Abstract
Calcineurin inhibitors have become a pillar of immunosuppressive treatment in solid organ transplant recipients. Several case reports have shown hypertrophic and dilated cardiomyopathy as an adverse effect to tacrolimus therapy. We present the case of a kidney transplant recipient woman who developed hypertrophic cardiomyopathy due to tacrolimus therapy.
Keywords: cardiomyopathy, graft rejection, graft survival, hypertrophic, kidney transplantation, sirolimus, tacrolimus
Hypertrophic cardiomyopathy, a rare, potentially life‐threatening adverse event of tacrolimus, has been identified in solid organ transplant recipients. Therefore, physicians must always pay attention to this drug for proper disease diagnosis.
1. INTRODUCTION
Solid organ transplantation is a complex procedure made possible by our better understanding of the immune system and technological advances in modern medicine. Transplantation carries an intrinsic risk of graft rejection that must be controlled. Therefore, immunosuppressive therapy is always initiated in these patients. 1 Among immunosuppressive drugs used in solid organ transplantation, calcineurin inhibitors (CNIs), including tacrolimus, have been shown to be effective and relatively safe option with low rates of transplant rejection for these patients. 1
However, patients taking tacrolimus are not free from adverse effects, some of which can be potentially life‐threatening if not identified early. Some important side effects reported in the medical literature include nephrotoxicity, hypertension, post‐transplant diabetes mellitus (PTMD), new‐onset diabetes after transplantation (NODAT), dyslipidemia, and modification of the cardiovascular risk profile. 2 Multiple electrolyte disorders, such as hyperkalemia, hypomagnesemia, hypercalciuria, and metabolic acidosis, have also been reported. 3 Cardiovascular adverse effects such as NODAT, dyslipidemia, and arterial hypertension with an increased risk of stroke, myocardial infarction, and heart failure have been described. 4
Tacrolimus‐induced cardiomyopathy is a rare but important cardiovascular adverse event that has been reported predominantly in pediatric transplant recipients. 5 However, it has also been reported in adult renal, hepatic, cardiac, and small bowel transplant recipients. 6 , 7 , 8 , 9 Hypertrophic cardiomyopathy due to tacrolimus therapy has been catalogued as non‐familial acquired cardiomyopathy by the European Society of Cardiology (ESC), 10 while the American Heart Association classifies it as secondary cardiomyopathy. 11
2. CASE DESCRIPTION
A 65‐year‐old woman with a history of arterial hypertension, diabetes mellitus, and chronic kidney disease secondary to polycystic renal disease underwent cadaveric donor kidney transplantation. Her mother had arterial hypertension and polycystic renal disease; Her father had arterial hypertension, and four of her seven siblings had polycystic renal disease.
Before kidney transplantation, an electrocardiogram and echocardiogram were normal. After transplantation, immunosuppressive therapy with tacrolimus was initiated for 5 years without novelty. The drug therapy was suspended for a short period of time when patient had medication‐related tremors. During this 5 years treatment, tacrolimus plasma levels remained within the range of 5.1–11.2 ng/ml (average 6.39 ng/ml).
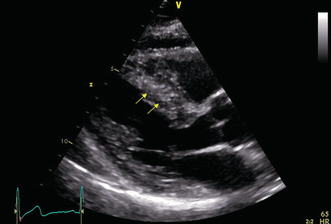
In the fifth year of treatment, the patient developed lipothymia, dyspnea during physical activity, asthenia, and adynamia. Therefore, she underwent echocardiography, which revealed findings consistent with obstructive hypertrophic cardiomyopathy: an interventricular septum thickness of 15 mm and a left ventricular posterior wall thickness of 11 mm (Figure 1). Continuous Doppler showed an end‐systolic gradient (64 mmHg) in the left ventricular outflow tract (LVOT), indicating the signs of left ventricular outflow tract obstruction (Figures 2 and 3).
FIGURE 1.

Longitudinal parasternal end‐diastolic plane: yellow arrows showing asymmetric septal hypertrophy (interventricular septum thickness, 15 mm)
FIGURE 2.

Apical mid‐systolic long‐axis view: systolic turbulence in the left ventricular outflow tract
FIGURE 3.

Continuous Doppler through the left ventricular outflow tract: an end‐systolic gradient of 64 mmHg
Cardiac magnetic resonance (CMR) was performed, which showed myocardial hypertrophy of the anterior wall of the left ventricle with a thickness of 14 mm and of the basal and middle segments of the interventricular septum with a thickness of 12 mm and 15 mm, respectively. Additionally, anterior systolic movement of the anterior mitral leaflet was observed because of anterior and septal myocardial hypertrophy, resulting in a significant decrease in the amplitude of the LVOT with an increase in the systolic gradient. In addition, the CMR neither showed segmental abnormalities in contractility or alterations in myocardial perfusion or at rest nor showed abnormalities in contrast enhancement. These CMR findings were consistent with those of asymmetric hypertrophic cardiomyopathy.
No significant variants were detected in a panel test for ACTC1 (sarcomere gene), FLNC, LAMP2, MYL2 (sarcomere gene), PRKAG2 (related to glycogen storage disease), TNNI3 (sarcomere gene), TTR, CSRP3, GLA (related to Fabry disease), MYBPC3 (sarcomere gene), MYL3, PTPN11, TNNT2 (sarcomere gene), DES, JPH2, MYH7 (sarcomere gene), PLN, TNNC1, and TPM1 (sarcomere gene) genes, which are associated with genetic hypertrophic cardiomyopathy.
Initially, surgical myectomy and cardiac resynchronization therapy were considered as treatment therapies to improve the patient's quality of life. Coronary arteriography was performed, which revealed severe coronary artery disease with critical coronary stenosis in the middle segment of the circumflex artery. The condition was successfully treated with angioplasty and a resolute onyx ™ drug‐eluting stent in this vessel (Figure 4). Severe coronary stenosis was found in the middle and distal segments of the right coronary artery and in the middle segment of the posterior descending coronary artery, which were successfully treated with angioplasty and two adjacent resolute onyx ™ drug‐eluting stents with overlapping edges (Figure 5).
FIGURE 4.

A. Left coronary arteriography in right anterior oblique (RAO) caudal view showing critical stenosis in the middle segment of the circumflex coronary artery (yellow arrow) B. Left coronary arteriography in RAO caudal view showing a resolute onyx ™ drug‐eluting stent in the middle segment of the circumflex coronary artery (yellow arrow). C. Left coronary arteriography in RAO caudal view showing adequate vessel reperfusion (yellow arrow)
FIGURE 5.

A. Right coronary arteriography in left anterior oblique (LAO) cranial view showing severe middle and distal stenosis of the right coronary artery and the middle segment of the posterior descending artery (yellow arrows) B. Right coronary arteriography in LAO cranial view showing two adjacent resolute onyx ™ drug‐eluting stents with overlapping edges in the middle and distal segments of the right coronary artery (yellow arrows). C. Right coronary arteriography in LAO cranial view showing adequate vessel reperfusion (yellow arrow)
Nevertheless, the patient's symptoms and echocardiographic findings worsened. Based on the medical literature, we presumed that tacrolimus caused obstructive cardiomyopathy in our patient; therefore, it was discontinued. After switching from immunosuppressive therapy to an mTOR inhibitor (sirolimus), the symptoms resolved after approximately 1 year, and the echocardiographic findings improved after 2 years (Figures 6 and 7).
FIGURE 6.

Control echocardiogram in parasternal long‐axis view of the left ventricle and inflow tract showing normal thickness of the mid septal segment with resolution of previously documented septal hypertrophy (yellow arrows)
FIGURE 7.

Control pulsed Doppler from the left ventricular outflow tract showing the absence of an abnormal late systolic gradient
3. DISCUSSION
The relationship between tacrolimus blood levels of above 15 ng/ml and increasing left ventricular wall thickness has been reported more frequently; however, this relationship was also significant at blood levels below 15 ng/ml, as in our case. 5 In addition, these patients usually present with nonspecific symptoms such as asthenia, adynamia, and lipothymia. A variety of signs and symptoms related to congestive heart failure include dyspnea, orthopnea, anorexia, fatigue, tachypnoea, tachycardia, pulmonary edema, and hepatomegaly. 8 , 9
3.1. Epidemiology
We reviewed the available medical literature in the major clinical databases (PubMed, Google Scholar, and SciELO). We found 18 articles, consisting of case reports and observational studies, which described hypertrophic cardiomyopathy as an adverse effect to tacrolimus therapy, occurring in a wide age range (Table 1). Although similar cases have been described in patients between the ages of 58 and 62 years, 6 , 8 , 12 there are reports of this adverse effect occurring in much younger patients. For example, Turska et al. reported a 17‐month‐old infant with hypertrophic cardiomyopathy, 13 and another case described a premature newborn with hypertrophic cardiomyopathy whose mother had received tacrolimus during pregnancy. 14 There were no differences between men and women in the occurrence of tacrolimus‐induced cardiomyopathy.
TABLE 1.
Some articles related to Cardiomyopathy secondary to Tacrolimus
| Age of publication ‐ Article title | Type of article | Number of study patients | Age | Gender F = Female M = Male B = Both | Tacrolimus indication | Treatment time | Tacrolimus average blood concentration or daily dose at diagnosis | Type of Cardiomyopathy | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 ‐ Dilated cardiomyopathy in an adult renal transplant recipient: recovery upon tacrolimus to sirolimus switch. | Case report | 1 | 66 years old | F | Kidney transplant | 8 months | 5–8 ng/ml | Dilated Cardiomyopathy | Switch to sirolimus | Symptoms and echocardiographic findings resolved |
| 2017 ‐ Tacrolimus‐associated dilated cardiomyopathy in adult patient after orthotopic liver transplant. | Case report | 1 | 59 years old | M | Hepatic transplant | 3 months | Below 15 ng/ml | Hypertrophic Cardiomyopathy progressing to dilated cardiomyopathy | Switch to cyclosporine | Echocardiographic findings remained unchanged after switching from tacrolimus to cyclosporine |
| 2015 ‐ Tacrolimus‐induced cardiomyopathy in an adult renal transplant recipient. | Case report | 1 | 62 years old | F | Kidney transplant | 6 months and 1 week | 7,9 ng/ml | Hypertrophic Cardiomyopathy | Switch to Betalacept | Symptoms and echocardiographic findings resolved |
| 2012 ‐ Tacrolimus‐related hypertrophic cardiomyopathy in an adult cardiac transplant patient. | Case report | 1 | 58 years old | M | Cardiac transplant | 7 years | 80–160 ng/ml | Hypertrophic Cardiomyopathy | Switch to Sirolimus | Symptoms and echocardiographic findings resolved |
| 2009 ‐ Reversible myocardial hypertrophy induced by tacrolimus in a pediatric heart transplant recipient. | Case report | 1 | 14 years old | F | Cardiac transplant | 5 years | 11–13 ng/ml | Hypertrophic Cardiomyopathy | The tacrolimus dosage was reduced to achieve a target blood concentration around 12 ng/ml | Symptoms and echocardiographic findings resolved |
| 2003 ‐ Multicenter prospective investigation on cardiovascular adverse effects of tacrolimus in kidney transplantations. | Cross‐sectional study | 68 (0 patients with Cardiomyopathy detected) | 33.4 years old (average age) | B | Kidney transplant | 24 weeks | Does not apply (no cardiomyopathy was detected) | No Cardiomyopathy was detected | Does not apply | Does not apply |
| 2001 ‐ Echocardiographic findings of hypertrophic cardiomyopathy in children after orthotopic liver transplantation. | Cross‐sectional study | 19 patients: 15 with Tacrolimus (2 developed Hypertrophic Cardiomyopathy) 4 with Cyclosporine | 4 years old (average age) | B | Hepatic transplant | Case 1: 1 month Case 2: 3 weeks | Both cases below 15 ng/ml | Obstructive Hypertrophic Cardiomyopathy | Case 1: Switch to Cyclosporine Case 2: Propranolol was started and Tacrolimus was continued | Case 1: Symptoms resolved and echocardiographic findings improved Case 2: Resolution of left ventricular outflow tract obstruction |
| 1998 ‐ Marked left ventricular hypertrophy in children on tacrolimus after orthotopic liver transplantation. | Case series | 2 | Case 1: 2 years old Case 2: 14 years old | B | Hepatic transplant | Without data | Case 1: 7–15 ng/ml Case 2: 7–13 ng/ml | Case 1: Hypertrophic Cardiomyopathy (postmortem diagnosis) Case 2: Hypertrophic Cardiomyopathy (postmortem diagnosis) | Case 1: Without treatment (postmortem diagnosis) Case 2: Without treatment (postmortem diagnosis) | Case 1: Died due to sepsis secondary to bacteremia (vancomycin‐resistant enterococcus) Case 2: Died due to multiorganic system failure with no clear diagnosis |
| 1995 ‐ Hypertrophic cardiomyopathy associated with tacrolimus in pediatric transplant patients. | Case series | 5 | Case 1: 21 months Case 2: 12 months Case 3: 7 months Case 4: 5 years Case 5: 15 months | Without data | Small bowel and hepatic transplant | 2 months | Case 1: 27,9 ng/ml Case 2: 26,6 ng/ml Case 3: 30,6 ng/ml Case 4: 11,5 ng/ml Case 5: 27,5 ng/ml | Hypertrophic Cardiomyopathy | Case 1: Switch to Cyclosporine and azathioprine was added to prednisone. Case 2: Switch to Cyclosporine and azathioprine was added to prednisone. Case 3: Switch to Cyclosporine and azathioprine was added to prednisone. Case 4: Switch to Cyclosporine and azathioprine was added to prednisone. Case 5: Remained on tacrolimus | Case 1: Symptoms and echocardiographic findings improved Case 2: Symptoms and echocardiographic findings improved Case 3: Symptoms and echocardiographic findings resolved Case 4: Without data Case 5: Without data |
| 2000 ‐ Sirolimus in pediatric gastrointestinal transplantation: The use of sirolimus for pediatric transplant patients with tacrolimus‐related cardiomyopathy. | Case series | 3 | Case 1: 12 years old Case 2: 6 years old | Without data | Case 1: Hepatic transplant Case 2: Hepatic and small bowel transplant | Case 1: 96 months Case 2: 15 months | Case 1: 10 mg day (daily dose) Case 2: 2 mg day (daily dose) Case 3: without data | Obstructive Hypertrophic Cardiomyopathy | Case 1: Switch to Sirolimus Case 2: Switch to Sirolimus | Case 1: Echocardiographic findings resolved Case 2: Global decrease in thickness of the interventricular septum with slow progressive increase in the posterior ventricular wall. However, global decrease in ventricular mass and addition of gradient |
| 2005 ‐ Reversal of tacrolimus‐related hypertrophic obstructive cardiomyopathy 5 years after kidney transplant in a 6‐year‐old recipient. | Case report | 1 | 6 months old | M | Kidney transplant | 5 years | 5–10 ng/ml | Hypertrophic Cardiomyopathy | Switch to Ciclosporyne | Echocardiographic findings resolved |
| 2007 ‐ Reversal of tacrolimus‐related hypertrophic cardiomyopathy after conversion to rapamycin in a pediatric live transplant recipient. | Case report | 1 | 17 months old | F | Hepatic transplant | 8 months | 14–16 ng/ml | Hypertrophic Cardiomyopathy | Switch to Rapamycin | Symptoms and echocardiographic findings resolved |
| 1995 ‐ Immunosuppressive drugs and hypertrophic cardiomyopathy. | Case series | 2 | Case 1: 39 years old Case 2: 2 years old | B | Case 1: Bone marrow transplant Case 2: Small bowel transplant | Case 1: 72 days Case 2: 40 days | Case 1: 24 ng/ml. Case 2: 40 ng/ml | Hypertrophic Cardiomyopathy | Case 1: Without data Case 2: Switch to Cyclosporine | Case 1: Does not apply (postmortem diagnosis in autopsy) Case 2: Symptoms and echocardiographic findings resolved |
| 2001 ‐ Lack of Tacrolimus‐Induced Cardiomyopathy. | Cross‐sectional study | 3609 patients who met entry criteria; 502 who had undergone ECHO's after transplantation; 171 with left ventricular hypertrophy; 6 patients with no underlying evident cause of LVH (secondary to tacrolimus) | 51 years old (average age) | B | Case 1: Renal transplant Case 2: Renal transplant Case 3: Renal transplant Case 4: Renal transplant Case 5: Renal transplant Case 6: Hepatic transplant | Case 1: 18 months Case 2: 1 month Case 3: 12 months Case 4: 24 months Case 5: 1 month Case 6: 5 months | Case 1: 0,8 ng/ml (plasma concentration) Case 2: 26,4 ng/ml Case 3: 1,8 ng/ml (plasma concentration) Case 4: 19, 6 ng/ml Case 5: 1,9 ng/ml (plasma concentration) Case 6: 14,4 ng/ml | Hypertrophic Cardiomyopathy | Case 1: Without data Case 2: Tacrolimus was continued Case 3: Without data Case 4: Without data Case 5: Without data Case 6: Without data | Case 1: Without data Case 2: Without data Case 3: Without data Case 4: Without data Case 5: Without data Case 6: Without data |
| 2017 ‐ Tacrolimus‐induced hypertrophic cardiomyopathy in a patient with dermatomyositis. | Letter to editor | 1 | 61 years old | F | Diffuse interstitial lung disease | 5 months | 6,6 ng/ml | Hypertrophic Cardiomyopathy | Switch to Cyclosporine | Symptoms and echocardiographic findings resolved |
| 2000 ‐ Tacrolimus and Myocardial Hypertrophy. | Cross‐sectional study | 32 | 5.75 years old (average age) | B | Hepatic transplant | Without data | 10–20 ng/ml | Hypertrophic Cardiomyopathy | Without data | Without data |
| 2018 ‐ Hypertrophic cardiomyopathy in preterm newborn with kidney transplanted mother. | Case report | 1 | Preterm birth newborn with 29 weeks of gestational age | M | Kidney transplant | 29 weeks and 6 days | 5,5 mg day (daily dose) | Hypertrophic Cardiomyopathy | Propranolol was started | Echocardiographic findings resolved |
| 2010 ‐Tacrolimus‐related hypertrophic cardiomyopathy in liver transplant recipients. | Prospective cohort study | 63 with Tacrolimus (1 developed Cardiomyopathy and 1 aortic stenosis and tricuspid regurgitation) 50 with Cyclosporine | Exposed group: 27.8 years old (average age) Control group: Without data | B | Hepatic transplant | Case 1: 3 months Case 2: 3 months | 10–20 ng/ml | Case 1: Hypertrophic Cardiomyopathy Case 2: Aortic and mitral stenosis, also tricuspid regurgitation | Case 1: Switch to Sirolimus Case 2: Tacrolimus dosage was reduced | Case 1: Died one month after diagnosis Case 2: Echocardiographic findings resolved |
3.2. Clinical presentation
Several cases presenting with dyspnea, fatigue, and symptoms and signs of obstructive hypertrophic cardiomyopathy have been described. 8 , 9 Our patient's main complaint was lipothymia, although other symptoms were also present. Less frequently, asymptomatic cases have been reported in which a new heart murmur was identified during follow‐up. 15 , 16 Although hypertrophic cardiomyopathy due to tacrolimus is the most frequent type of cardiomyopathy, few cases of dilated cardiomyopathy have also been described. 17 , 18
3.3. Therapeutic approach
Since hypertrophic myocardiopathy secondary to tacrolimus is a rare adverse effect, there are currently no guidelines for its treatment. However, several case reports and observational studies have shown reversal of this adverse effect with tacrolimus suspension and replacement with another immunosuppressive drug, such as cyclosporine or an mTOR inhibitor. 6 , 12 , 15 , 18 , 19 After switching from immunosuppressive therapy to sirolimus, the patient's symptoms and echocardiographic findings were improved.
Finally, we propose that a diagnosis based on the genetic origin of polycystic renal disease will allow personalized and better treatment of these patients. PKD1 and PKD2 mutations may predispose to primary cardiomyopathies, especially PKD1 mutations to hypertrophic obstructive cardiomyopathy. 20 PKD1 mutations were detected in 62.5% of patients with polycystic renal disease and hypertrophic cardiomyopathy in a cross‐sectional study. 20 Because a cellular pathway involving PKD1 and calcineurin has been described, calcineurin inhibition by tacrolimus may alter the feedback response and induce hypertrophy. 21 However, more observational and experimental studies are required to confirm this hypothesis.
4. CONCLUSION
Hypertrophic cardiomyopathy secondary to tacrolimus is an uncommon, potentially life‐threatening adverse effect, reported in solid organ transplant recipients who receive this calcineurin inhibitor. Therefore, physicians should pay attention to patients receiving tacrolimus, especially when symptoms are detected, to allow a proper diagnosis of cardiomyopathy and establish accurate management and treatment. This adverse effect has been observed to be reversible with tacrolimus suspension.
AUTHOR CONTRIBUTIONS
Guillermo Hernández Silva, Ricardo Giovanni Puerto Chaparro, and Javier Álvaro Martínez Melo conceived and designed the study and reviewed the manuscript. Cristian Orlando Porras Bueno, Javier Eduardo Martínez Rodríguez, and Sharon Julieth González Trillos wrote and reviewed the manuscript and was involved in data acquisition and analysis.
CONFLICT OF INTEREST
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
Hernández Silva G, Puerto Chaparro RG, Martínez Melo JÁ, Porras Bueno CO, Martínez Rodríguez JE, González Trillos SJ. Hypertrophic cardiomyopathy secondary to tacrolimus therapy in a kidney transplant patient: A case report and focused review of the literature. Clin Case Rep. 2022;10:e06539. doi: 10.1002/ccr3.6539
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author.
REFERENCES
- 1. Stolp J, Zaitsu M, Wood KJ. Immune tolerance and rejection in organ transplantation. Methods Mol Biol. 2019;1899:159‐180. doi: 10.1007/978-1-4939-8938-6_12 [DOI] [PubMed] [Google Scholar]
- 2. Noble J, Terrec F, Malvezzi P, Rostaing L. Adverse effects of immunosuppression after liver transplantation. Best Pract Res Clin Gastroenterol. 2021;54‐55:101762. doi: 10.1016/j.bpg.2021.101762 [DOI] [PubMed] [Google Scholar]
- 3. Farouk SS, Rein JL. The many faces of calcineurin inhibitor toxicity‐what the FK? Adv Chronic Kidney Dis. 2020;27(1):56‐66. doi: 10.1053/j.ackd.2019.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Robert N, Wong GW, Wright JM. Effect of cyclosporine on blood pressure. Cochrane Database Syst Rev. 2010;(1):CD007893. doi: 10.1002/14651858.CD007893.pub2 [DOI] [PubMed] [Google Scholar]
- 5. Nakata Y, Yoshibayashi M, Yonemura T, et al. Tacrolimus and myocardial hypertrophy. Transplantation. 2000;69(9):1960‐1962. doi: 10.1097/00007890-200005150-00039 [DOI] [PubMed] [Google Scholar]
- 6. Bowman LJ, Brennan DC, Delos‐Santos R, Larue SJ, Anwar S, Klein CL. Tacrolimus‐induced cardiomyopathy in an adult renal transplant recipient. Pharmacotherapy. 2015;35(12):1109‐1116. doi: 10.1002/phar.1666 [DOI] [PubMed] [Google Scholar]
- 7. Dehghani SM, Haghighat M, Imanieh MH, et al. Tacrolimus related hypertrophic cardiomyopathy in liver transplant recipients. Arch Iran Med. 2010;13(2):116‐119. [PubMed] [Google Scholar]
- 8. Liu T, Gao Y, Gao YL, et al. Tacrolimus‐related hypertrophic cardiomyopathy in an adult cardiac transplant patient. Chin Med J (Engl). 2012;125(7):1352‐1354. [PubMed] [Google Scholar]
- 9. Atkison P, Joubert G, Barron A, et al. Hypertrophic cardiomyopathy associated with tacrolimus in paediatric transplant patients. Lancet. 1995;345(8954):894‐896. doi: 10.1016/s0140-6736(95)90011-x [DOI] [PubMed] [Google Scholar]
- 10. Elliott P, Andersson B, Arbustini E, et al. Classification of the cardiomyopathies: a position statement from the european society of cardiology working group on myocardial and pericardial diseases. Eur Heart J. 2008;29(2):270‐276. doi: 10.1093/eurheartj/ehm342 [DOI] [PubMed] [Google Scholar]
- 11. Maron BJ, Towbin JA, Thiene G, et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association scientific statement from the council on clinical cardiology, heart failure and transplantation committee; quality of care and outcomes research and functional genomics and translational biology interdisciplinary working groups; and council on epidemiology and prevention. Circulation. 2006;113(14):1807‐1816. doi: 10.1161/CIRCULATIONAHA.106.174287 [DOI] [PubMed] [Google Scholar]
- 12. Noda K, Ukichi T, Furuya K, et al. Tacrolimus‐induced hypertrophic cardiomyopathy in a patient with dermatomyositis. Rheumatology (Oxford). 2017;56(11):2037‐2038. doi: 10.1093/rheumatology/kex310 [DOI] [PubMed] [Google Scholar]
- 13. Turska‐Kmieć A, Jankowska I, Pawłowska J, et al. Reversal of tacrolimus‐related hypertrophic cardiomyopathy after conversion to rapamycin in a pediatric liver transplant recipient. Pediatr Transplant. 2007;11(3):319‐323. doi: 10.1111/j.1399-3046.2006.00633.x [DOI] [PubMed] [Google Scholar]
- 14. Méndez‐Abad P, Zafra‐Rodríguez P. Miocardiopatía hipertrófica en un recién nacido pretérmino con madre trasplantada renal [hypertrophic cardiomyopathy in preterm newborn with kidney transplanted mother]. Arch Argent Pediatr. 2018;116(6):e749‐e752. doi: 10.5546/aap.2018.e749 [DOI] [PubMed] [Google Scholar]
- 15. Jarzembowski TM, John E, Panaro F, et al. Reversal of tacrolimus‐related hypertrophic obstructive cardiomyopathy 5 years after kidney transplant in a 6‐year‐old recipient. Pediatr Transplant. 2005;9(1):117‐121. doi: 10.1111/j.1399-3046.2005.00260.x [DOI] [PubMed] [Google Scholar]
- 16. Chang RK, McDiarmid SV, Alejos JC, Drant SE, Klitzner TS. Echocardiographic findings of hypertrophic cardiomyopathy in children after orthotopic liver transplantation. Pediatr Transplant. 2001;5(3):187‐191. doi: 10.1034/j.1399-3046.2001.00052.x [DOI] [PubMed] [Google Scholar]
- 17. Kakhi S, Phanish MK, Anderson L. Dilated cardiomyopathy in an adult renal transplant recipient: recovery upon tacrolimus to sirolimus switch: a case report. Transplant Proc. 2020;52(9):2758‐2761. doi: 10.1016/j.transproceed.2020.06.011 [DOI] [PubMed] [Google Scholar]
- 18. McLeod J, Wu S, Grazette L, Sarcon A. Tacrolimus‐associated dilated cardiomyopathy in adult patient after orthotopic liver transplant. J Investig Med High Impact Case Rep. 2017;5(2):2324709617706087. doi: 10.1177/2324709617706087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pappas PA, Weppler D, Pinna AD, et al. Sirolimus in pediatric gastrointestinal transplantation: the use of sirolimus for pediatric transplant patients with tacrolimus‐related cardiomyopathy. Pediatr Transplant. 2000;4(1):45‐49. doi: 10.1034/j.1399-3046.2000.00083.x [DOI] [PubMed] [Google Scholar]
- 20. Chebib FT, Hogan MC, El‐Zoghby ZM, et al. Autosomal dominant polycystic kidney patients may be predisposed to various cardiomyopathies. Kidney Int Rep. 2017;2(5):913‐923. doi: 10.1016/j.ekir.2017.05.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuo IY, Chapman AB. Polycystins, ADPKD, and cardiovascular disease. Kidney Int Rep. 2019;5(4):396‐406. doi: 10.1016/j.ekir.2019.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.


