction can be modified by host genetic factors, host nutritional status, inoculum, and route of infection, etc., it is possible to group pathogens based on the likelihood that they cause damage as a function of the magnitude of the host response (Table 3). The curves depict the fact that for any given pathogen the likelihood of damage is greatest at either or both extremes of the host response. Although all curves in Fig. 1 can be derived by modifying the class 3 curve, additional curves are needed to classify the pathogens for which damage generally occurs only at one extreme of the host response.
FIG. 1.
Six damage-response curves representing six classes of microbial pathogens. The y axis denotes the amount of damage to the host resulting from the host-pathogen interaction. The x axis denotes the magnitude of the host immune response. Variable refers to the fact that the amount of damage can vary depending on the individual host.
TABLE 2.
Examples of weak and strong responses that can be associated with host damagea
| Evaluation | Description of response
|
|
|---|---|---|
| Weak | Strong | |
| Quantitative | Insufficient number of immune effector cells and/or molecules to prevent host damage | Overproduction of inflammatory mediators that result in tissue fibrosis or promote malignant transformation |
| Qualitative | (i) Antibodies of specificities or isotype that do not mediate protection | (i) Antigenic mimicry |
| (ii) Th2 responses instead of Th1 responses for pathogens that require Th1 responses for containmentb | (ii) Eosinophilic inflammation in response to certain antigensc | |
| (iii) Antibody-mediated enhancement of disease | ||
The appropriateness of weak and strong responses must be considered in the context of specific pathogens.
Eosinophilic inflammatory responses may be useful for helminths but not certain fungi.
Th2 responses are associated with strong antibody responses whereas Th1 responses are proinflammatory (15, 25). Th2 responses to pathogens that require strong cellular inflammatory responses for containment and eradication may result in chronic and progressive infections. However, it is noteworthy that this view may be an oversimplification of a very complex process (1).
TABLE 3.
Classification of human pathogens based on damage as a function of immune responsea
| Class | Pathogen | Damage as a magnitude of the immune responseb
|
||
|---|---|---|---|---|
| Weak | Intermediate | Strong | ||
| 1 | Legionella pneumophila | Legionnaire’s disease | None | None |
| Pneumocystis carinii | Pneumonia | None | None | |
| Pseudallescheria boydii | Invasive sinusitis, pneumonia | None | None | |
| Staphylococcus epidermidis | Vascular infections | None | None | |
| 2 | Adenovirus | Pneumonia, disseminated infection | Upper respiratory infection, diarrhea, hemorrhagic cystitis | None |
| Alphaviruses | Encephalitis | Encephalitis | None | |
| Bacillus anthracis | Anthrax | Anthrax | None | |
| Blastomyces dermatitidis | Disseminated blastomycosis | Pneumonia | None | |
| Bordetella pertussis | Secondary pneumonia | Pertussis | None | |
| Borrelia burgdorferi | Persistence of infection with arthritis and meningitis | Lyme disease | None | |
| Brucella spp. | Brucellosis | Brucellosis | None | |
| Candida spp. | Mucocutaneous candidiasis | Vaginal candidiasis | None | |
| Clostridium tetani | Tetanus | Tetanus | None | |
| Clostridium botulinum | Botulism | Botulism | None | |
| Corynebacterium diphtheriae | Diphtheria | Diphtheria | None | |
| Cryptococcus neoformans | Meningoencephalitis | Primary complex in lung, pneumonia, meningoencephalitis | None | |
| Cryptosporidium spp. | Chronic diarrhea | Diarrhea | None | |
| Ebola virus | Hemorrhagic fever | Hemorrhagic fever | None | |
| Entamoeba histolytica | Amebiasis | Amebiasis | None | |
| Francisella tularensis | Disseminated infection | Tularemia | None | |
| Group B streptococcus | Invasive infection | Puerperal sepsis | None | |
| Haemophilus influenzae type b | Disseminated infection | Upper respiratory infection, meningitis | None | |
| Hemophilus ducreyi | Disseminated infection | Chancroid | None | |
| Hepatitis A virus | Hepatitis | Hepatitis | None | |
| JC virus | Hemorrhagic cystitis, ureteral stenosis, progressive multifocal leukoencephalopathy | Asymptomatic | None | |
| Listeria monocytogenes | Listeriosis | Listeriosis | None | |
| Molluscum virus | Disseminated molluscum | Molluscum contagiosum | None | |
| Neisseria gonorrhoeae | Disseminated infection | Urethritis, PID | None | |
| Neisseria meningitidis | Meningococcemia carrier state | Meningitis | None | |
| Paracoccidioides brasiliensis | Disseminated infection | Pneumonia | None | |
| Parvovirus | Aplastic and chronic anemia | Erythema infectiosum | None | |
| Plasmodium spp. | Malaria | Malaria | None | |
| Pseudomonas aeruginosa | Pneumonia, systemic infection | Diarrhea | None | |
| Rhinovirus | Upper respiratory infection | Upper respiratory infection | None | |
| Rickettsia rickettsii | Rocky mountain spotted fever | Rocky mountain spotted fever | None | |
| Rochalimaea spp. | Bacillary angiomatosis | Bacillary angiomatosis | None | |
| Rotavirus | Diarrhea | Diarrhea | None | |
| Streptococcus pneumoniae | Pneumonia, meningitis, pneumococcal sepsis | Pneumonia, meningitis | None | |
| Toxoplasma gondii | Toxoplasmosis | Cervical lymphadenopathy | None | |
| Trichomonas vaginalis | Trichomoniasis | Trichomoniasis | None | |
| Varicella-zoster virus | Disseminated infection | Varicella, dermatomal zoster | None | |
| Vibrio cholera | Diarrhea | Diarrhea | None | |
| Yersinia pestis | Septicemic, pneumonic and meningeal plague | Bubonic plague | None | |
| 3 | Chlamydia pneumoniae | Pneumonia | Pneumonia | Asthma(?), atherosclerosis(?) |
| Coccidioides immitis | Disseminated coccidioidomycosis | Pneumonia | Erythema nodosum, erythema multiforme | |
| Cytomegalovirus | Pneumonitis, hepatitis, retinitis | Mononucleosis | Guillain-Barré syndrome | |
| Escherichia coli O157:H7 | Diarrhea | Diarrhea | Hemolytic uremic syndrome | |
| Epstein-Barr virus | Hairy leukoplakia, lymphoproliferative disorders | Mononucleosis | Burkitt’s lymphoma, nasopharyngeal carcinoma | |
| Streptococcus pyogenes | Scarlet fever, erysipelas, toxic shock syndrome | Scarlet fever, erysipelas, toxic shock syndrome | Rheumatic fever glomerulonephritis | |
| Hepatitis B virus | Chronic infection | Hepatitis | Hepatocellular carcinoma | |
| Herpes simplex virus 2 | Genital herpes, disseminated herpes | Genital herpes, neonatal herpes, encephalitis | Cervical cancer | |
| Herpes simplex virus 1 | Gingivostomatitis, esophagitis, pneumonitis | Gingivostomatitis, encephalitis | Oropharingeal carcinoma | |
| Histoplasma capsulatum | Disseminated histoplasmosis | Primary complex in lung | Fibrosing mediastinitis | |
| Human immunodeficiency virus | AIDS | AIDS | Sjogren-like syndrome(?) | |
| Influenza virus | Influenza | Influenza | Reye syndrome(?), Guillain-Barré syndrome, transverse myelitis | |
| Leishmania spp. | Visceral leishmaniasis | Leishmaniasis | Glomerulonephritis | |
| Measles virus | Severe measles, giant cell pneumonia | Measles | Atypical measles, SSPE | |
| Mycobacterium tuberculosis | Pulmonary and disseminated tuberculosis | Primary complex in lung, latent infection | Scar carcinoma, constrictive pericarditis, fibrosing mediastinitis | |
| Papilloma virus | Warts, condyloma acuminata, neoplasia | Warts, condyloma acuminata, neoplasia | Neoplasia | |
| Respiratory syncytial virus | Severe pneumonia | Pneumonia, bronchiolitis | Hyperactive airways? | |
| Salmonella spp. | Salmonellosis chronic carrier | Enteric fever | Reiter syndrome | |
| Staphylococcus aureus | Suppurative infections, toxic shock syndrome, endovascular infections | Suppurative infections, endovascular infections | Toxic shock syndrome | |
| Treponema pallidum | Accelerated course | Primary and secondary syphilis | Obliterative endarteritis, tertiary syphilis | |
| Yersinia enterocolitica | Septicemia | Enterocolitis | Reactive polyarthritis | |
| 4 | Aspergillus spp. | Invasive aspergillosis | None | Allergic sinusitis, Farmer’s lung |
| Vaccinia virus | Vaccinia necrosum | None | Encephalitis | |
| 5 | Mycoplasma pneumoniae | Pneumonia | Pneumonia | Raynoud’s phenomenon, Guillain-Barré, erythema multiforme |
| Chlamydia trachomatis | Trachoma, perinatatal infections, lymphogranulama venereum | Trachoma, perinatatal infections, lymphogranulama venereum | Reiter’s syndrome, infertility, ectopic pregnancy, spontaneous abortions | |
| Mumps virus | Mumps | Mumps | Diabetes(?), encephalitis | |
| Campylobacter jejuni | Diarrhea | Diarrhea | Reiter’s syndrome | |
| Poliovirus | Poliomyelitis | Poliomyelitis | Postmyelitis syndrome | |
| Shigella spp. | Diarrhea | Diarrhea | Reiter’s syndrome | |
| Trematoda spp. | Schistosomiasis | Schistosomiasis | Portal and pulmonary hypertension, bladder neoplasia | |
| Trypanosoma spp. | Trypanosomiasis | Trypanosomiasis | Cardiomyopathy | |
| 6 | Helicobacter pylori | None associated | None associated | Gastric gastritis, carcinoma, lymphoma |
Not a complete list of all pathogens.
The terms weak, intermediate, and strong include qualitative and quantitative aspects of the host response. For examples of weak and strong responses see Table 2. The intermediate category is the most common type of response in a healthy population and the conditions listed under this category can occur in healthy individuals. SSPE, subacute sclerosing panencephalitis; PID, pelvic inflammatory disease.
Class 1: pathogens that cause damage only in situations of weak immune responses.
Class 1 microorganisms, which are usually considered opportunistic or commensal, are associated with disease only in individuals with impaired immune function and almost never cause symptomatic or clinically apparent infections in individuals with normal immunity. Class 1 microorganisms do not cause host damage in the setting of normal immune function because they have low intrinsic virulence. A prototypical class 1 microorganism is Pneumocystis carinii, which causes life-threatening pneumonia in patients with specific immunological deficits, particularly those with AIDS. Although disease due to P. carinii occurs only in individuals with impaired immunity, serological studies have shown specific antibodies in normal hosts, suggesting that exposure to P. carinii is commonplace. At present, there is no evidence that colonization of normal hosts with P. carinii elicits pathological changes or long-term sequelae. The damage associated with class 1 microorganisms can be either host and/or pathogen mediated. For example, in persons with AIDS, the mortality of P. carinii infection can be significantly reduced by the use of corticosteroids to reduce the host inflammatory response. Thus, the inflammatory consequences of P. carinii infection result in damage, even in the setting of severe immunological deficits. In contrast, the hyphal forms of Pseudallescheria boydii are invasive and cause direct tissue destruction in individuals with severely impaired immunity. Hence, for P. boydii, the host damage is primarily pathogen mediated.
Class 2: pathogens that cause damage either in hosts with weak immune responses or in the setting of normal immune responses.
Class 2 microorganisms cause host damage by both host- and pathogen-mediated mechanisms. These microorganisms have the capacity to cause serious infections in normal hosts but are frequently associated with more severe infections in hosts with impaired immune function. Some class 2 microorganisms, e.g., fungal species such as Candida albicans and Cryptococcus neoformans, may be viewed as opportunists because their prevalence is higher in groups with impaired immune function. However, the capacity of class 2 microorganisms to mediate disease in individuals with apparently normal immunity is indicative of the expression of microbial characteristics that promote their ability to evade normal host defenses that would otherwise eliminate them. A prototypical class 2 microorganism is Streptococcus pneumoniae, a causative agent of life-threatening pneumonia in normal hosts but more frequently associated with severe infections in individuals at the extremes of age and those with defects in humoral immunity and phagocytic function. In normal hosts, class 2 microorganisms may cause episodic infections, but they do not elicit immune responses that will continue to damage the host after the resolution of an acute infection. A subset of class 2 is the classical toxigenic bacterial pathogens, such as Corynebacterium diphtheriae (diphtheria), which damage the host through secreted toxins. In general, protection against toxins is mediated by neutralizing antibody, which binds to the toxin and interferes with toxin-mediated damage. Toxins produce damage rapidly, generally before the immune system can respond. Hence, there is no long-lasting immunity for many toxin-mediated diseases because the amount of toxin produced is presumably not sufficient to stimulate an antibody response. As a result, toxin-producing organisms tend to cause damage irrespective of the immune status of the host, and the damage-response curve for toxigenic pathogens is flat, reflecting the action of the pathogen on the host, in spite of normal immunity. An exception to this generalization is toxigenic Staphylococcus aureus, which produces a superantigen that causes damage by stimulating a T-cell response that can result in the development of toxic shock syndrome.
Class 3: pathogens that cause damage in the setting of appropriate immune responses and produce damage at both ends of the continuum of immune responses.
Class 3 microorganisms can cause disease by both host- and pathogen-mediated mechanisms. They can cause disease in normal hosts but are distinguished from class 2 microbes by their ability to cause significant damage in the setting of both weak or strong immune responses. A prototypical class 3 microorganism is Histoplasma capsulatum. In the majority of normal hosts, inhalation of conidia results in a pulmonary infection that can be asymptomatic or a flu-like illness, but some develop pneumonia. The organism is usually contained in the lungs but can become latent and then reactivate. In individuals with impaired immune function, both primary and reactivated H. capsulatum infections can disseminate and are fatal if untreated. In individuals with weak immune responses, the damage is mediated by the pathogen, resulting in a profuse proliferation of yeast cells in bone marrow, liver, and other organs. In individuals with strong immune responses, the damage in histoplasmosis is primarily mediated by an unmodulated immune response which produces a very strong inflammatory response to H. capsulatum antigens, resulting in chronic inflammation and progressive mediastinal fibrosis.
Class 4: pathogens that cause damage primarily at the extremes of both weak and strong immune responses.
Class 4 microorganisms make up a relatively small set of pathogens that cause symptomatic infections only in patients who have impaired immunity or protracted immune responses to the pathogen. A prototypical class 4 microorganism is Aspergillus fumigatus, which causes invasive aspergillosis in individuals with quantitative and qualitative deficiencies of polymorphonuclear leukocyte function, such as cancer patients undergoing myelosuppressive treatment (e.g., neutropenia) or individuals with chronic granulomatous disease (e.g., defective oxidative burst). Host damage in invasive aspergillosis is pathogen mediated and results from tissue necrosis and infarction as the result of hyphal invasion and elaboration of hydrolytic enzymes. However, some patients exposed to Aspergillus antigens mount an enhanced immune response that results in allergic sinusitis or bronchopulmonary aspergillosis. In this case, damage is mediated by an intense host immune response. Unlike class 3 pathogens, the normal immune responses to class 4 pathogens result in no detectable damage to the host.
Class 5: pathogens that cause damage across the spectrum of immune responses, but damage can be enhanced by strong immune responses.
Class 5 microorganisms tend to cause infections that result in pathogen-mediated damage but are associated with protracted or chronic damage resulting from an excessive or inappropriate immune response. Prototypical class 5 microorganisms include the enteric bacterial pathogens Shigella and Campylobacter spp., which usually cause self-limited gastrointestinal diseases resulting from either pathogen-mediated damage to the intestinal mucosa or pathogen-elicited host immune responses that produce intestinal inflammation. Although these infections can be severe in individuals with impaired immunity, most cases resolve without permanent damage to the gastrointestinal tract or other tissues. However, individuals with certain genetic backgrounds (e.g., HLA-B27 histocompatibility antigens) are at high risk for the development of a polyarticular arthritis known as Reiter’s syndrome. Although the pathogenesis of Reiter’s syndrome is not fully understood, it is thought to be the result of an immune response to microbial antigens which cross-reacts with host tissues to produce tissue damage.
Class 6: microorganisms that can cause damage only in conditions of strong immune responses.
Pathogen class 6 is largely a theoretical category which does not adequately define any known pathogen. It is included to encompass a growing list of diseases that may be shown to be the result of infectious microorganisms, such as Crohn’s and Whipple’s diseases. A microorganism that might meet class 6 criteria is Helicobacter pylori, a human pathogen recently discovered to be associated with peptic ulcer disease. H. pylori infection is usually asymptomatic, but some individuals develop chronic gastritis and peptic ulcer disease. Since neither condition is associated with impaired immune function, we consider the host response that results in chronic infection to be inappropriate. Damage in H. pylori infection is probably the result of both pathogen- and host-mediated processes.
MEDICAL INTERVENTIONS ALTER THE SHAPE OF THE DAMAGE-RESPONSE CURVE
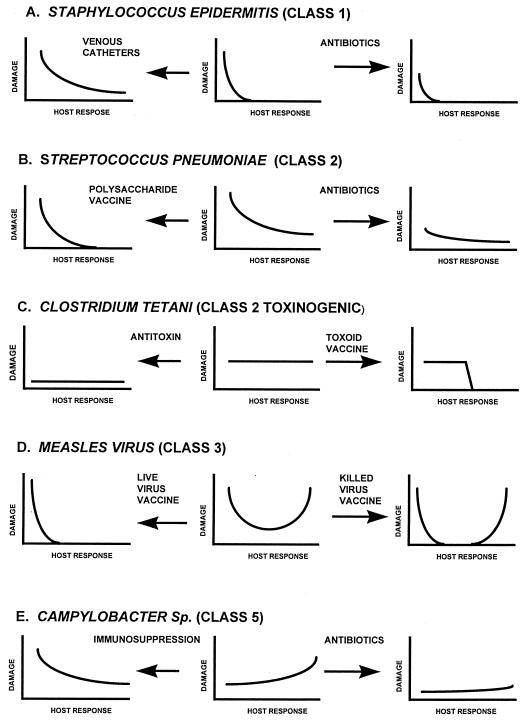
The damage-response curves provide a means to analyze the effect of medical interventions on the outcome of the host-pathogen interaction. Staphylococcus epidermidis is a significant pathogen in the setting of disruption of skin integrity, which weakens hosts defenses and permits access of the bacterium to the bloodstream and internal tissues (Fig. 2A). Hence the placement of an indwelling venous catheter in an otherwise normal individual allows this organism to cause intravascular infections and mediate damage. Administration of antibiotics may reduce the number of Staphylococcus epidermidis bacteria colonizing an intravascular device and reduce damage, but this may not succeed in preventing further damage or may lead to superinfection and damage to the host by another pathogen. Streptococcus pneumoniae causes pneumonia in normal hosts and severe infections in patients with impaired immunity (Fig. 2B). A polysaccharide vaccine for Streptococcus pneumoniae can elicit an antibody response that reduces the incidence of pneumonia in hosts that mount appropriate responses to the vaccine. Hence, the vaccine reduces the probability of sporadic pneumococcal infections and lowers the likelihood of damage in normal hosts who respond to vaccination (Fig. 2B). However, the vaccine is not very immunogenic in individuals with impaired immunity. Administration of antibiotics reduces the number of Streptococcus pneumoniae bacteria and consequently reduces the likelihood of damage in hosts with both weak and immune responses (Fig. 2B). Tetanus is a paralytic and spasmodic condition caused by a toxin elaborated by Clostridium tetani in tissue (Fig. 2C). A bout of tetanus does not elicit lasting immunity, presumably because the amount of toxin made is not sufficient to induce strong immune responses. Administration of preformed antibodies to tetanus can ameliorate the symptoms in patients regardless of their immune response to the toxin. Administration of an inactivated tetanus toxin (toxoid) induces antibody responses in normal hosts that prevent Clostridium tetani infections from causing tetanus. However, patients with impaired immunity who are given toxoid may not respond to the toxoid vaccine and are not protected (23). Measles is a childhood viral infection that has nearly been eradicated by an effective attenuated live virus vaccine. Measles is considered a class 3 pathogen because it can cause significant host damage in conditions of weak and strong immune responses (Fig. 2D). In the 1960’s, a killed virus vaccine which was associated with severe measles (atypical measles) in individuals who had little or no antibody response to vaccination was used. When these individuals were exposed to wild-type measles virus, they mounted strong antibody responses accompanied by a severe immunologic disease, and the pathogenesis of the latter was considered a manifestation of hypersensitivity to measles antigens in a partially immune host. In patients with weak immune responses, the vaccine virus itself can disseminate to produce fatal measles (23). Campylobacter spp. classically produce enteric diseases (Figure 2E). In hosts with impaired immune responses, Campylobacter spp. can disseminate and produce deep tissue infections resulting in considerable damage. Like the situation for Staphylococcus epidermidis and Streptococcus pneumoniae, antibiotic administration can reduce bacterial burden and consequently reduce damage in most hosts irrespective of their specific immune responses.
FIG. 2.
Examples of how the damage-response curves for five pathogens can be altered by medical interventions. Details of each intervention are discussed in the text.
CONCLUSIONS: THE DAMAGE-RESPONSE CLASSIFICATION CAN ACCOMMODATE NEW KNOWLEDGE
The utility of a conceptual framework ultimately lies in its ability to accommodate alterations in the variables used to formulate its construction. We have noted how a damage-response classification system can be used to analyze the effect of medical interventions on the probability of damage resulting from a host-pathogen interaction (Fig. 2). In addition, the different categories can be used to classify and reclassify microbes as new information becomes available. For example, the class 1 category can accommodate the increasing number of rare opportunistic microbes that are being described in patients with impaired immunity, and class 6 can accommodate medical diseases without known etiologies which may be redefined as infectious diseases. The assignments of the various pathogens to the specific classes was based on our interpretation of current knowledge and can be changed as new information becomes available (e.g., some class 2 pathogens would be reclassified as class 3 if associations are made between the infection and other forms of host damage). Hence, we recognize that for some pathogens, our assignments can be debated, and we encourage our colleagues to use this classification scheme to make revisions as they see fit.
A strength of the damage-response classification is that it does not depend on pathogen type and stresses the continuity of microbial pathogens in the context of a relevant outcome: damage as the prerequisite condition for disease. In this classification, pathogens are grouped by their ability to inflict damage as a function of host response irrespective of their phylogenetic derivation, biological kingdom, or previous classification. By merging the concepts that the host response contributes to pathogen-mediated damage and the classical view that pathogens have distinct characteristics which define their virulence, the damage-response classification permits a new approach to host-pathogen interactions that is not constrained by pathogen- and/or host-centered views of microbial pathogenesis. Furthermore, the damage-response classification permits the integration of widely diverse aspects of the host-pathogen interaction under one umbrella. For example, many types of infections have been associated with malignancies (22), but it is difficult to incorporate them within the traditional views of infectious disease. Since neoplastic transformation is a form of tissue damage, malignancies caused by microbes are part of the damage-response continuum, and microbes that are associated with malignancy can be classified as group 3, 4, or 5 pathogens (Table 3). Neoplasia can be the result of either pathogen-mediated damage (i.e., mutation, or transformation) or host-mediated damage (e.g., chronic inflammation). By this reasoning, it follows that microbial genes and products that promote malignant transformation can be classified as virulence factors. Recently, the central immunological principle of recognition based on discrimination between self and non-self has been challenged by the proposal that the immune system reacts primarily to danger signals posed by microbial pathogens (reviewed in references 18 and 20). Since microbe-mediated tissue damage is a danger signal, the damage-response framework can accommodate this versions of immunological thinking. Finally, the damage-response scheme provides a framework to guide microbial pathogenesis research by suggesting the identification of host and pathogen variables responsible for influencing the amount of damage resulting from host-pathogen interactions.
In summary, existing concepts of virulence and pathogenicity are inadequate because they do not account for the full complexity of microbial pathogenesis in hosts with and without impaired immunity. Here we propose that host-pathogen interactions can be analyzed using host damage as the common denominator for characterizing microbial pathogenicity and can provide a conceptual framework for incorporating the importance of the host response into the outcome of the host-microbe interaction. The versatility of this framework is shown by its capacity to accommodate the changes in pathogenicity that result from medical intervention. Given our incomplete knowledge of host-pathogen interactions, this framework should be considered a work in progress that will undoubtedly require modification and redefinition as new information accumulates.
ACKNOWLEDGMENTS
Both authors contributed equally to this work.
We thank Marta Feldmesser, Marcia Goldberg, and Reid Schwebach for critical reading of the manuscript.
A.C. was supported by NIH grants RO1-AI33774, RO1-AI3342, and RO1-59842-01 and a Burroughs Wellcome Development Therapeutics Award. L.P. was supported by NIH grant RO1-AI35370.
REFERENCES
- 1.Allen J E, Maizels R M. Th1-Th2: reliable paradigm or dangerous dogma. Immunol Today. 1997;18:387–392. doi: 10.1016/s0167-5699(97)01102-x. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong D. History of opportunistic infection in the immunocompromised host. Clin Infect Dis. 1993;17(Suppl. 2):S318–S321. doi: 10.1093/clinids/17.supplement_2.s318. [DOI] [PubMed] [Google Scholar]
- 3.Brubaker R R. Mechanisms of bacterial virulence. Annu Rev Microbiol. 1985;39:21–50. doi: 10.1146/annurev.mi.39.100185.000321. [DOI] [PubMed] [Google Scholar]
- 4.Deitsch K W, Moxon E R, Wellems T E. Shared themes of antigenic variation and virulence in bacterial, protozoal, and fungal infections. Microbiol Mol Biol Rev. 1997;61:281–293. doi: 10.1128/mmbr.61.3.281-293.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubos R J. The bacterial cell. London, United Kingdom: Harvard University Press; 1945. pp. 188–228. [Google Scholar]
- 6.Evans A S. Causation and disease: the Henle-Koch postulates revisited. Yale J Biol Sci. 1976;49:175–195. [PMC free article] [PubMed] [Google Scholar]
- 7.Ewald P W. The evolution of virulence. Sci Am. 1993;268:86–93. doi: 10.1038/scientificamerican0493-86. [DOI] [PubMed] [Google Scholar]
- 8.Falk I S. A theory of microbiologic virulence. In: Jordan E O, Falk I S, editors. The newer knowledge of bacteriology and immunology. Chicago, Ill: The University of Chicago Press; 1928. pp. 565–575. [Google Scholar]
- 9.Falkow S. Molecular Koch’s postulates applied to microbial pathogenicity. Rev Infect Dis. 1988;10(Supp 2):S274–S276. doi: 10.1093/cid/10.supplement_2.s274. [DOI] [PubMed] [Google Scholar]
- 10.Falkow S. What is a pathogen? ASM News. 1997;63:359–365. [Google Scholar]
- 11.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ford W W. Text-book of bacteriology. Philadelphia, Pa: W. B. Saunders Company; 1927. pp. 826–844. [Google Scholar]
- 13.Hacker J, Blum-Oehler G, Mühldorfer I, Tschäpe H. Pathogenicity islands of virulent bacteria: structure, function and impact on microbial evolution. Mol Microbiol. 1997;23:1089–1097. doi: 10.1046/j.1365-2958.1997.3101672.x. [DOI] [PubMed] [Google Scholar]
- 14.Harries E H R, Mitman M, Taylor I. Clinical practice in infectious diseases. Baltimore, Md: The Williams and Wilkins Co.; 1951. pp. 15–16. [Google Scholar]
- 15.Heinzel F P. Th1 and Th2 cells in the cure and pathogenesis of infectious diseases. Curr Opin Immunol. 1995;8:151–155. [Google Scholar]
- 16.Henderson B, Poole S, Wilson M. Bacterial modulins: a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoeprich P D. Host-parasite relationships and the pathogenesis of infectious disease. In: Hoeprich P D, editor. Infectious diseases. Philadelphia, Pa: Harper & Row; 1983. pp. 45–56. [Google Scholar]
- 18.Ingham H R, Sisson P R. Pathogenic synergism. Microbiol Sci. 1984;1:206–208. [PubMed] [Google Scholar]
- 19.Isenberg H D. Pathogenicity and virulence: another view. Clin Microbiol Rev. 1988;1:40–53. doi: 10.1128/cmr.1.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janeway C A, Goodnow C C, Medzhitov R. Immunological tolerance: danger—pathogen on the premises! Curr Biol. 1996;6:519–522. doi: 10.1016/s0960-9822(02)00531-6. [DOI] [PubMed] [Google Scholar]
- 21.Lipsitch M, Moxon E R. Virulence and transmissibility of pathogens: what is the relationship? Trends Microbiol. 1997;5:31–37. doi: 10.1016/S0966-842X(97)81772-6. [DOI] [PubMed] [Google Scholar]
- 22.Mackowiak P A. Microbial oncogenesis. Am J Med. 1987;82:79–97. doi: 10.1016/0002-9343(87)90381-0. [DOI] [PubMed] [Google Scholar]
- 23.Pirofski L, Casadevall A. The use of licensed vaccines for active immunization of the immunocompromised host. Clin Microbiol Rev. 1998;11:1–26. doi: 10.1128/cmr.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Read A F. The evolution of virulence. Trends Microbiol. 1994;2:73–81. doi: 10.1016/0966-842x(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 25.Romagnani S. Understanding the role of the Th1/Th2 cells in infection. Trends Microbiol. 1996;4:470–473. doi: 10.1016/s0966-842x(97)82906-x. [DOI] [PubMed] [Google Scholar]
- 26.Rosenow E C. Human pneumococcal opsonin and the anti-opsonic substance in virulent pneumococci. J Infect Dis. 1907;4:285–296. [Google Scholar]
- 27.Smith H. Microbial surfaces in relation to pathogenicity. Bacteriol Rev. 1977;41:475–500. doi: 10.1128/br.41.2.475-500.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith T. An attempt to interpret present-day uses of vaccines. JAMA. 1913;60:1591–1599. [Google Scholar]
- 29.Smith T. Parasitism and disease. New York, N.Y: Hafner Publishing Co.; 1934. pp. 112–129. [Google Scholar]
- 30.Sokurenko E V, Chesnokova V, Dykhuizen D, Ofek I, Wu X, Krogfelt K A, Struve C, Schembri M A, Hasty D L. Pathogenic adaptation of Escherichia coli by natural variation of the FimH adhesin. Proc Natl Acad Sci USA. 1998;95:8922–8926. doi: 10.1073/pnas.95.15.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparling P F. Bacterial virulence and pathogenesis: an overview. Rev Infect Dis. 1983;5(Suppl. 4):S637–S646. doi: 10.1093/clinids/5.supplement_4.s637. [DOI] [PubMed] [Google Scholar]
- 32.Watson D W, Brandly C A. Virulence and pathogenicity. In: Clifton C E, Raffel S, Barker H A, editors. Annual review of microbiology. Stanford, Calif: Annual Reviews, Inc.; 1949. pp. 195–220. [Google Scholar]
- 33.Wood W B, Davis B D. Host-parasite relations in bacterial infections. In: Davis B D, Dulbecco R, Eisen H N, Ginsberg H S, editors. Microbiology. Cambridge, Mass: Harper & Row; 1980. pp. 551–571. [Google Scholar]
- 34.Youmans G P, Paterson P Y, Sommers H M. The biologic and clinical basis of infectious diseases. Philadelphia, Pa: W. B. Saunders Co.; 1975. pp. 12–13. [Google Scholar]
- 35.Zinsser H. Infection and resistance. New York, N.Y: The Macmillan Company; 1914. pp. 1–27. [Google Scholar]