Abstract
The patient was a 55‐year‐old female patient who presented with sudden onset of left hemiplegia, facial hemiparesis, and hypoesthesia. She has received her first dose of the AstraZeneca COVID‐19 vaccine. This case indicates that vaccination may raise the hypercoagulable state even in a condition of post‐ICH and anticoagulant prophylaxis.
Keywords: cerebral venous sinus thrombosis, ChAdOx1 nCoV‐19 vaccine, intracerebral hemorrhage, Oxford‐AstraZeneca COVID‐19 vaccine
We reported a CVST case that occurred after ICH while she was on prophylactic UWH. She had received the AstraZeneca COVID‐19 vaccine earlier. In this case, we should consider different manifestations of vaccination side effects.
1. INTRODUCTION
Since the vaccination against COVID‐19 has been started, concerns and worries have been raised about the prothrombotic effects of the AstraZeneca (ChAdOx1 nCov‐19) vaccine. 1 There are few clinical reports of cerebral venous sinus thrombosis (CVST) due to this viral vector vaccine. 2 It was realized that thrombocytopenia alone or thrombosis without thrombocytopenia could be part of the thrombotic events. 3 After ruling out other causes of CVST, the vaccine could be a reason to induce CVST. 4 Till April 4, 2021, European Medicine Agency's Pharmacovigilance Risk Assessment Committee reported 169 cases of CVST from all 34 million people who received the AstraZeneca COVID‐19 vaccine. 5 CVST‐thrombosis with thrombocytopenia syndrome (TTS)after the AstraZeneca (ChAdOx1 nCov‐19) vaccine in Germany was 45. 6 In a new published systematic review, 144 patients were studied, and they reported that the most common thromboembolic events were cerebral venous sinus thrombosis (38.5%) and deep vein thrombosis/pulmonary embolism (21.1%). 7 It has been estimated that among CVST patients who have been reported, 44% have presented with intracerebral hemorrhage (ICH) or subarachnoid hemorrhage (SAH) in a condition named vaccine‐induced immune thrombotic thrombocytopenia (VITT). 2 Patients with VITT often present with CVST, nearly 1 month after vaccination. 8 , 9 Generally, the concurrency of CVST and ICH makes the prognosis for the outcome of the patients poor and the management of the patients more complicated. 10 Thus, it requires more attention to consider different manifestations of this new challenging side effects of the COVID‐19 vaccines. To the best of our knowledge and by reviewing previous reports, it is rare to develop CVST with a considerable delay after ICH occurrence. Most cases develop ICH secondary to CVST. 2 We report an occurrence of ICH in basal ganglia and thalamus after the first dose of the AstraZeneca (ChAdOx1 nCov‐19) vaccine. After ICH, the patient developed CVST while she was on prophylactic anticoagulant with normal platelet counts. The aim of reporting this case is to attract attention to the probable hypercoagulable state of vaccines in some patients, which may not fulfill the criteria of VITT and the prophylactic anticoagulants may not prevent this side effect.
2. PATIENT INFORMATION
The patient was a 55‐year‐old fit and non‐smoker woman who presented to the emergency department of our hospital with a sudden onset of left hemiplegia, facial hemiparesis, hypoesthesia, and a left frontotemporal headache accompanied by episodes of emesis from 2 h ago. The patient had a history of hypertension, which was non‐compliant with her antihypertensive drugs and a surgery history of hysterectomy 10 years ago. There was no history of taking any routine medications such as oral contraceptives (OCP) drugs or hormone replacement therapy (HRT), but the patient declared that 15 days ago, she had received her first dose of the ChAdOx1 nCov‐19 vaccine (AstraZeneca). Her Body Mass Index (BMI) was 24. The patient's blood pressure, heart rate, respiratory rate, and temperature were 142/76 mm Hg, 78 per minute, 14 per minute, and 37.6°C, respectively.
3. CLINICAL FINDINGS
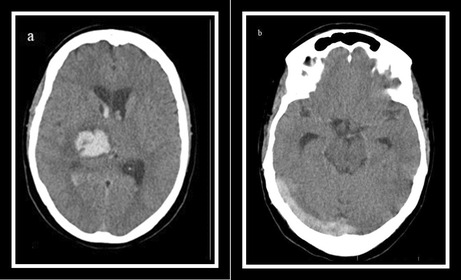
On the neurological examination, the patient was confused with a Glasgow Coma Score (GCS): 14/15. Pupils were of normal size, symmetrical, and reactive to light. Except for a left facial nerve paresis, and a left facial hemiparesthesis, other cranial nerves were unremarkable. The patient was hemiplegic, reducing the left upper and lower extremities muscles' tones and forces: 0/5. The left side of her body had a sensory impairment at the extremities. Also, on the left side, deep tendon reflexes were decreased, and the plantar reflex was upward (positive Babinski sign). The National Institutes of Health Stroke Score of the patient was 13, and based on that, the neurologist ordered an urgent brain Computed Tomography (CT), which revealed a hyperdense lesion at the right thalamus and basal ganglia with expansion to the lateral ventricles in favor of intraparenchymal and intraventricular hemorrhage (IVH; Figure 1A). The CT‐scan cuts at the level of transverse and sigmoid sinus demonstrate no evidence on behalf of CVST (Figure 1B).
FIGURE 1.
(A) Brain computed tomography (CT) scan at the time of admission demonstrating intra cranial hemorrhage at the right thalamus and basal ganglia with expansion to the lateral ventricles. (B) at the level of the transverse and sigmoid sinus, there is no evidence on behalf of CVST. (C) On the seventh day after admission, a brain CT scan showed the cord sign and (D) a good absorption process for his ICH.
4. DIAGNOSIS ASSESSMENT
Laboratory test investigations showed: hemoglobin (Hgb): 14.3 g/dl, white blood cell (WBC): 9.1 × 103 cells/mm3, platelet (Plt): 238 × 103 cells/mm3, prothrombin time (PT): 13 s, international normalized ratio (INR): 1, partial thromboplastin time (PTT): 38 s, alanine transaminase (ALT): 49 U/L, aspartate aminotransferase (AST): 36 U/L, alkaline phosphatase (ALP): 92 U/L, creatinin: 0.88 mg/dl, and lactate dehydrogenase (LDH): 250 U/L. Also, the polymerase chain reaction (PCR) test for COVID‐19 was negative, and there was no evidence favoring COVID‐19 in the chest CT scan. The patient's electrocardiography (ECG) was normal, and the ejection fraction (EF) was 55%.
5. THERAPEUTIC INTERVENTION
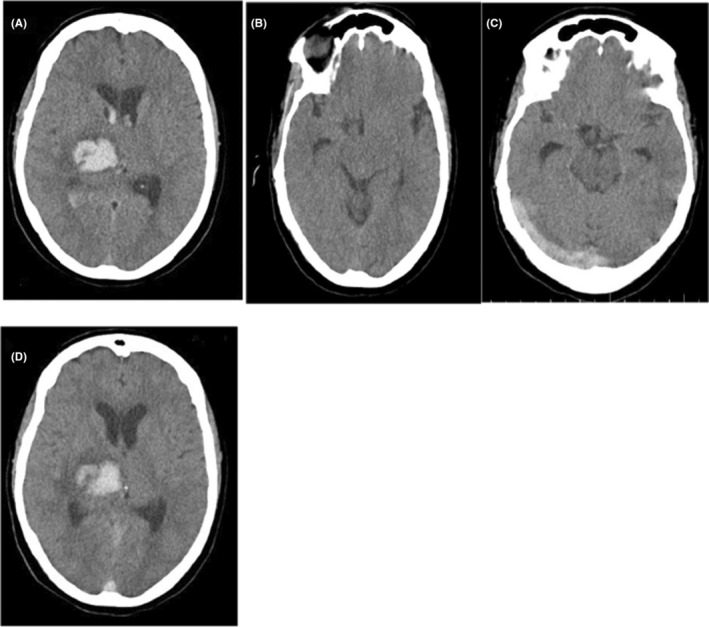
The patient was admitted to the Intensive Care Unit (ICU), and intravenous administration of acetaminophen and pantoprazole was initiated; losartan and amlodipine were also prescribed for controlling hypertension. Subsequently, the patient headache gradually improved. Three days after admission, we started subcutaneous administration of unfractionated weighted heparin (UWH; 5000 mg BID) as prophylaxis against thromboembolic events in ICH‐associated hemiplegia. CT scans were done on third and fifth day of admission. These CT scans showed an improvement in ICH. No other abnormalities were observed in the brain. On the seventh day of hospitalization, the patient's headache deteriorated again, while the patient no longer complained of headaches in the previous days. There were no additional focal neurological deficits compared with the patient's admission signs. We ordered a new CT scan in which the cord sign had appeared in addition to the previous ICH (Figure 1C,D). Brain magnetic resonance imaging (MRI) study (with and without contrast) and magnetic resonance venography (MRV) indicated thrombosis within the right transverse and right sigmoid and superior sagittal sinuses (Figure 2). Also, there was no evidence of a new acute ICH.
FIGURE 2.
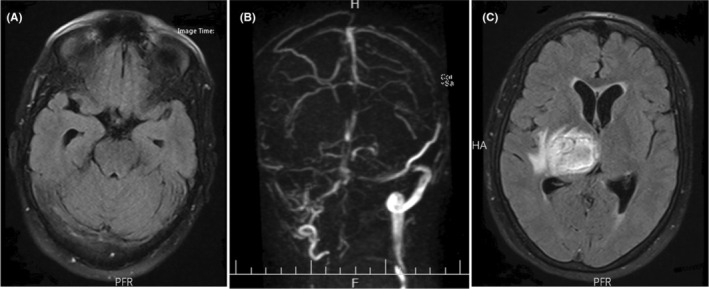
(A) Brain magnetic resonance imaging (MRI) showing signal intensity changes at the right transverse sinus on T2‐weighted FLAIR sequence. (B) Gadolinium‐enhanced MR venography (MRV) shows the filling defect of the right transverse and right sigmoid and superior sagittal sinuses. (C) T2‐weighted FLAIR sequence of brain MRI demonstrating hyperintense signal at the right thalamus and basal ganglia due to the patient's former ICH.
Complementary hematological test results showed: Antithrombin III: 117 (75–120), Protein C: 130 (70–140), Protein S: 99 (60–140), factor V Laden 171.2 (>120), D‐dimer: 5981 ng/ml, Fibrinogen: 3.8 g/dl, PT:13 s, INR:1.1, PTT:36 s, and normal level of Von Willebrand factor–cleaving protease (ADAMTS13). Other serological and immunological tests were normal for rheumatoid factor (RF), antinuclear antibody (ANA), anti‐ds‐DNA, anticardiolipin, anti phospholipid, anti‐beta‐2‐glycoprotein antibody, perinuclear and cytoplasmic anti‐neutrophilic cytoplasmic autoantibody (c‐ANCA and p‐ANCA). Also, the anti‐platelet factor 4 (PF4) antibody ELISA was negative. We changed the heparin dosage from prophylactic to a therapeutic range. Due to the patient's former ICH expansion risk during heparin therapy, we continuously controlled it with regular CT scans (Figure 3). A week later, the patient's headache gradually improved. We, therefore, discharged her in good general condition with a 2/5 of left extremities forces on the 17th day of hospitalization. Also, her anticoagulant drug was switched from UFH to Warfarin, and continuous physiotherapy, performed since admission, continued after discharge.
FIGURE 3.
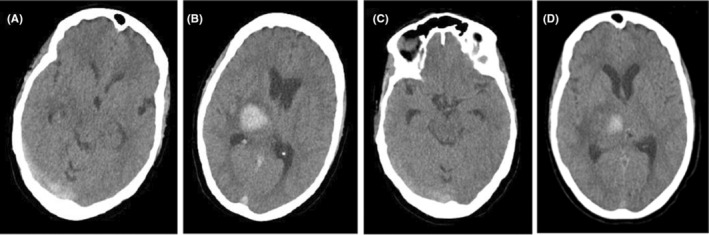
(A, B) Brain computed tomography (CT) scan on the 11th day of hospitalization showing relatively smaller cord sign and no expansion for ICH, respectively. (C, D) CT scan on the 15th day of hospitalization showed approximate disappearance of cord sign and smaller dimensions of ICH, respectively.
6. FOLLOW‐UP AND OUTCOMES
On the control CT scan, 2 months after discharge, the ICH of the thalamus and basal ganglia were completely absorbed, and there was no sign in favor of CVST. After 4 months of rehabilitation, the patient's left extremities force increased to 4/5, and the facial hemiparesis varnished.
7. DISCUSSION
We reported a case of intracerebral hemorrhage (ICH) and then cerebral venous and dural sinus thrombosis (CVST), followed by the ChAdOx1 nCov‐19 vaccine (AstraZeneca). Based on previous studies, almost 40% of all CVST patients develop an ICH concurrently or following their hospitalization. 11 , 12 Accompanying ICH and CVST is challenging when the patient is under antithrombotic agents treatment, making the patient's outcome a poor prgonosis. 13 Afifi et al. suggested that a mixture of different hemorrhages, especially the presence of both intraparenchymal and subarachnoidal hemorrhage (IPH and SAH) or sulcal SAH or small juxta‐cortical hemorrhage (JCH) could help diagnose CVST related ICH. 10 , 14 Based on the past medical history of our patient(hypertension), and the ICH involvement site (thalamus and basal ganglia), the hemorrhage was associated with hypertension‐related causes, which decreases the probability of CVST‐related ICH. Also, in contrast to their investigations, our patient's CVST was following a delay after ICH.
There are different known risk factors of CVST, including structural, hematological, immunological, serological, metabolical, drug‐related, and infectious reasons. 15 There were no history, signs, or symptoms favoring CVST risk factors. We have ruled out our patient's possible genetic hypercoagulable state inducers such as factor V Leiden and G20210A prothrombin gene mutation, protein C and S, and anti‐thrombin III deficiency. Also, the immunological laboratory test for antiphospholipid syndrome and other rheumatological diseases was negative. There are several reports of CVST or thrombosis at other sites, 4–28 days following vaccination with the SARS‐CoV‐2 vaccines such as ChAdOx1 nCov‐19 (Oxford–AstraZeneca) and Ad26.COV2.S (Janssen/Johnson & Johnson), has led to a restriction in usage of this in several countries, especially in their younger patients. 8 , 16 Based on these reports, we consider the AstraZeneca vaccine a risk factor for our patient's CVST. Interestingly, our patient developed CVST while she was on prophylactic unfractionated weighted heparin (UWH) to prevent thromboembolic events. Another similar complication of COVID‐19 viral vector vaccines is vaccine‐induced immune thrombotic thrombocytopenia (VITT), which mainly presents with headaches and other possible neurological symptoms and signs. The VITT's thrombotic event mainly consists of CVST, and the mean platelet count is between 5 and 127 × 103 cells/mm3. Also, antibodies against platelet factor 4 (PF4) are generally positive in VITT patients. 4 , 9 A meta‐analysis conducted by Sharifian‐Dorche et al. revealed that 38.8% of VITT patients developed ICH and SAH as a result of CVST. Their investigations show that the ICH and SAH mainly manifest concurrently or due to CVST. 2 Another interesting finding in our patients was that the CVST developed by a 7 days delay following the ICH, which is a rare occurrence. We did not detect antibodies against platelet factor 4 (PF4), and our patient's platelet counts were in the normal range. So, our patient did not fulfill the VITT criteria even in the probable VITT category.
Although it is known that the concomitant presence of ICH and CVST is not a contraindication for heparin therapy, 17 it is better to avoid Heparin in VITT treatment due to their common pathophysiological processes of producing anti‐PF4 with heparin‐induced thrombotic thrombocytopenia (HITT). 18 We used UFH followed by Warfarin to treat our patient, while the VITT diagnosis was ruled out, and we monitored the patient's ICH with the control CT scans to ensure that it did not expand.
8. CONCLUSION
Since the ChAdOx1 nCov‐19 vaccine (AstraZeneca) was presented for immunization against COVID‐19, several hypercoagulable states such as CVST have been reported. Several of them described a more rare condition named VITT, in which mainly the ICH occurs concurrently or secondary to CVST. We reported a rare case of CVST followed by a 7 days delay after ICH. This timeline transposed CVST occurrence after ICH without fulfilling the VITT criteria while the patient was on prophylactic UWH, making this case an educational report for all medical staff to consider different manifestations and probabilities of vaccination side effects. Also, it indicates that vaccination may raise the hypercoagulable state even in a condition of post‐ICH and anticoagulant prophylaxis, which might be explained by another pathophysiological process except for VITT. Hence, future studies can focus on this pro‐thrombotic causality of vaccines to make them safer with lower side effects.
AUTHOR CONTRIBUTIONS
Arsh Haj Mohamad Ebrahim Ketabforoush, Ghazale Molaverdi, and Nahid Abbasi Khoshsirat involved in study concept and design. Ghazale Molaverdi did acquisition of data. Arsh Haj Mohamad Ebrahim Ketabforoush, Ghazale Molaverdi Matineh Nirouei, and Nahid Abbasi Khoshsirat drafted the manuscript. Nahid Abbasi Khoshsirat, Arsh Haj Mohamad Ebrahim Ketabforoush, Ghazale Molaverdi, and Matineh Nirouei involved in critical revision of the manuscript for important intellectual content. Nahid Abbasi Khoshsirat, Arsh Haj Mohamad Ebrahim Ketabforoush, Ghazale Molaverdi, and Matineh Nirouei involved in administrative, technical, and material support. Nahid Abbasi Khoshsirat supervised the study.
FUNDING INFORMATION
This work has no funding.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
CONSENT
Written informed consent was obtained from the patient.
ACKNOWLEDGMENT
The authors want to thank the Clinical Research Development Unit (CRDU) of Shahid Rajaei Hospital, Alborz University of Medical Sciences, Karaj, Iran, for their support, cooperation, and assistance throughout this study.
Haj Mohamad Ebrahim Ketabforoush A, Molaverdi G, Nirouei M, Abbasi Khoshsirat N. Cerebral venous sinus thrombosis following intracerebral hemorrhage after COVID‐19 AstraZeneca vaccination: A case report. Clin Case Rep. 2022;10:e06505. doi: 10.1002/ccr3.6505
Arsh Haj Mohamad Ebrahim Ketabforoush and Ghazale Molaverdi should be considered joint first authors with equal contributions.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
REFERENCES
- 1. Voysey M, Costa Clemens SA, Madhi SA, et al. Single‐dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV‐19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397(10277):881‐891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sharifian‐Dorche M, Bahmanyar M, Sharifian‐Dorche A, Mohammadi P, Nomovi M, Mowla A. Vaccine‐induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID‐19 vaccination; a systematic review. J Neurol Sci. 2021;428:117607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Siddig A, Abbasher Hussien Mohamed Ahmed K, Hassan Haroun MS, et al. AstraZeneca COVID‐19 vaccine: a possible risk factor for ischemic stroke and cerebral venous sagittal sinus thrombosis: a case series. Clin Case Rep. 2022;10(7):e6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klok FA, Pai M, Huisman MV, Makris M. Vaccine‐induced immune thrombotic thrombocytopenia. Lancet Haematol. 2021;9:e73‐e80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hernández AF, Calina D, Poulas K, Docea AO, Tsatsakis AM. Safety of COVID‐19 vaccines administered in the EU: should we be concerned? Toxicol Rep. 2021;8:871‐879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wittstock M, Walter U, Volmer E, Storch A, Weber M‐A, Großmann A. Cerebral venous sinus thrombosis after adenovirus‐vectored COVID‐19 vaccination: review of the neurological‐neuroradiological procedure. Neuroradiology. 2022;64(5):865‐874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Matar RH, Than CA, Nakanishi H, et al. Outcomes of patients with thromboembolic events following coronavirus disease 2019 AstraZeneca vaccination: a systematic review and meta‐analysis. Blood Coagul Fibrinolysis. 2022;33(2):90‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolf ME, Luz B, Niehaus L, Bhogal P, Bäzner H, Henkes H. Thrombocytopenia and intracranial venous sinus thrombosis after “COVID‐19 vaccine AstraZeneca” exposure. J Clin Med. 2021;10(8):1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Purkayastha P, Mckechnie C, Kalkur P, Scully M. Rare case of COVID‐19 vaccine‐associated intracranial haemorrhage with venous sinus thrombosis. BMJ Case Rep. 2021;14(9):e245092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Afifi K, Bellanger G, Buyck P‐J, et al. Features of intracranial hemorrhage in cerebral venous thrombosis. J Neurol. 2020;267(11):3292‐3298. [DOI] [PubMed] [Google Scholar]
- 11. Ferro JM, Bousser M‐G, Canhão P, et al. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis – endorsed by the European Academy of Neurology. Eur J Neurol. 2017;24(10):1203‐1213. [DOI] [PubMed] [Google Scholar]
- 12. Saposnik G, Barinagarrementeria F, Brown RD, et al. Diagnosis and management of cerebral venous thrombosis. Stroke. 2011;42(4):1158‐1192. [DOI] [PubMed] [Google Scholar]
- 13. Pizzi MA, Alejos DA, Siegel JL, Kim BY, Miller DA, Freeman WD. Cerebral venous thrombosis associated with intracranial hemorrhage and timing of anticoagulation after hemicraniectomy. J Stroke Cerebrovasc Dis. 2016;25(9):2312‐2316. [DOI] [PubMed] [Google Scholar]
- 14. Ferro JM, Canhão P, Stam J, Bousser M‐G, Barinagarrementeria F. Prognosis of cerebral vein and dural sinus thrombosis: results of the International Study on Cerebral Vein and Dural Sinus Thrombosis (ISCVT). Stroke. 2004;35(3):664‐670. [DOI] [PubMed] [Google Scholar]
- 15. Alvis‐Miranda HH, Castellar‐Leones SM, Alcala‐Cerra G, Moscote‐Salazar LR. Cerebral sinus venous thrombosis. J Neurosci Rural Pract. 2013;4(4):427‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oldenburg J, Klamroth R, Langer F, et al. Diagnosis and management of vaccine‐related thrombosis following AstraZeneca COVID‐19 vaccination: guidance statement from the GTH. Hamostaseologie. 2021;41:184‐189. [DOI] [PubMed] [Google Scholar]
- 17. Einhäupl K, Bousser MG, De Bruijn S, et al. EFNS guideline on the treatment of cerebral venous and sinus thrombosis. Eur J Neurol. 2006;13(6):553‐559. [DOI] [PubMed] [Google Scholar]
- 18. Rizk JG, Gupta A, Sardar P, et al. Clinical characteristics and pharmacological management of COVID‐19 vaccine–induced immune thrombotic thrombocytopenia with cerebral venous sinus thrombosis: a review. JAMA Cardiol. 2021;6(12):1451‐1460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.


