Abstract
Background:
Human and animal exposure to bisphenol A (BPA) has been associated with adverse developmental and reproductive effects. The molecular mechanisms by which BPA exposure exerts its effects are not well-understood, even less known about its analogues bisphenol F (BPF). To address these knowledge gaps, we conducted an untargeted metabolome-wide association study (MWAS) to identify metabolic perturbations associated with BPA/BPF exposures in a pregnant African American cohort.
Methods:
From a subset of study participants enrolled in the Atlanta African American Maternal-Child cohort, we collected both urine samples, for targeted exposure assessment of BPA (N = 230) and BPF (N = 48), and serum samples, for high-resolution metabolomics (HRM) profiling (N = 230), during early pregnancy (8–14 weeks’ gestation). Using an established untargeted HRM workflow consisting of MWAS modeling, pathway enrichment analysis, and chemical annotation and confirmation, we investigated the potential metabolic pathways and features associated with BPA/BPF exposures.
Results:
The geometric mean creatinine-adjusted concentrations of urinary BPA and BPF were 0.85 ± 2.58 and 0.70 ± 4.71 μg/g creatinine, respectively. After false positive discovery rate correction at 20 % level, 264 and 733 unique metabolic features were significantly associated with urinary BPA and BPF concentrations, representing 10 and 12 metabolic pathways, respectively. Three metabolic pathways, including steroid hormones biosynthesis, lysine and lipoate metabolism, were significantly associated with both BPA and BPF exposure. Using chemical standards, we have confirmed the chemical identity of 16 metabolites significantly associated with BPA or BPF exposure.
Conclusions:
Our findings support that exposure to BPA and BPF in pregnant women is associated with the perturbation of aromatic amino acid metabolism, xenobiotics metabolism, steroid biosynthesis, and other amino acid metabolism closely linked to stress responses, inflammation, neural development, reproduction, and weight regulation.
Keywords: Bisphenol A, Bisphenol F, Urinary bisphenol metabolites, High-resolution metabolomics, Metabolic perturbations
1. Introduction
Bisphenol A (BPA) is a chemical produced and used in high volumes in industrial processes, with annually consumption of approximately 7.7 million metric tons globally (Newswire, 2016), including the synthesis of polycarbonate plastics for food and drink packaging, epoxy resins to coat metals, and less often the production of other resins such as polyester resins (Program, 2008; Program, 2010). Exposure to BPA is widespread, where ninety-three percent of participants in the National Health and Nutrition Examination Survey (NHANES) had detectable levels of BPA (NIEHS, 2020). Studies have evaluated levels of exposure to BPA chemicals in pregnant women finding detectable levels in almost all participants (Gerona et al., 2016; Machtinger et al., 2018). Evidence from epidemiological and animal studies implicates BPA in adverse outcomes, including effects on the brain, immune system, reproductive system, and metabolic processes (Rochester, 2013). Given that BPA exposure is prevalent in utero in developing fetuses (Ikezuki et al., 2002), many studies have reported a wide range of BPA-associated adverse reproductive and developmental effects, including endome-trial hyperplasia, miscarriages (Vandenberg et al., 2007), premature delivery, lower birth weight, childhood behavior and neurodevelopment (Rochester, 2013; Cantonwine et al., 2010; Miao et al., 2011). In particular, communities of color, especially African Americans (AA), are disproportionately exposed to high levels of BPA, where the median level of urinary BPA among black adults and children were statistically significantly higher compared to non-black population (Lehmler et al., 2018). At the same time, pregnant AA women and their infants are more likely to have higher risk of preterm birth delivery and experience disproportionately high rates of low birthweight (Ahern et al., 2003; Giscombé and Lobel, 2005).
The occurrence of bisphenol analogues has been well-documented in environmental compartments (wastewater, sediments or air), foods, consumer products, and human specimens, which has become more prevalent, creating the need to understand their biological activity (Pelch et al., 2017). However, investigations involving other analogues are still limited. Specifically, less is known about bisphenol F (BPF) and its human health impact and mechanisms of action. A systematic review of 32 studies found BPF to have endocrine disrupting properties similar to those associated with BPA (Rochester and Bolden, 2015). Recent biomarker studies suggest an association between BPF and adverse human health effects including maternal thyroid hormone level alteration, oxidative stress, inflammatory response, and neurotoxic and reproductive effects (Aker et al., 2018; Aung et al., 2019; Ferguson et al., 2019; Santoro et al., 2019). Among these health effects, cytotoxic and genotoxic effects of bisphenol analogues were reported to be greater than that of BPA. Studies suggested that BPF was effective on HepG2 cell DNA fragmentation at noncytotoxic concentrations (Cabaton et al., 2009), and exhibited greater genotoxic potentials than that of BPA in mutant chicken DT40 cells (Lee et al., 2013). Given the existing knowledge about the health effects of BPA, exposure to bisphenol analogues is of particular concern in pregnant populations, because of the potential to impact both maternal and child health through endocrine-disrupting activities in an adverse manner.
Despite these epidemiological observations, detailed molecular mechanisms and endogenous pathways underlying the BPA/BPF toxicity on health effects have not yet been fully established. High-resolution metabolomics (HRM) is an innovative analytical platform used to explore metabolic perturbations associated with environmental exposures, with the capacity of detecting tens of thousands of exogenous and endogenous metabolic features (Chen et al., 2020; Li et al., 2021; Liang et al., 2018; Lin et al., 2006). Previously, several animal metabolomics studies have revealed perturbed metabolic pathways associated with exposures to BPA and its analogues. Aromatic amino acids, including phenylalanine, tyrosine, and tryptophan, have been consistently reported, suggesting a structural homology to bisphenol compounds. Specifically, phenylalanine and tryptophan were found to increase in liver of male goldfish (Jordan et al., 2012), in urine of rats (Zeng et al., 2013), in liver of the fetuses of female mice (Susiarjo et al., 2017), and in extracts of zebrafish after BPA exposures (Yoon et al., 2017). Tyrosine was found to decrease in urine of rats (Zeng et al., 2013) and in liver of the fetuses of female mice (Susiarjo et al., 2017). While another study on Daphnia magna showed decreased concentration of metabolites associated aromatic amino acid metabolism after BPA and its analogues exposures (Oliveira Pereira et al., 2021). Additionally, the disruption of xenobiotic metabolism, sterol metabolism, folate metabolism upon exposures to BPA/BPF at cellular level have been reported in previous studies (Hercog et al., 2019; Kim and Park, 2019; Wang et al., 2020). These pathways have also been shown to be inolved in a series of physiological processes, including oxidative stress and immune response, and associated with developmental and reprodutive health (Anesi et al., 2019; Bailey et al., 2015).. However, few previous studies have used HRM to explore the metabolic effects of bisphenol A and its analogues on humans (Khan et al., 2017), and none of studies have focused on minor groups.
To understand the full scope of potential human health effects of bisphenol analogues and bridge the gaps of current inconsistencies between results, it is important to conduct hypothesis-generating studies to identify mechanistic perturbations as the first step. We hypothesized that maternal BPA/BPF exposures would be associated with perturbations in aromatic-structure-associated pathways including sterol, aromatic amino acid, and xenobiotic metabolism. To address these critical knowledge gaps, we conducted the untargeted metabolomics analysis to examine whether prenatal maternal urine BPA and BPF metabolite levels are associated with perturbations in the maternal metabolome in the Atlanta African American Maternal-Child cohort. By revealing potential molecular mechanisms underlying the impact of BPA/BPF exposures on human metabolome, our results would ultimately provide opportunities for early detection and intervention to mitigate the health burden associated with BPA/BPF among this minor population.
2. Methods
Study population –
The study population is a subset of women participating in the Atlanta African American Maternal-Child cohort (Corwin et al., 2017; Brennan et al., 2019), which recruits pregnant women between 18 and 40 years of age who identify as Black or African American when they present for prenatal care with a singleton pregnancy between 8 and 14 weeks gestation (verified by medical record) to clinics of two hospitals in Atlanta, Georgia, USA: Grady Memorial Hospital (public county hospital) and Emory University Hospital (a private hospital). For the present analysis, we retrieved information on participants enrolled in the cohort between 2014 and 2017 with available BPA or BPF exposure measurements and metabolomic assessment. Specifically, 230 individuals were included with both BPA exposure data and maternal high-resolution metabolic profiling available. BPF levels were measured on a subset of 48 participants by randomly selecting from these 230 participants. The study was approved by the Emory University Institutional Review Board (IRB ID 1017).
Data collection –
For the cohort study, survey and biological sample data are collected twice during pregnancy: at the first prenatal visit (8 to 14 gestational weeks) and the second prenatal visit (24 to 30 weeks). The urine samples and the blood samples were taken at the same time either at a clinic visit or at home. In this analysis, we focused on biological samples and survey data in 8–14 weeks gestation due to limited exposure data on second prenatal visit. Clinical data are also collected via maternal medical record abstraction after delivery. Measures relevant to the present analysis are described below and are described in depth elsewhere (Corwin et al., 2017).
Blood sample collection –
Maternal blood samples were collected at 8–14 weeks gestation for metabolomics measurement in this study. After sample collection, the samples were transported to the laboratory, processed to obtain the serum, and stored at −80 °C for future analyses.
Survey data –
Participants complete a sociodemographic survey based on maternal self-report and prenatal administrative record review to ascertain information on the participants’ age upon entry into the study, residential address, education, income (percent of federal poverty level), insurance status, marital status, and substance use (tobacco, alcohol, and marijuana) (Corwin et al., 2017).
Clinical medical record.
Medical chart abstraction is completed by the research team using a standardized chart abstraction tool to ascertain the following characteristics, conditions and birth outcomes: (1) Parity, categorized according to whether the woman had any prior birth or not and if prior births were term or preterm; (2) First prenatal body mass index (BMI), calculated from measured height and weight at the first prenatal visit between 8 and 14 weeks gestation and categorized according to accepted definitions (obesity ≥ 30 kg/m2, overweight 25–29.99 kg/m2, healthy weight 18.5–24.99 kg/m2, and underweight < 18.5 kg/m2); (3) Pregnancy outcomes, including gestational age at birth is determined from the delivery record using the best obstetrical estimate (American College of Obstetricians and Gynecologists, 2014) based upon the date of delivery in relation to the estimated date of confinement established by the 8–14 week prenatal visit. All participants received early pregnancy dating by last menstrual period (LMP) and/or early ultrasound, given enrollment criteria. (4) Type of Labor (spontaneous, induced, none) and mode of delivery (vaginal, C-section) along with indication for induction and/or C-section are also obtained and used to phenotype birth outcomes.
Exposure assessment –
The exposure assessment of BPA and BPF was conducted as part of the analysis at the Center for Children’s Health, the Environment, the Microbiome, and Metabolomics (C-CHEM2). Convenience, spot, clean-catch urinary samples were collected for BPA/BPF measurement. We used collection material lots prescreened for contamination and laboratory blanks were also included that indicated no contamination. All samples were randomized using a Fisher-Yates shuffling algorithm prior to analysis to reduce any potential batch effects (Fisher and Yates, 1943; Knuth, 1997). BPX analogues (BPA/BPF) were measured using a modification of the method by Zhou et al (Zhou et al., 2014). Briefly, a 1-mL aliquot of urine was spiked with isotopically labeled analogues of the target phenols then was subjected to an enzyme hydrolysis to liberate glucuronide-bound conjugates. The hydrolysate was extracted using an ABS Elut-NEXUS solid phase extraction column, eluting with acetonitrile and ethyl acetate. The extract was concentrated to dryness and reconstituted in mobile phase for analysis using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Analyte concentrations were calculated using isotope dilution calibration. Two quality control materials (one high and one low, one reagent blank, and one matrix blank sample were analyzed concurrently with each set of 24 unknown samples. Further quality assurance measures were included in the sample analyses including the analysis of NIST SRM 3672 and 3673 (one of each per 100 samples), inclusion of blinded quality controls (6 each of 2 materials per 100 samples), and bi-annual participation in the German External Quality Assessment Scheme (G-EQUAS). For values below the limit of detection (LODs), imputed values of LOD/√2 were used. (Hornung and Reed, 1990).
Creatinine was measured by diluting urine samples 1000-fold with water after spiking with its isotopically labeled analogue. Diluted samples were analyzed by liquid chromatography electrospray ionization coupled with tandem mass spectrometry. For creatinine, two ion transition were monitored (m/z 113.9 → m/z 44.2 and m/z 113.9 → 86) and only one ion transition was monitored for labeled creatinine (m/z 116.9 → m/z 47.2) (Kwon et al., 2012). Quantification was achieved using the method of isotopic dilution. Quality control/assurance included the concurrent measurement of calibrants, blanks and quality control materials and semi-annual certification by the GEQUAS program. The LODs were calculated as 3 s0. It was the lowest concentration where a signal-to-noise ratio was 3 when the blank level was zero. BPA and BPF values were then corrected using creatinine level to account for urinary dilution (Barr et al., 2005; O’Brien et al., 2017). Specifically, we used the standardization approach using the ratio of BPA/BPF concentration to the creatinine levels measured in the same sample. Unadjusted and creatinine-adjusted analyte concentrations were provided in ng/ml of urine and μg/g creatinine, respectively.
High Resolution Metabolomics –
Untargeted high-resolution metabolomics profiling was conducted at Emory Clinical Biomarker Laboratory using established protocol using maternal non-fasting serum samples (Liang et al., 2018; Go et al., 2015; Liang et al., 2019). Each serum sample were first treated with two sample volumes of ice-cold acetonitrile to precipitate proteins. The samples were then incubated on ice for 30 mins, centrifuged (at 14,000 g for 10 mins) to separate supernatant from precipitated proteins, and stored at 4 °C until analysis. Extractants were then analyzed in triplicate using liquid chromatography-high-resolution mass spectrometry (LC–HRMS) techniques (Dionex Ultimate 3000; ThermoFisher Orbitrap Fusion Tribrid). Two chromatography types were applied the hydrophilic interaction liquid chromatography (HILIC) (Waters XBridge BEH Amide XP HILIC column; 2.1 × 50 mm2, 2.6 μm particle size) with positive electrospray ionization (ESI) and reverse phase (C18) chromatography (Higgins Targa C18 2.1 × 50 mm2, 3 μm particle size) with negative ESI. For both types of chromatography, mobile phase flow rate was 0.35 mL/min for the first minute and increased to 0.4 mL/min for the final 4 min. Although the gradient elution starting at 60 % aqueous condition in C18 column might miss some metabolites, which could be separated between 100 % and 60 % aqueous, these metabolites are likely to be better detected in the HILIC column. Thus, applying two chromatography types in this study can enhance the coverage of metabolic feature for each sample (Go et al., 2015). Two quality control pooled reference plasma samples, including NIST 1950 (Simón-Manso et al., 2013) and pooled human plasma purchased from Equitech Bio were included at the beginning and end of each analytical batch for normalization, control for background noise, batch evaluation, and post hoc quantification. Following instrumental analyses of all samples, raw data files were converted to.mzXML files using ProteoWizard (Chambers et al., 2012) and extracted using apLCMS with modifications by xMSanalyzer (Uppal et al., 2013; Yu et al., 2009). Detected signals (referred to as metabolic features) were uniquely defined by their mass-to-charge ratio (m/z), retention time, and ion intensity. Compared to other technically advanced software packages, xMSanalyzer coupled with apLCMS can efficiently process large LCMS datasets, with several major technical improvements including the adaptive tolerance level searching rather than hard cutoff or binning, the use of non-parametric methods to fine-tune intensity grouping, the use of run filter to preserve weak signals and the model-based estimation of peak intensities for absolute quantification. To filter out the noise signals and optimize the metabolomics data quality, the data quality assurance/quality control process followed the same procedure as our previous studies (Go et al., 2015; Chang et al., 2021; Zhang et al., 2021; Tan et al., 2022; Liu et al., 2020). Only metabolic features detected in > 15 % of serum samples with median coefficient of variation (CV) among technical replicates < 30 % and Pearson correlation ρ > 0.7 among 3 replicates for each sample were included in further analyses. The intensities of the extracted metabolic features were then averaged across triplicates for future statistical analyses. The intensities of metabolic features were log2 transformed to normalize the metabolomics data for the following analysis.
Statistical Analysis –
We analyzed the associations between BPA and BPF urine concentrations (predictor) and metabolic feature intensities (outcome) using generalized multivariable linear regression models adjusting for maternal age, infant sex, first prenatal body mass index (BMI), tobacco use, alcohol use, marijuana use, socioeconomic status (income, percent of federal poverty level), and parity. Separate models were conducted for each metabolic feature, from each chromatography column (serum HILIC positive ESI, and serum C18 negative ESI) – a total of four separate sets of models. False positive discovery rate was controlled at 20 % using the Benjamini-Hochberg method (FDRB-H) (Benjamin and Hochberg, 1995). The correction was applied separately to each set of models. R (version 4.1.2) was used for the MWAS analysis.
The following is the model form used in the MWAS:
| (2) |
where Yij refers to the log base 2 intensity of metabolic feature j for participant i, β0 is the intercept, which refers to the predicted value of log base 2 intensity of metabolic feature when setting continuous variables to zero, and categorical variables to their corresponding reference groups. εij is residual random normal error, and BPXi is creatinine adjusted BPA or BPF concentration for participant i. The covariates that have potential to confound the association between urinary BPA/BPF and metabolites were controlled in the model, including infant sex (Sexofbabyi, categorical), maternal age (Agei, continuous), tobacco use during pregnancy (Tobaccoi, categorical), marijuana use during pregnancy (Marijuanai, categorical), parity (Parityi, continuous), first prenatal BMI (PrenatalBMIi, continuous), alcohol use during pregnancy (Alcoholi, categorical) and measures of socioeconomic status (income- percent of federal poverty level, Incomei, categorical).
Pathway enrichment analysis and chemical annotation –
We used mummichog (v1.0.10), a statistical application leveraging the organization of metabolic pathways and networks to predict the functional activity without upfront chemical identification. Since its development in 2013, mummichog has been adopted and used widely in hundreds of untargeted metabolomics applications. It is effective in linking the observed significant m/z feature to known metabolic network database to infer functional activity and pathway elicitation without prior identification. Briefly, Mummichog matches all the possible metabolites to the significant metabolic features (m/z), and searches for the pathways that can be constructed by these tentative chemicals. The significance of pathways can then be calculated by Fisher’s exact test on the null distribution, which is estimated by permutation where the features were randomly drawn from the list of all the extracted metabolic features (Li et al., 2013). Since mummichog only uses m/z to match metabolic features putatively to pathways, there are inevitable chances of increased risks of false positive discovery. To further minimize the possibility of false positive discovery, candidate pathways were re-run using a subset of 6 most common forms out of the 16 standard adduct forms in mummichog (for HILIC positive ion mode, only the following adducts were considered: M[+], M + H[+], M–H2O + H[+], M + Na[+], M + K[+], M + 2H[2+], and M(C13) + 2H[2+]; for C18 negative ion mode, only the following adducts were considered: M H[ ], M + Cl[ ], M + ACN-H[ ], M + HCOO[ ], M(C13)-H[ ], M H2O H[ ], and M + Na-2H[ ]). Specifically, in our analyses, each set of significant features from BPA and BPF associated MWAS models in the HILIC and C18 columns were used for pathway enrichment analyses. Due to the limited numbers of significant features detected when setting the FDR at the level of 5 %, which is not sufficient to conduct the pathway enrichment analysis via mummichog, we elected to use a less conservative corrected p-value threshold to decrease the possibility of false negatives. We then used the multiple testing corrected p-value at 0.2 (FDRB-H < 0.2) to select the significant features as the input for the pathway analysis.
Chemical annotation and confirmation were performed for metabolic features that were significantly associated (FDRB-H < 0.2) with BPA and/or BPF. Metabolic features were matched by m/z values for common adducts to the METLIN, ChemSpider, Human Metabolome Database, and Kyoto Encyclopedia of Genes and Genomes databases using a mass error threshold of 5 ppm (Uppal et al., 2013). To minimize further false positive matches, each matched feature was screened on their retention time, isotope patterns, and spectrum peak quality by examining the extracted ion chromatographs (EICs). Finally, the features passing the examination were annotated and confirmed using the Metabolomics Standards Initiative criteria described below (Schymanski et al., 2014; Sumner et al., 2007). The features whose m/z (±5 ppm difference), retention time (±30 s) and ion dissociation patterns (True peak: exhibiting clear gaussian peak shapes and signal-to-noise ratio above 3:1) (Yu and Jones, 2014) matched the authentic compounds analyzed in our laboratory under identical method and instrument parameters were assigned with level 1 confidence.
3. Results
Of the 230 participants with BPA exposure data, 86 (37.4 %) were recruited at Emory Midtown hospital clinics, and 144 (62.6 %) were from Grady Hospital clinics (Table 1). The mean age of participants was 25.8 ± 4.6 years, the mean prenatal BMI was 28.7 ± 7.1, the mean parity was 1.0 ± 1.0, and 38 (16.5 %) were married and 192 (83.5 %) were single. Among these participants, 33 (14.3 %) reported tobacco use, 17 (7.4 %) reported alcohol use, and 49 (21.3 %) reported marijuana use. The demographic distribution was similar for participants with BPF exposure data (Table 1).
Table 1.
Demographic characteristics of study population.
| Characteristic | Level | BPA Subset | BPF Subset |
|---|---|---|---|
| N | 230 | 48 | |
| Age, years (mean (SD)) | 25.8 (4.6) | 24.9 (3.7) | |
| Sex of Infant (%)* | Female | 113 (47.4) | 19 (39.6) |
| Male | 108 (47.0) | 27 (56.3) | |
| Parity (%) | 0 | 95 (41.3) | 24 (50.0) |
| 1 | 67 (29.1) | 13 (27.1) | |
| ≥ 2 | 68 (29.6) | 11 (22.9) | |
| First Prenatal BMI, kg/m2 (mean (SD)) | 28.7 (7.1) | 28.8 (8.2) | |
| Marital Status (%) | Single | 192 (83.5) | 45 (93.8) |
| Married | 38 (16.5) | 3 (6.2) | |
| Education (%) | 8th grade or less | 3 (1.3) | 1 (2.1) |
| Some high school | 33 (14.3) | 5 (10.4) | |
| Graduated high school | 98 (42.6) | 20 (41.7) | |
| Some college or technical school | 57 (24.8) | 19 (39.6) | |
| Graduated college | 24 (10.4) | 3 (6.2) | |
| Some graduate work or degree | 15 (6.5) | 3 (6.2) | |
| Income, percent of federal poverty level (FPL) (%) | <150 % FPL | 149 (64.8) | 32 (66.7) |
| Between 150 % and 300 % FPL | 36 (15.7) | 11 (22.9) | |
| >300 % FPL | 45 (19.6) | 5 (10.4) | |
| Tobacco Use (%) | No | 197 (85.7) | 41 (85.4) |
| Yes | 33 (14.3) | 7 (14.6) | |
| Alcohol Use (%) | No | 213 (92.6) | 45 (93.7) |
| Yes | 17 (7.4) | 3 (6.3) | |
| Marijuana Use (%) | No | 181 (78.7) | 41 (85.4) |
| Yes | 49 (21.3) | 7 (14.6) | |
| Other Drug Use (%) | No | 227 (98.7) | 48 (100.0) |
| Yes | 3 (1.3) | 0 (0.0) |
Some do not total to 100% due to missing data.
The method specifications, including LOD and relative standard deviation, of analytes (BPA, BPF and creatinine) are provided in Supplementary Table 1. The raw and creatinine adjusted maternal urinary BPA/BPF concentrations among study subjects are presented in Table 2. Pearson correlation coefficients between adjusted BPA and adjusted BPF is −0.07 (p = 0.64). Both raw and creatinine adjusted BPA had a higher detection rate and higher geometric mean compared to BPF. The geometric mean of raw maternal urinary BPA and BPF were 1.23 ± 2.94 ng/ml and 1.02 ± 4.61 ng/ml, respectively, and the geometric mean of creatinine adjusted maternal urinary BPA and BPF were 0.85 ± 2.58 μg/g creatinine and 0.70 ± 4.71 μg/g creatinine, respectively. Maternal urinary BPF concentration varied largely than BPA concentration among study subjects, with the maximum value of adjusted BPF ranging up to 467.67 μg/g and BPA up to 72.97 μg/g. Moreover, when comparing our study population of AA pregnant women to the adult U.S. population from 2013 to 2014 NHANES12, the median of both raw and creatinine adjusted BPA levels were slightly lower in AA pregnant women, but with higher IQR of raw BPA levels in AA pregnant women. The median of both raw and creatinine adjusted BPF levels were higher in AA pregnant women. Specifically, when comparing to the non-Hispanic black and female population in NHANES, the median of both raw and creatinine adjusted BPF levels and raw BPA levels were higher in our study population, and the median of creatinine adjusted BPA levels were lower in AA pregnant women.
Table 2.
BPA and BPF raw (ng/ml) and creatinine adjusted (μg/g) maternal urinary concentrations among study populations from Atlanta AA Maternal-Child cohort (2014–2017).
| N | % Detection | GM | GSD | P25 | P50 | P75 | P95 | Max | |
|---|---|---|---|---|---|---|---|---|---|
| BPA raw (ng/ml) | 230 | 82.17 | 1.23 | 2.94 | 0.55 | 1.20 | 2.72 | 6.53 | 139.69 |
| BPA adjusted (μg/g) | 0.85 | 2.58 | 0.47 | 0.79 | 1.41 | 4.45 | 72.97 | ||
| BPF raw (ng/ml) | 48 | 64.58 | 1.02 | 4.61 | 0.35 | 0.56 | 1.79 | 11.55 | 950.31 |
| BPF adjusted (μg/g) | 0.70 | 4.71 | 0.26 | 0.46 | 1.34 | 8.32 | 467.67 |
Acronym: GM: geometric mean; GSD: geometric standard deviation.
3.1. MWAS models
After data quality assurance and quality control, 16,481 and 13,043 metabolic features in HILIC positive ESI and C18 negative ESI, respectively, were included for final analyses. We ran four sets of MWAS models: two sets (HILIC and Reverse Phase C18) for each BPA (n = 230) and BPF (n = 48). For the HILIC column, the number of significant (FDRB-H < 0.2) features were approximately the same between BPA (Fig. 1A) and BPF (Fig. 1C) (207 vs 268, respectively). For the C18 column, a much larger number of significant features were found to be associated with BPF (Fig. 1B) than BPA (Fig. 1D) and (465 vs 57, respectively). A summary of the FDR-corrected MWAS results can be found in Supplementary Table S2.
Fig. 1.
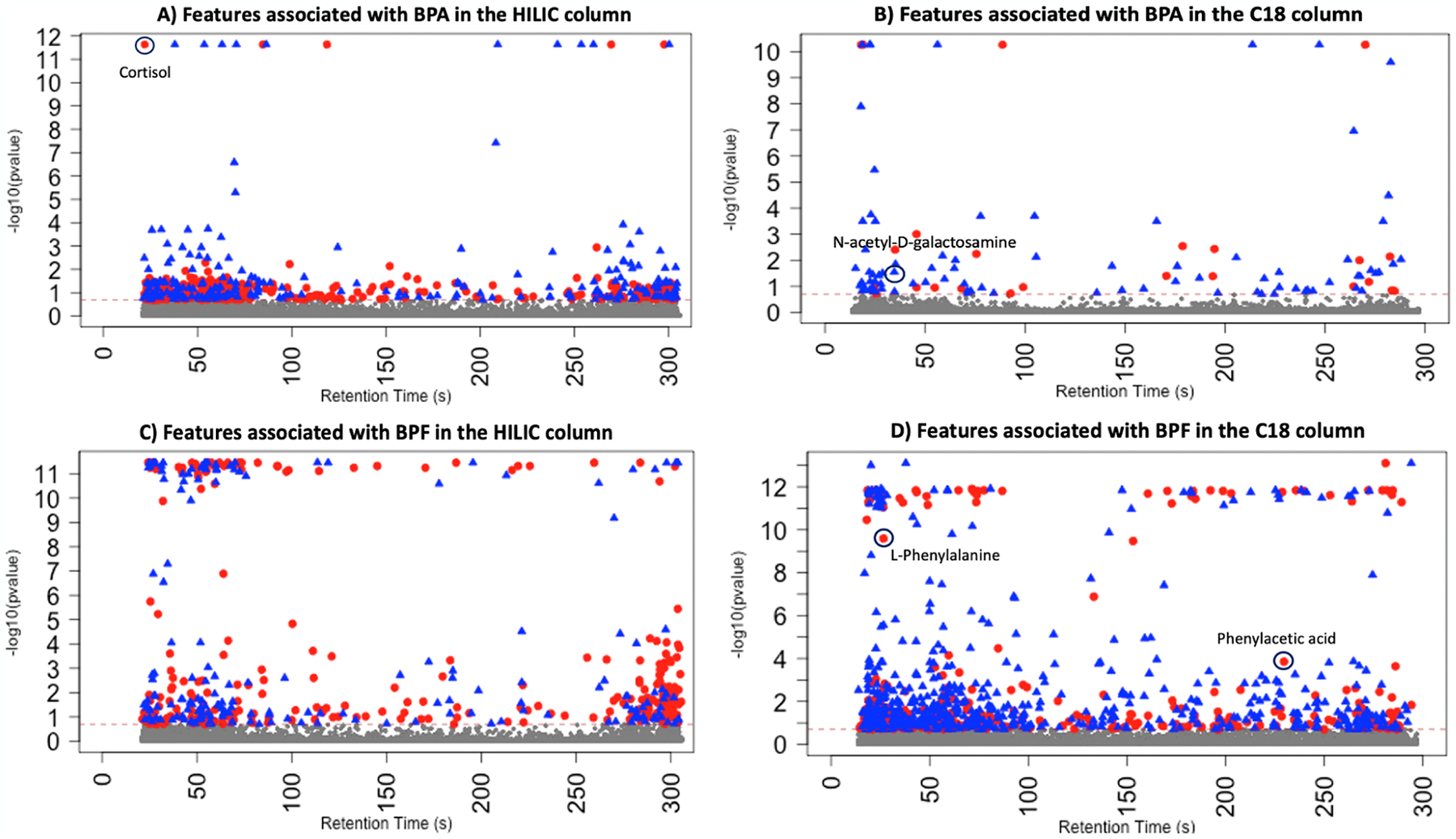
Significant metabolic features associated with bisphenol A (BPA) and bisphenol F (BPF). Red dots represent the features that were positively associated with BPA/BPF and blue dots represent the metabolic features that were negatively associated with BPA/BPF. Dashed line is the threshold −log10 (FDRB-H < 0.2). Note: Some metabolites identified in later chemical annotation and confirmation are labelled in the plots.
3.2. Pathway enrichment analysis
Pathway enrichment analyses were run separately for HILIC and C18 significant (FDRB-H < 0.2) metabolic features. A total of 9 metabolic pathways were significantly associated with BPA exposure (Supplementary Table S3), including C-21 steroid hormone biosynthesis and metabolism, lysine metabolism, dynorphin metabolism, and lipoate metabolism. Meanwhile, we also identified 9 metabolic pathways significantly associated with BPF (Supplementary Table S4), including xenobiotic metabolism, C-21 steroid hormone biosynthesis and metabolism, lipoate metabolism, and lysine metabolism. Notably, three metabolic pathways, C-21 steroid hormone biosynthesis and metabolism, lysine metabolism, and lipoate metabolism pathways, were significantly associated with both BPA and BPF exposure (Fig. 2, Supplementary Fig. S1).
Fig. 2.
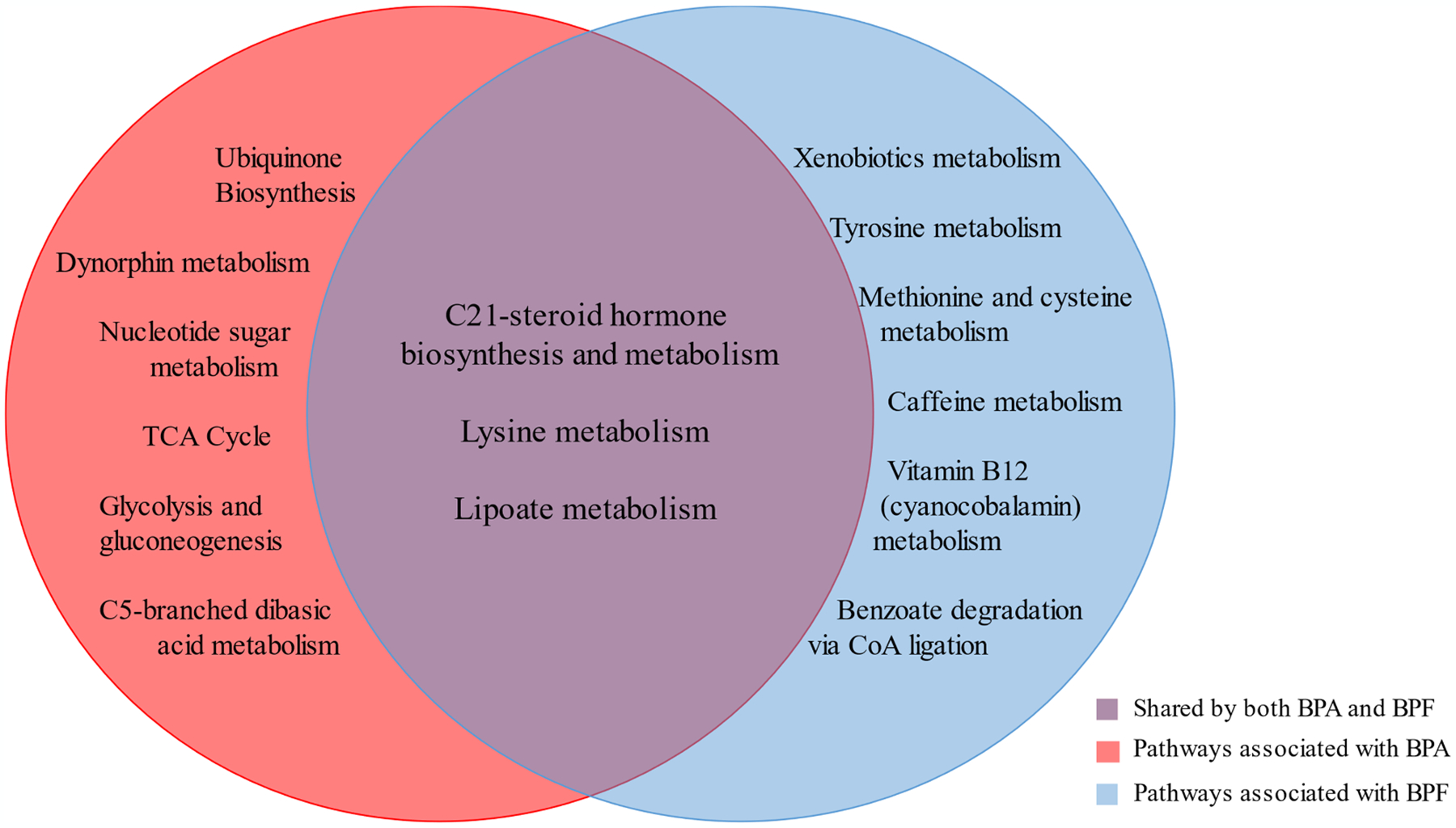
Significant metabolic pathways associated with bisphenol A (BPA) and/or bisphenol F (BPF).
3.3. Chemical annotation and confirmation
We conducted chemical annotation and confirmation of metabolic features significantly associated with BPA or BPF and confirmed 16 metabolites (Table 3; Supplementary Table S5; Supplementary Fig. S2) with Level One evidence. Seven were associated with BPA exposure, and nine were associated with BPF exposure (including one feature matched to three metabolites), with 6 detected in the HILIC column and 10 detected in the C18 column, respectively. Specifically, most of identified metabolic features were found to be positively associated with BPA, including tyramine (β = 0.16), L-carnitine (β = 0.02), N-acetyl-L-phenylalanine (β = 0.01), cortisol (β = 0.01) and bilirubin (β = 0.01), suggesting the disruption on aromatic amino acid metabolism/steroid hormone synthesis, and the hepatotoxicity effect of BPA. Many of the confirmed metabolites for BPF exposure are related to amino acid metabolism, including valine, tyramine, N-alpha-acetyl-L-asparagine, N-methyl-D-aspartic acid. Further, the results of enriched pathways and verified metabolites are closely linked and connected in an interrelated molecular network, which underlies the potential toxicant mechanisms and stress responses in relation to external bisphenol analogues exposures among pregnant AA women (Fig. 3). Specifically, the detailed associations of these 16 identified metabolites are provided in Supplementary Fig. S3/S4.
Table 3.
Chemical identity of metabolic features significantly associated with BPA or BPF.
| m/z | RT (s) | ppm error^# | RT (s) difference# | Metabolite | Adduct | Associated exposure | Coefficient* |
|---|---|---|---|---|---|---|---|
| 118.0863 | 45.6 | −4.1 | −16.5 | L-Valine | M + H | BPA | −0.15 |
| 138.0914 | 22.5 | −3.3 | −8.6 | Tyramine | M + H | BPA | 0.16 |
| 162.1125 | 42.3 | −3.0 | −30.0 | L-Carnitine | M + H | BPA | 0.02 |
| 208.0973 | 30.3 | 0.2 | 0.0 | N-acetyl-L-phenylalanine | M + H | BPA | 0.01 |
| 256.0581 | 27.2 | −2.7 | 4.2 | N-acetyl-D-galactosamine | M + Cl | BPA | −0.01 |
| 363.2164 | 26.0 | −2.0 | −3.8 | Cortisol | M + H | BPA | 0.01 |
| 585.2707 | 49.1 | −1.0 | 23.0 | Bilirubin | M + H | BPA | 0.01 |
| 108.0455 | 16.7 | 5.0 | −15.7 | 2-Aminophenol | M−H | BPF | 7.68E-04 |
| 135.045 | 227.9 | 2.7 | 1.5 | Phenylacetic acid | M−H | BPF | 9.82E-04 |
| 138.0196 | 269.9 | 3.3 | 24.0 | 6-Hydroxynicotinate | M−H | BPF | −1.44E-03 |
| 146.046 | 20.9 | 4.4 | 1.3 | N-methyl-D-aspartic acid | M−H | BPF | 5.32E-04 |
| 151.0401 | 250.4 | 3.6 | 7.6 | 4-Hydroxyphenylacetate 2′-4′-dihydroxyacetophenone 3-hydroxyphenylacetate |
M−H | BPF | −3.06E-03 |
| 164.0717 | 28.4 | −3.2 | 3.0 | L-Phenylalanine | M−H | BPF | 1.40E-03 |
| 217.0483 | 23.1 | −0.5 | 0.9 | Mannitol | M + Cl | BPF | −2.18E-03 |
Acronym: m/z – mass to charge ratio; RT – retention time.
ppm (parts per million) error is a quantity frequently used to report mass errors.
The coefficient represents the change in log-transformed metabolite intensity per 1-unit increase in urinary BPA or BPF level.
The difference refers to the ppm error and retention time difference between metabolites and authentic standards.
Fig. 3.
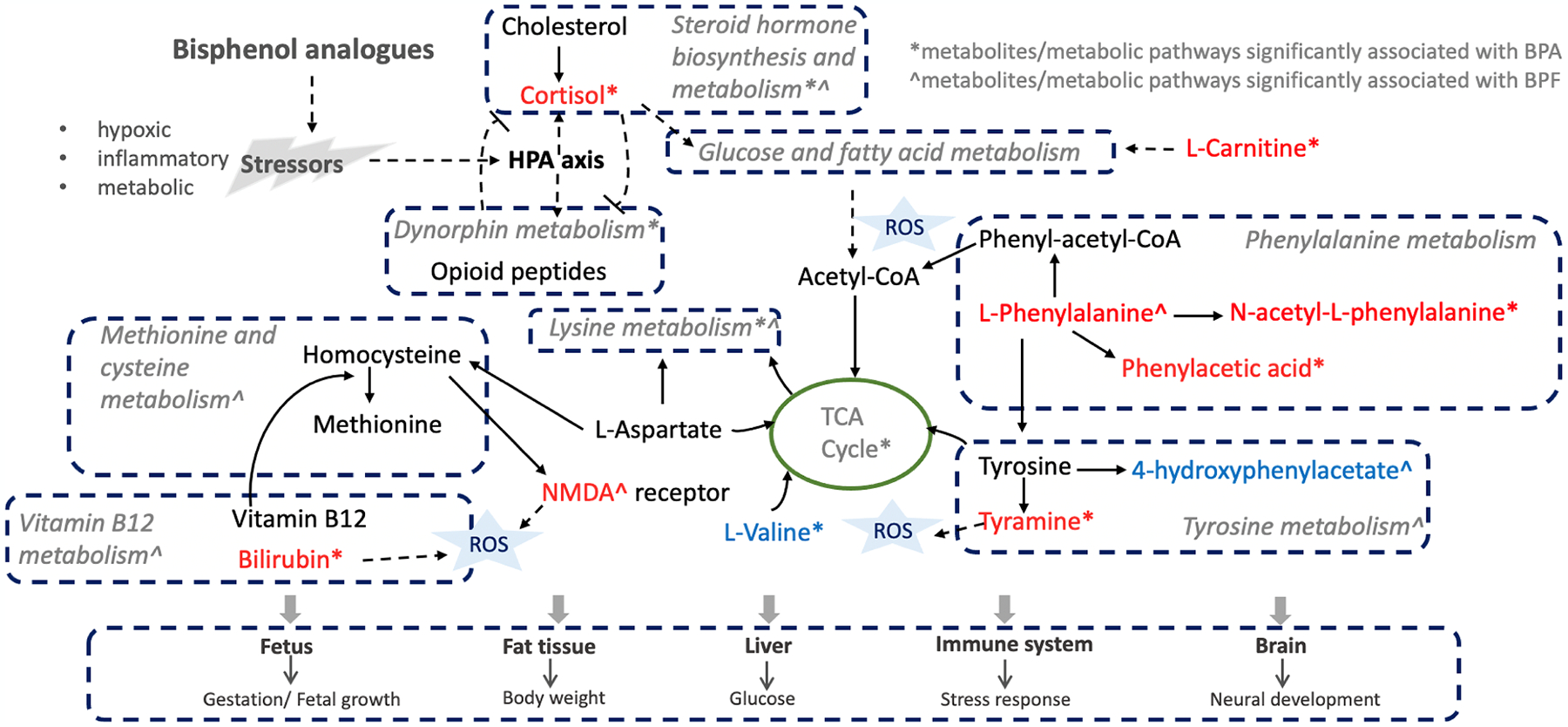
Potential molecular mechanisms underlying the effects of bisphenol analogues toxicity on pregnant women. Molecules in red denoted the metabolites confirmed and positively associated with BPA/BPF. Molecules in blue denoted the metabolites confirmed and negatively associated with BPA/BPF. Acronym: ROS, reactive oxygen species; NMDA: N-methyl-D-aspartic acid; HPA axis: hypothalamic-pituitaryadrenal axis.
4. Discussion
Untargeted metabolomics is an efficient bottom-up approach to explore metabolic changes associated with exposure to environmental toxicants and characterize human exposures to environmental contaminants. In the present study, we applied HRM to assess the internal metabolic profiles associated with environmental toxicants from an African American Maternal-Child cohort in Atlanta using a cross-sectional study design. We identified several biological pathways and metabolites associated with BPA and BPF that are closely involved in various endogenous processes, including stress responses, inflammation, neural development, reproduction, and weight regulation. To our knowledge, this is the first untargeted HRM application conducted for BPA and BPF among pregnant women.
The most pronounced and consistent findings from our current analysis were that prenatal exposure to BPA and BPF were both associated with perturbations in several key metabolic pathways, including biosynthesis of steroid hormones, lysine metabolism, tyrosine metabolism, lipoate metabolism, and methionine and cysteine metabolism. These metabolic pathways have been reported to be associated with inflammatory and stress response, neurotoxic effects, and energy storage in other BPA/BPF exposure metabolic profiling studies (Khan et al., 2017; Yue et al., 2019; Hainan et al., 2020; Ji et al., 2014; Li et al., 2018). Among these identified metabolic pathways, C21-steroid hormone biosynthesis and metabolism, lysine metabolism, and lipoate metabolism were found to be significantly associated with both BPA and BPF exposures. Specifically, C21-steroid hormone biosynthesis and metabolism involves the synthesis of hormones such as glucocorticoids, androgens, estrogens, and progestogens, which regulate key endogenous processes including metabolism, stress response, sexual differentiation, and reproduction (Schiffer et al., 2019). We also identified cortisol, an important metabolite involved in the C-21 steroid biosynthesis and metabolism. Cortisol is a glucocorticoid produced by the adrenal glands as regulated by the hypothalamic–pituitaryadrenal (HPA) axis (Schiffer et al., 2019), which increases plasma cortisol in response to acute stress during antenatal periods (Garrud and Giussani, 2019). It has been suggested that the high maternal cortisol levels during intra-uterine life may have consequences to the fetal immune system (Bertram and Hanson, 2002), which correlate negatively with birthweight, and arise in association with maternal stress and malnutrition (Reynolds, 2013). In our study, cortisol was found to be positively associated with BPA exposure, which is consistent with a previous study conducted by Giesbrecht et al., where dysregulation of cortisol levels was reported to be associated with urinary BPA in pregnant women (Giesbrecht et al., 2016). Interestingly, in that same cohort of mother-infant pairs, higher maternal levels of BPA were found to be associated with higher signal intensity for cortisol in female infants but lower signal intensity for cortisol in male infants (Giesbrecht et al., 2017). Further studies are needed to explore the effect of infant sex on the association of BPA to cortisol to explain these potential underlying mechanisms. Dynorphin metabolism is another top pathway for BPA and it has been associated with HPA axis. Recent animal studies suggested that perturbations in dynorphin peptide levels may influence signaling pathways of governing weight (Sainsbury et al., 2007; Ghule et al., 2020).
We also observed perturbations in several amino acid, vitamin, and cofactors metabolic pathways, including lipoate metabolism, lysine metabolism, tyrosine metabolism, methionine and cysteine metabolism, and vitamin B12 (cyanocobalamin) metabolism. Although the evidence on metabolic perturbations associated with BPA/BPF is limited, these findings were consistent with existing literature. Specifically, lipoate metabolism and lysine metabolism are two overlapping pathways associated with both BPA and BPF. Changes in lipoate metabolism and lysine metabolism on gut microbiota were observed after exposure to BPA in rodents (Javurek et al., 2016). Animal studies showed the protective response of lipoic acid on BPA induced adverse effect. Lipoic acid was found to mitigate the oxidative damage caused by BPA in liver and ovarian tissues (Avci et al., 2016), lower the BPA-induced mitochondrial toxicity through antioxidant mechanism or by direct free radical scavenging activity (El-Beshbishy et al., 2013), and prevent the neurotoxicity of BPA and endocrine disruptors (Khan et al., 2018). Moreover, in a metabolic pathway analysis of urinary samples of female children, researchers showed that biosynthesis of steroid hormones and several amino acid pathways including lysine degradation and tyrosine metabolism were affected in highly BPA-exposed children (Khan et al., 2017). Similar results were shown in human hepatocarcinoma cell line where after BPA/BPF exposures, key metabolites in lysine metabolism such as lysine and succinate significantly decreased (Yue et al., 2019). Animal studies on mice and Mytilus galloprovincialis also showed consistent results where BPA treatment affects lysine metabolism and several amino acid metabolites including lysine and tyrosine (Ji et al., 2014). Additionally, we observed metabolic perturbations in sulfur-containing amino acids pathways such as methionine and cysteine metabolism. Methionine and cysteine metabolism plays an important role in biological growth and redox balance, and it has been reported the significant changes in sulfur-containing amino acids after BPA exposure (Wang et al., 2018). Taken together, the prenatal exposure to bisphenol may be associated with a series of endogenous and metabolic response in reaction to the external stress (Fig. 3). The steroid hormone metabolism and dynorphin metabolism function under the regulation of HPA axis and collectively produce the endocrine molecules to regulate the downstream biological processes that are closely related to inflammation, placental condition, neural development, and fetal health. The concurrent response of a series of amino acid metabolism and derivatives of amino acid metabolites also play an important role in stress response and are potentially related to some health effects of pregnant women under BPA/BPF toxicity.
Importantly, we confirmed the chemical identifies of numerous metabolites enriched within these pathways and most of these metabolites exhibited positive associations with BPA/BPF exposures. In particular, tyrosine metabolism, one of the aromatic amino acid metabolisms, is the top pathway that enriched with most of these identified metabolites. Specifically, we observed a positive association between BPA and tyramine, which can cause the reactive oxidative species (ROS) formation inside mitochondria and act as a source of oxidative damage to mitochondrial DNA (Farooqui and Farooqui, 2016). Imbalance in levels of tyramine has been reported to be associated with Parkinson’s disease, depression, migraines, and increased blood pressure (Dhakal and Macreadie, 2020). Accumulating evidence suggests that early life exposure to BPA impacts neural development. Maternal BPA exposure during gestation is associated with hyperactivity and aggression in two-year-old girls (Braun et al., 2009) and with anxiety and depression in 3-year-old girls (Braun et al., 2011). Harley et al. reported that prenatal urinary BPA concentrations were associated with increased internalizing problems in boys. (Harley et al., 2013) These observed associations may contribute to revealing the potential mechanisms underlying the impact of prenatal BPA exposures on early neurodevelopment. We also identified 4-hydroxyphenylacetate, another metabolite enriched in tyrosine metabolism and positively associated with BPF, which is a downstream product of tyrosine. Evidence showed that higher maternal BPA during early to mid-pregnancy increased the levels of oxidized tyrosine moieties (Veiga-Lopez et al., 2015), which can lead to high oxidant rates, such as inflammation and be associated with preterm birth related diseases, such as perinatal asphyxia (Groenendaal et al., 2006), or chronic hypoxia (Escobar et al., 2013). It provides evidence of the induction of nitrosative stress by prenatal bisphenol analogues in pregnant mothers and further affect the offspring health.
We also confirmed the chemical identities of N-acetyl or N-methyl derivatives of several amino acids, including N-acetyl-L-phenylalanine, N-acetyl-D-galactosamine, 4-hydroxyphenylacetate, and N-methyl-D-aspartic acid. It has been noted that BPA exposure affects the alanine, aspartate, and glutamate metabolism in Sprague–Dawley (SD) rats, by the downregulation of N-acetyl-l-phenylalanine (Mao et al., 2021). The N-acetyl-l-phenylalanine is a hazardous amphipathic metabolite of phenylalanine, and higher levels of phenylalanine appears to exist in large amounts of patients with phenylketonuria (Wang et al., 2014). Elevated levels of phenylalanine and N-acetyl-L-phenylalanine have been reported in Alzheimer’s disease (AD) samples, which indicate that dysregulated phenylalanine metabolism may be an important pathogenesis for AD pathology formation (Liu et al., 2021). Phenylacetic acid is another identified chemical enriched in phenylalanine metabolism and positively associated with BPF exposures. It can promote ROS generation, stimulating secretion of tumor necrosis factor (TNF-α), one of the inflammatory cytokines, in human aortic endothelial cells in vitro (Pontiki and Hadjipavlou-Litina, 2018). We also identified bilirubin, a metabolite enriched in vitamins and cofactors metabolic pathway, and observed a positive association between bilirubin and BPA. Serum bilirubin has antioxidant properties and may serve as a biomarker of oxidative stress (Sedlak and Snyder, 2004), and the moderate levels of serum bilirubin can reduce free radicals in cells and prevent oxidative stress–related diseases, including coronary artery disease and myocar-dial infarction (Lin et al., 2006). Low levels of bilirubin were associated with poor maternal and infant outcomes in women diagnosed with pre-eclampsia (Breslin et al., 2013). Thus, the positive association we observed may be explained by the antioxidant effect of bilirubin to compensate for the oxidative effect of BPA, which may result in the low level of bilirubin in late pregnancy and further lead to the poor maternal and infant outcomes. We also identified the significant perturbation in vitamin B12 (cyanocobalamin) metabolism associated with BPF exposure, indicating the similar perturbation pattern of bisphenol analogues. Taken together, these results further support the potential oxidative stress effect of bisphenol analogues.
Additionally, we identified an exogenous metabolite associated with BPF exposures: 2-aminophenol. 2-aminophenol is an organic chemical compound that is usually found in cosmetics and personal care products and was found to be positively associated with BPF. This association may be explained by the usage of industrial and consumer products, as exposure to bisphenol analogues has been reported to associated with the use of cosmetics and related personal products (Mok et al., 2021).
4.1. Strengths and limitations
The strengths of our study include the use of targeted exposure assessment on urinary BPA and BPF and the application of novel HRM analysis to investigate the potential mechanisms underlying the toxicities of BPA and BPF on maternal metabolome among participants in the Atlanta African American Maternal-Child cohort. Through the metabolic profiling of disproportionately exposed environmental toxicants on AA pregnant women, it can provide us opportunities for future studies to develop early detection and reduce the related health burden among this minor population. Moreover, in this first MWAS study of BPA and BPF, we detected numerous significant metabolic perturbations in biological pathways associated with exposure to BPA and BPF. To minimize the risk of risk of false positive discovery, we took multiple approaches in the MWAS study, including rigorous controlling of multiple comparison bias, systematic chemical identification, and validation with reference standards. Specifically, we confirmed and verified 16 metabolites with Level One evidence, which were closely linked and connected in several inflammation, oxidative stress, and endocrine disruption related pathways.
Despite these promising findings, there are several limitations in this analysis. First, the sample size of the BPF analysis (n = 48) is limited since we conducted the analysis based upon the availability of bio-specimens with both untargeted metabolomics and targeted BPF measurements. This is a common challenge in proof-of-concept environmental metabolomics applications, and this study was a hypothesis-generating study for exploratory purpose. While there might be a risk of selection bias, we have compared the demographic pattern in the BPF analysis (n = 48, which is a subset of the participants with BPA measurements) to the BPA analysis (n = 230) and there were no statistically significant differences in any demographic characteristics or behavior risk factors (p > 0.05), including age, BMI, parity, infant sex, education, and substance use. Nevertheless, given the hypothesis-generating nature of the analysis, caution shall be taken when interpreting the study findings on the potential effects of BPA and BPF exposure on maternal metabolome that will need to be validated by future large-scale hypothesis testing studies. Second, we found more significant features associated with BPF, even with relatively smaller sample size compared to BPA. To minimize the potential impact of false positive, we used a multiple comparison correction to control false discovery rate at 20 % on MWA model result. Furthermore, BPF levels have much larger variances and wider range than BPA levels among study subjects, and such wider contrast of exposure levels may result in the larger difference detected in maternal metabolome, and thus more significant features could be detected to be associated with BPF. Third, due to the cross-sectional nature of the associations between BPA/BPF concentrations and metabolomic features, it is difficult to determine causal relationships. The single spot urine samples of exposures and one time point measurement of metabolomics may not reflect the long-term exposure status and limit the interpretation of metabolic changes associated with BPA/BPF during late pregnancy. However, given the exploratory nature of the study, these results will provide valuable insights and opportunities for future studies to develop sensitive biomarkers of BPA/BPF associated maternal perturbation during pregnancy. Moreover, since only BPA and BPF were included in the present study, it is possible that other bisphenol analogues, such as bisphenol S (BPS), or co-exposed chemicals may also yield similar results, and thus, it would be important to repeat these analyses for other bisphenol analogues that are commonly used in future analysis. Although we controlled for several important confounding factors, including age, infant sex, BMI, socioeconomic status (i.e., education level), and some behavioral risk factors (tobacco use, marijuana use and alcohol use), dietary variables were not considered in this study and non-fasting status may introduce measurement variation. Adjusting for more variables might cause issues of overadjustment. Also, we used pool standards and internal references in the metabolic profiling and followed a comprehensive metabolomics workflow to minimize the potential impact of non-fasting status, which has been shown to successfully analyze many non-fasting samples previously (Go et al., 2015). Finally, the findings from this pregnant African American women cohort might reduce generalizability to a broader population.
5. Conclusions
During pregnancy, the developing fetus undergoes critical stages of development and are exposed to toxicants passing through the placenta. Compounds such as BPA and BPF that may have systemic effects and perturb metabolic pathways could have adverse downstream health effects. In this first metabolome-wide association study of BPA/BPF, we demonstrated that urinary BPA/BPF levels were associated with perturbations in biological pathways closely linked to inflammation, oxidative stress, and endocrine disruption. We also confirmed the chemical identity of 16 metabolites significantly associated with BPA or BPF exposure with Level One evidence, which are closely linked to stress responses, inflammation, neural development, reproduction, and weight regulation. These findings support future hypothesis-testing investigations on potential molecular mechanisms underlying the impact of maternal bisphenol exposures on adverse health outcomes. It is also important to consider compounding effects of these metabolic changes in mothers and children in addition to the already disproportionate health burden facing by African American populations.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Health (NIH) research grants [R21ES032117, R01NR014800, R01MD009064, R24ES029490, R01MD009746], NIH Center Grants [P50ES02607, P30ES019776, UH3OD023318, U2CES026560], and Environmental Protection Agency (USEPA) center grant [83615301]. Additionally, we are grateful for our colleagues -Nathan Mutic, Cierra Johnson, Erin Williams, Priya D’Souza, Estefani Ignacio Gallegos, Nikolay Patrushev, Kristi Maxwell Logue, Castalia Thorne, Shirleta Reid, Cassandra Hall, and the clinical health care providers and staff at the prenatal recruiting sites for helping with data and sample collection and logistics and sample chemical analyses in the laboratory. We also thank the participants themselves for their contributions to this project. We would like to thank all members from the Environmental Metabolomics and Exposomics Research Group at Emory (EMERGE) for their valuable input and feedback on this project.
Footnotes
CRediT authorship contribution statement
Rachel Tchen: Formal analysis, Investigation, Writing – original draft, Writing – review & editing, Visualization. Youran Tan: Formal analysis, Investigation, Writing – review & editing, Visualization. Dana Boyd Barr: Methodology, Validation, Resources, Data curation, Writing – review & editing, Supervision, Funding acquisition. P. Barry Ryan: Methodology, Resources, Data curation, Writing – review & editing, Supervision, Funding acquisition. ViLinh Tran: Data curation, Methodology, Writing – review & editing. Zhenjiang Li: Data curation, Methodology, Writing – review & editing. Yi-Juan Hu: Data curation, Resources, Writing – review & editing. Alicia K. Smith: Data curation, Methodology, Writing – review & editing. Dean P. Jones: Methodology, Resources, Writing – review & editing, Funding acquisition. Anne L. Dunlop: Investigation, Resources, Data curation, Writing – review & editing, Project administration, Funding acquisition. Donghai Liang: Conceptualization, Methodology, Investigation, Writing – review & editing, Supervision, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107530.
Data availability
Data will be made available on request.
References
- Ahern J, Pickett KE, Selvin S, Abrams B, 2003. Preterm birth among African American and white women: a multilevel analysis of socioeconomic characteristics and cigarette smoking. J. Epidemiol. Community Health 57, 606–11 PMC1732558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aker AM, Johns L, McElrath TF, Cantonwine DE, Mukherjee B, Meeker JD, 2018. Associations between maternal phenol and paraben urinary biomarkers and maternal hormones during pregnancy: A repeated measures study. Environ. Int 113, 341–349. NIHMS936389.PMC5866216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anesi A, Rubert J, Oluwagbemigun K, Orozco-Ruiz X, Nöthlings U, Breteler MMB, Mattivi F, 2019. Metabolic profiling of human plasma and urine, targeting tryptophan, tyrosine and branched chain amino acid pathways. Metabolites, 9 PMC6918267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aung MT, Ferguson KK, Cantonwine DE, Bakulski KM, Mukherjee B, Loch-Caruso R, McElrath TF, Meeker JD, 2019. Associations between maternal plasma measurements of inflammatory markers and urinary levels of phenols and parabens during pregnancy: A repeated measures study. Sci. Total Environ 650, 1131–1140. NIHMS1506685.PMC6236678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avci B, Bahadir A, Tuncel OK, Bilgici B, 2016. Influence of α-tocopherol and α-lipoic acid on bisphenol-A-induced oxidative damage in liver and ovarian tissue of rats. Toxicol. Ind. Health 32, 1381–1390. [DOI] [PubMed] [Google Scholar]
- Bailey LB, Stover PJ, McNulty H, Fenech MF, Gregory JF 3rd, Mills JL, Pfeiffer CM, Fazili Z, Zhang M, Ueland PM, Molloy AM, Caudill MA, Shane B, Berry RJ, Bailey RL, Hausman DB, Raghavan R, Raiten DJ, 2015. Biomarkers of nutrition for development-folate review. J. Nutr 145, 1636s–80s PMC4478945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, Gonzalez AJ, Needham LL, Pirkle JL, 2005. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ. Health Perspect 113, 192–200. PMC1277864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram CE, Hanson MA, 2002. Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction 124, 459–467. [DOI] [PubMed] [Google Scholar]
- Braun JM, Yolton K, Dietrich KN, Hornung R, Ye X, Calafat AM, Lanphear BP, 2009. Prenatal bisphenol A exposure and early childhood behavior. Environ. Health Perspect 117, 1945–1952. PMC2799471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP, 2011. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics 128, 873–882. PMC3208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan PA, Dunlop AL, Smith AK, Kramer M, Mulle J, Corwin EJ, 2019. Protocol for the Emory University African American maternal stress and infant gut microbiome cohort study. BMC Pediatrics 19, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslin E, Kaufmann A, Quenby S, 2013. Bilirubin influences the clinical presentation of pre-eclampsia. Eur. J. Obstet. Gynecol. Reprod. Biol 170, 111–113. [DOI] [PubMed] [Google Scholar]
- Cabaton N, Dumont C, Severin I, Perdu E, Zalko D, Cherkaoui-Malki M, Chagnon MC, 2009. Genotoxic and endocrine activities of bis(hydroxyphenyl) methane (bisphenol F) and its derivatives in the HepG2 cell line. Toxicology 255, 15–24. [DOI] [PubMed] [Google Scholar]
- Cantonwine D, Meeker JD, Hu H, Sánchez BN, Lamadrid-Figueroa H, Mercado-García A, Fortenberry GZ, Calafat AM, Téllez-Rojo MM, 2010. Bisphenol a exposure in Mexico City and risk of prematurity: a pilot nested case control study. Environ. Health 9, 62. PMC2965706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers MC, Maclean B, Burke R, Amodei D, Ruderman DL, Neumann S, Gatto L, Fischer B, Pratt B, Egertson J, Hoff K, Kessner D, Tasman N, Shulman N, Frewen B, Baker TA, Brusniak MY, Paulse C, Creasy D, Flashner L, Kani K, Moulding C, Seymour SL, Nuwaysir LM, Lefebvre B, Kuhlmann F, Roark J, Rainer P, Detlev S, Hemenway T, Huhmer A, Langridge J, Connolly B, Chadick T, Holly K, Eckels J, Deutsch EW, Moritz RL, Katz JE, Agus DB, MacCoss M, Tabb DL, Mallick P, 2012. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol 30, 918–920. NIHMS374482.PMC3471674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Barr DB, Ryan PB, Panuwet P, Smarr MM, Liu K, Kannan K, Yakimavets V, Tan Y, Ly V, Marsit CJ, Jones DP, Corwin EJ, Dunlop AL, Liang D, 2021. Per- and polyfluoroalkyl substance (PFAS) exposure, maternal metabolomic perturbation, and fetal growth in African American women: A meet-inthe-middle approach. Environ. Int 158, 106964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Yang T, Walker DI, Thomas DC, Qiu C, Chatzi L, Alderete TL, Kim JS, Conti DV, Breton CV, Liang D, Hauser ER, Jones DP, Gilliland FD, 2020. Dysregulated lipid and fatty acid metabolism link perfluoroalkyl substances exposure and impaired glucose metabolism in young adults. Environ. Int 145, 106091. NIHMS1623960.PMC8009052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corwin EJ, Hogue CJ, Pearce B, Hill CC, Read TD, Mulle J, Dunlop AL, 2017. Protocol for the emory university African American vaginal, oral, and gut microbiome in pregnancy cohort study. BMC Pregnancy Childbirth 17, 161. PMC5455081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhakal S, Macreadie I, 2020. Tyramine and amyloid beta 42: a toxic synergy. Biomedicines, 8 PMC7345151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Beshbishy HA, Aly HA, El-Shafey M, 2013. Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol. Ind. Health 29, 875–887. [DOI] [PubMed] [Google Scholar]
- Escobar J, Teramo K, Stefanovic V, Andersson S, Asensi MA, Arduini A, Cubells E, Sastre J, Vento M, 2013. Amniotic fluid oxidative and nitrosative stress biomarkers correlate with fetal chronic hypoxia in diabetic pregnancies. Neonatology 103, 193–198. [DOI] [PubMed] [Google Scholar]
- Farooqui T, Farooqui AA, 2016. Trace amines and neurological disorders: Potential Mechanisms and Risk Factors. Academic Press. [Google Scholar]
- Ferguson KK, Lan Z, Yu Y, Mukherjee B, McElrath TF, Meeker JD, 2019. Urinary concentrations of phenols in association with biomarkers of oxidative stress in pregnancy: Assessment of effects independent of phthalates. Environ. Int 131, 104903. NIHMS1534598.PMC6728185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher RA, Yates F, 1943. Statistical tables for biological, agricultural and medical research. Oliver and Boyd Ltd, London. [Google Scholar]
- Garrud TAC, Giussani DA, 2019. Combined antioxidant and glucocorticoid therapy for safer treatment of preterm birth. Trends Endocrinol. Metab 30, 258–269. [DOI] [PubMed] [Google Scholar]
- Gerona RR, Pan J, Zota AR, Schwartz JM, Friesen M, Taylor JA, Hunt PA, Woodruff TJ, 2016. Direct measurement of Bisphenol A (BPA), BPA glucuronide and BPA sulfate in a diverse and low-income population of pregnant women reveals high exposure, with potential implications for previous exposure estimates: a cross-sectional study. Environ. Health 15, 50. PMC4828888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghule A, Rácz I, Bilkei-Gorzo A, Leidmaa E, Sieburg M, Zimmer A, 2020. Modulation of feeding behavior and metabolism by dynorphin. Sci. Rep 10, 3821. PMC7052232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesbrecht GF, Liu J, Ejaredar M, Dewey D, Letourneau N, Campbell T, Martin JW, 2016. Urinary bisphenol A is associated with dysregulation of HPA-axis function in pregnant women: Findings from the APrON cohort study. Environ. Res 151, 689–697. [DOI] [PubMed] [Google Scholar]
- Giesbrecht GF, Ejaredar M, Liu J, Thomas J, Letourneau N, Campbell T, Martin JW, Dewey D, 2017. Prenatal bisphenol a exposure and dysregulation of infant hypothalamic-pituitary-adrenal axis function: findings from the APrON cohort study. Environ. Health 16, 47. PMC5437646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giscombé CL, Lobel M, 2005. Explaining disproportionately high rates of adverse birth outcomes among African Americans: the impact of stress, racism, and related factors in pregnancy. Psychol. Bull 131, 662–683. NIHMS1612809.PMC7451246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go Y-M, Walker DI, Liang Y, Uppal K, Soltow QA, Tran V, Strobel F, Quyyumi AA, Ziegler TR, Pennell KD, 2015. Reference standardization for mass spectrometry and high-resolution metabolomics applications to exposome research. Toxicol. Sci 148, 531–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenendaal F, Lammers H, Smit D, Nikkels PG, 2006. Nitrotyrosine in brain tissue of neonates after perinatal asphyxia. Arch. Dis. Child. Fetal Neonatal Ed 91. F429–33 PMC2672757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainan J, Haishan L, Naining S, Baoliang X, Chan Z, Wentao L, Guolin S, 2020. Effect of bisphenol a on the plasma metabolic spectrum in mice based on quadrupole orbitrap mass spectrometry. Asian J. Ecotoxicol 71–80. [Google Scholar]
- Harley KG, Gunier RB, Kogut K, Johnson C, Bradman A, Calafat AM, Eskenazi B, 2013. Prenatal and early childhood bisphenol A concentrations and behavior in school-aged children. Environ. Res 126, 43–50. NIHMS506827. PMC3805756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hercog K, Maisanaba S, Filipič M, Sollner-Dolenc M, Kač L, Žegura B, 2019. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci. Total Environ 687, 267–276. [DOI] [PubMed] [Google Scholar]
- Hornung RW, Reed LD, 1990. Estimation of average concentration in the presence of nondetectable values. Appl. Occup. Environ. Hyg 5, 46–51. [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y, 2002. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod 17, 2839–2841. [DOI] [PubMed] [Google Scholar]
- Javurek AB, Spollen WG, Johnson SA, Bivens NJ, Bromert KH, Givan SA, Rosenfeld CS, 2016. Effects of exposure to bisphenol A and ethinyl estradiol on the gut microbiota of parents and their offspring in a rodent model. Gut Microbes 7, 471–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji C, Wei L, Zhao J, Wu H, 2014. Metabolomic analysis revealed that female mussel Mytilus galloprovincialis was sensitive to bisphenol A exposures. Environ. Toxicol. Pharmacol 37, 844–849. [DOI] [PubMed] [Google Scholar]
- Jordan J, Zare A, Jackson LJ, Habibi HR, Weljie AM, 2012. Environmental contaminant mixtures at ambient concentrations invoke a metabolic stress response in goldfish not predicted from exposure to individual compounds alone. J. Proteome Res 11, 1133–1143. [DOI] [PubMed] [Google Scholar]
- Khan A, Park H, Lee HA, Park B, Gwak HS, Lee HR, Jee SH, Park YH, 2017. Elevated metabolites of steroidogenesis and amino acid metabolism in preadolescent female children with high urinary bisphenol a levels: a high-resolution metabolomics study. Toxicol. Sci 160, 371–385. [DOI] [PubMed] [Google Scholar]
- Khan J, Salhotra S, Ahmad S, Sharma S, Abdi SAH, Banerjee BD, Parvez S, Gupta S, Raisuddin S, 2018. The protective effect of α-lipoic acid against bisphenol A-induced neurobehavioral toxicity. Neurochem. Int 118, 166–175. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Park YJ, 2019. Bisphenols and thyroid hormone. Endocrinol. Metab. (Seoul) 34, 340–348. PMC6935774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knuth DE, 1997. Seminumerical algorithms. The art of computer programming; 2. [Google Scholar]
- Kwon W, Kim JY, Suh S, In MK, 2012. Simultaneous determination of creatinine and uric acid in urine by liquid chromatography-tandem mass spectrometry with polarity switching electrospray ionization. Forensic Sci. Int 221, 57–64. [DOI] [PubMed] [Google Scholar]
- Lee S, Liu X, Takeda S, Choi K, 2013. Genotoxic potentials and related mechanisms of bisphenol A and other bisphenol compounds: a comparison study employing chicken DT40 cells. Chemosphere 93, 434–440. [DOI] [PubMed] [Google Scholar]
- Lehmler HJ, Liu B, Gadogbe M, Bao W, 2018. Exposure to bisphenol a, bisphenol f, and bisphenol s in U.S. adults and children: the national health and nutrition examination survey 2013–2014. ACS Omega 3, 6523–6532. PMC6028148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liang D, Ye D, Chang HH, Ziegler TR, Jones DP, Ebelt ST, 2021. Application of high-resolution metabolomics to identify biological pathways perturbed by traffic-related air pollution. Environ. Res 193, 110506. NIHMS1650541.PMC7855798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Lu Q, Ye J, Qin H, Long Y, Wang L, Ou H, 2018. Metabolic and proteomic mechanism of bisphenol A degradation by Bacillus thuringiensis. Sci. Total Environ 640–641, 714–725. [DOI] [PubMed] [Google Scholar]
- Li S, Park Y, Duraisingham S, Strobel FH, Khan N, Soltow QA, Jones DP, Pulendran B, 2013. Predicting network activity from high throughput metabolomics. PLoS Comput. Biol 9, e1003123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Moutinho JL, Golan R, Yu T, Ladva CN, Niedzwiecki M, Walker DI, Sarnat SE, Chang HH, Greenwald R, Jones DP, Russell AG, Sarnat JA, 2018. Use of high-resolution metabolomics for the identification of metabolic signals associated with traffic-related air pollution. Environ. Int 120, 145–154. NIHMS1004710.PMC6414207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D, Ladva CN, Golan R, Yu T, Walker DI, Sarnat SE, Greenwald R, Uppal K, Tran V, Jones DP, Russell AG, Sarnat JA, 2019. Perturbations of the arginine metabolome following exposures to traffic-related air pollution in a panel of commuters with and without asthma. Environ. Int 127, 503–513. NIHMS1526869. PMC6513706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JP, O’Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F, 2006. Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 114, 1476–1481. [DOI] [PubMed] [Google Scholar]
- Lin CY, Viant MR, Tjeerdema RS, 2006. Metabolomics: methodologies and applications in the environmental sciences. J. Pesticide Sci 31, 245–251. [Google Scholar]
- Liu KH, Nellis M, Uppal K, Ma C, Tran V, Liang Y, Walker DI, Jones DP, 2020. Reference standardization for quantification and harmonization of large-scale metabolomics. Anal. Chem 92, 8836–8844. NIHMS1660756.PMC7887762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Yang Q, Yu N, Cao Y, Wang X, Wang Z, Qiu WY, Ma C, 2021. Phenylalanine metabolism is dysregulated in human hippocampus with alzheimer’s disease related pathological changes. J. Alzheimers Dis 83, 609–622. [DOI] [PubMed] [Google Scholar]
- Machtinger R, Berman T, Adir M, Mansur A, Baccarelli AA, Racowsky C, Calafat AM, Hauser R, Nahum R, 2018. Urinary concentrations of phthalate metabolites, bisphenols and personal care product chemical biomarkers in pregnant women in Israel. Environ. Int 116, 319–325. NIHMS967196.PMC5983044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao L, Fang S, Zhao M, Liu W, Jin H, 2021. Effects of bisphenol a and bisphenol s exposure at low doses on the metabolome of adolescent male Sprague-Dawley rats. Chem. Res. Toxicol 34, 1578–1587. [DOI] [PubMed] [Google Scholar]
- Miao M, Yuan W, Zhu G, He X, Li DK, 2011. In utero exposure to bisphenol-A and its effect on birth weight of offspring. Reprod. Toxicol 32, 64–68. [DOI] [PubMed] [Google Scholar]
- Mok S, Jeong Y, Park M, Kim S, Lee I, Park J, Kim S, Choi K, Moon HB, 2021. Exposure to phthalates and bisphenol analogues among childbearing-aged women in Korea: Influencing factors and potential health risks. Chemosphere 264, 128425. [DOI] [PubMed] [Google Scholar]
- Newswire P, 2016. Bisphenol-A—A Global Market Overview. Retrieved October;25: 2020.
- NIEHS, 2020. Bisphenol A (BPA).
- O’Brien KM, Upson K, Buckley JP, 2017. Lipid and creatinine adjustment to evaluate health effects of environmental exposures. Curr. Environ. Health Rep 4, 44–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira Pereira EA, Labine LM, Kleywegt S, Jobst KJ, Simpson AJ, Simpson MJ, 2021. Metabolomics reveals that bisphenol pollutants impair protein synthesis-related pathways in daphnia magna. Metabolites 11. PMC8540811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelch KE, Wignall JA, Goldstone AE, Ross PK, Blain RB, Shapiro AJ, Holmgren SD, Hsieh JH, Svoboda D, Auerbach SS, Parham FM, Masten SA, Thayer KA, 2017. NTP research reports. NTP research report on biological activity of bisphenol a (BPA) structural analogues and functional alternatives: research report 4. Research Triangle Park (NC): National Toxicology Program; 2017. [PubMed] [Google Scholar]
- Pontiki E, Hadjipavlou-Litina D, 2018. Multi-target cinnamic acids for oxidative stress and inflammation: design, synthesis, biological evaluation and modeling studies. Molecules, 24 PMC6337588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Program, N.T., 2008. NTP-CERHR monograph on the potential human reproductive and developmental effects of bisphenol A. NIH Publication No. 08–5994. National Toxicology Program: Research Triangle Park, NC. [Google Scholar]
- Program, N.T., 2010. Bisphenol A (BPA).
- Reynolds RM, 2013. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis–2012 Curt Richter Award Winner. Psychoneuroendocrinology 38, 1–11. [DOI] [PubMed] [Google Scholar]
- Rochester JR, 2013. Bisphenol A and human health: a review of the literature. Reprod. Toxicol 42, 132–155. [DOI] [PubMed] [Google Scholar]
- Rochester JR, Bolden AL, 2015. Bisphenol S and F: a systematic review and comparison of the hormonal activity of bisphenol a substitutes. Environ. Health Perspect 123:643–50 [DOI] [PMC free article] [PubMed] [Google Scholar]; PMC4492270 analysis, or interpretation of data, nor were they involved in producing this work. J.R.R. and A.L.B. are employed by The Endocrine Disruption Exchange (TEDX), a U.S. 501(c)3 organization that occasionally provides consultation, legal assistance, or expert testimony on the topic of endocrine-disrupting chemicals. Neither the authors nor TEDX stand to gain or lose financially through publication of this article.
- Sainsbury A, Lin S, McNamara K, Slack K, Enriquez R, Lee NJ, Boey D, Smythe GA, Schwarzer C, Baldock P, Karl T, Lin EJ, Couzens M, Herzog H, 2007. Dynorphin knockout reduces fat mass and increases weight loss during fasting in mice. Mol. Endocrinol 21, 1722–1735. [DOI] [PubMed] [Google Scholar]
- Santoro A, Chianese R, Troisi J, Richards S, Nori SL, Fasano S, Guida M, Plunk E, Viggiano A, Pierantoni R, Meccariello R, 2019. Neuro-toxic and reproductive effects of BPA. Curr. Neuropharmacol 17, 1109–1132. PMC7057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer L, Barnard L, Baranowski ES, Gilligan LC, Taylor AE, Arlt W, Shackleton CHL, Storbeck KH, 2019. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol 194, 105439. PMC6857441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, Hollender J, 2014. Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environ. Sci. Technol 48, 2097–2098. [DOI] [PubMed] [Google Scholar]
- Sedlak TW, Snyder SH, 2004. Bilirubin benefits: cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 113, 1776–1782. [DOI] [PubMed] [Google Scholar]
- Simón-Manso Y, Lowenthal MS, Kilpatrick LE, Sampson ML, Telu KH, Rudnick PA, Mallard WG, Bearden DW, Schock TB, Tchekhovskoi DV, Blonder N, Yan X, Liang Y, Zheng Y, Wallace WE, Neta P, Phinney KW, Remaley AT, Stein SE, 2013. Metabolite profiling of a NIST Standard Reference Material for human plasma (SRM 1950): GC-MS, LC-MS, NMR, and clinical laboratory analyses, libraries, and web-based resources. Anal. Chem 85, 11725–11731. [DOI] [PubMed] [Google Scholar]
- Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TW, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ, Viant MR, 2007. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 3, 211–221. NIHMS504189.PMC3772505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susiarjo M, Xin F, Stefaniak M, Mesaros C, Simmons RA, Bartolomei MS, 2017. Bile acids and tryptophan metabolism are novel pathways involved in metabolic abnormalities in BPA-exposed pregnant mice and male offspring. Endocrinology 158, 2533–2542. PMC5551548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan Y, Barr DB, Ryan PB, Fedirko V, Sarnat JA, Gaskins AJ, Chang CJ, Tang Z, Marsit CJ, Corwin EJ, Jones DP, Dunlop AL, Liang D, 2022. High-resolution metabolomics of exposure to tobacco smoke during pregnancy and adverse birth outcomes in the Atlanta African American maternal-child cohort. Environ. Pollut 292, 118361. NIHMS1748945.PMC8616856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uppal K, Soltow QA, Strobel FH, Pittard WS, Gernert KM, Yu T, Jones DP, 2013. xMSanalyzer: automated pipeline for improved feature detection and downstream analysis of large-scale, non-targeted metabolomics data. BMC Bioinf. 14, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV, 2007. Human exposure to bisphenol A (BPA). Reprod. Toxicol 24, 139–177. [DOI] [PubMed] [Google Scholar]
- Veiga-Lopez A, Pennathur S, Kannan K, Patisaul HB, Dolinoy DC, Zeng L, Padmanabhan V, 2015. Impact of gestational bisphenol A on oxidative stress and free fatty acids: Human association and interspecies animal testing studies. Endocrinology 156:911–22 PMC4330308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lv H, Zhang A, Sun W, Liu L, Wang P, Wu Z, Zou D, Sun H, 2014. Metabolite profiling and pathway analysis of acute hepatitis rats by UPLC-ESI MS combined with pattern recognition methods. Liver Int. 34, 759–770. [DOI] [PubMed] [Google Scholar]
- Wang M, Rang O, Liu F, Xia W, Li Y, Zhang Y, Lu S, Xu S, 2018. A systematic review of metabolomics biomarkers for Bisphenol A exposure. Metabolomics 14, 45. [DOI] [PubMed] [Google Scholar]
- Wang W, Yu H, Qin H, Long Y, Ye J, Qu Y, 2020. Bisphenol A degradation pathway and associated metabolic networks in Escherichia coli harboring the gene encoding CYP450. J. Hazard. Mater 388, 121737. [DOI] [PubMed] [Google Scholar]
- Yoon C, Yoon D, Cho J, Kim S, Lee H, Choi H, Kim S, 2017. 1)H-NMR-based metabolomic studies of bisphenol A in zebrafish (Danio rerio. J. Environ. Sci. Health B 52, 282–289. [DOI] [PubMed] [Google Scholar]
- Yu T, Jones DP, 2014. Improving peak detection in high-resolution LC/MS metabolomics data using preexisting knowledge and machine learning approach. Bioinformatics 30, 2941–2948. PMC4184266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Park Y, Johnson JM, Jones DP, 2009. apLCMS–adaptive processing of high-resolution LC/MS data. Bioinformatics 25, 1930–1936. PMC2712336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue S, Yu J, Kong Y, Chen H, Mao M, Ji C, Shao S, Zhu J, Gu J, Zhao M, 2019. Metabolomic modulations of HepG2 cells exposed to bisphenol analogues. Environ. Int 129, 59–67. [DOI] [PubMed] [Google Scholar]
- Zeng J, Kuang H, Hu C, Shi X, Yan M, Xu L, Wang L, Xu C, Xu G, 2013. Effect of bisphenol A on rat metabolic profiling studied by using capillary electrophoresis time-of-flight mass spectrometry. Environ. Sci. Technol 47, 7457–7465. [DOI] [PubMed] [Google Scholar]
- Zhang X, Barr DB, Dunlop AL, Panuwet P, Sarnat JA, Lee GE, Tan Y, Corwin EJ, Jones DP, Ryan PB, Liang D, 2021. Assessment of metabolic perturbations associated with exposure to phthalates among pregnant African American women. Sci. Total Environ 151689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Kramer JP, Calafat AM, Ye X, 2014. Automated on-line column-switching high performance liquid chromatography isotope dilution tandem mass spectrometry method for the quantification of bisphenol A, bisphenol F, bisphenol S, and 11 other phenols in urine. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci 944, 152–156. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.


