This prespecified analysis of the Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE AF) randomized clinical trial investigates the association of commonly assessed risk factors with undiagnosed atrial fibrillation in patients with ischemic stroke from large- or small-vessel disease.
Key Points
Question
Are there commonly assessed risk factors associated with undiagnosed atrial fibrillation (AF) in patients with ischemic stroke attributed to large- or small-vessel disease?
Findings
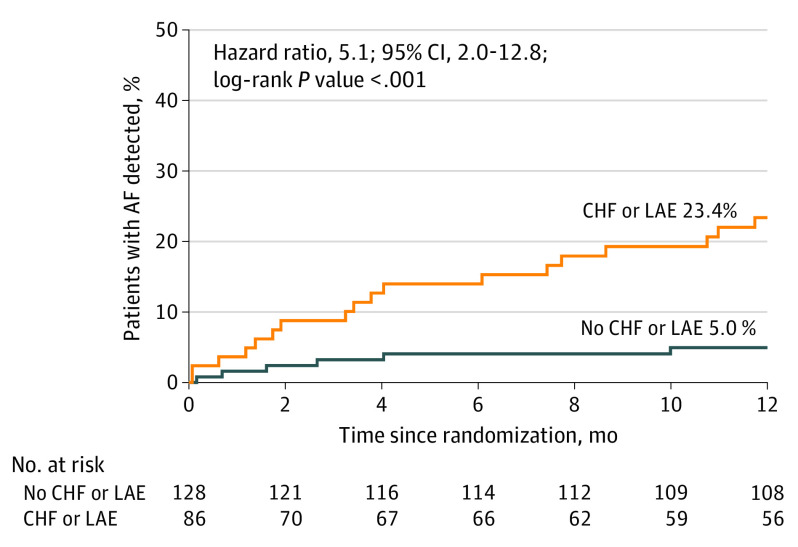
In this prespecified analysis of a randomized clinical trial that included 242 patients monitored with insertable cardiac monitors, the annual risk of detecting AF was significantly higher in patients with congestive heart failure and/or left atrial enlargement (23.4%) compared with patients without either condition (5.0%).
Meaning
If these findings are replicated in other cohorts, the associations of congestive heart failure and left atrial enlargement with AF may be useful when considering an insertable cardiac monitor in routine poststroke clinical care.
Abstract
Importance
The Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE AF) trial found that approximately 1 in 8 patients with recent ischemic stroke attributed to large- or small-vessel disease had poststroke atrial fibrillation (AF) detected by an insertable cardiac monitor (ICM) at 12 months. Identifying predictors of AF could be useful when considering an ICM in routine poststroke clinical care.
Objective
To determine the association between commonly assessed risk factors and poststroke detection of new AF in the STROKE AF cohort monitored by ICM.
Design, Setting, and Participants
This was a prespecified analysis of a randomized (1:1) clinical trial that enrolled patients between April 1, 2016, and July 12, 2019, with primary follow-up through 2020 and mean (SD) duration of 11.0 (3.0) months. Eligible patients were selected from 33 clinical research sites in the US. Patients had an index stroke attributed to large- or small-vessel disease and were 60 years or older or aged 50 to 59 years with at least 1 additional stroke risk factor. A total of 496 patients were enrolled, and 492 were randomly assigned to study groups (3 did not meet inclusion criteria, and 1 withdrew consent). Patients in the ICM group had the index stroke within 10 days before insertion. Data were analyzed from October 8, 2021, to January 28, 2022.
Interventions
ICM monitoring vs site-specific usual care (short-duration external cardiac monitoring).
Main Outcomes and Measures
The ICM device automatically detects AF episodes 2 or more minutes in length; episodes were adjudicated by an expert committee. Cox regression multivariable modeling included all parameters identified in the univariate analysis having P values <.10. AF detection rates were calculated using Kaplan-Meier survival estimates.
Results
The analysis included the 242 participants randomly assigned to the ICM group in the STROKE AF study. Among 242 patients monitored with ICM, 27 developed AF (mean [SD] age, 66.6 [9.3] years; 144 men [60.0%]; 96 [40.0%] women). Two patients had missing baseline data and exited the study early. Univariate predictors of AF detection included age (per 1-year increments: hazard ratio [HR], 1.05; 95% CI, 1.01-1.09; P = .02), CHA2DS2-VASc score (per point: HR, 1.54; 95% CI, 1.15-2.06; P = .004), chronic obstructive pulmonary disease (HR, 2.49; 95% CI, 0.86-7.20; P = .09), congestive heart failure (CHF; with preserved or reduced ejection fraction: HR, 6.64; 95% CI, 2.29-19.24; P < .001), left atrial enlargement (LAE; HR, 3.63; 95% CI, 1.55-8.47; P = .003), QRS duration (HR, 1.02; 95% CI, 1.00-1.04; P = .04), and kidney dysfunction (HR, 3.58; 95% CI, 1.35-9.46; P = .01). In multivariable modeling (n = 197), only CHF (HR, 5.06; 95% CI, 1.45-17.64; P = .05) and LAE (HR, 3.32; 1.34-8.19; P = .009) remained significant predictors of AF. At 12 months, patients with CHF and/or LAE (40 of 142 patients) had an AF detection rate of 23.4% vs 5.0% for patients with neither (HR, 5.1; 95% CI, 2.0-12.8; P < .001).
Conclusions and Relevance
Among patients with ischemic stroke attributed to large- or small-vessel disease, CHF and LAE were associated with a significantly increased risk of poststroke AF detection. These patients may benefit most from the use of ICMs as part of a secondary stroke prevention strategy. However, the study was not powered for clinical predictors of AF, and therefore, other clinical characteristics may not have reached statistical significance.
Trial Registration
ClinicalTrials.gov Identifier: NCT02700945
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia newly diagnosed after stroke and likely includes cases of preexisting AF that had escaped detection before stroke as well as new-onset AF after stroke or stroke-induced AF.1,2,3 The recent Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE AF) trial, which included participants with stroke due to large- or small-vessel disease, found AF detected by insertable cardiac monitors (ICMs) at a rate of 12.1% at 1 year.4 We sought to determine the association between commonly assessed risk factors and poststroke detection of new AF in the STROKE AF cohort.
Methods
Study Population
The STROKE AF trial has been previously described (Supplement 1).4,5 Briefly, at baseline (April 1, 2016-July 12, 2019), 496 patients with an index ischemic stroke classified by the enrolling investigator as being due to large- or small-vessel disease using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria6 were included from 33 clinical research sites in the US. In total, 492 patients were randomly assigned to groups (3 did not meet inclusion criteria, and 1 withdrew consent) (eFigure in Supplement 2). All participants provided written informed consent, and the study was approved by all relevant institutional review boards. Patients were 60 years or older or aged 50 to 59 years with at least 1 stroke risk factor.4,5 Participants were randomly assigned (1:1) to AF monitoring using an ICM (Reveal LINQ [Medtronic]) within 10 days of index stroke vs site-specific usual care. The ICM detects AF episodes of 2 minutes or longer, and first episodes of AF were adjudicated by a clinical events committee to confirm its diagnosis.
Statistical Analysis
This analysis was a prespecified ancillary outcome of the trial to identify variables associated with a first-detected AF episode through 12 months. Exposure variables were electrocardiographic and echocardiographic predictors of AF (Table 1). All participants randomly assigned to the ICM group were included, and only those with complete predictor data were included in the multivariable models.
Table 1. Univariate Analysis for Predictors of Atrial Fibrillation Detection at 12 Months in Participants of the Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE AF) Trial.
| Predictor | No. | Hazard Ratio (95% CI) | P value |
|---|---|---|---|
| Age/y | 240 | 1.05 (1.01-1.09) | .02a |
| Sex | 240 | 1.40 (0.66-2.97) | .39 |
| BMI | 240 | 1.04 (0.98-1.10) | .20 |
| Blood pressure | |||
| Diastolic | 240 | 0.98 (0.96-1.01) | .20 |
| Systolic | 240 | 1.01 (0.99-1.03) | .19 |
| CHA2DS2-VASc score/point | 240 | 1.54 (1.15-2.06) | .004a |
| Cerebral artery stenosis | 240 | 1.18 (0.35-3.91) | .79 |
| Chronic obstructive pulmonary disorder | 240 | 2.49 (0.86-7.20) | .09b |
| Congestive heart failure | 240 | 6.64 (2.29-19.24) | <.001a |
| Coronary artery disease | 240 | 0.98 (0.34-2.84) | .98 |
| Coronary artery bypass graft | 240 | 1.35 (0.32-5.68) | .69 |
| Coronary artery intervention | 240 | 0.80 (0.19-3.36) | .76 |
| Diabetes | 240 | 1.37 (0.63-2.95) | .43 |
| Heart rate | 238 | 1.00 (0.97-1.03) | .87 |
| Hypertension | 240 | 1.35 (0.47-3.90) | .58 |
| Left atrial diameter | 166 | 1.05 (0.99-1.11) | .08b |
| Left atrial enlargement | 214 | 3.63 (1.55-8.47) | .003a |
| Left atrial volume index (+10 mL/m2) | 142 | 2.30 (1.58-3.34) | <.001a |
| Myocardial infarction | 240 | 0.50 (0.07-3.66) | .49 |
| Peripheral vascular disease | 240 | 1.81 (0.63-5.24) | .27 |
| Kidney dysfunction | 240 | 3.58 (1.35-9.46) | .01a |
| Sleep apnea | 240 | 1.98 (0.68-5.73) | .21 |
| PR interval/ms | 218 | 1.00 (0.98-1.01) | .51 |
| QRS duration/ms | 219 | 1.02 (1.00-1.04) | .04a |
| QTc interval/ms | 219 | 1.00 (1.00-1.01) | .35 |
| RR interval/ ms | 180 | 1.00 (1.00-1.00) | .26 |
| Stroke/TIA prior to qualifying event | |||
| Stroke or stroke-related event | 240 | 0.76 (0.31-1.88) | .55 |
| Ischemic stroke, of known origin | 240 | 1.23 (0.49-3.04) | .66 |
| Transient ischemic attack | 240 | 0.76 (0.18-3.23) | .72 |
| Modified Rankin Score | 239 | 1.05 (0.82-1.34) | .71 |
| NIHSS | 240 | 1.02 (0.94-1.12) | .63 |
| Qualifying stroke infarction location | |||
| Brainstem | 240 | 1.17 (0.44-3.08) | .76 |
| Cerebellum | 240 | 0.33 (0.04-2.42) | .28 |
| Cerebral artery | |||
| Anterior | 240 | 1.25 (0.38-4.17) | .71 |
| Middle | 240 | 1.12 (0.52-2.38) | .77 |
| Posterior | 240 | 1.94 (0.82-4.59) | .13 |
| Qualifying stroke side (left vs right) | 240 | 1.00 (0.47-2.12) | >.99 |
| Qualifying stroke type (small vessel vs large vessel) | 242 | 1.14 (0.53-2.43) | .74 |
Abbreviations: BMI, body mass index; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
Significant at P ≤ .05.
Significant at P < .10 (cutoff for inclusion in multivariable models).
To address high rates (40.8%) of missing left atrial volume index (LAVI) values, a post hoc composite variable for left atrial enlargement (LAE) was created and used for the primary analysis. Participants were classified as having LAE if they met any of the following accepted criteria7,8,9:
LAVI greater than 28 mL/m2.
Male participant with LA diameter greater than 41 mm.
Female participant with LA diameter greater than or equal to 39 mm.
No measurements for LA volume or diameter, but LAE was documented in the echocardiography report.
LAE was classified as missing if none of this information was available. Variable selection for multivariable models was based on the outcomes of univariate models. Cox proportional-hazards regression models were fitted to various baseline characteristics for the prediction of AF. Predictors with P values <.10 in univariate models were included in a multivariable Cox model using a complete case data set. In all regression models, predictors were analyzed using 2-sided P values. A significance level of .05 was used in the multivariable Cox models, and hazard ratios (HRs) were calculated along with their 95% CIs. Data were analyzed from October 8, 2021, to January 28, 2022, using SAS software, version 9.4 (SAS Institute).
Results
The analysis included the 242 participants randomly assigned to the ICM group in the STROKE AF study. The mean (SD) age was 66.6 (9.3) years; 96 participants (40.0%) were women, and 144 (60.0%) were men (2 patients had missing baseline data and exited the study early). The eTable in Supplement 2 shows baseline characteristics for patients randomly assigned to ICM vs those with successful insertion (n = 221), and no meaningful differences were observed between the groups. Follow-up continued through August 2020 (from randomization to 12 months) for a mean (SD) duration of 11.0 (3.0) months. AF was detected in 27 patients in the ICM group (11.2%), and 26 first episodes (96.3%) were asymptomatic. None of the 7 patients who crossed over to the control group had AF detected.
Table 1 shows the univariate HR and 95% CI of AF detection at 12 months for each potential predictor. LAE was available for 214 participants (89.1%). Variables identified as univariate predictors of AF (based on a nominal P value <.10) included LAVI per 10-mL increments (HR, 2.30; 95% CI, 1.58-3.34; P < .001), LAE (HR, 3.63; 95% CI, 1.55-8.47; P = .003), chronic obstructive pulmonary disease (HR, 2.49; 95% CI, 0.86-7.20; P = .09), CHF (with preserved or reduced ejection fraction: HR, 6.64; 95% CI, 2.29-19.24; P < .001), kidney dysfunction (HR, 3.58; 95% CI, 1.35-9.46; P = .01), age (per 1-year increments: HR, 1.05; 95% CI, 1.01-1.09; P = .02), CHA2DS2-VASc score (per point: HR, 1.54; 95% CI, 1.15-2.06; P = .004), QRS duration (HR, 1.02; 95% CI, 1.00-1.04; P = .04), and LA diameter (per millimeter: HR, 1.05; 95% CI, 0.99-1.11; P = .08).
In the multivariable analysis (n = 197) shown in Table 2, only CHF (HR, 5.06; 95% CI, 1.45-17.64; P = .05) and LAE (HR, 3.32; 95% CI, 1.34, 8.19; P = .009) were associated with an increased likelihood of detecting AF during 12 months of monitoring, with a trend toward significance for QRS duration (HR, 1.02; 95% CI, 1.00-1.04; P = .06). There was no statistically significant interaction between CHF and LAE. The rate of AF detection at 12 months among patients with either CHF and/or LAE (40 of 142 patients) was significantly higher compared with patients with neither attribute (23.4% vs 5.0%; HR, 5.1; 95% CI, 2.0-12.8; P < .001) (Figure).
Table 2. Multivariable Analysis for Predictors of Atrial Fibrillation Detection at 12 Months in Participants of the Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE AF) Trial.
| Stroke AF complete case ICM group (N = 197a) | ||
|---|---|---|
| Predictor | Hazard ratio (95% CI) | P value |
| Age/y | 1.00 (0.94-1.06) | .98 |
| CHA2DS2-VASc score/point | 1.29 (0.83-2.02) | .26 |
| Chronic obstructive pulmonary disorder | 1.59 (0.41-6.19) | .51 |
| Congestive heart failure | 5.06 (1.45-17.64) | .05b |
| Left atrial enlargement | 3.32 (1.34-8.19) | .009b |
| QRS duration/ms | 1.02 (1.00-1.04) | .06 |
| Kidney dysfunction | 2.33 (0.76-7.18) | .14 |
Abbreviation: ICM, insertable cardiac monitor.
197 patients with ICM had complete case data for all predictors and outcome.
Significant at P ≤ .05.
Figure. Rate of Atrial Fibrillation (AF) Detection at 12 Months Among Patients With Congestive Heart Failure (CHF) or Left Atrial Enlargement (LAE).
Increased AF detection in patients with CHF and/or LAE in participants randomized to insertable cardiac monitor (ICM) in the Stroke of Known Cause and Underlying Atrial Fibrillation (STROKE AF) trial through 12 months compared with participants without either condition (23.4% vs 5%; P < .001).
Discussion
In this prespecified analysis of the STROKE AF randomized clinical trial of patients with ischemic stroke due to large- or small-vessel disease, those with CHF and/or LAE had an annual risk of AF that was substantially elevated compared with patients without CHF or LAE, with rates of 23.4% vs 5.0%, respectively. This translates to a number needed to monitor of just over 5 to detect AF in the first 12 months. Using an ICM to continuously monitor these patients also showed the rate of AF detection over time. Selecting individuals with risk factors such as CHF and LAE for monitoring could lead to higher rates of AF detection.
Most patients with ischemic stroke are treated with antiplatelet agents. Detection of AF after stroke is important regardless of whether it predated the index stroke because it often leads to an evidence-based change in therapy. However, the optimal management of patients with AF and symptomatic atherosclerotic disease is unknown. Likewise, the efficacy and safety of oral anticoagulant (OAC) therapy is not established in patients with large- and small-vessel atherosclerotic disease and coexisting AF. It is well established that antiplatelet therapy alone is inadequate for recurrent stroke prevention in AF.10 To answer these questions, randomized clinical trials are necessary.
An overemphasis on monitoring for AF only in patients with an index cryptogenic embolic stroke may be doing patients a disservice by failing to detect and intervene on clinically meaningful AF in patients with other index stroke subtypes. Given the high rates of recurrent stroke among patients in general, and particularly in those with AF, identifying the subset of patients with the greatest probability of future AF detection should be the focus rather than relying solely on the index stroke mechanism. This concept is supported by the nearly identical rates of AF detected by ICM at 1 year in the STROKE AF4 and Cryptogenic Stroke and Underlying AF (CRYSTAL AF)11 trials (12.1% and 12.4%, respectively), suggesting that stroke mechanism alone does not explain the likelihood of underlying AF.
Currently, it remains unclear whether OAC for poststroke AF detected by ICM is beneficial to prevent secondary strokes and what AF burden is sufficient to produce benefit from OAC. However, early detection of poststroke AF would allow for continued close monitoring to detect when patients cross the threshold to a clinically meaningful AF burden before a recurrent stroke occurs. Future studies are needed to determine the proper thresholds for initiating OAC therapy in patients with ICM-detected AF after stroke.
Limitations
Our study has several important limitations. Although the data were acquired prospectively and in a randomized clinical trial setting with adjudicated end points, the trial was not powered to detect clinical predictors of AF, and therefore, other clinical characteristics may not have reached statistical significance. Our limited sample size may explain why variables such as age and CHA2DS2-VASc score did not reach statistical significance in our modeling.
Conclusions
In summary, in this prespecified analysis of patients from the STROKE AF randomized clinical trial who were continuously monitored for AF, participants with CHF or LAE were at greater risk of having AF detected at 12 months than those without either and may represent an enriched population for monitoring with ICM. Although preliminary in nature, if the findings from our study are replicated in other cohorts, then the associations of CHF and LAE with AF may be useful when considering an ICM in routine poststroke clinical care.
Trial Protocol and Statistical Analysis Plan
eFigure. Patient Flow
eTable. Baseline Characteristics of STROKE AF Patients Randomized to ICM vs Those With Successful Insertion
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Hankey GJ, Jamrozik K, Broadhurst RJ, Forbes S, Anderson CS. Long-term disability after first-ever stroke and related prognostic factors in the Perth Community Stroke Study, 1989-1990. Stroke. 2002;33(4):1034-1040. doi: 10.1161/01.STR.0000012515.66889.24 [DOI] [PubMed] [Google Scholar]
- 2.Kallmünzer B, Breuer L, Kahl N, et al. Serious cardiac arrhythmias after stroke: incidence, time course, and predictors—a systematic, prospective analysis. Stroke. 2012;43(11):2892-2897. doi: 10.1161/STROKEAHA.112.664318 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Qian Y, Smerin D, Zhang S, Zhao Q, Xiong X. Newly detected atrial fibrillation after acute stroke: a narrative review of causes and implications. Cardiology. 2019;144(3-4):112-121. doi: 10.1159/000502971 [DOI] [PubMed] [Google Scholar]
- 4.Bernstein RA, Kamel H, Granger CB, et al. ; STROKE-AF Investigators . Effect of long-term continuous cardiac monitoring vs usual care on detection of atrial fibrillation in patients with stroke attributed to large- or small-vessel disease: the STROKE AF randomized clinical trial. JAMA. 2021;325(21):2169-2177. doi: 10.1001/jama.2021.6470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein RA, Kamel H, Granger CB, Kowal RC, Ziegler PD, Schwamm LH. Stroke of known cause and underlying atrial fibrillation (STROKE AF) randomized trial: design and rationale. Am Heart J. 2017;190:19-24. doi: 10.1016/j.ahj.2017.04.007 [DOI] [PubMed] [Google Scholar]
- 6.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial—TOAST, Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24(1):35-41. doi: 10.1161/01.STR.24.1.35 [DOI] [PubMed] [Google Scholar]
- 7.Bouzas-Mosquera A, Broullón FJ, Álvarez-García N, et al. Left atrial size and risk for all-cause mortality and ischemic stroke. CMAJ. 2011;183(10):E657-E664. doi: 10.1503/cmaj.091688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, et al. ; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology . Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79-108. doi: 10.1016/j.euje.2005.12.014 [DOI] [PubMed] [Google Scholar]
- 9.Patel DA, Lavie CJ, Milani RV, Ventura HO. Left atrial volume index predictive of mortality independent of left ventricular geometry in a large clinical cohort with preserved ejection fraction. Mayo Clin Proc. 2011;86(8):730-737. doi: 10.4065/mcp.2010.0682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diener HC, Hankey GJ, Easton JD, Lip GYH, Hart RG, Caso V. Nonvitamin K oral anticoagulants for secondary stroke prevention in patients with atrial fibrillation. Eur Heart J Suppl. 2020;22(suppl I):I13-I21. doi: 10.1093/eurheartj/suaa104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanna T, Diener HC, Passman RS, et al. ; CRYSTAL AF Investigators . Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370(26):2478-2486. doi: 10.1056/NEJMoa1313600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol and Statistical Analysis Plan
eFigure. Patient Flow
eTable. Baseline Characteristics of STROKE AF Patients Randomized to ICM vs Those With Successful Insertion
Nonauthor Collaborators
Data Sharing Statement