ABSTRACT
Background
Emerging data suggest that sodium disarrays including hyponatremia are potential risk factors for infection ensuing from impairments in host immunity, which may be exacerbated by coexisting conditions (i.e. mucosal membrane and cellular edema leading to breakdown of microbial barrier function). While dysnatremia and infection-related mortality are common in dialysis patients, little is known about the association between serum sodium levels and the risk of bloodstream infection in this population.
Methods
Among 823 dialysis patients from the national Biospecimen Registry Grant Program who underwent serum sodium testing over the period January 2008–December 2014, we examined the relationship between baseline serum sodium levels and subsequent rate of bloodstream infection. Bloodstream infection events were directly ascertained using laboratory blood culture data. Associations between serum sodium level and the incidence of bloodstream infection were estimated using expanded case mix–adjusted Poisson regression models.
Results
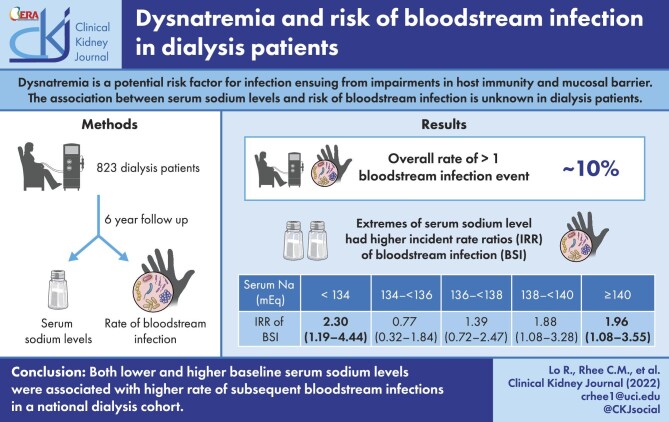
In the overall cohort, ∼10% of all patients experienced one or more bloodstream infection events during the follow-up period. Patients with both lower sodium levels <134 mEq/l and higher sodium levels ≥140 mEq/l had higher incident rate ratios (IRRs) of bloodstream infection in expanded case mix analyses (reference 136–<138 mEq/l), with adjusted IRRs of 2.30 [95% confidence interval (CI) 1.19–4.44], 0.77 (95% CI 0.32–1.84), 1.39 (95% CI 0.78–2.47), 1.88 (95% CI 1.08–3.28) and 1.96 (95% CI 1.08–3.55) for sodium levels <134, 134–<136, 138–<140, 140–<142 and ≥142 Eq/l, respectively.
Conclusions
Both lower and higher baseline serum sodium levels were associated with a higher rate of subsequent bloodstream infections in dialysis patients. Further studies are needed to determine whether correction of dysnatremia ameliorates infection risk in this population.
Keywords: bloodstream infection, dialysis, dysnatremia, hypernatremia, hyponatremia
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Dysnatremia is the most commonly encountered electrolyte disorder in clinical practice and is frequently comorbid to a wide range of medical disorders such as chronic kidney disease (CKD), heart failure and liver cirrhosis [1–3]. For example, in CKD patients there is a 2-fold higher prevalence of hyponatremia compared with non-CKD patients [3–5]. While the relationship between hyponatremia and increased mortality has been established primarily in patients with heart failure and liver cirrhosis [6–9], a growing body of literature has suggested that both hyponatremia and hypernatremia are also independent predictors of overall mortality in end-stage renal disease (ESRD) patients [3, 10–17]. However, whether there is a direct causal relationship between altered serum sodium levels and heightened mortality in ESRD patients remains to be established. A few studies have also begun to explore specific causes of mortality in hyponatremic ESRD patients, with one study by Mandai et al. [18] showing increased susceptibility to infection-related hospitalizations in hemodialysis patients with lower serum sodium levels. However, there has been a paucity of research examining the relationship between dysnatremia and specific types of infection in the dialysis patients, in whom bacteremia is among the most common causes of infection [19, 20] and a major source of hospitalizations, morbidity and mortality [21].
To address this knowledge gap, we sought to examine the relationship between serum sodium concentrations and the rate of bloodstream infections in a well-characterized cohort of dialysis patients from the national Biospecimen Registry Grant (BioReg) Program. We hypothesized that both hyponatremia and hypernatremia were independently associated with higher rates of bloodstream infections in dialysis patients.
MATERIALS AND METHODS
Study population
An observational study was conducted using data from dialysis patients in the BioReg Program with detailed patient information on sociodemographics, comorbidities, laboratory tests, dialysis treatment characteristics, clinical events and vital status. The BioReg parent cohort was comprised of 4023 maintenance hemodialysis patients from US-wide dialysis units within a large dialysis organization (DaVita, Denver, CO, USA) who provided informed consent and were prospectively enrolled and underwent specimen collections (including plasma, serum and whole blood) on a quarterly basis for up to 1 year. Following completion of the specimen collection phase, the biospecimens and patients’ corresponding deidentified clinical and outcome information were allocated to four academic centers (University of California Irvine, Harvard University, Johns Hopkins University and University of Tennessee Health Science Center). The present study was comprised of 976 adult dialysis patients from the University of California Irvine BioReg cohort who were followed over a 7-year period from January 2008 to December 2014. Patients in this cohort were included provided they were ≥18 years of age and underwent one or more serum sodium measurements. Patients were excluded if they did not undergo serum sodium measurement or had a nonsensical follow-up time value (Supplemental Fig. S1). The study was approved by the Institutional Review Committee of the University of California Irvine.
Exposure ascertainment
The objective of the study was to examine the association between baseline serum sodium levels and the rate of bloodstream infections. The primary exposure of interest was the baseline serum sodium concentration obtained from patients’ clinical data, which was divided into six categories: <134, 134–<136, 136–<138 (reference group), 138–<140, 140–<142 and ≥142 mEq/l. Among hemodialysis patients, serum samples were drawn predialysis within outpatient dialysis clinics and were transported to a single central laboratory for testing using automated and standardized methods, typically within 24 hours for sodium measurement.
Outcome ascertainment
The primary outcome of interest was bloodstream infection. Bloodstream infections were ascertained using laboratory data indicating the presence of a positive blood culture. At-risk time began the day after the baseline quarter of sodium measurement. Patients were censored for positive blood cultures or at the end of the study period (31 December 2014).
Statistical analyses
Baseline characteristics between exposure groups were compared using chi-squared, analysis of variance and Kruskal–Wallis tests, according to variable type. We first conducted logistic regression analyses to examine the association between relevant clinical characteristics with low serum sodium levels <134 mEq/l (versus ≥134 mEq/l). We then examined the relationship between baseline serum sodium level and the risk of bloodstream infection defined by incident rate ratios (IRRs) using Poisson regression models. Both logistic and Poisson regression analyses were conducted using four incremental levels of covariate adjustment:
Unadjusted model: included serum sodium level as the primary exposure of interest;
Case mix–adjusted model: included age, sex and race/ethnicity;
Expanded case mix–adjusted model: adjusted for covariates in the case mix–adjusted model, as well as dialysis vintage, Charlson Comorbidity Index (CCI), dialysis access and diabetes comorbidity status; and
Expanded case mix + laboratory–adjusted model: adjusted for covariates in the expanded case mix–adjusted model, as well as body mass index (BMI), serum albumin, dialysis adequacy [single-pool Kt/V (spKt/V)], serum creatinine, serum glucose and interdialytic weight gain (for hemodialysis patients).
We a priori defined the expanded case mix model as our primary model, which forced into the model core sociodemographic, dialysis treatment and comorbidity covariates. To explore the impact of other potential confounders, we also conducted expanded case mix + laboratory–adjusted models as sensitivity analyses given the high number of parameters relative to the number of bloodstream infection cases.
Effect modification of baseline serum sodium level and bloodstream infection across clinically relevant categories of sociodemographics, comorbidities, dialysis treatment characteristics and laboratory measures were explored through the addition of two-way interaction terms using likelihood ratio testing. There were no missing data for age, sex, race, dialysis vintage and diabetes status; remaining covariates had <1% missing values except for serum creatinine (3%), BMI (6%), spKt/V (7%), CCI score (13%), interdialytic weight gain (13%) and serum glucose (49%), which were addressed using multiple imputation. Analyses and figures were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA), Stata version 13.1 (StataCorp, College Station, TX, USA) and SigmaPlot version 12.5 (Systat Software, San Jose, CA, USA).
RESULTS
Baseline characteristics
Among the 823 patients who met eligibility criteria for the study cohort (798 hemodialysis patients, 16 peritoneal dialysis patients and 9 with unknown modality type), the mean ± standard deviation (SD), median [interquartile range (IQR)] and minimum–maximum of observed baseline serum sodium levels were 138 ± 3 mEq/l, 138 mEq/l (136–140) and 116–147 mEq/l, respectively. Among these patients, 9.5% had serum sodium levels consistent with hyponatremia (sodium ≤134 mEq/l) (Supplemental Fig. S2). Upon comparing baseline characteristics across strata of serum sodium levels (Table 1), we observed that, compared with patients in the highest sodium category (≥142 mEq/l), those in the lowest sodium category (≤134 mEq/l) were more likely to be non-Hispanic White, had a longer dialysis vintage, were more likely to have an arteriovenous fistula or graft, were more likely to have diabetes, had lower BMI and serum creatinine levels and had higher interdialytic weight gains and serum glucose levels. Conversely, patients in the lowest sodium category were less likely to be Black and have a catheter venous access. Both the lowest and highest sodium categories had a higher proportion of females to males compared with the intervening serum sodium categories.
Table 1:
Baseline characteristics according to serum sodium level in dialysis patients.
| Characteristics | Overall | <134 | 134-<136 | 136-<138 | 138-<140 | 140-<142 | ≥142 |
|---|---|---|---|---|---|---|---|
| Serum sodium (mEq/l) | |||||||
| Patients, n (%) | 823 | 78 (9.48) | 104 (12.64) | 180 (21.87) | 210 (25.52) | 162 (19.68) | 89 (10.81) |
| Age (years), mean ± SD | 60.0 ± 13.9 | 59.4 ± 12.9 | 59.8 ± 13.4 | 59.8 ± 13.8 | 61.3 ± 13.9 | 58.7 ± 14.5 | 60.4 ± 14.1 |
| Female, n | 46 | 55 | 45 | 48 | 43 | 44 | 51 |
| Race/ethnicity, n (%) | |||||||
| Non-Hispanic White | 39 | 49 | 39 | 38 | 45 | 29 | 40 |
| Black | 37 | 26 | 39 | 37 | 34 | 43 | 44 |
| Hispanic | 19 | 21 | 18 | 21 | 18 | 22 | 12 |
| Other | 4 | 5 | 3 | 4 | 3 | 6 | 3 |
| Diabetes, n | 53 | 65 | 51 | 56 | 49 | 55 | 43 |
| Vintage (months), mean ± SD | 48.7 ± 49.3 | 51.7 ± 46.3 | 47.3 ± 38.0 | 49.9 ± 52.0 | 50.8 ± 44.7 | 44.8 ± 53.2 | 47.3 ± 60.0 |
| CCI, median (IQR) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) | 5.0 (4.0–7.0) | 5.0 (4.0–7.0) | 6.0 (4.0–6.0) | 5.0 (4.0–7.0) | 5.0 (4.0–6.0) |
| Vascular access, n | |||||||
| AVF/AVG | 29 | 33 | 31 | 29 | 26 | 30 | 27 |
| Catheter | 21 | 17 | 17 | 21 | 16 | 23 | 31 |
| Unknown | 50 | 50 | 52 | 49 | 58 | 46 | 42 |
| BMI (kg/m2), mean ± SD | 29.7 ± 8.1 | 28.1 ± 7.6 | 28.5 ± 7.6 | 30.1 ± 8.0 | 30.1 ± 8.7 | 30.6 ± 8.0 | 29.1 ± 8.1 |
| Weight gain (kg), mean ± SD | 2.7 ± 1.6 | 3.1 ± 2.4 | 2.8 ± 1.0 | 2.8 ± 1.8 | 2.5 ± 1.6 | 2.5 ± 1.2 | 2.4 ± 1.2 |
| Laboratory tests, median (IQR) | |||||||
| Serum albumin (g/dl) | 3.9 (3.6–4.1) | 3.8 (3.5–3.9) | 3.9 (3.5–4.1) | 3.9 (3.7–4.2) | 3.9 (3.6–4.1) | 3.9 (3.7–4.1) | 3.9 (3.6–4.2) |
| spKt/V | 1.6 (1.4–1.8) | 1.6 (1.4–1.8) | 1.6 (1.4–1.8) | 1.6 (1.4–1.8) | 1.6 (1.4–1.8) | 1.6 (1.4–1.7) | 1.6 (1.4–1.7) |
| Serum creatinine (g/dl) | 8.0 (6.0–10.5) | 7.5 (5.8–9.7) | 8.1 (6.1–0.0) | 8.1 (6.2–10.9) | 8.2 (6.2–10.6) | 7.9 (6.2–10.5) | 8.0 (5.7–10.5) |
| Serum glucose (mg/dl) | 131 (102–183) | 164 (117–266) | 157 (109–219) | 139 (102–189) | 126 (96–167) | 126 (96–160) | 124 (88–166) |
AVF, arteriovenous fistula; AVG, arteriovenous graft.
Clinical characteristics associated with hyponatremia
In logistic regression analyses examining clinical characteristics associated with low serum sodium levels ≤134 mEq/l (versus >134 mEq/l) (Table 2), we observed that patients of Black race and with higher serum albumin levels were less likely to have hyponatremia across all levels of covariate adjustment. In contrast, patients with higher serum glucose levels and interdialytic weight gains were more likely to have hyponatremia across all levels of covariate adjustment.
Table 2:
Clinical characteristics associated with low serum sodium <134 mEq/l (versus ≥134 mEq/l) in dialysis patients, using logistic regression.
| Characteristics | Unadjusted, OR (95% CI) | Case mix, OR (95% CI) | Expanded case mix, OR (95% CI) | Expanded case mix + laboratory, OR (95% CI) |
|---|---|---|---|---|
| Age (Δ10 years) | 0.97 (0.82–1.14) | 0.92 (0.77–1.09) | 1.00 (0.75–1.33) | 1.03 (0.76–1.39) |
| Male (versus female) | 0.68 (0.43–1.09) | 0.65 (0.41–1.05) | 0.65 (0.40–1.06) | 0.55 (0.31–0.96) |
| Race/ethnicity (versus non-Hispanic White) | ||||
| Black | 0.52 (0.30–0.92) | 0.49 (0.28–0.88) | 0.47 (0.26–0.85) | 0.44 (0.23–0.83) |
| Hispanic | 0.85 (0.46–1.57) | 0.80 (0.43–1.51) | 0.76 (0.40–1.44) | 0.80 (0.41–1.57) |
| Other | 1.00 (0.34–3.01) | 1.06 (0.35–3.19) | 0.95 (0.31–2.88) | 1.11 (0.35–3.53) |
| Diabetes (versus non-diabetes) | 1.78 (1.09–2.89) | 1.72 (1.05–2.83) | 2.07 (1.13–3.80) | 1.85 (0.95–3.61) |
| Vintage (Δ6 months) | 1.01 (0.98–1.04) | 1.01 (0.99–1.04) | 1.01 (0.99–1.04) | 1.02 (0.99–1.05) |
| CCI score | 1.00 (0.88–1.15) | 1.04 (0.86–1.26) | 0.89 (0.69–1.14) | 0.84 (0.63–1.09) |
| Vascular access (versus AVF/AVG) | ||||
| Catheter | 0.68 (0.34–1.37) | 0.66 (0.33–1.34) | 0.70 (0.34–1.44) | 0.54 (0.24–1.20) |
| Unknown | 0.85 (0.50–1.44) | 0.86 (0.50–1.45) | 0.84 (0.49–1.44) | 0.81 (0.46–1.44) |
| BMI (Δ5 kg/m2) | 0.86 (0.73–1.01) | 0.84 (0.71–1.00) | 0.80 (0.67–0.96) | 0.76 (0.63–0.92) |
| Weight gain (Δ0.5 kg) | 1.08 (1.02–1.16) | 1.10 (1.03–1.18) | 1.10 (1.03–1.19) | 1.10 (1.01–1.19) |
| Serum albumin (Δ0.5 mg/dl) | 0.69 (0.55–0.87) | 0.67 (0.53–0.85) | 0.60 (0.46–0.78) | 0.65 (0.48–0.86) |
| spKt/V (Δ0.2) | 1.04 (0.92–1.16) | 0.99 (0.87–1.12) | 0.99 (0.87–1.12) | 0.97 (0.84–1.13) |
| Serum creatinine (Δ1.0 g/dl) | 0.94 (0.87–1.02) | 0.96 (0.88–1.05) | 0.94 (0.85–1.04) | 1.01 (0.90–1.13) |
| Serum glucose (Δ100 mg/dl) | 2.25 (1.60–3.17) | 2.19 (1.55–3.09) | 2.28 (1.58–3.30) | 2.44 (1.63–3.67) |
| Hemodialysis session length (Δ30 minutes) | 0.96 (0.81–1.13) | 0.96 (0.82–1.13) | 0.96 (0.81–1.14) | 1.00 (0.83–1.20) |
| Predialysis weight (Δ0.5kg) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 1.01 (1.00–1.02) |
| Post-dialysis weight (Δ0.5 kg) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 1.00 (0.99–1.00) | 1.01 (1.00–1.02) |
| Predialysis SBP (Δ20 mmHg) | 0.94 (0.80–1.11) | 0.93 (0.79–1.11) | 0.92 (0.78–1.10) | 0.95 (0.79–1.14) |
| Postdialysis SBP (Δ20 mmHg) | 0.98 (0.82–1.17) | 0.97 (0.81–1.16) | 0.95 (0.79–1.15) | 0.87 (0.71–1.06) |
AVF, arteriovenous fistula; AVG, arteriovenous graft; SBP, systolic blood pressure.
Serum sodium level and risk of bloodstream infection
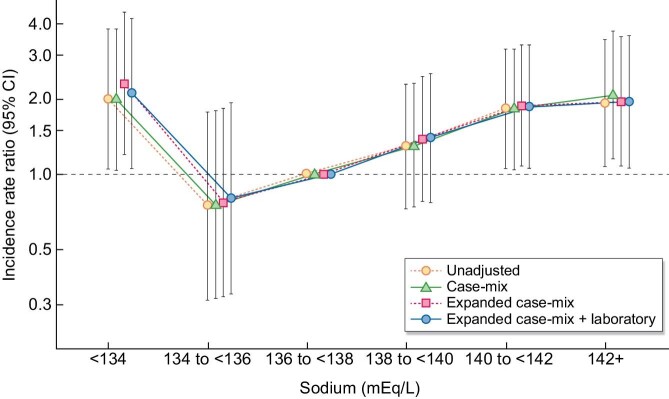
Patients contributed a total of 1976 patient-years of follow-up, during which time 141 total bloodstream infection events occurred among 83 patients (10.1%). The median at-risk time was 1.4 years (IQR 1.2–3.6). Across all levels of covariate adjustment, serum sodium levels <134 mEq/l and >140 mEq/l were associated with significantly higher IRRs of bloodstream infection compared with the reference sodium level of 136–<138 mEq/l (Fig. 1), exhibiting a J-shaped association between sodium concentration and rate of bloodstream infection. The primary expanded case mix model showed IRRs of 2.30 (95% CI 1.19–4.44), 0.77 (0.32–1.84), 1.39 (0.78–2.47), 1.88 (1.08–3.28) and 1.96 (1.08–3.55) for sodium categories <134, 134–<136, 138–<140, 140–<142 and ≥142 mEq/l, respectively (Supplemental Table S1). A similar pattern of findings were observed for unadjusted, case mix and expanded case mix + laboratory models.
Figure 1:
Association between baseline serum sodium level and IRRs of bloodstream infection risk in dialysis patients.
Serum sodium level and bloodstream infection risk across clinically relevant subgroups
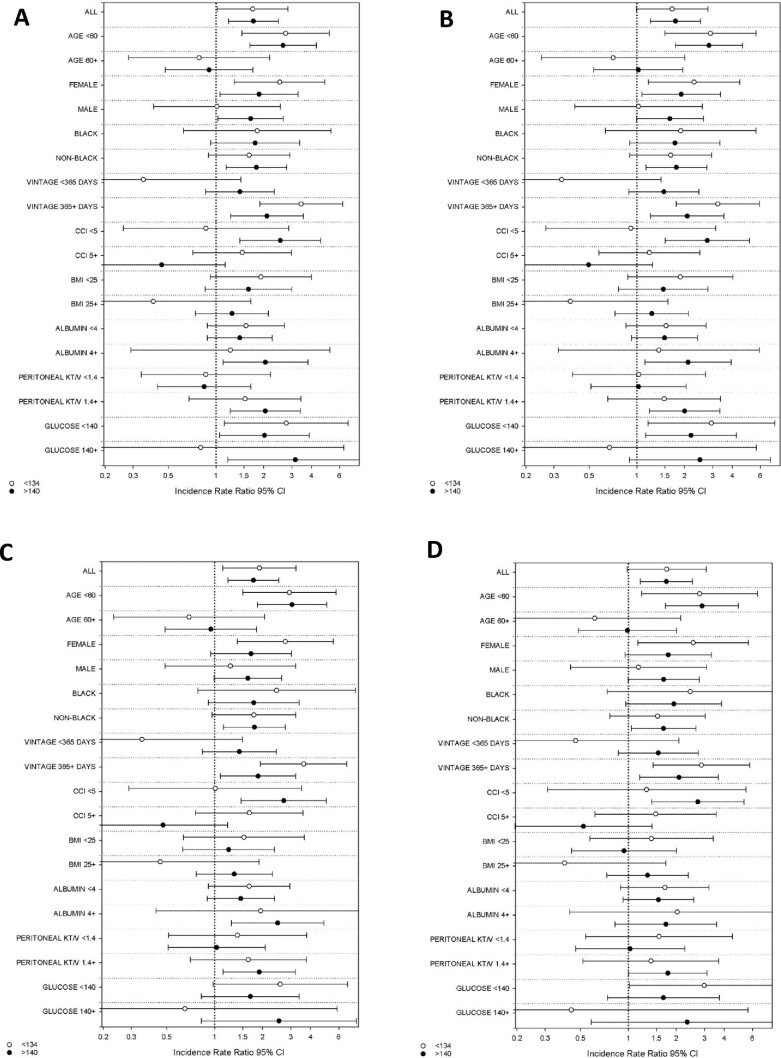
We then examined the relationship between serum sodium level, categorized as <134, 134–140 and >140 mEq/l (reference 134–140 mEq/l), and bloodstream infection rates across clinically relevant subgroups (Fig. 2 and Supplemental Table S2). In expanded case mix analyses we found there was effect modification of the serum sodium–bloodstream infection rate relationship on the basis of age and dialysis vintage, such that both lower and higher sodium levels <134 mEq/l and >140 mEq/l were each associated with a higher rate of infection in those of younger versus older age (<60 versus ≥60 years) and longer versus shorter dialysis vintage (≥1 versus <1 year) (P interaction values = .005 and .01, respectively). We also observed a differential sodium–bloodstream infection relationship across the level of comorbidity burden, such that higher sodium levels >140 mEq/l were associated with a higher rate of infection in those with lower versus higher CCI scores (<5 versus ≥5) (P interaction value = .007). We did not detect effect modification on the basis of sex, race, BMI, serum albumin, dialysis adequacy or serum glucose level (all P interaction values >.05).
Figure 2:
Association between baseline serum sodium level and bloodstream infection rate in dialysis patients across clinically relevant subgroups in (A) unadjusted, (B) case mix, (C) expanded case mix and (D) expanded case mix + laboratory–adjusted analyses.
Across all subgroups the nominal IRRs for risk of bloodstream infection for sodium levels <134 mEq/l were >1 in the expanded case mix models, except among patients who were ≥60 years of age, had a dialysis vintage <1 year, had a BMI ≥25 kg/m2 and had a serum glucose ≥140 mg/dl. Nominal associations for sodium levels <134 mEq/l were statistically significant among patients who were <60 years of age or who had a dialysis vintage ≥1 year. Conversely, in expanded case mix analyses, across all subgroups the nominal IRRs of bloodstream infection for sodium levels >140 mEq/l were >1 except among patients who were ≥60 years of age or had a CCI score ≥5. Nominal associations for sodium levels >140 mEq/l were statistically significant among patients who were <60 years of age, of non-Black race, with a dialysis vintage ≥1 year, CCI score <5, serum albumin ≥4 g/dl or spKt/V ≥1.4.
DISCUSSION
In this well-characterized, contemporary cohort of dialysis patients from the national BioReg Program, we found that both lower and higher serum sodium levels were independently associated with a >2-fold higher rate of bloodstream infection. These associations were robust across multiple levels of covariate adjustment that accounted for differences in sociodemographics, comorbidities, dialysis treatment characteristics and laboratory test profiles, including markers for nutritional and inflammatory status (e.g. serum albumin).
While multiple prior studies have demonstrated that derangements in serum sodium in dialysis patients have been associated with heightened mortality risk [3, 10–17], few have examined the risk of cause-specific mortality, thus the causal relationship between dysnatremia and mortality remains unclear. Despite the fact that cardiovascular mortality is the largest contributor to mortality in ESRD patients overall [22], one report from the HEMO study [16] suggests that the higher mortality risk in hyponatremic hemodialysis patients stems from noncardiovascular-related causes of death. These findings have prompted greater scrutiny of infection-related mortality (i.e. the second most common cause of death in dialysis patients overall [22]) as a potential adverse complication of hyponatremic dialysis patients.
To date, there have been few studies that have examined serum sodium concentrations with infection in the dialysis population. Among these is a study of 332 maintenance hemodialysis patients by Mandai et al. [18] that showed those with lower serum sodium levels in the lowest two tertiles were associated with a 2.4-fold higher risk of infection-related hospitalizations. In another study, by Chang et al. [23], of 441 incident peritoneal dialysis patients, each 1-mEq/l higher time-averaged serum sodium level was associated with a 33% lower risk of infection-related death. These findings were corroborated in a subsequent study of 1656 peritoneal dialysis patients by Qiu et al. [24], which showed that hyponatremia (defined as serum sodium <135 mEq/l) was associated with a higher risk of infection-related death among those who were ≥50 years of age. However, there remain major knowledge gaps with respect to the relationship between dysnatremia and the risk of specific types of infection in dialysis patients, including bacteremia as one of the dominant sources of infection in this population [19, 20].
To our knowledge, ours is the first study to granularly examine the association between fine gradations of serum sodium levels with subsequent bloodstream infection incidence rigorously ascertained by laboratory blood culture data among a large contemporary dialysis cohort. Given that prior research of serum sodium levels and other outcomes (i.e. all-cause mortality) in hemodialysis patients have shown that incrementally higher levels even in the high-normal range as well as incrementally lower levels in the low-normal range are associated with increasingly higher death risk, we sought to granularly examine serum sodium concentrations in order to more precisely determine the threshold at which a higher risk of bloodstream infections is observed [14]. While it is still unclear as to whether dysnatremia is an indicator of versus a direct contributor to increased susceptibility to infection, there may be several pathophysiological mechanisms by which alterations in serum sodium promote bloodstream infection. There is an increasing body of literature that has reported the negative impact of hypernatremia on immune cell function, particularly macrophages and T cell subsets. Hypersalinity has been shown to impact macrophage chemotaxis in vitro [25], as well as promote pro-inflammatory or M1 macrophage differentiation [26–28] while suppressing wound-healing M2 macrophage activity in vivo [29]. Hypersalinity has also been shown to promote differentiation of T helper 17 (Th17) cells [30, 31] and suppress the inhibitory action of regulatory T cells on Th17 cells [32], causing further dysregulation of the immune response and increased susceptibility for infectious processes. With respect to hyponatremia, changes in extracellular tonicity often drive fluid shifts into and out of intracellular compartments, resulting in cellular edema or shrinkage that may impair the inherent antimicrobial barrier and structure of mucosal surfaces [27, 28, 33].
The strengths of our study include its examination of a well-characterized dialysis cohort with detailed collection of sociodemographic, comorbidity, dialysis treatment and laboratory test data; more granular examination of serum sodium levels as compared with prior studies; and rigorous adjudication of bloodstream infection events using laboratory blood culture data. However, several limitations of the study bear mention. First, while we were able to adjust for a large number of confounders of the sodium–bloodstream infection association, we were unable to account for dietary factors (i.e. sodium and fluid intake) and differences in dialysate sodium concentrations, which may have resulted in residual confounding. Second, it is possible that some of the lower sodium values may have been observed in the context of hyperglycemia, which is an independent risk factor for infection. However, we accounted for serum glucose derangements in multivariable models, which showed a robust association between dysnatremia and bloodstream infection risk. Third, there were variable degrees of missingness for some of the covariates that may confound the serum sodium–bloodstream infection association (e.g. serum creatinine, glucose, etc.), which we sought to address with multiple imputation. Given that the laboratory data were obtained as part of routine clinical care from the dialysis clinics of a large national dialysis organization, it is possible that selection of certain laboratory measurements may have been at the discretion of the various clinics/providers and/or there may have been missed measurements among patients. Fourth, due to the limited availability of repeated serum sodium measurements, we were restricted to examine sodium levels measured at a single point in time, and further corollary studies are needed to determine the longitudinal impact of dysnatremia upon the risk of bloodstream infection in dialysis patients. Lastly, as with all observational research, we are unable to confirm a causal relationship between dysnatremia and bloodstream infection, and further studies are needed to determine if hypo- and hypernatremia are markers or mediators of infection in dialysis patients.
In summary, our study found that both lower and higher serum sodium levels are each associated with a heightened risk of bloodstream infection in a well-characterized national cohort of dialysis patients. Future studies are needed to define underlying mechanisms, establish whether sodium derangements predispose to other types of infections and determine whether correction of dysnatremia attenuates the risk for infection in this population.
Supplementary Material
ACKNOWLEDGEMENTS
We thank DaVita Clinical Research for providing the clinical data for this research. Portions of these data have been presented as an abstract at the 2017 American Society of Nephrology Kidney Week Meeting, 31 October–5 November, 2017, New Orleans, LA, USA.
Contributor Information
Robin H Lo, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA.
Kamyar Kalantar-Zadeh, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA; Tibor Rubin Long Beach Veterans Affairs Medical Center, Long Beach, CA, USA.
Amy S You, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA.
Juan Carlos Ayus, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA.
Elani Streja, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA; Tibor Rubin Long Beach Veterans Affairs Medical Center, Long Beach, CA, USA.
Christina Park, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA; University of Washington School of Public Health, Seattle, WA, USA.
Peter Sohn, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA.
Tracy Nakata, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA.
Yoko Narasaki, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA.
Steven M Brunelli, DaVita Clinical Research, Minneapolis, MN, USA.
Csaba P Kovesdy, Division of Nephrology, University of Tennessee Health Science Center, Memphis, TN, USA; Nephrology Section, Memphis Veterans Affairs Medical Center, Memphis, TN, USA.
Danh V Nguyen, Division of General Internal Medicine, University of California Irvine, Orange, CA, USA.
Connie M Rhee, Harold Simmons Center for Chronic Disease Research and Epidemiology, Division of Nephrology, Hypertension and Kidney Transplantation, University of California Irvine, Orange, CA, USA.
FUNDING
The authors are supported by research grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases [K24-DK091419 (to K.K.Z.), R44-116383 (to K.K.Z.), R01-DK092232 (to D.V.N.), R03-DK114642 (to C.M.R.), R01-DK122767 (to C.M.R.) and R01-DK124138 (to C.M.R. and K.K.Z.). Funders of this study did not have any role in the study design; collection, analysis and interpretation of data; writing of the report; or the decision to submit the report for publication.
AUTHORS’ CONTRIBUTIONS
R.H.L., A.S.Y. and C.M.R. were responsible for the research idea and study design and original draft preparation and editing. C.M.R. and K.K.Z. were responsible for data acquisition. C.M.R. was responsible for the investigation and supervision and mentorship. A.S.Y. and C.M.R. were responsible for the statistical analysis. All authors were responsible for review of the manuscript.
DATA AVAILABILITY STATEMENT
The data underlying this article cannot be shared publicly to protect the privacy of individuals who participated in the study.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
REFERENCES
- 1. Angeli P, Wong F, Watson Het al. Hyponatremia in cirrhosis: results of a patient population survey. Hepatology 2006;44:1535–42. 10.1002/hep.21412 [DOI] [PubMed] [Google Scholar]
- 2. Bettari L, Fiuzat M, Shaw LKet al. Hyponatremia and long-term outcomes in chronic heart failure—an observational study from the Duke Databank for Cardiovascular Diseases. J Card Fail 2012;18:74–81. 10.1016/j.cardfail.2011.09.005 [DOI] [PubMed] [Google Scholar]
- 3. Kovesdy CP, Lott EH, Lu JLet al. Hyponatremia, hypernatremia, and mortality in patients with chronic kidney disease with and without congestive heart failure. Circulation 2012;125:677–84. 10.1161/CIRCULATIONAHA.111.065391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis 2013;62:139–49. 10.1053/j.ajkd.2012.09.019 [DOI] [PubMed] [Google Scholar]
- 5. Liamis G, Rodenburg EM, Hofman Aet al. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med 2013;126:256–63. 10.1016/j.amjmed.2012.06.037 [DOI] [PubMed] [Google Scholar]
- 6. Borroni G, Maggi A, Sangiovanni Aet al. Clinical relevance of hyponatraemia for the hospital outcome of cirrhotic patients. Dig Liver Dis 2000;32:605–10. 10.1016/s1590-8658(00)80844-0 [DOI] [PubMed] [Google Scholar]
- 7. Gheorghiade M, Abraham WT, Albert NMet al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J 2007;28:980–88. 10.1093/eurheartj/ehl542 [DOI] [PubMed] [Google Scholar]
- 8. Heuman DM, Abou-Assi SG, Habib Aet al. Persistent ascites and low serum sodium identify patients with cirrhosis and low MELD scores who are at high risk for early death. Hepatology 2004;40:802–10. 10.1002/hep.20405 [DOI] [PubMed] [Google Scholar]
- 9. Lee DS, Austin PC, Rouleau JLet al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA 2003;290:2581–7. 10.1001/jama.290.19.2581 [DOI] [PubMed] [Google Scholar]
- 10. Hecking M, Karaboyas A, Saran Ret al. Predialysis serum sodium level, dialysate sodium, and mortality in maintenance hemodialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2012;59:238–48. 10.1053/j.ajkd.2011.07.013 [DOI] [PubMed] [Google Scholar]
- 11. Hecking M, Karaboyas A, Saran Ret al. Dialysate sodium concentration and the association with interdialytic weight gain, hospitalization, and mortality. Clin J Am Soc Nephrol 2012;7:92–100. 10.2215/CJN.05440611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCausland FR, Brunelli SM, Waikar SS.. Dialysate sodium, serum sodium and mortality in maintenance hemodialysis. Nephrol Dial Transplant 2012;27:1613–8. 10.1093/ndt/gfr497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nigwekar SU, Wenger J, Thadhani Ret al. Hyponatremia, mineral metabolism, and mortality in incident maintenance hemodialysis patients: a cohort study. Am J Kidney Dis 2013;62:755–62. 10.1053/j.ajkd.2013.02.367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rhee CM, Ravel VA, Ayus JCet al. Pre-dialysis serum sodium and mortality in a national incident hemodialysis cohort. Nephrol Dial Transplant 2016;31:992–1001. 10.1093/ndt/gfv341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sahin OZ, Asci G, Kircelli Fet al. The impact of low serum sodium level on mortality depends on glycemic control. Eur J Clin Invest 2012;42:534–40. 10.1111/j.1365-2362.2011.02613.x [DOI] [PubMed] [Google Scholar]
- 16. Waikar SS, Curhan GC, Brunelli SM.. Mortality associated with low serum sodium concentration in maintenance hemodialysis. Am J Med 2011;124:77–84. 10.1016/j.amjmed.2010.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rhee CM, Ayus JC, Kalantar-Zadeh K.. Hyponatremia in the dialysis population. Kidney Int Rep 2019;4:769–80. 10.1016/j.ekir.2019.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mandai S, Kuwahara M, Kasagi Yet al. Lower serum sodium level predicts higher risk of infection-related hospitalization in maintenance hemodialysis patients: an observational cohort study. BMC Nephrol 2013;14:276. 10.1186/1471-2369-14-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dalrymple LS, Mu Y, Romano PSet al. Outcomes of infection-related hospitalization in Medicare beneficiaries receiving in-center hemodialysis. Am J Kidney Dis 2015;65:754–62. 10.1053/j.ajkd.2014.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Suzuki M, Satoh N, Nakamura Met al. Bacteremia in hemodialysis patients. World J Nephrol 2016;5:489–96. 10.5527/wjn.v5.i6.489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fisher M, Golestaneh L, Allon Met al. Prevention of bloodstream infections in patients undergoing hemodialysis. Clin J Am Soc Nephrol 2020;15:132–51. 10.2215/CJN.06820619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. 2018. https://www.usrds.org/2018/view/Default.aspx [Google Scholar]
- 23. Chang TI, Kim YL, Kim Het al. Hyponatremia as a predictor of mortality in peritoneal dialysis patients. PLoS One 2014;9:e111373. 10.1371/journal.pone.0111373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Qiu Y, Ye H, Wang Yet al. Age difference in the association between hyponatremia and infection-related mortality in peritoneal dialysis patients. Blood Purif 2020;49:631–40. 10.1159/000505614 [DOI] [PubMed] [Google Scholar]
- 25. Muller S, Quast T, Schroder Aet al. Salt-dependent chemotaxis of macrophages. PLoS One 2013;8:e73439. 10.1371/journal.pone.0073439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jantsch J, Schatz V, Friedrich Det al. Cutaneous Na+ storage strengthens the antimicrobial barrier function of the skin and boosts macrophage-driven host defense. Cell Metab 2015;21:493–501. 10.1016/j.cmet.2015.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muller DN, Wilck N, Haase Set al. Sodium in the microenvironment regulates immune responses and tissue homeostasis. Nat Rev Immunol 2019;19:243–54. 10.1038/s41577-018-0113-4 [DOI] [PubMed] [Google Scholar]
- 28. Wilck N, Balogh A, Marko Let al. The role of sodium in modulating immune cell function. Nat Rev Nephrol 2019;15:546–58. 10.1038/s41581-019-0167-y [DOI] [PubMed] [Google Scholar]
- 29. Binger KJ, Gebhardt M, Heinig Met al. High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest 2015;125:4223–38. 10.1172/JCI80919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kleinewietfeld M, Manzel A, Titze Jet al. Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 2013;496:518–22. 10.1038/nature11868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu C, Yosef N, Thalhamer Tet al. Induction of pathogenic TH17 cells by inducible salt-sensing kinase SGK1. Nature 2013;496:513–7. 10.1038/nature11984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hernandez AL, Kitz A, Wu Cet al. Sodium chloride inhibits the suppressive function of FOXP3+ regulatory T cells. J Clin Invest 2015;125:4212–22. 10.1172/JCI81151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ayus JC, Caputo D, Bazerque Fet al. Treatment of hyponatremic encephalopathy with a 3% sodium chloride protocol: a case series. Am J Kidney Dis 2015;65:435–42. 10.1053/j.ajkd.2014.09.021 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared publicly to protect the privacy of individuals who participated in the study.