Abstract
Injectable bone biomaterials like bone cement should be designed and fabricated with certain biological criteria, which include: 1) recruitment and polarization of the macrophages from M1 (pro-inflammatory) to M2 (anti-inflammatory) phenotype, 2) enhance vascularization, and 3) activate osteogenic differentiation of bone marrow-derived stem cells to promote bone healing. So far, no injectable biomaterials could spontaneously regulate the entire bone healing process that involves inflammation, angiogenesis, and osteogenesis. Therefore, in this study, we designed bone cement comprised of strontium and copper-incorporated borosilicate glass (Sr/Cu-BSG) in the liquid phase of chitosan to modulate bone healing. In vitro studies showed that the controlled release of Sr and Cu ions up-regulated anti-inflammatory genes(IL-1Ra and TGF-β1) while down-regulating pro-inflammatory genes(IL-1β and IL-6) in macrophages at 3 days. Sr and Cu ions also increased the expressions of angiogenic genes (VEGF and bFGF) in HUVECs at 5 days and osteogenic genes (Runx-2, OCN, and OPN) in hBMSCs at 7, 14, and 21 days. 5Sr3Cu-BSG bone cement exhibited the best anti-inflammatory, angiogenic, and osteogenic properties among the bone cement groups with different Sr and Cu ratios. Short-term and long-term implantation of Sr/Cu-BSGs in femoral condylar bone defects of rats and rabbits confirmed the in vitro results, where the degradation rate of Sr/Cu-BSG matched the bone healing rate. Similar to in vitro, the 5Sr3Cu-BSG group also showed the highest bone formation in vivo. Excellent physical and chemical properties, along with its bone repairing ability, make the Sr/Cu-BSG bone cement a good candidate biomaterial for treating bone defects.
Keywords: Borosilicate glass bone cement, Immunomodulation, Angiogenesis, Osteogenesis
Graphical abstract
Highlights
-
•
The setting mechanism of Sr/Cu-BSG cement is ascertained.
-
•
Sr/Cu-BSG cement modulates immunity, angiogenesis, and osteogenesis to facilitate bone defect repair in vitro and in vivo.
-
•
Sr/Cu-BSG cement has excellent physical and chemical properties, biocompatibility, and bone repairing ability.
1. Introduction
Treatment methods for pathological fractures arising from infections, tumors, or osteoporosis remain elusive and are a clinical dilemma [1,2]. The minimally invasive surgery is often used to insert an implant into the bone defect site to strengthen and fasten the fracture surface. The success of this surgery heavily relies on the injectable biomaterials, with good syringeability, reasonable coagulation time, sufficient mechanical strength matching the adjacent bone, and their ability to induce new bone formation. However, for pathological fractures, the bone healing process is delayed. The imbalance between bone resorption and formation retards certain steps in bone remodeling, where inflammation prevents or delays the generation of new bone [3]. Therefore, modulation of inflammatory response is important for successfully repairing bone defects.
Implantation of a biomaterial can activate the foreign body reaction, comprised of macrophages [4], which are in charge of distinguishing and eliminating external threats, including bacteria, foreign particles, aged and damaged cells, deformed stromal cells, and tumor cells. However, a high inflammatory response to a biomaterial can inhibit bone formation. M1 macrophages secrete many pro-inflammatory factors, which activate osteoclastogenesis and intensify osteoclast activities, leading to bone resorption [5]. On the other hand, M2 macrophages have been reported to secrete cytokines that promote angiogenesis and osteogenesis, such as vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2 (BMP-2) [6,7]. Therefore, increasing the ratio of M2 macrophages usually leads to the formation of new bone. Many studies show that bioactive materials exhibit osteogenic properties by promoting the polarization of macrophages to M2. For example, Zhang et al. reported that strontium-substituted bioactive glass (Sr-SBG) induced the macrophages toward the M2 phenotype, which improved osteogenesis [8]. Another study by Lin et al. also showed that copper-incorporated bioactive glass-ceramics (Cu-BGC) regenerated cartilage by inhibiting the inflammatory response, inducing the macrophages towards the M2 [9]. These studies support that Sr/Cu-incorporated BSGs can generate new bone by regulating macrophage polarization.
Usually, angiogenesis precedes osteogenesis, where neovascularization eliminates metabolic waste and transmits oxygen and nutrients, which are conducive to the subsequent formation of new bone [10,11]. Histological studies found that osteoblasts and osteoprogenitor cells are densely populated near neovascularization in the defect area, confirming the spatial correlation between angiogenesis and osteogenesis [12]. Therefore, angiogenesis is an essential step for osteogenesis to take place. Cu and Sr ions have been found to enhance both angiogenesis and osteogenesis. Cu ions increased the proliferation of human endothelial cells (ECs) and promoted VEGF secretion in vitro [13,14]. In addition, Cu ions enhanced the osteogenic differentiation of MSCs [15]. Sr ions also showed a similar effect as Cu ions. Sr ions increased the expressions of angiogenic genes in ECs and osteogenic genes in MSCs, increasing the secretion of alkaline phosphatase (ALP) and osteoprotegerin (OPG) [[16], [17], [18], [19]].
Bioactive glass (BG) can successfully repair bone fractures and defects due to cysts, tumors, and periodontal infections [[20], [21], [22], [23]], and BGs can be doped with different elements, such as strontium, iron, copper, lithium, and manganese for different biological effects, such as immunomodulation, angiogenesis, and osteogenesis. The degradation rate of borosilicate glass (BSG) with a dual network containing [BO3] and [SiO4] can be adjusted by changing the molar ratios of B and Si, which is important to match the degradation rate of the implant and the bone healing rate. More importantly, BSGs have the potential to regulate inflammation, angiogenesis, and osteogenesis by releasing Sr and Cu ions.
An ideal bone cement should have the following properties: sufficient injectability, appropriate mechanical strengths, moderate biodegradability, excellent bone conductivity, and bone induction. PMMA, often used as the bone cement material, exhibits high plasticity. However, its mechanical strength and curing temperature are too high, and notably, PMMA lacks bone inductivity, which cannot be ignored. As of now, the construction of new bone remains a challenge. Recruitment and polarization of the macrophages from M1 to M2, vascularization, and activation of bone marrow stem cells are all essential in bone healing. Therefore, injectable biomaterials should be designed and fabricated to regulate this process. As shown in Fig. 1, considering the triple functions of Sr and Cu in modulating inflammation, angiogenesis, and osteogenesis, Sr/Cu-doped BSG bone cement was synthesized to not only reduce local inflammation but promote angiogenesis and osteogenesis. Syringeability, coagulation time, biological activity, and biodegradability of the Sr/Cu-doped BSG bone cement were measured in vitro. The modulation of RAW 264.7 macrophage polarization and the differentiation of human umbilical cord vein endothelial cells (HUVECs) and human bone marrow stem cells (hBMSCs) due to Sr and Cu ions release were also investigated in vitro. The bone cement was implanted in a rat femoral condylar bone defect model for 3–28 days to observe short-term and early onset of the inflammatory response, angiogenesis, and osteogenesis. The bone cement was also studied in a rabbit femoral condylar bone defect model for 8–16 weeks to observe the bone repair in the long term.
Fig. 1.
A schematic diagram of bone defect repair using Sr/Cu-BSG bone cement. (a) There are three main processes for the solidification of solid and liquid phases of bone cement: 1. The sol-gel transition of the chitosan solution, 2. The generated HA contacts and bridges with each other, 3. Formation of chemical bonds between the converted HA on the BSG surface and the chitosan. (b) Sr/Cu-BSG bone cement was implanted into the bone defect of the femoral condyle. Sr/Cu-BSG cement could repair bone defects by regulating immunity and promoting angiogenesis and osteogenesis.
2. Materials and methods
2.1. Fabrication of Sr/Cu-BSG cement and CaSO4 bone cement
The fabrication of the Sr/Cu-BSG bone cement method was adopted from a reference [24]. Sr/Cu-BSG bone cement was comprised of borosilicate glass (BSG) particles dispersed in the setting liquid. Five different composites of BSGs were made using 2 different strontium oxide (SrO; 0 and 5.0 mol% SrO substituted CaO) and 3 different copper oxide (CuO; 0, 1.0, and 3.0 mol% CuO doping into 100% 5Sr0Cu component) concentrations (Table 1). BSGs were produced using the standard melt quenching method. Briefly, the required concentrations of H3BO3, CaCO3, SiO2, Na2CO3, SrCO3, 4MgCO3·Mg(OH)2·5H2O, K2CO3 and NaH2PO4·2H2O (Sinopharm Chemical Reagent Co., Ltd., China) were mixed evenly and melted in a platinum/rhodium crucible in a muffle furnace for 120 min at 1200 °C. Next, the glass melt was poured into an ice slurry, which cooled the glass melt into glass raw materials. Finally, the raw glass materials were crushed, grounded, and sieved through an oxidation-resistant steel screen mesh to produce a fine powder with an average diameter of 40 μm or smaller. The composition of the BSG particles was measured using ICP (inductively-coupled plasma atomic emission spectroscopy; Optima 7000DV, Perkin Elmer, Waltham, USA). Five milligrams of BSG particles were incubated in 25 mL aqua regia in an autoclave with an environmental temperature of 190 °C and an atmospheric pressure of 20 MPa to make the standard solution for ICP testing. After 10 h of treatment to achieve complete dissolution, the ion concentrations were measured from the standard solution. The setting liquid was prepared by dissolving citric acid, chitosan (CS; degree of deacetylation ≥95%), and glucose (Aladdin Biochemical Technology Co., Ltd., China) in deionized (DI) water with the ratio of 5:1:10:30 by weight, respectively. Firstly, citric acid was dissolved in DI water. Then CS powder was dissolved in the acidic solution with magnetic stirring for about 30 min to acquire a clear and transparent CS solution. After that, the setting liquid was produced by continuously stirring for 1 h to dissolve glucose in the CS solution and was stored at 4 °C for future use. Cement slurries were acquired by mixing the BSG particles in the setting liquid by adding 2.0 g of BSG particles in 1 ml of the setting liquid for all cement groups. The Sr/Cu-BSG bone cement was named after the composition of the BSG powder: 5Sr0Cu-BSG, 5Sr1Cu-BSG, 5Sr3Cu-BSG, 0Sr1Cu-BSG, and 0Sr3Cu-BSG). The calcium sulfate (CaSO4) bone cement was prepared by Calcium sulfate hemihydrate powder (Aladdin, China)blend in 0.7%(w/v) sodium chloride solution (solid-liquid ratio = 1.0 g/ml).
Table 1.
Composition of BSG particles.
| Glass designation | Mole percent |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Na2O | K2O | MgO | CaO | SrO | CuO | SiO2 | B2O3 | P2O5 | |
| 5Sr0Cu-BSG | 6 | 8 | 8 | 17 | 5 | 0 | 4 | 50 | 2 |
| 5Sr1Cu-BSG | 6 | 8 | 8 | 17 | 5 | 1 | 4 | 50 | 2 |
| 5Sr3Cu-BSG | 6 | 8 | 8 | 17 | 5 | 3 | 4 | 50 | 2 |
| 0Sr1Cu-BSG | 6 | 8 | 8 | 22 | 0 | 1 | 4 | 50 | 2 |
| 0Sr3Cu-BSG | 6 | 8 | 8 | 22 | 0 | 3 | 4 | 50 | 2 |
2.2. Syringeability, initial coagulation time, compressive strength and setting temperature of Sr/Cu-BSG cement
The syringeability of the cement slurries was determined using a previously reported method [25]. After vigorous stirring to yield a uniform mixture, the cement slurry was added to a 5 mL syringe (Kindly Medical Devices Co., Ltd) with a needle diameter of 1.7 mm, without the needle covering the opening. The plunger was pushed with a pressure of 0.15 kN at a detection device velocity of 0.5 cm/min using a mechanical testing machine (ZQ-990LA, ZHIQU Test Machine Inc., China). Syringeability (S) was measured using the following equation (1), where M0 is the weight of the cement slurry in the syringe before injection, and M is the weight of the remaining cement slurry in the syringe after injection. M0 and M were weighed by an electronic balance(JE2002G/02, METTLER TOLEDO Co., Ltd, China). For the syringeability test, 3 samples were used as repeats, and the results are shown as a mean ± SD (standard deviation).
| (1) |
The initial coagulation time (ICT) was determined using Gilmore methods, according to the ASTM C266-2007 standard protocol. After mixing for 1 min, the cement slurries were perfused into a Teflon mold (2 cm in diameter and 0.5 cm in height) and set at a constant temperature of 37 °C. Again, the ICT test was repeated 3 times for each cement group, and the results are shown as a mean ± SD.
After placing the cement slurry in a chamber with a constant temperature of 37 °C for 1 day, the cured cement samples (0.6 cm in diameter and 1.2 cm in height) were tested for their compressive strength with a universal mechanical testing machine (ZQ-990LA) using a descending velocity of 1.0 mm/min. Four samples of each cement group were measured, and the results are shown as a mean ± SD.
The probe thermometer was used to measure the temperature change caused by the heat released during the setting process of Sr/Cu borosilicate bioactive glass cement. The solid-liquid ratio of the cement was 2g/1 ml, and the environmental temperature was 25.3 °C. 2 mL of cement paste was settled in a Teflon bottle after mixing evenly, and then the pH was measured using the pH electrode, which was inserted into the center of the bone cement paste about 1 cm deep. Three samples of each cement group were measured, and the results are shown as a mean ± SD.
2.3. Setting of the Sr/Cu-BSG cement
2 g of BSG glass particles (with particle size <40 μm) were added to 2 ml of the setting liquid. The weight of the BSG glass particles and the volume of the setting liquid were selected to amplify and accelerate the setting reaction. After vigorous mixing, the BSG particles were evenly dispersed in the setting liquid, giving a homogenous cement paste. At a reaction time of 24 h, the cement paste was lyophilized, taped to a metal test stage using a conductive tape, and sputtered with gold. The surface morphology of the paste was observed by an FE-SEM (field emission scanning electron microscope; Nova NanoSEM 450, FEI, Netherlands). The structural unit change of setting products were analyzed using the FTIR (EQUINOXSS/HYPERION2000, BRUKER, Germany) at a wavenumber range of 400–2000 cm−1 and scanned rate of 0.04 cm−1. The pH of the cement paste was also measured every min or so for the first 20 min of the reaction time using a pH microelectrode (Sentron, SI600 7600-010). As described before, to acquire the pH value of bone cement paste as precisely as possible, 2 mL of cement paste was first allowed to settle in a Teflon bottle after mixing evenly. Then the pH electrode was inserted into the bone cement paste about 1 cm deep. The pH values were recorded. The pH was measured for 3 samples of each cement group, and the results are shown as a mean ± SD.
2.4. Degradation and biological activity of Sr/Cu-BSG cement In vitro
Cylindrical-shaped cured cement specimens with 1 cm in diameter and 0.3 cm in height(the size was chosen based on our previous study [24]) were used to test the in vitro degradation rate and biological activity of the Sr/Cu-BSG cement. The cement specimens were placed in sterile polyethylene bottles and 25.1 ml of PBS was poured into the bottle (the volume of PBS based on the cement size was calculated based on a reference [26]). At different time points, the samples were taken out and dried at 80 °C. The weight loss of the cement was taken as the difference between the initial (without soaked) weight and the weight at the selected soaking time. The ionic concentrations of Sr, Cu, Mg and Ca in PBS were measured using ICP (inductively-coupled plasma atomic emission spectroscopy; Optima 7000DV, Perkin Elmer, Waltham, USA). Three samples were used as repeats, and the results are shown as a mean ± SD. pH was also measured at each time point after cooling the PBS to room temperature (n = 3). The Sr/Cu-BSG cement before and after immersion in PBS was taped to a metal test stage using a conductive tape and sputtered with gold to make the surface conductive. The surface morphology of the bone cement was then observed using FE-SEM (Field emission scanning electron microscope; Nova NanoSEM 450, FEI, Netherlands). XRD (x-ray diffraction; D8 ADVANCE A25X, Bruker, Germany) and FTIR were used to determine the conversion of hydroxyapatite (HA) from BSGs before and after degradation in PBS. The XRD measurement conditions were Cu Kα radiation (λ = 0.15406 nm) at a scanning rate of 10°/min (in the range of 10–80° 2θ). The FTIR conditions were the same as above in section 2.3.
2.5. In vitro response of RAW264.7, HUVECs, and hBMSCs to Sr/Cu-BSG bone cement
2.5.1. Cell culture and cement extract preparation
RAW 264.7 (CL-0191), HUVECs (CP-H082), and hBMSCs (CP-H166) were purchased from Procell Life Science & Technology Co., LTD. (Wuhan, China). The selected cell line was cultured in Dulbecco's Modified Eagle Medium (DMEM, HyClone, USA), Ham's F-12 Nutrient Mix (HyClone, USA), and MEM alpha modification (α-MEM, HyClone, USA), respectively, with the addition of 10% (v/v) fetal bovine serum (FBS; HyClone, USA) and 1% (v/v) penicillin-streptomycin (PS; Solarbio, China). Cells were cultured in an incubator (Thermo, USA) with standard cell culture conditions, 37 °C and 5% CO2. When the confluency reached about 80%, the cells were passaged using 0.25% trypsin (Solarbio, China), and passage numbers 6–8 were used in the experiment.
CaSO4 cement and the Sr/Cu-BSG samples were immersed in a cell culture medium with the sample weight to the cell culture medium volume ratio of 0.1 mg/ml according to the previous research [27]. CaSO4 cement and The Sr/Cu-BSG cement in a cell culture medium was constantly shaken (60 rpm, Blue pard, China) at 37 °C for 24 h to obtain the Sr/Cu-BSG extract medium. The extract medium was then centrifuged at 1000 rpm for 5 min (Eppendorf, Germany) and syringed through a 0.2 micron-rated filter.
2.5.2. Polarization and inflammatory gene expressions of RAW264.7
RAW 264.7 was cultured with the cement extract medium, and the effect of the cement extract on the polarization of RAW 264.7 was measured using flow cytometry. Briefly, RAW 264.7 was cultured in a 6-well plate with a seeding density of 2 x 105 cells/well (A = 9.6 cm2) in cell culture media (DMEM+10% FBS+1% PS). After 24 h, when RAW 264.7 cells adhered to the plate surface, the cell culture medium was displaced with the cement extract medium. The negative control group was RAW 264.7 cells cultured in a cell culture medium without cement extract. After 3 days of culture, RAW 264.7 was trypsinized and centrifuged at 1000 rpm for 5 min. Next, RAW 264.7 cells were re-suspended in 0.5–1 ml of PBS and fixed with 4% (w/v) formaldehyde at room temperature for 15 min. The fixed cells were washed in PBS twice, then centrifuged and re-suspended in PBS. Cells were incubated in a 1.5 ml centrifuge tube with FITC-conjugated CD80 (M1 Marker, 1:600, Thermo, 11-0801-82) and PE-conjugated CD206 (M1 Marker, 1:600, Thermo, 12-2061-82) at 4 °C for 30 min and centrifuged at 1000 rpm for 5 min. The supernatant was discarded, and the cells were re-suspended in PBS. The polarization state (M1/M2) of RAW 264.7 was analyzed using an Agilent FACS-Calibur cytometer (NovoCyte, USA) and software FlowJo X. Gene expression levels of pro-inflammatory genes, IL-1β and IL-6, and anti-inflammatory genes, IL-1Ra and TGF-β1, were measured using qRT-PCR after culturing RAW 264.7 in the cement extract medium for 3 days. Total RNA was extracted using the HiPure Total RNA Mini Kit (Magen), and then the RNA was reverse-transcribed to cDNA using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific). The target genes were analyzed by quantitative reverse transcription-polymerase chain reaction (qRT-PCR; Realstar green fast mixture, Genstar), where the primer sequence information is provided in Table 2.
Table 2.
Primers used for real time polymerase chain reaction (RT-PCR) analysis.
| Gene | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| IL-1β | TGGAGAGTGTGGATCCCAAG | GGTGCTGATGTACCAGTTGG |
| IL-6 | CTTGGGACTGATGCTGGTGA | TTGGGAGTGGTATCCTCTGTGA |
| IL-1Ra | CTCCAGCTGGAGGAAGTTAAC | CTGACTCAAAGCTGGTGGTG |
| TGF-β1 | CAGTACAGCAAGGTCCTTGC | ACGTAGTAGACGATGGGCAG |
| VEGF | CTACCTCCACCATGCCAAGT | CACACAGGATGGCTTGAAGA |
| bFGF | AAAAGGCAAGATGCAGGAGA | TTTTGCAGCCTTACCCAATC |
| FLT1 | GGGCTGAAACCATGTGCAAG | GCCAAAGATGCACTCCTCCT |
| KDR | GGCATGGGGTCTGTTCTGAA | TTGGCCAGGAGACACGTAAC |
| RUNX-2 | TGGTTCTGTCATGGCGGGTA | TCTCAGATCGTTGAACCTTGCTA |
| OPN | ACGCCGACCAAGGAAAACTC | GTCCATAAACCACACTATCACCTCG |
| OCN | CATGAGAGCCCTCACA | AGAGCGACACCCTAGAC |
| GAPDH | ATCCCATCACCATCTTCC | GAGTCCTTCCACGATACCA |
2.5.3. Tube formation assay and angiogenesis-related gene expressions of HUVECs
Tube formation ability of HUVECs was assessed after culturing in cement extract medium for 5 days. Briefly, the 96-well plate was coated with MatrigelTM Matrix (354230, BD) and placed in the incubator at 37 °C for 45 min. HUVECs were seeded in the 96-well plate at a density of 2.5 x 104 cells/well (A = 0.32 cm2) and placed in the incubator for 4 h. The cells were rinsed with PBS, fixed with 4% (w/v) paraformaldehyde (PFA), then stained with calcein. Bright-field and fluorescence images of the tube formation were taken using a fluorescence microscope (Olympus, Japan), and tube meshes, segments, and junctions were measured using Image J (NIH, USA). Three random areas within the bright-field image were chosen for measurement. Relative gene expression levels of angiogenic genes, VEGF, bFGF, FLT1, and KDR, were measured using qRT-PCR in HUVECs after culturing in a cell culture medium for 5 days. The primer sequence information is listed in Table 2.
2.5.4. Viability, proliferation, and differentiation of hBMSCs
hBMSCs were seeded in a 48-well plate with sterilized bone cement samples at a density of 5 x 104 cells/well (A = 0.95 cm2) in cell culture medium (α-MEM + 10% FBS + 1% PS). After 12 h of culture, hBMSCs were stained with live/dead assay (calcein and propidium iodide; L6037 M, Bioscience, Shanghai, China) to measure cell-material interaction. hBMSCs were seeded in a 48-well plate at a density of 5 x 104 cells/well (A = 0.95 cm2) in cell culture medium (α-MEM + 10% FBS + 1% PS). After 24 h, when about 80% of cells were attached to the plate, the cell culture medium was displaced with the cement extract medium. Cells cultured in a medium without cement extract were used as the blank control. After culturing hBMSCs in cement extract medium for 3 days, hBMSCs were stained with live/dead assay (calcein and propidium iodide; L6037 M, Bioscience, Shanghai, China) to measure cell viability. The nucleus and actin cytoskeleton was stained using DAPI and Phalloidin-488 to observe cell morphology.
Cell proliferation was measured using the CCK-8 assay. hBMSCs were seeded in a 96-well plate with a density of 5 x 103 cells/well. After 24 h, the medium was displaced with cement extract medium. Again, the blank control group was cells cultured in a cell culture medium only. Cells were cultured for either 1, 3, or 5 days in the cement extraction medium. Then 10% CCK-8 in fresh culture medium was added and cultured in the dark for 2 h. The OD value (absorbance) was measured at 450 nm using the Multiskan Spectrum Microplate Spectrophotometer (Thermo Scientific).
Alkaline phosphatase activity was measured to see the differentiation potential of hBMSCs treated with cement extracts. hBMSCs were cultured in a 48-well plate with density of 1 x 105 cells/well in α-MEM + 10% FBS medium. Osteogenic-induction medium was prepared by supplementing the medium with ascorbic acid (50 nM), dexamethasone (0.1 μM), and β-glyceryl phosphate (10 mM). Osteogenic-induction medium was used to make the cement extract medium using the same conditions (0.1 mg/ml of bone cement in osteogenic-induction medium for 24 h at 37 °C). After cells reached a confluency of 80%, the cell culture medium was changed to cement extract osteogenic-induction medium to induce the differentiation of hBMSCs. hBMSCs cultured in an osteogenic-induction medium without cement extract were used as the blank control group. After incubation of 7 days, the medium was discarded, and cells were washed twice with PBS. hBMSCs were then stained using the ALP detection kit (C3206, Beyotime Biotechnology), following the standard protocol given for this kit.
2.5.5. Expression of osteogenesis-related genes in hBMSCs
hBMSCs were cultured in a 6-well plate with density of 1 x 106 cells/well in α-MEM + 10% FBS. Then after cells reached the confluency of 80%, the cell culture medium was replaced with cement extract osteogenic-induction medium. The blank control group was the same as those in section 2.5.4. After incubation for 7, 14, and 21 days, the relative gene expression levels of osteogenesis-related genes, RUNX-2, OCN, and OPN, were measured using qRT-PCR. The primer sequence information is listed in Table 2.
2.6. Immunoregulation, angiogenesis, and osteogenesis using Sr/Cu-BSG bone cement In vivo
2.6.1. Animal experiment and surgical procedure
Two animal models, rats and rabbits with femoral condylar defects, were used. All animal experiments were conducted at Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences (CAS), located in Shenzhen, China, supervised by the Animal Research Committees of SIAT. A total of 36 female adult rats (weighing 250–280 g) and 72 female adult New Zealand white rabbits (weighing 3.5–4.0 kg) were used for short-term and long-term implantation experiments, respectively. Animals were kept and fed in a feeding room with a temperature of 22 °C and relative humidity of 40–70%. The alternating time of day and night was 12h/12h. Animals spent 7 days of an adjustment period in the feeding room before the experiment started. All animals were operated on under general anesthesia. 1% of pentobarbital sodium (Merck, Germany) was injected intraperitoneally for rats (40 mg/kg), and 3% of pentobarbital sodium was injected into the marginal auricular vein for rabbits (0.8 ml/kg).
Six different bone cement groups were implanted in animals. Six rats (n = 6 rats per cement group, total n = 36 rats) and twelve rabbits(n = 12 rabbits per cement group, total n = 72 rabbits) were used per cement group.The cement was implanted bilaterally into the femoral condyles of rats (n = 12 bone samples per cement group) and rabbits(n = 24 bone samples per cement group). Two rats were sacrificed at different implantation times of t = 3, 7, and 28 days (n = 2 rats for each time point per cement group). Four rabbits were sacrificed at different time points of t = 8, 12, and 16 weeks after the implantation (n = 4 rabbits for each time point per cement group). The surgical procedure was the same for both rats and rabbits. First, a lateral longitudinal incision was made on the knee joint. The outer skin and fascia of the knee joint were cut to make the later condyle of the femur visible. After the periosteum was removed, a cylindrical bone defect was made. For rats, a cylindrical bone defect with 2.5 mm in diameter and 5 mm in depth was drilled using a 2.5 mm Kuntscher pin. For rabbits, a cylindrical bone defect with 6 mm in diameter and 8 mm in depth was drilled using a 6 mm Kuntscher pin. The cured bone cement with the proper size was then implanted into the defect site in the metaphyseal region of the lateral condyle of both hind limbs. The calcium sulfate (CaSO4) bone cement (Aladdin, China) was used as the control group. After Sr/Cu-BSG bone cement was implanted, the muscles, fascia, and skin were sutured. Digital photos of the surgical procedures were show in Fig. S4. Benzylpenicillin potassium (Youxin Biotechnology Co., Ltd China) was injected intramuscularly into rats and rabbits for three consecutive days after the surgery to prevent perioperative infections. All animals were allowed to move, eat, and drink freely after the surgery.
Rats were killed by injecting excessive pentobarbital sodium at 3, 7, and 28 days of implantation. Rabbits were sacrificed using excessive pentobarbital sodium at 8, 12, and 16 weeks of implantation. The femurs were taken out at the end of each time point for analysis. In addition, blood and visceral samples were collected from the New Zealand rabbits in the 16-week group for in vivo biocompatibility testing.
2.6.2. Micro-computed tomography (μCT) analysis
The harvested femurs from rabbits were fixed in 4% PFA (BOSTER) for 24 h at normal atmospheric temperature and then scanned with micro-CT (SkyScan 1176, Bruker, Germany) with an 18 μm resolution. NRecon software (1.7.1.0, Bruker, Germany), DataViewer (1.4.4.0, SkyScan, Germany), CT Analyser (1.11.8.0, SkyScan, Germany) were used for image format conversion, image axial adjustment, and data analysis, respectively. The region of interest (ROI) was chosen from the scanogram of micro-CT for threshold segmentation to analyze various morphological characteristics of the bone-implant interface and calculate new bone volume to total bone volume (BV/TV) and bone mineral density (BMD) at the femoral condylar defect site.
2.6.3. Decalcified histological characterization
Rat and rabbit femurs were harvested, rinsed with PBS 3 times, and fixed with 4% PFA for 1 day. The femur was decalcified with neutral EDTA solution for 4 weeks and dehydrated using different gradients of ethanol (70%, 80%, 90%, 95%, and 100% volume fraction). The decalcified specimens were then sealed in a paraffin mold, which was sliced on a microtome and transferred to a slide to dry. Hematoxylin-eosin staining and Masson trichrome staining were used for histological analysis.
2.6.4. Undecalcified histological analysis
The harvested rabbit femurs were rinsed in PBS 3 times, fixed with 4% PFA for 1 day, and then dehydrated in different gradients of ethanol (the same as section 2.6.3.). The undecalcified specimens were embedded in polymethylmethacrylate (PMMA), then sliced with a thickness of about 40 μm. The specimens were polished along the sagittal plane using a hard tissue grinding disc [28]. The uncalcified specimens were stained using Goldner trichromatic and Methylene blue acid fuchsin staining. The percentage of bone directly in contact with the bone cement, referred to as the bone-implant contact (BIC) value, was calculated from stained tissue sections using CaseView software (3DHISTECH Ltd., Hungary).
2.6.5. Sequential fluorescent labeling of new bone mineralized deposition rate
New bone mineralized deposition rate (MAR), an indicator of the speed of bone mineralization and remodeling activity of osteoblasts, was determined by Tetracycline-calcein labeling of the undecalcified slices prepared as section 2.5.4. Briefly, rabbits were injected subcutaneously with two different fluorochromes, tetracycline hydrochloride (30 mg/kg, Aladdin, China) and calcein (5 mg/kg, Aladdin, China), 14 and 4 days before being sacrificed, respectively. MAR was calculated by measuring the furthest distance of tetracycline-calcein fluorescent labeling using the TISSUEFAXS VIEWER imaging system (TissueGnostics Technology Co., Ltd, China) and divided by the interval time of administration (10 days).
2.6.6. Immunohistochemical and immunofluorescence analysis
Immunohistochemistry staining (IHC) was used to detect IL-1β, IL-6, IL-1Ra, TGF-β1, VEGF, bFGF, VEGFR1 (FLT1), VEGFR2(KDR), a-SMA, RUNX-2, OCN, and OPN expressions. The decalcified slices from section 2.6.3 were soaked in 10 mM citrate buffer (pH of 6.0) at a quasi-boiling temperature of 95–99 °C for 10 min and then cooled to room temperature for 30 min. The slices were blocked with Tris Buffered Saline (TBST, ThermoFisher Scientific) containing 5% goat serum at normal atmospheric temperature for 60 min and then incubated overnight at 4 °C with primary antibodies. The antibodies were diluted as follows: IL-1β (1:200, BS-0812R, Bioss), IL-6 (1:200, BS-0379R, Bioss), IL-1Ra (1:200, ab175392, Abcam), TGF-β1 (1:200, BS-0086R, Bioss), VEGF (1:200, BS-1313R, Bioss), bFGF (1:150, BSM-34016R, Bioss), VEGFR1 (1:200, AF6204, Affinity), VEGFR2 (1:200, BS-0565R, Bioss), a-SMA (1:200, 14-9760-82, Invitrogen), RUNX-2 (1:400, BS-1134R, Bioss), OCN (1:400, 23418-1-ap, PTG), and OPN (1:200, BS-0026R, Bioss). After rinsing with TBST 3 times (5 min per wash), the slices were incubated with a biotin-labeled second antibody for 2 h. The colored markers produced by chromogenic enzymes were observed under The colored markers produced by chromogenic enzymes were observed under the Microscope slide scanner(Pannoramic MIDI, 3DHISTECH Ltd, Hungary). Immunofluorescence (IF) was used to detect CD86 and CD206 expressions. Sections were blocked with goat serum and incubated overnight at 4 °C with primary antibodies. The antibodies were diluted as follows: CD86 (1:100, 13395-1-AP, Proteintech) and CD206 (1:200, 18704-1-AP, Proteintech). Sections were further incubated with the corresponding secondary antibodies and stained with DAPI. Finally, the images of the sections were taken with the Microscope slide scanner(Pannoramic MIDI, 3DHISTECH Ltd, Hungary). A circle with a diameter of 1600 pixels centered on the bone cement was designated as the region of interest(ROI), as indicated by the white circle in Fig. 9, Fig. 10. IF and IHC were semi-quantified by calculating the fraction of positively-stained areas in ROI using Image J (1.80_172, NIH, USA).
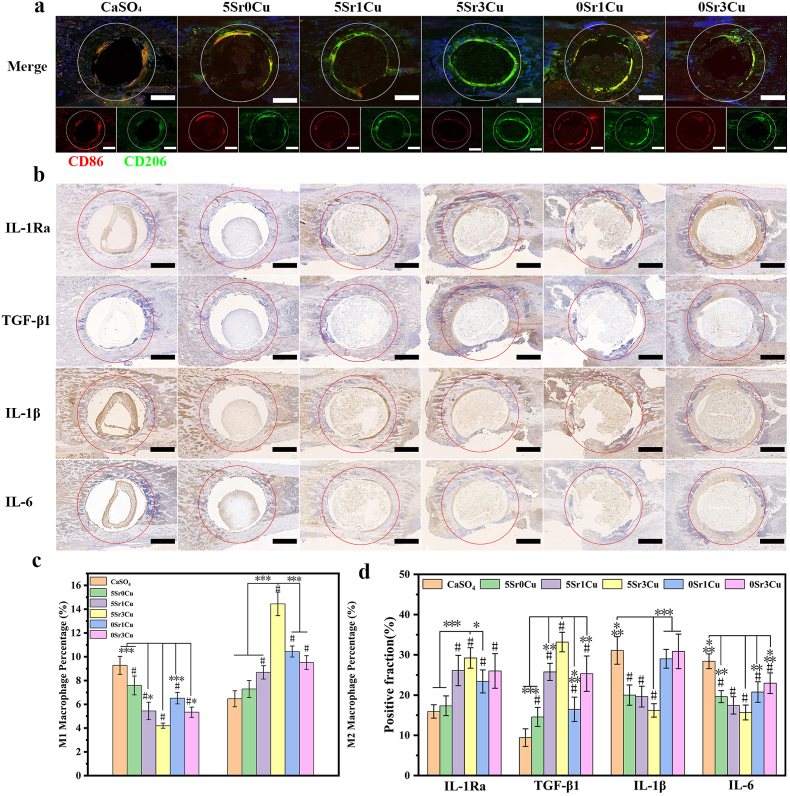
Fig. 9.
Immunomodulation with Sr/Cu-BSG bone cement implantation in rat femoral condylar defects for 3 days. Immunofluorescence staining (a) for the surface markers including CD86 (M1 phenotype macrophage) and CD206 (M2 phenotype macrophage) and Immunohistochemical staining (b) for the expression of inflammatory cytokine (IL-1Ra, TGF-β, IL-1β and IL-6) with cement implanted for 3 days; Semi-quantitative statistical data of M1 and M2 Macrophage Percentage (%)(c) and the expression of immunomodulation (d), as detected from immunofluorescence staining (a) and inflammatory cytokine (b). (Mean ± SD; n = 4; *significant difference compared with the 5Sr3Cu-BSG group, p < 0.05, **P < 0.01, ***P < 0.001; # significant difference compared with the CaSO4 group, p < 0.05).
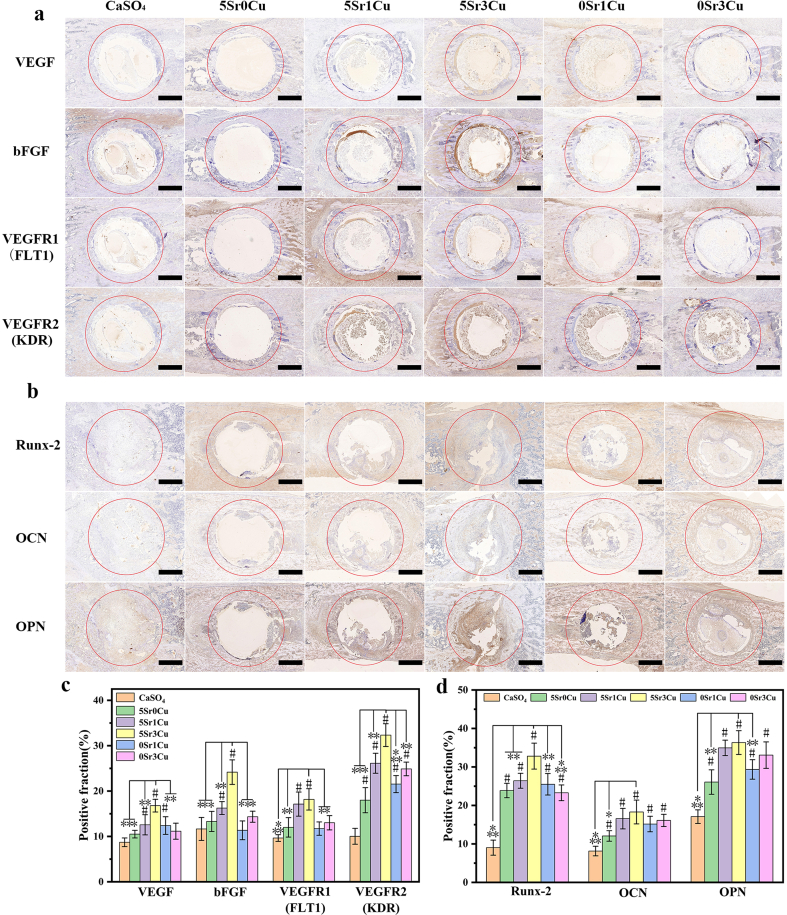
Fig. 10.
Immunohistochemical staining for the expression of angiogenic factors (VEGF, bFGF, FLT1 and KDR) with cement implanted for 7 days (a), and osteogenic factors (Runx-2, OCN and OPN) with implanted cement for 28 days (b); Semi-quantitative assays for the expression of angiogenic (c) and osteogenic (d) factors. (Mean ± SD; n = 3; *significant difference compared with the 5Sr3Cu-BSG group, p < 0.05, **P < 0.01, ***P < 0.001; # significant difference compared with the CaSO4 group, p < 0.05).
2.6.7. Statistical analysis
All quantitative data were presented as a mean ± SD. Statistical analysis was performed using one-way ANOVA and the student's t-test, with the significance level set at p < 0.05.
3. Results
3.1. Basic properties of Sr/Cu-BSG bone cement
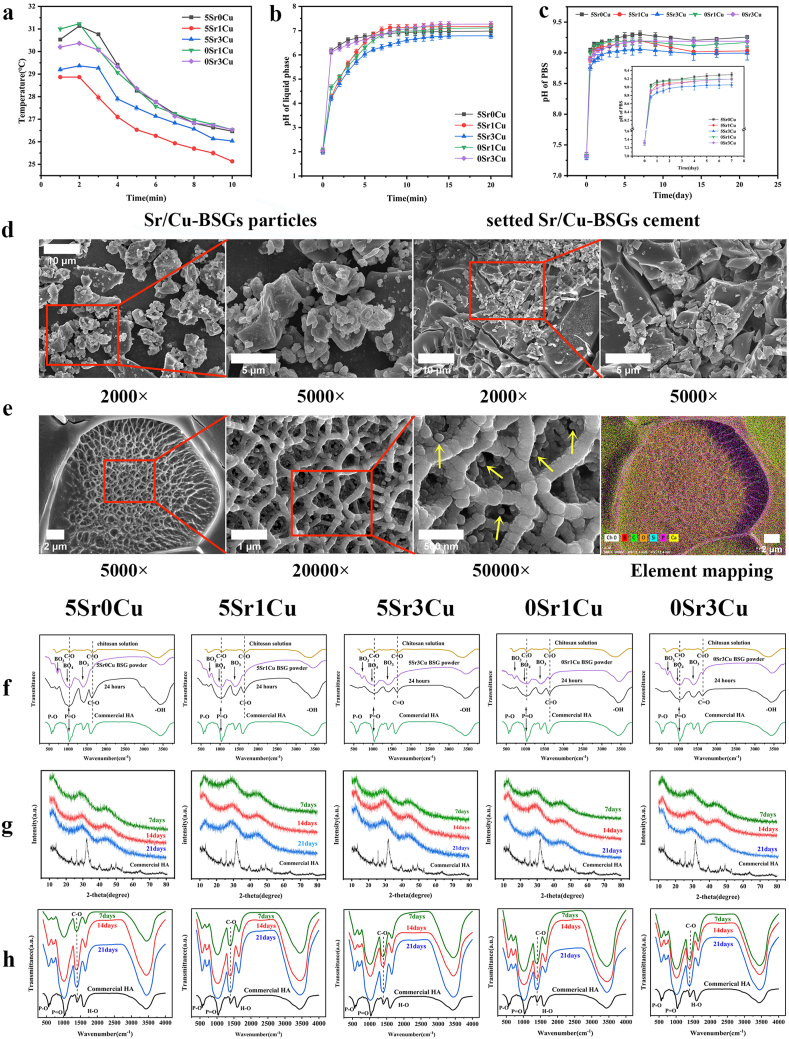
Table S1displays the actual composition of BSGs calculated from the ICP results. The actual composition of the BSGs roughly matched the designated composition. Table 3 demonstrates the handling properties, including syringeability, initial coagulation time, and compressive strength of Sr/Cu-BSG bone cement. The initial coagulation time increased as the Cu2+ concentration increased, from 14.22 ± 0.39 min for the 5Sr0Cu group to 23.16 ± 1.01 min for the 5Sr3Cu group. On the other hand, incorporating of Sr2+ ions increased the syringeability, from 84.53 ± 0.49% for the 5Sr3Cu group to 94.15 ± 0.76% for the 0Sr1Cu group. However, the compressive strength did not change proportionally to the concentrations of Sr or Cu ions. The 5Sr3Cu group showed the highest compressive strength of 15.62 ± 3.46 MPa, and the 0Sr1Cu group showed the lowest compressive strength of 11.31 ± 2.18 MPa. The compressive strength of all Sr/Cu-BSG groups was similar to that of human cancellous bone. As shown in Fig. 2a, the setting temperatures of the 5Sr0Cu, 0Sr1Cu, and 0Sr3Cu groups were higher than that of the 5Sr1Cu and 5Sr3Cu groups, which indicates that the strontium or copper incorporation could lower the setting temperature of the Sr/Cu-BSG bone cement group. The highest setting temperature was 31.1 °C(0Sr1Cu group), far lower than the human body temperature of 37 °C. Therefore, the setting of the Sr/Cu-BSG bone cement in vivo would not produce damage to the surrounding tissues due to excessive heat.
Table 3.
Syringeability, initial coagulation time and compressive strength of Sr/Cu-BSG cement.
| Cement designation | Injectability (%) | Initial setting time(min) | Compressive strength(MPa) |
|---|---|---|---|
| 5Sr0Cu-BSG | 88.13 ± 1.65 | 14.22 ± 0.39 | 15.50 ± 2.06 |
| 5Sr1Cu-BSG | 88.52 ± 2.30 | 21.47 ± 0.82 | 11.86 ± 2.03 |
| 5Sr3Cu-BSG | 84.53 ± 0.49 | 23.16 ± 1.01 | 15.62 ± 3.46 |
| 0Sr1Cu-BSG | 94.15 ± 0.76 | 18.36 ± 0.93 | 11.31 ± 2.18 |
| 0Sr3Cu-BSG | 92.34 ± 0.84 | 20.46 ± 1.31 | 12.41 ± 1.76 |
Fig. 2.
(a)Temperature variation curve of Sr/Cu-BSG bone cement paste during solidification (b)pH of the CS solution as a function of reaction time of the BSG particles (SL ratio = 1.0 g/ml), (c)pH of the solution as a function of immersion time of the cement in PBS. (d)SEM images of Sr/Cu-BSG particles distribution in bone cement.; (e)SEM and Element mapping images of the reaction product formed by immersing 0Sr1Cu-borosilicate glass particles in the setting liquid with SL ratio of 0.5 g/ml for 2 h (f)FTIR spectra of CS solution, borosilicate glass, and the reaction product between the BSG particles and the CS solution (SL ratio = 1.0) for reaction times of 24 h. X-ray diffraction patterns (g) and Fourier-transform infrared spectra (h) of Sr/Cu-BSG cement after immersion in PBS for 7, 14 and 21 days.
3.2. Setting mechanism of BSG bone cement
The initial pH value of the setting liquid was 2.01–2.06. When the BSG particles were immersed in the setting liquid, the ions (Ca2+, Na+, K+, Mg2+, and BO33−) released from the BSGs changed the pH of the setting liquid (Fig. 2b) [29]. The pH increased to 6.79–7.27 after the reaction time of 20 min and then plateaued to a stable value. pH value higher than 6.25 was reported to gel the CS-based acid sol solution [30]; therefore, the sol-gel transition could positively lead to the initial setting of the BSG-based bone cement as reported previously [31].
After the reaction at 37 °C for 2 h, the microstructure and elemental mapping images (Fig. 2c) revealed that the matrix of the reaction products was interlocked into a three-dimensional network, presumably due to the sol-gel transition of the CS triggered by the alkaline pH during the setting reaction [30,31]. Careful inspection showed the deposition of nanoparticles in the inner surface of the three-dimensional CS network, likely due to the nucleation and growth of Ca and PO43− released from the BSGs.
In Fig. 2d, when the solid-liquid phase of Sr/Cu-BSG bone cement was mixed, the glass particles (about 1–20 μm) were evenly distributed in the liquid phase. In SEM images of Sr/Cu-BSG bone cement, the particles of different sizes were glass particles, and the substance between the particles is the liquid phase of the Sr/Cu-BSG bone cement. As depicted in Fig. 2f, the vibration of the primary amide groups of the acetylated units of chitosan emerged at 1649 (cm−1) and C-O asymmetric stretching at 1097 (cm−1) for the setting liquid [32,33]. On the other hand, The FTIR spectrum of the BSGs showed one weak resonance of the B-O stretching of [BO4] at 700 (cm-1) and two broad and strong resonances at ∼800–1200 (cm-1) and ∼1200–1600 (cm-1), attributed to the B-O stretching vibration of [BO4] and B-O asymmetric vibration of [BO3], respectively [34,35]. After mixing the BSGs in the setting liquid for 24 h, the reaction product showed a new wavelength at 500–700 (cm−1), corresponding to the P-O functional groups of hydroxyapatite (HA), which shows that BSGs converted to HA. The FTIR spectrum also showed that the primary amide groups (at about 1649 cm−1) and C-O asymmetric stretching (at about 1097 cm−1) from chitosan shifted to a lower wavenumber, due to the chemical chelation between the BSGs and the chitosan, as reported previously.
3.3. Biodegradability and biological activity of Sr/Cu-BSG bone cement In vitro
The weight loss measurement showed that the co-incorporation of strontium and copper elements accelerated the biodegradation rate of the Sr/Cu-borosilicate glass cement(Fig. S1). Sr/Cu-BSG bone cement was immersed in PBS for 7, 14, and 21 days, then characterized by XRD (Fig. 2g). Commercial HA was used as a reference. After 7 days in PBS, a broad diffraction peak (at about 2 θ = 32°) emerged for all cement groups, which corresponded to the reference HA, indicating that BSGs converted to HA with a low degree of crystallization. After 21 days in PBS, a higher and sharper diffraction peak at about 2 θ = 32° emerged for all cement groups, showing that BSGs converted to HA with a higher degree of crystallinity. 5Sr3Cu cement group showed the sharpest and strongest diffraction peak at all times compared to other cement groups.
After immersion in PBS for 7–21 days, the FTIR spectra of the Sr/Cu-BSG bone cement showed major vibrational bands associated with HA, corresponding to the PO43− ν3 (band at ∼1040 cm−1) and PO43− ν4 (band at ∼600 cm−1) vibrations [36]. Furthermore, the intensity of these two bands increased proportionally with the immersion time, illustrating that the formation and crystallization of HA increased over time.
The pH curve for all cement groups exhibited a similar trend (Fig. 2c). Starting from 7.32, the pH of the PBS increased exponentially for the first 5 days of immersion and then increased more slowly between 5 and 7 days, reaching a plateau of 9.0–9.3 after 7 days. The pH values after 21 days of immersion were 9.25 ± 0.02, 9.03 ± 0.05, 9.00 ± 0.11, 9.17 ± 0.07, and 9.19 ± 0.07 for 5Sr0Cu, 5Sr1Cu, 5Sr3Cu, 0Sr1Cu, and 0Sr3Cu, respectively. As shown, the co-incorporation of Sr and Cu into the BSGs decreased the pH more than the single incorporation of either Sr or Cu, where 5Sr3Cu exhibited the lowest pH. Lower alkaline pH may be the cause of minimizing the inflammatory reactions in vivo, as reported previously [31].
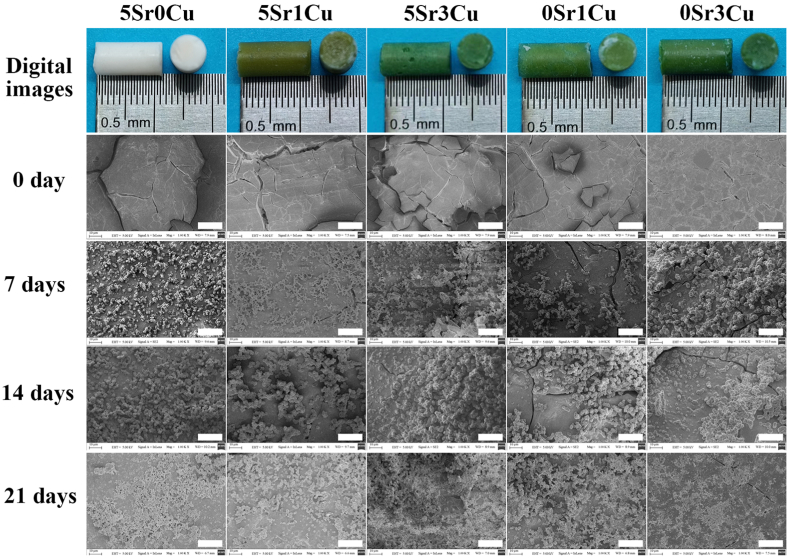
The cured cement showed a smooth surface with few cracks, which probably resulted from capillary drying stresses during specimen preparation before soaking in PBS, as depicted in Fig. 3. After soaking in PBS, BSGs degraded and transformed to HA [24], resulting in a layer of fine HA particles sedimented on the cement surface (Fig. 3). When the immersion time lasted long (21 days), the HA particle-deposited areas became larger and more apparent. The incorporation of the Sr and Cu ions did not change the microstructure of the bone cement.
Fig. 3.
Optical photograph of the cured 5Sr0Cu-BSG, 5Sr1Cu-BSG, 5Sr3Cu-BSG, 0Sr1Cu-BSG and 0Sr3Cu-BSG cement and their surface morphology before and after immersed in PBS for 7, 14 and 21 days, the scale bar at 20 μm.
Table 4 and Table S2 list the cumulative concentrations of Sr2+, Cu2+, Mg2+ and Ca2+ ions released from the bone cement into PBS at different immersion times. The maximum cumulative concentrations of Sr2+ after 7 days were 0.91 ± 0.17, 1.14 ± 0.04, and 1.16 ± 0.07 ppm for 5Sr0Cu, 5Sr1Cu, and 5Sr3Cu groups, respectively. Surprisingly, the prolonged immersion time did not result in a higher cumulative release of Sr2+. In fact, the cumulative concentrations of Sr2+ were lower when the immersion time was 14–21 days compared to 7 days, which may be due to a fraction of released Sr2+ incorporated into the HA crystal lattice, leading to the formation of a Sr-substituted HA, such as (Ca1−x Srx)10(PO4)6(OH)2 [37]. On the other hand, more Cu2+ ions were released when more CuO was incorporated into the BSGs, and so, 0Sr3Cu cement released the highest Cu2+. The cumulative concentrations of Cu2+ reached the maximum value as early as 7 days of immersion, and the prolonged immersion time to 14–21 days did not result in higher released Cu2+ concentrations (no statistical difference in the [Cu2+] among the cement groups immersed for 7, 14, and 21 days). The incorporation of Cu2+ accelerated the release of Sr2+ at all immersion times, whereas the incorporation of Sr2+ impeded the release of Cu2+, showing that the incorporation of SrO and CuO in BSGs had an opposite effect on each other on the release of ions. As shown in Table S2, the cumulative concentrations of Mg2+ ions increased as the immersion time increased, except for the 0Sr3Cu group which reached its maximum value at t = 14 days. However, the cumulative concentrations of Ca2+ ions for all cement groups were relatively steady during immersion, possibly due to the consumption of released Ca2+ ions to form hydroxyapatite (HA) [38].
Table 4.
Cumulative release of Sr2+ and Cu2+ from cement after immersed in PBS for 7, 14 and 21 days.
| Cement designation | concentration of Sr2+ |
concentration of Cu2+ |
||||
|---|---|---|---|---|---|---|
| 7 days | 14 days | 21 days | 7 days | 14 days | 21 days | |
| 5Sr0Cu-BSG | 0.91 ± 0.17 | 0.27 ± 0.03 | 0.37 ± 0.18 | 0 | 0 | 0 |
| 5Sr1Cu-BSG | 1.14 ± 0.04 | 0.31 ± 0.10 | 0.38 ± 0.10 | 22.19 ± 1.65 | 21.33 ± 1.78 | 20.71 ± 2.81 |
| 5Sr3Cu-BSG | 1.16 ± 0.07 | 0.38 ± 0.10 | 0.39 ± 0.11 | 71.04 ± 6.24 | 82.26 ± 5.87 | 79.08 ± 4.74 |
| 0Sr1Cu-BSG | 0 | 0 | 0 | 24.49 ± 2.40 | 24.29 ± 0.90 | 27.85 ± 2.10 |
| 0Sr3Cu-BSG | 0 | 0 | 0 | 80.54 ± 3.31 | 84.30 ± 4.75 | 86.99 ± 4.83 |
3.4. Polarization and inflammatory gene expression of RAW 264.7 In vitro
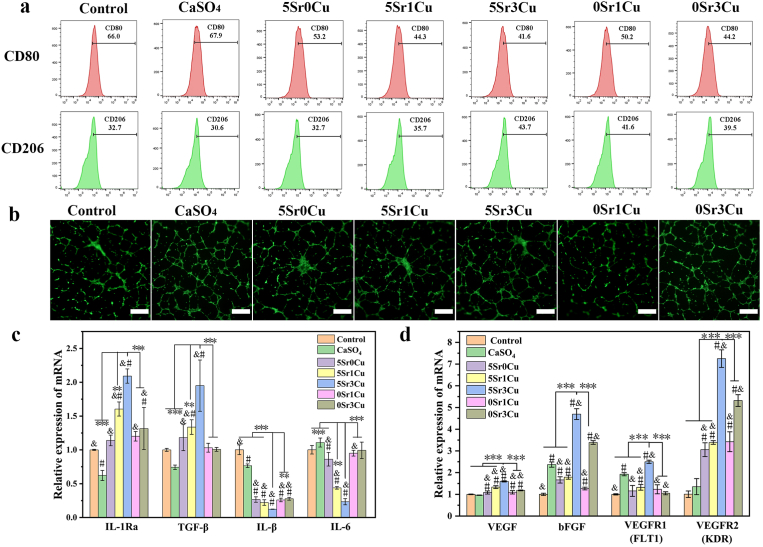
RAW 264.7 macrophages were cultured with cement extract medium for 3 days, and flow cytometry results (Fig. 4a) showed that the expression of the M1 phenotype marker, CD80, was significantly reduced when Sr and/or Cu were incorporated compared to the control group and CaSO4 group. Moreover, the expression of the M2 phenotype marker, CD206, was significantly higher for the cement extracts group than in the control group and CaSO4 group. Among the cement extract group, the 5Sr3Cu group demonstrated the lowest expression (41.6%) of CD80 and the highest expression (43.7%) of CD206. Fig. 4c shows the inflammatory gene expressions of RAW 264.7. Compared to the control group and CaSO4 group, cells cultured with the Sr/Cu-BSG bone cement extract medium showed significantly lower expressions of pro-inflammatory genes (IL-1β and IL-6) and higher expressions of anti-inflammatory genes (IL-1Ra and TGF-β1), where again, 5Sr3Cu groups showed the best outcome. The data indicate that Sr and Cu ions incorporated into BSGs synergistically affected the macrophages, reducing the inflammatory responses.
Fig. 4.
Inflammation and angiogenesis regulation in vitro. (a) Polarization of Raw 264.7 after cultured in cement extracts for 3 days was evaluated by the expressions of CD80 (M1) and CD206 (M2) using flow cytometry; (b) Tube formation assay(Calcein staining) of HUVECs after cultured in cement extracts for 5days, scale bar at 500 μm; (c) Pro-inflammatory (IL-1β and IL-6) and anti-inflammatory genes (IL-1Ra and TGF-β1) expression of Raw 264.7 cultured in cement extracts for 3 days; (d)Angiogenesis genes (VEGF, bFGF, FLT1 and KDR) expression of HUVECs after cultured in cement extracts for 5 days; cells cultured in medium without any cement extract was used as control; *significant difference compared with the 5Sr3Cu-BSG group, p < 0.05, **P < 0.01,***P < 0.001; # significant difference compared with the control group, p < 0.05; & significant difference compared with the CaSO4 group, p < 0.05.
3.5. Tube formation and angiogenesis-related gene expression of HUVECs In vitro
After culturing HUVECs in all cement extract medium for 5 days, angiogenic gene expressions of HUVECs were significantly up-regulated compared to the control group (Fig. 4d). Up-regulation of VEGF and bFGF enhanced the proliferation of HUVECs. Furthermore, the 5Sr3Cu group increased the gene expressions of VEGF, bFGF, FLT1, and KDR the highest among the bone cement groups. As shown in Fig. 4b, tube formations were profound for all cement groups after 5 days of culture. The structure parameters of tube formation, including meshes, junctions, and segments, were analyzed, and again, the 5Sr3Cu group showed the highest number of meshes, junctions, and segments (Fig. S3b), showing that Sr/Cu-BSG bone cement can enhance the angiogenesis by promoting the proliferation and tube formation of HUVECs.
3.6. Viability, proliferation, differentiation, and osteogenic gene expression of hBMSCs In vitro
Most of hBMSCs remained alive after 12 h of seeding time on all of the cement surface(Fig. S2). hBMSCs were cultured in cement extract medium for 1, 3, or 5 days. CCK-8 analysis revealed that cement extracts do not adversely affect the proliferation of hBMSCs (Fig. 5a). Live/dead assay analysis also showed that cells were viable for all cement extract groups (Fig. 5b). Furthermore, cytoskeleton staining (Fig. 5c) showed that hBMSCs exhibited normal cell morphology, spread out without any curl, shrinkage, or fragmentation. Overall, all Sr/Cu-BSG bone cement was biocompatible without showing any signs of cytotoxicity on hBMSCs.
Fig. 5.
(a) Proliferation of hBMSCs cultured with cement extracts for 1,3 and 5 days; (b) Live/Dead assay and (c) cytoskeleton staining of hBMSCs cultured cement extracts for 3 days; (d) ALP activity and (e) ALP staining of hBMSCs cultured with bone cement extracts in osteogenic-induction medium for 7 days, scale bar at 500 μm(b,e), scale bar at 200 μm(c); (f–h) Osteogenic genes expression of hBMSCs after cultured with cement extracts for 7, 14 and 21 day; cells cultured in medium without any cement extract was used as control. (Results were shown as Mean ± SD; n = 3; *significant difference compared with the 5Sr3Cu-BSG group, p < 0.05, **P < 0.01, ***P < 0.001; # significant difference compared with the control group, p < 0.05; & significant difference compared with the CaSO4 group, p < 0.05).
ALP activity (Fig. 5d) and ALP staining (Fig. 5e) showed that all cement extracts, except 0Sr3Cu, significantly promoted the ALP expressions in hBMSCs compared to the control group, which was cultured in cell culture media without bone cement. hBMSCs cultured in 5Sr3Cu-BSG extract medium showed the highest ALP expressions.
The osteogenic gene expressions (Runx-2, ALP, and OPN) of hBMSCs are shown in Fig. 5f–h. Compared to the control group and CaSO4 group, hBMSCs cultured in the Sr/Cu-BSG bone cement extract medium showed significantly higher osteogenic gene expressions. Specifically, 5Sr3Cu and 5Sr1Cu groups exhibited the highest expressions of Runx-2 and OPN than other cement extract groups after 7 days of culture. Moreover, 5Sr3Cu and 0Sr1Cu groups showed the highest expressions of OCN. After 2 and 3 weeks of culture, hBMSCs showed significantly higher expressions of Runx-2, OCN, and OPN for 5Sr3Cu and 5Sr1Cu groups compared to 5Sr0Cu, 0Sr1Cu, and 0Sr3Cu groups, indicating that both Sr and Cu ions need to be present. Overall, the 5Sr3Cu showed the highest expressions of Runx-2, OPN, and OCN at all time points, which meant that this ratio of Sr and Cu yielded the best result in inducing osteogenesis of hBMSCs.
3.7. Micro-Computed tomography (micro-CT) of bone repairing ability in rabbit femoral condylar defects
Biodegradability and osteogenic properties of Sr/Cu-BSG bone cement were analyzed in vivo in rabbit femoral condylar bone defects for 16 weeks using micro-CT. According to the sagittal images (Fig. 6a), the CaSO4 bone cement group showed high degradation, showing no obvious signs of new bone formation. However, the 3D reconstruction images of the Sr/Cu-BSG bone cement groups showed signs of bone formation, with a large quantity of new bone (yellow area) formed around the bone cement. Among the bone cement groups, 5Sr3Cu and 5Sr1Cu groups showed the highest new bone formation. A quantitative assay of the micro-CT images (Fig. 6c and d) showed that the CaSO4 group showed the lowest BV/TV (2.84 ± 0.53), BMD (26.49 ± 5.58), Tb.N() and Tb.Th() and the 5Sr3Cu-BSG group showed the highest BV/TV (18.92 ± 1.11) and BMD (319.88 ± 7.84) after 8 weeks of implantation. After 16 weeks of implantation, BV/TV and BMD values for the CaSO4 group were 4.13 ± 1.15 and 47.71 ± 18.36, respectively, while BV/TV and BMD values for the 5Sr3Cu-BSG group increased to 26.17 ± 2.07 and 354.98 ± 11.19, respectively. The 5Sr3Cu-BSG group also showed the highest Th.N and Tb.Th, while the CaSO4 group showed the lowest at each point of time. Similar to in vitro, the 5Sr3Cu-BSG group showed the best osteogenic capacity in rabbit femoral condylar defect models.
Fig. 6.
(a) Sagittal images and (b) 3D reconstructed images of the rabbit femoral condylar defects with cement implanted for 16 weeks, yellow area presenting new bone formation in 3D reconstructed images (b), and white scale bar at 3 mm; (c) Bone volume/total volume (BV/TV), (d) bone mineral density, (e) trabeculae number and (f) trabecular bone thickness of the rabbit femoral condylar defects determined from the micro CT images (results were expressed as mean ± SD, *significant difference compared with the 5Sr3Cu-BSG group, p < 0.05; **P < 0.01,***P < 0.001; # significant difference compared with the CaSO4 group, #p < 0.05).
3.8. Histological assay of bone repairing Capability in rabbit femoral condylar defects
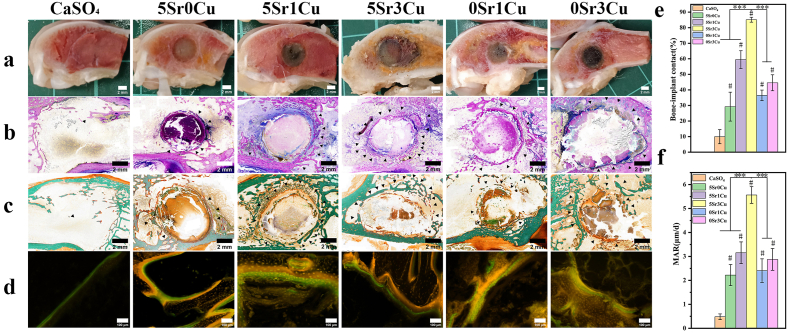
Rabbit femoral condyle defects after 8, 12, and 16 weeks of implantation with Sr/Cu-BSG bone cement were further evaluated using histological analysis. As depicted in hematoxylin-eosin (H&E) and Masson trichrome (MT) staining images (Fig. 7, S6, and S7), there was no evidence of implant rejection, tissue necrosis, or bacterial infection for all cement groups. After 8 weeks of implantation (Fig. S6), the CaSO4 bone cement group showed few new bone tissues, while the Sr/Cu-BSG bone cement groups exhibited relatively larger new bone tissues (areas stained blue using MT staining). The 5Sr3Cu group showed degradation of the material at the material-bone interface, and a small quantity of new bone had grown into the implant. After 12 and 16 weeks of implantation (Fig. 7 and S7), more cement was degraded, releasing more ions from the BSGs, which induced new bone and neovascularization (as shown with red arrows in the MT-stained images) in the bone defect area. The 5Sr3Cu group again showed more profound neovascularization and osteogenesis at the defect site than other cement groups at all time points.
Fig. 7.
Hematoxylin-eosin staining (HE) and Masson's trichrome staining (MST) of the rabbit femoral condylar defects with cement implanted for 16 weeks, where dense calcified new bone was stained blue, and cement implants were stained red (Triangles indicated new bone formation around the implant-bone interface; Red arrows indicated vascular formation; Red circle indicated the bone defect area of CaSO4 bone cement).
Methylene blue-acid fuchsin staining and Goldner trichromatic staining were used to distinguish new bone tissue around the implant-bone interface at 16 weeks of implantation [39,40]. As depicted in Fig. 8b, there was only little bone formation (crimson area) for the CaSO4 cement group, while there was significantly more new bone formation at the interface and in the defect site for all Sr/Cu-BSG bone cement groups. Methylene blue-acid fuchsin staining images showed that 5Sr3Cu bone cement groups exhibited significantly more new bone than other cement groups. Goldner trichromatic staining, as depicted in Fig. 8c, shows newly formed osteoids (orange-red area) near the cement and newly mineralized bone (green area) near the host bone. Again, the 5Sr3Cu group exhibited the highest osteoids and mineralized bone, concluding that 5Sr3Cu is the best cement group for fast bone repair and remodeling. Furthermore, the bone-implant contact area (Fig. 8e) was calculated from methylene blue-acid fuchsin and Goldner trichromatic staining, and the BICs for CaSO4, 5Sr0Cu, 5Sr1Cu, 5Sr3Cu, and 0Sr1Cu, and 0Sr3Cu after 16 weeks of implantation were 9.94, 29.24, 59.44, 85.13, 36.49, and 44.59%, respectively, where the 5Sr3Cu and 5Sr1Cu groups possessed the highest BIC values.
Fig. 8.
Osteogenesis of cement in rabbit femoral condylar defects at implantation time of 16 weeks; (a) Digital sagittal plane images, (b) methylene blue-acid fuchsin staining, (c) Goldner trichromatic staining and (d) tetracycline-calcein sequential fluorescent labeling of the rabbit femoral condylar defects; (e) bone-implant contact (BIC) determined from methylene blue-acid fuchsin staining images and (f) mineral apposition rate determined from tetracycline-calcein sequential fluorescent labeling images; New bone appeared in crimson and cement implants appeared in light red in methylene blue-acid fuchsin staining (b); Mineralized bone appeared in green and the osteoid appeared in orange red in Goldner trichromatic staining (c); Light orange area presented the Tetracycline fluorescence and light green area presented the Calcein fluorescence in fluorescent labeling of rabbit femoral condyle defects (d). Black arrowhead indicated areas for new bone formation (Mean ± SD; n = 4; *significant difference compared with the 5Sr3Cu-BSG group, p < 0.05, **P < 0.01, ***P < 0.001; # significant difference compared with the CaSO4 group, p < 0.05).
As depicted in Fig. 8d, there were no obvious areas stained with tetracycline-calcein fluorescent dyes for the CaSO4 group. However, the 5Sr3Cu and 5Sr1Cu groups showed the widest spacing of tetracycline-calcein fluorescent labeling, indicating a high bone mineralization rate and remodeling activity of osteoblasts. Moreover, the new bone mineralized deposition rate (MAR) was calculated from the average distance of the tetracycline-calcein fluorescent labeling, and the results show that MAR is significantly higher for the 5Sr3Cu group compared to other cement groups (Fig. 8f).
3.9. Immunofluorescence and immunohistochemical assay of cement implants in rat femoral condylar defects
Figs. 9 and 10 and Fig. S5 show the immunofluorescence, immunohistochemical, hematoxylin-eosin (H&E) and Masson trichrome (MT) staining and quantitative assay from rat femoral condylar defects implanted with either CaSO4 bone cement or Sr/Cu-BSG bone cement for 3, 7, or 28 days. The immunofluorescence and immunohistochemistry show positive staining of CD86, CD206, IL-1Ra, TGF-β1, IL-b, IL-6, VEGF, bFGF, FTL1, KDR, OPN, OCN, and Runx-2 for all cement groups. As shown in Fig. 9a, the immunofluorescence of the 5Sr3Cu-BSG group showed the lowest percentage(4.2%) of M1 macrophages (red, CD86) and the highest percentage(14.45%) of M2 macrophages (green, CD206) compared to other groups. The inflammatory, angiogenic, and osteogenic cytokines were mainly expressed at the bone cement and host bone interface. Semi-quantitation of the immunohistochemical staining showed that 5Sr1Cu and 5Sr3Cu groups exhibited the highest positive cells fraction of IL-1Ra and TGF-β1 (anti-inflammatory cytokines) and lowest expressions of IL-1 and IL-6 (pro-inflammatory cytokines) 3 days after the implantation (Fig. 9b). Furthermore, the angiogenic factors (VEGF, bFGF, FLT1, and KDR) after 7 days of implantation and the osteogenic factors (Rux-2, OCN, and OPN) after 28 days of implantation were found highest positive cells fraction for the 5Sr1Cu and 5Sr3Cu groups, compared to CaSO4, 5Sr0Cu, 0Sr1Cu, and 0Sr3Cu (Fig. 10a and b). As shown in Fig. S5, more mature bone tissues formed around the implant-host bone interface for the 5Sr1Cu and 5Sr3Cu groups at 28 days post-implantation. In conclusion, the co-incorporation of Sr and Cu ions, specifically the ratio of 5Sr3Cu, resulted in the best synergistic effect in regulating osteoimmunology, angiogenesis, and osteogenesis. Furthermore, the H&E staining assay revealed no pathological change for all Sr/Cu-BSG bone cement groups(Fig. S5).
4. Discussion
Bone tissues are highly organized structures arranged with Collagen I that provide the necessary mechanical support for the human body. Injectable biomaterials, which can be easily shaped to fill bone defects, instantaneously fasten and repair bone defects via minimally invasive surgery. For bone tissues of older people and osteoporotic patients, the high mechanical strength of biomaterials may cause stress shielding, thus raising the risk of fracture. In fact, these bones usually lack intrinsic bio-inductivity, and therefore, bone healing is significantly delayed. Therefore, borosilicate provides essential ions to modify the pathological bone microenvironment, where bone remodeling is unbalanced due to a higher number of activated osteoclasts. The long-term debates on bioceramics focus more on their brittleness and weak mechanical properties to support bone than their own biological activities to induce bone regeneration. So far, the biological mechanism of bioceramics on bone remodeling/regeneration is unclear. In this study, the dual network of borosilicate containing [BO3] and [SiO4] provides the versatile space to incorporate essential elements like Sr and Cu. We aim to deliver Sr and Cu ions to modulate immune cells like macrophages and osteogenic cells like hBMSCs.
4.1. Physicochemical properties of Sr/Cu-BSG cement
The self-setting of bone cement from borosilicate is achieved by the sol-gel transition of the CS solution, the rapid release of Ca2+ and PO43−, and the formation of chemical bonds between converted HA and CS. The last two steps are activated by the liquid phase of CS. The acid accelerated the BSG degradation, and the released Ca2+ and P5+ ions increased the saturation degree of HA. Therefore, the formation of HA is the initial setting mechanism of the bone cement. After implantation of the cement, a rigorous inflammatory response is triggered, possibly due to the different composition or structure of the cement from the host bone. Therefore, calcium phosphate is a good bone cement material since calcium phosphate is chemically similar to the inorganic composition of bone, which can possibly minimize the inflammatory response due to foreign material [41]. In addition, calcium phosphate as bone cement has an excellent self-setting time that fulfills the requirement of minimally invasive surgery. In this study, we hypothesized that the self-setting of BSGs could create a local microenvironment that can spontaneously recruit and polarize the macrophages towards the M2 phenotype due to the formation of HA. Calcium ions released from BSGs spontaneously precipitate with phosphate, raising the pH to a moderately alkaline level. Moderate alkaline pH has been reported to induce a positive osteo-immunomodulatory effect [42].
The compressive strength of the bone cement can shift based on the incorporation of Sr or Cu, possibly due to the degree of supersaturation. The compressive strengths of Sr/Cu-BSG bone cement (11–16 MPa) were very similar to that of human trabecular bone (2–12 mPa) [43]. Therefore, the cement prepared in this study is ideal for human cancellous bone defects. Furthermore, unlike conventional bone cement, such as PMMA-based cement, Sr/Cu-BSG bone cement significantly reduces the incidence of stress shielding, increasing the possibility of adjacent vertebral fractures [44]. Since bioactive BSG particles and CS are cohesive, bone cement is easily syringed out without any filter-pressing phenomenon [45]. The initial coagulation time increased when Cu concentration increased, where the initial coagulation time was only 14.21 min for the 5Sr0Cu (0 mol% CuO) and 23.1 min for the 5Sr3Cu (3 mol% CuO) (see Table 3). Even with a higher coagulation time for 5Sr3Cu, the time was within 10–25 min, ideal for clinical applications [46].
4.2. Sr/Cu-BSG bone cement triggered anti-inflammatory Immunoregulation and facilitated angiogenesis and osteogenesis In vitro and In vivo
Reconstruction of bone by implant materials depends on a series of systematic biological responses. For immunomodulation, active intervention of local immune response is critical by redirecting the polarization of macrophages from pro-inflammatory to anti-inflammatory phenotype (from M1 to M2), significantly enhancing osteogenesis. For angiogenesis, rapid vascularization of the defect area, providing sufficient nutrition and energy, is needed for cell recruitment and new tissue formation. For osteogenesis, the biomaterial (specifically ions released during the material degradation) should induce osteogenesis by enhancing the osteogenic differentiation of hBMSCs and increasing the osteoblast activity. These three goals need to reach in order to achieve successful bone regeneration.
The inflammatory reactions mainly inhibit the early stage of bone healing. At this stage, most macrophages express the M1 phenotype, which then leads to foreign body reactions, causing tissue fibrosis [47]. Continuous M1 phenotype of macrophages leads to chronic inflammatory response and hinders the normal process of bone repair, while phenotypic transformation to M2 leads to new bone formation. Therefore, Sr/Cu-BSG bone cement is a good candidate material to treat bone defects and fractures since Sr/Cu-BSGs can modulate the polarization of macrophages from M1 to M2, significantly increasing the expressions of anti-inflammatory cytokines like IL-1Ra and TGF-β1 while reducing pro-inflammatory cytokines like IL-1β and IL-6 (Fig. 4). Inhibition of inflammation has been reported to initiate the bone healing process [48]. Furthermore, HA converted from BSGs is chemically similar to that of bone, which makes Sr/Cu-BSG-based bone cement biocompatible, potentially reducing inflammation risen from foreign materials. M2 macrophages also secrete VEGF proteins [6,7], which could enhance endothelial cell proliferation and vessel formation.
Neovascularization provides necessary oxygen and nutrients and transports essential metabolites for osteogenesis. Neovascularization, which is the first stage of angiogenesis, usually starts at the bone-implant interface. Sr/Cu-BSG bone cement has been found to up-regulate the angiogenic gene expressions, including VEGF, bFGF, VEGFR1 (also known as FLT1), and VEGFR2 (KDR) (Fig. 4). Increased expressions of VEGF-VEGFR and bFGF caused the ECs to degrade the basement membrane to release the ECs, allowing them to migrate to the defect site and proliferate. Finally, new vascular lumens are formed, the capillary network densified, and neovascularization become mature [49]. Immunohistochemical staining of α-SMA (Fig. S8) showed the formation of blood vessels, supporting that Sr/Cu-BSG cement induced angiogenesis.
In the stage of osteogenesis, BMSCs are recruited to the bone-cement interface and distributed around the newly formed blood vessels [50]. bMSCs then differentiate into osteoblasts, which secrete and form new bone. Sr/Cu-BSG bone cement significantly enhanced the osteogenic differentiation of hBMSCs, up-regulating the expressions of Runx-2 (the earliest and most specific gene marker during bone formation) [51], OPN (an early gene marker showing osteogenic differentiation of hBMSCs) [52], and OCN (important gene marker showing late-stage differentiation and maturation of osteoblasts) [53] (Fig. 5). Based on previous studies, a moderate alkaline microenvironment (pH 7.8–8.0) at the bone-cement interface inhibited abnormally activated osteoclasts and enhanced the osteogenic differentiation of hBMSCs [54,55]. Overall, Sr/Cu-BSG bone cement successfully repaired bone defects by not only regulating the immune response of macrophages but also by enhancing angiogenesis and osteogenesis, as in other previous studies [[56], [57], [58]].
4.3. Long-term effect of Sr/Cu-BSG bone cement on biocompatibility and bone regeneration In vivo
The HE staining of visceral sections of rabbits with Sr/Cu-BSG cement showed that the visceral microstructure did not show any signs of tissue necrosis, inflammation, infections, or pseudo-tumors (Fig. S9). Blood routines and biochemical tests showed that all indexes were within the normal range, not raising any complications (Fig. S10 and S11). Therefore, we can safely conclude that Sr/Cu-BSG cement is biocompatible. CaSO4 and Sr/Cu-BSG bone cement were implanted to evaluate their ability to treat critical-sized defects in rabbits. Optimal orthopedic biomaterials should have the material degradation rate matching the bone healing rate [59,60]. Based on HE and Masson staining images (Fig. 7), the CaSO4 cement group showed no signs of healing even after 16 weeks of implantation. This result may be due to the fast degradation rate of CaSO4 with poor bone conductivity. On the other hand, Sr/Cu-BSG bone cement showed high bone ingrowth because its degradation rate matched the bone healing rate. Sr/Cu-BSG also showed good bone conductivity. In addition, bone defects with the 5Sr3Cu-BSG cement group showed the best peri-implant bone formation, with the highest BMD, BV/TV, MAR, and BIC compared to other bone cement groups (Fig. 6, Fig. 8). Although all Sr/Cu-BSG bone cement showed positive outcomes compared to CaSO4, 5Sr3Cu-BSG bone cement was far the best in immunomodulating the macrophages and enhancing angiogenesis and osteogenesis, possibly owing to the optimal concentrations of Sr and Cu ions released at this ratio [9,11,16,61,62]. The concentrations of Sr and Cu ions released from Sr/Cu-BSG bone cement resulted in a positive synergistic effect on three stages of bone repair: inflammation, angiogenesis, and osteogenesis. Therefore, Sr/Cu-BSG bone cement, specifically 5Sr3Cu-BSG bone cement, shows good promise as a bone cement material in orthopedic applications to treat bone defects/fractures.
5. Conclusion
In summary, the physical and chemical properties of the Sr/Cu-BSG bone cement met the clinical requirements. Sr/Cu-BSG regulated inflammation, angiogenesis, and osteogenesis. In vitro and in vivo studies showed that Sr/Cu-BSG bone cement successfully regenerated bone, repairing critical bone defects by modulating the polarization of macrophages to M2, facilitating rapid vascularization in the defect area, and enhancing osteogenesis by promoting osteogenic differentiation of hBMSCs. Long-term in vivo studies in rabbit femoral condylar bone defect models showed that Sr/Cu-BSGs were very biocompatible. They also degraded moderately, where the degradation rate of the material matched the bone formation rate. Compared to other Sr/Cu-BSGs, 5Sr3Cu-BSG bone cement showed the highest bone formation. Overall, the results showed that Sr/Cu-BSG bone cement has good physiochemical and biological properties and can be used as injectable bone cement to treat bone defects. For pathological fractures, Sr/Cu-BSG bone cement may restore the balance between bone resorption and formation and prevent chronic inflammation, providing a favorable microenvironment at the defect site for new bone formation.
Ethics approval and consent to participate
The animal experiments of this study were carried out based on the National Natural Science Foundation of China (approval No. 52072398 and IACUC No.SIAT-IACUC-200401-YYS-CX-A1220-01). All animal experiments in this study were consented and supervised by the Animal Research Committees of Shenzhen Institute of Advanced Technology, Chinese Academy of Science. All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the U.K. Animals (Scientific Procedures) Act 1986 and associated guidelines.
CRediT authorship contribution statement
Shuaijie Li: contributed to the experimental planning, performed the experiments, analyzed the data, and wrote the main manuscript text. Liyan Zhang: contributed to the in vitro experiment planning and analyzed the data. Chunyu Liu: contributed to the in vitro experiment planning and analyzed the data. Jua Kim: revised the manuscript. Kun Su: contributed to the in vitro experiment planning and analyzed the data. Tingli Chen: contributed to the in vitro experiment planning and analyzed the data. Limin Zhao: contributed to the in vivo experiment planning and analyzed the data. Xiaomei Lu: contributed to the in vivo experiment planning and analyzed the data. Hao Zhang: contributed to the in vitro experiment planning and analyzed the data. Yinglin Cui: contributed to the in vitro experiment planning and analyzed the data. Xu Cui: supervised the project, contributed to the intellectual input and edited the main manuscript text. Feng Yuan: supervised the project, contributed to the intellectual input and edited the main manuscript text. Haobo Pan: supervised the project, contributed to the experimental planning, provided funding and intellectual input and edited the main manuscript text. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declared that they have no conflicts of interest to this work.
Acknowledgements
This work was supported by the National Key R&D Program of China (Grant No. 2021YFC2400500 and 2018YFC1106300), the National Natural Science Foundation of China (Grant No. 52072398, 31870956, 32161160327, U2001221, 31771041, 81860385), the Frontier Science Key Research Programs of CAS (Grant No. QYZDB-SSW-JSC030), the Shenzhen Significant Strategy Layout Project (Grant No. JCYJ20200109114620793), Natural Science Foundation of Guangxi Province (Grant No. 2020GXNSFBA297154 and 2022GXNSFAA035472).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.10.021.
Contributor Information
Xu Cui, Email: xu.cui@siat.ac.cn.
Feng Yuan, Email: yuanfengtj@aliyun.com.
Haobo Pan, Email: hb.pan@siat.ac.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ruggieri P., Mavrogenis A.F., Casadei R., Errani C., Angelini A., Calabro T., Pala E., Mercuri M. Protocol of surgical treatment of long bone pathological fractures. Injury. 2010;41(11):1161–1167. doi: 10.1016/j.injury.2010.09.018. [DOI] [PubMed] [Google Scholar]
- 2.Ray N.F., Chan J.K., Thamer M., Melton L.J., III Medical expenditures for the treatment of osteoporotic fractures in the United States in 1995: report from the National Osteoporosis Foundation. J. Bone Miner. Res. 2010;12(1):24–35. doi: 10.1359/jbmr.1997.12.1.24. [DOI] [PubMed] [Google Scholar]
- 3.Hengartner N.E., Fiedler J., Ignatius A., Brenner R.E. IL-1β inhibits human osteoblast migration. Mol. Med. 2013;19:36–42. doi: 10.2119/molmed.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lam J., akeshita S.T., Barker J.E., Kanagawa O., Ross F.P., Teitelbaum S.L. TNF-α induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J. Clin. Invest. 2000;106(12):1481–1488. doi: 10.1172/JCI11176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champagne C.M., Takebe J., Offenbacher S., Cooper L.F. Macrophage cell lines produce osteoinductive signals that include bone morphogenetic protein-2. Bone. 2002;30(1):26–31. doi: 10.1016/S8756-3282(01)00638-X. [DOI] [PubMed] [Google Scholar]
- 7.Freytes D.O., Kang J.W., Marcos-Campos I., Vunjak-Novakovic G. Macrophages modulate the viability and growth of human mesenchymal stem cells. J. Cell. Biochem. 2013;114(1):220–229. doi: 10.1002/jcb.24357. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., Zhao F.J., Huang D.Q., Fu X.L., Li X., Chen X.F. Strontium-substituted submicrometer bioactive glasses modulate macrophage responses for improved bone regeneration. ACS Appl. Mater. Interfaces. 2016;8(45):30747–30758. doi: 10.1021/acsami.6b10378. [DOI] [PubMed] [Google Scholar]
- 9.Lin R.C., Deng C.J., Li X.X., Liu Y.Q., Zhang M., Qin C., Yao Q.Q., Wang L.M., Wu C.T. Copper-incorporated bioactive glass-ceramics inducing anti-inflammatory phenotype and regeneration of cartilage/bone interface. Theranostics. 2019;9(21):6300–6313. doi: 10.7150/thno.36120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y.C., Lim J., Teoh S.H. Review: development of clinically relevant scaffolds for vascularised bone tissue engineering. Biotechnol. Adv. 2013;31(5):688–705. doi: 10.1016/j.biotechadv.2012.10.003. [DOI] [PubMed] [Google Scholar]
- 11.Zhou Y.N., Wu Y.Q., Jiang X.Q., Zhang X.L., Xia L.G., Lin K.L., Xu Y.J. The effect of quercetin on the osteogenesic differentiation and angiogenic factor expression of bone marrow-derived mesenchymal stem cells. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0129605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rivron N.C., Liu J., Rouwkema J., de Boer J., van Blitterswijk C.A. Engineering vascularised tissues in vitro. Eur. Cell. Mater. 2008;15:27–40. doi: 10.22203/eCM.v015a03. [DOI] [PubMed] [Google Scholar]
- 13.Wu C.T., Zhou Y.H., Xu M.C., Han P.P., Chen L., Chang J., Yin X. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials. 2013;34(2):422–433. doi: 10.1016/j.biomaterials.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 14.Bose S., Fielding G., Tarafder S., Bandyopadhya A. Understanding of dopant-induced osteogenesis and angiogenesis in calcium phosphate ceramics. Trends Biotechnol. 2013;31(10):594–605. doi: 10.1016/j.tibtech.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodríguez J.P., Ríos S., González M. Modulation of the proliferation and differentiation of human mesenchymal stem cells by copper. J. Cell. Biochem. 2002;85(1):92–100. doi: 10.1002/jcb.10111. [DOI] [PubMed] [Google Scholar]
- 16.Marie P.J., Felsenberg D., Brandi M.L. How strontium ranelate, via opposite effects on bone resorption and formation, prevents osteoporosis. Osteoporos. Int. 2011;22(6):1659–1667. doi: 10.1007/s00198-010-1369-0. [DOI] [PubMed] [Google Scholar]
- 17.Mao L.X., Xia L.G., Chang J., Liu J.Q., Jiang L.Y., Wu C.T., Fang B. The synergistic effects of Sr and Si bioactive ions on osteogenesis, osteoclastogenesis and angiogenesis for osteoporotic bone regeneration. Acta Biomater. 2017;61:217–232. doi: 10.1016/j.actbio.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Jiang S.J., Wang X.H., Ma Y.H., Zhou Y.N., Liu L., Yu F., Fang B., Lin K.L., Xia L., Cai M. Synergistic effect of micro-nano-hybrid surfaces and Sr doping on the osteogenic and angiogenic capacity of hydroxyapatite bioceramics scaffolds. Int. J. Nanomed. 2022;17:783–797. doi: 10.2147/IJN.S345357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng S.L., Zhou G.Q., Luk K.D.K., Cheung K.M.C., Li Z.Y., Lam W.M., Zhou Z.J., Lu W.W. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the ras/MAPKSignaling pathway. Cell. Physiol. Biochem. 2009;23:165–174. doi: 10.1159/000204105. [DOI] [PubMed] [Google Scholar]
- 20.Rahaman M.N., Day D.E., Bal B.S., Fu Q., Jung S.B., Bonewald L.F., Tomsia A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011;7(6):2355–2373. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jones J.R. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2015;23(Supplement) doi: 10.1016/j.actbio.2015.07.019. S53-S82. [DOI] [PubMed] [Google Scholar]
- 22.Hench L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006;17(11):967–978. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 23.Pang L.B., Zhao R.L., Chen J., Ding J.X., Chen X.C., Chai W.W., Cui X., Li X.L., Wang D.P., Pan H.B. Osteogenic and anti-tumor Cu and Mn-doped borosilicate nanoparticles for syncretic bone repair and chemodynamic therapy in bone tumor treatment. Bioact. Mater. 2022;12:1–15. doi: 10.1016/j.bioactmat.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cui X., Zhang Y.D., Wang J.Y., Huang C.C., Wang Y.D., Yang H.S., Liu W.L., Wang T., Wang D.P., Wang G.C., Ruan C.S., Cheng D.F., Lu W.W., Huang W.H., Rahaman M.N., Pan H.B. Strontium modulates osteogenic activity of bone cement composed of bioactive borosilicate glass particles by activating Wnt/β-catenin signaling pathway. Bioact. Mater. 2020;5(2):334–347. doi: 10.1016/j.bioactmat.2020.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui X., Huang C.C., Chen Z.Z., Zhang M., Liu C.Y., Su K., Wang J.Y., Li L., Wang R.X., Li B., Chen D.F., Ruan C.S., Wang D.P., Lu W.W., Pan H.B. Hyaluronic acid facilitates bone repair effects of calcium phosphate cement by accelerating osteogenic expression. Bioact. Mater. 2021;6(11):3801–3811. doi: 10.1016/j.bioactmat.2021.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kokubo T., Takadama H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials. 2006;27(15):2907–2915. doi: 10.1016/j.biomaterials.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Westhauser F., Widholz B., Nawaz Q., Tsitlakidis S., Hagmann S., Moghaddam A., Boccaccini A.R. Favorable angiogenic properties of the borosilicate bioactive glass 0106-B1 result in enhanced in vivo osteoid formation compared to 45S5 Bioglass. Biomater. Sci. 2019;7(12):5161–5176. doi: 10.1039/c9bm01220f. [DOI] [PubMed] [Google Scholar]
- 28.Zou D.H., Zhang Z.Y., He J.C., Zhang K., Ye D.X., Han W., Zhou J., Wang Y.Y., Li Q.L., Liu X., Zhang X., Wang S.Y., Hu J.Z., Zhu C., Zhang W.J., Zhou Y., Fu H.H., Huang Y.L., Jiang X.Q. Blood vessel formation in the tissue-engineered bone with the constitutively active form of HIF-1α mediated BMSCs. Biomaterials. 2012;33(7):2097–2108. doi: 10.1016/j.biomaterials.2011.11.053. [DOI] [PubMed] [Google Scholar]
- 29.Liu X., Huang W.H., Fu H.L., Yao A.H., Wang D.P., Pan H.B., Lu W.W., Jiang X.Q., Zhang X.L. Bioactive borosilicate glass scaffolds: in vitro degradation and bioactivity behaviors. J. Mater. Sci. Mater. Med. 2009;20:1237–1243. doi: 10.1007/s10856-009-3691-7. [DOI] [PubMed] [Google Scholar]
- 30.Carreira A.S., Gonçalves F.A.M.M., Mendonça P.V., Gil M.H., Coelho J.F.J. Temperature and pH responsive polymers based on chitosan: applications and new graft copolymerization strategies based on living radical polymerization. Carbohydr. Polym. 2010;80(3):618–630. doi: 10.1016/j.carbpol.2009.12.047. [DOI] [Google Scholar]
- 31.Cui X., Zhang Y.D., Wang H., Gu Y.F., Li L., Zhou J., Zhao S.C., Huang W.H., Zhou N., Wang D.P., Pan H.B., Rahaman M.N. An injectable borate bioactive glass cement for bone repair: preparation, bioactivity and setting mechanism. J. Non-Cryst. Solids. 2016;432:150–157. doi: 10.1016/j.jnoncrysol.2015.06.001. [DOI] [Google Scholar]
- 32.Ding C.M., Song Q.Q., Ye S.M. Adsorption mechanism and preparation of chitosan-Zn2+ complex. J. East China Univ. Sci. Technol. 2003;29(3):315–317. http://en.cnki.com.cn/Article_en/CJFDTotal-HLDX200303023.htm [Google Scholar]
- 33.Shukur M.F., Azmi M.S., Zawawi S.M.M., Majid N.A., Illias H.A., Kadir M.F.Z. Conductivity studies of biopolymer electrolytes based on chitosan incorporated with NH4Br. Phys. Scripta. 2013;T157 doi: 10.1088/0031-8949/2013/T157/014049. [DOI] [Google Scholar]
- 34.Kamitsos E.I., Patsis A.P., Karakassides M.A., Chryssikos G.D. Infrared reflectance spectra of lithium borate glasses. J. Non-Cryst. Solids. 1990;126(1–2):52–67. doi: 10.1016/0022-3093(90)91023-K. [DOI] [Google Scholar]
- 35.Kamitsos E.I., Karakassides M.A., Chryssikos G.D. Vibrational spectra of magnesium-sodium-borate glasses. 2. Raman and mid-infrared investigation of the network structure. J. Phys. Chem. B. 1987;91(5):1073–1079. doi: 10.1021/j100289a014. [DOI] [Google Scholar]
- 36.Cui X., Huang W.H., Zhang Y.D., Huang C.C., Yu Z.X., Wang L., Liu W.L., Wang T., Zhou J., Wang H., Zhou N., Wang D.P., Pan H.B., N Rahaman M. Evaluation of an injectable bioactive borate glass cement to heal bone defects in a rabbit femoralcondyle model. Mater. Sci. Eng. C. 2017;73:585–595. doi: 10.1016/j.msec.2016.12.101. [DOI] [PubMed] [Google Scholar]
- 37.Pan H.B., Zhao X.L., Zhang X., Zhang K.B., Li L.C., Li Z.Y., Lam W.M., Lu W.W., Wang D.P., Huang W.H., Lin K.L., Chang J. Strontium borate glass: potential biomaterial for bone regeneration. J. R. Soc. Interface. 2010;7(48):1025–1031. doi: 10.1098/rsif.2009.0504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hench L.L. Bioceramics: from concept to clinic. J. Am. Ceram. Soc. 1991;74(7):1487–1510. doi: 10.1111/j.1151-2916.1991.tb07132.x. [DOI] [Google Scholar]
- 39.Xing H.L., Wang X., Xiao S.S., Zhang G.L., Li M., Wang P.H., Shi Q., Qiao P.Y., Ling L., Liu H.C. Osseointegration of layer-by-layer polyelectrolyte multilayers loaded with IGF1 and coated on titanium implant under osteoporotic condition. Int. J. Nanomed. 2017;12:7709–7720. doi: 10.2147/IJN.S148001. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kanczler J.M., Ginty P.J., Barry J.J.A., Clarke N.M.P., Howdle S.M., Shakesheff K.M., Oreffo R.O.C. The effect of mesenchymal populations and vascular endothelial growth factor delivered from biodegradable polymer scaffolds on bone formation. Biomaterials. 2008;29(12):1892–1900. doi: 10.1016/j.biomaterials.2007.12.031. [DOI] [PubMed] [Google Scholar]
- 41.Apelt D., Theiss F., El-Warrak A.O., Zlinszky K., Bettschart-Wolfisberger R., Bohner M., Matter S., Auer J.A., von Rechenberg B. In vivo behavior of three different injectable hydraulic calcium. Biomaterials. 2004;25(7–8):1439–1451. doi: 10.1016/j.biomaterials.2003.08.073. [DOI] [PubMed] [Google Scholar]
- 42.Wu H., Yin Y., Hu X.B., Peng C., Liu Y., Li Q.X., Huang W.D., Huang Q.L. Effects of environmental pH on macrophage polarization and osteoimmunomodulation. ACS Biomater. Sci. Eng. 2019;5(10):5548–5557. doi: 10.1021/acsbiomaterials.9b01181. [DOI] [PubMed] [Google Scholar]
- 43.Goldstein S.A. The mechanical properties of trabecular bone: dependence on anatomic location and function. J. Biomech. 1987;20(11–12):1055–1061. doi: 10.1016/0021-9290(87)90023-6. [DOI] [PubMed] [Google Scholar]
- 44.Taylor R.S., Taylor R.J., Fritzell P. Balloon kyphoplasty and vertebroplasty for vertebral compression fractures. Spine. 2006;31(23):2747–2755. doi: 10.1097/01.brs.0000244639.71656.7d. [DOI] [PubMed] [Google Scholar]
- 45.Khairoun I., Boltong M.G., Driessens F.C., Planell J.A. Some factors controlling the injectability of calcium phosphate bone cements. J. Mater. Sci. Mater. Med. 1998;9(8):425–428. doi: 10.1023/A:1008811215655. [DOI] [PubMed] [Google Scholar]
- 46.Chen L., Zhai D., Huan Z.G., Ma N., Zhu H.B., Wu C.T., Chang J. Silicate bioceramic/PMMA composite bone cement with distinctive physicochemical and bioactive properties. RSC Adv. 2015;5(47):37314–37322. doi: 10.1039/c5ra04646g. [DOI] [Google Scholar]
- 47.Remes A., Williams D.F. Immune response in biocompatibility. Biomaterials. 1992;13(11):731–743. doi: 10.1016/0142-9612(92)90010-L. [DOI] [PubMed] [Google Scholar]
- 48.Xie L.X., Wang G.M., Wu Y.Z., Liao Q., Mo S., Ren X.X., Tong L.P., Zhang W., Guan M., Pan H.B., Chu P.K., Wang H.Y. Programmed surface on poly(aryl-ether-ether-ketone) initiating immune mediation and fulfilling bone regeneration sequentially. Innovation. 2021;2(3) doi: 10.1016/j.xinn.2021.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cross M.J., Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol. Sci. 2001;22(4):201–207. doi: 10.1016/S0165-6147(00)01676-X. [DOI] [PubMed] [Google Scholar]
- 50.Bragdon B., Lam S., Aly S., Femia A., Clark A., Hussein A., Morgan E.F., Gerstenfeld L.C. Earliest phases of chondrogenesis are dependent upon angiogenesis during ectopic bone formation in mice. Bone. 2017;101:49–61. doi: 10.1016/j.bone.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang X., Aubin J.E., Inman R.D. Molecular and cellular biology of new bone formation: insights into the ankylosis of ankylosing spondylitis. Curr. Opin. Rheumatol. 2003;15(4):387–393. doi: 10.1097/00002281-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 52.Sodek J., Ganss B., McKee M.D. Osteopontin, Crit. Rev. Oral Biol. Med. 2000;11(3):279–303. doi: 10.1177/10454411000110030101. [DOI] [PubMed] [Google Scholar]
- 53.Beck G.R., Jr., Zerler B., Moran E. Phosphate is a specific signal for induction of osteopontin gene expression. Proc. Natl. Acad. Sci. U.S.A. 2000;97(15):8352–8357. doi: 10.1073/pnas.140021997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W.L., Dan X.L., Lu W.W., Pan H.B. In: Developments and Applications of Calcium Phosphate Bone Cements. Springer Series in Biomaterials Science and Engineering. Liu C.S., He H.Y., editors. Springer; Singapore: 2018. Importance of biomaterials in vivo microenvironment pH (μe-pH) in the regeneration process of osteoporotic bone defects; pp. 473–495. [DOI] [Google Scholar]
- 55.Liu W.L., Dan X.L., Lu W.W., Zhao X.L., Ruan C.S., Wang T., Cui X., Zhai X.Y., Ma Y.F., Wang D.P., Huang W.H., Pan H.B. Spatial distribution of biomaterial microenvironment pH and its modulatory effect on osteoclasts at the early stage of bone defect regeneration. ACS Appl. Mater. Interfaces. 2019;11(9):9557–9572. doi: 10.1021/acsami.8b20580. [DOI] [PubMed] [Google Scholar]
- 56.Bai L., Du Z.B., Du J.J., Yao W., Zhang J.M., Weng Z.M., Liu S., Zhao Y., Liu Y.L., Zhang X.Y., Huang X.B., Yao X.H., Crawford R., Hang R.Q., Huang D., Tang B., Xiao Y., Hang R.Q. A multifaceted coating on titanium dictates osteoimmunomodulation and osteo/angio-genesis towards ameliorative osseointegration. Biomaterials. 2018;162:154–169. doi: 10.1016/j.biomaterials.2018.02.010. [DOI] [PubMed] [Google Scholar]
- 57.Wang M., Yu Y.M., Dai K., Ma Z.Y., Liu Y., Wang J., Liu C.S. Improved osteogenesis and angiogenesis of magnesium-doped calcium phosphate cement via macrophage immunomodulation. Biomater. Sci. 2016;4(11):1574–1583. doi: 10.1039/c6bm00290k. [DOI] [PubMed] [Google Scholar]
- 58.Yang C., Zhao C.C., Wang X.Y., Shi M.C., Zhu Y.L., Jing L.G., Wu C.T., Chang J. Stimulation of osteogenesis and angiogenesis by micro/nano hierarchical hydroxyapatite via macrophage immunomodulation. Nanoscale. 2019;11(38):17699–17708. doi: 10.1039/C9NR05730G. [DOI] [PubMed] [Google Scholar]
- 59.Burg K.J.L., Porter S., Kellam J.F. Biomaterial developments for bone tissue engineering. Biomaterials. 2000;21(23):2347–2359. doi: 10.1016/S0142-9612(00)00102-2. [DOI] [PubMed] [Google Scholar]
- 60.Hubbell J.A. Biomaterials in tissue engineering. Nat. Biotechnol. 1995;13(6):565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 61.Reginster J.Y. Strontium ranelate in osteoporosis. Curr. Pharmaceut. Des. 2002;8(21):1907–1916. doi: 10.2174/1381612023393639. [DOI] [PubMed] [Google Scholar]
- 62.Buehler J., Chappuis P., Saffar J.L., Tsouderos Y., Vignery A. Strontium ranelate inhibits bone resorption while maintaining bone formation in alveolar bone in monkeys. Bone. 2001;29(2):176–179. doi: 10.1016/S8756-3282(01)00484-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.