Abstract
Two patients with history of cardiac ablation presented with massive hemoptysis secondary to pulmonary vein stenosis. They underwent successful pulmonary vein angioplasty and stenting. Although the second patient ended up having a lobectomy, the successful opening of the Left Superior Pulmonary Vein helped sparing the superior lobe and lingula.
Keywords: angioplasty, cardiac ablation, massive hemoptysis, pulmonary vein stenosis
A low threshold should be kept for pulmonary vein stenosis as considerable etiology of hemoptysis episodes, including the massive ones. Previous cardiac ablation is the key to the diagnosis, even if it was performed several years earlier.
1. INTRODUCTION
Pulmonary vein stenosis (PVS) is a rare cardiovascular disorder characterized by the blockage of the venous return from the lung to the left atrium. Etiologies of PVS are either congenital, secondary to an underlying mediastinal pathology (mediastinal granulomatous disease, fibrosing mediastinitis, mediastinal tumor), or iatrogenic (post‐cardiac ablation, lung transplant, or lobectomy). 1 Cardiac ablation has become by far the most common of all etiologies in the adult population with the increased use of this technology for the treatment of arrhythmias. 2
Pulmonary vein stenosis is an uncommon and often overlooked etiology of hemoptysis. Massive hemoptysis is even a rarer presentation. 3 The management of the PVS secondary to cardiac ablation is not standardized. We hereby discuss our experience with two patients with unusual presentation of PVS‐induced massive hemoptysis who were treated with pulmonary vein stenting.
The hospital ethics committee approved this work and the patients consented to the anonymous publication of their cases.
2. CASE DESCRIPTION
2.1. Patient 1
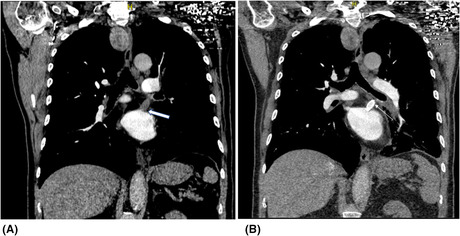
A 52‐year‐old man underwent cardiac ablation for Atrial fibrillation in February 2020. He is known to have hypertension, diabetes, and dyslipidemia and takes Apixaban 5 mg PO twice daily. He had recurrent intermittent episodes of mild hemoptysis that started in August 2020 and resolved upon cessation of the anticoagulant in September of the same year. In the meanwhile, we excluded several etiologies of hemoptysis such as tuberculosis, vasculitis, and other auto‐immune diseases. In November 2020, he presented to our hospital with massive hemoptysis. The coughed blood was dark red and filled half a cup every hour. Chest CT Angiography showed ground glass opacities in the left lower lobe and clots in the left bronchus without identification of the bleeding source. The patient remained hemodynamically stable and saturated well on a nasal cannula with 3 L/min of oxygen. Although the bleeding source was not identified, however, an urgent embolization of the left lower bronchial artery was performed, given the suspicion raised based on the CT finding. However, it failed to control the bleeding. Given his history of cardiac ablation, a cardiac CT scan was done and showed a 99% occlusion of the Left superior Pulmonary vein (LS‐PV) (Figure 1A), and the patient was sent for angioplasty of the obstructed pulmonary vein (PV).
FIGURE 1.
Cardiac CT (A) and Chest CT angiography (B) for the patient 1 before and after the angioplasty of the obstructed left superior pulmonary vein
We entered through the right femoral vein and the left atrium was reached via transseptal puncture using a 9 Fr SL0 catheter and BRK1 needle. All steps were done under TEE guidance. The obstructed vein was identified and accessed. We then balloon dilated the stenosis with a 2 × 15 Euphora balloon followed by a 4 × 15 NC balloon. We then placed a 9 × 27 ev3 balloon expandable Medtronic stent. Excellent final result with 0% residual stenosis. TEE confirmed a well‐expanded stent. The bleeding ceased immediately and dual antiplatelets were started on the same day. The patient was discharged home on Day 7. A follow‐up CT angiography was done 1 month after the procedure and showed patency of the stent and no evidence of active bleeding (Figure 1B). There was no recurrence of the bleed on clinical follow‐up after 18 months.
The dual anti‐platelets were continued for 1 month then switched to Apixaban 5 mg twice daily and Aspirin 81 mg daily.
2.2. Patient 2
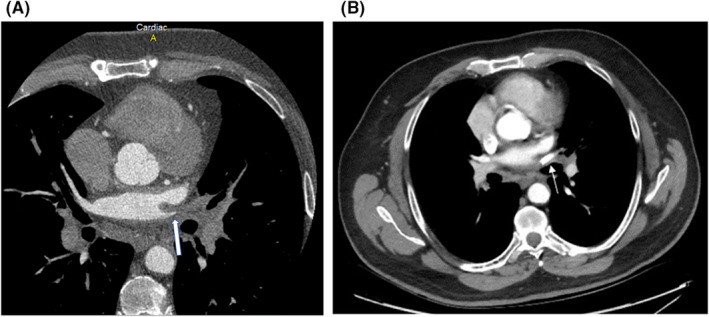
A 73‐year‐old man underwent cardiac ablation for Atrial fibrillation on two occasions (2015 and 2018). During both ablations, fluoroscopic and intracardiac ultrasound guidance along with 3D mapping techniques were used. Before attempting the second ablation in 2018, his PVs were checked and were all patent. Besides Atrial fibrillation, he has also chronic obstructive pulmonary disease and dyslipidemia. In March 2020, he started to have recurrent episodes of blood‐tinged sputum associated with cough, treated as bronchitis by his primary physician. The bleeding recurred in July 2020; the volume estimated by the patient was around half a cup a day. A workup was done and ruled out tuberculosis as well as pulmonary embolism, vasculitis, and other auto‐immune diseases. Chest CT angiography and bronchoscopy described the location of the bleed to be from the left lung but failed to identify the bleeding source, however, embolization of the left bronchial artery was attempted, and the bleeding stopped. Eight months following the embolization, the patient presented with massive hemoptysis as he was coughing around half a cup volume of dark red blood every hour. CT angiography showed obstruction of both superior and inferior left Pulmonary veins (Figure 2A), findings that were missed on the previous CTs. Bronchoscopy showed an engorged varicose vein at the level of the left main bronchus (Figure 3). TEE identified a faint flow at the level of LS‐PV. No flow could be identified at the level of the Left inferior PV. The patient underwent a PV venosplasty under fluoroscopic and TEE guidance. We placed an 8Fr short sheath in the right common femoral vein, then we used an SL0 catheter and BRK1 needle to do a transeptal puncture under TEE guidance. We used an 0.014 PT12 wire to cross the LS‐PVS. We then used a 4 × 15 balloon to dilate the stenosis, then we placed a 0.035 Benson wire then a 6 × 27 EV3 balloon expandable Medtronic stent. The Left lower PV obstruction could not be crossed. The final angiogram showed the excellent result of the LS‐PV angioplasty. Hemoptysis ceased and dual antiplatelets were started following the procedure. Two days later, he developed a new onset of severe hemoptysis that we believe is secondary to the persistent obstruction of the left inferior PV. The Antiplatelets were stopped for 5 days, and the patient underwent video‐assisted thoracoscopic surgery for left lower lobe lobectomy (Figure 4). The Post‐op course was smooth. The patient was extubated on the same day and discharged home after 2 weeks of hospitalization. He took dual antiplatelets for a total of 1 month, then switched to Aspirin 81 mg daily plus Apixaban 5 mg Twice daily. There was neither recurrence of the hemoptysis on the clinical follow‐up done every 3 months up to 18 months nor a sign of stent thrombosis on CT angiography done at 6 months after the procedure (Figure 2B). The patient was left with mild dyspnea on exertion that improved gradually.
FIGURE 2.
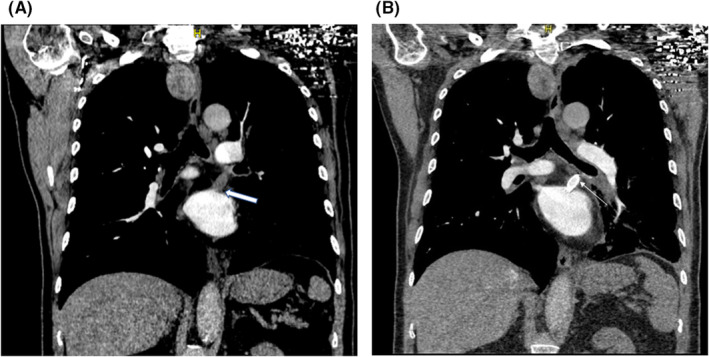
CT angiography for the patient 2 before (A) and after (B) the angioplasty of the obstructed left superior pulmonary vein
FIGURE 3.
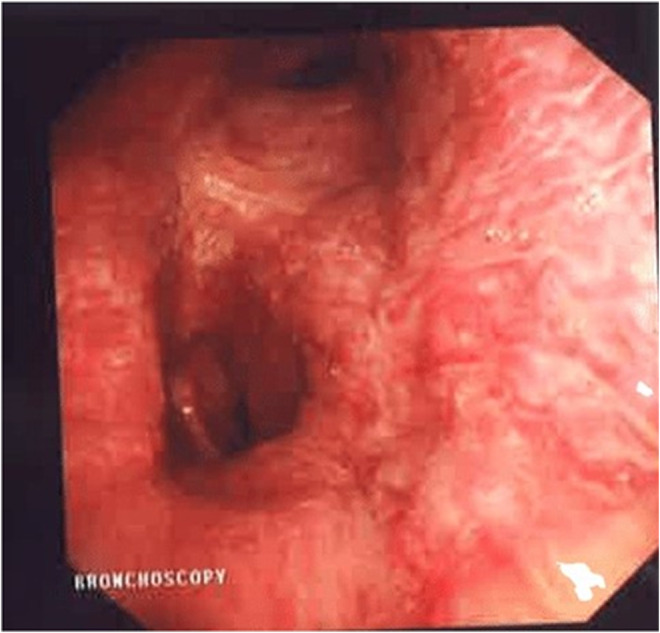
Bronchoscopic view of the engorged varicose veins of the left main bronchus mucosa for patient 2
FIGURE 4.
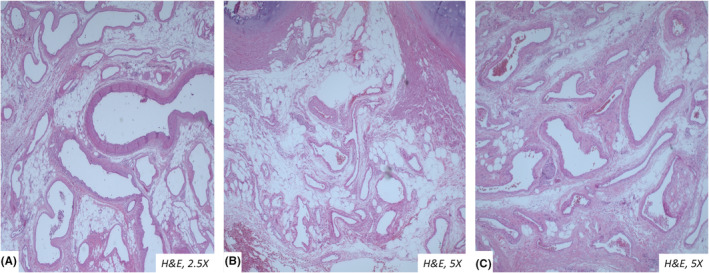
Microscopic view of the tortuous veins in the lobectomy section of the patient 2
3. COMMENT
Physicians should keep a low threshold for suspicion of PVS when patients present with respiratory symptoms following cardiac ablation, as early diagnosis can change the management towards a less invasive procedure. The timing of occurrence of the PVS is unpredictable and can occur several years following the cardiac ablation. Clinical and radiological follow‐ups are important to ensure the patency of the stent.
AUTHOR CONTRIBUTIONS
Laura Akiki: Conceptualization; data curation; project administration; validation; visualization; writing – original draft; writing – review and editing. Dima Siblani: Conceptualization; data curation; project administration; validation; visualization; writing – original draft; writing – review and editing. Wajdy Abi Saleh: Conceptualization; supervision; validation; writing – review and editing. Clara Chamoun: Conceptualization; supervision; validation; visualization. Labib Abouzahr: Conceptualization; supervision; validation. Wassim Shatila: Conceptualization; supervision; validation; writing – review and editing.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
None.
CONSENT
Written informed consent was obtained from the patient to publish this report by the journal's patient consent policy.
ACKNOWLEDGMENT
We would like to thank Dr. Elie Chammas and Dr. Wadih Ayoub who were essential members of the cardiology team tacking care of the patients. We would also like to thank Dr Selim Nasser and Dr Aya Soubra the clinical pathologists who contributed to the success of this work.
Akiki L, Siblani D, Abi Saleh W, Chamoun C, Abouzahr L, Shatila W. Pulmonary vein stenosis presenting as massive hemoptysis, treated with pulmonary veins angioplasty. Clin Case Rep. 2022;10:e06584. doi: 10.1002/ccr3.6584
All authors approved the version of the submitted manuscript. They take public responsibility for appropriate portions of the content and they agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1.(1)Braun S, Platzek I, Zöphel K, et al. Haemoptysis due to pulmonary venous stenosis. Eur Respir Rev. 2014;23(132):170‐179. doi: 10.1183/09059180.00003713 Erratum in: Eur Respir Rev. 2014 Sep;23(133):399. PMID: 24881072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(2)Latson LA, Prieto LR. Congenital and acquired pulmonary vein stenosis. Circulation. 2007;115(1):103‐108. doi: 10.1161/CIRCULATIONAHA.106.646166 [DOI] [PubMed] [Google Scholar]
- 3.(3)Lee JY, Chon GR, Park JH, Kang BJ, Shim TS, Jo KW. Massive hemoptysis due to pulmonary vein stenosis following catheter ablation for atrial fibrillation. Respir Care. 2015;60(3):e52‐e55. doi: 10.4187/respcare.03336 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


