Abstract
Acquired cystic lung disease in premature infants is a serious respiratory complication, and pulmonary interstitial emphysema (PIE) has been widely reported. We report a rare case of giant pulmonary bulla in an infant treated with bullectomy where chest computed tomography was useful in directing treatment.
Keywords: acquired cystic lung disease, giant pulmonary bulla, pulmonary interstitial emphysema, very low‐birthweight infant
Acquired cystic lung disease in premature infants is a serious respiratory complication, and pulmonary interstitial emphysema (PIE) has been widely reported. We report a rare case of giant pulmonary bulla in an infant treated with bullectomy where chest computed tomography was useful in directing treatment.
1. BACKGROUND
Acquired cystic lung disease in premature infants is a serious respiratory complication. 1 , 2 This includes various pathologies such as pulmonary interstitial emphysema (PIE), air leak syndrome, 3 , 4 , 5 bronchial obstruction/stenosis, 6 bronchiectasis, 7 pneumatocele, 8 and bulla. 9 Among acquired cystic lung diseases, most reported cases are of PIE. However, bullous lung diseases are rare in infants. Since the treatment strategy for these pathologic conditions is different, it is important to establish a correct diagnosis. Here, we report a rare case of giant pulmonary bulla in a very low‐birthweight infant and highlight the utility of computed tomography (CT) in deciding the therapeutic strategy.
2. CASE PRESENTATION
A male infant (525 g) with no congenital pulmonary cystic disease detected on fetal screening echocardiography was born at 23 weeks of gestation with low Apgar scores of 1, 3, and 6 at 1, 5, and 10 min, respectively. After birth, the patient was intubated and received synchronized intermittent mandatory ventilation. Initial chest radiograph at birth was consistent with respiratory distress syndrome (RDS) and absence of congenital cystic lesions, including congenital bronchial atresia and congenital lobar emphysema (Figure 1A). The patient was then treated with surfactant. On 11th day, he developed pneumothorax on the right side, which required placement of a chest drain, and radiographic findings showed that pneumothorax had improved. On 24th day, the lung cyst appeared and gradually expanded in the right upper lobe but there was no persistent lung infection based on the clinical course and laboratory findings (Figure 1B). Due to the recurrence of pneumothorax, the mechanical ventilator mode was changed to neurally adjusted ventilatory assist or high‐frequency oscillatory ventilation to reduce ventilator‐induced lung injury. On 76th day, the patient was extubated and continued to receive non‐invasive respiratory support. However, the lung cysts gradually expanded.
FIGURE 1.
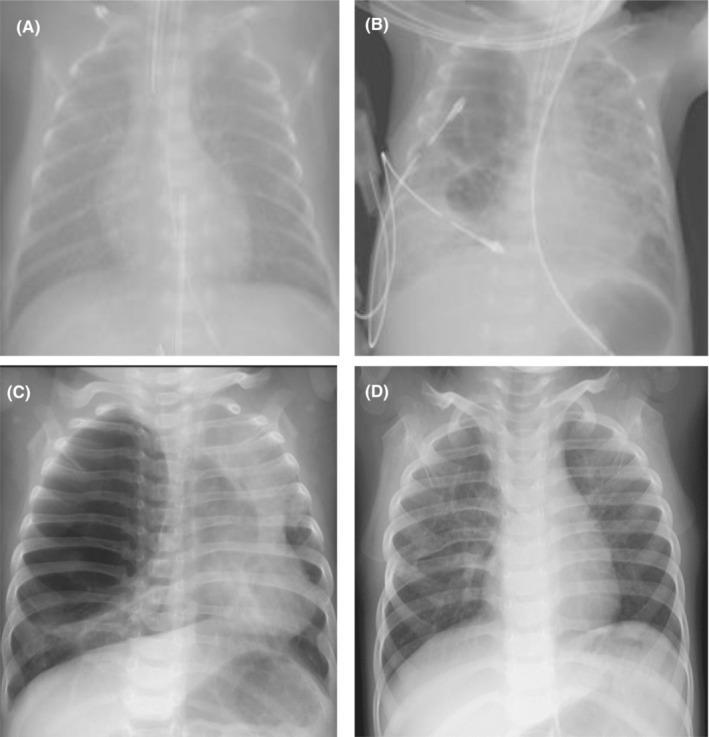
Radiographs. Congenital cystic lesions were ruled out by radiographs at birth. (A) Chest drain being inserted during pneumothorax at Day 24. (B) A chest radiograph at 9 months of age reveals a giant cyst with a diameter of 80 × 60 mm, causing left mediastinal left shift and left lung atelectasis. (C) At age 3 years, absence of worsening of emphysematous lesions was observed (D).
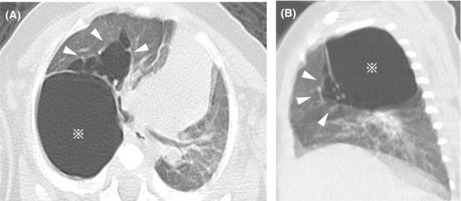
Chest CT performed at 5 months of age revealed two types of cystic lesions. The first was a huge solitary cyst with an empty interior and smooth outline below the pleura. The second type was multicystic lesions of various sizes with septations located adjacent to the first. Due to mass effects, there was a left mediastinal shift with left lung atelectasis (Figure 2A,B). These cysts were diagnosed as giant pulmonary bulla with PIE.
FIGURE 2.
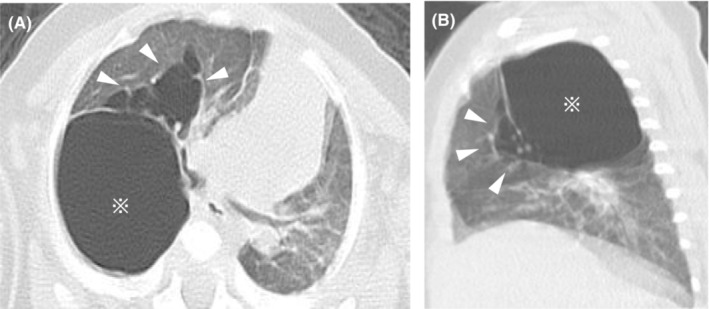
Chest computed tomography performed at 5 months of age demonstrates a large 30 × 25 mm monocystic space with a clear rim (asterisk) and multiple cystic air spaces with a line‐and‐dot pattern (white arrowheads) in the upper right lobe (A, B).
In the interim, his respiratory condition remained stable with oxygen therapy and his body weight gradually increased. He was discharged at 6 months with the introduction of home oxygen therapy via nasal cannula (0.5 L/min). At the time of discharge, the patient was still small in stature, and surgical intervention was not immediately considered in anticipation of future normal lung growth.
At 9 months of age, he was admitted due to acute bronchitis caused by human metapneumovirus. A chest radiograph showed further expansion of the giant pulmonary bulla triggered by the respiratory infection (Figure 1C). He was very poorly oxygenated, with an oxygenation index 10 of 22.3 and arterial oxygen partial pressure/inhalation oxygen fraction of 50.6 mmHg, and required high‐pressure control with a mean airway pressure of 18 cm H2O. His clinical condition could not be controlled by medical treatment as he presented with respiratory failure and obstructive shock. While PIE is expected to spontaneously regress, the giant pulmonary bulla was thought to be the main cause of respiratory failure. However, the policy, in this case, was to preserve respiratory function as much as possible for the growth and development of the infant. Therefore, open chest cystectomy was chosen instead of lobectomy with PIE. The giant pulmonary bulla was incised, and a few air leakage sites were sutured directly. The cyst sac was empty without remnants of the lung tissues. The visceral pleura was then reinforced using a residual cyst wall. Histopathologic examination of the excised bulla wall revealed that it was derived from the fibrous tissue and was located within the subpleural lung parenchyma. Histological examination showed that the cyst was a consistent finding as a bulla. (Figure 3).
FIGURE 3.
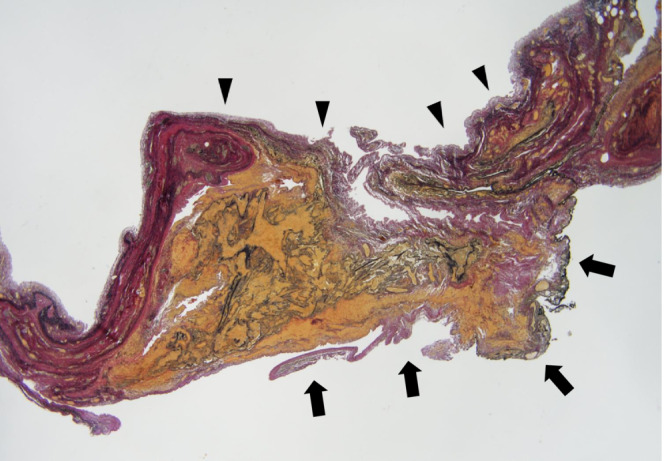
Histopathologic analysis of the excised bulla wall (black arrows), visceral pleura (black arrowheads), and lung parenchyma. The wall of the bulla is located below the visceral pleura adjacent to the lung parenchyma and is formed by fibrous tissue (Elastica van Gieson staining, ×200).
After the operation, the patient had no respiratory problems and did not need respiratory support and radiographic examination confirmed that the emphysematous lesions had resolved (Figure 1D). He is now 3 years old with good growth.
3. DISCUSSION AND CONCLUSIONS
A bulla is defined radiologically as an emphysematous space with a diameter of 1 cm or more and is morphologically located in the subpleural lung parenchyma. 11 Reid et al. proposed a classification for bulla. 11 Type I bulla are characterized pathologically as narrow‐necked empty sacs with a clear linear outline in the absence of vascular or airway remnants. 11 The outer surface of the bulla is composed of visceral pleura, while its inner surface is composed of fibrous tissue, derived from the pleura and the destroyed pulmonary tissue. 11 In the present case, the expanded cyst was a monocyst with a clear rim of the wall on CT. The cystic wall histologically contained fibrous tissue and was located within the subpleural lung parenchyma. The cyst was diagnosed as a Reid type I bulla based on both radiologic and histopathologic findings excluding the possibility of other diseases. The symptomatic expanded cyst met the following criteria of a giant pulmonary bulla: (1) upper lobe involvement, (2) occupying at least one‐third of the hemithorax, and (3) compressing the surrounding lung parenchyma. 12 On the contrary, multiple cystic spaces were adjacent to the giant pulmonary bulla. The mechanism of PIE is likely from air leakage into the perivascular and peribronchial spaces due to the high airway pressure of mechanical ventilation. 3 , 4 , 5 This PIE may spread centrifugally along the bronchovascular sheath or lymphatic channels. Air leakage develops into expanded airspaces of the terminal bronchiole and alveolar septal destruction. 3 , 4 , 5 These showed extra‐alveolar air accumulation and a line‐and‐dot pattern, which is a specific sign of PIE on CT.
In the management of acquired cystic lung disease, PIE may be reversible. Conservative treatment by selective intubation, selective bronchial obstruction, or decubitus positioning is accepted as the initial management. 3 , 4 , 5 However, giant pulmonary bulla can be considered for surgery. 13 , 14 Based on the good outcome of our case, cystectomy was considered to be safe and effective as it can leave sufficient residual lung in small infants.
In the present case, the chest radiograph could not sufficiently evaluate the structure of the acquired cystic lung disease. However, CT can be utilized to evaluate the positioning of the cyst wall and internal structure of an acquired cystic lesion at a higher resolution.
In conclusion, most case reports of acquired cystic lung disease are PIE, and bulla are rarely involved in acquired cystic lung disease. To determine the therapeutic strategy, CT is useful when chest radiography is equivocal on the evaluation of acquired cystic lung disease.
AUTHOR CONTRIBUTIONS
TS, YH, DW, KK, and NO interpreted the patient data regarding the lung disease. TO performed histological examination. AS performed radiological diagnosis of the bulla. TS and KK involved in major contributors in writing the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
INFORMED CONSENT
Written informed consent was obtained from the parents of the patient for publication purposes of this case report and any accompanying images. A copy of the written consent is available for review by the Chief Editor of the Journal.
ACKNOWLEDGMENT
We would like to thank the members of the Department of Neonatology, Paediatrics, Paediatric Surgery, Pathology, and Radiology.
Shinohara T, Hasebe Y, Watanabe D, et al. Giant pulmonary bulla causing respiratory compromise in a very low‐birthweight infant. Clin Case Rep. 2022;10:e06577. doi: 10.1002/ccr3.6577
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Elron E, Sheinfeld T, Konen O, Klinger G. Unilateral multi‐cystic lung disease in a preterm infant. Harefuah. 2020;159(10):735‐738. [PubMed] [Google Scholar]
- 2. Sacks GD, Chung K, Jamil K, Garg M, Dunn JC, DeUgarte DA. Surgical salvage of acquired lung lesions in extremely premature infants. Pediatr Surg Int. 2014;30(5):573‐576. doi: 10.1007/s00383-014-3482-1 [DOI] [PubMed] [Google Scholar]
- 3. Srinivasan R, Ali H, Harigopal S. Persistent pulmonary interstitial emphysema presenting as solitary lung cyst in a preterm infant. BMJ Case Rep. 2012;2012:bcr2012007516. doi: 10.1136/bcr-2012-007516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matta R, Matta J, Hage P, Nassif Y, Mansour N, Diab N. Diffuse persistent interstitial pulmonary emphysema treated by lobectomy. Ann Thorac Surg. 2011;92(4):e73‐e75. doi: 10.1016/j.athoracsur.2011.04.071 [DOI] [PubMed] [Google Scholar]
- 5. Rao J, Hochman MI, Miller GG. Localized persistent pulmonary interstitial emphysema. J Pediatr Surg. 2006;41(6):1191‐1193. doi: 10.1016/j.jpedsurg.2006.01.071 [DOI] [PubMed] [Google Scholar]
- 6. Seid AB, Canty TG. The anterior cricoid split procedure for the management of subglottic stenosis in infants and children. J Pediatr Surg. 1985;20(4):388‐390. doi: 10.1016/s0022-3468(85)80224-4 [DOI] [PubMed] [Google Scholar]
- 7. Tonson la Tour A, Spadola L, Sayegh Y, et al. Chest CT in bronchopulmonary dysplasia: clinical and radiological correlations. Pediatr Pulmonol. 2013;48(7):693‐698. doi: 10.1002/ppul.22714 [DOI] [PubMed] [Google Scholar]
- 8. Rocha G, Flôr‐de‐Lima F, Azevedo I, Guimarães H. Severe bronchopulmonary dysplasia with large pneumatoceles in an extreme preterm newborn. Rev Port Pneumol. 2017;23(3):170‐172. doi: 10.1016/j.rppnen.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 9. Firinci F, Duman N, Ates O, et al. Case of acquired lobar emphysema mimicking pneumothorax in a neonate. East Mediterr Health J. 2013;19(11):960‐961. doi: 10.26719/2013.19.11.960 [DOI] [PubMed] [Google Scholar]
- 10. Muniraman HK, Song AY, Ramanathan R, et al. Evaluation of oxygen saturation index compared with oxygenation index in neonates with hypoxemic respiratory failure. JAMA Netw Open. 2019;2(3):e191179. doi: 10.1001/jamanetworkopen.2019.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bullae RL. The Pathology of Emphysema. Lloyd‐Luke Medical Books; 1967. [Google Scholar]
- 12. Aramini B, Ruggiero C, Stefani A, Morandi U. Giant bulla or pneumothorax: how to distinguish. Int J Surg Case Rep. 2019;62:21‐23. doi: 10.1016/j.ijscr.2019.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Krishnamohan P, Shen KR, Wigle DA, et al. Bullectomy for symptomatic or complicated giant lung bullae. Ann Thorac Surg. 2014;97(2):425‐431. doi: 10.1016/j.athoracsur.2013.10.049 [DOI] [PubMed] [Google Scholar]
- 14. Ghattas C, Barreiro TJ, Gemmel DJ. Giant bullae emphysema. Lung. 2013;191(5):573‐574. doi: 10.1007/s00408-013-9495-z [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


