Abstract
Recent findings suggest that both peripheral and central auditory system dysfunction occur in the prodromal stages of Alzheimer Disease (AD), and therefore may represent early indicators of the disease. In addition, loss of auditory function itself leads to communication difficulties, social isolation and poor quality of life for both patients with AD and their caregivers. Developing a greater understanding of auditory dysfunction in early AD may shed light on the mechanisms of disease progression and carry diagnostic and therapeutic importance. Herein, we review the literature on hearing abilities in AD and its prodromal stages investigated through methods such as pure-tone audiometry, dichotic listening tasks, and evoked response potentials. We propose that screening for peripheral and central auditory dysfunction in at-risk populations is a low-cost and effective means to identify early AD pathology and provides an entry point for therapeutic interventions that enhance the quality of life of AD patients.
Keywords: Auditory cortex, Mild cognitive impairment, Audiometry, Evoked potential, Dichotic
1. Introduction
Alzheimer’s Disease (AD) is a neurodegenerative disease of increasing prevalence and primarily affects the elderly population. It is characterized by loss of cognitive function across multiple domains including early loss of episodic memory, loss of executive control, language and behavioral dysregulation (McKhann et al., 2011). Current estimates identify 4.7 million individuals diagnosed with AD in the United States and that number is expected to increase to 13.8 million by the year 2050 (Hebert et al., 2013). Thus, interest in potential treatment and prevention of the disease has gained significant attention.
The defining neuropathological features seen in AD include deposition of amyloid plaques (Glenner and Wong, 1984; Masters et al., 1985), abnormal phosphorylation of microtubule-associated proteins referred to as neurofibrillary tangles (Grundke-Iqbal et al., 1986), and atrophy of the medial temporal lobe structures (Scheltens et al., 1992). The auditory midbrain and forebrain are also targets of AD pathology. Early autopsy-based studies revealed amyloid plaques and neurofibrillary tangles in the central nucleus of the inferior colliculus, the ventral division of the medial geniculate body, and primary and secondary auditory cortical areas. More peripheral auditory processing regions such as the cochlea and cochlear nucleus, showed little AD pathology (Sinha et al., 1993; Sinha et al., 1996), suggesting that patients with AD-related pathology may show selective deficits in higher auditory cognitive function.
Mild cognitive impairment (MCI) is considered to be an intermediate stage between normal cognitive function (for age) and AD. Patients with MCI may present with both memory and non-memory cognitive complaints that do not significantly affect normal functional activities (Roberts and Knopman, 2013). Furthermore, MCI is often separated into multiple subgroups including amnestic, non-amnestic, single-domain, and multiple-domain (Morris and Cummings, 2005). Amnestic MCI patients are important to AD research because they have a high conversion rate to AD compared to age-matched controls, show a neuropathology similar to that seen in AD, and they represent a potential earlier therapeutic intervention point (Morris and Cummings, 2005; Petersen et al., 2001; Petersen et al., 1999).
Current diagnostic approaches for MCI and AD rely on neuropsychological testing demonstrating early episodic memory dysfunction and the absence of a competing diagnosis based on serologic and imaging workup (McKhann et al., 1984). A series of other biomarkers have been proposed to assist in the diagnosis of AD and prediction of progression from MCI to AD such as cerebrospinal fluid (CSF) amyloid beta and tau (Sunderland et al., 2003), multivariate CSF and serum markers (Llano et al., 2017; Llano et al., 2012), and magnetic resonance imaging (MRI) and positron-emission tomography (PET) imaging markers (Herholz et al., 2002; Klunk et al., 2004; Schuff et al., 2009). The cost and invasiveness (in the case of CSF) of these biomarkers limits their use in routine clinical practice. Given the predilection of AD to affect temporal lobe structures which house the auditory cortex and related subfields, auditory neurophysiological measurements coupled with audiological assessments may provide a unique opportunity to detect and track the severity of this neurodegenerative disease.
Both past and recent evidence suggest that pathological changes in the human auditory system can be objectively measured and potentially assist in differentiating between normal ageing-related cognitive decline, MCI, and AD (Hardy et al., 2016; Taljaard et al., 2016; Vecchio and Maatta, 2011). For example, non-invasive and low-cost paradigms such as pure tone audiometry (PTA), speech-in-noise tests, dichotic listening tasks, and auditory event-related potentials have elucidated neural correlates to many of the pathological findings seen in MCI and AD (reviewed below). An additional consideration is evaluation of auditory comprehension in MCI and AD (MacDonald et al., 2001; Markova et al., 2017; Welland et al., 2002), alteration in which produces a significant social burden for AD patients and their caregivers. Herein, the literature supporting the use of audiological assessments and auditory evoked potentials in the diagnosis of AD and its prodromal stages are reviewed.
2. Audiological assessments
2.1. Peripheral hearing in MCI and AD
PTA is designed to measure hearing thresholds in subjects based upon their response to pure tone stimuli and is generally considered to be a measurement of peripheral hearing function (Walker et al., 2013). The primary use of PTA is to establish perceptual hearing thresholds, typically across frequencies ranging from 125 Hz to 8 kHz, and is used clinically to diagnose the type and degree of hearing impairment. Although PTA is typically assumed to reflect changes in peripheral auditory function, it should be noted that in many conditions including ageing-related hearing loss and acoustic trauma that involves cochlear neuropathy, normal PTA results may be obtained. Subjects with co-chlear neuropathy and normal PTA often complain of difficulties with speech comprehension and are referred to as having “hidden hearing loss” (Liberman and Kujawa, 2017; Pienkowski, 2017). On the contrary, data from animal studies and human case reports have documented changes in PTA after central lesions (Heffner and Heffner, 1986; Musiek et al., 1994). Although these data suggest that elevations in perceptual thresholds using PTA are not 100% sensitive and/or specific to cochlear damage, PTA is currently the most widely used tool to test for peripheral hearing loss and is relatively simple to use in cognitively-impaired listeners. As such, throughout this review PTA will be considered to be a biomarker of cochlear function.
Presbycusis, or ageing-related hearing loss, is a common finding among cognitively normal older adults as well as AD patients and is primarily thought to be caused by ageing-related dysfunction at the level of the cochlea (Gates and Mills, 2005), although central mechanisms also play a key role (Humes et al., 2012; Jerger et al., 1995; Mudar and Husain, 2016). The classical taxonomy of cochlear pathologies and their PTA correlates was developed by Schuknecht (1964). This work originally outlined four possible types of presbycusis based on audiograms of geriatric patients and microscopic pathological analysis of the temporal bone: a “sensory” type characterized by loss of the outer hair cells, a “neural” type defined by loss of spiral ganglion cells, a “metabolic” type related to strial atrophy, and a “conductive” type relating to basilar membrane stifiness or spiral ligament atrophy. More recent data suggest that many patients do not fit neatly into any one of these four types of presbycusis and are defined as either “mixed” or “indeterminate” (Huang and Tang, 2010).
It should be noted that presbycusis and AD share many common risk factors such as obesity, diabetes, and hypertension (Agrawal et al., 2009; Fransen et al., 2008; Gates et al., 1993; Kivipelto et al., 2002; Kivipelto et al., 2005; Luchsinger et al., 2005) suggesting that micro-vascular and/or metabolic compromise may contribute to these disorders and may, in part, be responsible for their temporal coincidence. In fact, multiple studies suggest that association between presbycusis and cognitive decline is an expression of common pathophysiological mechanisms that occur in the ageing brain (Anstey et al., 2001; Golub et al., 2017; Lindenberger and Baltes, 1994; Quaranta et al., 2014; Taljaard et al., 2016). For example, Lindenberger and Baltes (1994) found that more than 90% of the ageing-related change in cognition was related to sensory variables. In a study involving 894 participants between 70 and 98 years of age, Anstey et al. (2001) found that 78.46% of the ageing-related variance in cognition was shared by the sensory function. Since the correlation between cognitive function and visual and hearing acuity was similar in magnitude, these simultaneous deficits in sensory and cognitive functions were thought to reflect a generalized widespread ageing-related neural degeneration.
Furthermore, numerous studies have found an association between presbycusis and cognitive decline. For example, Lin et al. (2011b) provided evidence of the correlation between hearing and cognitive loss in the Baltimore Longitudinal Study of Aging. A total of 639 volunteers were followed from 1958 to 2008 and had PTA and cognitive testing done biennially. It was found that patients with mild hearing loss had a hazard ratio of 1.89 for incident dementia, while patients with moderate hearing loss had a hazard ratio of 3.00, and the hazard ratio for patients with severe hearing loss (n = 6) was 4.94. Additionally, Lin et al. (2011a) also reported that greater hearing loss was negatively associated with scores on the Mini Mental State Exam. Notably, a sub-study of the Baltimore Longitudinal Study performed by Lin et al. (2014) reported brain volume changes in hearing impaired individuals measured by MRI and showed that subjects with hearing impairment had a marked increase in the rate of volume loss in whole brain and regional volumes in the right temporal lobe compared to subjects with normal hearing. These results were robust to adjustment for multiple confounds and were consistent with voxel-based analyses, which also implicated greater volume loss on the right than left temporal regions. In addition, patients with hearing impairment showed greater rates of whole brain volume decline. The authors posited that altered central auditory processing associated with peripheral hearing impairment may lead to loss of volume in the temporal lobe and contribute to the observed deficits in semantic memory and other cognitive functions characteristic of AD.
A recent meta-analysis by Taljaard et al. (2016) investigated the relationship between peripheral hearing loss and cognitive function and examined the effect of treating hearing loss in hearing aid or cochlear implant users. The authors reported on 33 studies that included 602 participants with hearing impairment that was left untreated, 672 participants who received treatment for their hearing impairment, 176 control subjects, and 4260 individuals who demonstrated varying levels of hearing impairment both with and without treatment. This meta-analysis also confirmed that cognition is significantly poorer in individuals with hearing impairment and is worse in individuals who have not received treatment for their hearing loss. More specifically, they found that participants with superior hearing ability showed superior performance across multiple cognitive domains. However, the effect sizes were small for all domains and the authors posited that these studies may be confounded by task effects since cognitive tasks that involve hearing are more difficult for people who are hearing impaired to complete, drawing upon greater cognitive resources, compared to controls.
In the context of AD, most studies that have prospectively examined hearing changes in AD subjects have reported no significant differences in peripheral hearing between MCI, AD, and age-matched control subjects (Gates et al., 2008; Idrizbegovic et al., 2011; Rahman et al., 2011; Strouse et al., 1995). An exception is the large (over 2000 subjects) study by Heywood et al. (2017), which showed an association between hearing loss (measured with the whispered voice test) and AD that was independent of ageing (Heywood et al., 2017). However, the whispered voice test may be more dependent on cognitive factors than PTA (Groen, 1973). It is important to note that the studies not showing an association between AD and peripheral hearing loss were focused on identifying central auditory dysfunction, not peripheral deficits. and they also had much smaller sample sizes, ranging from 10 to 150 participants. Taken together, these data suggest though common ageing-related changes may produce peripheral hearing loss and subclinical cognitive changes, the impact of peripheral hearing loss on the development of clinically-significant AD is relatively small.
2.2. Overview: dichotic listening tasks in MCI and AD
Dichotic listening tasks are commonly employed in audiological assessments to examine central auditory function. There are a few variations of these tests such as dichotic presentation of consonant-vowels (CV) syllables, synthetic sentence identification with ipsilateral competing message (SSI-ICM) synthetic sentence identification with contralateral competing message (SSI-CCM), dichotic sentence identification (DSI), and dichotic digits identification (DDI; also referred to as dichotic digits test [DDT]). In all forms of these paradigms, a different, competing message is presented to each ear and the listener is asked to focus on specific messages within the clutter. The main differences between these four assessments lie in the specific stimulus being presented. Consonant-vowel syllables combine different consonants, such as/p/,/t/, or/b/with the vowel/a/, SSI and DSI use sentences, and DDI/ DDT uses digits alone. Studies have shown that impairment in dichotic listening tasks may suggest temporal and frontal lobe dysfunction (Hugdahl et al., 2003; Jancke and Shah, 2002). Recent work has also shown that performance on dichotic listening tasks is strongly correlated to more general cognitive impairment on the Repeatable Battery for the Assessment of Neuropsychological Status (RBANS) in aged subjects at risk for dementia, though correlations with more traditional AD biomarkers (CSF amyloid, tau, and volumetric measures) were weak (Tuwaig et al., 2017).
Multiple studies have suggested that central auditory dysfunction may be an early indicator of AD pathology and have used dichotic listening tasks to identify impairment. Mohr et al. (1990) were one of the first groups to document central auditory impairment in AD. They investigated 22 patients with AD using a dichotic listening paradigm where word pairs were presented to each ear that were either of no relation to one another or matched either semantically (e.g., cloud/sun) or phonemically (e.g., cold/car). In addition, the number of word pairs presented in each test varied from one-pair, two-pairs, or three-pairs. Subjects initially were required to recall any of the words presented (free-recall) followed by an alternately varied left ear recall or right ear recall condition. They found that the participants’ performance accuracy on all of the tasks combined was lower in AD patients compared to the control group. Furthermore, while control subjects demonstrated the well-known left ear preference under left ear recall and enhanced right ear advantage under right ear recall compared to free recall, the AD patients were less able to shift order of recall specified by the perimenters, suggesting an inability to shift attention. However, words that were matched either semantically or phonemically imposed fewer task demands in both groups and showed smaller right ear advantages. Interestingly, there was an absence of an interaction between group effects suggesting that loss of auditory processing in AD is not global.
Strouse et al. (1995) tested both peripheral and central auditory function in 10 patients with mild to moderate AD and 10 control subjects. The authors used immittance audiometry, pure-tone and speech audiometry, and otoacoustic emissions to test peripheral auditory function and SSI-ICM, DDT, DSI, pitch patterns, and duration patterns for central auditory function. They found that the AD group did not differ from controls based upon peripheral auditory thresholds, except that AD patients had a lower average hearing sensitivity for low frequency tones presented in the left ear. However, AD patients performed significantly worse on DDT, DSI, and SSI-ICM than control subjects.
Gates et al. (2008) designed a study consisting of 17 patients with AD, 64 with mild memory impairment without dementia, and 232 age-matched controls and created a protocol consisting of PTA, tympanometry, word recognition testing, SSI, DSI, and DDT. Both the AD group and the memory impaired without dementia group scored significantly worse on the pure tone threshold testing in either ear compared to controls. Therefore, adjustments for pure-tone thresholds were implemented to evaluate group scores on central auditory processing tests which found that combined performance on DSI, SSI, and DDT was a good predictor of level of memory impairment for both groups. The experimenters measured performance at two different testing thresholds, < 80% composite score and < 50% composite score. Using the < 80% threshold, composite scores showed a 97.5% sensitivity and 28.9% specificity for discrimination between control subjects and those with either mild memory impairment or AD. Decreasing to the < 50% threshold showed an 80.2% sensitivity and 78.4% specificity. Most notably, data from the study showed control groups performing the best, AD patients the worst, and memory impaired without dementia patients showed intermediate performance illustrating a strong relationship between early memory loss and tests of central auditory function. Furthermore, the SSI test showed the largest difference in means between the two target groups which suggests that this protocol may be the most sensitive to change when memory impairment without dementia has progressed to a diagnosis of AD. The results for DSI and SSI-ICM were replicated by Edwards et al. (2017) in MCI patients.
Gates et al. (2011) followed patients from their original study three years later and tested them again using SSI, DSI, and DDT. All 17 of the patients previously diagnosed with AD and 22 cognitively normal participants who dropped out of the study were excluded from the follow-up study. At the 3-year follow up, all three of the listening paradigms showed the incident dementia group performing significantly worse than the nondemented group. Of these paradigms, DSI in free report mode strongly predicted the risk of diagnosis of AD from memory impaired without dementia and control subjects (adjusted hazard ratios = 9.9 and 6.8 for the severe and moderate central auditory dysfunction groups, respectively). Although DDT and SSI both showed impairments in the dementia group, neither were significant predictors of AD diagnosis.
2.3. Other listening tests
Idrizbegovic et al. (2011) investigated auditory impairment in MCI and AD using PTA, DDT, speech in quiet (SPIQ), and speech in noise (SPIN) testing and compared these patient populations to controls and individuals with subjective memory complaints (SMC). SPIN testing is similar to dichotic testing in that listeners are asked to focus on an auditory stimulus with a competing stimulus, but differs in that the two are not necessarily presented to different ears. The investigators found no differences in performance between the three groups based on pure tone threshold, SPIQ, and SPIN testing (Idrizbegovic et al., 2011). DDT ex- showed significance between groups with AD scoring in the worst category and MCI in the intermediate range. In addition, a significant right ear advantage was observed in the AD and MCI groups, but not the SMC group. When right ear advantages were compared among groups, the differences showed statistical significance between the AD group and both MCI and SMC groups; however, the right ear advantage was not significant between the MCI and SMC groups. Patients in the AD group performed worse on dichotic tests of the left ear, thus enhancing the right ear advantage. These data were similar to results found by Strouse et al. (1995).
The same patients were followed 1.5 years later and tested again using the same paradigms, and only the AD group showed a decline of DDT performance in the free report paradigm of the left ear. There were no significant changes in the directed report performance in either the left or right ear. Furthermore, the MCI and SMC groups showed a slight improvement in their DDT scores suggesting a training effect. Most notably, Idrizbegovic et al. (2013) identified that during this period the 4 patients that developed AD showed scores on DDT that were very similar to the scores found in the original AD group, suggesting that DDT may carry prognostic significance.
Rahman et al. (2011) tested central auditory processing in MCI patients using DDT in addition to administering PTA, immittance, selective auditory attention test (SAAT), pitch pattern sequence (PPS) and Goldman-Fristoe-Woodcock (GFW) testing. A total of 150 patients with MCI and 150 age-matched controls participated in the study. They found that MCI and control subjects did not differ in their performance on basic audiological tests. However, the MCI patients scored significantly lower than control groups on SAAT, DDT left ear, PPS, and GFW testing. When scores from DDT, PPS, and recognition memory were combined they showed a sensitivity and specificity for diagnosis of MCI of 81.2% and 93.2% respectively. Finally, Rahman et al. (2011) posited that the increased right ear advantage seen in MCI patients may be attributable to deficits of auditory information transfer between the left and right hemisphere through the corpus callosum. As described previously, normal elderly patients show a right ear advantage in the DDT, but this advantage is more significant in MCI patients (Aimoni et al., 2014; Lee et al., 2016; Rahman et al., 2011).
2.4. Summary: audiological assessment
Overall, the data from audiological assessments of MCI and AD subjects suggest that AD pathology is associated with primarily central, rather than peripheral, auditory dysfunction. This conclusion is based on the relative preservation of pure tone thresholds in patients with MCI and AD. However, early changes are seen in measures of central auditory function, such as the findings from various forms of dichotic listening tasks. Diagnostically, the dichotic listening protocol that yielded the best sensitivity and specificity for MCI was used by Rahman et al. (2011) which combined scores from DDT, PPS, and recognition memory with a sensitivity of 81.2% and 93.2% respectively. An additional and intriguing set of findings is that patients with AD and MCI exhibit an enhanced right ear advantage compared to age-matched controls who also show right ear advantage, but of smaller magnitude. The enhanced right ear advantage seen in AD and MCI patients could be due to a higher degree of dysfunction in the right temporal lobe or corpus callosum as evidenced by studies performed on patients with lesions in the splenium of the corpus callosum (Pollmann et al., 2002) and patients with hearing loss who demonstrated an accelerated rate of volume decline in the right temporal lobe (Lin et al., 2014). More research is needed to confirm a pathophysiological model that ties together cortical functional loss with the observed deficits.
3. Electrophysiological measurements
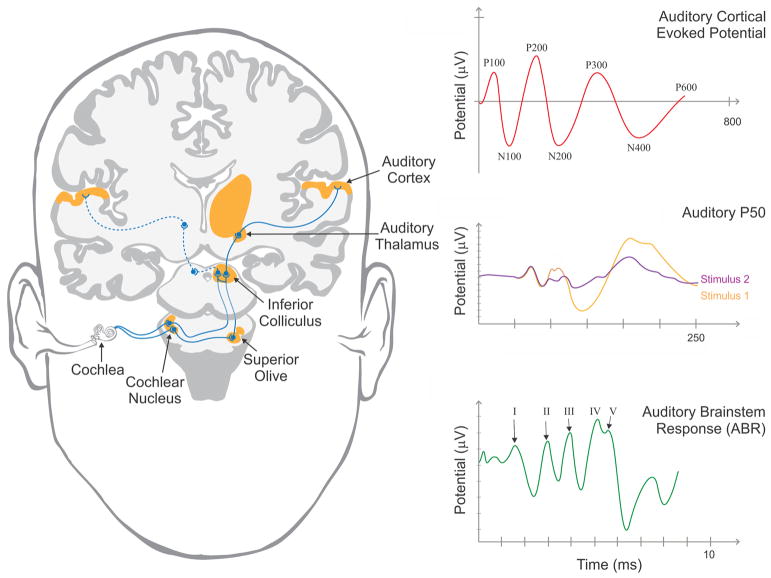
Electroencephalography (EEG) is a relatively inexpensive, non-invasive electrophysiological technique that yields temporally sensitive measures of neural function. EEG activity time-locked to the presentation of auditory stimuli, referred to the auditory evoked potential, has been used to study peripheral and central auditory system in MCI and AD. The auditory brainstem response (ABR) is defined as a series of voltage peaks recorded within the first 10 milliseconds following auditory stimulus, such as a click or vowel sound, and has been shown to measure transmission of auditory sensory information from the auditory nerve to the inferior colliculus (See Fig. 1; (Rowe, 1981)). Although age-related changes in the ABR have been documented in the literature (Konrad-Martin et al., 2012), historically MCI and AD patients have not shown pathological changes in the ABR (Grimes et al., 1987; Rowe, 1981). However, recent evidence has emerged suggesting pathological changes in short-latency ( < 10 milliseconds), presumably brainstem-generated, auditory evoked potentials which will be discussed below (Bidelman et al., 2017).
Fig 1.
Left) Diagram of the human auditory system illustrating the presumed generators of auditory evoked potentials. Right) Representative images of the auditory brainstem response (bottom), thought to be generated from the auditory brainstem and midbrain, P50 response (middle), thought to be generated from auditory subcortical generators, demonstrating responses to initial (orange) and subsequent (purple) stimuli and cortically-based (top) auditory evoked potentials.
The majority of the research outlining pathological changes observed in the auditory system of patients with MCI and AD have examined cortical activity commonly referred to as event-related potentials (ERPs). ERPs reflect the summation of post-synaptic potentials that are timed-locked to certain events of interest and are capable of detecting subtle neurophysiological alterations that often precede structural brain changes. ERPs are often divided into two broad categories: early, “sensory” waveforms that depend largely on the stimulus characteristics and peak within the first 100–150 milliseconds and later, “cognitive” waveforms that reflect cognitive processing (Sur and Sinha, 2009). The primary ERPs investigated within the scope of MCI and AD are the mismatch negativity (MMN), P50 (P1), N100 (N1), N200 (N2), P300 (P3), N400, and P600 waveforms (Fig. 1).
3.1. Mismatch negativity (MMN)
MMN is an auditory event-related potential reflecting stimulus discrimination. To elicit this response, the classic “oddball” paradigm is used where infrequent deviant sounds are randomly embedded in a sequence of standard sounds (Pekkonen, 2000). The process is thought to be an index of pre-attentive sensory memory or nondeclarative implicit memory because attention is not required to observe its response (Key et al., 2005). There is evidence in the literature that the MMN is not attenuated or delayed in AD when using tasks with short inter-stimulus intervals (Kazmerski et al., 1997; for review see; Pekkonen, 2000). Furthermore, Pekkonen (2000) posited that degenerative changes in AD are often seen in the mesial temporal lobe and are relatively spared in the primary auditory cortex which is one source of the MMN and a possible explanation for its preservation in AD.
3.2. P50 (P1)
The P50 waveform, often described as the most positive peak between 40 and 75 milliseconds after the conditioning stimulus, is responsible for “sensory gating” which is crucial to an individual’s ability to selectively attend to salient stimuli (Sur and Sinha, 2009). Brain structures associated with this waveform include the thalamus and thalamic reticular nucleus as well as the temporal cortex (Key et al., 2005; Korzyukov et al., 2007; Krause et al., 2003). Multiple studies have shown increased raw amplitude of P50 waveforms in both MCI (Golob et al., 2007; Golob et al., 2002; Irimajiri et al., 2005) and AD (Cancelli et al., 2006; Golob and Starr, 2000)suggesting dysfunctional sensory gating in these conditions.
Cancelli et al. (2006) investigated whether an auditory sensory gating deficit may be present in AD and if the acetylcholinesterase inhibitor, donepezil, would improve this potential deficit. These researchers evaluated 18 probable AD patients and 15 age-matched controls using a conditioning-testing paradigm where two identical auditory stimuli (clicks) were presented 500 milliseconds apart. Healthy subjects normally show suppression of the second (test) P50 wave compared to the first (conditioning) wave, and the ratio of these two (P50t/c) is used as an index of sensory gating. This paradigm was first performed in drug naïve patients and then again both one month after donepezil therapy and three months after therapy. As expected, the study found that AD patients showed both an increased P50t/c ratio anand a larger P50 amplitude compared to controls, suggesting diminished sensory gating in these subjects. Donepezil induced a significant reduction in P50 amplitude and latency, but did not affect the P50t/c ratio. In addition, 10 mg donepezil was more effective in decreasing P50 amplitude relative to the 5 mg dose. Due to the failure of donepezil in restoring sensory gating function in AD patients, the authors speculated that P50 gating in AD may not be linked to a cholinergic deficit. In addition, based upon research that showed that donepezil enhances the release of dopamine in vitro and that dopamine reduced both P50 amplitude and latency, they posited that donepezil may contribute to sensory gating by diminishing cortical excitability and the P50 waveform through a dopaminergic mechanism.
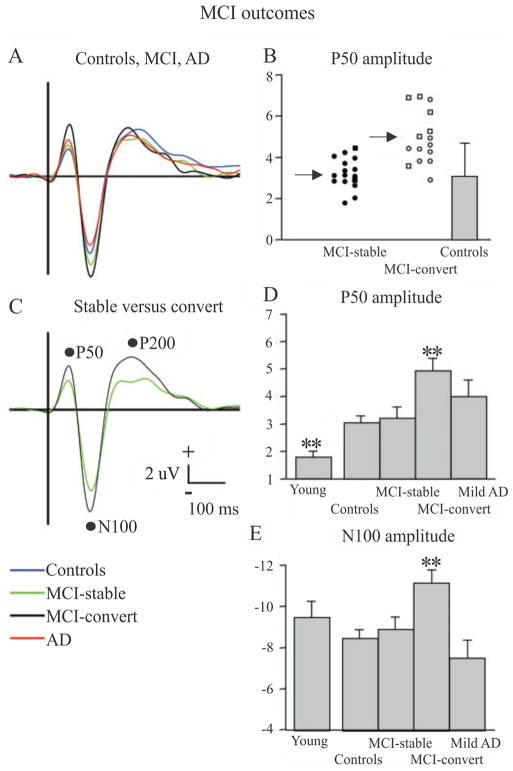
Golob et al. (2007) examined a total of 134 subjects from 1999 to 2005 using a target detection or oddball task where subjects were asked to press a button if they heard a 2000 Hz target tone and to ignore a 1000 Hz non-target tone. Subjects were split into one of 5 groups: young (n = 22), age-matched controls (n = 44), amnestic MCI-single domain (MCI-SD; n = 28), amnestic MCI-multiple domain (MCI-MD; n = 13), and mild AD (n = 14) and ERPs were recorded via EEG. The patients with MCI were further categorized into two other groups, MCI-stable and MCI-convert, based upon whether they converted to dementia by the end of the study (tested up to five years later). The researchers discovered that not only did P50 amplitude increase with normal ageing, it also showed additional increases in MCI as a function of both initial diagnosis (MD > than SD) and outcome (MCI-convert > MCI-stable), as shown in Fig. 2. Furthermore, using P50 amplitude as a predictor of MCI converting to dementia showed a sensitivity of 81% and a specificity of 73%. The amplitude and latency of other evoked potentials (N100, P200, and P300) were measured, but did not enhance prediction outcomes.
Fig. 2.
ERPs in response to 1000 Hz tones (“non-targets”, presented with a probability of 0.8) across five groups: young controls, aged controls, MCI-stable (do not convert to AD at up to 5 year follow-up), MCI-convert (convert to AD at up to 5 year follow-up) and AD. A) ERP waveforms for all four groups and C) for MCI-stable versus MCI-convert. B, D, E) Plots of average P50 [B and D] and N100 [E] across the different groups (**p < 0.01). In [B], circle = MCI-single domain and square = MCI-multiple domain. Error bars = standard deviation.
Reproduced with permission from Golob et al., 2007.
3.3. N200 (N2)
The N2 waveform is typically described as a large negative peak between 180 and 350 milliseconds after stimulus onset measured over fronto-central scalp areas. The functional significance of the N2 has been widely debated and has been reported to be associated with selective pre-attentive stimulus evaluation and discrimination (Bennys et al., 2007) to response inhibition, stimulus evaluation, and conflict monitoring (Donkers and van Boxtel, 2004; Folstein and Van Petten, 2008). Measures of N2 latency show the most promise as a marker of cognitive deterioration (e.g., Bennys et al., 2007; Papaliagkas et al., 2008; Papaliagkas et al., 2009a,b; for review see Howe, 2014), although some have also found alterations in N2 amplitude (e.g., Bennys et al., 2011; Papaliagkas et al., 2011). Bennys et al. (2007) examined N2 changes related to an auditory oddball paradigm in 30 mild-to-moderate AD patients, 20 individuals with MCI, and 10 healthy controls. They found that N2 latency differentiated AD patients from controls with 70% sensitivity and 100% specificity, and AD patients from individuals with MCI with 75% sensitivity and 70% specificity. In a later study, Bennys et al. (2011) examined longitudinal changes in N2 in 71 individuals with MCI and 31 age-matched healthy controls at baseline and a one-year follow-up. At follow-up, individuals with MCI were categorized as MCI-stable or MCI-convert based on whether they remained cognitively stable or converted to dementia. MCI-convert had increased N2 latency at baseline compared to controls, and decreased N2 amplitudes compared to both MCI-stable individuals and controls. The researchers found that the baseline N2 amplitude showed a sensitivity of 80% and a specificity of 62% for differentiating MCI-convert from MCI-stable.
In another longitudinal study, Papaliagkas et al. (2008) examined 91 individuals with MCI and 30 age-matched healthy controls at baseline and an average follow-up of 14 months. Individuals with MCI were categorized as MCI-stable or MCI-convert at follow-up. They found that baseline N2 latency was significantly prolonged in MCI-convert compared to MCI-stable and controls, but that N2 latency did not differentiate MCI-stable from controls. The baseline N2 latency showed 100% sensitivity and 94% specificity for MCI-convert. In a later study using a subgroup of the participants from Papaliagkas et al. (2008), the researchers compared 22 individuals with MCI and 30 age-matched healthy controls at baseline, a 14 month follow-up, and a 23 month follow-up (Papaliagkas et al., 2011). They found increased baseline N2 latencies and amplitudes for MCI compared to controls. However, at both follow-ups, individuals with MCI did not show a difference in N2 latency from baseline, but did show a decrease in N2 amplitude from baseline. These findings suggest that N2 latency might be useful in identifying those who are at risk of converting to dementia, whereas N2 amplitude might be useful in monitoring longitudinal decline.
3.4. P300 (P3)
The P300 wave is typically described as the largest positive peak in the latency range of 250–400 milliseconds in the EEG waveform measured over the parietal areas. Shorter P300 latencies and larger waveforms are typically associated with superior cognitive performance (Sur and Sinha, 2009). An early study by Goodin et al. (1978) showed decreased P300 amplitude and increased latency in AD in response to a 1000 Hz standard tone and a 2000 Hz target tone in the classic oddball task. Both Polich et al. (1990) and Polich and Corey-Bloom (2005) replicated these results and found that P300 differences between AD patients and controls were largest for relatively easy tasks. Golob and Starr (2000) also described increased P300 latency in AD, but did not report decreased P300 amplitude. While investigating ERPs in MCI patients, Golob et al. (2002) reported significantly longer P300 latencies and Golob et al. (2007) replicated this observation and suggested that P300 latency increases with disease severity as evidenced by further prolongation in AD patients compared to amnestic MCI-single domain patients.
Frodl et al. (2002) also examined the P300 ERP in MCI and AD patients using an “odd-ball” paradigm in which 20% of the tones were targets (100 sinusoidal tones, 1000 Hz) and 80% were standards (400 sinusoidal tones, 500 Hz) presented at 80 dB SPL. They separated the waveform into two components, temporo-superior (TS-P300) and temporo-basal (TB-P300), based upon a protocol created by Hegerl and Frodl-Bauch (1997). Twenty-six patients with MCI, 30 patients with AD, and 26 age-matched controls were subjected to neuropsychological testing and the ERP paradigm. Results of the study provided evidence of significantly diminished amplitudes of the temporo-basal dipoles in AD patients when compared to both MCI patients and the control group. In addition, AD patients showed significantly prolonged latency of temporo-superior dipoles compared to the control group alone. Using these two measurements as predictive factors yielded a sensitivity of 90.0% and specificity of 79.1% for distinguishing AD patients from controls. TB-P300 latencies were increased in length in the left hemisphere compared to the right in AD patients. Furthermore, the amplitudes of the TS-P300 response were equal in both hemispheres of AD patients, but MCI and control subjects demonstrated larger amplitudes in the right hemisphere when compared to the left. The authors posited the deficits observed in the TS-P300 response were consistent with patterns of degeneration seen in the temporal and parietal lobe of AD brains. MCI patients did not differ from control subjects on these tests, suggesting that the paradigm may not be useful in early detection of pre-clinical levels of AD.
3.5. N400/P600
The N400 waveform of the ERP is defined as a negative wave presenting 300–550 milliseconds after stimulus onset and has been shown to be sensitive to semantic manipulations (Kutas and Federmeier, 2011; Kutas and Hillyard, 1980). For instance, previous studies have shown that N400 amplitude is smaller for words that are semantically congruous with their context and larger when words are incongruent with their context (Olichney et al., 2002). The N400 waveform is larger over the parietal and temporal regions in the right hemisphere and is likely to arise from multiple generators found in the parahippocampal gyrus, medial temporal structures near the hippocampus and amygdala, and lateral temporal region (Key et al., 2005). Studies suggest that disruption of semantic analysis of words in AD may alter the presentation of the N400 waveform (Ford et al., 1996; Iragui et al., 1996) such that semantically congruous words do not show a decrease in the N400 waveform.
Olichney et al. (2002) designed a study that investigated the N400 and another waveform that plays a role in semantic analysis referred to as P600 or late positive component, which occurs between 550 and 800 milliseconds, in individuals with MCI. The group described the phenomena that words that are encoded deeply and able to be remembered often correlate with an increase in the P600 amplitude. However, if the list is preceded by a semantic context which would increase the likelihood of the word to reappear, the P600 amplitude is subsequently decreased. Fourteen patients with MCI and 14 age-matched controls underwent neuropsychological testing and an ERP protocol. Subjects were read aloud a category statement each followed by a visually presented target word. As expected, in normal controls, ERPs to congruous targets showed a late P600 which was attenuated with repetition. MCI patients, on the other hand, demonstrated a diminished repetition effect. Notably, no congruous word repetition effect was observed among seven individuals who later developed probable AD. In addition, MCI patients who did not convert to probable AD were almost entirely responsible for any repetition effect measured within the MCI group. Finally, the P600 repetition effect was significantly correlated with several neuropsychological measures such as the California Verbal Learning Test, Consortium to Establish a Registry for AD (CERAD) word list, and Dementia Rating Scale (DRS) memory subscale. Olichney et al. (2006) repeated this protocol with AD patients and found that using P600 and N400 as classifiers yielded a 100% sensitivity and 82% specificity for the disease. Additionally, Olichney et al. (2008) examined patients with MCI and found that disrupted P600/N400 repetition effect predicted an over 80% likelihood of development of dementia within 3 years. In contrast, those with normal repetition effects had only an 11–27% likelihood of converting in the same time frame. A similar pattern of abnormal P600/N400 response was found by Olichney et al. (2013) in pre-AD patients compared to robust normal elderly.
3.6. Combined Brainstem/Cortical response
Bidelman et al. (2017) examined dual brainstem and cortical responses using a novel protocol defined in (Bidelman, 2015) by measuring the frequency following response (FFR) and cortical speech ERP. Eight patients with MCI and 15 age-matched controls were evaluated in this study and were subjected to one block of 200 active trials where they were presented with/u/to/a/sounds and forced to choose which sound they heard by pressing a button a keyboard. The results showed that patients with MCI had larger FFRs than controls, suggesting a hypersensitive response to the experimental paradigm. Contrary to previous studies, the experimenters found no effect of group on P1 amplitude which they attributed to decreased sensitivity of the P1 waveform to the stimuli in the novel paradigm due to increased duration of the stimuli, poor definition of the P1 amplitude across studies, and its strong dependence on neural synchrony. However, cortical N1-P2 amplitudes in the 100–200 milliseconds window were enhanced in patients with MCI. Finally, when brainstem and cortical ERPs were combined the researchers were able to diagnose MCI with an accuracy of 80%. The FFR finding in this study suggests that examining brainstem response in addition to cortical evoked response using novel metrics aside from traditional ABR might unravel alterations in the auditory system in MCI and AD that may have been missed in earlier studies.
3.7. Summary of electrophysiological findings
Similar to audiological assessments, evoked potential studies across multiple timescales and levels of the auditory system suggest that AD pathology is strongly associated with central auditory dysfunction. The multiple electrophysiological measures that are impaired in patients with MCI and AD are summarized in Table 1. Alterations have been seen from the auditory brainstem (FFR) to the auditory cortex, though no studies provided evidence of changes in the MMN response suggesting that immediate, echoic memory may still be intact in AD (Key et al., 2005; Pekkonen, 2000). The alterations observed in P50, N200, and P300 in studies that used simple stimuli such as pure tones that are not language specific, unlike studies of the N400/P600, may have broad diagnostic implications globally.
Table 1.
Summary of studies of auditory ERPs in MCI and AD patients.
| Waveform | Description | Changes in MCI | Changes in AD | References |
|---|---|---|---|---|
| ABR | Series of peaks recorded within the first 10 milliseconds following an auditory stimulus. Correlated with transmission of auditory sensory information from the auditory nerve to the inferior colliculus. | None | None | AD: Grimes et al. (1987), Rowe (1981) |
|
| ||||
| MMN | ERP recorded in response to infrequent deviant sound. Measurement of automatic detection of changes in stimulus related to pre-attentive sensory memory. | None | None | AD: Kazmerski et al. (1997), Pekkonen (2000) |
| P50 | Positive peak between 40 and 75 milliseconds representing function of the reticular activating system and thalamus in ‘sensory gating.’ Increases in amplitude associated with | Increased amplitude | Increased amplitude | MCI: Golob et al. (2007), Golob et al. (2002), Irimajiri et al. (2005). AD: Cancelli et al. (2006), Golob and Starr (2000) |
| N200 | Negative peak between 180–350 milliseconds elicited in response to presentation of an infrequent stimulus during an oddball paradigm or novel stimuli. | Increased latency | Increased latency | MCI: Bennys et al. (2007), Bennys et al. (2011), Cintra et al. (2017), Papaliagkas et al. (2008), Papaliagkas et al. (2009), Papaliagkas et al. (2011), AD: Bennys et al. (2007), Caravaglios et al. (2008), Cintra et al. (2017) |
| P300 | Positive peak between 250–400 milliseconds elicited in response to an unexpected stimulus. Produced by activity of multiple structures in the medial temporal lobe. Larger amplitudes reffective of greater attention and shorter latency associated with superior mental performance. | Increased latency | Increased latency (except Golob and Starr which showed decreased latency) Decreased amplitude* | MCI: Golob et al. (2002) and Golob et al. (2007) AD: Goodin et al. (1978), Polich et al. (1990), Golob and Starr (2000), Frodl et al. (2002), Polich and Corey-Bloom (2005) |
| N400 | Negative wave between 300–550 milliseconds arising from multiple generators found in both medial and lateral temporal regions. Related to semantic processing demands and demonstrates reduction in amplitude with presentation of semantically congruous words. | Lack of decrease in amplitude to semantically congruous stimulus | Lack of decrease in amplitude to semantically congruous stimulus | MCI: Olichney et al. (2002), Olichney et al. (2008), and Olichney et al. (2013). AD: Ford et al. (1996), Iragui et al. (1996), Olichney et al. (2006) |
| P600 | Positive wave between 550–800 milliseconds arising from multiple generators in the medial temporal lobe and paralimbic cortical regions. Implicated as an index of memory encoding that demonstrates reduction in amplitude to semantically primed or repeated words. | Lack of decrease in amplitude to semantically congruous stimulus | Lack of decrease in amplitude to semantically congruous stimulus | MCI: Olichney et al. (2002), Olichney et al. (2008), and Olichney et al. (2013). AD: Olichney et al. (2006) |
| FFR/N1-P2 | Dual protocol measuring neuroelectric activity in the auditory brainstem (FFR) and cortical ERP’s simultaneously. | Increased FFR response and N1-P2 amplitudes | N/a | MCI: Bidelman et al. (2017) |
4. Pathophysiological mechanisms and implications for AD and conclusion
AD is a devastating illness characterized by cognitive losses caused by insults to multiple brain structures including the hippocampus and temporal cortex, as well as the frontal and parietal lobes. Both peripheral and central auditory dysfunction have been implicated in the disease from prodromal AD to advanced stages of the illness. Although there is widespread atrophy of the AD brain, specific structures seem to be at greater risk than others early in the disease, particularly in central auditory structures. From the research reviewed here, an interesting pattern of pathological findings has emerged such that loss of top-down modulation of incoming auditory stimuli and loss of sensory gating ability appear to be hallmarks of AD pathology. The dichotic listening tasks are particularly useful in investigating top-down modulation as they require participant to select between competing auditory stimuli. Further evidence can be found by examining the P50 waveform responsible for sensory gating which is crucial to an individual’s ability to selectively attend to salient stimuli and protect the brain from information overload (Sur and Sinha, 2009). As described above, the P50 amplitude has been found to be pathologically enhanced in MCI and AD, suggestive of impairment to sensory modulation resulting in overload. In addition, the changes in N200 and P300 in AD described above may reflect alterations in auditory attention and effectiveness of selection in these patients.
Previous investigators have speculated that hearing loss itself may lead to later cognitive impairment (Wayne and Johnsrude, 2015). This supposition is supported by epidemiological studies linking peripheral hearing loss with cognitive impairment (Gurgel et al., 2014; Lin et al., 2011b; Teipel et al., 2015) and animal studies whereby noise exposure known to damage the cochlea can lead to more general cognitive dysfunction (Cernak et al., 2001; Zheng et al., 2011a; Zheng et al., 2011b), as well as alterations to structures outside of the auditory system, such as the hippocampus (Goble et al., 2009; Kraus et al., 2010). Although there is ample evidence that peripheral hearing loss leads to changes in the central auditory system (reviewed in Eggermont, 2017; Lesicko and Llano, 2017; Syka, 2002), the clinical data reviewed here suggest that the audiological deficits seen in AD are not directly related to mala-daptive plastic changes driven by loss of peripheral hearing. This is because AD and MCI patients demonstrate poor performance across multiple central auditory tasks in the setting of normal or near-normal PTAs. This interpretation would be consistent with the anatomical data demonstrating a predilection for AD pathology to be found in higher central auditory structures (Sinha et al., 1993; Sinha et al., 1996). An alternative interpretation of the audiological data is that some degree of cochlear synaptopathy is present in AD patients (i.e., “hidden hearing loss”), preserving PTA values, but impacting speech-in-noise performance. Future work involving postmortem examination of cochlear synaptic pathology in conjunction with AD pathology will help to differentiate these two possibilities.
Given the high diagnostic and predictive accuracy of central processing audiological and neurophysiological metrics described above, combined with their low-cost and non-invasiveness, a comprehensive audiological and evoked-potential assessment should be considered for all MCI and AD patients. In addition, unlike other biomarkers, audiological assessments provide a therapeutic entry point by revealing functional gaps in the patient’s ability to communicate. Further, given the increasing evidence that auditory-based therapeutic interventions, such as music-based therapy, can increase cognitive reserve and memory function (Alain et al., 2014; Sarkamo et al., 2014; Simmons-Stern et al., 2010), audiological and evoked-potential based tools may be useful for monitoring progress and response to therapeutic interventions.
Current research is highly suggestive of a relationship between central auditory dysfunction and subsequent cognitive deterioration. Future studies should focus on establishing an easily replicable audiological and evoked potential protocol capable of stratifying at-risk patients prior to the manifestation of symptoms. Given the high rates of ageing-related hearing loss in the geriatric population (Lin et al., 2011c; Zhan et al., 2010), referral of all individuals with presbycusis for cognitive evaluations would lead to unnecessary screenings. A more costeffective approach would be to focus on patients with more AD-specific patterns, such as those patients with preferential dysfunction in dichotic listening tasks compared to PTAs. Therefore, and optimal approach would be to develop methods to increase the specificity of such analyses to further hone in on specific vulnerable populations. Once at-risk populations are identified, specific interventions to target central auditory dysfunction can be implemented to enhance their communication abilities and possibly their more general cognitive abilities.
References
- Agrawal Y, Platz EA, Niparko JK. Risk factors for hearing loss in US adults: data from the National Health and Nutrition Examination Survey, 1999–2002. Otol Neurotol. 2009;30:139–145. doi: 10.1097/MAO.0b013e318192483c. [DOI] [PubMed] [Google Scholar]
- Aimoni C, Prosser S, Ciorba A, Menozzi L, Soavi C, Zuliani G. Speech audiometry tests in noise are impaired in older patients with mild cognitive impairment: a pilot study. J Int Adv Otol. 2014;10(3):228–233. [Google Scholar]
- Alain C, Zendel BR, Hutka S, Bidelman GM. Turning down the noise: the benefit of musical training on the aging auditory brain. Hear Res. 2014;308:162–173. doi: 10.1016/j.heares.2013.06.008. [DOI] [PubMed] [Google Scholar]
- Anstey KJ, Luszcz MA, Sanchez L. A reevaluation of the common factor theory of shared variance among age, sensory function: and cognitive function in older adults. J Gerontol B Psychol Sci Soc Sci. 2001;56:3–11. doi: 10.1093/geronb/56.1.p3. [DOI] [PubMed] [Google Scholar]
- Bennys K, Portet F, Touchon J, Rondouin G. Diagnostic value of event-related evoked potentials N200 and P300 subcomponents in early diagnosis of Alzheimer's disease and mild cognitive impairment. J Clin Neurophysiol. 2007;24:405–412. doi: 10.1097/WNP.0b013e31815068d5. [DOI] [PubMed] [Google Scholar]
- Bennys K, Rondouin G, Benattar E, Gabelle A, Touchon J. Can event-related potential predict the progression of mild cognitive impairment. J Clin Neurophysiol. 2011;28:625–632. doi: 10.1097/WNP.0b013e31823cc2d3. [DOI] [PubMed] [Google Scholar]
- Bidelman GM, Lowther JE, Tak SH, Alain C. Mild cognitive impairment is characterized by deficient brainstem and cortical representations of speech. J Neurosci. 2017;37:3610–3620. doi: 10.1523/JNEUROSCI.3700-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidelman GM. Towards an optimal paradigm for simultaneously recording cortical and brainstem auditory evoked potentials. J Neurosci Methods. 2015;241:94–100. doi: 10.1016/j.jneumeth.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Cancelli I, Cadore IP, Merlino G, Valentinis L, Moratti U, Bergonzi P, Gigli GL, Valente M. Sensory gating deficit assessed by P50/Pb middle latency event related potential in Alzheimer's disease. J Clin Neurophysiol. 2006;23:421–425. doi: 10.1097/01.wnp.0000218991.99714.ee. [DOI] [PubMed] [Google Scholar]
- Cernak I, Wang Z, Jiang J, Bian X, Savic J. Cognitive deficits following blast injury-induced neurotrauma: possible involvement of nitric oxide. Brain Inj. 2001;15:593–612. doi: 10.1080/02699050010009559. [DOI] [PubMed] [Google Scholar]
- Cintra MTG, Avila RT, Soares TO, Cunha LCM, Silveira KD, de Moraes EN, Simas KR, Fernandes RB, Goncalves DU, de Rezende NA, Bicalho MAC. Increased N200 and P300 latencies in cognitively impaired elderly carrying ApoE epsilon-4 allele. Int J Geriatr Psychiatry. 2017:e221–e227. doi: 10.1002/gps.4773. [DOI] [PubMed] [Google Scholar]
- Donkers FC, van Boxtel GJ. The N2 in go/no-go tasks reflects conflict monitoring not response inhibition. Brain Cogn. 2004;56:165–176. doi: 10.1016/j.bandc.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Edwards JD, Lister JJ, Elias MN, Tetlow AM, Sardina AL, Sadeq NA, Brandino AD, Harrison Bush AL. Auditory processing of older adults with probable mild cognitive impairment. J Speech Lang Hear Res. 2017;60:1427–1435. doi: 10.1044/2016_JSLHR-H-16-0066. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Acquired hearing loss and brain plasticity. Hear Res. 2017;343:176–190. doi: 10.1016/j.heares.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Folstein JR, Van Petten C. Influence of cognitive control and mismatch on the N2 component of the ERP: a review. Psychophysiology. 2008;45:152–170. doi: 10.1111/j.1469-8986.2007.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, Woodward SH, Sullivan EV, Isaacks BG, Tinklenberg JR, Yesavage JA, Roth WT. N400 evidence of abnormal responses to speech in Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1996;99:235–246. doi: 10.1016/0013-4694(96)95049-x. [DOI] [PubMed] [Google Scholar]
- Fransen E, Topsakal V, Hendrickx JJ, Van Laer L, Huyghe JR, Van Eyken E, Lemkens N, Hannula S, Maki-Torkko E, Jensen M, Demeester K, Tropitzsch A, Bonaconsa A, Mazzoli M, Espeso A, Verbruggen K, Huyghe J, Huygen PL, Kunst S, Manninen M, Diaz-Lacava A, Steffens M, Wienker TF, Pyykko I, Cremers CW, Kremer H, Dhooge I, Stephens D, Orzan E, Pfister M, Bille M, Parving A, Sorri M, Van de Heyning P, Van Camp G. Occupational noise, smoking, and a high body mass index are risk factors for age-related hearing impairment and moderate alcohol consumption is protective: a European population-based multicenter study. J Assoc Res Otolaryngol. 2008;9:264–276. doi: 10.1007/s10162-008-0123-1. discussion 261–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodl T, Hampel H, Juckel G, Burger K, Padberg F, Engel RR, Moller HJ, Hegerl U. Value of event-related P300 subcomponents in the clinical diagnosis of mild cognitive impairment and Alzheimer's Disease. Psychophysiology. 2002;39:175–181. doi: 10.1017/S0048577202010260. [DOI] [PubMed] [Google Scholar]
- Gates GA, Mills JH. Presbycusis. Lancet. 2005;366:1111–1120. doi: 10.1016/S0140-6736(05)67423-5. [DOI] [PubMed] [Google Scholar]
- Gates GA, Cobb JL, D'Agostino RB, Wolf PA. The relation of hearing in the elderly to the presence of cardiovascular disease and cardiovascular risk factors. Arch Otolaryngol Head Neck Surg. 1993;119:156–161. doi: 10.1001/archotol.1993.01880140038006. [DOI] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, Feeney MP, McCurry SM, Larson EB. Central auditory dysfunction in older persons with memory impairment or Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2008;134:771–777. doi: 10.1001/archotol.134.7.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates GA, Anderson ML, McCurry SM, Feeney MP, Larson EB. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2011;137:390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Goble TJ, Moller AR, Thompson LT. Acute high-intensity sound exposure alters responses of place cells in hippocampus. Hear Res. 2009;253:52–59. doi: 10.1016/j.heares.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Starr A. Effects of stimulus sequence on event-related potentials and reaction time during target detection in Alzheimer's disease. Clin Neurophysiol. 2000;111:1438–1449. doi: 10.1016/s1388-2457(00)00332-1. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Johnson JK, Starr A. Auditory event-related potentials during target detection are abnormal in mild cognitive impairment. Clin Neurophysiol. 2002;113:151–161. doi: 10.1016/s1388-2457(01)00713-1. [DOI] [PubMed] [Google Scholar]
- Golob EJ, Irimajiri R, Starr A. Auditory cortical activity in amnestic mild cognitive impairment: relationship to subtype and conversion to dementia. Brain. 2007;130:740–752. doi: 10.1093/brain/awl375. [DOI] [PubMed] [Google Scholar]
- Golub JS, Luchsinger JA, Manly JJ, Stern Y, Mayeux R, Schupf N. Observed hearing loss and incident dementia in a multiethnic cohort. J Am Geriatr Soc. 2017;65:1691–1697. doi: 10.1111/jgs.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodin DS, Squires KC, Starr A. Long latency event-related components of the auditory evoked potential in dementia. Brain. 1978;101:635–648. doi: 10.1093/brain/101.4.635. [DOI] [PubMed] [Google Scholar]
- Grimes AM, Grady CL, Pikus A. Auditory evoked potentials in patients with dementia of the Alzheimer type. Ear Hear. 1987;8:157–161. doi: 10.1097/00003446-198706000-00005. [DOI] [PubMed] [Google Scholar]
- Groen JJ. Pure tone audiometry and whispered voice test: conformities and differences in tests results. ORL J Otorhinolaryngol Relat Spec. 1973;35:65–70. doi: 10.1159/000275089. [DOI] [PubMed] [Google Scholar]
- Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci U S A. 1986;83:4913–4917. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurgel RK, Ward PD, Schwartz S, Norton MC, Foster NL, Tschanz JT. Relationship of hearing loss and dementia: a prospective, population-based study. Otol Neurotol. 2014;35:775–781. doi: 10.1097/MAO.0000000000000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CJ, Marshall CR, Golden HL, Clark CN, Mummery CJ, Griffiths TD, Bamiou DE, Warren JD. Hearing and dementia. J Neurol. 2016;263:2339–2354. doi: 10.1007/s00415-016-8208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert LE, Weuve J, Scherr PA, Evans DA. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology. 2013;80:1778–1783. doi: 10.1212/WNL.0b013e31828726f5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffner HE, Heffner RS. Effect of unilateral and bilateral auditory cortex lesions on the discrimination of vocalizations by Japanese macaques. J Neurophysiol. 1986;56:683–701. doi: 10.1152/jn.1986.56.3.683. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Frodl-Bauch T. Dipole source analysis of P300 component of the auditory evoked potential: a methodological advance? Psychiatry Res. 1997;74:109–118. doi: 10.1016/s0925-4927(97)03129-6. [DOI] [PubMed] [Google Scholar]
- Herholz K, Salmon E, Perani D, Baron JC, Holthoff V, Frolich L, Schonknecht P, Ito K, Mielke R, Kalbe E, Zundorf G, Delbeuck X, Pelati O, Anchisi D, Fazio F, Kerrouche N, Desgranges B, Eustache F, Beuthien-Baumann B, Menzel C, Schroder J, Kato T, Arahata Y, Henze M, Heiss WD. Discrimination between Alzheimer dementia and controls by automated analysis of multicenter FDG PET. Neuroimage. 2002;17:302–316. doi: 10.1006/nimg.2002.1208. [DOI] [PubMed] [Google Scholar]
- Heywood R, Gao Q, Nyunt MSZ, Feng L, Chong MS, Lim WS, Yap P, Lee TS, Yap KB, Wee SL, Ng TP. Hearing loss and risk of mild cognitive impairment and dementia: findings from the Singapore longitudinal ageing study. Dement Geriatr Cogn Disord. 2017;43:259–268. doi: 10.1159/000464281. [DOI] [PubMed] [Google Scholar]
- Howe AS. Meta-analysis of the endogenous N200 latency event-related potential subcomponent in patients with Alzheimer's disease and mild cognitive impairment. Clin Neurophysiol. 2014;125:1145–1151. doi: 10.1016/j.clinph.2013.10.019. [DOI] [PubMed] [Google Scholar]
- Huang Q, Tang J. Age-related hearing loss or presbycusis. Eur Arch Otorhinolaryngol. 2010;267:1179–1191. doi: 10.1007/s00405-010-1270-7. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Bodner T, Weiss E, Benke T. Dichotic listening performance and frontal lobe function. Brain Res Cogn Brain Res. 2003;16:58–65. doi: 10.1016/s0926-6410(02)00210-0. [DOI] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH, Wingfield A. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol. 2012;23:635–666. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idrizbegovic E, Hederstierna C, Dahlquist M, Kampfe Nordstrom C, Jelic V, Rosenhall U. Central auditory function in early Alzheimer's disease and in mild cognitive impairment. Age Ageing. 2011;40:249–254. doi: 10.1093/ageing/afq168. [DOI] [PubMed] [Google Scholar]
- Idrizbegovic E, Hederstierna C, Dahlquist M, Rosenhall U. Short-term longitudinal study of central auditory function in Alzheimer's disease and mild cognitive impairment. Dement Geriatr Cogn Dis Extra. 2013;3:468–471. doi: 10.1159/000355371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iragui V, Kutas M, Salmon DP. Event-related brain potentials during semantic categorization in normal aging and senile dementia of the Alzheimer's type. Electroencephalogr Clin Neurophysiol. 1996;100:392–406. [PubMed] [Google Scholar]
- Irimajiri R, Golob EJ, Starr A. Auditory brain-stem, middle- and long-latency evoked potentials in mild cognitive impairment. Clin Neurophysiol. 2005;116:1918–1929. doi: 10.1016/j.clinph.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Jancke L, Shah NJ. Does dichotic listening probe temporal lobe functions? Neurology. 2002;58:736–743. doi: 10.1212/wnl.58.5.736. [DOI] [PubMed] [Google Scholar]
- Jerger J, Chmiel R, Wilson N, Luchi R. Hearing impairment in older adults: new concepts. J Am Geriatr Soc. 1995;43:928–935. doi: 10.1111/j.1532-5415.1995.tb05539.x. [DOI] [PubMed] [Google Scholar]
- Kazmerski VA, Friedman D, Ritter W. Mismatch negativity during attend and ignore conditions in Alzheimer's disease. Biol Psychiatry. 1997;42:382–402. doi: 10.1016/S0006-3223(96)00344-7. [DOI] [PubMed] [Google Scholar]
- Key AP, Dove GO, Maguire MJ. Linking brainwaves to the brain: an ERP primer. Dev Neuropsychol. 2005;27:183–215. doi: 10.1207/s15326942dn2702_1. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Helkala EL, Laakso MP, Hanninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Ann Intern Med. 2002;137:149–155. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]
- Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kareholt I, Winblad B, Helkala EL, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Arch Neurol. 2005;62:1556–1560. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- Konrad-Martin D, Dille MF, McMillan G, Griest S, McDermott D, Fausti SA, Austin DF. Age-related changes in the auditory brainstem response. J Am Acad Audiol. 2012;23:18–35. doi: 10.3766/jaaa.23.1.3. quiz 74–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzyukov O, Pflieger ME, Wagner M, Bowyer SM, Rosburg T, Sundaresan K, Elger CE, Boutros NN. Generators of the intracranial P50 response in auditory sensory gating. Neuroimage. 2007;35:814–826. doi: 10.1016/j.neuroimage.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus KS, Mitra S, Jimenez Z, Hinduja S, Ding D, Jiang H, Gray L, Lobarinas E, Sun W, Salvi RJ. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause M, Hoffmann WE, Hajos M. Auditory sensory gating in hippocampus and reticular thalamic neurons in anesthetized rats. Biol Psychiatry. 2003;53:244–253. doi: 10.1016/s0006-3223(02)01463-4. [DOI] [PubMed] [Google Scholar]
- Kutas M, Federmeier KD. Thirty years and counting: finding meaning in the N400 component of the event-related brain potential (ERP) Annu Rev Psychol. 2011;62:621–647. doi: 10.1146/annurev.psych.093008.131123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutas M, Hillyard SA. Reading senseless sentences: brain potentials reflect semantic incongruity. Science. 1980;207:203–205. doi: 10.1126/science.7350657. [DOI] [PubMed] [Google Scholar]
- Lee SJ, Park KW, Kim LS, Kim H. Effects of noise level and cognitive function on speech perception in normal elderly and elderly with amnestic mild cognitive impairment. Cogn Behav Neurol. 2016;29:68–77. doi: 10.1097/WNN.0000000000000092. [DOI] [PubMed] [Google Scholar]
- Lesicko AM, Llano DA. Impact of peripheral hearing loss on top-down auditory processing. Hear Res. 2017;343:4–13. doi: 10.1016/j.heares.2016.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC, Kujawa SG. Cochlear synaptopathy in acquired sensorineural hearing loss: manifestations and mechanisms. Hear Res. 2017;349:138–147. doi: 10.1016/j.heares.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, Metter EJ, An Y, Zonderman AB, Resnick SM. Hearing loss and cognition in the Baltimore Longitudinal Study of Aging. Neuropsychology. 2011a;25:763–770. doi: 10.1037/a0024238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Metter EJ, O'Brien RJ, Resnick SM, Zonderman AB, Ferrucci L. Hearing loss and incident dementia. Arch Neurol. 2011b;68:214–220. doi: 10.1001/archneurol.2010.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Thorpe R, Gordon-Salant S, Ferrucci L. Hearing loss prevalence and risk factors among older adults in the United States. J Gerontol A Biol Sci Med Sci. 2011c;66:582–590. doi: 10.1093/gerona/glr002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin FR, Ferrucci L, An Y, Goh JO, Doshi J, Metter EJ, Davatzikos C, Kraut MA, Resnick SM. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindenberger U, Baltes PB. Sensory functioning and intelligence in old age: a strong connection. Psychol Aging. 1994;9:339–355. doi: 10.1037//0882-7974.9.3.339. [DOI] [PubMed] [Google Scholar]
- Llano DA, Li J, Waring JF, Ellis T, Devanarayan V, Witte DG, Lenz RA. Cerebrospinal fluid cytokine dynamics differ between Alzheimer disease patients and elderly controls. Alzheimer Dis Assoc Disord. 2012;26:322–328. doi: 10.1097/WAD.0b013e31823b2728. [DOI] [PubMed] [Google Scholar]
- Llano DA, Bundela S, Mudar RA, Devanarayan V. Alzheimer's Disease Neuroimaging, I., 2017. A multivariate predictive modeling approach reveals a novel CSF peptide signature for both Alzheimer's Disease state classification and for predicting future disease progression. PLoS One. 2017;12:e0182098. doi: 10.1371/journal.pone.0182098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luchsinger JA, Reitz C, Honig LS, Tang MX, Shea S, Mayeux R. Aggregation of vascular risk factors and risk of incident Alzheimer disease. Neurology. 2005;65:545–551. doi: 10.1212/01.wnl.0000172914.08967.dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald MC, Almor A, Henderson VW, Kempler D, Andersen ES. Assessing working memory and language comprehension in Alzheimer's disease. Brain Lang. 2001;78:17–42. doi: 10.1006/brln.2000.2436. [DOI] [PubMed] [Google Scholar]
- Markova J, Horvathova L, Kralova M, Csefalvay Z. Sentence comprehension in Slovak-speaking patients with Alzheimer's disease. Int J Lang Commun Disord. 2017;52:456–468. doi: 10.1111/1460-6984.12284. [DOI] [PubMed] [Google Scholar]
- Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of alzheimer's disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on alzheimer's disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr E, Cox C, Williams J, Chase TN, Fedio P. Impairment of central auditory function in Alzheimer's disease. J Clin Exp Neuropsychol. 1990;12:235–246. doi: 10.1080/01688639008400970. [DOI] [PubMed] [Google Scholar]
- Morris JC, Cummings J. Mild cognitive impairment (MCI) represents early-stage Alzheimer's disease. J Alzheimers Dis. 2005;7:235–239. doi: 10.3233/jad-2005-7306. discussion 255–262. [DOI] [PubMed] [Google Scholar]
- Mudar RA, Husain FT. Neural alterations in acquired age-Related hearing loss. Front Psychol. 2016;7:828. doi: 10.3389/fpsyg.2016.00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek FE, Baran JA, Pinheiro ML. Neuroaudiology: Case Studies. Singular Pub. Group; San Diego, Calif: 1994. [Google Scholar]
- Olichney JM, Morris SK, Ochoa C, Salmon DP, Thal LJ, Kutas M, Iragui VJ. Abnormal verbal event related potentials in mild cognitive impairment and incipient Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2002;73:377–384. doi: 10.1136/jnnp.73.4.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Iragui VJ, Salmon DP, Riggins BR, Morris SK, Kutas M. Absent event-related potential (ERP) word repetition effects in mild Alzheimer's disease. Clin Neurophysiol. 2006;117:1319–1330. doi: 10.1016/j.clinph.2006.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Taylor JR, Gatherwright J, Salmon DP, Bressler AJ, Kutas M, Iragui-Madoz VJ. Patients with MCI and N400 or P600 abnormalities are at very high risk for conversion to dementia. Neurology. 2008;70:1763–1770. doi: 10.1212/01.wnl.0000281689.28759.ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Pak J, Salmon DP, Yang JC, Gahagan T, Nowacki R, Hansen L, Galasko D, Kutas M, Iragui-Madoz VJ. Abnormal P600 word repetition effect in elderly persons with preclinical Alzheimer's disease. Cogn Neurosci. 2013;4:143–151. doi: 10.1080/17588928.2013.838945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaliagkas V, Kimiskidis V, Tsolaki M, Anogianakis G. Usefulness of event-related potentials in the assessment of mild cognitive impairment. BMC Neurosci. 2008;9:107. doi: 10.1186/1471-2202-9-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papaliagkas VT, Anogianakis G, Tsolaki MN, Koliakos G, Kimiskidis VK. Prediction of conversion from mild cognitive impairment to Alzheimer's disease by CSF cytochrome c levels and N200 latency. Curr Alzheimer Res. 2009a;6:279–284. doi: 10.2174/156720509788486626. [DOI] [PubMed] [Google Scholar]
- Papaliagkas VT, Anogianakis G, Tsolaki MN, Koliakos G, Kimiskidis VK. Progression of mild cognitive impairment to Alzheimer's disease: improved diagnostic value of the combined use of N200 latency and beta-amyloid(1-42) levels. Dement Geriatr Cogn Disord. 2009b;28:30–35. doi: 10.1159/000229023. [DOI] [PubMed] [Google Scholar]
- Papaliagkas VT, Kimiskidis VK, Tsolaki MN, Anogianakis G. Cognitive event-related potentials: longitudinal changes in mild cognitive impairment. Clin Neurophysiol. 2011;122:1322–1326. doi: 10.1016/j.clinph.2010.12.036. [DOI] [PubMed] [Google Scholar]
- Pekkonen E. Mismatch negativity in aging and in Alzheimer's and Parkinson's diseases. Audiol Neurootol. 2000;5:216–224. doi: 10.1159/000013883. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58:1985–1992. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- Pienkowski M. On the etiology of listening difficulties in noise despite clinically normal audiograms. Ear Hear. 2017;38:135–148. doi: 10.1097/AUD.0000000000000388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Corey-Bloom J. Alzheimer's disease and P300: review and evaluation of task and modality. Curr Alzheimer Res. 2005;2:515–525. doi: 10.2174/156720505774932214. [DOI] [PubMed] [Google Scholar]
- Polich J, Ladish C, Bloom FE. P300 assessment of early Alzheimer's disease. Electroencephalogr Clin Neurophysiol. 1990;77:179–189. doi: 10.1016/0168-5597(90)90036-d. [DOI] [PubMed] [Google Scholar]
- Pollmann S, Maertens M, von Cramon DY, Lepsien J, Hugdahl K. Dichotic listening in patients with splenial and nonsplenial callosal lesions. Neuropsychology. 2002;16:56–64. doi: 10.1037//0894-4105.16.1.56. [DOI] [PubMed] [Google Scholar]
- Quaranta N, Coppola F, Casulli M, Barulli MR, Panza F, Tortelli R, Capozzo R, Leo A, Tursi M, Grasso A, Solfrizzi V, Sobba C, Logroscino G. The prevalence of peripheral and central hearing impairment and its relation to cognition in older adults. Audiol Neurootol. 2014;19(Suppl 1):10–14. doi: 10.1159/000371597. [DOI] [PubMed] [Google Scholar]
- Rahman TT, Mohamed ST, Albanouby MH, Bekhet HF. Central auditory processing in elderly with mild cognitive impairment. Geriatr Gerontol Int. 2011;11:304–308. doi: 10.1111/j.1447-0594.2010.00684.x. [DOI] [PubMed] [Google Scholar]
- Roberts R, Knopman DS. Classification and epidemiology of MCI. Clin Geriatr Med. 2013;29:753–772. doi: 10.1016/j.cger.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe MJ., 3rd The brainstem auditory evoked response in neurological disease: a review. Ear Hear. 1981;2:41–51. doi: 10.1097/00003446-198101000-00008. [DOI] [PubMed] [Google Scholar]
- Sarkamo T, Tervaniemi M, Laitinen S, Numminen A, Kurki M, Johnson JK, Rantanen P. Cognitive, emotional, and social benefits of regular musical activities in early dementia: randomized controlled study. Gerontologist. 2014;54:634–650. doi: 10.1093/geront/gnt100. [DOI] [PubMed] [Google Scholar]
- Scheltens P, Leys D, Barkhof F, Huglo D, Weinstein HC, Vermersch P, Kuiper M, Steinling M, Wolters EC, Valk J. Atrophy of medial temporal lobes on MRI in probable Alzheimer's disease and normal ageing: diagnostic value and neuropsychological correlates. J Neurol Neurosurg Psychiatry. 1992;55:967–972. doi: 10.1136/jnnp.55.10.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Woerner N, Boreta L, Kornfield T, Shaw LM, Trojanowski JQ, Thompson PM, Jack CR, Jr, Weiner MW, Disease Alzheimer's, Neuroimaging I. MRI of hippocampal volume loss in early Alzheimer's disease in relation to ApoE genotype and biomarkers. Brain. 2009;132:1067–1077. doi: 10.1093/brain/awp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuknecht HF. Further observations on the pathology of presbycusis. Arch Otolaryngol. 1964;80:369–382. doi: 10.1001/archotol.1964.00750040381003. [DOI] [PubMed] [Google Scholar]
- Simmons-Stern NR, Budson AE, Ally BA. Music as a memory enhancer in patients with Alzheimer's disease. Neuropsychologia. 2010;48:3164–3167. doi: 10.1016/j.neuropsychologia.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha UK, Hollen KM, Rodriguez R, Miller CA. Auditory system degeneration in Alzheimer's disease. Neurology. 1993;43:779–785. doi: 10.1212/wnl.43.4.779. [DOI] [PubMed] [Google Scholar]
- Sinha UK, Saadat D, Linthicum FH, Jr, Hollen KM, Miller CA. Temporal bone findings in Alzheimer's disease. Laryngoscope. 1996;106:1–5. doi: 10.1097/00005537-199601000-00001. [DOI] [PubMed] [Google Scholar]
- Strouse AL, Hall JW, 3rd, Burger MC. Central auditory processing in Alzheimer's disease. Ear Hear. 1995;16:230–238. doi: 10.1097/00003446-199504000-00010. [DOI] [PubMed] [Google Scholar]
- Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA. 2003;289:2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- Sur S, Sinha VK. Event-related potential: an overview. Ind Psychiatry J. 2009;18:70–73. doi: 10.4103/0972-6748.57865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syka J. Plastic changes in the central auditory system after hearing loss, restoration of function, and during learning. Physiol Rev. 2002;82:601–636. doi: 10.1152/physrev.00002.2002. [DOI] [PubMed] [Google Scholar]
- Taljaard DS, Olaithe M, Brennan-Jones CG, Eikelboom RH, Bucks RS. The relationship between hearing impairment and cognitive function: a meta-analysis in adults. Clin Otolaryngol. 2016;41:718–729. doi: 10.1111/coa.12607. [DOI] [PubMed] [Google Scholar]
- Teipel S, Fritze T, Ovari A, Buhr A, Kilimann I, Witt G, Pau HW, Doblhammer G. Regional pattern of dementia and prevalence of hearing impairment in Germany. J Am Geriatr Soc. 2015;63:1527–1533. doi: 10.1111/jgs.13561. [DOI] [PubMed] [Google Scholar]
- Tuwaig M, Savard M, Jutras B, Poirier J, Collins DL, Rosa-Neto P, Fontaine D, Breitner JCS, Group PAR. Deficit in central auditory processing as a biomarker of pre-Clinical alzheimer's disease. J Alzheimers Dis. 2017;60:1589–1600. doi: 10.3233/JAD-170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio F, Maatta S. The use of auditory event-related potentials in Alzheimer's disease diagnosis. Int J Alzheimers Dis. 2011;2011:653173. doi: 10.4061/2011/653173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JJ, Cleveland LM, Davis JL, Seales JS. Audiometry screening and interpretation. Am Fam Physician. 2013;87:41–47. [PubMed] [Google Scholar]
- Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev. 2015;23:154–166. doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Welland RJ, Lubinski R, Higginbotham DJ. Discourse comprehension test performance of elders with dementia of the Alzheimer type. J Speech Lang Hear Res. 2002;45:1175–1187. doi: 10.1044/1092-4388(2002/095). [DOI] [PubMed] [Google Scholar]
- Zhan W, Cruickshanks KJ, Klein BE, Klein R, Huang GH, Pankow JS, Gangnon RE, Tweed TS. Generational differences in the prevalence of hearing impairment in older adults. Am J Epidemiol. 2010;171:260–266. doi: 10.1093/aje/kwp370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Hamilton E, McNamara E, Smith PF, Darlington CL. The effects of chronic tinnitus caused by acoustic trauma on social behaviour and anxiety in rats. Neuroscience. 2011a;193:143–153. doi: 10.1016/j.neuroscience.2011.07.026. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Hamilton E, Stiles L, McNamara E, de Waele C, Smith PF, Darlington CL. Acoustic trauma that can cause tinnitus impairs impulsive control but not performance accuracy in the 5-choice serial reaction time task in rats. Neuroscience. 2011b;180:75–84. doi: 10.1016/j.neuroscience.2011.02.040. [DOI] [PubMed] [Google Scholar]