ABSTRACT
The physical exam is changing. Many have argued that the physical exam of the 21st century should include point-of-care ultrasound (POCUS). POCUS is being taught in medical schools and has been endorsed by the major professional societies of internal medicine. In this review we describe the trend toward using POCUS in medicine and describe where the practicing nephrologist fits in. We discuss what a nephrologist's POCUS exam should entail and we give special attention to what nephrologists can gain from learning POCUS. We suggest a ‘nephro-centric’ approach that includes not only ultrasound of the kidney and bladder, but of the heart, lungs and vascular access. We conclude by reviewing some of the sparse data available to guide training initiatives and give suggested next steps for advancing POCUS in nephrology.
Keywords: nephrology education, POCUS, sonography, ultrasound, ultrasonography
INTRODUCTION
When looking at the evolution of the physical exam, the advances have been few and far between. The first came when Rene Laennec invented the stethoscope in 1816 [1]. Laennec not only invented a device and wrote a manual that would revolutionize medicine, he physically made the device that became synonymous with the medical profession. He was a relentless marketer, and it is said that up until his death in 1826 of tuberculosis, every stethoscope in existence had been made by him. Until recently, there have not been many advancements in the physical exam. Now, point-of-care ultrasound (POCUS) is revolutionizing physical exam the way the stethoscope did ˃200 years ago.
Sound waves were first identified thanks to the echolocation abilities of bats. Then, piezoelectric crystals were discovered that can transform mechanical energy into electrical energy. These crystals were able to send and receive sound waves. This technological advancement became practical with the use of underwater radar to identify the sunken Titanic. From there the technology grew into the ultrasounds we are familiar with today.
Ultrasound came into the hands of clinicians in the 1940s and began to be used regularly in the 1960s and 1980s by obstetricians and intensivists, respectively [2]. While the ultrasound is not a new device, it has only recently become small and affordable enough to become freely available to all physicians, including nephrologists. Calls to incorporate ultrasound into the physical exam have gone as far back as 1988 [3]. This argument has gained more traction recently, with some arguing that POCUS should be the fifth pillar of physical exam—inspection, palpation, percussion, auscultation and insonation [4]. POCUS is distinct from consultative ultrasonography where images are interpreted by a physician who is not directly involved in the clinical care of the patient. A POCUS exam is a real-time exam that is an extension of the physical exam. A POCUS exam is performed and interpreted by the treating physician at the time of the clinical encounter, often to answer a clinical question. In this review, we will discuss the broader trend toward POCUS in medicine and give special attention to how the nephrology community has begun to adopt this practice. We will conclude by describing some of the educational initiatives to teach POCUS in nephrology and describe suggested next steps.
WHERE IS THE MEDICINE WORLD IN TERMS OF POCUS?
There is mounting evidence that POCUS can augment the physical exam and reduce diagnostic uncertainty among emergency department, critical care and internal medicine doctors [5, 6]. US medical schools have taught POCUS for years. A national survey in 2014 found that one-third of schools had a formal POCUS program [7]. That number has subsequently grown. These medical school curricula include sonographic assessment of volume status as well as basic imaging of the kidneys [8, 9]. Indeed, some medical schools are even giving pocket ultrasound devices at white coat ceremonies instead of stethoscopes [10]. Internal medicine residency training programs have also taken up POCUS, but at a slower rate. Almost all residents agree that POCUS is a useful skill, but almost 80% of internal medicine residents receive little to no ultrasound training during residency [11, 12]. In 2019, the Alliance for Academic Internal Medicine, the American College of Physicians and the Society of Hospital Medicine, each released position statements endorsing the use of POCUS for the diagnosis and management of medically ill patients [13–15]. These statements follow a precedent for POCUS that has been firmly established by both emergency medicine and critical care medicine. In both specialties, POCUS is a core part of training by the American College of Graduate Medical Education. Nephrologists and nephrology trainees will soon be practicing in an environment where our colleagues from other disciplines know how to use an ultrasound probe. If nephrologists do not incorporate POCUS into their clinical decisions, we will be left behind.
what DO NEPHROLOGISTS HAVE TO GAIN FROM POCUS?
A small but growing number of nephrologists have advocated for incorporating POCUS into nephrology practice. Figure 1 depicts the applicability of POCUS throughout the continuum of kidney disease.
FIGURE 1:
POCUS through the continuum of kidney disease.
Kidneys/bladder
First, and most importantly, POCUS can be used to image the kidneys. There are few experiences more gratifying than identifying obstructive uropathy and treating it with an indwelling urethral catheter hours before a formal sonogram could be performed. Indeed, even after placement, a repeat sonogram should be performed; the authors have all had experience with misplaced indwelling urethral catheter seen on ultrasound. A meta-analysis of emergency room physicians has shown that trainees can learn to accurately identify obstructive uropathy with POCUS, leading to more timely care [16]. Sibley et al. [17] sought to measure the diagnostic accuracy of POCUS to identify hydronephrosis. They looked at 413 patients where a radiologist reported on either computed tomography or ultrasound findings related to renal colic. Ultrasound-trained, blinded emergency department (ED) attendings and residents then performed POCUS on these patients. They found that the sensitivity and specificity of POCUS for hydronephrosis among patients with renal colic were 77.1 and 71.8, respectively. This increased with an increasing degree of hydronephrosis and, interestingly, operator experience did not affect diagnostic accuracy. In a post-hoc analysis, they found that the presence of hydronephrosis on POCUS was associated with a 3.2-times increased odds of requiring urgent intervention. Other meta-analyses have similarly corroborated this moderate diagnostic ability while others have shown a 94% specificity [16, 18]. Despite the wide range of sensitivity and specificity, the lack of radiation exposure and timely nature of POCUS is invaluable to the treating physician. A randomized controlled trial compared ED-performed POCUS to radiology POCUS and found no difference in high-risk diagnoses and showed a lower radiation exposure and shorter ED length of stay [17]. No similar trials have looked at nephrologist-performed ultrasound, but we believe that with adequate training nephrologists would achieve results similar to those of ED physicians.
Work at Emory University has shown that nephrologist-performed ultrasound can yield high-quality information about kidney pathology [19, 20]. Nephrologist-performed ultrasound findings of increased echogenicity correlate strongly with tubular atrophy and inflammation [19]. Nephrologist-performed ultrasound can also accurately measure kidney size in autosomal dominant polycystic kidney disease when compared with MRI [20]. Additionally, cortical echogenicity combined with size has been shown to be a predictor of histopathologic findings and can be used to help guide decisions about the diagnostic yield of biopsies, potentially saving patients from procedural complications [19]. The workup and monitoring of acute kidney injury (AKI) with ultrasound is a burgeoning field, and while it requires more study, POCUS can yield invaluable insights into the cause and treatment of AKI [21].
POCUS of the kidney is making strides in training as well, with refinement in the approach as more nephrologists are involved. Koratala [22] has published widely on nephrologist-performed kidney POCUS and has described an acronym called SECONDS, which stands for size, echogenicity, collecting system, outline, notable lesions, Doppler and surroundings. This intuitive approach can be taught to nephrologists and nephrology trainees.
Volume assessment
Nephrologists can benefit from more than just kidney ultrasounds. Nephrologists are called upon to be the masters of volume assessment. POCUS has a tremendous ability to help nephrologists make important decisions about volume status. Dyspnea is a common symptom in patients with kidney disease. Imagine a dialysis patient complaining to you about worsening dyspnea—you might ask them about fever and cough, chest pain and palpitations. The history would take you a long way, but the history would need to be supplemented by a physical exam. Ultimately you want to answer the question: Will my patient benefit from additional ultrafiltration or is there something else going on? A comprehensive review by Bhagra et al. [5] compiled the sensitivity and specificity for a variety of physical exam findings and compared them with POCUS findings. Overall, traditional physical exam findings such as egophony, rales and jugular vein distention had good specificity but limited sensitivity, while POCUS evaluation of the heart and lungs generally had superior sensitivity and similar specificity . Especially when looking for more critical issues such as pericardial effusion or pneumothorax, the assurance of POCUS is a necessary supplement to the physical exam.
Whether in the hospital or the dialysis unit, there is literature available to support the use of POCUS for volume assessment in nephrology practice. B-lines seen on lung ultrasound have been shown to correlate with extravascular lung water and disappear in real-time with ultrafiltration [23–25]. The number of B-lines correlates with mortality in many diseases, including heart failure and coronavirus disease 2019 [26, 27]. Lung ultrasound (LUS)-guided protocols have been shown to be useful to reduce blood pressure, but more studies are needed to determine if these protocols can reduce mortality in dialysis patients [28]. Similarly, ultrasound of the inferior vena cava (IVC) has been used by nephrologists to assess volume status in dialysis patients to ensure patients can tolerate fluid removal [29]. There is some evidence that inferior vena cava ultrasound (IVCUS)-guided protocols can reduce patient symptoms [30]. These techniques are not difficult to learn and implement; LUS can be learned in a short 4-h training session [31]. Likewise, IVCUS can be taught to both nephrologists and dialysis nurses alike in a short training session [32].
In the hospital setting, POCUS-based volume assessment can help identify patients at risk for intradialytic hypotension. da Hora Passos et al. [33] demonstrated an association between LUS and IVCUS and intradialytic hypotension in critically ill patients. Khanin et al. [34] similarly found an association between dry lungs on LUS and the likelihood of intradialytic hypotension. Kaptein and Kaptein [35, 36] looked at IVCUS in critically ill patients and found that a small collapsible IVC is associated with a lower likelihood of achieving the desired ultrafiltration goal. Taken together, these findings help support clinical decision making with ultrasound and ultrafiltration. There are currently randomized prospective trials under way to look at B-line assessment and adjustment of ultrafiltration with outpatient HD patients as well.
The intravascular fluid status in obese and edematous patients is often debated where a physical exam is difficult. The internal jugular vein (IJV) is often very difficult to assess in this population. Ultrasound removes this difficulty, removing the interobserver variability of IJV assessment and making it more accurate [37]. The assessment of the IJV with ultrasound, with or without the IVC, has been shown to correlate well with directly measured central venous pressure [38]. Indeed, in our own practice, these measurements have helped to correctly identify patients who would not benefit from fluid removal during dialysis, and lead to better patient care [39].
Focused cardiac ultrasound is a critical piece of assessing volume status and hemodynamics, however, there is comparatively little data on focused cardiac ultrasound when compared with studies looking at just IVC or LUS alone. Thus, while we present the findings of IVC and LUS trials, we feel it is important to emphasize the need for focused cardiac ultrasound in any fluid exam [40]. An important part of any nephrologist’s job is to evaluate dyspnea. A prospective study of focused cardiac ultrasound with LUS in the ED and emergency response unit in the hospital found that POCUS increased diagnostic certainty and accuracy in short-of-breath patients [41, 42]. In a large study of 2683 patients, POCUS was sensitive for ruling out heart failure [42]. In evaluating hypotension, whether in a case of dialysis or AKI, it is important for a nephrologist to be able to use POCUS. Shokoohi et al. [43] performed cardiac POCUS prospectively in 118 patients in the ED with hypotension, and found that POCUS reduced uncertainty by 27% and management was changed in a quarter of patients. There was a high concordance between the ultrasound diagnosis for the etiology of hypotension as compared with the expert chart review [6]. Velez et al. [44] performed IVCUS in patients with presumed hepatorenal syndrome and found that IVCUS changed management in ∼40% of cases. Lohani et al. [45] trained a nephrology fellow and medical student to assess left ventricular ejection fraction (EF) by POCUS on 52 dialysis patients. Compared with expert review, POCUS was 93% sensitive for a decreased EF, with a negative predictive value of 95%, although specificity was limited to 51%.
Pericardial effusion and tamponade are feared complications of advanced kidney failure. Traditional physical exam is poor at identifying pericardial effusion, but POCUS is sensitive and specific [46]. The use of ultrasound in this examination cannot be understated, and indeed the only cases of lawsuits regarding POCUS are when it is not performed [47].
Hemodialysis access
Nephrologists can also use ultrasound for temporary vascular access assessment. In placing a dialysis catheter, ultrasound guidance is now the standard of care [48]. However, going beyond that, LUS can be performed after placement and is highly sensitive to rule out a subsequent pneumothorax [49]. Indeed, there has been a growing trend to use ultrasound alone to assess the placement of central venous catheters (both dialysis and nondialysis catheters) and complications rather than X-ray [48, 50, 51]. POCUS can be used throughout the spectrum of dialysis access—from preoperative planning to access creation and maintenance. Preoperative or intraoperative ultrasound provides an objective, noninvasive assessment of both venous and arterial systems without exposure to radiation or potentially nephrotoxic contrast and may help to maximize arteriovenous fistula (AVF) placement. Traditionally, preoperative mapping is a detailed, comprehensive vascular examination performed by specially trained radiology technicians, but it is now increasingly being done at the point-of-care by trainees and physicians. In a retrospective analysis, vascular mapping by surgical residents at the time of initial consult decreased the time to dialysis access creation, bypassing the need for formal ultrasound [52]. In other studies, surgeon-performed ultrasound optimized AVF placement by decreasing rates of primary failure, enhancing anastomotic options and allowing for prompt intervention when needed [51, 53].
Once an AVF is placed, nephrologists and staff can be trained to use POCUS to evaluate an AVF for maturity and also to guide cannulation [54]. Although anecdotal data suggest that ultrasound-guided cannulation may minimize AVF infiltration and the subsequent sequelae, such as prolongation of catheter dependence, and the use of POCUS helps cannulation of difficult, deep and stented AVFs [55, 56], these benefits have not yet been published in a randomized trial. However, in a single-center quality improvement study, the authors showed that the use of serial ultrasound assessment in new AVFs at the point of care in dialysis units by dialysis staff to evaluate maturity and guide cannulation resulted in a marked decrease in infiltration rates [57]. The routine assessment of AVFs with POCUS and formal duplex studies has also been shown to help predict the successful cannulation and maturation of AVFs [58, 59].
Where is the nephrology community in terms of POCUS training?
Figure 2 shows the growing trend toward teaching ultrasound in nephrology fellowships over time. Berns and O'Neill [60] surveyed nephrology fellows and training program directors in 2008. They found that ultrasound teaching in fellowship programs was low but the interest was high. At that time, 8% of programs performed diagnostic kidney ultrasounds. Later, in 2016 and 2017, Sachdeva et al. [61] and Rope et al. [62] reported that 13% and 12%, respectively, of fellows reported having training in kidney ultrasound. In contrast, a more recent publication by Moore et al. [63] in 2021 reported that 38% of graduating nephrology fellows had some degree of ultrasound training. The survey by Moore et al. [63] was the first to ask explicitly about POCUS for volume assessment and they found that more than half of trainees and program directors felt this was important to the practice of nephrology.
FIGURE 2:
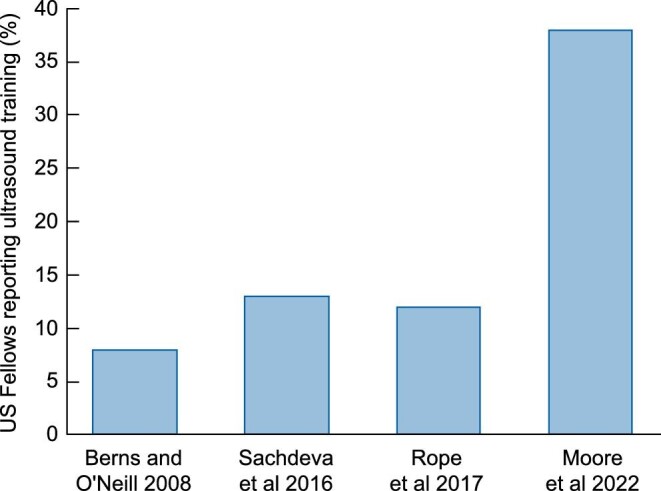
Results of nephrology trainee surveys over time.
It is very clear that POCUS is essential to the practice of nephrology and that our colleagues in other disciplines will expect us to be up-to-date with this monumental change in the practice of medicine. The optimum process for teaching nephrology trainees is not known. Table 1 shows that a number of curricula have been published including by Mullangi et al. [64] and Koratala et al. [65]. None of these curricula have yet been promoted by national societies. By comparison, other fields such as critical care and hospital medicine have had their respective professional societies endorse pathways to certification [66, 67]. A lesson can be learned from our critical care colleagues. In 2017, Greenstein et al. [68] reported on five consecutive POCUS courses that were held at the national American College of Chest Physicians headquarters in Glenview, IL., USA. Each course took place over 3 days and included a total of 363 students. They found a significant improvement between pre- and posttest scores and also high scores on clinical skills exams. This model of using focused courses at a national society was adopted by the Society of Hospital Medicine and American College of Physicians. Each offers a pathway for certification after follow-up studies at the learner’s home institution. For nephrology, the American Society of Diagnostic and Interventional Nephrology is presently working on a similar certification and the International Society of Nephrology has online modules available [69].
Table 1.
Published POCUS educational curricula in nephrology
| Author | Year | Duration of course | Country | Faculty | Students | Didactics | Hands-on | Ultrasound skills taught | Evaluation of student | Required follow-up studies | Learning evaluation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nunes et al. [72] | 2016 | 16 h over 2 days | Brazil | Nephrologists | Nephrology trainees and residents | Yes | Mix of live scanning and simulation software | Kidney, focused cardiac, lung, central venous access | Pre- and posttest knowledge, OSCE | Not specified | Pre- and posttests showed significant improvement, very good OSCE scores |
| Mullangi et al. [64] | 2019 | 8 h didactics, 10 h hands-on, 8 h image review, 30 h independent practice | USA | Non-nephrologist | Nephrology trainees | Yes | Mix of online image interpretations and live scanning | Kidney, focused cardiac, lung, dialysis access | Pre- and posttest confidence, OSCE | Not specified | High degree of learner confidence following course |
| Koratala et al. [65] | 2019 | 10 h of didactics over 6 months with live scanning sessions | USA | Nephrologist | Nephrology trainees | Yes | At least 10 supervised scans on live patients to ensure basic proficiency | Kidney, focused cardiac, lung, central venous access | Quality review of all saved images | 40–50 per exam type | Not specified |
| Moses et al. [73] | 2020 | 4 h of flipped classroom didactics with 4 h of hands-on sessions | USA | Nephrologists | Nephrology trainees | Yes | Mix of online image interpretations and live scanning, standardized patients | Kidney, focused cardiac, lung, fluid exam | Pre- and posttest knowledge, OSCE | Not specified | Pre- and posttests showed significant improvement, 100% pass of OSCEs |
| Young et al. [74] | 2022 | 2 h | Canada | Nephrologists and non-nephrologists | Nephrology trainees | No | Live scanning of healthy patients and patients with kidney disease | Kidney | Not specified | Goal was 50, but not achieved | High degree of student satisfaction but low adoption of skills in practice |
OSCE, objective structured clinical examination.
There has been declining interest in nephrology as a career among graduating internal medicine residents. In 2021, 27% of the available fellowship spots in the USA went unfilled [70]. Jhaveri et al. [71] asked graduating medical residents, ‘Why not nephrology’? ‘No role model’ and ‘not enough procedures’ were among the top reasons why graduating residents had negative views of nephrology. A nephrologist with a hand-carried ultrasound device can rapidly enhance patient care by identifying kidney disease, accurately assessing volume status and dialysis access. This, hands-on and proactive approach to patient care is likely to make internal medicine residents take notice, and lead to more enthusiasm for the subject.
As POCUS comes of age in nephrology, the next step is to make training and certification accessible to all nephrologists and to actively promote the use of POCUS by our professional societies. Nephrologists are already widely accepted by other fields as the masters of volume and the protector of nephrons, thus it is time for nephrologists to move into the future with an ultrasound probe in hand.
Contributor Information
Daniel W Ross, Donald and Barbara Zucker School of Medicine at Hofstra-Northwell, Division of Kidney Diseases and Hypertension, Great Neck, NY, USA.
Andrew A Moses, Donald and Barbara Zucker School of Medicine at Hofstra-Northwell, Lenox Hill Division of Nephrology, New York, NY, USA.
Vandana Dua Niyyar, Emory University, Division of Nephrology, Woodruff Memorial Research Building, Atlanta GA, USA.
CONFLICT OF INTEREST STATEMENT
D.R. and A.M. have nothing to disclose. V.N. has served as a consultant for Medtronic and Eversana. The results in this article have not been published previously in whole or in part.
REFERENCES
- 1. Walker HK. The origins of the history and physical examination. In: Walker HK, Hall WD, Hurst JW, eds. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed.LeBoston: Butterworths, 1990. http://www.ncbi.nlm.nih.gov/books/NBK458/ (31 December 2021, date last accessed). [PubMed] [Google Scholar]
- 2. Kane D. A brief history of musculoskeletal ultrasound: ‘From bats and ships to babies and hips.’ Rheumatology (Oxford) 2004; 43: 931–933. [DOI] [PubMed] [Google Scholar]
- 3. Filly RA. Ultrasound: the stethoscope of the future, alas. Radiology 1988; 167: 400. [DOI] [PubMed] [Google Scholar]
- 4. Narula J, Chandrashekhar Y, Braunwald E. Time to add a fifth pillar to bedside physical examination: inspection, palpation, percussion, auscultation, and insonation. JAMA Cardiol 2018; 3: 346–350. [DOI] [PubMed] [Google Scholar]
- 5. Bhagra A, Tierney DM, Sekiguchi Het al. Point-of-care ultrasonography for primary care physicians and general internists. Mayo Clin Proc 2016; 91: 1811–1827. [DOI] [PubMed] [Google Scholar]
- 6. Shokoohi H, Boniface KS, Pourmand Aet al. Bedside ultrasound reduces diagnostic uncertainty and guides resuscitation in patients with undifferentiated hypotension. Crit Care Med 2015; 43: 2562–2569. [DOI] [PubMed] [Google Scholar]
- 7. Dinh VA, Fu JY, Lu Set al. Integration of ultrasound in medical education at united states medical schools: a national survey of directors’ experiences. J Ultrasound Med 2016; 35: 413–419. [DOI] [PubMed] [Google Scholar]
- 8. Rempell JS, Saldana F, DiSalvo Det al. Pilot point-of-care ultrasound curriculum at Harvard Medical School: early experience. West J Emerg Med 2016; 17: 734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nelson BP, Narula S, Argulian Eet al. Including insonation in undergraduate medical school curriculum. Ann Glob Health 2019; 85: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. An East Coast First for the Lewis Katz School of Medicine Class of 2025. Lewis Katz School of Medicine at Temple University. https://medicine.temple.edu/news/temple-medicine-students-butterfly-ultrasounds (2 January 2022, date last accessed). [Google Scholar]
- 11. Rosana M, Asmara OD, Pribadi RRet al. Internal medicine residents’ perceptions of point-of-care ultrasound in residency program: highlighting the unmet needs. Acta Med Indones 2021; 53: 299–307. [PubMed] [Google Scholar]
- 12. Olgers TJ, Ter Maaten JC. Point-of-care ultrasound curriculum for internal medicine residents: what do you desire? A national survey. BMC Medical Education 2020; 20: 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. LoPresti CM, Jensen TP, Dversdal RKet al. Point-of-care ultrasound for internal medicine residency training: a position statement from the alliance of academic internal medicine. Am J Med 2019; 132: 1356–1360. [DOI] [PubMed] [Google Scholar]
- 14. Soni NJ, Schnobrich D, Mathews BKet al. Point-of-care ultrasound for hospitalists: a position statement of the society of hospital medicine. Am J Hosp Med 2019; 14: E1–E6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American College of Physicians. Our Statement in Support of Point-of-Care Ultrasound in Internal Medicine . https://www.acponline.org/meetings-courses/focused-topics/point-of-care-ultrasound-pocus-for-internal-medicine/acp-statement-in-support-of-point-of-care-ultrasound-in-internal-medicine (7 March 2022, date last accessed). [Google Scholar]
- 16. Wong C, Teitge B, Ross Met al. The accuracy and prognostic value of point-of-care ultrasound for nephrolithiasis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med 2018; 25: 684–698. [DOI] [PubMed] [Google Scholar]
- 17. Sibley S, Roth N, Scott Cet al. Point-of-care ultrasound for the detection of hydronephrosis in emergency department patients with suspected renal colic. Ultrasound J 2020; 12: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanno T, Kubota M, Sakamoto Het al. The efficacy of ultrasonography for the detection of renal stone. Urology 2014; 84: 285–288. [DOI] [PubMed] [Google Scholar]
- 19. Moghazi S, Jones E, Schroepple Jet al. Correlation of renal histopathology with sonographic findings. Kidney Int 2005; 67: 1515–1520. [DOI] [PubMed] [Google Scholar]
- 20. Bhutani H, Smith V, Rahbari-Oskoui Fet al. A comparison of ultrasound and magnetic resonance imaging shows that kidney length predicts chronic kidney disease in autosomal dominant polycystic kidney disease. Kidney Int 2015; 88: 146–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moses AA, Fernandez HE. Ultrasonography in acute kidney injury. POCUS J 2022; 7: 35–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Koratala A. Point-of-care renal ultrasound: the SECONDS checklist. Clin Exp Nephrol 2022; 26: 486–487. [DOI] [PubMed] [Google Scholar]
- 23. Jiang C, Patel S, Moses Aet al. Use of lung ultrasonography to determine the accuracy of clinically estimated dry weight in chronic hemodialysis patients. Int Urol Nephrol 2017; 49: 2223–2230. [DOI] [PubMed] [Google Scholar]
- 24. Noble VE, Murray AF, Capp Ret al. Ultrasound assessment for extravascular lung water in patients undergoing hemodialysis. Time course for resolution. Chest 2009; 135: 1433–1439. [DOI] [PubMed] [Google Scholar]
- 25. Reisinger N, Koratala A. Quantitative lung ultrasonography for the nephrologist: applications in dialysis and heart failure. Kidney360 2022; 3: 176–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dubón-Peralta EE, Lorenzo-Villalba N, García-Klepzig JLet al. Prognostic value of B lines detected with lung ultrasound in acute heart failure. A systematic review. J Clin Ultrasound 2022; 50: 273–283. [DOI] [PubMed] [Google Scholar]
- 27. Ji L, Cao C, Gao Yet al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Critical Care 2020; 24: 700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Loutradis C, Sarafidis PA, Ekart Ret al. The effect of dry-weight reduction guided by lung ultrasound on ambulatory blood pressure in hemodialysis patients: a randomized controlled trial. Kidney Int 2019; 95: 1505–1513. [DOI] [PubMed] [Google Scholar]
- 29. Kaptein MJ, Kaptein JS, Oo Zet al. Relationship of inferior vena cava collapsibility to ultrafiltration volume achieved in critically ill hemodialysis patients. Int J Nephrol Renovasc Dis 2018; 11: 195–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brennan JM, Ronan A, Goonewardena Set al. Handcarried ultrasound measurement of the inferior vena cava for assessment of intravascular volume status in the outpatient hemodialysis clinic. Clin J Am Soc Nephrol 2006; 1: 749–753. [DOI] [PubMed] [Google Scholar]
- 31. House DR, Amatya Y, Nti Bet al. Lung ultrasound training and evaluation for proficiency among physicians in a low-resource setting. Ultrasound J 2021; 13: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Steinwandel U, Gibson N, Towell Aet al. Can a renal nurse assess fluid status using ultrasound on the inferior vena cava? A cross-sectional interrater study. Hemodialysis Int 2018; 22: 261–269. [DOI] [PubMed] [Google Scholar]
- 33. da Hora Passos R, Caldas J, Ramos JGRet al. Ultrasound-based clinical profiles for predicting the risk of intradialytic hypotension in critically ill patients on intermittent dialysis: a prospective observational study. Crit Care 2019; 23: 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Khanin Y, Hirsch JS, Stalbow Det al. Intradialytic hypotension in critically ill patients on hemodialysis with A-line versus B-line pattern on lung ultrasonography. Kidney Int Rep 2021; 6: 1969–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaptein MJ, Kaptein EM. Inferior vena cava collapsibility index: clinical validation and application for assessment of relative intravascular volume. Adv Chronic Kidney Dis 2021; 28: 218–226. [DOI] [PubMed] [Google Scholar]
- 36. Kaptein YE, Kaptein EM. Comparison of subclavian vein to inferior vena cava collapsibility by ultrasound in acute heart failure: a pilot study. Clin Cardiol 2022; 45: 51–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Istrail L, Stepanova M. Novel point-of-care ultrasound (POCUS) technique to modernize the JVP exam and rule out elevated right atrial pressures. medRxiv 2021; 10.1101/2021.10.14.21264891. [DOI] [Google Scholar]
- 38. Jassim HM, Naushad VA, Khatib MYet al. IJV collapsibility index vs IVC collapsibility index by point of care ultrasound for estimation of CVP: a comparative study with direct estimation of CVP. Open Access Emerg Med 2019; 11: 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. International Society of Nephrology Academy . ISN Point of Care Ultrasound (POCUS) Quick Cases by Andrew Moses. https://academy.theisn.org/isn/2020/isn-pocus-quick-cases/312374/andrew.moses.isn.point.of.care.ultrasound.28pocus29.quick.cases.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dpocus (8 April 2022, date last accessed). [Google Scholar]
- 40. International Society of Nephrology Academy . POCUS Curriculum by Faculty - Nathaniel Reisinger, Basics of Echocardiography for the Nephrologist. https://academy.theisn.org/isn/2021/pocus-curriculum/314419/andrew.moses.the.fluid.exam.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dpocus (8 April 2022, date last accessed). [Google Scholar]
- 41. Blans MJ, Bousie E, van der Hoeven JGet al. A point-of-care thoracic ultrasound protocol for hospital medical emergency teams (METUS) improves diagnostic accuracy. Ultrasound J 2021; 13: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zanobetti M, Scorpiniti M, Gigli Cet al. Point-of-care ultrasonography for evaluation of acute dyspnea in the ED. Chest 2017; 151: 1295–1301. [DOI] [PubMed] [Google Scholar]
- 43. Shokoohi H, Boniface KS, Zaragoza Met al. Point-of-care ultrasound leads to diagnostic shifts in patients with undifferentiated hypotension. Am J Emerg Med 2017; 35: 1984.e3–1984.e7. [DOI] [PubMed] [Google Scholar]
- 44. Velez JCQ, Petkovich B, Karakala Net al. Point-of-care echocardiography unveils misclassification of acute kidney injury as hepatorenal syndrome. Am J Nephrol 2019; 50: 204–211. [DOI] [PubMed] [Google Scholar]
- 45. Lohani S, Annadanam S, Martire Cet al. Point-of-care ultrasound for evaluation of systolic heart function in outpatient hemodialysis units. Kidney Med 2021; 3: 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hanson MG, Chan B. The role of point-of-care ultrasound in the diagnosis of pericardial effusion: a single academic center retrospective study. Ultrasound J 2021; 13: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Stolz L, O'Brien KM, Miller MLet al. A review of lawsuits related to point-of-care emergency ultrasound applications. West J Emerg Med 2015; 16: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Saugel B, Scheeren TWL, Teboul JL. Ultrasound-guided central venous catheter placement: a structured review and recommendations for clinical practice. Crit Care 2017; 21: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Husain LF, Hagopian L, Wayman Det al. Sonographic diagnosis of pneumothorax. J Emerg Trauma Shock 2012; 5: 76–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ablordeppey EA, Drewry AM, Beyer ABet al. Diagnostic accuracy of central venous catheter confirmation by bedside ultrasound versus chest radiography in critically ill patients: a systematic review and meta-analysis. Crit Care Med 2017; 45: 715–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Montrief T, Auerbach J, Cabrera Jet al. Use of point-of-care ultrasound to confirm central venous catheter placement and evaluate for postprocedural complications. J Emerg Med 2021; 60: 637–640. [DOI] [PubMed] [Google Scholar]
- 52. Gray K, Korn A, Zane Jet al. Ultrasound vein and artery mapping by general surgery residents during initial consult can decrease time to dialysis access creation. Ann Vasc Surg 2018; 49: 285–288. [DOI] [PubMed] [Google Scholar]
- 53. Jennings WC, Parker DE. Creating arteriovenous fistulas using surgeon-performed ultrasound. J Vasc Access 2016; 17: 333–339. [DOI] [PubMed] [Google Scholar]
- 54. Niyyar VD. Ultrasound-based simulation for cannulation in outpatient hemodialysis units: an educational protocol. J Vasc Access 2021; 22: 585–589. [DOI] [PubMed] [Google Scholar]
- 55. Schoch M, Bennett PN, Currey Jet al. Point-of-care ultrasound use for vascular access assessment and cannulation in hemodialysis: a scoping review. Semin Dial 2020; 33: 355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schoch M, Bennett PN, Currey Jet al. Point-of-care ultrasound-guided cannulation versus standard cannulation in hemodialysis vascular access: a controlled random order crossover pilot feasibility study. J Vasc Access 2022; doi: 10.1177/11297298211069821. [DOI] [PubMed] [Google Scholar]
- 57. Dua Niyyar V, Buch K, Rawls Fet al. Effectiveness of ultrasound-guided cannulation of AVF on infiltration rates: a single center quality improvement study. J Vasc Access 2021; doi: 10.1177/11297298211034280. [DOI] [PubMed] [Google Scholar]
- 58. Etkin Y, Talathi S, Rao Aet al. The role of duplex ultrasound in assessing AVF maturation. Ann Vasc Surg 2021; 72: 315–320. [DOI] [PubMed] [Google Scholar]
- 59. Robbin ML, Greene T, Allon Met al. Prediction of arteriovenous fistula clinical maturation from postoperative ultrasound measurements: findings from the Hemodialysis Fistula Maturation Study. J Am Soc Nephrol 2018; 29: 2735–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Berns JS, O'Neill WC. Performance of procedures by nephrologists and nephrology fellows at U.S. nephrology training programs. Clin J Am Soc Nephrol 2008; 3: 941–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sachdeva M, Ross DW, Shah HH. Renal ultrasound, dialysis catheter placement, and kidney biopsy experience of US nephrology fellows. Am J Kidney Dis 2016; 68: 187–192. [DOI] [PubMed] [Google Scholar]
- 62. Rope RW, Pivert KA, Parker MGet al. Education in nephrology fellowship: a survey-based needs assessment. J Am Soc Nephrol 2017; 28: 1983–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Moore CA, Ross D, O'Neill WC. Point-of-care ultrasound training for nephrologists: a national survey of nephrology fellows. In: Vol Kidney Week Edition. J Am Soc Nephrol 2021; 356. [Google Scholar]
- 64. Mullangi S, Sozio SM, Hellmann DBet al. Integrative point-of-care ultrasound curriculum to impart diagnostic skills relevant to nephrology. Am J Kidney Dis 2019; 73: 894–896. [DOI] [PubMed] [Google Scholar]
- 65. Koratala A, Segal MS, Kazory A. Integrating point-of-care ultrasonography into nephrology fellowship training: a model curriculum. Am J Kidney Dis 2019; 74: 1–5. [DOI] [PubMed] [Google Scholar]
- 66. Marik PE, Mayo P. Certification and training in critical care ultrasound. Intensive Care Med 2008; 34: 215–217. [DOI] [PubMed] [Google Scholar]
- 67. POCUS Certificate of Completion. https://www.hospitalmedicine.org/clinical-topics/ultrasound/pocus-certificate-of-completion/ (8 March 2022, date last accessed). [Google Scholar]
- 68. Greenstein YY, Littauer R, Narasimhan Met al. Effectiveness of a critical care ultrasonography course. Chest 2017; 151: 34–40. [DOI] [PubMed] [Google Scholar]
- 69. International Society of Nephrology Academy. ISN POCUS Curriculum. https://academy.theisn.org/isn/2021/pocus-curriculum/314419/andrew.moses.the.fluid.exam.html?f=listing%3D0%2Abrowseby%3D8%2Asortby%3D1%2Asearch%3Dpocus (8 April 2022, date last accessed). [Google Scholar]
- 70. National Resident Matching Program. Fellowship Data & Reports . https://www.nrmp.org/match-data-analytics/fellowship-data-reports/ (25 April 2022, date last accessed). [Google Scholar]
- 71. Jhaveri KD, Sparks MA, Shah HHet al. Why not nephrology? A survey of US internal medicine subspecialty fellows. Am J Kidney Dis 2013; 61: 540–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Nunes AA, Júnior JMP, Rodrigues ATet al. Development of skills to utilize point-of-care ultrasonography in nephrology practice. J Bras Nefrol. 2016; 38: 38: 209–214. [DOI] [PubMed] [Google Scholar]
- 73. Moses AA, Fernandez HE. Point-of-care ultrasound education in nephrology during the COVID-19 pandemic. J Am Soc Nephrol 2021: 356–357. [Google Scholar]
- 74. Young A, Imbeault B, Goffi Aet al. Integrating point of care ultrasound into nephrology fellowship training: insights from a pilot program. POCUS J 2022; 7: 5–6. [DOI] [PMC free article] [PubMed] [Google Scholar]



