Chronic kidney disease (CKD) has been recognized as a leading public health burden worldwide driven by the increasing prevalence of diabetes mellitus, hypertension, obesity, and aging, but effective treatment options are limited.1 Renal fibrosis is the pathological feature of CKD regardless of underlying causes. Therefore, a clearer understanding of the mechanism is essential for defining potential therapeutic target for CKD.
Tubule epithelial cells are the most populous cell type which are vulnerable to a variety of injury stimuli. In recent years, the signals sending by injured tubules in the process of renal fibrosis has attracted increasing attention. Single-cell RNA-sequencing technologies have revealed the appearance of maladaptive or failed repaired proximal tubule clusters which express proinflammatory and profibrotic cytokines.2,3 Those injured tubules secrete cytokines, such as CXCL1 which interacts with CXCR2+ basophils, contributing to the development of renal fibrosis.4 Tubular cells undergoing autophagy release FGF2 as paracrine factor leading to fibroblast activation and renal fibrosis.5 In addition to cytokines, tubules also communicate with macrophages or fibroblast via secreting extracellular vesicles carrying functional cargoes such as TGF-β1 mRNA, CCL2 mRNA and Osteopontin protein.6, 7, 8 Therefore, the communication of altered renal tubules with interstitial cells indeed contributed to the process of kidney fibrosis.
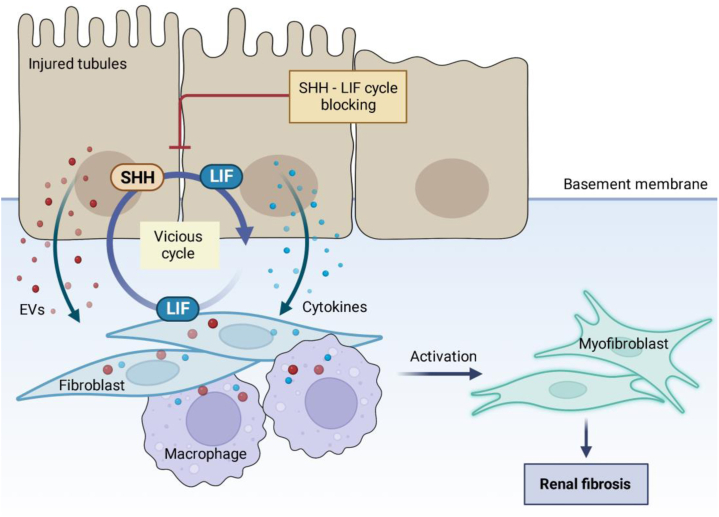
In the recent issue of eBiomedicine, Xu and colleagues uncovered a previously unrecognized vicious cycle mediated by leukemia inhibitory factor (LIF)- Sonic Hedgehog (SHH) signaling between fibroblast and tubule epithelial cells, leading to the development of renal fibrosis9 (Fig. 1). They observed that LIF was prominently increased among the IL6 family members in both kidneys of CKD patients and mouse models induced by ischemia-reperfusion injury (IRI) and unilateral ureteral obstruction (UUO). Importantly, LIF has the strongest association with the severity of tubulointerstitial fibrosis and the decline of renal function. In a large prospective cohort of CKD patients, they found that baseline urine LIF levels predicted the risk of CKD progression to end stage renal disease.
Fig. 1.
The communication of tubules and interstitial cell in kidney fibrosis. Injured tubules communicate with interstitial fibroblast and immune cells with paracrine factors including cytokines, extracellular vesicles (EVs) to amplify the pro-fibrotic responses. Xu et al. identified a vicious cycle mediated by leukemia inhibitory factor (LIF)- Sonic Hedgehog (SHH) signaling between fibroblast and tubule epithelial cells. LIF elicits a pro-fibrotic response in both fibroblasts and renal tubular cells, which may represent a potential therapeutic target and biomarker for renal fibrosis.
In mechanism, Xu et al. demonstrated a pro-fibrotic role of LIF in myofibroblast activation via ERK and STAT3 pathway, and a similar fibrosis response was induced in tubules by LIF. Interestingly, fibroblasts-derived LIF augment SHH expression in renal tubules which in turn promoted LIF expression in fibroblasts. Therefore, fibroblasts and renal tubular cells communicated with LIF-SHH signaling which formed a vicious cycle in pro-fibrotic response. To confirm the pro-fibrotic role of LIF in vivo, lentiviral overexpression of LIF were introduced into renal parenchyma which accelerated renal fibrosis in UUO model. At the same time, they showed that renal fibrosis was alleviated significantly through knockdown of LIF receptor or by using LIF-neutralizing antibody in animal models. Thus, experiments with both gain- and loss-of-function in vivo strongly supported the pro-fibrotic role of LIF in renal fibrosis. Besides, LIF promoted macrophage phenotype transition toward pro-fibrotic phenotype. Taken together, LIF expression on fibroblasts contributes importantly to renal fibrosis, and its communication with tubules and macrophages accelerated pro-fibrotic responses.
Previous study showed that LIF was involved in tubular regeneration after acute kidney injury.10 However, sustained activation of LIF signaling may contribute to the pathogenesis of renal fibrosis. Given the different roles of LIF in acute and chronic kidney injury, more refined studies are needed to establish the optimal strategies of targeting LIF considering the intervention dosage and stages after kidney injury. Nevertheless, this study demonstrated a new paracrine viscous cycle mediated by LIF-SHH signaling between tubules and fibroblasts in renal fibrosis. Therapeutically inhibiting LIF to disrupt this cycle may represent a novel approach of therapy, and urinary LIF may be a useful non-invasive biomarker for renal fibrosis with potential to monitor the progress of CKD.
Contributors
Literature search: A.R.S. and L.L.L.; Graph preparation: A.R.S.; Data interpretation: L.L.L.; Writing: A.R.S. and L.L.L. Both authors read and approved the final version of the manuscript.
Declaration of interests
The authors declare no conflict of interest.
Acknowledgements
Lv LL was supported by grants from the National Natural Science Foundation of China (82122011, 81970616).
References
- 1.Lv J.C., Zhang L.X. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol. 2019;1165:3–15. doi: 10.1007/978-981-13-8871-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Balzer M.S., Doke T., Yang Y.W., et al. Single-cell analysis highlights differences in druggable pathways underlying adaptive or fibrotic kidney regeneration. Nat Commun. 2022;13:4018. doi: 10.1038/s41467-022-31772-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gerhardt L.M.S., Liu J., Koppitch K., Cippa P.E., McMahon A.P. Single-nuclear transcriptomics reveals diversity of proximal tubule cell states in a dynamic response to acute kidney injury. Proc Natl Acad Sci U S A. 2021;118 doi: 10.1073/pnas.2026684118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doke T., Abedini A., Aldridge D.L., et al. Single-cell analysis identifies the interaction of altered renal tubules with basophils orchestrating kidney fibrosis. Nat Immunol. 2022;23:947–959. doi: 10.1038/s41590-022-01200-7. [DOI] [PubMed] [Google Scholar]
- 5.Livingston M.J., Shu S., Fan Y., et al. Tubular cells produce FGF2 via autophagy after acute kidney injury leading to fibroblast activation and renal fibrosis. Autophagy. 2022:1–22. doi: 10.1080/15548627.2022.2072054. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borges F., Melo S., Özdemir B., et al. TGF-β1-containing exosomes from injured epithelial cells activate fibroblasts to initiate tissue regenerative responses and fibrosis. J Am Soc Nephrol. 2013;24:385–392. doi: 10.1681/ASN.2012101031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lv L.L., Feng Y., Wen Y., et al. Exosomal CCL2 from tubular epithelial cells is critical for albumin-induced tubulointerstitial inflammation. J Am Soc Nephrol. 2018;29:919–935. doi: 10.1681/ASN.2017050523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S., Zhang M., Li J., et al. β-catenin-controlled tubular cell-derived exosomes play a key role in fibroblast activation via the OPN-CD44 axis. J Extracell Vesicles. 2022;11 doi: 10.1002/jev2.12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu S.Y.X., Chen Q., Liu Z., et al. Leukemia inhibitory factor is a therapeutic target for renal interstitial fibrosis. EBioMedicine. 2022 doi: 10.1016/j.ebiom.2022.104312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshino J., Monkawa T., Tsuji M., Hayashi M., Saruta T. Leukemia inhibitory factor is involved in tubular regeneration after experimental acute renal failure. J Am Soc Nephrol. 2003;14:3090–3101. doi: 10.1097/01.asn.0000101180.96787.02. [DOI] [PubMed] [Google Scholar]