ABSTRACT
Background
Recently, several pharmaceutical companies have developed new medium cut-off (MCO) dialyzers for expanded hemodialysis (HDx). This study aimed to compare the safety and efficacy of four MCO dialyzers, against each other and versus high-flux hemodialysis (HD) and post-dilution hemodiafiltration (HDF).
Methods
A prospective study was carried out on 23 patients who underwent six dialysis sessions: two sessions with the FX80 Cordiax in HD and HDF, and four HDx sessions with the Phylther 17-SD, Vie-18X, Elisio HX19 and Theranova 400 dialyzers. The reduction ratios (RRs) of urea, creatinine, β2-microglobulin, myoglobin, kappa free immunoglobulin light chain (κFLC), prolactin, α1-microglobulin, α1-acid glycoprotein, lambda (λFLC) and albumin were compared. Dialysate albumin loss was also measured.
Results
The differences in efficacy between the evaluated dialyzers were minimal in small molecules and even up to the size of β2-microglobulin. The main differences were found between myoglobin, κFLC, prolactin, α1-microglobulin and λFLC RRs, in which all four MCO dialyzers, with similar efficacy, were clearly superior to HD and slightly inferior to HDF treatment. Albumin losses in the dialysate with HD dialyzers were <1 g and between 1.5 and 2.5 g in HDx and HDF. The global removal score values were similar in all four HDx treatments, and again significantly higher than those with HD.
Conclusions
The results of the four MCO dialyzers evaluated in this study showed good efficiency, with no significant performance differences between them while being completely safe in terms of albumin loss. Likewise, the study confirms the superiority of HDx over high-flux HD with an efficacy close to that of post-dilution HDF.
Keywords: dialyzer performance, expanded hemodialysis, hemodiafiltration, medium cut-off, safety
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Expanded hemodialysis with medium cut-off (MCO) membranes was designed to improve the permeability of the dialyzers. These can only be used in the hemodialysis (HD) modality and could provide an alternative to hemodiafiltration (HDF) since they achieve similar solute-removal performance to post-dilution HDF [1–3]. This is due to the tailored cut-off of the membrane pores combined with an internal architecture that allows MCO membranes to achieve increased removal capacities for middle molecules and large middle molecules compared with standard HD treatments [2].
Scientific evidence of the superiority of post-dilution HDF versus high-flux HD in overall and cardiovascular survival has been demonstrated [4–7]. Consequently, it can currently be considered the standard conventional HD treatment [8].
Initially, very few alternatives were available for expanded HD (HDx). However, several pharmaceutical companies have recently developed new MCO dialyzers, which have obtained the CE mark, thus increasing their therapeutic possibilities. Unfortunately, few studies have compared their efficacy and their safety issues.
The safety of MCO dialyzers is ensured by restricting pore sizes to limit albumin losses below 5 g per session [9, 10]. In this regard, most published studies report that MCO membranes lead to a higher albumin loss than HD and show inconsistent results compared with HDF [11–16]. However, the albumin loss could be considered clinically tolerable in all cases, as long as it is used in HD modality.
To evaluate these new MCO dialyzers, this study compared the safety issues and efficacy of four of them and also compared them against high-flux HD and post-dilution HDF treatments. Removal of a wide range of molecular weight molecules was assessed, and safety was evaluated with blood and dialysate albumin loss.
MATERIALS AND METHODS
The study included 23 patients, of whom 15 were male and 8 were female, with a mean age of 68.6 ± 12 years (range 46–90 years) on a regular HD program. Sixteen patients had an autologous arteriovenous fistula as vascular access, six a tunneled catheter and one a prosthetic arteriovenous graft. The causes of end-stage kidney disease were chronic glomerulonephritis (four patients), hypertensive kidney disease (three patients), interstitial nephritis (three patients), systemic disease (two patients), diabetic kidney disease (three patients), autosomal dominant polycystic kidney disease (two patients), urological (one patient) and undiagnosed nephropathy (four patients). The anticoagulants used during dialysis were low molecular-weight heparin (tinzaparin) in 57% of the patients and unfractionated heparin in 26%; the remaining 17% of the patients were dialyzed without heparin. Net fluid removal was prescribed according to the patients’ clinical needs. Patients with a urine volume >50 mL per day were excluded. Every included patient provided informed consent. The study was approved by the local Ethics Committee and was conducted according to the Declaration of Helsinki.
Each patient received six different sessions with their usual parameters [dialysis duration 288 ± 17 min, blood flow rate (Qb) 439 ± 26 mL/min and dialysate flow rate (Qd) 400 mL/min plus additional dialysate for the replacement volume in HDF]:
high-flux FX80 CordiaxTM, helixone, Fresenius Medical Care, in HD;
MCO Phylther 17-SDTM, polyphenylene, Medtronic, in HD (HDx);
MCO Vie-18XTM, polysulfone, Asahi, in HD (HDx);
MCO Elisio HX19TM, polyethersulfone, Nipro, in HD (HDx);
MCO Theranova 400TM, polyarylethersulfone, Baxter, in HD (HDx);
high-flux FX80 CordiaxTM, helixone, Fresenius Medical, in post-dilution HDF.
Patients were dialyzed with 5008 Fresenius monitors, and dialyzers were automatically primed. Online HDF was performed with post-dilution infusion and automatic infusion flow. The order of the different treatment sessions was randomly assigned. The dialyzer characteristics are summarized in Table 1. Blood and dialysis fluid samples for analyses were taken from each patient in the same dialysis session of the week.
Table 1.
In vitro dialyzer performance
| FX80 Cordiax HD/HDF | Phylther 17SD HDx | Vie-18X HDx | Elisio HX19 HDx | Theranova 400 HDx | |
|---|---|---|---|---|---|
| Membrane brand | Helixone FMC | Polyphenylene Medtronic | Polysulfone Asahi | Polyethersulfone Nipro | Polyaryilethersulfone Baxter |
| KUF (mL/h/mmHg) | 64 | 53 | 87.9 | 75 | 48 |
| Wall thickness (µm) | 35 | 30 | 45 | 40 | 35 |
| Internal diameter (µm) | 185 | 200 | 185 | 200 | 180 |
| SC B2m | 0.90 | 0.93 | 0.90 | 1.0 | 1.0 |
| SC myoglobin | 0.50 | 0.70 | 0.80 | 0.86 | 0.9 |
| SC albumin | 0.001 | <0.02 | <0.01 | 0.0024 | 0.008 |
| Surface (m2) | 1.8 | 1.7 | 1.8 | 1.9 | 1.7 |
| Sterilization | Steam | Steam | Gamma radiation | Gamma radiation | Steam |
KUF, ultrafiltration coefficient; B2m, β2-microglobulin; SC, sieving coefficient; NA, not available.
The dialysis parameters collected in each session were as follows: real duration, dialyzer, Qb, Qd, recirculation index measured by the temperature module, arterial pressure, venous pressure, transmembrane pressure, relative hematocrit difference between the start and the end of the dialysis session automatically calculated by the blood volume monitor biosensor, initial and final body weight, the volume of blood processed, and replacement volume.
Laboratory measurements included concentrations of urea (60 Da), creatinine (113 Da), β2-microglobulin (11 800 Da), myoglobin (17 200 Da), prolactin (23 000 Da), α1-microglobulin (33 000 Da), α1-acid glycoprotein (41 000 Da) and albumin (66 000 Da) in serum at the beginning and at the end of each session to calculate the percentage reduction ratio (RR) of these solutes. Free immunoglobulin light chains (FLCs) were also measured, kappa FLC (κFLC) with a molecular weight of 22 500 Da and lambda FLC (λFLC) with a molecular weight of 45 000 Da.
The final concentration of β2-microglobulin, myoglobin, prolactin, κFLC, α1-microglobulin, α1-acid glycoprotein, λFLC and albumin were corrected for the degree of hemoconcentration and the distribution volume (approximate extracellular volume) according to Bergström and Wehle [17]: post-dialysis concentration correction = post-dialysis concentration/[1 + ((pre-dialysis weight – post-dialysis weight)/(0.2 × dry weight))].
Urea and creatinine were measured by molecular absorption spectrometry, albumin and β2-microglobulin were measured by immunoturbidimetric, and myoglobin and prolactin were measured by indirect enzyme immunoassay; all of them were performed in an Atellica Solution analyzer (Siemens Healthineers, Tarrytown, NY, USA). Finally, α1-acid glycoprotein, α1-microglobulin, κFLC and λFLC were measured by immunonephelometry using the BNII analyzer (Siemens Healthineers).
A proportional part of the dialysis fluid was collected throughout the treatment to quantify albumin loss employing a reverse perfusion pump.
A global removal score [14] was also calculated with the following formula: ((ureaRR + β2-mRR + myoglobinRR + prolactinRR + α1-microglobulinRR + α1-acid glycoproteinRR – albuminRR)/6).
The results are expressed as the arithmetic mean ± standard deviation. Quantitative parameters were analyzed with the Student’s t-test for paired data. Parametric data were analyzed with ANOVA for repeated data, followed by Bonferroni's post hoc test. P < .05 was considered statistically significant. Analyses were performed using SPSS software version 23 (SPSS, Chicago, IL, USA).
RESULTS
Assessment of the behavior and tolerance of the dialyzers
We observed proper tolerance to every used filter, with no adverse reactions observed in the connection or disconnection during the HD or HDF sessions in the studied population. Replacement fluid in online post-dilution HDF was 30.4 ± 4.1 L (range 24–37 L) with helixone HDF treatment.
There were no differences in dialysis parameters: Qb, total blood processed, vascular access recirculation, real session duration, initial weight, final weight, weight gain, initial and final hematocrit measured by the dialysis monitor, arterial pressure, and venous pressure (Table 2). As expected, the transmembrane pressure was significantly higher in HDF sessions than in the other sessions in HD.
Table 2.
Comparison of dialysis parameters in the six study sessions
| FX80 Cordiax HD | Phylther 17SD HDx | Vie-18X HDx | Elisio HX19 HDx | Theranova 400 HDx | FX80 Cordiax HDF | |
|---|---|---|---|---|---|---|
| Blood processed (L) | 124.6 ± 9.6 | 123.6 ± 9.9 | 124.2 ± 9.9 | 123.8 ± 9.5 | 124.2 ± 9.8 | 123.6 ± 9.7 |
| Recirculation (%) | 14.3 ± 5.5 | 14.9 ± 4.1 | 13.7 ± 4.6 | 15.4 ± 4.6 | 15.3 ± 4.8 | 14.2 ± 4.3 |
| Real dialysis time (min) | 283.3 ± 16.7 | 282.9 ± 17.8 | 283.0 ± 18.1 | 282.2 ± 17.3 | 282.4 ± 17.6 | 281.2 ± 17.6 |
| Initial weight (kg) | 69.7 ± 17.5 | 70.2 ± 17.6 | 70.0 ± 17.4 | 69.8 ± 17.4 | 70.0 ± 17.6 | 69.9 ± 17.6 |
| Final weight (kg) | 67.3 ± 17.1 | 67.7 ± 17.2 | 67.4 ± 17.2 | 67.2 ± 17.0 | 67.4 ± 17.3 | 67.5 ± 17.4 |
| Weight gain (kg) | 2.44 ± 1.06 | 2.59 ± 1.05 | 2.60 ± 0.81 | 2.56 ± 0.92 | 2.63 ± 0.99 | 2.38 ± 0.96 |
| Initial hematocrit (%) | 30.8 ± 4.9 | 29.7 ± 4.7 | 30.9 ± 4.7 | 30.8 ± 4.3 | 30.6 ± 4.5 | 30.9 ± 4.8 |
| Final hematocrit (%) | 36.2 ± 5.5 | 35.7 ± 5.8 | 36.7 ± 5.8 | 36.5 ± 5.3 | 36.9 ± 5.6 | 36.9 ± 6.2 |
| Arterial pressure. (mmHg) | –229 ± 24 | –227 ± 22 | –230 ± 22 | –229 ± 22 | –228 ± 25 | –228 ± 25 |
| Venous pressure. (mmHg) | 199 ± 23 | 192 ± 24 | 197 ± 23 | 202 ± 28 | 201 ± 21 | 200 ± 23 |
| TMP (mmHg) | 30.0 ± 5.1 | 31.4 ± 5.0 | 29.2 ± 3.5 | 29.0 ± 4.2 | 28.5 ± 2.6 | 191.3 ± 32.1a |
| Substitution volume (L) | Not applicable | Not applicable | Not applicable | Not applicable | Not applicable | 30.4 ± 4.1 |
P < .001 vs all others dialysis treatments. TMP, transmembrane pressure. Data are presented as mean ± standard deviation.
Small-sized molecules
The Kt obtained by ionic dialysance was 61.5 ± 5.8 L with FX80 in HD, 58.2 ± 5.4 L with Phylther (P < .001 versus all other five study situations), 63.8 ± 5 9 L with Vie 18X, 64.2 ± 6.5 L with Elisio 19HX, 63.4 ± 6.3 L with Theranova 400 and 68.7 ± 7.6 L with FX80 in HDF (P < .001 compared with FX80 in HD, Phylther and Theranova; P < .01 versus VieX and Elisio HX).
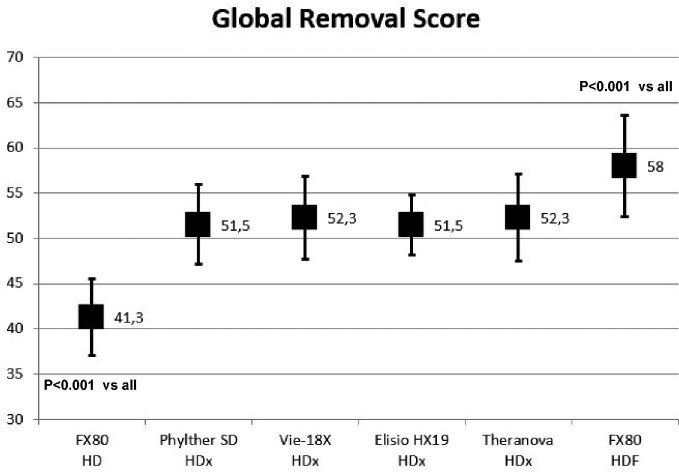
However, concerning urea and creatinine RR, the only statistically significant difference was observed in urea and creatinine RR between MCO Phylther 17-SD and the other three MCO dialyzers and FX80 Cordiax in HDF treatment (Fig. 1).
FIGURE 1:
Comparison of urea and creatinine reduction ratios in all study situations (ANOVA for repeated data).
Medium-sized molecules
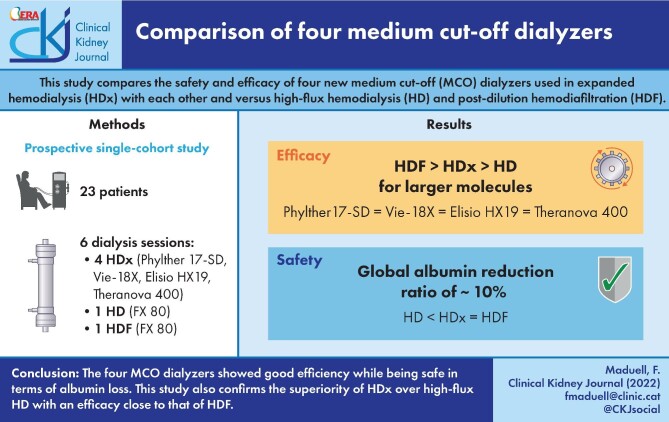
The RR of β2-microglobulin with HDF treatments was significantly higher than those obtained with the four MCO dialyzers and high-flux HD treatments. Among MCO dialyzers, the Phylther 17-SD had a slightly lower RR than the other three MCO dialyzers (Fig. 2).
FIGURE 2:
Comparison of β2-microglobulin, myoglobin and prolactin RRs in all study situations (ANOVA for repeated data).
There were no significant differences in the myoglobin and prolactin RR between four HDx treatments, and all of them were significantly higher than those obtained with HD treatments (Fig. 2). In addition, HDF treatments were significantly higher than those obtained with all MCO and HD treatments (Fig. 2).
In the high molecular weight range, there were no significant differences in the α1-microglobulin and α1-acid glycoprotein RR between the four MCO dialyzers. The α1-microglobulin RR with HDF and all HDx treatments were significantly higher than those obtained in helixone HD treatments (Fig. 3). The α1-acid glycoprotein RR in HDF was significantly higher than those obtained with FX80 in HD and Elisio HX in HDx treatments (Fig. 3).
FIGURE 3:
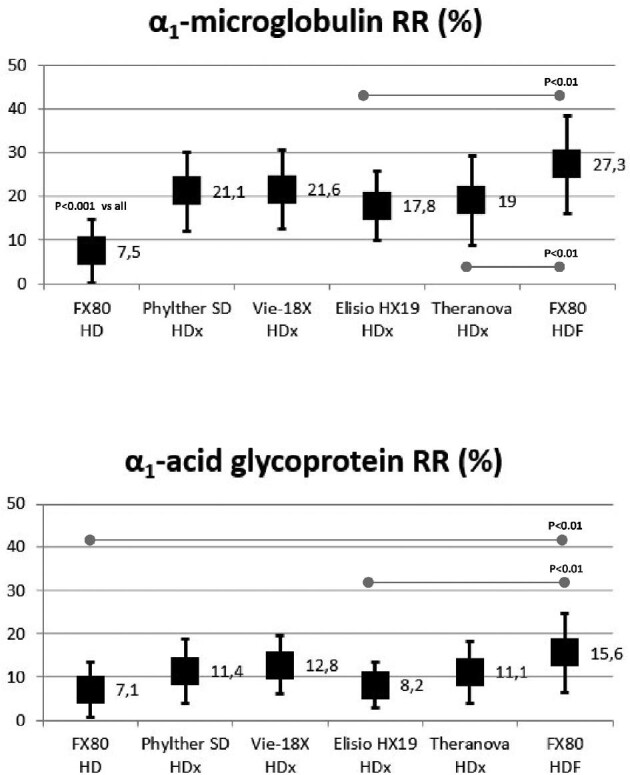
Comparison of α1-microglobulin and α1-acid glycoprotein RRs in all study situations (ANOVA for repeated data).
Free immunoglobulin light chain removal
The values of κFLC RRs varied between 60% and 85%, and the values of λFLC RR varied between 20% and 60%. There were no significant differences in the κFLC RR between the four HDx treatments. There were similar results in the λFLC RR between the four HDx treatments; only λFLC RR obtained with Phylther SD was slightly higher than Elisio HX. All κFLC and λFLC RR obtained with the four HDx treatments were significantly higher than those obtained with the HD treatment (Fig. 4). In addition, the κFLC and λFLC RR were also significantly higher with HDF than with HD and HDx treatments (Fig. 4).
FIGURE 4:
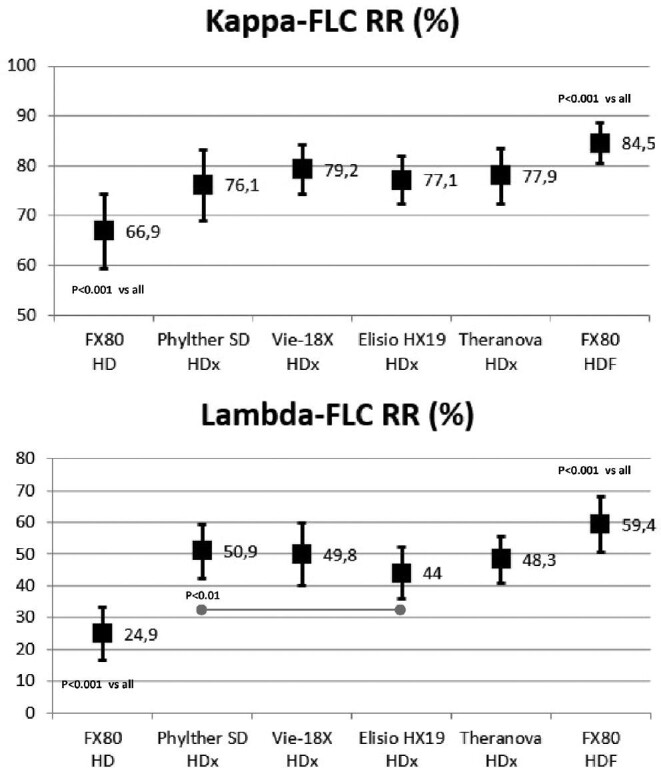
Comparison of κFLC and λFLC RRs in all study situations (ANOVA for repeated data).
Albumin loss in blood and dialysate
There were no significant differences in blood albumin RR in all the studied situations: 9.8 ± 4.7% with helixone in HD, 9.6 ± 5.2% with Phylther 17-SD in HDx, 9.5 ± 4.9% with Vie-18X in HDx, 8.7 ± 4.4% with Elisio HX19 in HDx, 9.0 ± 6.2% with Theranova in HDx and 10.4 ± 5.9% with FX80 in HDF.
The mean amount of dialysate albumin loss was <3 g in all situations, and significant differences were observed between the studied situations (Fig. 5). Albumin losses with HD were <1 g, with HDx were between 1.5 and 2.0 g, and were 2.5 g with HDF treatments (Fig. 5).
FIGURE 5:
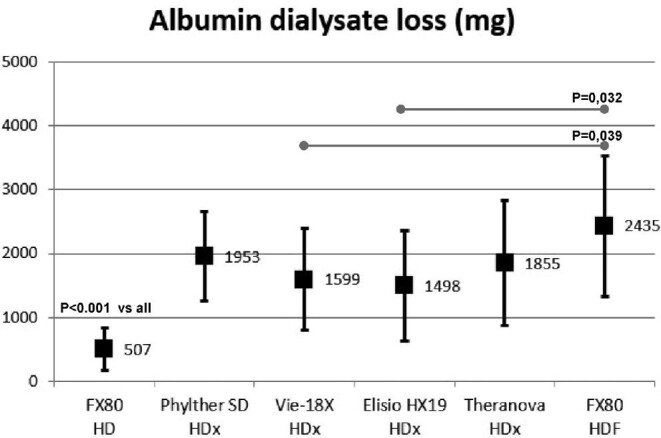
Comparison of dialysate albumin loss in all study situations (ANOVA for repeated data).
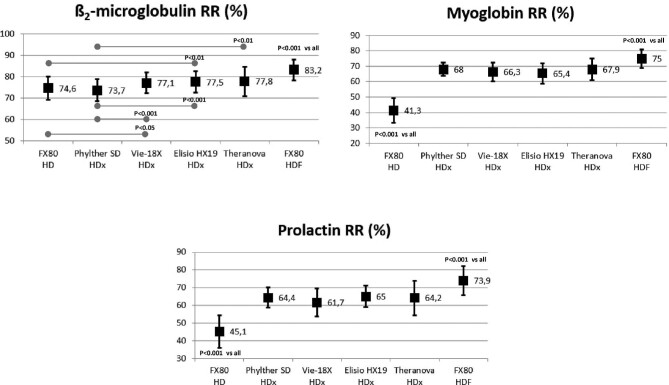
Global removal score
There were no differences between the four MCO dialyzers. The highest Global Removal Score (GRS) values were obtained in HDF treatment and were statistically superior to HD and HDx treatments. The GRS values with HDx treatments were statistically higher than those obtained with HD (Fig. 6).
FIGURE 6:
Comparison of GRSGRS in all study situations (ANOVA for repeated data).
DISCUSSION
The present study is, to our knowledge, the first to compare the efficiency and safety issues of four MCO dialyzers in HDx treatments and to confirm that all these dialyzers respond to the MCO membrane classification, with no significant performance differences between them. They all showed similar albumin RR in blood and dialysate albumin loss inferior to 3 g per dialysis session. Likewise, the study confirms the solute removal superiority with HDx over high-flux HD and an efficacy close to post-dilution HDF, showing HDx as the alternative that came closest to post-dilution HDF, as previously published in the literature [16–21], and reinforcing the importance of both the choice of the dialyzer and the treatment modality to obtain optimal performance.
Since 2017, based on the results published by Kirsch et al. [11] on the performance of different medium- and high cut-off membranes, the first MCO membrane (Theranova, Baxter) was marketed and incorporated into clinical practice increasing the therapeutic scenario in hemodialysis. During the following years, several studies were published showing that this MCO dialyzer could provide higher removal performance than that with high-flux HD [13, 18–21], and could be an alternative to HDF, since this specific HD, called expanded HD, could achieve similar efficacy to that achieved by HDF [1, 22–24]. Appreciating the importance that this new class of dialyzers could have in dialysis treatment, several pharmaceutical companies developed new MCO dialyzers, some of them obtaining in 2021 the CE mark (Asahi and Nipro), expanding the range of treatment possibilities. Finally, for more than 10 years, Medtronic already had a generation of polyphenylene dialyzers sterilized with steam, which retains the original size of the pores and therefore behaves like an MCO membrane [15].
The term expanded HD was proposed to define a treatment where diffusion and convection are conveniently combined inside a hollow-fiber dialyzer equipped with an MCO membrane [25]. This is due to the tailored cut-off of the membrane pores with an increased size and an adequate internal diameter that favor internal backfiltration, combined with an internal architecture that allows MCO membranes to achieve increased removal capacities for middle molecules and large middle molecules compared with standard HD treatments [2]. This new type of dialyzer has been called medium cut-off in Europe and super high-flux or sharp cut-off membrane in Japan [25–27]. Interest in this type of dialyzer has grown in recent years due to the efficacy and results obtained without an increase in safety issues, currently placing HDx, along with HDF, as the most effective options for dialysis treatment. This is the main reason why many of the pharmaceutical companies have or are developing them in order to incorporate these dialyzers into their portfolio.
At the current time, there are no clear and concise criteria to define whether a dialyzer pertains to the MCO group; however, they have some common aspects such as the enlarged pore size (something not usually shown in the technical issue) which determines the molecular weight retention onset and molecular weight cut-off sieving curves [9]. The internal architecture of the dialyzer assures a good combination between diffusion and convection. In fact, the optimization of the backfiltration of several dialyzers is not linked to the classic reduction of the internal diameter of the fibers as happens in the Theranova one (180 μm), but through keeping the inner diameter of the fibers and also lengthening the fibers, which particularly compensates backfiltration.
The differences in efficacy between the evaluated dialyzers were minimal in small molecules and even up to the size of β2-microglobulin. The differentiating profile was found in the 15 and 30 kDa molecules (myoglobin, prolactin and α1-microglobulin). In this study, all MCO dialyzers, with similar efficacy in GRS, were notably superior to high-flux HD treatments by around 60%, though 15% inferior to HDF treatments. These results confirm previously published studies [11–16, 18–21], establishing the current position of HDx as clearly superior to high-flux HD and very close to post-dilution HDF.
Both kappa and lambda light chains, 22 and 45 kDa, respectively, could be considered good differential markers of depurative efficacy, especially in the 40–45 kDa molecular weight range, where there are no clear differentiating markers. The RR of κFLC with all MCO dialyzers were 12%–15% superior to HD and 10% inferior to HDF treatments. However, the RR of λFLC with all MCO dialyzers were 90%–100% superior to HD and 10% inferior to HDF treatments. Kirsch et al. [11] found that both FLC RR were superior with MCO treatment than with high-flux HD and post-dilution HDF. Zickler et al. [28] and the a tRial Evaluating Mid cut-Off Value membrane clearance of Albumin and Light chains in HaemoDialysis patients study [29] reported that the MCO dialyzer effectively reduced κFLC and λFLC, compared with high-flux HD following 12 and 2 weeks of treatment, respectively.
The safety of MCO dialyzers is ensured by restricting pore sizes to limit albumin losses below 5 g per session [9, 10]. In this regard, most published studies report that MCO membranes lead to a higher albumin loss than HD and show inconsistent results compared with HDF [1, 2, 9, 13, 16, 24]. The mass of solute collected in the dialysate corresponds only to its elimination by diffusion and convection, obviating the adsorption one, which cannot be quantified routinely; however, to assess the overall loss of albumin (adsorption, diffusion and convection), the albumin RR in the blood was reported. In this study, all four dialyzers in HDx treatment maintained a safe behavior, with a global albumin RR of around 10% and a mean of dialysate albumin loss between 1.5 and 2.0 g per session—higher than HD treatments as has been described, but slightly lower compared with HDF (mean albumin loss 2.4 g). Although albumin loss could be considered clinically tolerable in all study treatments, it is necessary to mention that all MCO membranes should only be used in HD mode to avoid increased albumin losses with a progressive reduction in serum levels [30].
A limitation of the study is that the efficacy was assessed by removal of uremic toxins using RR calculation; however, there was no long-term clinical follow-up with these dialyzers. The advantage of assessing efficacy with the RR allows a final result to be obtained that includes the parameters of each dialyzer. The performance of dialysis therapy is usually evaluated by solute removal, and the quality of dialysis therapies should be assessed by clear outcome studies such as randomized control trials [27]. Other concerns would be that the differences in the surface area, the membrane thickness and the type of membrane influence the purifying efficacy; however, the differences in membrane surface area were <0.2 m2; moreover, in a previous study [31] no significant differences were found between Theranova 400 and 500 (1.7 vs 2.0 m2).
In conclusion, the results of the four MCO dialyzers evaluated in this study showed good efficiency with no significant performance differences between them. All four MCO dialyzers showed similar albumin RR in blood and clinically acceptable dialysate albumin loss. Likewise, the study confirms the superiority of treatment with HDx over high-flux HD and efficacy very close to that of post-dilution HDF. This study helps assess the increase in the availability of MCO dialyzers, which will likely increase even more in the coming years.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to express our gratitude to all participating patients, as well as to all the staff of the Dialysis Section of Hospital Clínic of Barcelona for their collaboration in this study and enthusiasm.
Contributor Information
Francisco Maduell, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
José Jesús Broseta, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
Diana Rodríguez-Espinosa, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
Jimena del Risco, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
Lida María Rodas, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
Marta Arias-Guillén, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
Manel Vera, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
Néstor Fontseré, Department of Nephrology, Hospital Clínic Barcelona, Barcelona, Spain.
Maria del Carmen Salgado, Department of Biochemistry, Hospital Clínic Barcelona, Barcelona, Spain.
Nayra Rico, Department of Biochemistry, Hospital Clínic Barcelona, Barcelona, Spain.
FUNDING
This work has received no private or public funding.
AUTHORS’ CONTRIBUTIONS
F.M. conceived the study. J.J.B., D.R.-E., J.R., LM.R., M.A.-G., M.V., N.F., M.C.S. and N.R. acquired the data. F.M. and J.J.B. analyzed the data, made the figures and drafted the paper. All authors have revised the drafts and approved the final one.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author, F.M., upon reasonable request.
CONFLICT OF INTEREST STATEMENT
The authors declare no financial support for the project. F.M. has received consultancy fees and lecture fees from Baxter, Fresenius Medical Care, Medtronic, Nipro, Toray and Vifor. The other authors declare no conflicts of interest.
REFERENCES
- 1. Maduell F, Rodas L, Broseta JJet al. Medium cut-off dialyzer versus eight hemodiafiltration dialyzers: comparison using a global removal score. Blood Purif 2019;48:167–74 [DOI] [PubMed] [Google Scholar]
- 2. Boscheti-de-Fierro A, Voigt M, Storr Met al. Extended characterization of a new class of membranes for blood purification: the high cut-off membranes. Int J Artif Organs 2013;36:455–63 [DOI] [PubMed] [Google Scholar]
- 3. Lindgren A, Fjellstedt E, Christensson A.. Comparison of hemodialysis using a medium cutoff dialyzer versus hemodiafiltration: a controlled cross-over study. Int J Nephrol Renovasc Dis 2020;13:273–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Maduell F, Moreso F, Pons Met al. ESHOL study group. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol 2013;24:487–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mostovaya IM, Blankestijn PJ, Bots MLet al. , on behalf of the EUDIAL group. Clinical evidence on hemodiafiltration: a systematic review and a meta-analysis. Semin Dial 2014;27:119–27 [DOI] [PubMed] [Google Scholar]
- 6. Mercadal L, Franck JE, Metzger Met al. Hemodiafiltration versus hemodialysis and survival in patients with ESRD: the French Renal Epidemiology and Information Network (REIN) registry. Am J Kidney Dis 2015;68:247–55 [DOI] [PubMed] [Google Scholar]
- 7. See EJ, Hedley J, Agar JWMet al. Patient survival on haemodiafiltration and haemodialysis: a cohort study using the Australia and New Zealand Dialysis and Transplant Registry. Nephrol Dial Transplant 2019;34:326–38 [DOI] [PubMed] [Google Scholar]
- 8. Maduell F. Hemodiafiltration versus conventional hemodialysis: should “conventional” be redefined? Semin Dial 2018;31:625–32 [DOI] [PubMed] [Google Scholar]
- 9. Boschetti-De-Fierro A, Voigt M, Storr Met al. MCO membranes: enhanced selectivity in high-flux class. Sci Rep 2015;5:18448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Potier J, Queffeulou G, Bouet J.. Are all dialyzers compatible with the convective volumes suggested for postdilution online hemodiafiltration? Int J Artif Ogans 2016;39:460–70 [DOI] [PubMed] [Google Scholar]
- 11. Kirsch AH, Lyko R, Nilsson LGet al. Performance of hemodialysis with novel medium cut-off dialyzers. Nephrol Dial Transplant 2017;32:165–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. García-Prieto A, Vega A, Linares Tet al. Evaluation of the efficacy of a medium cut-off dialyser and comparison with other high-flux dialysers in conventional haemodialysis and online haemodiafiltration. Clin Kidney J 2018;11: 742–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Belmouaz M, Bauwens M, Hauet Tet al. Comparison of the removal of uraemic toxins with medium cut-off and high-flux dialysers: a randomized clinical trial. Nephrol Dial Transplant 2020;35:328–35 [DOI] [PubMed] [Google Scholar]
- 14. Thammathiwat T, Tiranathanagul K, Limjariyakul Met al. Super high-flux hemodialysis provides comparable effectiveness with high-volume postdilution online hemodiafiltration in removing protein-bound and middle-molecule uremic toxins: a prospective cross-over randomized controlled trial. Ther Apher Dial 2021;25:73–81 [DOI] [PubMed] [Google Scholar]
- 15. Maduell F, Rodas L, Broseta Jet al. High permeability alternatives to current dialyzers performing both high-flux hemodialysis and postdilution online hemodiafiltration. Artif Organs 2019;43:1014–21 [DOI] [PubMed] [Google Scholar]
- 16. Cozzolino M, Magagnoli L, Ciceri Pet al. Effects of a medium cut-off (Theranova®) dialyser on haemodialysis patients: a prospective, cross-over study. Clin Kidney J 2019;14:382–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bergström J, Wehle B.. No change in corrected β2-microglobulin concentration after cuprophane hemodialysis. Lancet 1987;1:628–9 [DOI] [PubMed] [Google Scholar]
- 18. Weiner DE, Falzon L, Skoufos Let al. Efficacy and safety of expanded hemodialysis with the theranova 400 dialyzer: a randomized controlled trial. Clin J Am Soc Nephrol 2020;15:1310–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krishnasamy R, Hawley CM, Jardine MJet al. A trial evaluating mid cut-off value membrane clearance of albumin and light chains in hemodialysis patients: a safety device study. Blood Purif 2020;49:468–78 [DOI] [PubMed] [Google Scholar]
- 20. Sevinc M, Hasbal NB, Yilmaz Vet al. Comparison of circulating levels of uremic toxins in hemodialysis patients treated with medium cut-off membranes and high-flux membranes: Theranova in Sisli Hamidiye Etfal (THE SHE) randomized control study. Blood Purif 2020;49:733–42 [DOI] [PubMed] [Google Scholar]
- 21. Rambabova Bushljetik I, Trajceska L, Biljali Set al. Efficacy of medium cut-off dialyzer and comparison with standard high-flux hemodialysis. Blood Purif 2021;50:492–8 [DOI] [PubMed] [Google Scholar]
- 22. Belmouaz M, Diolez J, Bauwens Met al. Comparison of hemodialysis with medium cut-off dialyzer and on-line hemodiafiltration on the removal of small and middle-sized molecules. Clin Nephrol 2018;89:50–6 [PubMed] [Google Scholar]
- 23. Cordeiro ISF, Cordeiro L, Wagner CSet al. High-flux versus high-retention-onset membranes: in vivo small and middle molecules kinetics in convective dialysis modalities. Blood Purif 2020;49:8–15 [DOI] [PubMed] [Google Scholar]
- 24. Lindgren A, Fjellstedt E, Christensson A.. Comparison of hemodialysis using a medium cutoff dialyzer versus hemodiafiltration: a controlled cross-over study. Int J Nephrol Renovasc Dis 2020;13:273–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ronco C, Marchionna N, Brendolan Aet al. Expanded haemodialysis: from operational mechanism to clinical results. Nephrol Dial Transplant 2018;33Suppl3: iii41–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mineshima M. The past, present and future of the dialyzer. Contrib Nephrol 2015;185:8–14 [DOI] [PubMed] [Google Scholar]
- 27. Masakane I, Sakurai K.. Current approaches to middle molecule removal: room for innovation. Nephrol Dial Transplant 2018;33 (suppl_3): iii12–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zickler D, Schindler R, Willy Ket al. Medium cut-off (MCO) membranes reduce inflammation in chronic dialysis patients-A randomized controlled clinical trial. PLoS One 2017;12:e0169024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Krishnasamy R, Hawley CM, Jardine MJet al. A trial evaluating mid cut-off value membrane clearance of albumin and light chains in hemodialysis patients: a safety device study. Blood Purif 2020;49:468–78 [DOI] [PubMed] [Google Scholar]
- 30. Cuvelier C, Tintillier M, Migali Get al. Albumin losses during hemodiafiltration: all dialyzers are not created equal - a case report. BMC Nephrol 2019;20:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Maduell F, Rodasa L, Broseta JJ et al. Evaluation of the influence of the surface membrane and blood flow in medium «cut-off» (MCO) dialyzers. Nefrologia 2019;39:623–8. doi: 10.1016/j.nefro.2019.02.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, F.M., upon reasonable request.