Summary
Background
Ensuring menstrual cup safety is paramount, yet a menstrual cup safety assessment scheme is lacking. This paper presents a quadripartite scheme, showing how it can be applied.
Methods
The Tampax Menstrual Cup was evaluated in the safety assessment scheme: (1) Biocompatibility and chemical safety of cup constituents. Extractables were obtained under different use condition; exposure-based risk assessments (EBRA) were conducted for extractables exceeding thresholds of toxicological concern. (2) Physical impact to vaginal mucosa. After physical evaluations, the Tampax Cup and another cup were assessed in a randomised double-blinded, two-product, two-period cross-over clinical trial (65 women, mean age 34.2 years). (3) Impact to vaginal microbiota (in vitro mixed microflora assay and evaluation of vaginal swabs). (4) In vitro growth of Staphylococcus aureus and toxic shock syndrome toxin-1 (TSST-1) production.
Findings
Biocompatibility assessments and EBRA of cup constituents showed no safety concerns. In the randomised clinical trial, all potentially product-related adverse effects were mild, vaginal exams were unremarkable, no clinically relevant pH changes occurred, post-void residual urine volume with and without cup were similar, and self-reported measures of comfort along with reports of burning, itching and stinging between cups were comparable. Cup use had no effect on microbial growth in vitro or in the 62 subjects who completed the trial or on in vitro TSST-1 production.
Interpretation
The quadripartite safety assessment scheme allows evaluation of menstrual cup safety. The Tampax Cup is safe and well-tolerated upon intended use. As with all feminine hygiene products, post-market safety surveillance confirmed this conclusion.
Funding
By Procter & Gamble.
Keywords: Feminine hygiene products, Biocompatibility, Extractables, Vaginal microbiome/flora, Vaginal tolerability, Toxic shock syndrome
Research in context.
Evidence before this study
Menstrual cups are receptacles placed in the vagina to collect menstrual flow. When made of durable materials, they are amenable to repeated use. While menstrual cups have been available for many decades, their use is becoming increasingly popular, motivated by the woman's desire to use more environmentally friendly and/or reusable menstrual products. Ensuring the safety of menstrual cups is paramount. Earlier studies addressing specific endpoints generally indicated no hazard concerns upon menstrual cup use. Nonetheless, a menstrual cup safety assessment scheme is currently lacking.
Added value of this study
The authors of this paper have developed a quadripartite safety assessment scheme for menstrual cups, which is described in detail herein. Further, the safety assessment scheme is applied for the evaluation of the Tampax Menstrual Cup. All of the evaluations confirm the safety of the Tampax Cup when used as intended.
Implications of the available evidence
Application of the quadripartite safety assessment scheme ensures the safety of the menstrual cup. The comprehensive database shows that the Tampax Cup is safe and well-tolerated upon intended use. This conclusion has been confirmed during post-market safety surveillance.
Introduction
Menstrual cups are receptacles placed in the vagina to collect menstrual flow.1 When made of durable materials, they are amenable to repeated use. While menstrual cups have been available for many decades,2,3 their use is becoming increasingly popular and is expected to continue to rise over the next couple of years, motivated in part by the women's desire to use more environmentally friendly and/or reusable menstrual products.4,5 This potential increase in use highlights the importance of outlining a framework for intravaginal device safety assessment that is consistent with the expectations of regulators and augmented with current state-of-the-art scientific approaches.
On account of their intravaginal use, the United States Food and Drug Administration (U.S. FDA) Center for Devices and Radiological Health categorises menstrual cups as Class II medical devices,1 recently exempted from premarket notification requirements.6 Class II medical devices (i.e. here: gynaecological therapeutic devices) include permanent use, surface devices with mucosal membrane exposure, like the menstrual cup. The U.S. FDA has issued Guidance for Industry and FDA Staff on the Use of the International Standardization Organisation (ISO) 10993 Standards Series on the Biological Evaluation of Medical Devices.7 The ISO 10993 standards series themselves do not prescribe any specific testing but are employed world-wide as a framework to establish regulatory compliance needed for initiating clinical investigations of the particular medical device, or for obtaining global market clearance, as applicable.8, 9, 10 In addition, the U.S. FDA offers guidance to industry for other intravaginal devices (i.e. tampons) with clear requirements to address preclinical toxicology and microbiology11; however, such guidance does not exist for devices that are exempt from premarket notification.
Over the past several decades, the safety of menstrual cups has been studied to varying degrees. In 1962, Karnaky conducted a clinical assessment of a pliable rubber menstrual cup, which included evaluation of vaginal pH, examination of the vaginal walls, collection of vaginal smears and gram stains as well as culturing of vaginal secretions.3 No meaningful changes were noted, confirming early preliminary work done by Liswood in 1959 that concluded that menstrual cups were a safe and sanitary option for women.2 In 2011, North and Oldham expanded the safety approach reporting both preclinical and clinical data as well as 10 years-worth of post-marketing safety surveillance data for a commercially available menstrual cup.12 Preclinical studies focused on U.S. FDA guideline-required13 in vitro and in vivo studies (no irritation, mutagenicity or toxicity reported),12 and clinical data included gynaecological health evaluation via urinalysis, pelvic examination, vaginal pH and microscopic wet mount all of which showed no adverse effects upon cup use.12 Van Eijk and colleagues conducted a systematic review and meta-analysis which focused on menstrual blood leakage but included safety outcome measures such as adverse events, vaginal abrasions and effects on vaginal microflora and on the reproductive, digestive or urinary tracts, showing that menstrual cups are a safe option for women, but also that “good quality studies in this field are needed”.14
These studies lay the foundation for the safety assessment of menstrual cups, yet a comprehensive assessment paradigm that covers all potentially relevant aspects of the safety assessment of intravaginal devices has not yet been published either in the scientific literature or in regulatory guidance. To address this shortage, Procter & Gamble developed and present here a safety assessment scheme for intravaginal devices that covers all relevant aspects of safety assessment. The scheme includes four components, i.e. the assessment of (1) the biocompatibility and chemical safety of the cup constituents; (2) the physical impact to the vaginal mucosa; (3) the impact to vaginal microbiota; and (4) the growth of Staphylococcus aureus and risk for toxic shock syndrome (Fig. 1).
Fig. 1.
The quadripartite safety assessment scheme developed by Procter & Gamble to establish the safety of feminine hygiene products involving intravaginal usage.
Details on the rationale to develop the four components, as well as their structure, are presented in the methods section, with information on the regulatory background as well as methodological details provided as Supplementary Information. In addition, this manuscript shows how the safety assessment scheme was applied to assess the safety of the recently developed Tampax Menstrual Cup. This includes analytical data on eluting chemicals to address biocompatibility endpoints, in vitro data to evaluate chemical irritation, in vitro evaluation of cup use on a consortium of vaginal relevant microorganisms and the risk for toxic shock syndrome, as well as in-use clinical data on cup tolerability and the impact of cup use on the vaginal microbiome (investigated by culture-independent molecular assessment).
Methods
Ethics
This clinical trial was reviewed and approved (2 August 2018) by an independent institutional review body (IntegReview IRB; now Advarra, Columbia, MD, USA (www.advarra.com [accessed 25 August 2022]); IRB numbers IRB00008463, IRB00003657, IRB00004920, IRB00001035, IRB00006075) and conducted in compliance with the applicable Federal Regulations and the International Conference on Harmonization, Guidelines for Good Clinical Practice, E6. Further, the clinical trial was registered on www.clinicaltrials.gov (registration number NCT05411796). All subjects signed an informed consent document prior to enrolment. Subject enrolment began on 7 August 2018 and the last subject completed the study on 14 November 2018.
The biocompatibility and chemical safety of the cup constituents
Concept of the biocompatibility assessment
The Tampax Menstrual Cup is comprised of one single material, i.e. the liquid silicone rubber QP1-40 (supplied by Dow Corning at the time the present studies were performed).15, 16, 17 The Tampax Cup has a recommended use period of one year, and its intended use (as indicated on the product insert) includes boiling the cup for five to seven min prior to the initial use and at the end of each menstrual cycle, prior to storing the cup. Additionally, intermittent cleaning of the cup every 12 h using soap and water, or wipes if water is unavailable, is recommended during the menstrual cycle. Most manufacturers instruct users to empty the cup every four to 12 h depending on menstrual flow. To clean the cup with wipes, the commercial packaging of the Tampax Cup includes single wipe sachets of the Always® Fresh & Clean Feminine Wipes in some regions. These feminine wipes contain a soft, nonwoven, cloth like substrate made from regenerated cellulose and pulp and a water based (>97%) cleansing lotion containing emulsifying agents, skin conditioners, pH buffers and preservative.
Generally, menstrual cups can be in contact with mucosal tissue for greater than 30 days cumulatively, over a women's menstrual lifetime (five to seven days/cycle, thirteen cycles/year for approximately 40 years). Due to this cumulative exposure to a mucosal membrane, the biocompatibility and chemical safety of the eluting cup constituents needs to be established. The rationale described below was applied to assess the biocompatibility of the Tampax Cup to comply with the effective regulatory provisions while at the same time avoiding new animal testing.
The supplier of the silicone rubber conducted multiple biocompatibility tests on the raw material to address the various relevant endpoints to achieve (the highest) Class VI medical grade classification following the U.S. Pharmacopeia criteria for the classification of plastics18 (see Supplementary Information SI-1 for details on the regulatory background). These proprietary biocompatibility data were made available to Procter & Gamble to assess the biocompatibility of the Tampax Cup. However, since the biocompatibility data had been collected for the raw material (silicone), an informative approach was required to demonstrate their relevance for the article in its final finished form (i.e. the Tampax Cup). This was achieved by chemical analysis of both the raw material and the cup.
Generally, medical devices made from liquid silicone rubber are medical plastic materials. Regulatory guidance, such as the U.S. FDA7 guidance on use of ISO 10993-1, requests that the safety assessment of medical devices should be performed on the extractables since it is the leachable constituents that can come into contact with the body tissues (see Supplementary Information SI-1 for relevant provisions).7,18 To obtain extractables, the raw material (silicone) and the final, finished article (Tampax Cup) were subjected to different conditions of use. Since true leachable conditions (i.e. use of actual menses) or simulated use (i.e. artificial menses) were less-practical options, the extractable conditions used included exaggerated and accelerated conditions (ethanol/water at various times and temperatures) to gain this comprehensive comparison. Further, extractables were gained from a cup after wiping with Always Feminine Wipes.
The resulting extracts were analysed by ultra-high performance liquid chromatography (UHPLC) coupled to a charged aerosol detector (CAD) and high-resolution mass spectrometry to determine potential differences between the raw material and the Tampax Cup. A lack of ‘unique’ or ‘common but significantly higher’ chemical entities in the extraction fluid of the Tampax Cup as compared to the raw material, provided sufficient evidence that the process of manufacturing the cup had not changed the chemical profile of the raw material. In that case, the biocompatibility data available for the raw material, together with its long-lasting history of safe use, could be used for the risk assessment of the cup under the selected extraction conditions.19
Preparation of extracts
Silicone QP 1-40 LSR was obtained from the supplier, i.e. Dow Corning (USA) at the time the studies were performed. The final, finished Tampax Menstrual Cup and the Always Feminine Wipes were used as produced by Procter & Gamble (USA), i.e. as they are commercially available (this applies to all four components of the safety assessment scheme).
The extracts and chromatographic profiles were prepared by Procter & Gamble Corporate Functions Analytical (Mason OH, USA). Replicate (n = 3) extractables were obtained from both the raw material (silicone) and from the final, finished article (Tampax Cup) following a number of different conditions meant to be consistent with ISO 10993 standards and to represent a range of potential scenarios including:
-
(1)
Extraction under physiologically relevant, yet exaggerated use conditions (37 °C for 72 h),
-
(2)
Extraction under exaggerated conditions (50 °C for 2 h), and
-
(3)
Extraction under accelerated conditions (121 °C for 1 h).
Further, replicate (n = 5) extractables were gained from the Tampax Cup under conditions reflecting its recommended use, i.e.:
-
(4)
After 13 consecutive boiling cycles (per cycle: 5-min boiling, removal from water and cooling for 20 min; chromatographic profiling after seven and 13 boiling cycles; each: n = 5), and
-
(5)
After one boiling cycle followed by wiping the inside and outside of the cup with two Always Feminine Wipes (n = 5).
Scenario 4 considers that the Tampax Cup has a recommended use period of one year and that its intended use includes boiling the cup prior to the initial use and at the end of each menstrual cycle. Assuming a 28-day menstrual cycle, the Tampax Cup was submitted to 13 boiling sessions. Scenario 5 covers the intermittent cleaning of the Tampax Cup with Always Feminine Wipes to assess whether this yielded any extractables posing a safety concern.
All extractions were performed in 50:50 water/ethanol; see Supplementary Information SI-2 for details on the extraction procedures.
Generation of chromatographic profiles of the extracts
Chromatographic profiles of the extracts were generated on the platform previously described by Sica and colleagues.20 In brief, the extractables were separated using UHPLC followed by UV detection, CAD and high-resolution mass spectrometry. The Thermo ACCELA™ 1250 pump was used as UHPLC main pump and HPLC make-up pump. Further, the Thermo VEO™ RS CAD and the Thermo Orbitrap Elite™ mass spectrometer were used (all equipment: Thermo Fisher Scientific Inc., USA).
Five CAD reference standards (tryptophan, the tripeptide Val-Tyr-Val, kaempferol, reserpine and terfenadine; Sigma Aldrich, USA) were used for quantification by CAD, each at five concentrations ranging from 500 to 7500 ng/mL to generate the average response factor for quantification. All five standards passed the set criteria of a percent relative error (%RE) ≤ 25% and R2 ≥ 0.95, which passes the guidelines in the validation plan. The quality control standards passed with average %RE of −8.4. 8.4, −7.7, −4.2, −7.0, and −9.2 (%RE ≤ 25) for all five standards over an average of seven replicate quality control injections.
Evaluation of chromatographic profiles applying the threshold of toxicological concern (TTC) as analytical limit
For CAD peak analysis, the data for each sample were processed separately, the areas under each peak were tabulated and the triplicate samples were averaged.
The respective chromatograms for the raw material (silicone) and the final, finished article (Tampax Cup) were compared to determine:
-
•
If the chromatogram of the cup extract exhibited any ‘unique’ peaks, which were not present in that of the silicone, or
-
•
If common peaks were significantly higher in the chromatogram of the cup extract than in that of the silicone (‘common, but significantly higher’ peaks), and
-
•
If the chromatograms of the cup extracts under the two conditions of recommended use (Scenarios 4 and 5 in Preparation of extracts) exhibited any ‘unique’ or ‘common, but higher’ peaks as compared to the unused final, finished cup.
If common peaks were significantly higher for the cup than for the silicone, this indicated higher concentrations of the respective substance in the extract of the cup than in that of the raw material. Significance was defined here if the average area of a specific peak for the sample (Tampax Cup) minus the standard deviation of that peak for the sample was greater than the average area of that peak for the control (silicone QP1-40) plus the standard deviation of that peak for the control.
A threshold of toxicological concern (TTC)21 of 1.5 μg/day (0.025 μg/kg body weight (bw)/day for a 60-kg adult) was applied to calculate the analytical screening limit to determine whether the area under a CAD peak indicated that the concentration of the corresponding substance required further analytical work to identify and quantify the eluting chemical. This TTC value is consistent with U.S. FDA guidance7,22; it assumes that the corresponding substance could be genotoxic, and it “corresponds to a theoretical 10−5 excess lifetime risk of cancer”.22 Please see Supplementary Information SI-1 for scientific background to the TTC concept and details on its regulatory applicability.
Together with TTC value of 1.5 μg/day (0.025 μg/kg bw/day), the calculation of the analytical limit for the menstrual cup considered current habits and practices data and default exposure assumptions (i.e. 100% transfer, 100% absorption, daily use):
| X μg/cup = 0.025 μg/kg bw/day x 50 kg bw/2 cups day |
| X = 0.63 μg/cup |
Hence, an analytical screening level of 0.63 μg/cup was established for the evaluation of the chromatographic profiles. In all chromatograms, peaks above this limit were quantified and identified by high-resolution mass spectrometry.
Risk assessment of final, finished cup and of cup after 13 boiling cycles
As a follow up to the comparative assessment of the chromatographic profiles of the raw material and the Tampax Cup, risk assessment was performed for all substances corresponding to peaks above the analytical screening limit.
As presented in The biocompatibility and chemical safety of the cup constituents and Exposure-based risk assessment of the chemicals eluting from the menstrual cup, there were no ‘unique’ or ‘common but significantly higher’ chemical entities in the extraction fluid of the final, finished Tampax Cup for both the physiologically relevant yet exaggerated and accelerated conditions, or for the cup after 13 boiling cycles. Therefore, the biocompatibility data available for the raw material, together with its long-lasting history of safe use, could be used for the risk assessment of the cup under these extraction conditions.19 Accordingly, Exposure-based risk assessment of the chemicals eluting from the menstrual cup describes how read-across from biocompatibility data available for the raw material was performed, a scenario that would not have been possible if chromatographic differences between the raw material and the cup were noted.
The proprietary biocompatibility data available for the liquid silicone rubber included data on cytotoxicity, intracutaneous activity, acute systemic toxicity, and effects upon implantation. For the endpoints of irritation and sensitisation, the QP 1-40 data from the supplier were based on read-across from silicone QP 1-20 and QP 1-70, i.e. two liquid silicone test articles of lower and higher durometer.
Risk assessment of extractables after boiling cup once followed by wiping with two wipes
For all extractables obtained after boiling the cup once followed by wiping with two Always Feminine Wipes that exceeded the analytical screening limit of 0.63 μg/cup, chemical characterisation and risk assessment were performed further considering the database available for the extractable constituents of the raw material.
Given the difference in dose metrics (μg/kg body weight (bw)/day and μg/cm2), the assessment approach was different for systemic endpoints and local effects. For the endpoints of cytotoxicity, acute systemic toxicity and local effects after implantation, a weight-of-evidence approach was applied to evaluate the corresponding data for the extractable constituents of the raw material. Further, cytotoxicity was addressed in the in vitro EpiVaginal™ assay (Testing for vaginal irritation and cytotoxicity potential).
For the endpoints of genotoxicity, subchronic/chronic repeated dose toxicity, developmental and reproductive toxicity, chronic toxicity and carcinogenicity, exposure-based risk assessments using the TTC concept and the multi-step risk assessment process implemented originally by the U.S. National Research Council23,24 and later by the U.S. Environmental Protection Agency25 and others, were performed. These are applicable for a broad spectrum of systemic endpoints.26,27
Exposures to chemicals eluting from the Tampax Cup after boiling and wiping with Always Feminine Wipes were estimated using the following equations:
-
•
Systemic exposure (μg/kg bw/day): M x F x E x T x Ab/bw (see Table 1 for parameters and default values)
-
•
Dermal exposure (μg/cm2/day): M x F x E x T x Ab/SA (see Table 1 for parameters and default values)
Table 1.
Exposure estimates: parameters and default values.
| Abbreviation | Parameter | Default valuea |
|---|---|---|
| M | Mass per cup | Determined by chemical analysis |
| F | Frequency of use | 2 cups/day (1 cup for 12 h, 2 cups/day) |
| E | Exposure duration | 100% (default to everyday use although exposure is only 5–7 days/month during menstruation) |
| T | Transfer to mucosal tissue | 100% |
| Ab | Mucosal absorption | 100% |
| Bw | Body weight | 50 kg |
| SA | Surface area | 70 cm2 |
Default values can be refined with chemical specific and/or analytical data.
The parameters used to estimate exposure are based on habits and practices (Concept of the biocompatibility assessment), labelling instructions and default conservative exposure values. Systemic exposures are based on body weight and expressed as μg of eluting chemical per kilogramme of body weight, whereas local effects (e.g. sensitisation) are expressed as μg of eluting chemical per cm2 surface area. Use of the default values likely leads to a conservative estimate of exposure by assuming every day, continuous cup use (exposure is likely only 5–7 days per month during menstruation) and 100% dermal absorption of all eluting chemicals. Further, unless chemical specific analytical data are available, no refinements are made to account for a decrease in eluting materials with repeated cup use, a likely phenomenon with a reusable device.
The exposure estimations were applied to determine if the level of exposure to the respective extractable compared to the appropriate reference dose or risk value provided a sufficient margin of safety for genotoxicity, subchronic/chronic repeated dose toxicity, developmental and reproductive toxicity, chronic toxicity and carcinogenicity to support its presence in the extraction fluid.
If chemical specific reference doses or risk values were unavailable, the appropriate TTC value as outlined by Kroes and colleagues21 was used for the comparison. This approach considered databases of existing toxicological data on chemicals to establish a human exposure threshold value below which there is very low probability of an appreciable risk to human health.21,28 (Thereby, the TTC approach was used in two different ways to assess the chemical safety of the eluting cup constituents, i.e. first to set the analytical screening limit for the evaluation of the CAD peaks (Evaluation of chromatographic profiles applying the threshold of toxicological concern (TTC) as analytical limit) and second as a pragmatic risk assessment tool.) Specifically, evaluation of each data-deficient chemical included assignment into one of the three Cramer Classes,29 i.e.:
-
•
Class I – substances with simple chemical structures and for which efficient modes of metabolism exist, suggesting a low order of toxicity; TTC: 30 μg/kg bw/day × 60 kg = 1800 μg/day
-
•
Class II – substances which possess structures that are less innocuous than Class I substances, but do not contain structural features suggestive of toxicity like those in Class III; TTC: 9 μg/kg bw/day × 60 kg = 540 μg/day
-
•
Class III – substances with chemical structures that permit no strong initial presumption of safety or may even suggest significant toxicity or have reactive functional groups; TTC: 1.5 μg/kg bw/day × 60 kg = 90 μg/day
Although the TTC has been proposed for sensitisation,30, 31, 32, 33 no harmonised approach has been accepted globally by regulators and the TTC concept is currently not applicable for local endpoints.26,27,34 Therefore, data for eluting chemicals were reviewed to determine the potential for sensitisation. If sensitisation data were not available or were inconclusive or suggested the potential for sensitisation, the chemical was evaluated for structural alerts, and the estimated exposure (in μg/cm2) was compared to an appropriate reference value. This evaluation further considered the available data for the raw material that had been derived by read-across from similar silicones and the available database for the respective extractable.
Testing for vaginal irritation and cytotoxicity potential
In vitro assays using the EpiVaginal™ VEC-100 three-dimensional-tissue model (MatTek Corporation, USA; see https://www.mattek.com/products/epivaginal/ for technical specifications [accessed 27 August 2022]) were performed at the Institute for In Vitro Sciences, Inc., Gaithersburg MD (USA) to evaluate the vaginal irritation potential (as well as cytotoxicity) of the Tampax Cup and the accompanying Always Feminine Wipes. The in vitro EpiVaginal™ assay evaluates if test material exposure affects the viability of human vaginal ectocervical (VEC) cells.35,36 The measure of cell viability used is the reduction of the tetrazolium salt MTT to a blue formazan precipitate by the viable, metabolically competent cells.37
The cup was tested after solvent extraction using (1) sesame oil and (2) 0.9% saline. To prepare the solvent extracts, the test articles were placed into a sterile 125-mL container and 5 mL solvent was added per gram of test article. These extracts were then incubated at 37 ± 1 °C with continuous agitation for 24 ± 2 h. Following the extraction period, the extracts were allowed to cool to room temperature, vortexed for 30 s, and the supernatant containing the extractables transferred to sterile dry containers.
The wipe was tested using (1) an approx. 8-mm punch and (2) the liquid expressed from the wipe. For the latter, a freshly unwrapped wipe (Always Feminine Wipes are commercially available individually wrapped) was placed into a 30-mL syringe, the plunger was depressed, and the liquid collected into a sterile 15-mL conical tube.
Triton®-X-100 (1%; Fisher Scientific, USA) was used as positive control. Sesame oil alone and 0.9% saline alone served as solvent controls, and sterile deionised water (Quality Biological, Inc., USA) as exposure time control.
All test articles and the controls were tested in duplicate (applying 100 μL each or the 8-mm punch) at four exposure times (4, 8, 16, and 24 h) with the exception of the positive control, which was tested at 0.5, 1, and 2 h. All treated tissues were incubated for the appropriate exposure times under standard culture conditions (37 ± 1 °C; 5 ± 1% CO2 in air).
Upon completion of the respective exposure times, reduction of MTT (Sigma, USA) was measured by determining the absorbance (optical density (OD)) at 550 nm (OD550) with a Molecular Devices Vmax microplate reader (Molecular Devices LLC, San Jose, California), correcting for the OD550 value of a concurrent blank control.
For the test article in sesame oil, the OD550 value was additionally corrected for its potential to directly reduce MTT in the absence of viable cells (see Supplementary Information SI-3 for details of the MTT test, of the preliminary experiments addressing direct MTT reduction and of the OD550 calculations).
The exposure time response curves were graphed, and the exposure time necessary to decrease the tissue viability to 50% (ET50)35 was calculated. ET50 values > 24 h were assessed as indicating absence of vaginal irritation potential. The findings from the EpiVaginal™ assay supplemented the findings from the visual vaginal examination during the clinical trial, which also scored for erythema, i.e. irritation potential (Randomised clinical trial).
The physical impact to the vaginal mucosa
Visual inspection
The Tampax Menstrual Cup was evaluated visually for material conformity as well as sharp edges. Further, its size, dimensions, and compression forces were compared to two other commercially available menstrual cups as well as to the average size of the human vagina.38,39 These preliminary assessments served to provide a first indication that no detrimental physical effects were to be expected from use of the cup. The Supplementary Information SI-4 provides details on the Cup Compression Test Method.
Randomised clinical trial
A randomised, double-blinded, two-product, two-period cross-over clinical trial was conducted at Synexus, Cincinnati, OH (USA) to evaluate the tolerability of the Tampax Cup as compared to another commercially available menstrual cup (in the following: the Other Cup); see Supplementary Information SI-5 for inclusion and exclusion criteria for the randomised clinical trial.
The study design included use of the Tampax Cup during one of two menstrual cycles and use of the Other Cup during the other menstrual cycle. Subjects were randomly assigned to one of the two treatment sequences for the two-period crossover study. The sequence of use assigned to each woman was not disclosed to either the women or the professional staff (i.e. subjects and staff were blinded), and products were not branded. However, products were not identical in appearance.
This clinical trial served to assess vaginal tolerability of the Tampax Cup based on gynaecological measures and to identify if any adverse events evolved during cup use. Assessments included visual vaginal examinations (erythema, abrasions, ulcerations, lacerations, vaginal discharge), vaginal pH measurements, and measurement of the post-void residual urine volume (PVR) to determine if the physical presence of the menstrual cup in the vagina impeded bladder emptying as a risk factor for urinary tract infections. Further, vaginal swabs were taken for evaluations of the vaginal microbiome and the experience of cup use was collected via a questionnaire to better understand product-related sensations and comfort.
Prior to cup use, the impact of the cups on PVRs was assessed with a subset of the subjects (n = 22) using a three-dimensional bladder scanner (Bladder Scan BVI 3000, Verathon Inc., Bothell, Washington, USA). A gynaecologist conducted the vaginal examination. Urine void volumes and PVRs were measured before cup insertion (baseline) and while using the Tampax Cup or the Other Cup, in a randomised cross-over. Cups were typically worn approximately 2 h, non-menstrually, for these examinations.
Based on guidance from a consulting urogynaecologist, normal PVR was defined as:
-
•
Either a PVR less than 1/3 of the total volume (voided volume + PVR = total volume)
-
•
Or a PVR less than 100 mL
A change in PVR was considered possibly clinically meaningful if PVR was normal at baseline and abnormal following cup use.
Subjects were given one of two menstrual cups to use during their next menstrual cycle. The subjects completed a daily diary entry for each cup wearing and a monthly questionnaire to inform on product-related sensations and comfort. Subjects reported the time point of insertion and removal of the cup allowing calculation of overall wear times. Evaluations distinguished between wear times that were consistent with product labelling instructions (less than 12 h) versus wear times of 12 h or more.
Subjects returned to the site within 72 h of their last cup wear for an assessment of vaginal health conducted by the site medical director physician using a lighted speculum. Erythema was graded at six different sites of the vagina (i.e. labia majora, introitus, lower, middle, and upper vaginal wall, including fornix, and cervix) on a scale of 0–4. Abrasion, lacerations, and ulcerations were graded on a 0–2 scale at each of the six sites. Vaginal pH was measured using pH paper, and vaginal discharge was assessed as normal or abnormal. If discharge was abnormal, colour and consistency were evaluated. Vaginal swabs were obtained for microbiota assessment (Clinical trial – assessment of the vaginal microbiota). This procedure was repeated for a total of two consecutive menstrual cycles. Subjects were asked about health and compliance at each visit. Throughout the study, adverse events reported by the subject, observed by the staff, or recorded by any laboratory tests were assessed as a further measure of safety and tolerability.
Statistics
Base size for the clinical trial was based on expert advice from Synexus to protect for an unknown drop-out rate for a menstrual device unfamiliar to most subjects and on experience from previous studies testing other intravaginal devices (i.e. tampons) which showed that a sample size of 90 women produced a standard error of 2% for incidence rates around 5%. This variation was deemed sufficient for the endpoints of interest. Prior to statistical analysis, all data were checked for accuracy, completeness and compliance to protocol. Statistical analyses for all findings from the clinical trial were based on data from the “intent to treat” population and were performed using PC SAS Release 9.4 (https://support.sas.com/software/94/ [accessed 27 August 2022]) providing summary descriptive statistics for all parameters. Any data reported as ‘unable to evaluate’ were treated as missing and excluded from the analysis. All findings were tabulated for each visit/treatment, including frequencies for normal vs. abnormal classifications, frequencies and characteristics of vaginal discharge, frequencies of erythema scores, and frequencies for abrasion, ulceration, and lacerations. (Depending on the distributions, categories may have been collapsed prior to statistical analysis.) Wear times, pH, and diary rating data were evaluated with a linear mixed model (LMM) for repeated measures with fixed cup and random subject effects. A similar model with a binary distribution was used to evaluate the binary diary data (reports of burning, itching, stinging and discomfort). Post-use ratings with three categorical responses (No, Slight, Yes) were analysed with a Cochran–Armitage trend test. While there were no formal hypotheses in the study, some comparisons of the cups were conducted for learning purposes. PVR data were summarised for each subject as absolute values and as change from baseline.
The impact to vaginal microbiota
The Tampax Menstrual Cup was designed to minimise microbial risk through purposeful material selection, appropriate design, quality manufacturing procedures, and use instructions (see Supplementary Information SI-6 for details).
In vitro mixed microflora assay
An in vitro mixed microflora assay was conducted by Advanced Testing Laboratory, Inc., Cincinnati OH (USA) to determine if 48-h exposure to the Tampax Cup had a bactericidal or bacteriostatic effect on a consortium of six microorganisms. This assay was originally developed in collaboration between Procter & Gamble and Microbiologists Specialists, Inc. (Houston TX, USA), since standardised test methods addressing the potential impact of intravaginal feminine hygiene articles on the vaginal microbiome are unavailable. The six microorganisms included in the in vitro mixed microflora assay (Lactobacillus gasseri, Gardnerella vaginalis, Prevotella bivia, Escherichia coli, S. aureus, Candida albicans) were selected to simulate the heterogenous nature of the healthy vaginal microbiome while also including organisms that may give rise to infection or disease (see, e.g. Larsen and Galask).40
Both the final, finished Tampax Cup and the Other Cup were assessed to determine their potential impact on the vaginal microflora. Generally, the in vitro mixed microflora assay includes a preparatory stage, i.e. the preparation of freezer stocks and confirmatory identification of each organism, followed by three experimental stages, i.e. (I) the preparation of the stock inoculum by plating, incubation of individual organisms on specified media, and measurement of cell densities; (II) co-inoculation of each control and test product followed by incubation under anaerobic conditions; (III) plating of organisms on selective media following sample collection from control and test products. Below, these stages are briefly described. A detailed test protocol of the in vitro mixed microflora assay is provided as Supplementary Information SI-7.
Pre-stage
Preparation of freezer stock and confirmatory identification of each organism.
Lyophilised ATCC stocks of the six microorganisms, representative of the vaginal microbiome or known vaginal pathogens (L. gasseri, G. vaginalis, P. bivia, E. coli, S. aureus, C. albicans), were reconstituted and incubated in initial broth culture at 35 ± 2 °C for 24 or 48 ± 2 h in aerobic or anaerobic atmosphere, as appropriate (Table 2). At the end of incubation, each organism was dispensed in 20% v/v sterile glycerol and stored at −70 °C. Confirmatory identification (matrix-assisted laser desorption/ionisation-time of flight, serial number 269944.00291, Bruker, USA) was performed on freezer stock of each microorganism.
Table 2.
Specifications by organism within the vaginally defined consortium.
| Organism | ATCC strain | Broth | Atmosphere | Incubation |
|---|---|---|---|---|
| Lactobacillus gasseri | 9857 | De Man, Rogosa and Sharpe Media | Anaerobic | 35 ± 2 °C, 48 ± 2 h |
| Gardnerella vaginalis | 14018 | Microbiological Tryptone Glucose Extract | Anaerobic | 35 ± 2 °C, 24 ± 2 h |
| Prevotella bivia | 29303 | Reinforced Clostridial Broth | Anaerobic | 35 ± 2 °C, 24 ± 2 h |
| Escherichia coli | 53498 | Tryptic Soy Broth | Aerobic | 35 ± 2 °C, 24 ± 2 h |
| Staphylococcus aureus | 33589 | Tryptic Soy Broth | Aerobic | 35 ± 2 °C, 24 ± 2 h |
| Candida albicans | 62376 | Sabouraud Dextrose Broth | Aerobic | 35 ± 2 °C, 24 ± 2 h |
ATCC: American Type Culture Collection.
See Supplementary Information SI-7 for details on suppliers of broths, numbers of plates and volume of broth required for each organism. For example, L. gasseri requires 10 plates and a larger volume where other organisms require 1–2 plates and 9 mL.
Experimental stage I
Preparation of stock inoculum by plating, incubation of the individual organisms on specified media, and measurement of cell densities.
Twenty-four to 48 h prior to the start of the experiment, a frozen stock vial of each microorganism was thawed, dispensed into tubes containing broth media, and vortexed before plating onto selective agar plates (Table 2). Incubation times and specified atmospheric requirements for each organism are noted in Table 2. Each organism was individually collected by swabbing each plate with a cotton-tipped swab and transferred into tubes containing Genital Tract Secretion Medium (VDM-PS, used as a dilution and growth medium in this experiment). An optical density measurement was performed for each organism by spectrophotometric evaluation at a wavelength of 425 nm (OD425nm; PerkinElmer UV/VIS Spectrometer LAMBDA 35, PerkinElmer, USA). Based upon the measured OD425nm, each microorganism preparation was diluted with VDM-PS to achieve the cell density required for the assay (Table 3).
Table 3.
Dilution requirements and starting inoculum concentration in flask.
| Organism | %T | Dilution needed after %T dilution | In flask (CFU/mL) | Volume added per flask |
|---|---|---|---|---|
| Lactobacillus gasseri | ∼0.1 | No Dilution Needed | 108 | 20 mL |
| Gardnerella vaginalis | 41–43 | Dilute 1:10 in VDM-PS | 105 | 2 mL |
| Prevotella bivia | 39–41 | Dilute 1:10 in VDM-PS | 105 | 2 mL |
| Escherichia coli | 34–36 | Dilute 1:10 in VDM-PS | 105 | 2 mL |
| Staphylococcus aureus | 15–17 | Dilute 1:100 in VDM-PS | 104 | 2 mL |
| Candida albicans | 0.5–0.8 | Dilute 1:100 in VDM-PS | 104 | 2 mL |
%Transmittance tracker; CFU: Colony forming unit; VDM-PS: Genital tract secretion medium (vaginal defined medium).
The composition of the vaginal defined medium was previously described by Geshnizgani and Onderdonk.41 To enhance the growth and recovery of P. bivia and G. vaginalis,42 the vaginal defined medium was supplemented with peptone by reducing the concentration of dextrose to 1.0 g/L, supplementing with 5.0 g/L peptone and adjusting to pH 6.5 ± 0.2, yielding VDM-PS. Pilot studies confirmed that VDM-PS supported the growth of both the individual organisms and the mixed inoculum under anaerobic conditions (data not shown).
Experimental stage II
Co-inoculation of each control and test product followed by incubation under anaerobic conditions for up to 48 h (Fig. 2).
Fig. 2.
Overview of the in vitro mixed microflora assay: Preparation of co-inoculum (from stock inocula), test product incubation, selective microorganism growth and subsequent colony counting.
Five sterile vented flasks were used, i.e. duplicate test product flasks, one control product flask, one flask for the negative control (VDM-PS plus the consortium), and one flask for the positive control (VDM-PS plus the consortium plus betadine solution (0.3% povidone iodine)).
VDM-PS was added to each sterile vented flask under anaerobic conditions followed by sequential addition of aliquots of appropriately diluted concentrations of each of the six representative vaginal microorganisms (see Experimental stage I). Test products (Tampax Cup and Other Cup) were then added to the flasks containing the microorganism consortium (T = 0 h). All inoculated flasks were incubated under anaerobic conditions at 35 ± 2 °C for 24 or 48 h with rotation at 150 rpm using a platform shaker.
Experimental stage III
Plating of organisms on selective media following sample collection from control and test product broth with colony counting.
Sodium thiosulfate was added to the sample vial of the positive control (containing iodine) at the collection timepoints (T = 0 h, T = 24 ± 2 h, T = 48 ± 2 h) prior to adding the 10 mL aliquot from the flask to ensure neutralisation of the iodine. For the remaining sample flasks, 10 mL aliquot were removed from each flask, placed into a sterile dilution tube ‘0’ and serially diluted (1:10) in saline and plated onto agar plates. Before plating onto the Human Blood Bilayer Tween, 1 mg Lysostaphin was added to each of the initial ‘0’ tubes (positive control, negative control, product control and product test flasks), vortexed and incubated at 35 ± 2 °C for 10 min before dilution and plating onto Human Blood Bilayer Tween. The Lysostaphin treatment step serves to simplify the colony counting of L. gasseri and G. vaginalis on Human Blood Bilayer Tween by ‘lysing’ the S. aureus thereby minimising S. aureus overgrowth of the much smaller colonies of L. gasseri and G. vaginalis.
After 48 h of selective growth (24 h for E. coli; Table 4), the colony count of each test flask and control flask were recorded and adjusted by the dilution factor to determine the numbers of colony forming units (CFU/mL). All CFU counts were log normalised (log10) to facilitate data comparisons. Differences were calculated as the absolute deviation from the mean CFU (log10) for the product-containing flasks and those of the negative control. A perturbation of the microflora by ≥ 2 log change from the microorganism-containing negative control (consortia only) after 48-h test product exposure was defined as indicating failure of the test product in meeting microbial safety requirements. The 2-log criterion was based on work by Onderdonk and colleagues.43 In the present study, culture-based quantitative and qualitative methods were used to investigate the fluctuation of the microbial community during the menstrual cycle of healthy women. Onderdonk and colleagues found that the standard deviation of the culture-based method (measured in log CFU/g) ranged from 1.04 to 1.43.43 In addition, the variations of the mean aerobic and anaerobic counts (log CFU/g) through menstruation could range from >1 to < 2 log CFU/g and yet health was maintained. Therefore, a <2 log CFU/g change is considered normal variability and not clinically significant because to date, no clinical importance of such modest changes in microbial numbers has been demonstrated.
Table 4.
Incubation under the appropriate growth conditions.
| Organisms | Selective medium agar | Atmosphere | Incubation |
|---|---|---|---|
| Lactobacillus gasseri | Human Blood Bilayer Tween | Anaerobic | 35 ± 2 °C, 48 ± 2 h |
| Gardnerella vaginalis | Human Blood Bilayer Tween | Anaerobic | 35 ± 2 °C, 48 ± 2 h |
| Prevotella bivia | Laked Blood Kanamycin-Vancomycin | Anaerobic | 35 ± 2 °C, 48 ± 2 h |
| Escherichia coli | MacConkey Medium | Aerobic | 35 ± 2 °C, 24 ± 2 h |
| Staphylococcus aureus | Mannitol Salt Agar | Aerobic | 35 ± 2 °C, 48 ± 2 h |
| Candida albicans | Inhibitory Mould Agar with chloramphenicol and gentamycin | Aerobic | 35 ± 2 °C, 48 ± 2 h |
All selective media were obtained from Remel Microbiology Products (Fisher Thermo Scientific, USA).
Before plating on Human Blood Bilayer Tween, the respective aliquots were treated with 2 μg Lysostaphin (Sigma–Aldrich, USA) for 10 min at 37 °C. Pilot studies demonstrated that this procedure prevented the overgrowth of S. aureus on HBT without affecting growth of G. vaginalis and L. gasseri (data not shown).
Clinical trial – assessment of the vaginal microbiota
Microbiological assessments of vaginal swabs taken during the randomised, double-blinded clinical trial (Randomised clinical trial) served to determine how use of the Tampax Cup affects the composition and/or diversity of the vaginal microbiota as compared to baseline (upon subject arrival at site and prior to study cup usage) and use of the Other Cup.
Subjects were randomly assigned to one of two treatment sequences (AB or BA) for the two-period crossover study such that half of the subjects used the Tampax Cup first followed by the Other Cup and the other half used the Other Cup first and then the Tampax Cup. Vaginal swabs were taken at baseline and within 72 h after each of the two menstrual cycles. Information on the day of the menstrual cycle was not collected and the baseline visit was not pre-determined or standardised across the subject population. Hence, for each subject, there were three recordings, i.e. at baseline, after use of the Tampax Cup, and after use of the Other Cup (with the sequence of the two latter recordings depending on the assigned treatment sequence). While the vaginal microbiome is relatively stable, factors like menstrual cycle, pregnancy, use of contraceptives or antibiotics and diet can affect the composition.44, 45, 46, 47 Therefore, subjects were asked to use at least one form of birth control and were excluded from study participation for recent antibiotic or antifungal use. Further, throughout the trial, subjects were asked to refrain from use of antibacterial soap and/or any vaginal/perianal product, as well as from genital hair removal. Also, subjects were asked to refrain from vaginal intercourse for 48 h prior to the visit at which the swabs were taken, and to refrain from bathing within 24 h and showering within 12 h of the visit.
An initial analysis of the vaginal microbiota was conducted by Rocio Navarro Garcia, Research and Testing Laboratories (RTL) Genomics (Lubbock, TX, USA) and the final analysis by the authors of this study. The data analysis methodology followed that described by RTL Genomics48 and Teufel and colleagues.49
The spectrum of bacteria present in the samples was analysed by culture-independent molecular assessment. In brief, amplicons of the target regions of the 16S ribosomal RNA (rRNA) genes present in the samples were produced by polymerase chain reaction (PCR), followed by determination and classification of the gene sequences of the amplicons.
For the PCR reaction, DNA was loaded into 10 μL Quanta PerfeCTa qPCR ToughMix (QuantaBio, USA) and run on a Roche 480 LightCyler® (Roche Life Science) with the following cycling conditions: one cycle at 50 °C for 2 min, one cycle at 95 °C for 10 min, 35 cycles at 95 °C for 15 s and at 60 °C for 1 min, and, finally, one cycle at 40 °C for 30 s, with the limit of detection being above 30 cycles. Samples were amplified for 16s Vi-V3 region sequences using a forward and reverse primer: Forward MS28F: GAGTTTGATCNTGGCTCAG, Reverse 519R:GTNTTACNGCGGCKGCTG.
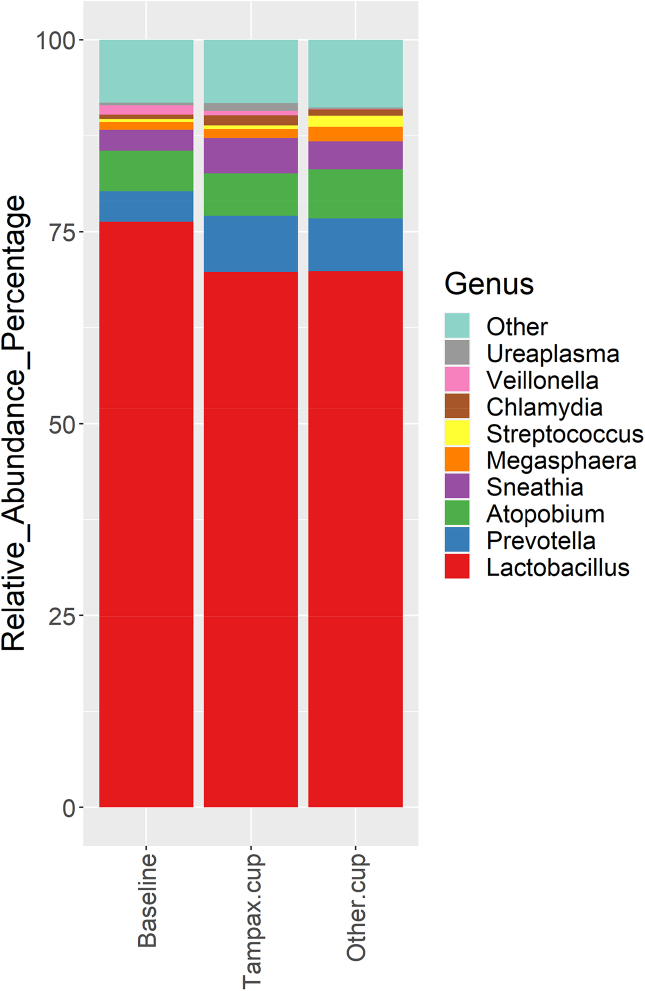
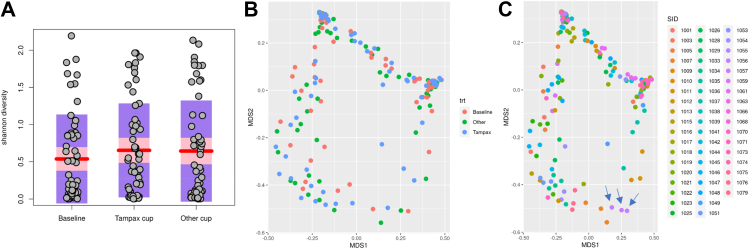
The data analysis pipeline consists of two major stages, the denoising and chimera detection stage and the microbial diversity analysis stage.48 QIIME™, version 1.9 (Quantitative Insights into Microbial Ecology; http://qiime.org/), and Mothur (https://mothur.org/ [both websites accessed 27 August 2022]) were used to obtain taxonomic identification for microbiome profiling using GreenGene version gg_13_8 database.49 The identity of the different bacterial species reported as the best match for each read was recorded, the number of reads assigned to each of the taxa counted, and their relative abundance in the respective sample calculated. All bioinformatics were performed using the R Vegan Package.50 Shannon diversity51,52 was reported as a representative alpha diversity measurement reflecting the microbial diversity within each sample. Cleaned data included the relative abundances of 70 genus for all subjects (presented in tabular form). These evaluations served to determine if/how the spectrum of microorganisms differed by product.
Further, Multi-Dimensional Scaling (MDS) calculated using Bray–Curtis similarities53 served to evaluate beta diversity,54 i.e.
-
1.
How each sampling point (i.e. baseline and within 72 h after use of the Tampax Cup and Other Cup, respectively) compared against the other sampling points, and
-
2.
How the three recordings for one subject (baseline, Tampax Cup, Other Cup) compared against the respective recordings for all other subjects.
Pairwise Wilcoxon Rank Sum tests and Adonis tests55 were performed to test microbial differences among different populations to establish the p values. Thereby, it was determined if changes of the vaginal microbiota were predominantly caused by use of a specific cup, or if they were rather accountable to inter-individual differences. The clinical design sought to decrease variability across women in terms of medication, pregnancy and sample collection times within a cycle.
The growth of S. aureus and risk for toxic shock syndrome
The effect of the Tampax Menstrual Cup on growth of S. aureus MN8 and production of toxic shock syndrome Toxin 1 (TSST-1) was assessed by P. M. Schlievert, Department of Microbiology and Immunology, Carver College of Medicine, University of Iowa, Iowa City, IA (USA). The applied methodology follows that originally described by Schlievert and Blomster,56 with further details on its application to assess intravaginal menstrual and contraceptive products provided by Schlievert.57 In brief, the method includes exposing the test article to cultures of 107 S. aureus/mL of Todd Hewitt broth. After 18 h, the supernatants, which will contain any TSST-1 produced, are collected and serially diluted. These dilutions of the TSST-1 preparations are reacted against antisera (produced by the hyper-immunisation of rabbits) in Ouchterlony immunodiffusion assays58 to establish a toxin titre (TSST-1 μg/mL). TSST-1 concentrations were also determined by Western immunoblot analysis. S. aureus MN8 growth was determined by colony counts (CFU/mL). Means and standard deviations were determined for all samples tested (n = 5 per test group). Student's t-test was used for comparison of means and p < 0.05 was considered significantly different.
Role of the funding source
The Procter & Gamble Company provided the funding relevant to the conduct of the studies described herein as well as funding the hiring of a scientific writer to assist in the preparation of the manuscript. The funders, however, played no role in the programme design, data collection, data analyses or data interpretation. The authors alone are responsible for the writing of and content of this manuscript.
Results
The biocompatibility and chemical safety of the cup constituents
Chromatograms of silicone versus cup upon extraction at 37 °C for 72 h, at 50 °C for 2 h, and at 121 °C for 1 h
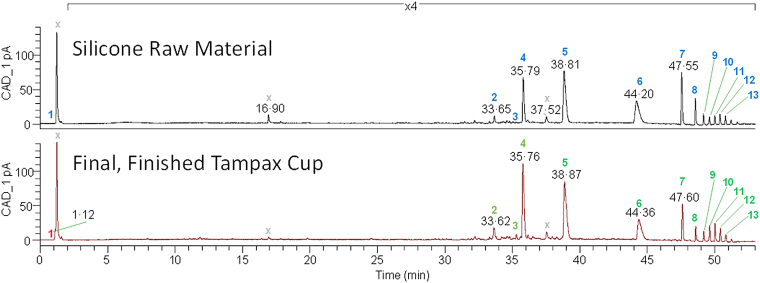
For all samples (i.e. the extractables of both silicone QP 1-40 and the Tampax Cup obtained after extraction at (1) 37 °C for 72 h, (2) 50 °C for 2 h, and (3) 121 °C for 1 h), the CAD peaks that were above the TTC (0.63 μg/material, i.e. TTC of 0.025 μg/kg bw/day x 50 kg bw/2 cups/day, which equates to 1260 ng/mL for the extracts in 0.5 mL of solvent) were compared. For all three sets of conditions, there were no new peaks between the control (QP 1-40) and the samples (menstrual cups). Any peaks that were shown as significantly increased were further characterised and assessed. For instance, after extraction at 50 °C for 2 h, there were no new or significantly increased peaks with the exception of a salt/solvent cluster peak, which was only noted in the Tampax Cup samples as discussed below (Fig. 3). This initial analysis enabled the commitment to use the simpler extraction methodology of a single extraction temperature and time (i.e. 50 °C for 2 h).
Fig. 3.
Chromatograms of the silicone raw material extract versus the final, finished Tampax Cup upon extraction at 50 °C for 2 h. Peaks denoted with an X are either below the threshold of toxicological concern (TTC) in both the silicone raw material and the final, finished Tampax Cup or in the solvent and/or blank control. Green numbers indicate peaks of the silicone raw material > peaks of the final, finished Tampax Cup; red numbers indicate peaks of the silicone raw material < peaks of the final, finished Tampax Cup. ‘>’ and ‘<’ were determined overall with the standard deviation of multiple replicates. There are no peaks in the chromatogram of the cup that are not also present in the chromatogram of the silicone. Chromatograms obtained under different extraction conditions (not shown) were similar qualitatively and quantitatively.
Thirteen CAD peaks above the TTC were observed for the silicone QP 1-40 and the cup. All constituents were lower for the cup when compared to the silicone with the exception of one peak (i.e. Peak 1). Peak 1 was found in the chromatograms of the cup extract but not in those of the silicone and was identified as Na+, Mg+ and Ca2+ solvent salt clusters that are irrelevant from a toxicological perspective. The remaining 12 peaks were identified as siloxanes of varying chain length ranging from n = 5 to n = 15 repeating units (Table 5). While these data reflect the chromatograms obtained after extraction at 50 °C for 2 h, the other extraction conditions (37 °C for 72 h and 121 °C for 1 h) yielded concordant results (data not shown).
Table 5.
Identification of peaks from chromatographs of extractables from raw material and final, finished Tampax Cup (extraction at 50 °C for 2 h).
| Peak # | Raw material (μg/g ± SD) (n = 3) |
Final, finished Tampax Cup (μg/g ± SD) (n = 3) |
Final, finished Tampax Cup (μg/cup) a,b | Exposureb (μg/kg bw/day)b,c | Identification molecular formula |
Structure | Confidenced | |
|---|---|---|---|---|---|---|---|---|
| 1 | <LOD | 0.19 ± 0.1 | 6.18 | 0.17 | Na+, Mg2+, Ca2+ solvent salt clusters | Partial | ||
| 2 | 0.23 ± 0.06 | 0.14 ± 0.02 | 3.41 | 0.14 | Siloxane e C14H44O8Si7 |
 |
n = 5 | Tentative |
| 3 | 0.038 ± 0.004 | 0.031 ± 0.003 | 0.72 | 0.03 | Silicone-containing constituent | Partial | ||
| 4 | 1.5 ± 0.7 | 0.95 ± 0.1 | 22.37 | 0.89 | Siloxane e C16H50O9Si8 |
 |
n = 6 | Tentative |
| 5 | 3.1 ± 1 | 1.1 ± 0.1 | 25.56 | 1.02 | Siloxane e C18H56O10Si9 |
 |
n = 7 | Matched |
| 6 | 2.3 ± 0.8 | 0.56 ± 0.05 | 12.99 | 0.52 | Siloxane e C20H62O11Si10 |
 |
n = 8 | Matched |
| 7 | 1.6 ± 0.5 | 0.29 ± 0.04 | 7.03 | 0.28 | Siloxane e C22H68O12Si11 |
 |
n = 9 | Matched |
| 8 | 0.70 ± 0.2 | 0.11 ± 0.03 | 2.98 | 0.12 | Siloxane e C24H74O13Si12 |
 |
n = 10 | Matched |
| 9 | 0.31 ± 0.1 | 0.073 ± 0.02 | 1.98 | 0.08 | Siloxane e C26H80O14Si13 |
 |
n = 11 | Matched |
| 10 | 0.21 ± 0.09 | 0.10 ± 0.04 | 2.98 | 0.12 | Siloxane e C28H86O15Si14 |
 |
n = 12 | Matched |
| 11 | 0.20 ± 0.08 | 0.11 ± 0.05 | 3.41 | 0.14 | Siloxane e C30H92O16Si15 |
 |
n = 13 | Matched |
| 12 | 0.24 ± 0.07 | 0.088 ± 0.04 | 2.73 | 0.11 | Siloxane e C32H98O14Si16 |
 |
n = 14 | Matched |
| 13 | 0.20 ± 0.04 | 0.049 ± 0.02 | 1.47 | 0.06 | Siloxane e C34H104O14Si17 |
 |
n = 15 | Matched |
| Combined daily exposure for all constituents (μg/kg bw/day) | 3.5 | |||||||
Abbreviations: bw, Body weight; LOD, Limit of detection; n, Number of repeat units.
This column is a calculation to convert μg/g to μg/cup using a menstrual cup weight of 21.3 g and amount of extractable in the final finished Tampax Cup +1 SD.
Note that the “μg/cup” and “μg/kg bw/day” values already have one standard deviation added to represent a “worse-case scenario” value for the safety assessment.
This column is a conversion calculation using the formula X (μg/cup) x 2 cups/day, 50 kg. This calculation assumes 100% absorption of the constituent across the vaginal membrane into the systemic circulation and everyday exposure throughout the menstrual cycle.
Level of confidence classifications: (i) A partial identification denotes the determination of one or more functional groups OR a proposed molecular formula. (ii) A tentative identification is a proposed structure derived by using commonly accepted mass spectrometry interpretation practices AND a secondary piece of mass spectral data, published material composition, or chromatographic information that supports the proposed chemical identification. (iii) A matched compound identification is valid when a proposed compound chemical identification can be justified by one of the following: (a) using a published or user library spectral match AND the identification can be deemed as reasonable given additional knowledge of the sourcing or composition of the sample; (b) OR a molecular formula derived from using accurate mass AND the product ion spectrum is consistent with the proposed identification AND the identification can be deemed as reasonable given additional knowledge of the sourcing or composition of the sample.
It was not determined whether the siloxane was linear or cyclic.
Exposure-based risk assessment of the chemicals eluting from the menstrual cup
Since limited systemic toxicity data are available for the varying chain length siloxanes identified, the TTC concept was utilised to derive an appropriate risk value. Siloxanes as a chemical class are, by default, grouped in Cramer Class III (1.5 μg/kg bw/day or 90 μg/day)29 due to the presence of a silicon atom. When this TTC value is compared to the estimated, combined daily exposure of all siloxanes found in the extract of the cup (3.5 μg/kg bw/day), the margin of safety is less than 1. However, this is deemed acceptable for the following reasons. The exposure calculations to estimate the total potential exposure to siloxanes (3.5 μg/kg bw/day; Table 5) were derived using exaggerated, non-physiologically relevant extraction conditions (50 °C for 2 h) and assumed everyday exposure to the menstrual cup eluting chemicals during a menstrual cycle. The default estimate of exposure assumes that the woman is exposed to 3.5 μg/kg bw/day each day as there is no refinement for decreased eluting chemical concentration with repeat use, a phenomenon that is likely with a durable device. Hence, this likely resulted in a conservative, over-estimate of exposure. Additionally, the recent analysis by Schmitt and colleagues,59 which demonstrated that the Cramer Class III designation is protective for the organosilicon chemistry, also showed that the “5th percentile of this dataset was 13-fold higher than the 5th percentile for Cramer Class III compounds reported by Munro et al. (1996)60 and more than 8 times higher than the corresponding values derived in the COSMOS TTC project”.59 This suggests an additional layer of conservatism in the risk assessment of siloxanes eluting from the menstrual cup.
In conclusion, there were no safety concerns for the differences in chemical entities above the TTC between the silicone (raw material) and the Tampax Cup (final, finished article). Also, the extractions performed under physiologically relevant, yet exaggerated conditions (37 °C for 72 h) and those performed under accelerated conditions (121 °C for 1 h) generated no new constituents for either of the materials. Thus, it was determined that the process of manufacturing the cup did not change the chemical profile of what could be extracted from the raw material under conditions considered to be more aggressive than real use case. Therefore, the biocompatibility studies conducted by Dow on the silicone material were used to bridge to the final, finished device.
Evaluation after intended use conditions
The profile peaks after seven and 13 boiling cycles, respectively, were quantified, and all peaks above the TTC were compared. There were no additional peaks above the TTC for either the seven- or 13-boiling cycle cups that had not been recorded for the final, finished cup (The biocompatibility and chemical safety of the cup constituents). All peaks that were above the TTC showed no statistical differences between the final, finished cup and the cups after seven and 13 boiling cycles, respectively (data not shown). Hence, the extractable profile for the Tampax Cup did not change after up to 13 boiling cycles, and no further evaluations were needed at this point of the analysis.
The chromatographic profiling of the cup after one boiling cycle and wiping with two wipes yielded 13 constituents (Table 6) quantified above the TTC and unique to the wiping process (i.e. peaks not observed in a non-boiled, non-wiped menstrual cup). Table 6 and Supplementary Information SI-8 details these 13 constituents that are unique to this experiment (extraction at 50 °C for 2 h), including their identification, concentration/cup, and exposure-based risk assessment. Three reference standards, which were ingredients on the wipe, were obtained for peak matching, i.e. sorbitan caprylate, Cremophor Rb 410, and PEG 40 Hydrogenated Castor Oil, and the respective chromatograms were prepared (data not shown). Each of these standards is a mixture of components and accounted for most of the peaks above the TTC. There were no other peaks that were a result of wiping the cup that could not be attributed to the cup or wipe. No new chemistry nor degradation occurred to create any additional species above the TTC.
Table 6.
Identification of eluting chemicals from Tampax Cup after one boiling cycle followed by wiping with two Feminine Always Wipes.
| No. | Identification | CAS Number | Molecular formula | Structure | Confidence a |
|---|---|---|---|---|---|
| 1 | Valine | 516-06-3 | C5H11O2N |  |
Matched |
| 2 | EDTA | 60-00-4 | C10H16O8N2 | 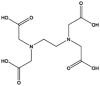 |
Matched |
| 3 | Sugar-like constituents | NA | C6H12O5 C12H22O9 C10H18O9 – C50H82O42 |
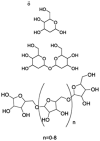 |
Tentative |
| 4 | Citric acid | 77-92-9 | C6H8O7 |  |
Matched |
| 5 | PEG 40 Hydrogenated Castor Oil | 61788-85-0 | Mixture | Mixture | Reference |
| 6 | Sorbitan caprylate | 60177-36-8 | C14H26O6 |  |
Reference |
| 7 | Isosorbide monocaprylate | 49553-31-3 | C14H24O5 | 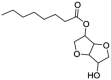 |
Reference |
| 8 | Dioctanoate sorbitan | 152261-28-4 | C22H40O7 |  |
Reference |
| 9 | 12-hydroxystearic acid | 106-14-9 | C18H36O3 | Tentative | |
| 10 | Diethylene glycol mono (12-hydroxystearate) | 122105-30-0 | C22H44O5 | Reference | |
| 11 | Trioctanoate sorbitan | 94131-37-0 | C22H44O5 |  |
Reference |
| 12 | 11-Carboxy-1-hexylundecyl 12-hydroxystearate | 218291-10-2 | C36H70O5 | Reference | |
| 13 | Triethylene glycol di (12-hydroxystearate) | 124615-57-2 | C42H82O8 | Reference |
Abbreviations: EDTA, Ethylene diamine tetra-acetic acid; PEG, Polyethylene glycol.
Colour legend: Structure components highlighted in green indicate the connectivity of the two moieties, often at one of the hydroxy groups. However, the exact hydroxy group cannot be determined without a standard.
Level of confidence classifications: (i) A partial identification denotes the determination of one or more functional groups OR a proposed molecular formula. (ii) A tentative identification is a proposed structure derived by using commonly accepted mass spectrometry interpretation practices AND a secondary piece of mass spectral data, published material composition, or chromatographic information that supports the proposed chemical identification. (iii) A matched compound identification is valid when a proposed compound chemical identification can be justified by one of the following: (a) using a published or user library spectral match AND the identification can be deemed as reasonable given additional knowledge of the sourcing or composition of the sample; (b) OR a molecular formula derived from using accurate mass AND the product ion spectrum is consistent with the proposed identification AND the identification can be deemed as reasonable given additional knowledge of the sourcing or composition of the sample. (iv) A reference identification occurs when the requirements of Matched identification are met AND there exists a mass spectral and retention time match to a reference standard containing the purported material.
Exposure-based risk assessment of the extractables after boiling cup once followed by wiping with two wipes
The Supplementary Information SI-8 summarises the exposure-based risk assessments performed for all 13 quantified constituents above the TTC. In brief, none of the risk assessments indicated any safety concerns for the intended use scenario ‘boiling cup once followed by wiping with two Feminine Always Wipes’.
Assessment of irritation potential
In the in vitro EpiVaginal™ assay, ET50 values > 24 h were recorded for all test materials, (1) Tampax Cup in sesame oil; (2) Tampax Cup in saline; (3) the punch of an Always Feminine Wipe; and (4) the liquid expressed from a further wipe. There was some reduction in viability for the EpiVaginal™ tissues treated with the liquid expressed from the wipes (53.5% viability at 16 h, 77.7% viability at 24 h). Hence, the response curve for the liquid indicates some toxicity compared to the punch. This was anticipated given the difference in the liquid volume when using the punch (which includes the wipe and the lotion) vs the liquid (i.e. the lotion alone). From a risk assessment perspective, the former is more indicative of the clinical use situation. Therefore, these results are considered more relevant.
The physical impact to the vaginal mucosa
Visual inspection
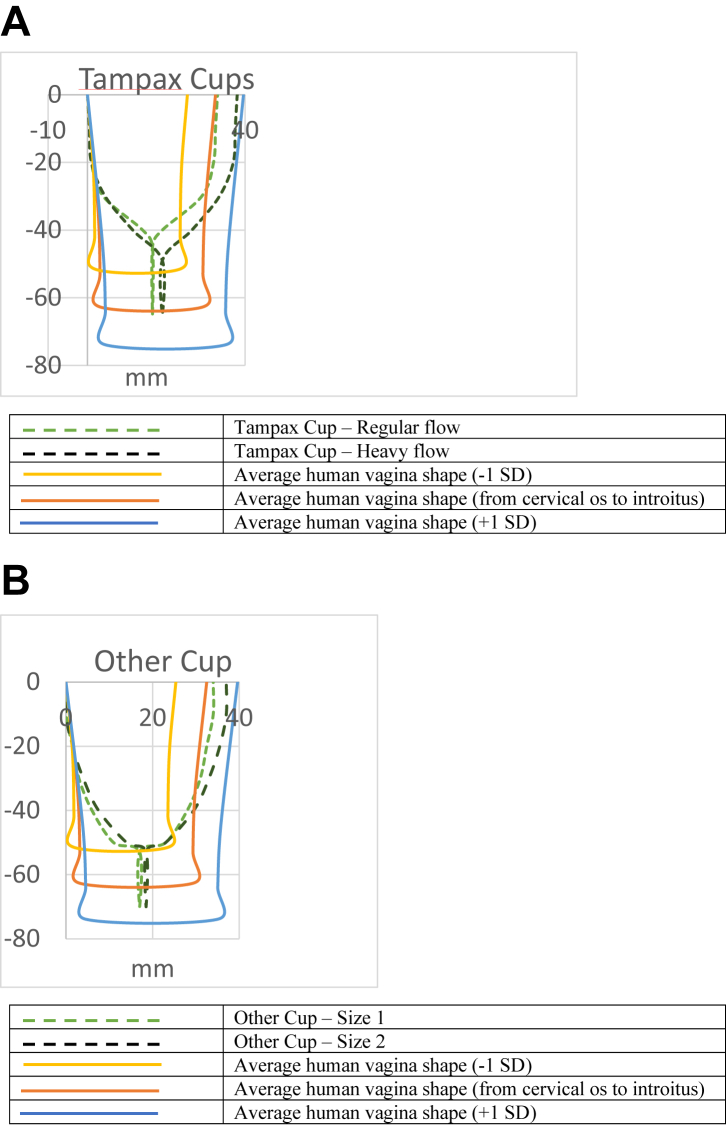
In the visual inspection for material conformity, the Tampax Cup was assessed as reasonably smooth. It was comparable in size, dimensions, and compression forces to the Other Cup (Fig. 4, Panel A: Tampax Cup; Panel B: Other Cup). Therefore, it is reasonable to expect that the two cups will have a similar adverse effect profile during the clinical trial (Randomised clinical trial) in terms of mechanical irritation.
Fig. 4.
Dimensions (mm) of (Panel A) the final, finished Tampax Cup and (Panel B) the Other Cup as compared to the dimensions of the human vagina (average, 5th and 25th percentiles).
Randomised clinical trial
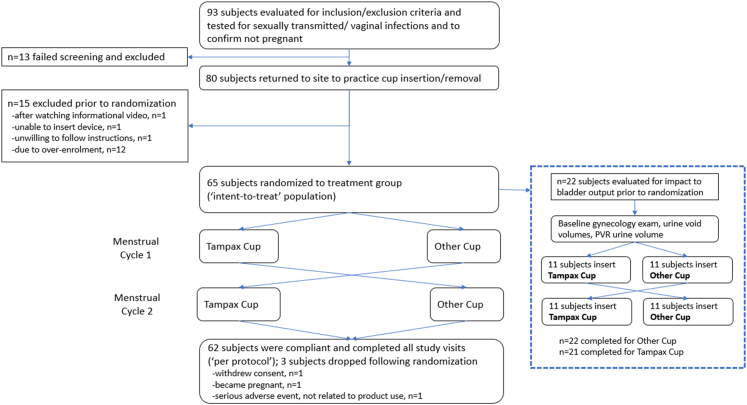
Ninety-three menstruating females were evaluated at an enrolment visit for adherence of inclusion and exclusion criteria (see Supplementary Information SI-5 for inclusion and exclusion criteria). Specifically, they were tested to be free from sexually transmitted and vaginal infections (bacterial vaginosis, Candida spp., Trichomonas vaginalis, Chlamydia trachomatis, Neisseria gonorrhea). Further, urine tests confirmed they were not pregnant. Vaginal swabs were obtained for microbiota analysis (Section Clinical trial – assessment of the vaginal microbiota). Thirteen subjects failed screening and were excluded. The 80 subjects who met the enrolment criteria returned to the site to practice cup insertion and removal (not during menstruation) (Fig. 5).
Fig. 5.
Design of the randomised, double-blinded, two-product, two-period crossover clinical trial. Abbreviation: PVR, Post-void residual.
Fifteen subjects were dropped prior to randomisation (one each after watching the informational video, because she was unable to insert the menstrual cup, and because she was unwilling to follow study instructions; and a further 12 subjects due to over-enrolment). Sixty-five subjects were randomised to test the cups. These subjects constitute the ‘intent-to-treat population’, i.e. the entirety of eligible subjects who are enrolled into the study, randomised to treatment, and are given their assigned test products for use. Three subjects were dropped after randomisation (one each withdrew consent, became pregnant, and had a serious adverse event of the respiratory tract, which was not product-related). Sixty-two subjects completed the study (‘per-protocol’ population).
Adverse events were recorded in the ‘intent-to-treat’ population (65 subjects). All potentially product-related adverse events were mild (i.e. categorised as ‘possible’ or ‘probable’ by the principal investigator). No subject withdrew from the study due to a cup-related adverse event. All menstrual/vaginal related adverse events were not unexpected for menstruating women or cup users. The type and severity of adverse events were comparable between the Tampax Cup and the Other Cup.
Table 7 presents the demographics of the intent-to-treat population. The age of the subjects ranged from 19 to 49 years (average 34.2 years). The mean weight was 82.7 kg (range 44.8–139.2 kg). According to the 2015–2016 National Health and Nutrition Examination Survey database,61 the average body weight for U.S. women is 77.6 kg. The body mass index ranged from 18 to 49 (average 30.5). Sixty-five % of the subjects were White, 29% Black, 3% Asian, 1.5% American Indian or Alaskan Native, and 1.5% Multiracial. Sixty subjects were generally tampon users and 5 subjects were cup users, with 94% of all subjects typically having a moderate flow and 6% a heavy flow.
Table 7.
Clinical trial: Demographics of the intent-to-treat population (n = 65).
| Measures | Result |
|---|---|
| Ethnicity (number of individuals (percent)) | |
| Hispanic/Latino | 2 (3.1%) |
| Not Hispanic or Latino | 63 (96.9%) |
| Race (number of individuals (percent)) | |
| American Indian or Alaskan Native | 1 (1.5%) |
| Asian | 2 (3.1%) |
| Black or African American | 19 (29.2%) |
| Multiracial | 1 (1.5%) |
| White/Caucasian | 42 (64.6%) |
| Smoking status (number of individuals (percent)) | |
| Current smoker | 11 (16.9%) |
| Former smoker (>10 years ago) | 1 (1.5%) |
| Former smoker (<10 years ago) | 11 (16.9%) |
| Never smoked | 42 (64.6%) |
| Pregnancy (number of individuals (percent)) | |
| Negative | 65 (100%) |
| Age (years) | |
| Mean | 34.2 |
| Median | 33.0 |
| Min - Max | 19.0–49.0 |
| Height (cm) | |
| Mean | 164.5 |
| Median | 163.8 |
| Min - Max | 152.0–180.1 |
| Weight (kg) | |
| Mean | 82.7 |
| Median | 82.0 |
| Min - Max | 44.8–139.3 |
| Body Mass Index (no unit) | |
| Mean | 30.5 |
| Median | 31.0 |
| Min – Max | 18.0–49.0 |
| Previous menstrual hygiene protection use (no unit) | |
| Tampon | 60 (92%) |
| Menstrual cup | 5 (8%) |
All baseline scores for erythema, abrasions, lacerations, and ulcerations were 0. All post-use scores were 0 for both cups, except at the cervix for the Other Cup. Two subjects had an erythema score of 2 and 3, respectively, at the cervix while using the Other Cup, which the Investigator considered to be ‘doubtfully related to product use’.
The average baseline vaginal pH was 4.38; the average pH for the Tampax Cup was 4.76 and for the Other Cup 4.80. The mean pH was not statistically significantly different between the two cups (p = 0.755, LMM F test). However, for both cups, the mean pH was statistically significantly higher than baseline (p < 0.05, LMM F test). There was no evidence of post-use abnormal vaginal discharge for either cup.
The daily diaries yielded the following findings (see Supplementary Information SI-9 for details): Average wear time was 8.6 h for the Other Cup and 8.9 h for the Tampax Cup. No statistically significant difference in the number of changes per day between the two cups was noted. Reports of burning, itching, and stinging were infrequent (≤0.4% of cup uses), and there was no statistically significant difference in the occurrence of such reports between the two cups. Products were also not statistically significantly different on discomfort questions, except for Tampax Cups having statistically significantly more perceived insertion discomfort than the Other Cup. Tampax Cups trended better on wearing comfort as compared to the Other Cup. Overall, there were more insertion and removal discomfort comments for both cups on the first day of the menstrual cycle; there was more insertion discomfort for the 25–34 years age group; and wearing discomfort was greater for shorter wear times and lower flow. Finally, removal discomfort was impacted by body weight with more discomfort reported by subjects at lower body weights.
As regards wear time, 30 subjects (of the total of 65) had at least one cup use of 12 or more hours. Also, for 11 subjects all wear times were 12 h or more. This corresponded to a total of 120 cup uses (19%) extending over 12 h or more, whereas 502 cup uses (81%) were less than 12 h. The findings for the subjects with cup uses with extended wear times were consistent with shorter wear times showing that the Tampax Cup was also well-tolerated at extended wear time (12 or more hours) with no impact on vaginal health endpoints.
As per monthly questionnaire (see Supplementary Information SI-9 for details), the ratings of parameters that provide some indication of safety [i.e. perceived comfort (bloating/cramping) and perceived changes in bladder and bowel habits] were not statistically significantly different between the two cups. Most subjects experienced no change in bloating or cramping compared to their usual menstrual cycle. For the subjects reporting a change, the majority of subjects reported experiencing less cramping or bloating (‘somewhat’ or ‘much’ less). Most subjects reported no impact on urination or bowel movements (87.3–98.4%). However, 1.6% and 7.8% subjects reported impacts on bowel movements and urination, respectively, upon use of the Other Cup, and 9.5% and 12.7% subjects reported impacts on bowel movements and urination, respectively, upon use of the Tampax Cup. Thusly, statistically significantly more subjects reported bowel movement changes for the Tampax Cup vs the Other Cup (p < 0.324, Cochran–Armitage test).
The gynaecological examination yielded no clinically meaningful findings. One subject had a 4-mm superficial laceration on the inner side of the labium minorum due to a fingernail cut, which was not product-related.
One subject wearing the Tampax Cup met the criteria for possibly clinically meaningful changes in PVR. Notably, however, she was the first subject for such testing, and there were some time delays from void volume collection to PVR measurements. Also, based on baseline PVR, site staff suspected the subject showed signs of urinary retention even though the subject denied history of urinary issues or urinary tract infections. The change from baseline PVR did not differ between Tampax Cup and the Other Cup. Seven of 22 women had higher residual volumes than baseline with both cups, and five of 22 women had lower residual volumes than baseline with both cups. In the remaining 10 subjects with data for both cups, results differed for the two cups. Four subjects showed increased PVR for the Tampax Cup and decreased PVR for the Other Cup, and 4 subjects showed decreased PVR for the Tampax Cup and increased PVR for the Other Cup when compared to baseline. One subject had equal baseline PVR and PVR for the Tampax Cup with an increased PVR for the Other Cup. One subject had PVR data only for the Other Cup, which was lower than baseline. Importantly, there were no reported urinary tract infections during the conduct of the study.
The impact to vaginal microbiota
In vitro mixed microflora assay
Both the Tampax Cup and the Other Cup met the criteria of ≤2 Log difference of product at both 24 and 48 h compared to the microorganism-containing negative control (consortium control). Most values were <0.5 Log10 change over the consortium control. The only organism that showed >1 Log increase at 48 h compared to the consortium control was G. vaginalis for the Other Cup (−1.76). The positive control containing povidone iodine produced results as expected. Therefore, the Tampax Menstrual Cup had no effect on microbial growth versus consortium control, with findings being comparable to the Other Cup (see Supplementary Information SI-10 for details).
Clinical trial – assessment of the vaginal microbiota
Of the 62 subjects who completed the clinical trial (Section Randomised clinical trial), 55 had a complete swab sample set for analysis (i.e. swabs taken at baseline and within 72 h after each of the two menstrual cycles).
Tables 8 and 9 and Fig. 6 present the vaginal microbiota composition of individual bacteria genera with average relative abundance >0.5% recorded in the vaginal swabs of women at baseline, i.e. before use of any cup, and within 72 h of use of either the final, finished Tampax Cup or the Other Cup. Clearly, Lactobacillus was the dominant genus across all samples. There were no observable statistically significant differences of these vaginal bacteria genus between (a) baseline and (b) either cup group; or between (a) the Tampax Cup group and (b) the Other Cup group. Observed changes in the composition and the diversity of the vaginal bacteria community did not produce clinically meaningful effects.
Table 8.
The vaginal microbiota composition at different sample points; P values.
| Taxon name (genus) | Kruskal–Wallis test |
Pairwise Wilcoxon test |
Pairwise t-test |
||||
|---|---|---|---|---|---|---|---|
| Test groupa | Tampax Cup vs. Baseline | Other Cup vs. Baseline | Other Cup vs. Tampax Cup | Tampax Cup vs. Baseline | Other Cup vs. Baseline | Other Cup vs. Tampax Cup | |
| Lactobacillus | 0.98 | 0.35 | 0.29 | 0.12 | 0.39 | 0.40 | 0.99 |
| Prevotella | 0.84 | 0.48 | 0.32 | 0.47 | 0.19 | 0.21 | 0.87 |
| Atopobium | 0.85 | 0.22 | 0.24 | 0.86 | 0.92 | 0.70 | 0.74 |
| Sneathia | 0.99 | 0.70 | 1.00 | 0.64 | 0.45 | 0.66 | 0.72 |
| Megasphaera | 0.91 | 1.00 | 0.58 | 0.19 | 0.83 | 0.31 | 0.40 |
| Streptococcus | 0.34 | 0.42 | 0.71 | 0.14 | 0.81 | 0.31 | 0.36 |
| Chlamydia | 0.32 | 0.37 | 0.31 | 0.50 | 0.32 | 0.63 | 0.57 |
| Veillonella | 0.49 | 0.62 | 0.89 | 0.61 | 0.63 | 0.33 | 0.32 |
| Ureaplasma | 0.9 | 0.79 | 0.31 | 0.57 | 0.48 | 0.66 | 0.39 |
Individual bacteria genera with average relative abundance of >0.5% (characterised by 16S rRNA gene sequencing) were recorded in the vaginal swabs of women at baseline and within 72 h of use of either the Tampax Cup or the Other Cup.
The Kruskal–Wallis test results compares vaginal microbiota composition results at baseline and after use of both the Tampax Cup and Other Cup to look for differences.
Table 9.
The vaginal microbiota composition at different sample points; relative abundance.
| Taxon name; genus | Average |
25% Quantile |
Median (50% Quantile) |
75% Quantile |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BL | TC | OC | BL | TC | OC | BL | TC | OC | BL | TC | OC | |
| Lactobacillus | 76.29 | 69.78 | 69.8 | 56.38 | 26.57 | 24.91 | 99.07 | 99.31 | 98.63 | 99.73 | 99.86 | 99.8 |
| Prevotella | 3.95 | 7.32 | 6.84 | 0 | 0 | 0 | 0.01 | 0 | 0 | 0.4 | 3.11 | 4.64 |
| Atopobium | 5.28 | 5.51 | 6.39 | 0 | 0 | 0 | 0 | 0 | 0 | 0.85 | 3.24 | 3.1 |
| Sneathia | 2.75 | 4.65 | 3.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0.02 | 0.36 | 0.02 |
| Megasphaera | 1.05 | 1.15 | 1.87 | 0 | 0 | 0 | 0 | 0 | 0 | 0.56 | 0.37 | 0.77 |
| Streptococcus | 0.39 | 0.5 | 1.43 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.01 |
| Chlamydia | 0.59 | 1.29 | 0.85 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.07 | 0.03 |
| Veillonella | 1.17 | 0.55 | 0.02 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ureaplasma | 0.35 | 1.01 | 0.22 | 0 | 0 | 0 | 0 | 0 | 0 | 0.04 | 0.06 | 0.04 |
Abbreviations: BL, Baseline; KW, Kruskal–Wallis; OC, Other Cup; TC, Tampax Cup.
Individual bacteria genera with average relative abundance of >0.5% (characterised by 16S rRNA gene sequencing) were recorded in the vaginal swabs of women at baseline and within 72 h of use of either the Tampax Cup or the Other Cup.
Fig. 6.
The vaginal microbiota composition. Individual bacteria genera with average relative abundance >0.5% (characterised by 16S rRNA gene sequencing) were graphed in the vaginal swabs of women at baseline and within 72 h of use of either the final, finished Tampax Cup or the Other Cup.
Fig. 7 shows diversity differences among different sampling points or from different subjects. No significant differences in Shannon diversity51,52 were noted among three sampling points of baseline, after Tampax Cup usage or after Other Cup uses (Fig. 7a). Fig. 7b presents the MDS analysis, calculated using Bray–Curtis similarities, serving to evaluate how one sampling point (i.e. baseline, within 72-h after use of the Tampax Cup, within 72-h after use of Other Cup) compared against the other sampling points. All three treatment types were distributed randomly across the plot. Thus, no significant changes in vaginal bacteria community structure were observable due to sampling points.
Fig. 7.
Diversity analysis of the vaginal microbiota. (a) Average Shannon diversity measurement showed no significant differences among 3 sampling points (Baseline, after “Tampax Cup” usage or after “Other Cup” usage). Each dot in the bar graph represents the Shannon diversity measurement from one sample Wilcoxon test p value: 0.11 for Baseline vs. Tampax Cup; 0.18 for Baseline vs. Other Cup; 0.74 for Tampax Cup vs. Other Cup. The red line indicates the mean, pink area indicates the 95% confidence interval and purple area indicates 1 SD (standard deviation) of the mean. (b) Beta diversity by Bray Curtis Distance: Multi-Dimensional Scaling analysis to evaluate sample differences among 3 different sample points (Baseline, Tampax Cup, Other Cup). Treatment type: Red dots: Baseline; blue dots: Final, finished Tampax Cup; Green dots: Other Cup. Each data point represents an entire swab sample compromising many organisms. Each vaginal bacteria community (characterised by 16S rRNA gene sequencing) is plotted against all other communities. If one of the colours (red, blue, green), i.e., sampling points, would cluster, this would demonstrate significant changes in community composition. However, all three sampling points are distributed randomly across the plot (Adonis test p value = 1.00). (c) Beta diversity by Bray Curtis Distance: Multi-Dimensional Scaling analysis to evaluate subject difference from 3 samples collected for one subject (Baseline, Tampax Cup, Other Cup) compared against all other subjects. Subject identification: Each subject (n = 55) has been assigned an individual colour, and for each subject, the swabs at baseline and within 72 h of use of the Tampax Cup or the Other Cup are presented. Thus, there are three dots with one specific colour for each subject. The three swabs for one subject are plotted against all swabs for all other subjects. If the dots for one subject cluster, this demonstrates that subject identification affects the composition of the vaginal bacteria community (characterised by 16S rRNA gene sequencing). It clearly shows that the three swabs for one subject (i.e., three dots with the same colour; blue arrows provide an example from one subject) cluster together (Adonis test p value = 0.001).
Fig. 7c presents the MDS, calculated using Bray-Curtis53 similarities, serving to evaluate how the three swabs for one subject (baseline, Tampax Cup, Other Cup) compared against the respective swabs for all other subjects. The plot clearly shows that for each subject, the respective swabs are clustering together with an Adonis test p value of 0.001. This demonstrates that vaginal bacteria community composition differs between different subjects.
Together, Fig. 7a–c demonstrate that any vaginal bacteria community differences observed are likely due to inter-individual differences in community composition as opposed to use of either the Tampax Cup or the Other Cup. Overall, menstrual cup wear did not induce clinically meaningful changes in the composition and the diversity of the vaginal bacteria community. Observed differences in vaginal microbiome between baseline and post use are explained by statistically significant subject-to-subject variability. These data support the in-use tolerability observed in clinical study of menstrual cups.
The growth of S. aureus and risk for toxic shock syndrome
The in vitro shake flask assay was performed to test the Tampax Cup and the Other Cup along with a medium control as well as the currently marketed Tampax Pearl and competitor tampons, as benchmark controls. Growth of S. aureus MN8 was not affected by either article (Table 10). Also, all articles showed significantly lower TSST-1 μg/mL concentrations when compared to the medium control tested by double immunodiffusion and Western immunoblot analysis (statistical significance evaluated by t-Test; Table 10). Additionally, both the Tampax Cup and the Other Cup showed significantly lower TSST-1 concentrations than the medium control and the currently marketed competitor tampon control.57 These data support the conclusion that the Tampax Menstrual Cup did not increase the growth of S. aureus and TSST-1 toxin production in vitro when compared to currently marketed products.
Table 10.
Effect of menstrual cup on growth of TSS Staphylococcus aureus MN8 and TSST-1 production in the in vitro Shake Flask Assay.
| Product | Growth of TSS Staph. aureus MN8 |
TSST-1 production (μg/mL) |
||
|---|---|---|---|---|
| CFU/mL | Log CFU/mL | Western blots | Double immunodiffusion | |
| Other Cup | 1.1E+10 | 10.04 | 1.33 | 1.2 |
| 1.2E+10 | 10.08 | 1.89 | 1.2 | |
| 9.8E+09 | 9.99 | 0.53 | 1.2 | |
| 1.0E+10 | 10.00 | 0.39 | 1.2 | |
| 1.3E+10 | 10.11 | 2.02 | 1.2 | |
| Mean | 1.1E + 10 | 10.05 | 1.23 | 1.2 |
| SD | Not determined | 0.05 | 0.67 | 0 |
| t-Test | 0.007633 | 0.002855 | ||
| Tampax Cup | 1.0E+10 | 10.00 | 1.21 | 1.2 |
| 1.3E+10 | 10.11 | 1.38 | 1.2 | |
| 9.6E+09 | 9.98 | 0.94 | 1.2 | |
| 9.8E+09 | 9.99 | 1.74 | 1.2 | |
| 9.8E+09 | 9.99 | 0.98 | 1.2 | |
| Mean | 1.0E + 10 | 10.02 | 1.25 | 1.2 |
| SD | Not determined | 0.05 | 0.29 | 0 |
| t-Test | 0.007633 | 0.002855 | ||
| Currently marketed competitor tampon | 1.3E+10 | 10.11 | 50.00 | 38.4 |
| 1.1E+10 | 10.04 | 122.00 | 76.8 | |
| 1.1E+10 | 10.04 | 410.00 | 307.2 | |
| 1.4E+10 | 10.15 | 373.00 | 307.2 | |
| 1.2E+10 | 10.08 | 307.00 | 307.2 | |
| Mean | 1.2E + 10 | 10.09 | 252.40 | 207.36 |
| SD | Not determined | 0.04 | 141.66 | 122.88 |
| t-Test | 0.015399 | 0.016499 | ||
| Control (no product) | 1.1E+10 | 10.04 | 11.70 | 9.6 |
| 1.2E+10 | 10.08 | 23.00 | 19.2 | |
| 9.7E+09 | 9.99 | 54.00 | 19.2 | |
| 1.3E+10 | 10.11 | 22.00 | 19.2 | |
| 1.4E+10 | 10.15 | 56.00 | 38.4 | |
| Mean | 1.2E + 10 | 10.07 | 33.34 | 21.12 |
| SD | Not determined | 0.06 | 18.13 | 9.41 |
Abbreviations: CFU, Colony forming unit; SD, Standard deviation; TSS(T), Toxic shock syndrome (toxin).
All tests were performed in replicate (n = 5). Data analysed via Student's t-test. Bold values indicate Mean and SD of individual datapoints as well as outcomes of T Test.
Discussion
This research article has pursued two aims, i.e. first, to describe the safety assessment scheme developed by Procter & Gamble to establish the safety of feminine hygiene products involving intravaginal use, and second, to apply this scheme for the safety assessment of the recently developed Tampax Menstrual Cup. The safety assessment scheme includes four main components that should be considered: (1) the biocompatibility and chemical safety of the cup constituents; (2) the physical impact to the vaginal mucosa; (3) the impact to vaginal microbiota; and (4) the growth of S. aureus and risk for toxic shock syndrome.
Components 1 and 2 fulfil the requirements in the U.S. FDA Guidance on the Use of ISO 10993-17 that are related to assessments of biocompatibility and physical impact:
“… the potential risks from a biocompatibility perspective should be identified. Such risks might include chemical toxicity, unacceptable biological response to physical characteristics of the device, and aspects of manufacturing and processing that could alter the physicochemical characteristics of the device, which could lead to changes in the biocompatibility response”.7
Components 3 and 4 fulfil the requirements in the U.S. FDA Guidance on Menstrual Tampons and Pads: Information for Premarket Notification Submissions (510(k))s11 that are related to preclinical microbiology and clinical studies:
“For tampon materials, we recommend that you demonstrate that the tampon, in its final manufactured form, does not: (1) enhance the growth of Staphylococcus aureus; (2) increase the production of TSST-1; (3) alter the growth of normal vaginal microflora”,11 and:
“While, in general, clinical studies will not be needed for most menstrual tampons and pads, FDA may recommend that you collect clinical data for [inherently new] menstrual tampons (including applicators, if present) or pads …”.11
Since menstrual cups are exempt from the premarket notification submissions,1 the requirements from the U.S. FDA guidance on menstrual tampons and pads11 are not mandatory for menstrual cups. Further, the safety assessment scheme includes a clinical study that is generally “not needed” following this guidance. Therefore, the safety assessment scheme developed by Procter & Gamble goes beyond what is legally mandated in the USA. This serves to enhance the confidence in the safety of the tested menstrual cups. Further, in selecting methodologies to address its four components, the safety assessment scheme has been designed to avoid the need for animal testing thereby contributing to the 3Rs principle to replace, reduce and refine animal testing.62
While the safety assessment scheme has been described here as it is used to assess the safety of menstrual cups, it can also be applied, with minor modifications, to assess other feminine hygiene articles for intravaginal use. Generally, however, the assessment scheme should at minimum consider the four basic components. Minor modifications may be necessary e.g. to adapt testing conditions to the evaluation of adsorbent materials such as used in tampons and to take into consideration other factors, such as habits and practices of device use, that may influence the extent of testing that is needed.
Below, insight on the scientific relevance and applicability of the four components of the safety assessment scheme for feminine hygiene products involving intravaginal use is discussed in further detail. This discussion also considers the findings from this research article relating to the safety of the tested Tampax Menstrual Cup.
For biocompatibility and chemical safety, extractables were obtained from both the raw material (silicone) and from the final, finished article (Tampax Cup) following different conditions meant to be consistent with ISO 10993 standards and to represent a range of potential scenarios. Data generated from extractable testing were used to augment supplier disclosure documents to build a weight-of-evidence position for chemical safety assessment. Testing included extraction under physiologically relevant yet exaggerated use conditions (37 °C for 72 h), extraction under exaggerated conditions (50 °C for 2 h), and extraction under accelerated conditions (121 °C for 1 h). The extractables within each set showed no significant differences from a chemical and/or toxicological point of view. Thereby, additional chemical characterisation work using leachable and/or simulated use conditions was not needed, and further assessment focused on the extractables obtained under moderate conditions. This approach was not only more technically feasible but also arguably adds an additional degree of conservatism and enhances the standardisation of the risk assessment approach.19
However, the approach selected here should not be generalised. Whenever a new raw material and/or a new final, finished article is evaluated, it is important to apply different extraction conditions representing a range of potential scenarios to allow identifying possible differences in the chromatographic profiling. To further reflect recommended use conditions, extractables were also gained from the Tampax Cup after up to 13 consecutive boiling cycles and after one boiling cycle followed by wiping the inside and outside of the cup with two Always Feminine Wipes (that accompany the commercial packaging of the Tampax Cup in some regions). Again, for other articles such specific use conditions may need to be adapted, as necessary.
The TTC concept21 plays a pivotal role in the evaluation of the biocompatibility and chemical safety of the extractable constituents of the cup and wipes, and it is used in two different ways in the safety assessment scheme. First, a TTC value is applied in the evaluation of the chromatographic profiles, and second a TTC value that is appropriate for the given extractable constituent is applied during exposure-based risk assessment.
Regarding the use of a TTC value in the evaluation of the chromatographic profiles, a TTC of 1.5 μg/day was used following the limit for cumulative exposures (>10 years) to mutagenic impurities laid down in the International Conference on Harmonisation (ICH) M7(R1) Guidance, which was adopted by the U.S. FDA first in 2015 and again in 2018.22 The safety assessment scheme foresees that all extractables that are present in the extraction fluid above this TTC value are identified and quantified and then submitted to exposure-based risk assessment.
In performing the exposure-based risk assessment, it is first assessed if chemical-specific data are available for the given extractable. If so, it is determined if the level of exposure, compared to the appropriate reference dose or risk value, provides a sufficient margin of safety to support its presence in the extraction fluid. If such toxicity data are unavailable, the level of exposure to the respective extractable is compared to an appropriate TTC value and the margin of safety is determined. This serves to eliminate the need for animal testing while at the same time ensuring the chemical safety of the menstrual cup and its constituents.
The TTC concept has been available for more than two decades (see e.g. Munro and colleagues).60 However, it was only in 2016 that the U.S. FDA formally adopted it for the safety assessment of medical devices.63 In this guidance, it was stated, for the first time: “If data are not available to evaluate the safety of a compound, then the concept of Threshold of Toxicological Concern (TTC) can be used to assess some endpoints”.63
Generally, the TTC concept may be used to assess the endpoints of genotoxicity, subchronic/chronic repeated dose toxicity, developmental and reproductive toxicity, chronic toxicity, and carcinogenicity. The U.S. FDA guidance of 201663 refers to the ICH M7 guidance that has since been adopted by the U.S. FDA22 for information on use of the TTC and structure activity relationship modelling to address genotoxicity and carcinogenicity within a risk management process. The formal and more broadly accepted adoption of the TTC concept supports the use of TTC as both a mechanism to set the appropriate analytical threshold for chemical identification and quantification and as a conservative approach to assess the safety of constituents of feminine hygiene articles for intravaginal use if chemical specific toxicity data are unavailable. Thereby, application of the TTC concept replaces the need to apply the ISO 10993-17 process to assess safety for eluting chemicals with limited or no known toxicity data [no/ lowest observed adverse effect level (NOAEL/ LOAEL), etc.] or for chemicals which are identified by chemical class but not by a definitive structure. The acceptance of TTC by various agencies and committees34,64, 65, 66 supports the view that the TTC concept is a conservative approach to assess the safety of constituents of feminine hygiene articles for intravaginal use if chemical specific toxicity data are unavailable.
The TTC concept is currently not applicable for local endpoints.26,27,34 Therefore, the in vitro EpiVaginal™ assay35,36 was selected as preclinical test to evaluate the vaginal irritation and cytotoxicity potential of the Tampax Cup and the accompanying Always Feminine Wipes while at the same time preventing animal testing. The wipes were tested both using a punch and the liquid expressed from a wipe. While the findings provided no indications for safety concerns related to the cup, there was some reduction in viability for the EpiVaginal™ tissues treated with the liquid expressed from a wipe (53.5% viability at 16 h and 77.7% viability at 24 h). Hence, the reduction in viability was more pronounced after the shorter exposure period. Presumably, this points to variability in the test results. Nonetheless, the reduced viability caused by the liquid expressed from a wipe is of subordinate relevance for the overall safety assessment of the wipes (or the cup). First, the assessment of the punch is more indicative of the clinical use situation. Second, the EpiVaginal™ assay was performed as preclinical test to support progress to the clinical trial. The clinical data then showed no findings on vaginal mucosal tissue upon use of the cup. These clinical data are overriding for the overall safety assessment. Of note, the study design of the EpiVaginal™ assay could be augmented by testing for cellular activation, as measured by Interleukin-1b or Interleukin-8 as indicators of inflammation, for situations where a clinical study is not warranted but additional confidence in the irritation profile of the eluting materials is needed.
As regards physical impact to the vaginal mucosa, there were few potentially product-related adverse events in the clinical trial, and all of these were mild in severity, and none were unexpected or unusual for menstruating women or cup users. No subject withdrew from the study due to a cup-related adverse event. The use experience and sensorial results further corroborate the conclusion that the Tampax Cup is well-tolerable. Increased PVR is considered an indirect indicator of increased risk for urinary tract infection. There was no clinically meaningful impact of cup use on PVR based on the lack of any consistent pattern of change in PVR. Importantly, no subjects reported urinary tract infection throughout the conduct of this study (Section Randomised clinical trial). Some subjects did perceive an impact to urination and bowel movements. However, this is also not uncommon during menstruation and during the use of feminine hygiene articles involving intravaginal use. Generally, Tampax Cups trended to have more unfavourable perception of discomfort with insertion and removal but trended better on wearing comfort as compared to the Other Cup. It is not unexpected to have comments of insertion/wear/removal discomfort for menstrual cups (and other internally worn devices) and overall, we consider the Tampax Cup as comparable in tolerability to the other marketed cup tested in this study. We continue to monitor in market data as part of our end-to-end safety programme.
For both cups, there were minor changes in the pH value from baseline (approx. 0.4); however, the pH measurements for the Tampax Cup and Other Cup were comparable. Data reported in the literature show normal variability in vaginal pH throughout the menstrual cycle with higher pH during menses when blood is present in the vagina and lower pH, typically between 4 and 4.5, at other times during the cycle.67 In the present study, it is possible that vaginal pH samples, taken within 72 h of last product use, were obtained when vaginal pH remained elevated, while baseline vaginal pH measurements were taken at a time in the cycle when vaginal pH is expected to be lower. The day of menstrual cycle at baseline visit was not determined. This, along with a lack of precision associated with use of pH strips, could explain the minor differences between the baseline, Tampax Cup and Other Cup pH levels. Importantly, no changes in vaginal discharge or in the microbiome community were noted with use of either cup further supporting the lack of clinical relevance of these pH changes.
Assessments of the implications of the wear time on cup tolerability formed an important aspect of the clinical trial. Average wear time was 8.9 h for the Tampax Cup (and 8.6 h for the Other Cup). While the majority of cup uses (81%) were less than 12 h, 30 subjects (of the total of 65) had at least one cup use of 12 or more hours, and for 11 subjects (17%) all wear times were 12 h or more. The findings for the subjects and cup uses with extended wear times were consistent with shorter wear times. This showed that the Tampax Cup was also well-tolerated at extended wear time with no impact on vaginal health endpoints. This supports the conclusion that use of the Tampax Menstrual Cup is well-tolerated and safe regardless of the use practice (> 12 hours) of the individual woman.
Regarding assessments of the impact to vaginal microbiota, the in vitro mixed microflora assay developed in collaboration between Procter & Gamble and Microbiologists Specialists, Inc. (Houston TX, USA has been published here for the first time in the peer review scientific literature. Therefore, a detailed test protocol has been included as Supplementary Information SI-7. The in vitro mixed microflora assay was developed as standardised test methods addressing the potential impact of intravaginal feminine hygiene articles on the vaginal microbiome were unavailable. Indeed, we have successfully used data from the in vitro mixed microflora assay in the context of U.S. FDA 510K requirements for tampons.11 The six microorganisms included in the assay were selected to simulate the heterogenous nature of the healthy vaginal microbiome while also including organisms of that may give rise to infection or disease. Also, the proportions of the six microorganisms were selected to resemble their relative abundance in the vaginal microbiome.43
Species of Lactobacillus or other acid producing bacteria are generally the most abundant microorganism present in the healthy vagina.67, 68, 69 At the time this assay was developed approximately 20 years ago, little was known about the human microbiome. Culture-dependent techniques were the only technologically sound and accurate tools available to study various micro-communities; more advanced techniques such as 16S rRNA gene sequencing were years away from regular use. We chose L. gasseri because it was a strain derived from the vaginal tract and readily available through the American Type and Culture Collection (ATCC). We continue to use L. gasseri (ATCC 9857) because it is one of the more commonly found commensal Lactobacillus species in the vagina,69,70 is fairly easy to isolate and maintain in the laboratory environment and is not known to carry pathogenic potential like Lactobacillus iners.71,72 Some microorganisms included in the consortium are potentially pathogenic, i.e. G. vaginalis and P. bivia are present in high concentrations in bacterial vaginosis; E. coli is commonly associated with urinary tract infections; S. aureus, is the bacterium associated with menstrual toxic shock syndrome, and C. albicans is the yeast associated with vaginal candidiasis.42,69,73 The proportions of the six microorganisms in the final co-inoculum are approx. 108 organisms/mL for L. gasseri,74 approx. 105 organisms/mL for G. vaginalis,75,76 P. bivia,76,77 and E. coli,73,76,78,79 respectively, and approx. 104 organisms/mL for S. aureus80 and C. albicans,73,78,79,81 respectively.
While the mixed microflora assay does not broadly represent the diversity of the vaginal microbiome composition, especially the condition of dysbiosis or non-optimal composition, it does inform on the potential impact of the medical device on a consortium of vaginally relevant organisms and thus enables further clinical testing. In the case of the Tampax Cup, the in vitro mixed microflora assay, that yielded no unfavourable findings, served as preclinical test to support progress to the clinical trial.
The assessment of the vaginal swabs taken during the clinical trial showed that any community differences observed were rather caused by subject-to-subject variability than by use of either the Tampax Cup or the Other Cup. The spectrum of bacteria present in the samples was analysed by culture-independent molecular assessment by determining and classifying the gene sequences of amplicons of the target regions of the 16S rRNA gene. The 16S rRNA gene, as a critical component of cell function, is highly conserved across prokaryotes; changes in the 16S rRNA gene sequence mark evolutionary distance and relatedness of organisms.82 Comparative assessments of 16S rRNA gene sequences allow determining the spectrum of bacteria present in a sample, up to the genus level.82 In the last decade, next-generation sequencing of the 16S rRNA gene amplicons, such as used in the present study, has facilitated high-throughput assessments of bacterial samples, including those taken by vaginal swabbing.49,69,83, 84, 85
Previously, Forney and colleagues86 and Ravel and colleagues69 described that the vaginal bacteria communities of 396 asymptomatic North American women representing four ethnic groups (white, black, Hispanic, and Asian) clustered into five groups: Four clusters were dominated by L. iners, Lactobacillus crispatus, L. gasseri, or Lactobacillus jensenii, whereas the fifth had lower proportions of lactic acid bacteria and higher proportions of strictly anaerobic organisms (i.e. a ‘diverse community’). The proportions of each community group varied among the four ethnic groups in a statistically significant manner. Nonetheless, over all ethnic groups, the vaginal bacteria communities that were dominated by L. iners and L. crispatus, respectively, as well as the diverse community were by far the most frequent.69,86 Similarly, most of the subjects included in the clinical trial presented here (n = 55) exhibited a vaginal bacterial community that was dominated by L. crispatus (n = 21), L. iners (n = 14) or the ‘diverse community’ (n = 16). For three subjects, L. gasseri was predominant, whereas L. jensenii was predominant for one subject (Supplementary Information SI-11). In the heat maps presented in the Supplemental Figs. SI-11.1, SI-11.2 and SI-11.3, each column reflects one subject from the clinical trial (n = 55), and each row the different taxa, with columns being clustered by vaginal bacteria community. These heat maps confirm the dynamics of subject-to-subject variability and provide further clarity that any community differences observed are likely attributed to these dynamics rather than use of either the Tampax Cup or the Other Cup.
It should be noted that the primary focus of the clinical study was to assess the overall safety of the Tampax Cup. Therefore, the clinical trial did not control for all aspects that would allow for an in-depth evaluation of the vaginal microbiome: First, the day of menstrual cycle at baseline visit was not pre-determined. Since the composition of the vaginal microbiome fluctuates throughout the menstrual cycle, the influence of day of menstrual cycle on the vaginal microbiome could not be considered in the evaluation of the vaginal swabs. Second, sexual activity, that might also affect the vaginal microbiome, was unrestricted at baseline. While the clinical trial was not designed with these details in mind, the data, which show lack of statistically significant changes at baseline and between the two test products, do add to the weight-of-evidence already presented and imply that there is no indication for an impact of cup use on the vaginal microbiome.
Toxic shock syndrome was first described by Todd and colleagues87 as a very rare, but potentially life-threatening disease caused by specific S. aureus strains that produce a specific exotoxin, which has since been called TSST-1. Early on, toxic shock syndrome was associated with use of tampons during menstruation and more recently has been noted with other vaginally used products such as diaphragms, intrauterine devices, pessaries, contraceptive sponges and menstrual cups.88 As a risk-mitigating measure, the U.S. FDA requires user labelling for menstrual tampons to alert consumers to the potential for toxic shock syndrome89; however, this same requirement has not been implemented for menstrual cups.
By comparison, in the U.S. FDA Guidance on Menstrual Tampons and Pads: Information for Premarket Notification Submissions (510(k)s),11 there are no reference methods or recognised standards for testing whether tampons (or menstrual cups) enhance the growth of S. aureus or increase the production of TSST-1. However, this guidance does provide a list of published methods, including the methodology described by Schlievert and Blomster.56 This method was selected for the Procter & Gamble safety assessment scheme since it has a long history of use (see e.g. Schlievert)57 and an acceptance with the U.S. FDA.
The data generated in the in vitro shake flask assay for the Tampax Cup showed no effect on S. aureus growth and TSST-1 toxin production. These data, along with the favourable mixed microflora assay results, provide some confidence that the Tampax Cup has a similar risk profile with regards to toxic shock syndrome as currently marketed products. However, due to the extremely low incidence of toxic shock syndrome,87 it is difficult to fully dimension the risk through in vitro or clinical testing alone, and as such close monitoring in market is essential. Additionally, to further mitigate the risk and assure safe use, women are appropriately informed of the signs and symptoms of toxic shock syndrome through package labelling, use instructions, and via other outlets (e.g. company website) and, importantly, they are instructed what to do should they experience signs or symptoms of toxic shock syndrome. This labelling is not required by the U.S. FDA or in any European Union member state today, but this information is available online and is included on pack and in package inserts for all Tampax Menstrual Cups. Following the marketing of the Tampax Cup, post-market surveillance serves to corroborate the overall conclusion that its use does not increase the risk of toxic shock syndrome.
The safety assessment conclusion has been confirmed during post-market safety surveillance (2018 to present) where consumer reported data, collected, processed, and analysed, through a standardised process is comparable in terms of incidence and severity to the adverse event profile established for menstrual cups in the published medical literature2,3,5,12,14 or e.g. in the U.S. FDA Manufacturers and User Facility Device Experience (MAUDE) Database.90
In conclusion, this research article has described the safety assessment scheme developed by Procter & Gamble to establish the safety of feminine hygiene products involving intravaginal use. While focus of this article was on the safety assessment of menstrual cups, Procter & Gamble also uses this safety assessment scheme, or versions thereof, for most other feminine hygiene products, e.g. tampons, pads, adult incontinence products. The safety assessment scheme includes four components, (1) the biocompatibility and chemical safety of the cup constituents; (2) the physical impact to the vaginal mucosa; (3) the impact to vaginal microbiota; and (4) the growth of S. aureus and risk for toxic shock syndrome. For any given feminine hygiene product, the extent of testing will depend on the type of product and its specific use.
Further, this research article has served to present data that were collected in applying this quadripartite scheme for the safety assessment of the recently developed Tampax Menstrual Cup. Generally, the presented data show that the Tampax Cup is safe for use. Under several, also exaggerated extraction conditions, no extractables were obtained from the cup that differed from those of the raw material (silicone). Boiling the cup for up to 13 boiling cycles as well as boiling it once followed by wiping it with Always Feminine Wipes also did not yield any extractables posing safety concerns. All extractables either did not present safety concerns per se or were obtained at concentrations providing a sufficient margin of safety as compared to the relevant TTC. Further, the physical impact to the vaginal mucosa was deemed acceptable (and comparable to that caused by other currently marketed cups) as the few potentially product-related adverse effects were mild in severity and none were unexpected or unusual. Extended wear times of 12 h or more also did not affect the tolerability or safety of the Tampax Cup. Finally, the Tampax Cup did not adversely affect the vaginal microbiota (as assessed both in the in vitro mixed microflora assay and using vaginal swabs taken during the clinical trial) nor did it increase the risk for toxic shock syndrome beyond that caused by other currently marketed products.
Taken together, the comprehensive database shows that the Tampax Menstrual Cup does not pose a safety concern and is well-tolerated upon intended use. This conclusion has been confirmed during post-market safety surveillance.
Contributors
All authors contributed to preparation and review of text and approved the final version of the manuscript. VPS, KLK, JMP and TRB participated in study design and data interpretation of chemical characterisation work. VPS and KLK provided technical oversight for analytical chemical characterisation work, analysed samples, and reviewed data. MAF provided technical oversight and data generation/interpretation for the in vitro mixed microflora assay. JLSS designed and provided oversight, as well as data interpretation, of the clinical study. AGT and PH provided advice on the interpretation of genomic data and drafted manuscript figures. UGS, as a freelance scientific consultant and scientific writer, prepared the current manuscript. KEW and JMAN conceived overall programme design, oversaw acquisition and analysis of data (thereby, KEW and JMAN accessed and verified all data) and provided technical oversight of the current manuscript. All authors read and approved the final version of the manuscript. KEW and JMAN were responsible for the decision to submit the manuscript.
Data sharing statement
All the data generated or analysed from these studies are included in this published article. All data supporting the findings in these studies are also available from the corresponding author upon request.
Declaration of interests
VPS, MAF, AGT, JLSS, PH, KLK, JMP, TRB, JMAN and KEW are employed by The Procter & Gamble Company (P&G), a consumer products corporation that produces and markets menstrual cups. These authors received no funding in cash or kind for their contribution to this manuscript (beyond the salaries paid) but do hold P&G company stock. UGS was hired by P&G to draft the current manuscript and received payment for working hours. The authors alone are responsible for the writing of and contents of this manuscript.
Acknowledgements
Mike Laufersweiler (Procter & Gamble) is thanked for the evaluation of eluting, data-deficient chemicals, and assignment to the appropriate TTC class. Rocio Navarro Garcia (Research and Testing Laboratories Genomics, Lubbox, TX, USA) is thanked for vaginal microbiota analysis. Michael Noss, MD and Margaret Rhee, MD (Synexus, Cincinnati, OH, USA) and Stacey Carpenter (Procter & Gamble) are thanked for oversight and conduct of the clinical study. Lisa Bohman, Lisa Bowman, Yu Wang and Dionne Swift (Procter & Gamble) are thanked for management and statistical analysis of data generated in the clinical study. Gertrude-Emilia Costin and Adam Gamson (Institute for In Vitro Sciences, Inc., Gaithersburg, MD, USA) are thanked for the oversight, conduct and reporting of the EpiVaginal™ study. Catherine Davis, PhD (Creighton University, Omaha, NE, USA) as well as Alice Weissfeld and Ernest Trevino (Microbiology Specialists Incorporated, Houston, TX, USA) are thanked for method development of the mixed microflora assay. Microbiologist Patrick Schlievert, PhD (University of Iowa, Iowa City, IA, USA) is thanked for the generation of TSST-1 shake flask data.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104339.
Appendix A. Supplementary data
References
- 1.U.S. Code of Federal Regulations . 2019. Title 21 Food and Drugs, Chapter I Food and Drug Administration Department of Health and Human Services; Subchapter H Medical Devices; Part 884 Obstetrical and Gynecological Therapeutic Devices. 21CFR884.5400. [Google Scholar]
- 2.Liswood R. Internal menstrual protection; use of a safe and sanitary menstrual cup. Obstet Gynecol. 1959;13(5):539–543. [PubMed] [Google Scholar]
- 3.Karnaky K.J. Internal menstrual protection with the rubber menstrual cup. Obstet Gynecol. 1962;19:688–691. [PubMed] [Google Scholar]
- 4.Stewart K., Powell M., Greer R. An alternative to conventional sanitary protection: would women use a menstrual cup? J Obstet Gynaecol. 2009;29(1):49–52. doi: 10.1080/01443610802628841. [DOI] [PubMed] [Google Scholar]
- 5.Howard C., Rose C.L., Trouton K., et al. FLOW (finding lasting options for women): multicentre randomized controlled trial comparing tampons with menstrual cups. Can Fam Physician. 2011;57(6):e208–e215. [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Food and Drug Administration . 2021. Product classification; cup, menstrual.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=4133 [Google Scholar]
- 7.U.S. FDA. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Devices and Radiological Health. Center for Biologics Evaluation and Resesarch . U.S. FDA; 2020. Use of international standard ISO 10993-1, “biological evaluation of medical devices – Part 1: evaluation and testing within a risk management process”. Guidance for Industry and FDA Staff. [Google Scholar]
- 8.French-Mowat E., Burnett J. How are medical devices regulated in the European Union? J R Soc Med. 2012;105(Suppl 1):S22–S28. doi: 10.1258/jrsm.2012.120036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamph S. Regulation of medical devices outside the European Union. J R Soc Med. 2012;105(Suppl 1):S12–S21. doi: 10.1258/jrsm.2012.120037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernard M., Jubeli E., Pungente M.D., Yagoubi N. Biocompatibility of polymer-based biomaterials and medical devices – regulations, in vitro screening and risk-management. Biomater Sci. 2018;6(8):2025–2053. doi: 10.1039/c8bm00518d. [DOI] [PubMed] [Google Scholar]
- 11.U.S. FDA. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Devices and Radiological Health . U.S. FDA; 2005. Guidance on menstrual tampons and pads: information for premarket notification submissions (510(k)s). Guidance for Industry and FDA Staff. [Google Scholar]
- 12.North B.B., Oldham M.J. Preclinical, clinical and over-the-counter postmarketing experience with a new vaginal cup: menstrual collection. J Women Health. 2011;20(2):303–311. doi: 10.1089/jwh.2009.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toxicology Subgroup of the Tripartite Sub-Committee on Medical Devices . 1986. Tripartite Biocompatibility Guidance for Medical Devices.https://journals.sagepub.com/doi/pdf/10.3109/10915818809019524 [Google Scholar]
- 14.Van Eijk A.M., Zulaika G., Lenchner M., et al. Menstrual cup use, leakage, acceptability, safety, and availability: a systematic review and meta-analysis. Lancet Public Health. 2019;4:e376–e393. doi: 10.1016/S2468-2667(19)30111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dow Corning. 2015. Liquid Silicone Rubber: Global Product Selection Guide – Expand Your Possibilities.https://www.dow.com/content/dam/dcc/documents/en-us/catalog-selguide/95/95-12/95-1226-01-liquid-silicone-rubber-product-selection-guide.pdf?iframe=true Form No. 95-1226A-01. [Google Scholar]
- 16.DuPont . DuPont; 2020. Chemical equivalency certificate. DOW CORNINGTM QP1-40 liquid silicone rubber kit; signed by Todd Ostergaard, Product Stewardship and Regulatory Manager, Sustainability Leader, Transportation and Industrial Business.https://www.knowde.com/stores/dupont/documents/206807 [Google Scholar]
- 17.DuPont. Healthcare Solutions . 2020. DuPontTM LiveoTM. QP1-2XX Liquid Silicone Rubber Materials.https://www.dupont.com/content/dam/dupont/amer/us/en/transportation-industrial/public/documents/en/QP1-2XX%20Liquid%20Silicone%20Rubber%20Materials_001-20384-CDP0820_A4-digital.pdf Form Number 001-20384-CDP0820. [Google Scholar]
- 18.United States Pharmacopeia – National Formulary 2020. http://www.uspbpep.com/usp29/v29240/usp29nf24s0_c88.html General Chapter <88> Biological reactivity tests, in vivo.
- 19.Sica V.P., Krivos K.L., Kiehl D.E., Pulliam C.J., Henry I.D., Baker T.R. The role of mass spectrometry and related techniques in the analysis of extractable and leachable chemicals. Mass Spectrom Rev. 2020;39:212–226. doi: 10.1002/mas.21591. [DOI] [PubMed] [Google Scholar]
- 20.Sica V.P., Mahony C., Baker T.R. Multi-detector characterization of grape seed extract to enable in silico safety assessment. Front Chem. 2018;6:334. doi: 10.3389/fchem.2018.00334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroes R., Renwick A.G., Cheeseman M., et al. European branch of the International Life Sciences Institute. Structure-based thresholds of toxicological concern (TTC): guidance for application to substances present at low levels in the diet. Food Chem Toxicol. 2004;42(1):65–83. doi: 10.1016/j.fct.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 22.U.S. FDA. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Drug Evaluation and Research. Center for Biologics Evaluation and Research . U.S. FDA; 2018. ICH Guideline M7 (R1) on assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. Guidance for Industry. [Google Scholar]
- 23.National Research Council . The National Academies Press; Washington, DC: 1983. Risk Assessment in the Federal Government: Managing the Process. [DOI] [PubMed] [Google Scholar]
- 24.National Research Council . The National Academies Press; Washington, DC: 2009. Science and Decisions: Advancing Risk Assessment. [DOI] [PubMed] [Google Scholar]
- 25.U.S. Environmental Protection Agency . U.S. EPA; 2014. Framework for human health risk assessment to inform decision making. April 5, 2014. EPA/100/R-14/001. [Google Scholar]
- 26.Yang C., Barlow S.M., Muldoon Jacobs K.L., et al. Thresholds of Toxicological Concern for cosmetics-related substances: new database, thresholds, and enrichment of chemical space. Food Chem Toxicol. 2017;109(Pt 1):170–193. doi: 10.1016/j.fct.2017.08.043. [DOI] [PubMed] [Google Scholar]
- 27.Patlewicz G., Wambaugh J.F., Felter S.P., Simon T.W., Becker R.A. Utilizing threshold of toxicological concern (TTC) with high throughput exposure predictions (HTE) as a risk-based prioritization approach for thousands of chemicals. Comput Toxicol. 2018;7:58–67. doi: 10.1016/j.comtox.2018.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroes R., Kleiner J., Renwick A. The threshold of toxicological concern concept in risk assessment. Toxicol Sci. 2005;86(2):226–230. doi: 10.1093/toxsci/kfi169. [DOI] [PubMed] [Google Scholar]
- 29.Cramer G.M., Ford R.A., Hall R.L. Estimation of toxic hazard – a decision tree approach. Food Chem Toxicol. 1978;16:255–276. doi: 10.1016/s0015-6264(76)80522-6. [DOI] [PubMed] [Google Scholar]
- 30.Nishijo T., Api A.M., Gerberick G.F., et al. Application of the dermal sensitization threshold concept to chemicals classified as high potency category for skin sensitization assessment of ingredients for consumer products. Regul Toxicol Pharmacol. 2020;117:1–8. doi: 10.1016/j.yrtph.2020.104732. [DOI] [PubMed] [Google Scholar]
- 31.Roberts D.W., Api A.M., Safford R.J., Lalko J.F. Principles for identification of high potency category chemicals for which the dermal sensitisation threshold (DST) approach should not be applied. Regul Toxicol Pharmacol. 2015;72:683–693. doi: 10.1016/j.yrtph.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Safford R.J., Aptula A.O., Gilmour N. Refinement of the Dermal Sensitisation Threshold (DST) approach using a larger dataset and incorporating mechanistic chemistry domains. Regul Toxicol Pharmacol. 2011;60:218–224. doi: 10.1016/j.yrtph.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 33.Safford R.J., Api A.M., Roberts D.W., Lalko J.F. Extension of the Dermal Sensitisation Threshold (DST) approach to incorporate chemicals classified as reactive. Regul Toxicol Pharmacol. 2015;72(3):694–701. doi: 10.1016/j.yrtph.2015.04.020. [DOI] [PubMed] [Google Scholar]
- 34.Scientific Committee on Consumer Safety (SCCS) Scientific Committee on Health and Environmental Risks (SCHER) Scientific Committee on Emerging and Newly Identified Health Risks (SCENIHR) European Commission, Directorate-General for Health & Consumers; 2012. Opinion on the use of the threshold of toxicological concern (TTC) approach for human safety assessment of chemical substances with focus on cosmetics and consumer products. SCCP/1171/08. [Google Scholar]
- 35.Ayehunie S., Cannon C., Lamore S., et al. Organotypic human vaginal-ectocervical tissue model for irritation studies of spermacides, microbicides, and feminine-care products. Toxicol Vitro. 2006;20(5):689–698. doi: 10.1016/j.tiv.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 36.Ayehunie S., Cannon C., Larosa K., Pudney J., Anderson D.J., Klausner M. Development of an in vitro alternative assay method for vaginal irritation. Toxicol. 2011;279(1–3):130–138. doi: 10.1016/j.tox.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berridge M.V., Tan A.S., McCoy K.D., Wang R. The biochemical and cellular basis of cell proliferation assays that use tetrazolium salts. Biochemica. 1996;4:14–19. [Google Scholar]
- 38.Barnhart K.T., Izquierdo A., Pretorius E.S., Shera D.M., Shabbout M., Shaunik A. Baseline dimensions of the human vagina. Hum Reprod. 2006;21(6):1618–1622. doi: 10.1093/humrep/del022. [DOI] [PubMed] [Google Scholar]
- 39.Luo J., Betschart C., Ashton-Miller J.A., DeLancey J.O. Quantitative analyses of variability in normal vaginal shape and dimension on MR images. Int Urogynecol J. 2016;27(7):1087–1095. doi: 10.1007/s00192-016-2949-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen B., Galask R.P. Vaginal microbial flora: composition and influences of host physiology. Ann Intern Med. 1982;96:926–930. doi: 10.7326/0003-4819-96-6-926. [DOI] [PubMed] [Google Scholar]
- 41.Geshnizgani A.M., Onderdonk A.B. Defined medium simulating genital tract secretions for growth of vaginal microflora. J Clin Microbiol. 1992;30:1323–1326. doi: 10.1128/jcm.30.5.1323-1326.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pybus V., Onderdonk A.B. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: potential significance for bacterial vaginosis. J Infect Dis. 1997;175(2):406–413. doi: 10.1093/infdis/175.2.406. [DOI] [PubMed] [Google Scholar]
- 43.Onderdonk A.B., Zamarchi G.R., Walsh J.A., Mellor R.D., Muñoz A., Kass E.H. Methods for quantitative and qualitative evaluation of vaginal microflora during menstruation. Appl Environ Microbiol. 1986;51(2):333–339. doi: 10.1128/aem.51.2.333-339.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Song S.D., Acharya K.D., Zhu J.E., et al. Daily vaginal microbiota fluctuations associated with natural hormonal cycle, contraceptives, diet, and exercise. mSphere. 2020;5(4) doi: 10.1128/mSphere.00593-20. 005933-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moosa Y., Kwon D., de Oliveira T., Wong E.B. Determinants of vaginal microbiota composition. Front Cell Infect Microbiol. 2020;10:467. doi: 10.3389/fcimb.2020.00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xu J., Bian G., Zheng M., et al. Fertility factors affect the vaginal microbiome in women of reproductive age. Am J Reprod Immunol. 2020;83(4) doi: 10.1111/aji.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaur H., Merchant M., Haque M.M., Mande S.S. Crosstalk between female gonadal hormones and vaginal microbiota across various phases of women's gynecological lifecycle. Front Microbiol. 2020;11:551. doi: 10.3389/fmicb.2020.00551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Genomics R.T.L. 2019. Data Analysis Methodology for Microbial Diversity. Version 2.3.2.http://www.rtlgenomics.com/docs/Data_Analysis_Methodology.pdf [Google Scholar]
- 49.Teufel A., Howard B., Hu P., Carr A.N. Characterization of the microbiome in the infant diapered area: Insights from healthy and damaged skin. Exp Dermatol. 2020 doi: 10.1111/exd.14198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oksanen J., Simpson G.L., Blanchet F.G., et al. 2020. Package ‘vegan’. Community Ecology Package.https://cran.r-project.org/web/packages/vegan/vegan.pdf [Google Scholar]
- 51.Shannon C.E. A mathematical theory of communication. Bell System Tech J. 1948;27:379–423. [Google Scholar]
- 52.Sherwin W.B. Entropy, or information, unifies ecology and evolution and beyond. Entropy. 2018;20:727. doi: 10.3390/e20100727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bray J.R., Curtis J.T. An ordination of upland forest communities of southern Wisconsin. Ecolog Monogr. 1957;27:325–349. [Google Scholar]
- 54.Whittaker R.H. Evolution and measurement of species diversity. Taxon. 1972;21(2–3):213–251. [Google Scholar]
- 55.Anderson M.J. A new method for non-parametric multivariate analysis of variance. Austral Ecol. 2001;26(1):32–46. [Google Scholar]
- 56.Schlievert P.M., Blomster D.A. Production of Staphylococcal pyrogenic exotoxin Type C: influence of physical and chemical factors. J Infect Dis. 1983;147(2):236–242. doi: 10.1093/infdis/147.2.236. [DOI] [PubMed] [Google Scholar]
- 57.Schlievert P.M. Effect of non-absorbent intravaginal menstrual/contraceptive products on Staphylococcus aureus and production of the superantigen TSST-1. Eur J Clin Microbiol Infect Dis. 2020;39(1):31–38. doi: 10.1007/s10096-019-03685-x. [DOI] [PubMed] [Google Scholar]
- 58.Ouchterlony O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1962;6:30–54. doi: 10.1159/000313795. [DOI] [PubMed] [Google Scholar]
- 59.Schmitt B.G., Jensen E., Laufersweiler M.C., Rose J.L. Threshold of Toxicological Concern: extending the chemical space by inclusion of a highly curated dataset for organosilicon compounds. Regul Toxicol Pharmacol. 2021;127:1–13. doi: 10.1016/j.yrtph.2021.105074. [DOI] [PubMed] [Google Scholar]
- 60.Munro I.C., Ford R.A., Kennepohl E., Sprenger J.G. Thresholds of toxicological concern based on structure-activity relationships. Drug Metab Rev. 1996;28(1–2):209–217. doi: 10.3109/03602539608994000. [DOI] [PubMed] [Google Scholar]
- 61.National Health and Nutrition Examination Survey database 2022. https://www.cdc.gov/nchs/nhanes/index.htm
- 62.Russell WMS, Burch RL. The principles of humane experimental technique. London, UK: Methuen. Reprinted by UFAW, 1992: 8 Hamilton Close, South Mimms, Potters Bar, Herts EN6 3QD England. 238:1959.
- 63.U.S. FDA. U.S. Department of Health and Human Services. Food and Drug Administration. Center for Devices and Radiological Health . U.S. FDA; 2016. Use of International Standard ISO 10993-1, "Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process. Guidance for Industry and FDA Staff. [Google Scholar]
- 64.Scientific Committee on Consumer Safety . October 2018. Notes of guidance for the testing of cosmetic ingredients and their safety evaluation; 10th revision – SCCS/1602/18; adopted at the SCCS plenary meeting on 24-25. [Google Scholar]
- 65.European Medicines Agency . Committee for Medicinal Products for Human Use (CHMP), Committee for Medicinal Products for Veterinary Use (CVMP); 2014. Guideline on the setting of health-based exposure limits for use in risk identification in the manufacture of different medicinal products in shared facilities. EMA/CHMP/ CVMP/ SWP/169430/2012. [Google Scholar]
- 66.European Food Safety Authority European Food Safety Authority Scientific Committee: guidance on the use of the Threshold of Toxicological Concern approach in food safety assessment (More SJ, Bampidis V, Benford D, Bragard C, Halldorsson TI, Hernández-Jerez AF et al.) EFSA J. 2019;17(6):5708. doi: 10.2903/j.efsa.2019.5708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Eschenbach D.A., Thwin S.S., Patton D.L., et al. Influence of the normal menstrual cycle on vaginal tissue, discharge, and microflora. Clin Infect Dis. 2000;30(6):901–907. doi: 10.1086/313818. [DOI] [PubMed] [Google Scholar]
- 68.Boris S., Barbes C. Role played by lactobacilli in controlling the population of vaginal pathogens. Microbes Infect. 2000;2:543–546. doi: 10.1016/s1286-4579(00)00313-0. [DOI] [PubMed] [Google Scholar]
- 69.Ravel J., Gajer P., Abdo Z., et al. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA. 2011;108(Suppl 1):4680–4687. doi: 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pan M., Hidalgo-Cantabrana C., Goh Y.H., Sanozky-Dawes R., Barrangou R. Comparative analysis of Lactobacillus gasseri and Lactobacillus crispatus isolated from human urogenital and gastrointestinal tract. Front Microbiol. 2020;10:1–17. doi: 10.3389/fmicb.2019.03146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zheng N., Guo R., Wang J., Zhou W., Ling Z. Contribution of Lactobacillus iners to the vaginal health and disease: a systematic review. Front Cell Infect Microbiol. 2021;11:1–12. doi: 10.3389/fcimb.2021.792787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mass General Press Release. Research identify potential approaches to modify vaginal microbiome. 2022. https://www.massgeneral.org/news/press-release/Researchers-identify-potential-approaches-to-modify-vaginal-microbiome [Google Scholar]
- 73.Baron E.J., Cassell G.H., Duffy L.B., et al. In: Cumulative techniques and procedures in clinical microbiology (Cumitech 17A) Baron E.J., editor. American Society for Microbiology (ASM); Washington DC: 1993. Cumitech 17A. Laboratory diagnosis of female genital infections. [Google Scholar]
- 74.Redondo-Lopez V., Cook R.L., Sobel J.D. Emerging role of Lactobacilli in the control and maintenance of the vaginal bacterial microflora. Rev Infect Dis. 1990;12(5):856–872. doi: 10.1093/clinids/12.5.856. [DOI] [PubMed] [Google Scholar]
- 75.Onderdonk A.B., Wissemann K.W. In: Vulvovaginitis. Elsner P., Martins J., editors. Marcel Dekker; New York, NY: 1993. Normal vaginal microflora; pp. 285–304. [Google Scholar]
- 76.Hiroshige M., Kawazoe K., Izumi K., Sato Y., Tamaya T. Studies on the clinical implications of anaerobes, especially Prevotella bivia in obstetrics and gynecology. J Infect Chemother. 1998;4:177–187. [Google Scholar]
- 77.Coolen M.J.L., Post E., Davis C.C., Forney L.J. Characterization of microbial communities found in the human vagina by analysis of terminal restriction fragment length polymorphisms of 16S rRNA genes. Appl Env Microbiol. 2005;71(12):8729–8737. doi: 10.1128/AEM.71.12.8729-8737.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shehin S.E., Jones M.B., Hochwalt A.E., Sarbaugh F.C., Nunn S. Clinical safety-in-use study of a new tampon design. Infect Dis Obstet Gynecol. 2003;11:89–99. doi: 10.1080/10647440300025504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chow A.W., Percival-Smith R., Bartlett K.H., Goldring A.M., Morrison B.J. Vaginal colonization with Escherichia coli in healthy women. Determination of relative risks by quantitative culture and multivariate statistical analysis. Am J Obstet Gynecol. 1986;154(1):120–126. doi: 10.1016/0002-9378(86)90406-0. [DOI] [PubMed] [Google Scholar]
- 80.Johnson S.R., Petzold C.R., Galask R.P. Qualitative and quantitative changes of the vaginal microbial flora during the menstrual cycle. Am J Reprod Immunol Microbiol. 1985;9(1):1–5. doi: 10.1111/j.1600-0897.1985.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 81.Barousse M.M., Van Der Pol B.J., Fortenberry D., Fidel P.L. Vaginal yeast colonization, prevalence of vaginitis, and associated local immunity in adolescents. Sex Transm Infect. 2004;80:48–53. doi: 10.1136/sti.2002.003855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Clarridge J.E., 3rd Impact of 16S rRNA gene sequence analysis for identification of bacteria on clinical microbiology and infectious diseases. Clin Microbiol Rev. 2004;17(4):840–862. doi: 10.1128/CMR.17.4.840-862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hamady M., Walker J.J., Harris J.K., Gold N.J., Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods. 2008;5(3):235–237. doi: 10.1038/nmeth.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sanschagrin S., Yergeau E. Next-generation sequencing of 16S ribosomal RNA gene amplicons. J Vis Exp. 2014;29(90) doi: 10.3791/51709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gliniewicz K., Schneider G.M., Ridenhour B.J., et al. Comparison of the vaginal microbiomes of premenopausal and postmenopausal women. Front Microbiol. 2019;10:193. doi: 10.3389/fmicb.2019.00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Forney L.J., Gajer P., Williams C.J., et al. Comparison of self-collected and physician-collected vaginal swabs for microbiome analysis. J Clin Microbiol. 2010;48(5):1741–1748. doi: 10.1128/JCM.01710-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Todd J., Fishaut M., Kapral F., Welch T. Toxic-shock syndrome associated with phage-group-I Staphylococci. Lancet. 1978;2(8100):1116–1118. doi: 10.1016/s0140-6736(78)92274-2. [DOI] [PubMed] [Google Scholar]
- 88.Schlievert P.M., Davis C.C. Device-associated menstrual toxic shock syndrome. Clin Microbiol Rev. 2020;33(3) doi: 10.1128/CMR.00032-19. 3000322–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.U.S. Code of Federal Regulations . 21CFR884.430; 2019. Title 21 Food and Drugs. Chapter I Food and Drug Administration Department of Health and Human Services; Subchapter H Medical Devices; Part 801 Labeling. User labeling for menstrual tampons. [Google Scholar]
- 90.U.S. Food and Drug Administration . 2022. Manufacturers and User Facility Device Experience (MAUDE) Database.https://www.fda.gov/medical-devices/mandatory-reporting-requirements-manufacturers-importers-and-device-user-facilities/about-manufacturer-and-user-facility-device-experience-maude [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.