Abstract
Aspartame (ASP) is probably the best known artificial sugar substitute that is used widely in food. Many experimental studies have reported the toxicity of long‐term administration of ASP in various organ tissues. However, there is little evidence available about the nature and mechanisms of the adverse effects of long‐term consumption of ASP on the cardiovascular system. This study was conducted to evaluate the possible effects of ASP on heart tissue. For this study 36 mature male mice were divided into one control group and three groups which received respectively 40 mg/kg, 80 mg/kg and 160 mg/kg ASP orally, for 90 days. ASP at the doses of 80 and 160 mg/kg increased the serum content of malondialdehyde (MDA), but decreased serum nitric oxide (NO), creatine kinase (CK) and CK‐MB, as well as blood superoxide dismutase (SOD) levels. Serum level of total anti‐oxidant capacity (TAC) in blood was also reduced in serum at the dose of 80 mg/kg. Histochemical staining, including Periodic acid‐Schiff, Masson's trichrome and Verhoeff‐van Gieson staining, indicated that ASP at doses of 80 and 160 mg/kg reduced glycogen deposition and decreased the number of collagen and elastic fibres in the cardiac tissue. The cardiac expression of pro‐apoptotic genes, including P53, Bax, Bcl‐2 and Caspase‐3, was modulated at the dose of 160 mg/kg. Moreover, transcription of Caspase‐3 was up‐regulated at the dose of 80 mg/kg. In conclusion, long‐term consumption of ASP any higher than the acceptable daily intake (40 mg/kg) appears to act by promoting oxidative stress, has the potential to alter both histopathological and biochemical parameters, and induces P53‐dependent apoptosis in cardiac tissue.
Keywords: aspartame, heart, mice, non‐nutritive sweeteners, oxidative stress, toxicity
1. INTRODUCTION
Aspartame (ASP), a synthetic methyl ester of the aspartic acid/phenylalanine dipeptide, is one of the best known non‐nutritive artificial sweeteners and is used worldwide. Due to its intense sweetness, cheapness and clean sugar‐like taste, ASP is the favoured sweetening additive for manufacturers and is used in various bakery goods, beverages, dietary supplements and even pharmaceutical products. 1 , 2 , 3 Nowadays, ASP is included commonly and consumed as part of low‐caloric products when people are attempting weight loss and/or are diabetic. 4 , 5 The acceptable daily intake (ADI) of ASP for both human adults and children is 40 mg/kg in Europe and 50 mg/kg in the United States. However, since many products contain ASP, consumers can inadvertently consume larger amounts than those recommended for ADI, which may result in serious health complications. 2 Hence, the safety of ASP has recently come under the spotlight. 1 It has been demonstrated ASP may cause neurobehavioral and neurodegenerative disorders, hormonal alterations, as well as allergies and skin problems. Besides, it may have carcinogenic, genotoxic and amyloidogenic properties. 2
During digestion in the body, ASP is hydrolysed into phenylalanine, aspartic acid and methanol (50%, 40% and 10%, respectively). 6 Methanol is well‐recognized as a cause of molecular and cellular damage. 7 Methanol intoxication results in the generation of reactive oxygen species (ROS), which in turn leads to DNA fragmentation and protein denaturation. 2 The elevation of methanol levels was detected after administration of ASP in humans and experimental animals. 6 , 8 Many empirical studies in recent years have reported that long‐term administration of ASP induces oxidative stress in several tissues such as liver, 9 , 10 , 11 , 12 kidneys, 13 , 14 prostate, 15 adrenal glands, 16 testes, 17 , 18 , 19 central nervous system, 5 , 20 haematopoietic, 21 , 22 and lymphoid tissues. 23 , 24 The results revealed that consumption of ASP for a long period leads to attenuation of the anti‐oxidant system, biochemical and histopathological alterations, and down‐regulation as well as up‐regulation of anti‐apoptotic and pro‐apoptotic genes, respectively, in these tissues.
Cardiovascular diseases are one of the major causes of death worldwide. 25 Although these complex disorders have multifactorial aetiology, the occurrence of oxidative stress due to excessive generation of ROS plays an important role in the pathophysiology of cardiovascular disease. 26 Nowadays, the pathologic mechanism of the redox signalling pathway is well‐established in induction and progression of various cardiovascular disorders, such as cardiomyocyte apoptosis, cardiac hypertrophy, myocardial ischaemia–reperfusion, heart failure, chemotherapy‐induced cardiotoxicity, atrial fibrillation and diabetic cardiomyopathy. 27 Despite the presence of strong experimental evidence on the association of long‐term consumption oxidative damage in many tissues, data on the cardiovascular system are limited. Choudhary et al. have reported that intake of ASP for a long period is associated with attenuation of the cardiac anti‐oxidant system in a rat model. 28 Hence, this study was designed to better clarify the pathologic mechanism of long‐term ASP consumption‐induced oxidative stress in the cardiovascular system.
2. MATERIALS AND METHODS
2.1. Chemicals
Aspartame, chloroform, ethanol, trichloroacetic acid (TCA), thiobarbituric acid (TBA) and hydrochloric acid (HCA) were obtained from Sigma‐Aldrich (USA). Total antioxidant capacity (TAC) and Superoxide dismutase (SOD) detection kit were purchased from RanDox Co. A creatine kinase (CK) detection kit was provided by Pishtaz Teb Co. A CK‐MB detection kit was purchased from Pars Azmun Co. RevertAid First Strand cDNA Synthesis Kit was provided by Fermentas Co. All other histochemical kits were purchased from Shimi Pazhouhesh Asia Co. RNX‐ Plus Solution SinaClon BioScience Co.
2.2. Animals
Thirty‐six NMRI male mice at age 10–12 weeks, with a bodyweight of 20–30 g, were obtained from Pasteur Institute (Karaj, Iran). They were housed in polycarbonate cages under a standard condition of illumination (12 h light/12 h darkness), temperature (20–25°C) and humidity (50%–60%) with free access to water and a standard pellet diet (Tehran pellet, Iran) during the study. The animals were adapted to the new environment for 2 weeks before the beginning of the study. All experiments conducted on animals in this study were in accordance with the guidance of the ethical committee for research on laboratory animals of the University of Tehran (7,506,025/6/24). Both the international guidelines for the animals' welfare and the comparable local regulations for experimenting were respected during the study.
2.3. Experimental design
Following 2 weeks of adaptation, the animals were first weighed (initial body weight, BW) and then divided into a control group and three experimental groups (n = 8). The animals in the control group received normal saline (0.5 ml/day). The three experimental groups received 40, 80 and 160 mg/kg/day of ASP, respectively. All animals were treated by gavage for 90 days (Figure 1). The dosages and duration of the treatment in the present study have been designed based on earlier studies. 29 , 30 The body weight of animals in all experimental groups was measured.
FIGURE 1.
Schematic view for grouping of the animals and treatment time for euthanasia.
2.4. Tissue sampling, BW , BW alterations (BWA) and heart weight (HW) measurement
Following 90 days, the animals were anaesthetised (induced by 5% ketamine and 2% xylazine), and the final BW of all animals was measured. BWA was calculated by subtraction of initial BW from final BW. Next, the blood samples were taken from the heart; the serum was separated by centrifugation (3000 g, 3 min) and stored at −20°C. Then, the animals were euthanized. The heart was dissected out and fixed in previously prepared 10% natural buffered formalin. Before fixation, the heart weight (HW) of all experimental animals was measured.
2.5. Biochemical assays for evaluating oxidative stress biomarkers
Serum values of TAC, SOD activity of hemolysate as well as serum activity of CK and CK‐MB were detected by commercial kits based on the manufacturer's instructions. These data were expressed as mmol/ml, U/g Hb and IU/L, respectively. The serum nitric oxide (NO) was measured by Griess reaction. 31 The serum NO levels were expressed as mmol/L. Malondialdehyde (MDA) level as an indicator of lipid peroxidation in serum was assessed. 32 Briefly, one volume of serum was mixed with two volumes of a stock solution of 15% w/v TCA, 0.375% w/v TBA and 0.25 moL/L HCA. Following a heating/cooling process, the solution was centrifuged at 1000 g for 10 min, and subsequently, the absorbance of the cleared solution was read at 535 nm against a blank solution. MDA content was estimated using 1.56 × 105 mol−1 cm−1 as the molar absorbance coefficient. MDA levels were presented as mmol/ml.
2.6. Histopathological and histochemical evaluations
The collected sample tissues were fixed with 10% natural buffered formalin for 48 h, dehydrated, cleared and embedded in paraffin wax. Sections of 5–7 μm thickness were prepared and subsequently stained with haematoxylin and eosin (H&E), Periodic acid‐Schiff (PAS, for glycogen), Masson's trichrome (MTC, for Collagen fibres) and Verhoeff‐van Gieson stain (VVG, for elastic fibres) were performed by standard methods for morphological observation.
2.7. RNA isolation and cDNA synthesis
Briefly, 50 mg of frozen heart tissue samples collected from each animal were homogenized in 2 ml of PBS and mixed with 1 ml of ice‐cold RNX™ –PLUS solution. The mixture was vortexed for 5–10 s and incubated at room temperature for 5 min. Then, 200 μl of Chloroform were added to the tube and mixed well for 15 s by handshaking. After incubation on ice for 5 min, the mixture was centrifuged at 12,000 g at 4°C for 15 min. Next, the obtained aqueous phase was transferred to a new RNase‐free 1.5 ml tube. An equal volume of Isopropanol was added, and after gently mixing, the solution was incubated on ice for 15 min. After centrifugation of the mixture at 12,000 g at 4°C for 15 min, the supernatant was discarded, and 1 ml 75% ethanol was added to the pellet. The tube was vortexed to dislodge the pellet and then centrifuged at 4°C for 8 min. at 7500 g. Subsequently, the supernatant was removed and the pellet was alloewed to dry at room temperature for a few minutes. After this step, 50 μl of DEPC‐treated water were added, and the tube was placed in a 55°C water bath for 10 min to improve dissolution of the pellet. Finally, using a NanoDrop® 2000c Spectrophotometer (Thermo Scientific), the quality and quantity of isolated RNA were evaluated. The RNA was, then transcribed to cDNA using a RevertAid First Strand cDNA Synthesis Kit (Fermentas) according to the instruction manual. In brief, 1 μg RNA was mixed with oligo 1 ml (dT) primer into a sterile nuclease‐free tube, and the mixture reached 12 ml using free‐DNase water. After gently vortex and incubation at 65°C for 5 min, 4 ml 5 reaction buffer, 1 ml RNAse inhibitor, 2 ml 10‐mM dNTP mix and 1 ml M‐MuLV Reverse Transcriptase was added to the mixture. The tube was gently vortexed and incubated for 60 min at 42°C. Eventually, the mixture was heated at 70°C for 5 min aimed to terminate the reaction.
2.8. Quantitative polymerase chain reaction (PCR)
To measure mRNA expression of Bcl‐2, Bax, Caspase‐3, P53 and GAPDH (as a housekeeping gene) Real‐time PCR technique was performed. The specific forward and reverse primers that were used for PCR amplification were summarized in Table 1. Briefly, 0.4 μl of each specific primer and 5 μl of each cDNA sample were added to PCR tubes containing SYBR‐Green Master Mix (7.5 μl) and nuclease‐free water (5.5 μl). Quantitative PCR analysis was performed using Power SYBR Green PCR master mix (Applied Biosystems) and analysed in the StepOnePlus real‐time PCR system (Applied Biosystems). The cycling conditions were adjusted according to the manufacturer's instructions: primary denaturation at 95°C for 15 min followed by 40 cycles of 95°C for 25 s and 68, 61, 58 and 62°C (for Bcl‐2, Bax, Caspase‐3 and P53, respectively) for 1 min and eventually 70°C for 1 min. The fold changes in gene expression were calculated using the 2‐ΔΔCt formula. 33
TABLE 1.
The forward (F) and reverse (R) primer sequences of the gene of interest for PCR amplification
| Target Gene | Primer Sequence (50‐ > 30) | Amplified product (bp) |
|---|---|---|
| Bcl‐2 | F: CTGGTGGACAACATCGCTCTG | 226 |
| R: GGTCTGCTGACCTCACTTGTG | ||
| Bax | F: TGCAGAGGATGATTGCTGAC | 176 |
| R: GATCAGCTCGGGCACTTTAG | ||
| Caspase‐3 | F: AGTTGGACCCACCTTGTGAG | 298 |
| R: AGTCTGCAGCTCCTCCACAT | ||
| P53 | F: ACATAGTGTGGTGGTGCCCT | 152 |
| R: ACCTCAAAGCTGTTCCGTCC | ||
| GAPDH | F: GAACATCATCCCTGCATCCA | 68 |
| R: CCAGTGAGCTTCCCGTTCA |
2.9. Statistical analysis
Data were analysed using spss program version 19.0 (spss Inc). All results are presented as Mean ± standard deviation (SD). Differences between quantitative histological and biochemical data were analysed with one‐way ANOVA, followed by the Tukey test post‐hoc. The p‐value of < .05 was considered statistically significant.
3. RESULTS
3.1. Effect of ASP on BW, BWA and HW
After 90 days, no significant differences were observed between the groups in total BW. However, the total BW alteration demonstrated a significant increase in the 160 mg/kg group compared to the control group. Also, our results on heart weight and heart weight (HW)/BW ratio (%) showed no significant differences between the experimental groups compared to the control group (Table 2).
TABLE 2.
Effect of different doses of ASP on total BW, BWA, HW and HW/BW ratio changes in different groups
| Parameters | Control | 40 mg/kg | 80 mg/kg | 160 mg/kg |
|---|---|---|---|---|
| BW (g) | 36.12 ± 2.82 | 36.31 ± 3.06 | 36.38 ± 3.67 | 37.31 ± 3.01 |
| BWA (g) | 4.62 ± 0.67 | 5.24 ± 1.56 | 5.58 ± 2.53 | 6.97 ± 1.15 a |
| HW (g) | 0.204 ± 0.035 | 0.205 ± 0.029 | 0.218 ± 0.038 | 0.217 ± 0.043 |
| HW /BW ratio (%) | 0.572 ± 0.121 | 0.566 ± 0.081 | 0.608 ± 0.133 | 0.583 ± 0.102 |
Note: All data are represented as mean ± SD (n = 8).
Represent significant (p < .05) differences compared to the control group.
3.2. Effect of ASP on oxidative stress biomarkers
The results showed a significant increase in MDA levels in 80 and 160 mg/kg but not 40 mg/kg compared to the control group (Figure 2A). TAC assay indicated a significant decrease in animals that received a long‐term intake of 160 mg/kg in comparison to the control group (Figure 2B). Animals that received 40 and 80 mg/kg did not show significant differences compared to the control and 160 mg/kg groups. Our findings from the NO assay demonstrated a significant increase in NO levels in the groups that received 80 and 160 mg/kg, while the data from the group 40 mg/kg had no significant difference compared to the control group (Figure 2C). Blood activity of SOD was significantly decreased between 80 and 160 mg/kg groups in comparison to each other and the control group. However, the 40 mg/kg group showed no significant differences compared to the control group (Figure 2D). The results of CK and CK‐MB assays showed that animals that received doses of 80 and 160 mg/kg had a significant increase in activity of these enzymes compared to the control group but 40 mg/kg did not show any significant differences compared to the control and 80 mg/kg groups (Figure 2E,F).
FIGURE 2.
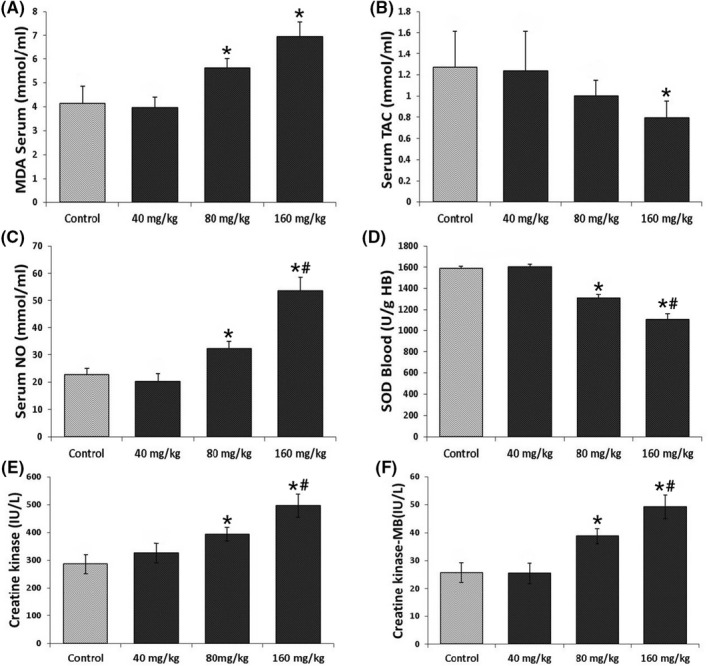
Levels of (A) Serum MDA, (B) Serum TAC, (C) Serum NO, (D) Blood SOD, (E) CK and (F) CK‐MB. All data are represented as mean ± SD (n = 8). *Represent significant (p < .05) differences compared to the control group. #Represent significant (p < .05) differences compared to the 80 mg/kg group.
3.3. mRNA Expression of Bcl‐2, Bax, Caspase‐3 and P53
The results of Real‐time PCR are illustrated in Figure 3. Long‐term consumption of ASP at the dose of 160 mg/kg significantly decreased mRNA expression of Bcl‐2, while the other doses did not significantly change when compared to the control group (Figure 3A). The Bax mRNA expression was significantly up‐regulated by long‐term intake of ASP at the dose of 160 mg/kg. At doses of 80 and 160 mg/kg, the mRNA expression of Caspase‐3 was significantly elevated compared to each other and the control group. Also, the mRNA expression of P53 was significantly increased at 160 mg/kg of ASP compared to the control group.
FIGURE 3.
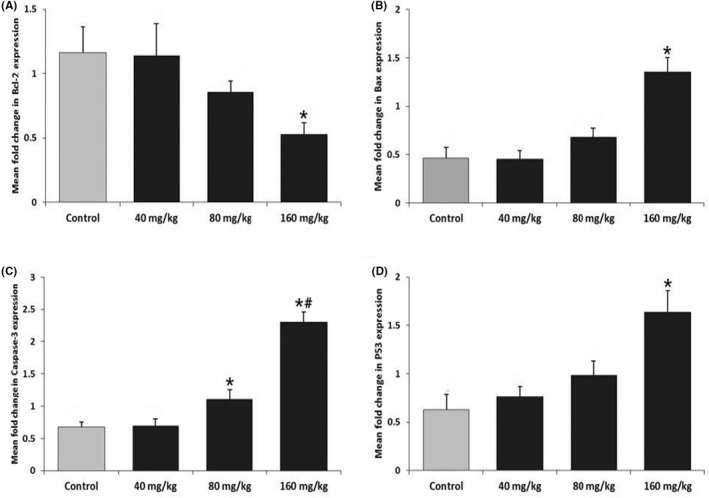
Effect of long‐term intake of ASP on Bcl‐2 (A), Bax (B), Caspase‐3 (C) and P53 (D) mRNA gene expression in cardiac tissue. All data are represented as mean ± SD (n = 8). *Represent significant (p < .05) differences compared to the control group. #Represent significant (p < .05) differences compared to the 80 mg/kg group.
3.4. Histopathological and histochemical findings
Histopathological and histochemical evaluations showed that cardiac tissue had a normal structure in control and 40 mg/kg ASP groups. The animals which received 160 mg/kg of ASP exhibited nuclear deformation, diffuse oedema, congestion, fibrosis and necrosis. Animals in the 80 mg/kg group also showed similar histopathologic alterations but there were less than that in the 160 mg/kg group (Figure 4). PAS staining indicated that the groups that received 80 and 160 mg/kg for a long period exhibited low and very low amounts of glycogen deposits in comparison to the control group, respectively; while the groups that received 40 mg/kg did not show remarkable differences in comparison to the control group. Our findings in MTC staining showed high amounts of collagen fibres deposits in the 160 mg/kg group compared to the control group. However, the 80 mg/kg group indicated lower amounts of collagen fibres deposits, while the 40 mg/kg group showed no notable difference compared to the control group. VVG staining demonstrated a higher number of elastic fibres in 80 and 160 mg/kg groups compared to the control group. However, the group which received 40 mg/kg had no remarkable differences compared to the control group (Figure 4).
FIGURE 4.
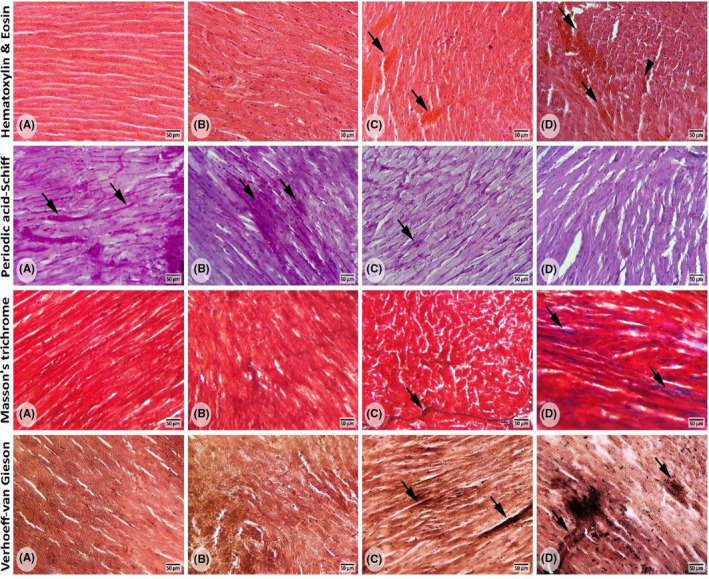
Cross‐sections from cardiac tissues: (A) Represents the control group; (B) represents the 40 mg/kg group; (C) represents the 80 mg/kg group; (D) represents the 160 mg/kg group. H&E staining; (A) normal appearances of the cardiac structure. (B) no remarkable difference in the cardiac structure between the control and 40 mg/kg group was seen. (C and D) represent the cardiac injuries including diffused oedema, necrosis, haemorrhage and congestion (arrows) as well as hyaline exudates (head arrows) following long‐term intake of ASP at doses 80 and 160 mg/kg. PAS staining; (A–C) normal PAS reaction (arrows) is present in cardiac cells. (D) negative PAS reaction in cardiac cells and faint PAS‐stained cytoplasm. MTC staining; (A and B) there was no difference in the number of collagen fibres between the control group and the 40 mg/kg group. (C and D) an increase in the number of collagen fibres (arrows) is seen in these groups. VVG staining; (A and B) there was no difference in the number of elastic fibres between the control group and the 40 mg/kg ASP.
4. DISCUSSION
In summary, no significant alterations were found in experimental parameters evaluated in this study following 90 days of intake of ASP at the dose of 40 mg/kg. However, doses of 80 and 160 mg/kg, resulted in the elevation of MDA, NO, CK and CK‐MB levels as well as a decrease in the activity of SOD in serum, and up‐regulation of gene expression of caspase‐3 in cardiac tissue. In addition, at the dose of 160 mg/kg, serum levels of TAC and cardiac expression of the Bcl‐2 gene were decreased, while expression of Bax and P53 genes was increased.
This study confirmed the role of ASP as a chemical stressor in the cardiovascular system, which was demonstrated by the elevation of oxidative stress biomarkers. Our results indicated that TAC levels and activity of SOD were significantly decreased in the serum and erythrocytes of animals, respectively. In line with our findings, Choudhary et al. have demonstrated a decrease in enzymatic and non‐enzymatic anti‐oxidants in heart tissue in rats after 90 days' consumption of ASP at recommended ADI. 28 These changes might be associated with ASP‐derived metabolites, primarily methanol. During methanol intoxication, it converts to formaldehyde and subsequently formic acid accompanied by forming ROS. 34 Prokic et al. have reported an increase in superoxide anion (O2−) and hydrogen peroxide (H2O2) concentrations following 6 weeks of consumption of ASP. SOD participates in scavenging of O2−. Therefore, the decrease of SOD might be related to over‐scavenging of O2− free radicals formed due to methanol intoxication in the body after consumption of ASP. Also, as intake of ASP leads to protein carbonylation and reduction of protein thiol, decreasing in serum TAC can result from the destruction of SOD and other thiol antioxidants. 35
NO is a derivative of L‐Arginine and plays many physiological and pathological roles in the body. 36 NO can interact with intracytoplasmic and intra‐mitochondrial iron as well as an inhibitor of DNA synthesis and cellular respiration. 12 Elevation of NO level in the 80 and 160 mg/kg groups reflects high production and anti‐oxidant enzyme dysfunction following long time consumption of ASP.
Increasing mitochondrial ROS generation was associated with the degree of contractile dysfunction, and it has been proposed that this relationship was causal. 37 Furthermore, cell injury causes the propagation of cytokines and produces ROS from the tissues, which in turn may cause lipid peroxidation. 28
Malondialdehyde is one of the most often used lipid peroxidation end products in cardiovascular studies. Serum assays are regarded as an index of oxidative degradation of polyunsaturated lipids. 38 Our results showed a significant increase in MDA levels at 80 and 160 mg/kg groups, which represent cardiovascular oxidative damage and subsequent lipid peroxidation following long‐term consumption of ASP. Similarly, Choudhary et al. 28 have reported an elevation of MDA in cardiac tissue after long‐term consumption of ASP. Prokic et al. 35 also have found that daily intake of 40 mg/kg of ASP increases MDA level in erythrocytes.
Moreover, ASP causes overproduction of free radicals, affects cell survival and leads to cardiac dysfunction. Consequently, it results in deformation in size and shapes of nuclei, which presents as necrosis and subsequent fibrosis in cardiac tissues. Furthermore, some evidence of bleeding and blood congestion was presented, which might be due to the harmful effects of ASP on the cardiac tissue. 39
In this study, we also revealed an increase in levels of CK and CK‐MB in animals that received ASP at doses of 80 and 160 mg/kg for 90 days. These results are in line with Choudhary et al.’s findings, which reported elevation of these enzymes following 90 days of intake of ASP in a folate‐deficient rat model. 28 These enzymes are bound to the myocardial contraction system, and any severe damage to the myocardium results in the release of these enzymes into the serum. 40 Hence, the increase in levels of CK and CK‐MB in serum might be due to cardiac damages resulting from ASP metabolism. 28
On the other hand, a marked increase in heart mRNA expression of genes like P53, Bax and Caspase‐3, as well as a decrease in Bcl‐2 was found in the animals that received 160 mg/kg of ASP compared to the control animals. P53 has a key role in the maintenance of genome stability. 41 , 42 P53 modulates the repair process and survivability of injured cells as well as the elimination of severely damaged cells. 41 In the latter condition, P53 leads to cell apoptosis, especially via the intrinsic apoptotic pathway. 41 , 42 , 43 Activation of P53 up‐regulates gene transcription of various pro‐apoptotic proteins which encode BH‐3 only proteins such as Bax. 42 , 43 P53 facilitates oligomerization and mitochondrial translocation of BAX. Besides, it inhibited the anti‐apoptotic function of Bcl‐2. 41 These events result in the release of apoptogenic factors following the disruption of mitochondrial membranes and consequently activate the caspase cascade. 41 , 42 , 43 Similar to our findings, other studies have reported up‐regulation of pro‐apoptotic and also down‐regulation of apoptotic genes. Iyaswamy et al. have demonstrated down‐regulation of Bcl2 expression and up‐regulation of Bax and caspase 3‐and 9 genes in a liver folate‐deficient rat model after long‐term consumption of ASP. 10 Anbara et al. have shown that high dose intake of ASP for a long period resulted in a decrease of mRNA expression of Bcl‐2 while increased mRNA expression of P53, Bax and Caspase‐3 in testis tissue of mice model. 18 Our results and findings from the above studies are consistent in finding that long‐term consumption of ASP induced P53‐dependent apoptosis in experimental animals.
The histopathological evaluations in this study revealed considerable changes at the cellular level in the heart tissue. Nuclear deformation, diffused oedema, blood congestion, necrosis and early fibrosis were found in tissue samples of animals at high doses of ASP following 90 days of consumption. Figure 5 graphically summarizes these study findings. Histopathological alterations may be the results of pathological damages at subcellular levels following ASP metabolism in the body. Trocho et al. 34 have reported that alternation in tissues, proteins and nucleic acids in both mitochondrial DNA and nucleic DNA is due to excess methanol formation following ASP intake. Gudadhe et al. 44 have observed myocardial hypertrophy and an increase in heart weight in ASP administrated mice as a consequence of myocardial matrix elevation. Also, Al‐Eisa et al. 45 reported a similar observation in Wistar rats and attributed it to the antioxidant system imbalance, including ROS and NO augmentation and attenuation of antioxidant enzyme activity. In line with the above reports, we revealed an imbalance in the antioxidant system and up‐regulation of apoptotic genes that can lead to cellular damage and morphological alterations. Similar pathological changes were also reported in other tissues, such as the brain, liver and kidneys in several studies. 9 , 10 , 14
FIGURE 5.
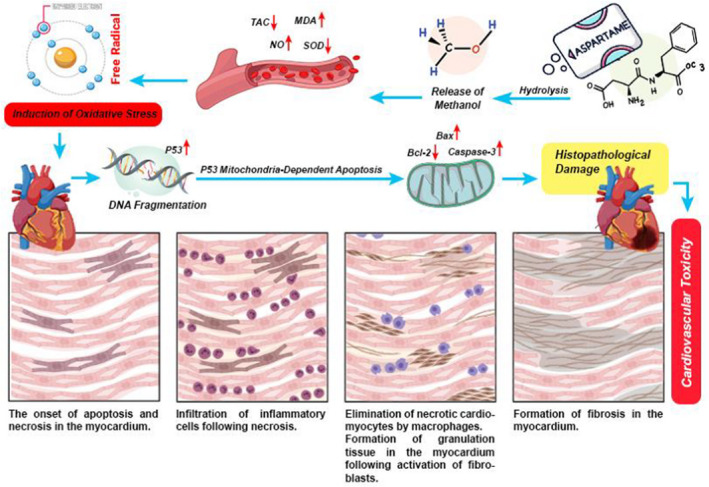
Infographic summary of pathologic mechanism of cardiovascular toxicity following a long‐term intake of ASP at high doses. Hydrolysis of ASP in the gut results in releasing and absorption of methanol. An increase in blood levels of methanol leads to an imbalance between free radicals and antioxidants (oxidative stress statute). Induction of oxidative stress leads to molecular and cellular damages in cardiac tissue which causes cell death (necrosis and apoptosis). Myocardial cell death can lead to myocardial inflammation and fibrosis.
We found cardiovascular toxicity in mice fed high doses of ASP, at either 80 or 160 mg/kg daily, following a long period of intake. However, the main question is whether consumption of ASP by humans results in similar adverse effects or not. As mentioned earlier, although the ADI of ASP for both humans is 40 mg/kg in Europe and 50 mg/kg in the United States, the development of products containing non‐nutritional sweeteners would increase the risk of consuming more than approved doses. 46 Also, oxidation of methanol and consequently its detoxification following administration of ASP is four times faster in mice and rats than in humans. 6 Hence, humans are more vulnerable to methanol poisoning than rodent species. 47 Therefore, results provided from an experimental rodent model in the present study, might be extrapolatable to humans. Nevertheless, further clinical investigations of long‐term metabolic and histological impacts in human consumers are needed to evaluate the possibility of cardiovascular toxicity induction following long‐term consumption of ASP.
In conclusion, our findings revealed that long‐term intake of ASP at 40 mg/kg did not have toxic effects on the cardiovascular system in mice. However, long‐term ASP consumption of 80 mg/kg or more can evoke oxidative stress and changes in Caspase‐3 gene expression, while consumption of 160 mg/kg leads to adverse biochemical as well as histochemical alterations, and induction of apoptosis in cardiac tissue in a murine model. Given the heightened vulnerability to methanol toxicity in humans compared to mice, further research is needed to ascertain whether increased oxidative stress and cardiac toxicity might also be observed in humans who consume ASP over a period of years, even within current ADI levels.
FUNDING INFORMATION
This study was self‐funded.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
ACKNOWLEDGEMENT
The authors appreciate the Department of Basic Sciences, Faculty of Veterinary Medicine, University of Tehran.
Anbara H, Kian M, Darya G‐H, Sheibani MT. Long‐term intake of aspartame‐induced cardiovascular toxicity is reflected in altered histochemical parameters, evokes oxidative stress, and trigger P53‐dependent apoptosis in a mouse model. Int J Exp Path. 2022;103:252‐262. doi: 10.1111/iep.12458
REFERENCES
- 1. Choudhary AK, Pretorius E. Revisiting the safety of aspartame. Nutr Rev. 2017;75(9):718‐730. doi: 10.1093/nutrit/nux035 [DOI] [PubMed] [Google Scholar]
- 2. Czarnecka K, Pilarz A, Rogut A, et al. Aspartame‐true or false? Narrative review of safety analysis of general use in products. Nutrients. 2021;13(6):1957 . doi: 10.3390/nu13061957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marinovich M, Galli CL, Bosetti C, Gallus S, La Vecchia C. Aspartame, low‐calorie sweeteners and disease: regulatory safety and epidemiological issues. Food Chem Toxicol. 2013;60:109‐115. doi: 10.1016/j.fct.2013.07.040 [DOI] [PubMed] [Google Scholar]
- 4. Alleva R, Borghi B, Santarelli L, et al. In vitro effect of aspartame in angiogenesis induction. Toxicol in Vitro. 2011;25(1):286‐293. doi: 10.1016/j.tiv.2010.09.002 [DOI] [PubMed] [Google Scholar]
- 5. Iyaswamy A, Kammella AK, Thavasimuthu C, et al. Oxidative stress evoked damages leading to attenuated memory and inhibition of NMDAR‐CaMKII‐ERK/CREB signalling on consumption of aspartame in rat model. J Food Drug Anal. 2018;26(2):903‐916. doi: 10.1016/j.jfda.2017.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ranney RE, Oppermann JA, Muldoon E, McMahon FG. Comparative metabolism of aspartame in experimental animals and humans. J Toxicol Environ Health. 1976;2(2):441‐451. doi: 10.1080/15287397609529445 [DOI] [PubMed] [Google Scholar]
- 7. Choudhary AK, Devi RS. Serum biochemical responses under oxidative stress of aspartame in wistar albino rats. Asian Pac J Trop Dis. 2014;4:S403‐S410. doi: 10.1016/s2222-1808(14)60478-3 [DOI] [Google Scholar]
- 8. Davoli E, Cappellini L, Airoldi L, Fanelli R. Serum methanol concentrations in rats and in men after a single dose of aspartame. Food Chem Toxicol. 1986;24(3):187‐189. doi: 10.1016/0278-6915(86)90227-9 [DOI] [PubMed] [Google Scholar]
- 9. Adaramoye OA, Akanni OO. Effects of long‐term administration of aspartame on biochemical indices, lipid profile and redox status of cellular system of male rats. J Basic Clin Physiol Pharmacol. 2016;27(1):29‐37. doi: 10.1515/jbcpp-2014-0130 [DOI] [PubMed] [Google Scholar]
- 10. Iyaswamy A, Wankhar D, Loganathan S, Shanmugam S, Rajan R, Rathinasamy S. Disruption of redox homeostasis in liver function and activation of apoptosis on consumption of aspartame in folate deficient rat model. J Nutr Intermed Metab. 2017;8:41‐50. doi: 10.1016/j.jnim.2017.06.002 [DOI] [Google Scholar]
- 11. Lebda MA, Tohamy HG, El‐Sayed YS. Long‐term soft drink and aspartame intake induces hepatic damage via dysregulation of adipocytokines and alteration of the lipid profile and antioxidant status. Nutr Res. 2017;41:47‐55. doi: 10.1016/j.nutres.2017.04.002 [DOI] [PubMed] [Google Scholar]
- 12. Ashok I, Sheeladevi R. Oxidant stress evoked damage in rat hepatocyte leading to triggered nitric oxide synthase (NOS) levels on long term consumption of aspartame. J Food Drug Anal. 2015;23(4):679‐691. doi: 10.1016/j.jfda.2014.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alwaleedi S. Alterations in antioxidant defense system in hepatic and renal tissues of rats following aspartame intake. J Appl Biol Biotechnol. 2016;4:46‐52. doi: 10.7324/jabb.2016.40207 [DOI] [Google Scholar]
- 14. Kumar Choudhary A, Selvaraj S, Sheela DR. Aspartame induce modification in membrane bound and antioxidant enzymes in liver and kidney of Wistar albino rats. Curr Nutr Food Sci. 2015;10(4):275‐287. doi: 10.2174/1573401310666141024215431 [DOI] [Google Scholar]
- 15. Morovvati H, Khaksar Z, Sheibani MT, Anbara H, Kafiabad MA, Moradi HR. Effect of aspartame on histological and histometrical structure of prostate gland in adult mice. Qom Univ Med Sci J. 2019;12(12):14‐27. doi: 10.29252/qums.12.12.2 [DOI] [Google Scholar]
- 16. Morovvati H, Anbara H, Sheibani M, Koohi M, Hasanzadeh A. The effect of long‐term exposure to aspartame on histomorphometric and histochemical adrenal gland in adult NMRI mice. Armaghane Danesh. 2019;24(2):150‐169. [Google Scholar]
- 17. Anbara H, Sheibani MT, Razi M. Long‐term effect of aspartame on male reproductive system: evidence for testicular Histomorphometrics, Hsp70‐2 protein expression and biochemical status. Int J Fertil Steril. 2020;14(2):91‐101. doi: 10.22074/ijfs.2020.6065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Anbara H, Sheibani MT, Razi M, Kian M. Insight into the mechanism of aspartame‐induced toxicity in male reproductive system following long‐term consumption in mice model. Environ Toxicol. 2021;36(2):223‐237. doi: 10.1002/tox.23028 [DOI] [PubMed] [Google Scholar]
- 19. Ashok I, Poornima PS, Wankhar D, Ravindran R, Sheeladevi R. Oxidative stress evoked damages on rat sperm and attenuated antioxidant status on consumption of aspartame. Int J Impot Res. 2017;29(4):164‐170. doi: 10.1038/ijir.2017.17 [DOI] [PubMed] [Google Scholar]
- 20. Lebda MA, Sadek KM, El‐Sayed YS. Aspartame and soft drink‐mediated neurotoxicity in rats: implication of oxidative stress, apoptotic signaling pathways, electrolytes and hormonal levels. Metab Brain Dis. 2017;32(5):1639‐1647. doi: 10.1007/s11011-017-0052-y [DOI] [PubMed] [Google Scholar]
- 21. Abhilash M, Varghese MV, Paul MVS, Alex M, Nair RH. Effect of long‐term intake of aspartame on serum biochemical parameters and erythrocyte oxidative stress biomarkers in rats. Comp Clin Pathol. 2014;24(4):927‐933. doi: 10.1007/s00580-014-2013-8 [DOI] [Google Scholar]
- 22. Arbind K, Devi SR, Sundareswaran L. Role of antioxidant enzymes in oxidative stress and immune response evaluation of aspartame in blood cells of wistar albino rats. Int Food Res J. 2014;21(6):2263. [Google Scholar]
- 23. Choudhary AK, Devi RS. Effects of aspartame on hsp70, bcl‐2 and bax expression in immune organs of Wistar albino rats. J Biomed Res. 2016;30(5):427‐435. doi: 10.7555/JBR.30.20140097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choudhary AK, Rathinasamy SD. Effect of long intake of aspartame on ionic imbalance in immune organs of immunized wistar albino rats. Biomed Aging Pathol. 2014;4(3):243‐249. doi: 10.1016/j.biomag.2014.03.001 [DOI] [Google Scholar]
- 25. Flora GD, Nayak MK. A brief review of cardiovascular diseases, associated risk factors and current treatment regimes. Curr Pharm des. 2019;25(38):4063‐4084. doi: 10.2174/1381612825666190925163827 [DOI] [PubMed] [Google Scholar]
- 26. Senoner T, Dichtl W. Oxidative stress in cardiovascular diseases: still a therapeutic target? Nutrients. 2019;11(9):2090. doi: 10.3390/nu11092090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. D'Oria R, Schipani R, Leonardini A, et al. The role of oxidative stress in cardiac disease: from physiological response to injury factor. Oxid Med Cell Longev. 2020;2020:5732956. doi: 10.1155/2020/5732956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Choudhary AK, Sundareswaran L, Sheela DR. Aspartame induced cardiac oxidative stress in Wistar albino rats. Nutri Clin et Métab. 2016;30(1):29‐37. doi: 10.1016/j.nupar.2016.01.071 [DOI] [Google Scholar]
- 29. Onaolapo AY, Onaolapo OJ, Nwoha PU. Aspartame and the hippocampus: revealing a bi‐directional, dose/time‐dependent behavioural and morphological shift in mice. Neurobiol Learn Mem. 2017;139:76‐88. doi: 10.1016/j.nlm.2016.12.021 [DOI] [PubMed] [Google Scholar]
- 30. Finamor I, Perez S, Bressan CA, et al. Chronic aspartame intake causes changes in the trans‐sulphuration pathway, glutathione depletion and liver damage in mice. Redox Biol. 2017;11:701‐707. doi: 10.1016/j.redox.2017.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131‐138. doi: 10.1016/0003-2697(82)90118-x [DOI] [PubMed] [Google Scholar]
- 32. Asri‐Rezaei S, Nourian A, Shalizar‐Jalali A, et al. Selenium supplementation in the form of selenium nanoparticles and selenite sodium improves mature male mice reproductive performances. Iran J Basic Med Sci. 2018;21(6):577‐585. doi: 10.22038/IJBMS.2018.26023.6397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C[T]) method. Methods. 2001;25(4):402‐408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 34. Trocho C, Pardo R, Rafecas I, et al. Formaldehyde derived from dietary aspartame binds to tissue components in vivo. Life Sci. 1998;63(5):337‐349. doi: 10.1016/s0024-3205(98)00282-3 [DOI] [PubMed] [Google Scholar]
- 35. Prokic MD, Paunovic MG, Matic MM, et al. Prooxidative effects of aspartame on antioxidant defense status in erythrocytes of rats. J Biosci. 2014;39(5):859‐866. doi: 10.1007/s12038-014-9487-z [DOI] [PubMed] [Google Scholar]
- 36. Snyder SH, Bredt DS. Biological roles of nitric oxide. Sci Am. 1992;266(5):68‐71, 74–77. doi: 10.1038/scientificamerican0592-68 [DOI] [PubMed] [Google Scholar]
- 37. Seddon M, Looi YH, Shah AM. Oxidative stress and redox signalling in cardiac hypertrophy and heart failure. Heart. 2007;93(8):903‐907. doi: 10.1136/hrt.2005.068270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Menazza S, Canton M, Sorato E, Boengler K, Schulz R, Di Lisa F. Old and new biomarkers of oxidative stress in heart failure. Drug Disco Today Ther Strateg. 2012;9(4):e189‐e198. doi: 10.1016/j.ddstr.2013.11.003 [DOI] [Google Scholar]
- 39. Othman SI, Jumah MB. Histopathological effect of aspartame on liver and kidney of mice. Int J Pharmacol. 2019;15(3):336‐342. doi: 10.3923/ijp.2019.336.342 [DOI] [Google Scholar]
- 40. Jacob R, Khan M. Cardiac biomarkers: what is and what can be. Indian J Cardiovasc Dis Women WINCARS. 2018;3(4):240‐244. doi: 10.1055/s-0039-1679104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Amaral JD, Xavier JM, Steer CJ, Rodrigues CM. The role of p53 in apoptosis. Discov Med. 2010;9(45):145‐152. [PubMed] [Google Scholar]
- 42. Schuler M, Green DR. Mechanisms of p53‐dependent apoptosis. Biochem Soc Trans. 2001;29(Pt 6):684‐688. doi: 10.1042/0300-5127:0290684 [DOI] [PubMed] [Google Scholar]
- 43. Shen Y, White E. p53‐dependent apoptosis pathways. Adv Cancer Res. 2001;82:55‐84. doi: 10.1016/s0065-230x(01)82002-9 [DOI] [PubMed] [Google Scholar]
- 44. Gudadhe DR, Talhar SS, Bokariya P, Shende MR, Tarnekar AM. Histo‐morphometric demonstration of cardiotoxic effects of aspartame on mice. IOSR J Pharm. 2013;3(2):30‐33. doi: 10.9790/3013-32203033 [DOI] [Google Scholar]
- 45. Al‐Eisa RA, Al‐Salmi FA, Hamza RZ, El‐Shenawy NS. Role of L‐carnitine in protection against the cardiac oxidative stress induced by aspartame in Wistar albino rats. PLoS One. 2018;13(11): e0204913. doi: 10.1371/journal.pone.0204913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gardner C, Wylie‐Rosett J, Gidding SS, et al. Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation. 2012;126(4):509‐519. doi: 10.2337/dc12-9002 [DOI] [PubMed] [Google Scholar]
- 47. Johlin FC, Fortman CS, Nghiem DD, Tephly TR. Studies on the role of folic acid and folate‐dependent enzymes in human methanol poisoning. Mol Pharmacol. 1987;31(5):557‐561. [PubMed] [Google Scholar]


