ABSTRACT
Background
Whether fracture rates, overall and by fracture site, vary by cause of kidney failure in patients receiving dialysis is unknown.
Methods
Using the US Renal Data System, we compared fracture rates across seven causes of kidney failure in patients who started dialysis between 1997 and 2014. We computed unadjusted and multivariable adjusted proportional sub-distribution hazard models, with fracture events (overall, and by site) as the outcome and immunoglobulin A nephropathy as the reference group. Kidney transplantation and death were competing events.
Results
Among 491 496 individuals, with a median follow-up of 2.0 (25%, 75% range 0.9–3.9) years, 62 954 (12.8%) experienced at least one fracture. Patients with diabetic nephropathy, vasculitis or autosomal polycystic kidney disease (ADPKD) had the highest (50, 46 and 40 per 1000 person-years, respectively), and patient with lupus nephritis had the lowest (20 per 1000 person-years) fracture rates. After multivariable adjustment, diabetic nephropathy [hazard ratio (HR) 1.43, 95% confidence interval 1.33–1.53], ADPKD (HR 1.37, 1.26–1.48), vasculitis (HR 1.22, 1.09–1.34), membranous nephropathy (HR 1.16, 1.02–1.30) and focal segmental glomerulosclerosis (FSGS) (HR 1.13, 1.02–1.24) were associated with a significantly higher, and lupus nephritis with a significantly lower (HR 0.85, 0.71–0.98) fracture hazard. The hazards for upper extremity and lower leg fractures were significantly higher in diabetic nephropathy, ADPKD, FSGS and membranous nephropathy, while the hazard for vertebral fracture was significantly higher in vasculitis. Our findings were limited by the lack of data on medication use and whether fractures were traumatic or non-traumatic, among other factors.
Conclusions
Fracture risk, overall and by fracture site, varies by cause of end-stage kidney disease. Future work to determine underlying pathogenic mechanisms contributing to differential risks might inform more tailored treatment strategies. Our study was limited by lack of data regarding numerous potential confounders or mediators including medications and measures or bone biomarkers.
Keywords: dialysis, end-stage kidney disease, fracture
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Adults with kidney failure have multiple risk factors for bone fragility, including abnormal mineral and vitamin D metabolism, secondary hyperparathyroidism, malnutrition, decreased muscle strength, reduced physical activity [1] and use of psychoactive medications that may increase fall risk [2]. As a result, patients with advanced chronic kidney disease (CKD) experience significantly higher rates of fracture than patients with normal or near normal kidney function [3–7]. The overall incidence of fracture among patients receiving dialysis is estimated to be 17-fold higher than in the general population [8], with fractures occurring an average of 16 and 13 years earlier in men and women on dialysis as compared with men and women in the general population, respectively [9]. Further, fracture events are associated with a 2.5-fold higher mortality rate in individuals on dialysis compared with the general population [8].
Beyond factors common to CKD more generally, features unique to certain causes of kidney disease might differentially affect bone turnover or mineralization, including: systemic inflammation [10–12] in many types of kidney disease; exposure to corticosteroids [13, 14] or calcineurin inhibitors [15] in glomerular diseases; urinary loss of vitamin D in diseases associated with nephrotic-range proteinuria [16, 17]; and alterations in bone mechano-sensing from dysfunctional polycystin in autosomal dominant polycystic kidney disease (ADPKD) [18, 19]. Further, these disease-specific factors might differentially act on cortical (dense outer bone layer) or trabecular (porous central) bone, predisposing to increased fracture risk at cortical or trabecular rich sites [13, 20]. Finally, pace of kidney disease progression prior to starting dialysis, or age at the onset of kidney disease, can vary by cause of kidney failure, resulting in differing severities and durations of CKD mineral bone disorder (CKD-BMD) prior to dialysis initiation [5, 6].
Whether fracture rates, overall and by fracture site, differ by cause of kidney failure once patients start dialysis has not been the focus of any prior study. Identifying any such differences may inform understanding of underlying pathogenic mechanisms and identify high-risk patient groups to target for personalized medical management or enrollment in clinical trials. The objectives of this study were to: (i) determine rates of first fracture after starting dialysis, according to cause of kidney failure; (ii) identify associations between cause of kidney failure and fracture risk at specific sites (hip, lower femur, upper extremity, lower leg or vertebral); and (iii) evaluate whether any observed associations between cause of kidney failure and fracture risk attenuate as time from dialysis initiation extends.
MATERIALS AND METHODS
Study design and population
This retrospective cohort study was conducted using the United States Renal Data System (USRDS), an extensive database that contains records for virtually all patients who initiate dialysis or undergo kidney transplantation in the USA and incorporates Medicare Institutional (Part A) and Physician/Supplier (Part B) insurance claims for eligible patients [21]. We included all adult patients (≥18 years) who initiated hemodialysis or peritoneal dialysis in the USA between 1 January 1997 and 30 June 2014, with end-of study date 30 September 2015. The date 30 September 2015 was chosen for end of follow-up because starting 1 October 2015, coding switched to a different system [from International Classification of Diseases (ICD)-9 to ICD-10]. We were concerned that the change from ICD-9 to ICD-10 in the USA could contribute to coding errors or misclassification. We included patients with any of the following causes of kidney failure: focal segmental glomerulosclerosis (FSGS), immunoglobulin A (IgA) nephropathy, membranous nephropathy, lupus nephritis, vasculitis, diabetic nephropathy or ADPKD. Membranoproliferative glomerulonephritis was not included as a glomerular disease subgroup of interest due to the wide heterogeneity in this group, which would make study findings difficult to interpret. We excluded patients with recorded aged >100 years, missing sex, missing first kidney failure treatment modality, residence outside of the continental USA (i.e. Alaska, Hawaii or the American Territories), and those who had a pre-emptive kidney transplant prior to receiving maintenance dialysis. Finally, because we required Medicare insurance (for which most patients become eligible after receiving dialysis for 90 days) to capture fracture events using Medicare claims, we excluded patients who died, discontinued dialysis, received a kidney transplant, or did not have Medicare Parts A & B as their primary insurance by the 90th day after dialysis initiation.
Exposure and covariates
Cause of kidney failure (primary exposure of interest), demographic characteristics, comorbidities, laboratory values and the Quételet (body mass) index (BMI) were obtained from patient and medevid files derived from Centers for Medicare & Medicaid Services (CMS) Medical Evidence Reports (Form CMS-2728) submitted by nephrologists within 45 days of a patient starting dialysis. Selection of a glomerular disease subtype on the Medical Evidence Report was reported to have a high positive predictive value (>90%) but low sensitivity (≤30%) for detecting biopsy-confirmed glomerular disease diagnoses in a previous validation study [22]. The initial end-stage kidney disease (ESKD) treatment modality was obtained from the rxhist60 file. We included residential ZIP code as a proxy for patient-level socioeconomic status, using data from the US Census Bureau. We used Year 2000 Decennial Census data or American Community Survey (ACS) 5-year data (2007–11) for patients starting dialysis prior to or after 2006, respectively [23, 24].
We selected a set of covariates for model adjustment based on clinical significance. We considered temporal (year of dialysis initiation), demographic, socioeconomic and clinical factors, measured at baseline (day of dialysis initiation), that might confound the association between primary cause of kidney failure and fracture rates. Demographic factors included age, sex, race (Black, White, Asian, other), Hispanic ethnicity (yes/no), geographic region (Northeast, Midwest, South, West) and the following neighborhood-level socioeconomic factors: % unemployed among residents 16 years or older; % with less than a high-school education among residents aged 25 years or older; proportion living below the federal poverty line; median household income; and median rent. Clinical factors at dialysis initiation included: comorbidities (diabetes, heart failure, coronary heart disease, cerebrovascular disease, hypertension, chronic obstructive pulmonary disease, current or recent smoking, cancer, peripheral vascular disease, needing assistance with daily activities and inability to transfer), laboratory values [serum albumin, estimated glomerular filtration rate (eGFR) and hemoglobin], dialysis modality [hemodialysis or peritoneal dialysis] and BMI. We calculated eGFR from reported serum creatinine and demographics at dialysis initiation, using the 2012 CKD Epidemiology Collaboration equation [25].
Outcome variables
The primary endpoint was a composite of first hip, lower femur, vertebral, upper extremity or lower leg fracture, with follow-up time starting on Day 91 after dialysis initiation. Secondary endpoints included individual components of the composite outcome. We ascertained fracture events from Medicare Institutional (Part A) and Physician/Supplier (Part B) files and using ICD-9, Clinical Modification (ICD-9-CM) codes (Supplementary data, Table S1). Fractures were defined using different algorithms as different fracture types are treated differently and reflected differently in claims [26]. Fractures of the hip, femur, lower leg and upper extremity were identified if a corresponding diagnosis code appeared in an inpatient claim or both a diagnosis code and a procedure code appeared in the same line of a Part B physician claim. Hip and femur fractures were identified from a Part B claim and required the service site to be an inpatient hospitalization or emergency department visit. Vertebral fractures were identified if the diagnosis code appeared in an inpatient claim. We also required that no fracture claim for the same site could occur within 180 days of a previous claim.
We treated kidney transplantation and death as competing events. Censoring, for patients who had not yet experienced a fracture or a competing event, occurred at the first of: loss of Medicare A & B as primary insurance; survival to 5 years and 90 days after dialysis initiation; or end-of study (31 December 2014).
Statistical analyses
We summarized baseline characteristics, stratified by cause of kidney failure, using means with standard deviations or medians with 25%, 75% ranges for continuous variables and counts with proportions for categorical variables. We calculated unadjusted fracture event rates as the number of events per 1000 person-years. We computed age/sex and age/sex/race standardized rates, adjusted to the 2015 US population, using US Census Bureau 2015 American Community Survey 5-Year Estimates [27]. For lupus nephritis, no fractures were observed in non-White men aged 65–75 years, so we calculated age- and race-standardized fracture rates were for women instead of calculating age/sex/race standardized rates in the full group. We also calculated and plotted the cumulative incidence of fracture, death and kidney transplantation (i.e. primary outcome and competing events) following dialysis initiation by cause of ESKD.
To evaluate whether any between-group differences in fracture rate shortly after starting dialysis might dissipate the longer a patient remains on dialysis, we calculated first fracture incidence rates for each year after dialysis initiation among patients still at risk for experiencing a first fracture at the beginning of the year, i.e. persons who are still alive, without having experienced a censoring or competing event in the year(s) prior.
To account for the competing risks of kidney transplantation and death, we used proportional sub-distribution hazard models (Fine and Gray) to compute sub-distribution hazard ratios and 95% confidence intervals (CIs) [28]. The sub-distribution hazard is defined as the probability of the outcome event occurring at time t, given that the patient had not yet experienced an outcome event, but may have experienced a competing event (transplantation or death), up to time t. Thus, the sub-distribution hazard ratio (HR) can be used to directly answer the question of whether patients with comparator causes of ESKD are more likely to experience a fracture event when compared with those with the reference cause of ESKD [29]. IgA nephropathy was selected as the reference group, as we expected patients with IgA nephropathy to have a comparatively low fracture rate, as a result of infrequent/remote corticosteroid exposure and a lower comorbidity burden.
As a companion analysis, we censored patients at time of death or kidney transplantation and used Cox proportional hazards regression to obtain cause-specific HRs (unadjusted and fully adjusted models only). For both sub-distribution hazard and cause-specific hazard models, we stratified by year of dialysis initiation.
For all analyses, we added covariates to regression models sequentially. Model 1 (unadjusted model) included cause of ESKD, stratified by year. Model 2 added demographic and socioeconomic characteristics. Model 3 added comorbidities and, dialysis modality. Model 4 added laboratory values and Model 5 (fully adjusted model) added BMI. Model 6 included Model 1 adjusted for age and sex only.
We used log(-log) plots of 1 – cumulative incidence function and plots of Loess smoothed scaled Schoenfeld residuals to examine the proportional hazards assumption [29, 30].
Missing data
The frequency of missing covariate data ranged from <1% (e.g. Hispanic ethnicity) to 24% (serum albumin) and 32% of patients had at least one variable with missing data. Missing data were similarly distributed across all categories of the exposure variable. We assumed these data to be missing at random and used multiple imputation through the joint modeling approach to generate 33 imputed data sets [31]. Besides the event indicator and the Nelson–Aalen estimate of the cumulative hazard, the imputation model also included all variables in the fully adjusted model.
Statistical analyses were performed using a combination of SAS version 9.4 (SAS Institute, Inc., Cary, NC), R version 3.1.2, and Stata version 13.1 (StataCorp., 2013, Stata Statistical Software: Release 13, College Station, TX, USA).
A Stanford University School of Medicine Institutional Review Board (IRB) approved the study as minimal risk, as all data were de-identified (IRB protocol number 17 904).
RESULTS
Patient characteristics
Creation of the study cohort (n = 491 496) is summarized in Fig. 1. Patients with lupus nephritis were the youngest (mean age 42 ± 16 years) and had the highest proportion of women (81.2%) and persons identified as being of Black race (53.5%). In contrast, patients with vasculitis were the oldest (66 ± 16 years) and had the highest proportion of persons identified as being of White race (89.2%). Use of peritoneal dialysis as a first ESKD treatment modality was highest in IgA nephropathy (19.6%) and lowest in diabetic nephropathy (7.2%) and vasculitis (7.5%). Patients with IgA nephropathy, lupus nephritis and ADPKD had comparatively low comorbidity frequencies, whereas patients with diabetic nephropathy had the highest comorbidity burden, the highest mean BMI (29.8 ± 7.9 kg/m2) and were most likely to be non-ambulatory (6.8%) (Table 1).
Figure 1:
Cohort selection.
Table 1:
Baseline characteristics of US patients with ESKD attributed to glomerular disease, diabetic nephropathy or ADPKD, who initiated hemodialysis or peritoneal dialysis in the US 1997–2014.a
| IgAN | FSGS | Membranous nephropathy | Lupus nephritis | Vasculitis | Diabetic nephropathy | ADPKD | |
|---|---|---|---|---|---|---|---|
| (N = 5028) | (N = 16 555) | (N = 3860) | (N = 7743) | (N = 4964) | (N = 438 516) | (N = 14 830) | |
| Age, in years | |||||||
| <40 | 1672 (33.3) | 3979 (24.0) | 424 (11.0) | 3982 (51.4) | 390 (7.9) | 20 670 (4.7) | 1090 (7.3) |
| 40–59 | 1569 (31.2) | 4891 (29.5) | 1056 (27.4) | 2478 (32.0) | 953 (19.2) | 126 130 (28.8) | 6033 (40.7) |
| 60–69 | 779 (15.5) | 3006 (18.2) | 841 (21.8) | 699 (9.0) | 1171 (23.6) | 130 003 (29.6) | 3316 (22.4) |
| 70+ | 1008 (20.0) | 4679 (28.3) | 1539 (39.9) | 584 (7.5) | 2450 (49.4) | 161 713 (36.9) | 4391 (29.6) |
| Mean (SD) | 50.7 (18.5) | 55.5 (18.6) | 62.3 (15.9) | 42.0 (16.3) | 65.8 (15.6) | 64.2 (12.9) | 60.4 (14.1) |
| Median (25%, 75% range) | 49.2 (34.2, 67.2) | 57.2 (40.2, 71.2) | 66.2 (51.2, 74.2) | 39.2 (28.2, 52.2) | 69.2 (58.2, 77.2) | 65.2 (56.2, 73.2) | 60.2 (49.2, 71.2) |
| Gender | |||||||
| Female | 1686 (33.5) | 6311 (38.1) | 1355 (35.1) | 6289 (81.2) | 2414 (48.6) | 212 271 (48.4) | 6871 (46.3) |
| Male | 3342 (66.5) | 10 244 (61.9) | 2505 (64.9) | 1454 (18.8) | 2550 (51.4) | 226 245 (51.6) | 7959 (53.7) |
| Race | |||||||
| White | 3946 (78.5) | 10 110 (61.1) | 2707 (70.1) | 3258 (42.1) | 4429 (89.2) | 293 477 (66.9) | 12 077 (81.4) |
| Black | 424 (8.4) | 5965 (36.0) | 1043 (27.0) | 4144 (53.5) | 393 (7.9) | 124 020 (28.3) | 2381 (16.1) |
| Asian | 509 (10.1) | 306 (1.8) | 69 (1.8) | 234 (3.0) | 59 (1.2) | 10 305 (2.3) | 259 (1.7) |
| Other | 124 (2.5) | 119 (0.7) | 29 (0.8) | 76 (1.0) | 66 (1.3) | 8749 (2.0) | 83 (0.6) |
| Ethnicity | |||||||
| Non-Hispanic | 4372 (87.0) | 15 248 (92.1) | 3529 (91.4) | 6563 (84.8) | 4552 (91.7) | 367 553 (83.8) | 13 477 (90.9) |
| Hispanic | 629 (12.5) | 1241 (7.5) | 313 (8.1) | 1143 (14.8) | 395 (8.0) | 69 148 (15.8) | 1307 (8.8) |
| Missing | 27 (0.5) | 66 (0.4) | 18 (0.5) | 37 (0.5) | 17 (0.3) | 1815 (0.4) | 46 (0.3) |
| Region in the USA | |||||||
| North-east | 966 (19.2) | 3129 (18.9) | 775 (20.1) | 1184 (15.3) | 932 (18.8) | 70 608 (16.1) | 2744 (18.5) |
| Mid-west | 1130 (22.5) | 3904 (23.6) | 918 (23.8) | 1383 (17.9) | 1372 (27.6) | 95 356 (21.7) | 3274 (22.1) |
| South | 1765 (35.1) | 7096 (42.9) | 1646 (42.6) | 3955 (51.1) | 1825 (36.8) | 196 709 (44.9) | 6281 (42.4) |
| West | 1167 (23.2) | 2426 (14.7) | 521 (13.5) | 1221 (15.8) | 835 (16.8) | 75 843 (17.3) | 2531 (17.1) |
| Dialysis modality | |||||||
| Hemodialysis | 4044 (80.4) | 14 107 (85.2) | 3366 (87.2) | 6809 (87.9) | 4594 (92.5) | 406 745 (92.8) | 12 350 (83.3) |
| Peritoneal dialysis | 984 (19.6) | 2448 (14.8) | 494 (12.8) | 934 (12.1) | 370 (7.5) | 31 771 (7.2) | 2480 (16.7) |
| Comorbidities | |||||||
| Non-ambulant | 75 (1.5) | 331 (2.0) | 111 (2.9) | 188 (2.4) | 184 (3.7) | 29 932 (6.8) | 253 (1.7) |
| Atherosclerotic heart disease | 540 (10.7) | 2548 (15.4) | 686 (17.8) | 567 (7.3) | 747 (15.0) | 124 071 (28.3) | 1985 (13.4) |
| Cancer | 214 (4.3) | 1027 (6.2) | 287 (7.4) | 136 (1.8) | 299 (6.0) | 18 948 (4.3) | 734 (4.9) |
| Congestive heart failure | 675 (13.4) | 3087 (18.6) | 838 (21.7) | 1339 (17.3) | 931 (18.8) | 175 006 (39.9) | 1866 (12.6) |
| COPD | 256 (5.1) | 1258 (7.6) | 345 (8.9) | 242 (3.1) | 553 (11.1) | 37 863 (8.6) | 888 (6.0) |
| Cerebrovascular disease | 208 (4.1) | 948 (5.7) | 272 (7.0) | 481 (6.2) | 287 (5.8) | 49 656 (11.3) | 992 (6.7) |
| Diabetes mellitus | 673 (13.4) | 2650 (16.0) | 643 (16.7) | 725 (9.4) | 835 (16.8) | 376 105 (85.8) | 1504 (10.1) |
| Hypertension | 4252 (84.6) | 14 069 (85.0) | 3230 (83.7) | 6285 (81.2) | 3640 (73.3) | 369 967 (84.4) | 12 562 (84.7) |
| Previous or current smoking | 349 (6.9) | 1455 (8.8) | 305 (7.9) | 392 (5.1) | 253 (5.1) | 22 084 (5.0) | 1237 (8.3) |
| Peripheral vascular disease | 235 (4.7) | 1101 (6.7) | 275 (7.1) | 304 (3.9) | 394 (7.9) | 86 962 (19.8) | 775 (5.2) |
| Alcohol use | 91 (1.8) | 299 (1.8) | 54 (1.4) | 44 (0.6) | 47 (0.9) | 3636 (0.8) | 157 (1.1) |
| Amputation | 25 (0.5) | 51 (0.3) | 21 (0.5) | 28 (0.4) | 20 (0.4) | 13 360 (3.0) | 35 (0.2) |
| Inability to transfer | 30 (0.6) | 117 (0.7) | 39 (1.0) | 75 (1.0) | 70 (1.4) | 11 823 (2.7) | 89 (0.6) |
| Need assistance with daily activities | 94 (1.9) | 429 (2.6) | 137 (3.5) | 243 (3.1) | 291 (5.9) | 32 482 (7.4) | 315 (2.1) |
| Institutionalized (assisted living, nursing home, other) | 61 (1.2) | 268 (1.6) | 88 (2.3) | 106 (1.4) | 184 (3.7) | 21 084 (4.8) | 181 (1.2) |
| BMI, kg/m2 | |||||||
| Underweight (<18.5) | 169 (3.4) | 655 (4.0) | 141 (3.7) | 582 (7.5) | 254 (5.1) | 11 897 (2.7) | 695 (4.7) |
| Normal (18.5–25) | 1737 (34.5) | 5186 (31.3) | 1391 (36.0) | 3070 (39.6) | 2077 (41.8) | 113 912 (26.0) | 5675 (38.3) |
| Overweight (25–30) | 1520 (30.2) | 5835 (35.2) | 1064 (27.6) | 1944 (25.1) | 1109 (22.3) | 177 703 (40.5) | 3781 (25.5) |
| Obese (>30) | 1467 (29.2) | 4459 (26.9) | 1148 (29.7) | 1923 (24.8) | 1414 (28.5) | 124 236 (28.3) | 4229 (28.5) |
| BMI, mean (SD) | 28.0 (7.2) | 29.0 (8.2) | 27.6 (7.0) | 26.8 (7.6) | 26.5 (6.7) | 29.8 (7.9) | 27.1 (6.9) |
| BMI, median (25%, 75% range) | 26.6 (23.1, 31.4) | 27.3 (23.3, 32.9) | 26.2 (22.9, 30.7) | 25.2 (21.6, 30.2) | 25.3 (22.1, 29.4) | 28.4 (24.2, 33.8) | 25.8 (22.5, 30.3) |
| Missing BMI | 135 (2.7) | 420 (2.5) | 116 (3.0) | 224 (2.9) | 110 (2.2) | 10 768 (2.5) | 450 (3.0) |
| Laboratory values | |||||||
| eGFR, mL/min/1.73m2 | |||||||
| Mean (SD) | 7.6 (3.8) | 7.7 (3.9) | 8.2 (4.2) | 8.3 (4.4) | 7.7 (4.1) | 9.4 (4.4) | 7.6 (3.5) |
| Median (25%, 75% range) | 6.9 (5.0, 9.6) | 7.0 (5.0, 9.6) | 7.5 (5.3, 10.1) | 7.4 (5.3, 10.3) | 6.9 (4.9, 9.6) | 8.6 (6.3, 11.6) | 7.0 (5.1, 9.4) |
| Missing | 46 (0.9) | 144 (0.9) | 52 (1.3) | 107 (1.4) | 46 (0.9) | 5440 (1.2) | 85 (0.6) |
| Hemoglobin, g/dL | |||||||
| Mean (SD) | 9.8 (1.8) | 10.0 (1.8) | 10.0 (1.8) | 9.3 (1.8) | 9.6 (1.7) | 9.8 (1.6) | 10.3 (1.9) |
| Median (25%, 75% range) | 9.9 (8.7, 11.0) | 10.0 (8.8, 11.1) | 9.9 (8.8, 11.1) | 9.2 (8.1, 10.4) | 9.5 (8.5, 10.6) | 9.7 (8.7, 10.8) | 10.3 (9.1, 11.5) |
| Missing | 559 (11.1) | 1828 (11.0) | 413 (10.7) | 827 (10.7) | 529 (10.7) | 46 492 (10.6) | 1723 (11.6) |
| Albumin, g/dL | |||||||
| Mean (SD) | 3.3 (0.7) | 3.2 (0.8) | 2.8 (0.8) | 2.9 (0.8) | 3.0 (0.7) | 3.1 (0.7) | 3.6 (0.6) |
| Median (25%, 75% range) | 3.4 (2.9, 3.8) | 3.3 (2.8, 3.8) | 2.9 (2.2, 3.5) | 2.9 (2.3, 3.4) | 3.0 (2.5, 3.4) | 3.1 (2.6, 3.5) | 3.7 (3.3, 4.1) |
| Missing | 1200 (23.9) | 3974 (24.0) | 881 (22.8) | 1881 (24.3) | 1185 (23.9) | 113 905 (26.0) | 3670 (24.7) |
| Neighborhood-level socioeconomics | |||||||
| % Unemployment | |||||||
| Mean (SD) | 7.8 (4.7) | 8.3 (4.8) | 8.1 (4.6) | 9.2 (5.0) | 7.5 (4.2) | 8.7 (4.9) | 7.8 (4.6) |
| Median (25%, 75% range) | 7.0 (4.7, 9.8) | 7.3 (4.9, 10.6) | 7.1 (4.9, 10.2) | 8.2 (5.6, 11.8) | 6.7 (4.5, 9.5) | 7.9 (5.3, 11.1) | 6.9 (4.6, 9.8) |
| % Below poverty | |||||||
| Mean (SD) | 14.5 (9.3) | 15.8 (10.0) | 15.2 (9.7) | 18.1 (10.4) | 13.5 (8.7) | 17.0 (10.3) | 14.5 (9.2) |
| Median (25%, 75% range) | 12.5 (7.6, 19.1) | 13.7 (8.1, 21.3) | 13.2 (7.7, 20.5) | 16.5 (9.8, 24.5) | 11.7 (6.9, 18.0) | 15.2 (9.1, 22.8) | 12.6 (7.5, 19.3) |
| % Below high school | |||||||
| Mean (SD) | 18.8 (11.4) | 19.3 (11.0) | 19.2 (11.0) | 22.1 (12.0) | 17.0 (10.1) | 21.8 (12.4) | 18.6 (11.2) |
| Median (25%, 75% range) | 16.3 (10.4, 25.0) | 17.4 (10.8, 25.8) | 17.4 (10.8, 25.3) | 20.2 (12.8, 29.3) | 15.0 (9.7, 22.3) | 19.5 (12.5, 28.8) | 16.5 (10.3, 24.7) |
| Median rent, US$ | |||||||
| Mean (SD) | 700.5 (329.9) | 666.6 (313.3) | 661.1 (313.3) | 667.7 (304.0) | 692.5 (320.3) | 656.8 (309.8) | 676.4 (318.1) |
| Median (25%, 75% range) | 657.0 (465.0, 877.0) | 627.0 (432.0, 837.0) | 622.0 (424.0, 831.0) | 635.0 (442.0, 837.0) | 648.0 (468.0, 866.0) | 620.0 (425.0, 825.0) | 637.0 (442.0, 849.0) |
| Missing | 37 (0.7) | 95 (0.6) | 26 (0.7) | 38 (0.5) | 43 (0.9) | 2891 (0.7) | 114 (0.8) |
| Median household income, US$ | |||||||
| Mean (SD) | 47 420.9 (18 498.6) | 45 038.3 (18 650.8) | 45 344.8 (18 526.4) | 42 511.2 (17 184.0) | 48 487.4 (18 805.8) | 43 516.4 (17 569.1) | 46 796.1 (18 946.5) |
| Median (25%, 75% range) | 43 750.0 (34 923.0, 56 188.0) | 41 012.0 (32 393.0, 53 333.0) | 41 119.5 (32 967.0, 53 315.0) | 39 040.0 (30 774.0, 50 322.0) | 44 300.0 (35 647.0, 57 140.0) | 39 818.0 (31 561.0, 51 258.0) | 42 458.0 (33 934.0, 55 088.0) |
aAnd remained on hemodialysis or peritoneal dialysis, with Medicare A & B as primary insurance, at study baseline (91 days after dialysis initiation). All values represent number (%) unless otherwise stated. COPD, chronic obstructive pulmonary disease; IgAN, immunoglobulin A nephropathy.
Unadjusted fracture rates, by cause of kidney failure
Overall, 62 954 (12.8%) patients experienced at least one fracture over a median follow-up of 2.0 (25%, 75% range 0.9–3.9) years, while 15 528 (3%) had two fractures and 3884 (0.8%) had three or more fractures (Table 2). Patients with lupus nephritis had the lowest fracture rate, both overall (20 per 1000 persons per year) and at individual fracture sites (Table 2). At the other end of the spectrum, patients with diabetic nephropathy or vasculitis had the highest fracture rates (50 and 46 per 1000 persons per year, respectively). Examining individual fracture sites, patients with diabetic nephropathy had the highest rates of lower femur and upper extremity or lower leg fractures (43 and 24 per 1000 persons per year, respectively) while those with vasculitis had the highest rates of hip and vertebral fractures (28 and 10 per 1000 persons per year, respectively).
Table 2:
Fracture event starting 90 days after dialysis initiation, among US patients with ESKD attributed to glomerular disease, diabetic nephropathy or ADPKD.
| Number of events | Years at risk | Event rate (per 1000 person years) | Age and sex standardized event rate (per 1000 persons per year) | Age, sex and race standardized event rate (per 1000 persons per year) | |
|---|---|---|---|---|---|
| Composite (any) fracture | |||||
| IgA nephropathy | 390 | 14 794 | 26 | 26 | 27 |
| FSGS | 1500 | 55 567 | 27 | 23 | 25 |
| Membranous nephropathy | 397 | 12 460 | 32 | 21 | 31 |
| Lupus nephritis | 520 | 26 123 | 20 | 25 | N/Aa |
| Vasculitis | 637 | 13 786 | 46 | 27 | 25 |
| Diabetic nephropathy | 57 607 | 1 162 982 | 50 | 41 | 47 |
| ADPKD | 1903 | 48 131 | 40 | 31 | 31 |
| Hip fracture | |||||
| IgA nephropathy | 205 | 15 226 | 13 | 10 | 11 |
| FSGS | 763 | 57 260 | 13 | 13 | 13 |
| Membranous nephropathy | 240 | 12 821 | 19 | 11 | 13 |
| Lupus nephritis | 213 | 26 848 | 8 | 11 | N/Aa |
| Vasculitis | 394 | 14 274 | 28 | 13 | 13 |
| Diabetic nephropathy | 26 623 | 1 226 930 | 22 | 14 | 17 |
| ADPKD | 965 | 50 522 | 19 | 13 | 13 |
| Lower femur fracture | |||||
| IgA nephropathy | 25 | 15 554 | 16 | 2 | 2 |
| FSGS | 90 | 58 300 | 15 | 1 | 1 |
| Membranous nephropathy | 27 | 13 162 | 20 | 1 | 1 |
| Lupus nephritis | 35 | 27 195 | 13 | 1 | N/Aa |
| Vasculitis | 28 | 14 949 | 19 | 1 | 1 |
| Diabetic nephropathy | 5366 | 1 258 173 | 43 | 4 | 5 |
| ADPKD | 83 | 52 080 | 16 | 1 | 1 |
| Upper extremity or lower leg fracture | |||||
| IgA nephropathy | 175 | 15 154 | 12 | 12 | 12 |
| FSGS | 702 | 56 778 | 12 | 12 | 13 |
| Membranous nephropathy | 165 | 12 828 | 13 | 10 | 18 |
| Lupus nephritis | 267 | 26 636 | 10 | 11 | N/Aa |
| Vasculitis | 212 | 14 534 | 15 | 11 | 20 |
| Diabetic nephropathy | 28 785 | 1 206 674 | 24 | 23 | 26 |
| ADPKD | 1029 | 49 697 | 21 | 18 | 18 |
| Vertebral fracture | |||||
| IgA nephropathy | 70 | 15 478 | 5 | 4 | 4 |
| FSGS | 217 | 58 164 | 4 | 3 | 3 |
| Membranous nephropathy | 61 | 13 107 | 5 | 3 | 4 |
| Lupus nephritis | 84 | 27 137 | 3 | 5 | N/Aa |
| Vasculitis | 143 | 14 789 | 10 | 5 | 4 |
| Diabetic nephropathy | 7379 | 1 256 187 | 6 | 4 | 5 |
| ADPKD | 302 | 51 718 | 6 | 4 | 4 |
aAge/sex/race standardized rates not calculable for lupus nephritis. Among women with lupus nephritis, the age and race-standardized rates were 13, 13, 1, 14 and 4 per 1000 persons per year for composite, hip, lower femur, extremity (upper extremity or lower leg) and vertebral fractures, respectively.
Highest and lowest event rate per fracture outcome in bold.
Supplementary data, Fig. S1 displays the cumulative incidences of fracture and the competing events of death and kidney transplantation following dialysis initiation, by cause of kidney failure.
Age-, sex- and race-standardized fracture rates, by cause of kidney failure
After adjusting for age and sex, the composite fracture rate was lowest in membranous nephropathy (21 events per 1000 persons per year), remained highest in diabetic nephropathy (41 events per 1000 persons per year) and was now second highest in ADPKD (31 events per 1000 persons per year). With further adjustment for race (age/sex/race-adjusted rates not calculable for lupus nephritis), the composite fracture rate was lowest in vasculitis or FSGS (25 events per 1000 persons per year), highest in diabetic nephropathy (47 events per 1000 persons per year), and second highest in ADPKD or membranous nephropathy (31 events per 1000 persons per year).
Multivariable-adjusted fracture rates, by cause of kidney failure
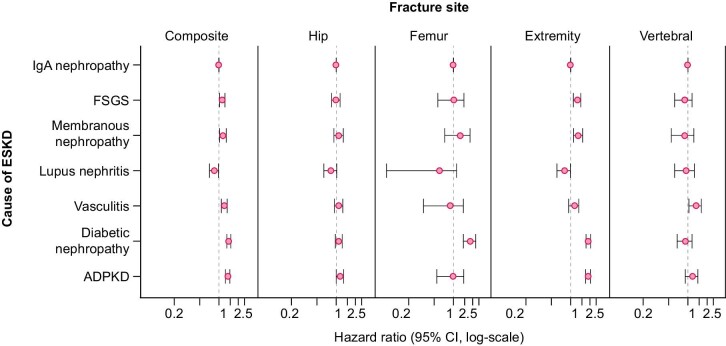
Comparing fracture hazards in IgA nephropathy (reference group) with the six comparator causes of kidney failure, adjusted hazards (Model 5) for the composite fracture outcome were significantly higher in patients with diabetic nephropathy (HR 1.43, 95% CI 1.33–1.53), ADPKD (HR 1.37, 1.26–1.48), vasculitis (HR 1.22, 1.09–1.34), membranous nephropathy (HR 1.16, 1.02–1.30) or FSGS (HR 1.13, 1.02–1.24) and were significantly lower in lupus nephritis (HR 0.85, 95% CI 0.71–0.98) (Tables 3, Fig. 2). Because standardized fracture rates for lupus nephritis could only be adjusted for age and sex, Model 6 was added and showed similar findings to other models.
Table 3:
Sub-distribution HRs (95% CI) for fracture risk starting 90 days after dialysis initiation in US patients with ESKD attributed to one of five glomerular diseases, diabetic nephropathy or ADPKD compared with IgA nephropathy.
| IgA nephropathy | FSGS | Membranous nephropathy | Lupus nephritis | Vasculitis | Diabetic nephropathy | ADPKD | |
|---|---|---|---|---|---|---|---|
| Composite (any) fracture | |||||||
| Model 1 | ref | 1.13 (1.02–1.24) | 1.27 (1.13–1.41) | 0.84 (0.71–0.97) | 1.65 (1.53–1.78) | 1.66 (1.56–1.76) | 1.63 (1.52–1.73) |
| Model 2 | ref | 1.14 (1.03–1.25) | 1.18 (1.04–1.32) | 0.88 (0.75–1.01) | 1.26 (1.13–1.38) | 1.44 (1.34–1.54) | 1.39 (1.28–1.49) |
| Model 3 | ref | 1.13 (1.02–1.24) | 1.17 (1.03–1.31) | 0.87 (0.74–1.01) | 1.23 (1.10–1.35) | 1.44 (1.33–1.54) | 1.37 (1.27–1.48) |
| Model 4 | ref | 1.13 (1.02–1.24) | 1.16 (1.02–1.29) | 0.86 (0.73–0.99) | 1.22 (1.10–1.35) | 1.41 (1.31–1.51) | 1.38 (1.27–1.49) |
| Model 5 | ref | 1.13 (1.02–1.24) | 1.16 (1.02–1.30) | 0.85 (0.71–0.98) | 1.22 (1.09–1.34) | 1.43 (1.33–1.53) | 1.37 (1.26–1.48) |
| Model 6 | ref | 1.13 (1.02–1.24) | 1.19 (1.06–1.33) | 0.88 (0.76–1.02) | 1.28 (1.15–1.40) | 1.41 (1.31–1.51) | 1.39 (1.28–1.50) |
| Hip fracture | |||||||
| Model 1 | ref | 1.08 (0.93–1.24) | 1.45 (1.27–1.64) | 0.65 (0.46–0.84) | 1.94 (1.77–2.11) | 1.43 (1.29–1.56) | 1.54 (1.39–1.69) |
| Model 2 | ref | 1.00 (0.85–1.16) | 1.14 (0.95–1.32) | 0.92 (0.73–1.11) | 1.20 (1.03–1.36) | 1.06 (0.93–1.20) | 1.19 (1.04–1.34) |
| Model 3 | ref | 0.99 (0.84–1.15) | 1.12 (0.94–1.31) | 0.92 (0.73–1.11) | 1.16 (0.99–1.33) | 1.06 (0.92–1.20) | 1.18 (1.03–1.32) |
| Model 4 | ref | 0.99 (0.83–1.14) | 1.09 (0.90–1.28) | 0.90 (0.71–1.09) | 1.15 (0.98–1.31) | 1.04 (0.90–1.18) | 1.19 (1.04–1.34) |
| Model 5 | ref | 1.00 (0.85–1.15) | 1.10 (0.92–1.29) | 0.83 (0.64–1.02) | 1.10 (0.94–1.27) | 1.10 (0.97–1.24) | 1.15 (1.00–1.30) |
| Model 6 | ref | 0.99 (0.85–1.15) | 1.05 (0.86–1.23) | 0.78 (0.59–0.97) | 1.20 (1.04–1.37) | 1.06 (0.92–1.20) | 1.16 (1.01–1.31) |
| Lower femur fracture | |||||||
| Model 1 | ref | 1.04 (0.60–1.49) | 1.32 (0.78–1.87) | 0.89 (0.37–1.40) | 1.09 (0.55–1.63) | 2.33 (1.93–2.72) | 1.08 (0.63–1.52) |
| Model 2 | ref | 1.05 (0.60–1.49) | 1.38 (0.83–1.92) | 0.63 (0.12–1.14) | 0.95 (0.41–1.49) | 2.13 (1.74–2.53) | 0.97 (0.52–1.41) |
| Model 3 | ref | 1.04 (0.60–1.49) | 1.36 (0.82–1.91) | 0.62 (0.10–1.13) | 0.91 (0.37–1.45) | 1.99 (1.59–2.39) | 0.97 (0.52–1.42) |
| Model 4 | ref | 1.03 (0.58–1.47) | 1.29 (0.74–1.83) | 0.59 (0.07–1.10) | 0.88 (0.34–1.42) | 1.89 (1.49–2.29) | 0.99 (0.54–1.44) |
| Model 5 | ref | 1.01 (0.57–1.46) | 1.28 (0.73–1.82) | 0.61 (0.09–1.12) | 0.89 (0.34–1.43) | 1.83 (1.43–2.23) | 1.00 (0.55–1.45) |
| Model 6 | ref | 1.04 (0.59–1.48) | 1.36 (0.82–1.90) | 0.68 (0.17–1.19) | 1.01 (0.47–1.55) | 2.14 (1.74–2.53) | 0.99 (0.55–1.44) |
| Upper extremity or lower leg fracture | |||||||
| Model 1 | ref | 1.17 (1.01–1.34) | 1.16 (0.95–1.38) | 0.97 (0.78–1.16) | 1.18 (0.98–1.38) | 1.82 (1.67–1.96) | 1.95 (1.79–2.11) |
| Model 2 | ref | 1.28 (1.11–1.44) | 1.32 (1.11–1.53) | 0.77 (0.58–0.96) | 1.32 (1.11–1.53) | 1.92 (1.77–2.07) | 1.89 (1.73–2.05) |
| Model 3 | ref | 1.28 (1.11–1.44) | 1.31 (1.10–1.52) | 0.78 (0.59–0.97) | 1.11 (0.91–1.31) | 1.93 (1.78–2.08) | 1.87 (1.71–2.03) |
| Model 4 | ref | 1.28 (1.11–1.45) | 1.33 (1.12–1.54) | 0.78 (0.59–0.97) | 1.13 (0.93–1.33) | 1.93 (1.78–2.08) | 1.86 (1.70–2.02) |
| Model 5 | ref | 1.27 (1.10–1.44) | 1.32 (1.11–1.54) | 0.80 (0.61–0.99) | 1.14 (0.93–1.34) | 1.90 (1.74–2.05) | 1.87 (1.71–2.03) |
| Model 6 | Ref | 1.26 (1.10–1.33) | 1.33 (1.12–1.45) | 0.76 (0.57–0.95) | 1.26 (1.06–1.46) | 1.87 (1.72–2.02) | 1.88 (1.72–2.04) |
| Vertebral fracture | |||||||
| Model 1 | ref | 0.91 (0.64–1.18) | 1.08 (0.74–1.42) | 0.77 (0.45–1.08) | 1.99 (1.71–2.28) | 1.15 (0.91–1.38) | 1.42 (1.16–1.68) |
| Model 2 | ref | 0.90 (0.63–1.17) | 0.93 (0.59–1.28) | 0.99 (0.68–1.31) | 1.39 (1.10–1.67) | 0.94 (0.70–1.18) | 1.16 (0.90–1.43) |
| Model 3 | ref | 0.90 (0.63–1.17) | 0.92 (0.58–1.27) | 0.99 (0.67–1.31) | 1.36 (1.07–1.64) | 0.94 (0.70–1.18) | 1.16 (0.90–1.42) |
| Model 4 | ref | 0.89 (0.62–1.16) | 0.96 (0.64–1.28) | 0.89 (0.55–1.24) | 1.34 (1.05–1.63) | 0.91 (0.67–1.15) | 1.18 (0.92–1.44) |
| Model 5 | ref | 0.89 (0.62–1.16) | 0.90 (0.55–1.24) | 0.94 (0.62–1.26) | 1.33 (1.04–1.62) | 0.92 (0.68–1.16) | 1.17 (0.91–1.43) |
| Model 6 | Ref | 0.90 (0.63–1.17) | 0.94 (0.60–1.29) | 0.92 (0.61–1.24) | 1.47 (1.18–1.76) | 0.94 (0.70–1.17) | 1.22 (0.96–1.48) |
Model 1: Cause of ESKD.
Model 2: Model 1 + demographic and socioeconomic characteristics, i.e. age, gender, race, Hispanic, region, socioeconomic status.
Model 3: Model 2 + clinical characteristics, i.e. comorbidities and dialysis modality.
Model 4: Model 3 + laboratory values, i.e. eGFR, hemoglobin and albumin.
Model 5: Model 4 + BMI.
Model 6: Model 1 + age, gender.
All models stratified by incidence year of ESKD.
Significant findings in bold (p < 0.05).
Figure 2:
Adjusted HRs for fracture risk starting 90 days after dialysis initiation in US patients with ESKD attributed to one of five glomerular diseases, diabetic nephropathy or ADPKD compared with IgA nephropathy.
Site-specific fracture rates, by cause of kidney failure
We observed no significant differences in hip fracture rates according to cause of kidney failure. In fully adjusted models (Table 3), patients with diabetic nephropathy were the only group with a significantly higher hazard for lower femur fractures (HR 1.83, 95% CI 1.43–2.23), while those with vasculitis were the only group with a significantly higher hazard for vertebral fractures (HR 1.33, 95% CI 1.04–1.62). Multiple comparator groups had higher hazards for upper extremity or lower leg fractures, including: diabetic nephropathy (HR 1.90, 95% CI 1.74–2.05), ADPKD (1.87, 1.71–2.03), membranous nephropathy (1.32, 1.11–1.54) and FSGS (1.27, 1.10–1.44). At the other end of the spectrum, patients with lupus nephritis had numerically lower adjusted hazards for fracture at all individual sites but the difference was only statistically significant for upper extremity or lower leg fractures (HR 0.80, 95% CI 0.61–0.99).
Annual fracture rates after dialysis initiation, by cause of kidney failure
Sex- and age-adjusted fracture rates according to time since dialysis initiation are depicted in Fig. 3. In general, fracture rates appear to increase with longer dialysis vintage. Diabetic nephropathy was associated with the highest fracture rate at all time points, including in the first year following dialysis initiation; in contrast, fracture rates in ADPKD were comparatively low in the first year following dialysis initiation and increased rapidly over time such that rates are almost as high as in diabetic nephropathy by the fourth year following dialysis initiation. Finally, rates in vasculitis and lupus nephritis were comparatively high in the first year following dialysis initiation, lagging only behind diabetic nephropathy, and remained relatively stable over time.
Figure 3:
Age- and sex- adjusted incidence rates (per 1000 person years) of first fracture for each year after dialysis initiation (starting 90 days after dialysis initiation) by cause of kidney failure.
Sensitivity analyses
In general, cause-specific HRs were similar to sub-distribution HRs, indicating that the competing risks of death and transplantation did not materially influence the association between cause of kidney failure and fracture (Supplementary data, Table S2).
DISCUSSION
In this cohort study of 491 496 patients who initiated dialysis in the USA between 1997 and 2014, rates of first fracture after starting dialysis varied significantly by cause of kidney failure, even after adjusting for between-group differences in sociodemographic and clinical characteristics. Patients with diabetic nephropathy or ADPKD had 43% and 37% higher fracture hazards, respectively, when compared with patients with IgA nephropathy, while risk was lowest in lupus nephritis.
Focusing on individual fracture sites, vasculitis was associated with a significantly higher hazard for vertebral fractures and diabetic nephropathy was associated with a significantly higher hazard for lower femoral fractures; we found no significant associations between cause of kidney failure and hip fracture risk. Extremity (upper extremity or lower leg) fracture hazards showed the largest variability by cause of kidney failure and were significantly higher in diabetic nephropathy, membranous nephropathy, FSGS and ADPKD, and significantly lower in lupus nephritis, when compared with IgA nephropathy.
Patients on dialysis are a heterogeneous population, differing not only by cause of kidney failure, but also by duration of kidney disease prior to kidney failure, prior and current drug exposures, comorbidities and functional status. We hypothesized that differences in these factors would contribute to differential fracture rates according to cause of kidney failure. We were not surprised to identify diabetic nephropathy as being strongly associated with higher fracture risk, even after multivariable adjustment for available sociodemographic and clinical factors. Diabetes is a well-recognized risk factor for fracture, even in the absence of overt kidney disease, with proposed mechanisms including direct effects of hyperglycemia on bone health, visual impairment, autonomic neuropathy, and impaired ambulation due to peripheral vascular disease or peripheral neuropathy [19]. The higher fracture rates observed in ADPKD were more surprising. However, pre-clinical studies have suggested that bone defects can result from loss of polycystin 1 and polycystin 2, and a recent report identified impaired bone formation even early in the disease process in patients with ADPKD [32].
We also hypothesized that cause of kidney failure might differentially influence fracture risk at specific sites. Given that corticosteroids are known to preferentially affect trabecular bone [33], we hypothesized that diseases commonly treated with corticosteroids might be associated with higher fracture rates at trabecular-rich sites, i.e. vertebral and hip fractures. Our finding of a higher hazard for vertebral fractures uniquely among patients with vasculitis might support this hypothesis, as patients with rapidly progressive glomerulonephritis or systemic vasculitis might be most likely to have had recent or current exposure to corticosteroids at the time of dialysis initiation. Our finding that patients with vasculitis or lupus nephritis had comparatively high fracture rates, that lagged only behind rates in diabetic nephropathy, in the first year after dialysis initiation might also support a role for recent corticosteroid exposure contributing to fracture risk in these disease groups. Conversely, secondary hyperparathyroidism results in disproportionate loss of cortical bone [34, 35]. We determined that patients with diabetic nephropathy or ADPKD experienced the highest overall rates of fracture, particularly at cortical-rich sites (upper extremity and lower leg), which we hypothesize might be explained by long-standing exposure to hyperparathyroidism in the setting of kidney disease that is typically slowly progressive.
Our finding that patients with lupus nephritis had the lowest overall and site-specific fracture rates, even after multivariable adjustment, were somewhat unexpected, especially considering that patients with lupus nephritis are known to have higher fracture rates when compared with the general population [36]. While we cannot explain this finding, we wonder whether it might reflect different prescription patterns of bone-protective medications and overall bone care in younger patients exposed to glucocorticoids. Alternatively, incomplete adjustment for unmeasured confounding factors including physical activity may be more relevant in this younger patient group.
This study has several limitations. First, data regarding numerous potential confounders or mediators of our observed association between cause of kidney failure and fracture risk are not routinely available in USRDS data, including: levels of phosphate, parathyroid hormone, and other parameters of bone and mineral metabolism; current or prior exposure to medications including corticosteroids or bone-protective agents; and measures of disease activity (e.g. hemoglobin A1C, markers of active lupus or vasculitis, markers of active nephrosis). Second, we lacked data on duration of kidney disease prior to dialysis initiation. Third, we lacked measurements of muscle function, balance or other determinants of falls, which in turn lead to fractures (although we were able to adjust for assistance with daily activities and ability to transfer). Accordingly, a causal relation between cause of kidney failure and fracture risk cannot be assumed, nor can the relative contribution from disease- and treatment-related factors to observed findings be explained. Fourth, data on high energy (traumatic) versus low energy (non-traumatic) fractures were not available in our cohort. Fifth, few validation studies have assessed fracture diagnoses using administrative claims data. Vertebral fractures in particular are often under diagnosed, especially at the time of incident fracture.
Nevertheless, this study also has important strengths. It is the first study, to our knowledge, to compare fracture rates across cause of kidney failure groups, and in doing so challenges the current research and public health reporting paradigm to group all causes of kidney failure—or at least all glomerular causes of kidney failure—together when reporting fracture risks in kidney failure populations. This study also has important clinical and research implications, and supports individualized approaches to fracture prevention strategies that consider cause of kidney failure as an important fracture risk determinant. Finally, as a population-based study, findings are broadly applicable to all patients receiving dialysis in the USA.
In summary, cause of kidney failure should be considered when evaluating fracture risk in patients receiving dialysis. Findings from this study can inform the counseling of patients regarding their absolute risk of fracture after starting dialysis. Future research could explore potential mechanisms contributing to differential risks.
Supplementary Material
ACKNOWLEDGEMENTS
This manuscript was reviewed and approved for publication by an officer of the NIDDK. Data reported herein were supplied by the United States Renal Data System (USRDS). Interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as official policy or interpretation of the US government.
Contributor Information
Susan Ziolkowski, Department of Medicine, Division of Nephrology, Stanford University School of Medicine, Stanford, CA, USA.
Sai Liu, Department of Medicine, Division of Nephrology, Stanford University School of Medicine, Stanford, CA, USA.
Maria E Montez-Rath, Department of Medicine, Division of Nephrology, Stanford University School of Medicine, Stanford, CA, USA.
Michelle Denburg, Department of Pediatrics, Perelman School of Medicine at the University of Pennsylvania, Division of Nephrology, Children's Hospital of Philadelphia, Philadelphia, PA, USA.
Wolfgang C Winkelmayer, Department of Medicine, Selzman Institute for Kidney Health, Section of Nephrology, Baylor College of Medicine, Houston, TX, USA.
Glenn M Chertow, Department of Medicine, Division of Nephrology, Stanford University School of Medicine, Stanford, CA, USA.
Michelle M O'Shaughnessy, Department of Medicine, Division of Renal Medicine, Cork University Hospital, Cork, Ireland.
AUTHORS’ CONTRIBUTIONS
S.Z. and M.M.O. conceived the study. S.Z., M.M.O., S.L. and M.E.M.-R. collected and analyzed the data. S.Z., M.M.O. and S.L. wrote the manuscript with input from all authors. All authors provided critical feedback and helped shape the research, analysis and manuscript. All authors approved the final version of the manuscript.
FUNDING
This work was supported by the National Institutes of Health grant number K24DK085446 (G.M.C. and M.E.M.-R.).
DATA AVAILABILITY STATEMENT
The data underlying this article were provided by the United States Renal Data System (USRDS) under a data use agreement. The data files are available from the USRDS after approval of a research proposal and signed data use agreement.
CONFLICT OF INTEREST STATEMENT
The authors report no conflict of interest.
REFERENCES
- 1. Nickolas TL, Leonard MB, Shane E.. Chronic kidney disease and bone fracture: a growing concern. Kidney Int 2008;74:721–31. 10.1038/ki.2008.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vangala C, Niu J, Montez-Rath MEet al. Selective serotonin reuptake inhibitor use and hip fracture risk among patients on hemodialysis. Am J Kidney Dis 2020;75:351–60. 10.1053/j.ajkd.2019.07.015 [DOI] [PubMed] [Google Scholar]
- 3. Jadoul M, Albert JM, Akiba Tet al. Incidence and risk factors for hip or other bone fractures among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Kidney Int 2006;70:1358–66. 10.1038/sj.ki.5001754 [DOI] [PubMed] [Google Scholar]
- 4. Daya N, Voskertchian A, Schneider ALCet al. Kidney function and fracture risk: the Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 2016;67:218–26. 10.1053/j.ajkd.2015.06.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naylor KL, McArthur E, Leslie WDet al. The three-year incidence of fracture in chronic kidney disease. Kidney Int 2014;86:810–8. 10.1038/ki.2013.547 [DOI] [PubMed] [Google Scholar]
- 6. Kim SM, Long J, Montez-Rath Met al. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res 2016;31:1803–9. 10.1002/jbmr.2862 [DOI] [PubMed] [Google Scholar]
- 7. Maravic M, Ostertag A, Urena-Torres Pet al. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int 2014;25:159–65. 10.1007/s00198-013-2435-1 [DOI] [PubMed] [Google Scholar]
- 8. Coco M, Rush H.. Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 2000;36:1115–21. 10.1053/ajkd.2000.19812 [DOI] [PubMed] [Google Scholar]
- 9. Jamal SA. Bone mass measurements in men and women with chronic kidney disease. Curr Opin Nephrol Hypertens 2010;19:343–8. 10.1097/MNH.0b013e328338f520 [DOI] [PubMed] [Google Scholar]
- 10. Kwan Tat S, Padrines M, Théoleyre Set al. IL-6, RANKL, TNF-alpha/IL-1: interrelations in bone resorption pathophysiology. Cytokine Growth Factor Rev 2004;15:49–60. [DOI] [PubMed] [Google Scholar]
- 11. Kudo O, Fujikawa Y, Itonaga Iet al. Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol 2002;198:220–7. 10.1002/path.1190 [DOI] [PubMed] [Google Scholar]
- 12. Ahuja SS, Zhao S, Bellido Tet al. CD40 ligand blocks apoptosis induced by tumor necrosis factor alpha, glucocorticoids, and etoposide in osteoblasts and the osteocyte-like cell line murine long bone osteocyte-Y4. Endocrinology 2003;144:1761–9. 10.1210/en.2002-221136 [DOI] [PubMed] [Google Scholar]
- 13. van Staa TP, Leufkens HG, Abenhaim Let al. Oral corticosteroids and fracture risk: relationship to daily and cumulative doses. Rheumatology (Oxford) 2000;39:1383–9. 10.1093/rheumatology/39.12.1383 [DOI] [PubMed] [Google Scholar]
- 14. Majumdar SR, Morin SN, Lix LMet al. Influence of recency and duration of glucocorticoid use on bone mineral density and risk of fractures: population-based cohort study. Osteoporos Int 2013;24:2493–8. 10.1007/s00198-013-2352-3 [DOI] [PubMed] [Google Scholar]
- 15. Kanda J, Izumo N, Furukawa Met al. Effects of the calcineurin inhibitors cyclosporine and tacrolimus on bone metabolism in rats. Biomed Res 2018;39:131–9. 10.2220/biomedres.39.131 [DOI] [PubMed] [Google Scholar]
- 16. Freundlich M, Bourgoignie JJ, Zilleruelo Get al. Bone modulating factors in nephrotic children with normal glomerular filtration rate. Pediatrics 1985;76:280–5. [PubMed] [Google Scholar]
- 17. Grymonprez A, Proesmans W, Van Dyck Met al. Vitamin D metabolites in childhood nephrotic syndrome. Pediatr Nephrol 1995;9:278–81. [DOI] [PubMed] [Google Scholar]
- 18. Jankowska M, Haarhaus M, Qureshi ARet al. Sclerostin–A debutant on the autosomal dominant polycystic kidney disease scene? Kidney Int Rep 2017;2:481–5. 10.1016/j.ekir.2017.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Oei L, Rivadeneira F, Zillikens MCet al. Diabetes, diabetic complications, and fracture risk. Curr Osteoporos Rep 2015;13:106–15. 10.1007/s11914-015-0260-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wetzsteon RJ, Shults J, Zemel BSet al. Divergent effects of glucocorticoids on cortical and trabecular compartment BMD in childhood nephrotic syndrome. J Bone Miner Res 2009;24:503–13. 10.1359/jbmr.081101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. US Renal Data System . 1997-2014 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda, MD:National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 1997–2014. [Google Scholar]
- 22. Layton JB, Hogan SL, Jennette CEet al. Discrepancy between Medical Evidence Form 2728 and renal biopsy for glomerular diseases. Clin J Am Soc Nephrol 2010;5:2046–52. 10.2215/cjn.03550410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. United States Census Bureau . The 2007-2011 ACS 5-Year summary file technical documentation. http://www2.census.gov/acs2011_5yr/summaryfile/ACS_2007-2011_SF_Tech_Doc.pdfhttp://www2.census.gov/programs-surveys/acs/tech_docs/subject_definitions/2014_ACSSubjectDefinitions.pdf (13 August 2016, date last accessed) [Google Scholar]
- 24. United States Census Bureau . American Community Survey and Puerto Rico Community Survey 2014 subject definitions. http://www2.census.gov/programs-surveys/acs/tech_docs/subject_definitions/2014_ACSSubjectDefinitions.pdf (13 August 2016, date last accessed) [Google Scholar]
- 25. Levey AS, Stevens LA, Schmid CHet al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. https://doi.org/150/9/604 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wetmore JB, Liu J, Wirtz HSet al. Geovariation in fracture risk among patients receiving hemodialysis. Clin J Am Soc Nephrol 2016;11:1413–21. 10.2215/CJN.11651115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. US Census Bureau American Community Survey . American Community Survey 5-year estimates, table S0101. 2015. www.census.gov (20 February 2020, date last accessed) [Google Scholar]
- 28. Fine JP, Gray RJ.. A proportional hazards model for the subdistribution of a competing risk. J Am Statist Assoc 1999;94:496–509 [Google Scholar]
- 29. Lau B, Cole SR, Gange SJ.. Competing risk regression models for epidemiologic data. Am J Epidemiol 2009;170:244–56. 10.1093/aje/kwp107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schoenfeld D. Partial residuals for the proportional hazards regression model. Biometrika 1982;69:239–41. [Google Scholar]
- 31. Montez-Rath ME, Winkelmayer WC, Desai M.. Addressing missing data in clinical studies of kidney diseases. Clin J Am Soc Nephrol 2014;9:1328–35. 10.2215/cjn.10141013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gitomer B, Pereira R, Salusky IBet al. Mineral bone disease in autosomal dominant polycystic kidney disease. Kidney Int 2021;99:977–85. 10.1016/j.kint.2020.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reid IR. Glucocorticoid effects on bone. J Clin Endocrinol Metab 1998;83:1860–2. 10.1210/jcem.83.6.4911 [DOI] [PubMed] [Google Scholar]
- 34. Dempster DW, Müller R, Zhou Het al. Preserved three-dimensional cancellous bone structure in mild primary hyperparathyroidism. Bone 2007;41:19–24. 10.1016/j.bone.2007.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Silverberg SJ, Shane E, de la Cruz Let al. Skeletal disease in primary hyperparathyroidism. J Bone Miner Res 1989;4:283–91. 10.1002/jbmr.5650040302 [DOI] [PubMed] [Google Scholar]
- 36. Tedeschi SK, Kim SC, Guan Het al. Comparative fracture risks among United States Medicaid enrollees with and those without systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1141–6. 10.1002/art.40818 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article were provided by the United States Renal Data System (USRDS) under a data use agreement. The data files are available from the USRDS after approval of a research proposal and signed data use agreement.