ABSTRACT
Background
Chronic kidney disease (CKD) is an increasing global health problem, but little is known about the age- and sex-specific prevalence of CKD and the associated risk factors in low- and middle-income populations. We examined the age- and sex-specific prevalence of CKD and the associated risk factors in a population-based study of 9 million Chinese adults.
Methods
The study involved a cross-sectional survey of 9 461 631 adults, >18 years of age, who were recruited in 2017 from 31 provinces in the Meinian Onehealth screening survey. All participants had plasma creatinine measured by standard methods and CKD was defined if the estimated glomerular filtration rate (eGFR) was <60 ml/min/1.73 m2.
Results
Overall, among 9.5 million adults [mean age 41 years (standard deviation 13.1)], 88 271 (1.26%) had CKD. The prevalence rate of CKD was 1.20%, 0.04% and 0.02% for stage 3, 4 and 5, respectively. After adjustment for the proportion and prevalence of urban and rural areas, the overall prevalence rate of CKD was 1.07%, indicating that ∼14 million Chinese adults have CKD. The prevalence of CKD increased 3-fold for each 10-year increment in age (1.15%, 3.05% and 13.02% at age 50–59, 60–69 and >70 years, respectively) and was 1.8-fold higher in women than men. The prevalence of CKD was higher in the Southwest region {1.68% [95% confidence interval (CI) 1.12–2.24]} but lower in the Northwest region [0.84% (95% CI 0.61–1.07)] than other regions. If proteinuria is also used as a diagnostic criterion, the prevalence rate increased to 2.16%. Stepwise logistic regression analysis demonstrated that body mass index; history of hypertension, cardiovascular disease or diabetes; and levels of systolic blood pressure, triglycerides, fasting glucose and uric acid were independent risk factors for CKD.
Conclusion
CKD is an important public health problem in Chinese adults and this study highlights the need for public health strategies to detect and reduce modifiable risk factors for prevention of CKD.
Keywords: chronic kidney disease, cross-sectional study, prevalence, public health, risk factor
INTRODUCTION
The prevalence of chronic kidney disease (CKD) worldwide has increased substantially in recent decades. CKD is an important public health problem that is associated with higher risks of cardiovascular mortality and end-stage renal disease [1]. Secular trends in economic growth in China have been accompanied by an ageing population and changes in lifestyle and socio-economic circumstances, all of which may exacerbate the burden of CKD [2].
During recent decades, the prevalence of CKD has increased exponentially in China [1, 3]. A cross-sectional survey in 2009–2010 involving a nationally representative sample of 47 204 Chinese adults from 13 provinces reported that the overall prevalence of CKD was 10.8% [95% confidence interval (CI) 10.2–11.3] and estimated that 119.5 million Chinese adults had CKD [1]. The China Kidney Disease Network (CK-NET) 2015 Annual Data Report review of hospitalized adult patients reported that patients with CKD accounted for 4.8% of the overall hospitalized population [3]. The prevalence of CKD differs between different geographic regions in China [1, 4–6], perhaps reflecting the variable prevalence of lifestyle and economic circumstances, but the possibility of differences in the sampling methods, screening procedures and diagnostic criteria used cannot be excluded [1, 4–8]. Previous national surveys were unable to assess any geographical differences in the prevalence of CKD due to their limited sample size and regions included [1, 4–6].
The aims of the present report were to estimate the age- and sex-specific prevalence of CKD in Chinese adults overall and separately by diverse regions in a nationally representative cross-sectional study of 11 903 244 Chinese white-collar workers in 2017 and to examine the separate and joint effects of associated risk factors for CKD in this population.
MATERIALS AND METHODS
Study design and population
The Meinian Onehealth screening survey recruited employed adults from >700 health screening centres in all provinces in China. All adult participants (≥18 years of age) had information collected using interviewer-administered questionnaires on demographic information and medical history and had physical measurements recorded and blood samples collected.
Information was collected between January 2017 and December 2017. Data were collected in examination centres in the participants’ residential area. All participants provided information on their sociodemographic status, personal and family medical history [e.g. hypertension, diabetes and cardiovascular disease (CVD)] and lifestyle using interview-administered questionnaires. All study investigators and staff members completed a training program about the aims and methods for the study. A manual of procedures was circulated and detailed instructions for administration of the questionnaires, recording of blood pressure and anthropometric measurements and collection of biological samples were provided. Participants were asked to provide a blood sample and urine spot sample after an overnight fast. Ethics approval was obtained from the ethics committee of Peking University.
Biochemical measurement and assessment of CKD
Total urinary protein and urinary albumin and creatinine concentrations were measured using the fresh morning urine spot samples. Total urinary protein was assessed using a semiquantitative method (i.e. dipstick) and urinary albumin was assessed using a quantitative method (i.e. immunoturbidimetric method). The methods were the same in all centres. We defined proteinuria as total urinary protein >1 g/l (++ or more), which is a strong predictor of CKD [9]. Urinary creatinine was measured using the Jaffe method [10]. Serum creatinine (SCr) was measured using the isotope dilution mass spectrometry–traceable Jaffe method on venous blood samples collected after an overnight fast [10]. Renal function was classified using estimated glomerular filtration rate (eGFR), calculated using the 2009 Chronic Kidney Disease Epidemiology Collaboration equation [11]. Reduced renal function was defined as an eGFR <60 ml/min/1.73 m2: eGFR = 175 × SCr − 1.234 × age − 0.179 [if female, × 0.79], where SCr is the serum creatinine concentration (in mg/dl) and age is in years. Individuals were classified as having CKD if the eGFR was <60 ml/min/1.73 m2.
Assessment of covariates
Covariates collected in the study included age and gender, body mass index (BMI), blood pressure (BP), heart rate (HR), uric acid (UA), triglycerides (TG), total cholesterol (TC), high-sensitivity C-reactive protein, region and gross domestic product (GDP). Body weight and height were measured by standard methods. BMI was calculated as weight in kilograms divided by the square of height in meters. Obesity was defined as a BMI ≥28 kg/m2 and overweight as a BMI of 24–27.9 kg/m2. Systolic BP, diastolic BP and HR were measured using a digital automatic BP monitor (HBP-9020, OMRON, Kyoto, Japan) after participants had been seated for at least 5 minutes. BP and HR were measured twice, with a 5-minute interval between measurements. The mean of two consecutive BP and HR readings were recorded. Fasting blood glucose was measured enzymatically using a glucose oxidase method. TC, TG and UA were assessed by an autoanalyser at the central laboratory of the health screening centres. Diabetes was defined if fasting plasma glucose was >7.0 mmol/l or if participants had a self-reported history of diabetes.
Statistical analysis
Continuous variables were presented as mean and standard deviation (SD) for normally distributed variables and categorical variables were presented as proportions. Mean and 95% confidence intervals (CIs) were estimated using SD values. Differences in mean values between the different groups were compared using the analysis of variance and the Student–Newman–Keuls test.
The prevalence of CKD was estimated by age, sex and levels of socio-economic status. In addition to crude prevalence, the prevalence estimates and comparisons were weighted to represent the total adult population in China. Furthermore, the adjusted prevalence of CKD was reported in different geographical regions of China (Supplementary Figure 1). Choropleth maps were produced using R version 4.1.1 software (R Foundation for Statistical Computing, Vienna, Austria) to visually examine the geographical variation in prevalence of CKD across China. Considering that the participants in our study come from urban areas, we also adjusted for the proportion and prevalence of urban (49.68% and 2.3%, respectively) and rural areas (50.32% and 1.6%, respectively) from a previous study [1] to obtain national results of the overall CKD prevalence.
Logistic regression models were used to assess the associations of risk factors with the risk of CKD, yielding crude and multivariable adjusted odds ratios (ORs) and their 95% CIs. Covariates included in the multivariable logistic regression models included age (10-year age groups), sex, history of CVD (yes versus no), hypertension (yes versus no), diabetes (yes versus no), region, lipids, BP, HR and UA. Analyses also included cubic spline regression to examine a possible non-linear relation between CKD and covariates including systolic BP, HR, BMI, TG concentration, low-density lipoprotein (LDL) cholesterol concentration and UA. The likelihood ratio test was used to test for non-linearity. P-values <.05 were considered statistically significant. All statistical analyses were conducted using SAS software version 9.1.4 (SAS, Cary, NC, USA).
RESULTS
Characteristics of all study participants
A total of 9 461 631 participants completed the interview and physical examination in 2017. The distribution of selected characteristics of the study population is shown in Table 1. Patients with CKD, as compared with non-CKD participants, were older; more likely to be female; had higher levels of BMI, BP, TC, TG and fasting glucose; and a history of diabetes, CVD or hypertension. Likewise, older people, females and participants with higher metabolic risk factors had lower eGFR levels (Supplementary Table 1).
Table 1.
Selected characteristics of study participants stratified by presence or absence of CKD
| Characteristics | Total | CKD | Non-CKD | P-value |
|---|---|---|---|---|
| Participants, n | 9 461 631 | 88 271 | 9 373 360 | |
| Demographics | ||||
| Age (years), mean (SD) | 41.1 (13.3) | 64.2 (14.1) | 40.9 (13.1) | <.001 |
| Female, n (%) | 4 402 939 (46.5) | 41 651 (47.2) | 4 361 288 (46.5) | <.001 |
| Live in the north, n (%) | 3 806 293 (40.2) | 31 754 (36.0) | 3 774 539 (40.3) | <.001 |
| Prior diseases, n (%) | ||||
| Diabetes | 467 410 (5.11) | 14 663 (16.8) | 452 747 (5.00) | <.001 |
| Hypertension | 1 922 436 (21.9) | 47 290 (56.0) | 1 875 146 (21.6) | <.001 |
| CVD | 52 693 (0.72) | 3040 (4.63) | 49 653 (0.69) | <.001 |
| Metabolic risk factors, mean (SD) | ||||
| Systolic BP (mmHg) | 123 (18.1) | 140 (22.8) | 123 (18.0) | <.001 |
| Diastolic BP (mmHg) | 75.2 (12.0) | 79.7 (13.6) | 75.2 (12.0) | <.001 |
| Heart rate (bpm) | 73.0 (9.16) | 73.2 (10.1) | 73.0 (9.15) | <.001 |
| BMI (kg/m2) | 24.0 (3.62) | 24.9 (3.42) | 23.9 (3.62) | <.001 |
| LDL (mmol/l) | 2.70 (0.82) | 2.90 (0.92) | 2.70 (0.82) | <.001 |
| HDL (mmol/l) | 1.36 (0.34) | 1.37 (0.36) | 1.36 (0.34) | <.001 |
| TC (mmol/l) | 4.80 (0.98) | 5.14 (1.14) | 4.79 (0.97) | <.001 |
| TG (mmol/l) | 1.53 (1.25) | 1.83 (1.36) | 1.52 (1.24) | <.001 |
| Fasting glucose (mg/dl) | 5.16 (1.24) | 5.75 (1.83) | 5.16 (1.23) | <.001 |
| Kidney function, mean (SD) | ||||
| UA (µmol/l) | 331 (95.0) | 399 (113) | 330 (94.6) | <.001 |
| Creatinine (µmol/l) | 69.4 (18.3) | 124 (72.9) | 68.8 (16.1) | <.001 |
| eGFR (ml/min/1.73 m2) | 106 (17.9) | 51.2 (9.86) | 106 (17.2) | <.001 |
| Proteinuria, n (%)a | 120 196 (1.36) | 11 088 (13.6) | 109 108 (1.25) | <.001 |
CKD was defined as an eGFR <60 ml/min/1.73 m2.
Hypertension was defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or a self-reported history of hypertension.
Diabetes was defined as fasting glucose ≥7.0 mmol/l or haemoglobin A1c ≥6.5% or a self-reported history of diabetes.
CVD was defined as a self-reported history of coronary heart disease.
Proteinuria was defined as a total urinary protein concentration >1 g/l (++ or more).
Prevalence of CKD and mean plasma creatinine concentrations and eGFR
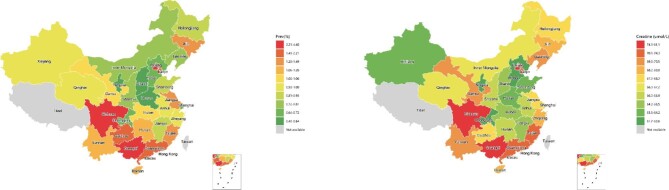
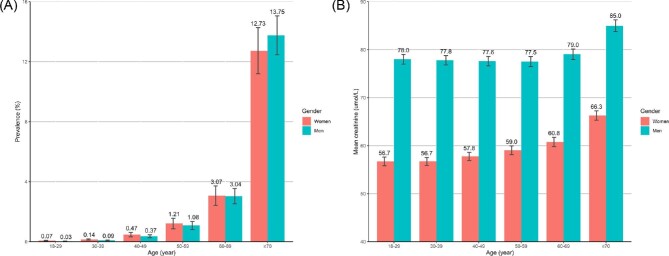
The prevalence of CKD increased 3-fold for each 10-year increment in age (1.15%, 3.05% and 13.02% at age 50–59, 60–69 and ≥70 years, respectively) and was 1.8-fold higher in women than men. Overall, 1.26% (95% CI 1.07–1.46) had CKD and the prevalence was higher in women than in men: 1.33% (95% CI 1.10–1.56) versus 1.20% (95% CI 1.0–1.38), respectively (Table 2). After adjustment for the proportion and prevalence in urban and rural areas, the overall prevalence rate of CKD was 1.07%. Extrapolating these results to the Chinese population suggests that ∼14.44 million adults >18 years of age had CKD (including 7.42 million women and 7.02 million men). The prevalence of CKD varied substantially by geographic region within China (Figure 1 and Supplementary Figure 2a). The characteristics of participants by regions in the Meinian Onehealth study are also shown in Supplementary Table 2 and Supplementary Figure 2b. Overall, individuals who were overweight (1.60%) and obese (1.44%) had a higher prevalence of CKD than those with normal weight (1.04%) (P < .001). Likewise, patients with diagnosed diabetes had higher age- and sex-standardized prevalence of CKD (4.46% versus 1.13%) and higher fasting creatinine levels [70.1 (SD 26.2) versus 68.3 (SD 17.8) µmol/l] than did those with undiagnosed diabetes (P < .001). The age- and sex-standardized prevalence of CKD also increased exponentially with increasing age, with the highest prevalence of CKD recorded in those >70 years of age (13.2% in total, 13.75% in men and 12.73% in women) (Figure 2a). Among participants 18–69 years of age, the age-specific prevalence of CKD was higher in women than in men. However, men had a higher prevalence of CKD than women among participants >70 years of age (Figure 2a). Men consistently demonstrated higher levels of creatinine (Figure 2b) and lower levels of eGFR (Supplementary Figure 3) than did women across all age groups. Individuals living in the Southern [2.35% (95% CI 0.91–3.79)] and Southwestern [1.68% (95% CI 1.12–2.24)] regions of China had much higher age- and sex-standardized prevalence of CKD. In particular, individuals living in the Sichuan and Guangxi provinces had the highest age- and sex-standardized prevalence of CKD (Figure 1a) and the highest levels of serum creatinine (Figure 1b). However, Northwest China had the lowest age- and sex-standardized prevalence of CKD [0.84% (95% CI 0.61–1.07)].
Table 2.
Age- and sex-standardized prevalence of CKD and mean values of creatinine in the overall population and in subgroups
| CKD prevalence (%), mean (95% CI) | Creatinine (µmol/l), mean (SD) | |||||
|---|---|---|---|---|---|---|
| Characteristics | Participants, n | Cases, n | Crude | Adjusted | Crude | Adjusted |
| Overall | 9 461 631 | 88 271 | 0.93 (0.79–1.07) | 1.26 (1.07–1.46) | 69.4 (18.3) | 68.4 (18.3) |
| Sex | ||||||
| Women | 4 402 939 | 41 651 | 0.95 (0.76–1.14) | 1.33 (1.10–1.56) | 58.4 (13.2) | 58.1 (13.5) |
| Men | 5 058 692 | 46 620 | 0.92 (0.80–1.04) | 1.20 (1.03–1.38) | 78.9 (16.8) | 78.3 (16.9) |
| BMI | ||||||
| <18.5 | 399 211 | 1775 | 0.44 (0.37–0.52) | 0.66 (0.55–0.77) | 64.2 (16.9) | 63.1 (18.5) |
| 18.5–24.9 | 4 937 477 | 38 283 | 0.78 (0.65–0.90) | 1.04 (0.85–1.22) | 67.8 (17.9) | 66.8 (17.7) |
| 25.0–29.9 | 2 567 817 | 30 412 | 1.18 (1.00–1.37) | 1.60 (1.34–1.86) | 72.4 (18.4) | 71.7 (18.4) |
| ≥30 | 460 127 | 5196 | 1.13 (0.96–1.30) | 1.44 (1.21–1.67) | 71.9 (18.9) | 71.5 (18.4) |
| Age (years) | ||||||
| 18–29 | 2 159 516 | 1185 | 0.05 (0.04–0.07) | 0.05 (0.04–0.06) | 69.3 (16.8) | 67.5 (16.3) |
| 30–39 | 2 739 774 | 3455 | 0.13 (0.09–0.16) | 0.12 (0.09–0.14) | 69.1 (17.4) | 67.5 (16.9) |
| 40–49 | 2 028 848 | 8969 | 0.44 (0.33–0.55) | 0.42 (0.30–0.53) | 68.7 (18.2) | 68.1 (17.9) |
| 50–59 | 1 531 715 | 17 447 | 1.14 (0.91–1.37) | 1.15 (0.85–1.44) | 69.0 (18.9) | 68.4 (18.9) |
| 60–69 | 747 543 | 23 043 | 3.08 (2.58–3.58) | 3.05 (2.50–3.61) | 70.1 (20.8) | 69.8 (22.0) |
| ≥70 | 254 235 | 34 172 | 13.4 (12.0–14.9) | 13.2 (11.9–14.6) | 77.1 (26.3) | 75.1 (26.2) |
| Region | ||||||
| North | 1 194 424 | 13 964 | 1.17 (0.77–1.57) | 1.42 (0.95–1.89) | 70.1 (17.4) | 69.4 (17.4) |
| East | 2 878 302 | 18 377 | 0.64 (0.41–0.87) | 1.01 (0.74–1.29) | 67.1 (17.7) | 67.6 (18.1) |
| Central | 1 392 312 | 10 690 | 0.77 (0.56–0.98) | 0.94 (0.65–1.24) | 65.1 (17.6) | 65.1 (17.5) |
| South | 1 313 092 | 13 148 | 1.00 (0.50–1.51) | 2.35 (0.91–3.79) | 73.2 (19.0) | 73.2 (20.1) |
| Southwest | 1 326 450 | 22 387 | 1.69 (1.22–2.16) | 1.68 (1.12–2.24) | 75.5 (19.5) | 70.3 (19.6) |
| Northwest | 589 818 | 3987 | 0.68 (0.50–0.85) | 0.84 (0.61–1.07) | 66.4 (16.2) | 65.6 (17.6) |
| Northeast | 767 233 | 5718 | 0.75 (0.61–0.88) | 0.98 (0.71–1.26) | 69.3 (17.4) | 68.8 (17.1) |
| Hypertension | ||||||
| Yes | 1 922 436 | 47 290 | 2.46 (2.14–2.78) | 3.61 (3.08–4.13) | 72.3 (22.2) | 72.2 (23.1) |
| No | 6 846 931 | 32 894 | 0.48 (0.39–0.58) | 0.57 (0.46–0.67) | 68.6 (16.9) | 67.4 (16.5) |
| Diabetes | ||||||
| Yes | 467 410 | 14 663 | 3.14 (2.73–3.54) | 4.46 (3.89–5.03) | 70.1 (23.5) | 70.1 (26.2) |
| No | 8 683 406 | 72 446 | 0.83 (0.70–0.97) | 1.13 (0.96–1.31) | 69.3 (18.0) | 68.3 (17.8) |
Prevalence of CKD and mean values of creatinine show significant differences across all category groups (P < .001). Diabetes was defined as fasting glucose ≥7.0 mmol/l or haemoglobin A1c ≥6.5% or a self-reported history of diabetes.
FIGURE 1:
The geographic distribution of CKD (eGFR ≤60 ml/min/1.73 m2) and plasma creatinine concentrations among Chinese adults.
FIGURE 2:
The age- and sex-specific prevalence of CKD (eGFR ≤60 ml/min/1.73 m2) and mean levels of creatinine among Chinese adults Adjusted prevalence of (A) eGFR <60 ml/min/1.73 m2 and (B) means of creatinine. Bars are 95% CIs.
The age- and sex-standardized mean creatinine was 68.4 µmol/l (SD 18.3) among the overall population. Men had significantly higher levels of creatinine than women [78.3 µmol/l (SD 16.9) versus 58.1 (SD 13.5)] (Table 2). Mean levels of creatinine also increased with increasing age. Individuals with diabetes, hypertension or higher BMI had significantly higher levels of creatinine. Individuals living in southern China had higher mean levels of creatinine than those living in northern China, but those living in Central and Northwest China had the lowest levels of creatinine. Importantly, Sichuan and Guangxi Provinces had the highest levels of serum creatinine (Figure 1b).
Distribution of creatinine and eGFR values among Chinese adults
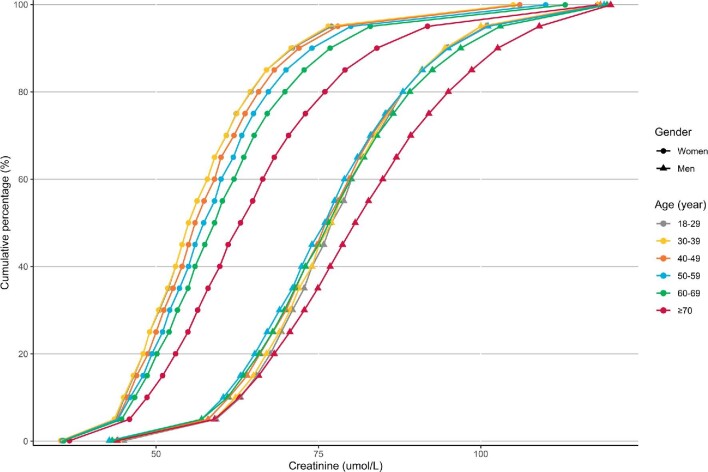
To estimate the age-adjusted percentiles of the distribution of creatinine and eGFR values among Chinese adults, 9 461 631 participants were included. Figure 3 shows the age-adjusted cumulative probability distributions for plasma creatinine concentrations among men and women. Women had significantly higher median levels of serum creatinine than men (P < .001). The differences in medians by age group were also statistically significant (all overall P-values <.001). Among women >70 years of age, the mean creatinine was 66.3 µmol/l (95% CI 65.9–66.6), followed by participants 60–69 years old [60.8 µmol/l (95% CI 60.7–60.9)], 50–59 years old [59.0 µmol/l (95% CI 58.9–59.1), 40–49 years [57.8 µmol/l (95% CI 57.7–57.8)], 30–39 years [56.7 µmol/l (95% CI 56.7–56.8)] and 18–29 years [56.7 µmol/l (95% CI 56.7–56.8)]. Similar trends were observed for men.
FIGURE 3:
The age-adjusted distribution of creatinine values among Chinese adults by sex in 2017. The percentiles of plasma creatinine concentrations were assessed in 9 461 631 participants.
Prevalence or mean values of indicators of kidney function by disease stage
Table 3 shows the prevalence or mean values of indicators of kidney damage at different stages of CKD. The adjusted prevalence of CKD (eGFR <60 ml/min/1.73 m2) was 1.20% (95% CI 1.01–1.39), 0.04% (95% CI 0.04–0.05) and 0.02% (95% CI 0.02–0.02) at stage 3, 4 and 5, respectively. The adjusted prevalence of proteinuria was 11.2% (95% CI 9.90–12.5), 53.8% (95% CI 50.2–57.5) and 72.4% (95% CI 66.9–77.8) at stage 3, 4 and 5, respectively. If proteinuria is also used as a diagnostic criterion, the prevalence rate increased to 2.16%. Women had a higher prevalence of kidney damage [1.27% (95% CI 1.04–1.50)] than did men [1.13% (95% CI 0.96–1.31)] at stage 3, with similar prevalences at stages 4 and 5.
Table 3.
Prevalence or mean values of markers of kidney function by disease stage of CKD
| CKD stage | 1 | 2 | 3 | 3a | 3b | 4 | 5 | |
|---|---|---|---|---|---|---|---|---|
| eGFR (ml/min/1.73 m2) | >90 | 60–89 | 30–59 | 45–59 | 30–44 | 15–29 | <15 | Total |
| Overall | ||||||||
| N | 7 780 977 | 1 592 383 | 83 873 | 73 115 | 10 759 | 3001 | 1397 | 9 461 631 |
| % (95% CI) | 82.7 (81.0–84.4) | 16.1 (14.5–17.6) | 1.20 (1.01–1.39) | 1.03 (0.86–1.20) | 0.17 (0.15–0.20) | 0.04 (0.04–0.05) | 0.02 (0.02–0.02) | 100 |
| Proteinuria (%), mean (95% CI) | 1.28 (1.10–1.46) | 2.65 (2.35–2.94) | 11.2 (9.90–12.5) | 8.95 (7.91–9.99) | 24.7 (22.0–27.5) | 53.8 (50.2–57.5) | 72.4 (66.9–77.8) | 1.65 (1.45–1.85) |
| CKD (%). mean (95% CI) | – | – | 1.20 (1.01–1.39) | 1.03 (0.86–1.20) | 0.17 (0.15–0.20) | 0.04 (0.04–0.05) | 0.02 (0.02–0.02) | 1.26 (1.07–1.46) |
| Creatinine (µmol/l), mean (SD) | 64.3 (13.5) | 85.0 (14.5) | 110 (20.2) | 105 (15.1) | 137 (24.7) | 218 (49.7) | 563 (234) | 68.4 (18.3) |
| eGFR (ml/min/1.73 m2), mean (SD) | 113 (13.1) | 79.9 (7.72) | 52.3 (6.67) | 54.5 (4.00) | 39.4 (4.10) | 23.9 (4.20) | 8.89 (3.39) | 107 (18.4) |
| Female | ||||||||
| N | 3 742 988 | 618 300 | 40 111 | 35 804 | 4307 | 1045 | 495 | 4 402 939 |
| % (95% CI) | 84.6 (82.9–86.3) | 14.1 (12.6–15.6) | 1.27 (1.04–1.50) | 1.10 (0.90–1.31) | 0.17 (0.14–0.20) | 0.04 (0.03–0.04) | 0.02 (0.01–0.02) | 100 |
| Proteinuria (%), mean (95% CI) | 0.98 (0.84–1.12) | 1.94 (1.70–2.19) | 7.50 (6.33–8.66) | 5.64 (4.68–6.59) | 19.9 (16.7–23.1) | 46.8 (41.8–51.8) | 67.7 (58.1–77.3) | 1.22 (1.08–1.37) |
| CKD (%), mean (95% CI) | – | – | 1.27 (1.04–1.50) | 1.10 (0.90–1.31) | 0.17 (0.14–0.20) | 0.04 (0.03–0.04) | 0.02 (0.01–0.02) | 1.33 (1.10–1.56) |
| Creatinine (µmol/l), mean (SD) | 54.8 (8.29) | 73.4 (9.68) | 97.2 (12.8) | 94.0 (8.80) | 118 (14.2) | 189 (34.5) | 489 (192) | 58.1 (13.5) |
| eGFR (ml/min/1.73 m2), mean (SD) | 115 (12.9) | 79.5 (7.95) | 52.5 (6.40) | 54.5 (3.99) | 39.8 (4.03) | 23.7 (4.13) | 8.74 (3.28) | 109 (18.5) |
| Male | ||||||||
| N | 4 037 989 | 974 083 | 43 762 | 37 311 | 6451 | 1956 | 902 | 5 058 692 |
| % (95% CI) | 80.8 (79.0–82.6) | 18.0 (16.3–19.6) | 1.13 (0.96–1.31) | 0.96 (0.81–1.11) | 0.18 (0.16–0.20) | 0.05 (0.04–0.05) | 0.02 (0.02–0.02) | 100 |
| Proteinuria (%), mean (95% CI) | 1.57 (1.34–1.80) | 3.17 (2.81–3.52) | 15.2 (13.7–16.7) | 12.6 (11.4–13.8) | 29.0 (25.8–32.2) | 58.8 (54.4–63.1) | 75.6 (70.4–80.9) | 2.05 (1.79–2.30) |
| CKD (%), mean (95% CI) | – | – | 1.13 (0.96–1.31) | 0.96 (0.81–1.11) | 0.18 (0.16–0.20) | 0.05 (0.04–0.05) | 0.02 (0.02–0.02) | 1.20 (1.03–1.38) |
| Creatinine (µmol/l), mean (SD) | 74.0 (10.6) | 93.8 (11.0) | 123 (18.4) | 117 (10.9) | 154 (19.6) | 240 (47.8) | 629 (247) | 78.3 (16.9) |
| eGFR (ml/min/1.73 m2), mean (SD) | 111 (13.0) | 80.2 (7.53) | 52.1 (6.94) | 54.6 (4.01) | 39.0 (4.14) | 24.1 (4.25) | 9.02 (3.48) | 105 (18.1) |
Prevalence and means are adjusted for age and gender.
Multivariable risk assessment
In the multivariable analyses, being female, older age, a history of prior CVD or hypertension, elevated systolic BP, elevated HR, elevated high-density lipoprotein (HDL), elevated TG, elevated UA and residence in southern China were all significantly associated with a higher risk of CKD. However, a lower risk for CKD was observed for those with a higher BMI, diastolic BP, LDL and fasting glucose, which may reflect the effects of reverse causality (Table 4). The spline regression models showed a linear positive association for BMI, systolic BP, HR and UA (for non-linearity, P < .001). However, a U-shaped association was observed for diastolic BP and TC and a J-shaped association for fasting glucose (Supplementary Figure 4).
Table 4.
Factors associated with CKD in the Meinian Onehealth study
| CKD | Creatinine | eGFR | ||||
|---|---|---|---|---|---|---|
| Factors | OR (95% CI) | P-value | β (SE) | P-value | β (SE) | P-value |
| Age, per 10-year increment | 3.05 (3.02–3.09) | <.001 | 0.712 (0.006) | <.001 | −7.350 (0.006) | <.001 |
| Female | 1.81 (1.77–1.85) | <.001 | −17.09 (0.018) | <.001 | 1.064 (0.018) | <.001 |
| North residence | 0.56 (0.55–0.58) | <.001 | −3.616 (0.015) | <.001 | 4.073 (0.015) | <.001 |
| BMI (kg/m2) | 0.93 (0.91–0.93) | <.001 | −0.363 (0.008) | <.001 | 0.217 (0.008) | <.001 |
| Stroke (yes/no) | 1.37 (1.26–1.48) | <.001 | 1.473 (0.138) | −0.466 (0.022) | <.001 | |
| Coronary heart disease (yes/no) | 1.32 (1.27–1.40) | <.001 | 1.581 (0.077) | <.001 | −1.523 (0.076) | <.001 |
| Diabetes (yes/no) | 1.35 (1.31–1.39) | <.001 | −2.262 (0.033) | <.001 | 1.857 (0.032) | <.001 |
| Hypertension (yes/no) | 1.51 (1.47–1.56) | <.001 | 0.577 (0.023) | <.001 | −0.466 (0.022) | <.001 |
| Systolic BP (mmHg) | 1.03 (1.02–1.05) | <.001 | −0.198 (0.010) | <.001 | 0.212 (0.010) | <.001 |
| Diastolic BP (mmHg) | 0.90 (0.89–0.91) | <.001 | −0.283 (0.009) | <.001 | 0.153 (0.009) | <.001 |
| HR (bpm) | 1.05 (1.04–1.06) | <.001 | −0.112 (0.006) | <.001 | 0.262 (0.006) | <.001 |
| HDL (mmol/l) | 1.11 (1.10–1.12) | <.001 | 0.448 (0.008) | <.001 | −0.613 (0.007) | <.001 |
| LDL (mmol/l) | 0.94 (0.92–0.95) | <.001 | −0.011 (0.010) | .261 | 0.104 (0.010) | <.001 |
| TC (mmol/l) | 1.00 (0.98–1.01) | .880 | 0.407 (0.011) | <.001 | −0.566 (0.011) | <.001 |
| TG (mmol/l) | 1.08 (1.07–1.10) | <.001 | 0.041 (0.008) | <.001 | −0.251 (0.008) | <.001 |
| Fasting glucose (mg/dl) | 0.97 (0.96–0.98) | <.001 | −0.046 (0.007) | <.001 | −0.020 (0.007) | .005 |
| UA (µmol/l) | 2.18 (2.15–2.20) | <.001 | 3.201 (0.008) | <.001 | −2.882 (0.008) | <.001 |
Data are presented as multivariable-adjusted OR (95% CI).
ORs were calculated with the use of multinomial logit models.
All covariables listed were included in the model simultaneously.
Hypertension was defined as systolic BP ≥140 mmHg or diastolic BP ≥90 mmHg or a self-reported history of hypertension.
Diabetes was defined as fasting glucose ≥7.0 mmol/l or haemoglobin A1c ≥6.5% or a self-reported history of diabetes.
SE: standard error.
DISCUSSION
Overall, among 9 461 631 participants (including 7.42 million women and 7.02 million men), 88 271 individuals (1.07% of the adult population of China) had CKD. Individuals living in southern China had the highest prevalence of CKD, particularly those living in Sichuan and Guizhou Provinces. Overall, 21.9% of the participants had hypertension and 5.11% had diabetes, which are important risk factors for CKD. The findings, which were based on a contemporary survey of 9 million adults in China from diverse regions and socio-economic backgrounds, provide reliable estimates of the burden of CKD in Chinese adults who are able to work.
Previous national or regional studies reported an increasing prevalence of CKD in Chinese adults [1, 3–6]. For example, a cross-sectional survey was conducted in 2009–2010 involving a sample of 47 204 Chinese adults from 13 provinces and demonstrated that the adjusted prevalence of eGFR <60 ml/min/1.73 m2 was 1.7% [1], which was higher than that observed in the present study (1.07%), and estimated that 14.44 million adults had CKD. However, previous national or regional surveys were constrained by small sample sizes and a limited number of regions studied and were unable to assess geographic differences in the prevalence of CKD across China [1, 4–6]. In this largest nationwide population-based study covering 31 provinces and municipalities, we observed that the prevalence of CKD differed substantially between geographic regions within China. Consistent with previous reports, we observed a high prevalence of CKD in Southwest China [1], but North and Central China were associated with a low prevalence of CKD. Such heterogeneity may be related to variability in lifestyles and socio-economic circumstances or differences in the study population, sampling methods, screening procedures and diagnostic criteria used [1, 4–8]. Moreover, the Chinese National Health and Nutrition Examination Survey reported that people in Southwest China consumed high levels of smoked meat, which are high in sodium and potassium [12]. Such an unhealthy diet may increase the risk of microalbuminuria and end-stage renal disease in the general population and substituting alternative sources of protein may reduce the incidence of renal disease [13]. In addition to potential effects of diet or lifestyle factors, differences in genetics in different areas of China may also contribute to the geographic differences in the prevalence of CKD [14]. Therefore, further investigations are required to elucidate the genetic and environmental factors and their complex interplay in the aetiology of the development of CKD in Chinese adults.
The prevalence of kidney disease has increased rapidly in recent decades in Asian populations, mostly due to the ageing population and rapid changes in lifestyle and cardiometabolic risk factors [2]. In the present study, elevated BP, HR, LDL, TG and UA, overall obesity and diabetes were each strongly associated with increased levels of creatinine and a higher prevalence of CKD. The associated higher risks of vascular complications and premature death caused by kidney disease result in a substantial economic and healthcare burden. Reliable reports from observational studies have shown that adverse lifestyle habits such as smoking and drinking, physical inactivity and unhealthy diets influence the progression of kidney disease [2]. Therefore, public health strategies to reduce alcohol intake and smoking and increase physical activity and adopting more healthy eating habits may help to mitigate the development of proteinuria and kidney disease [2]. Randomized trials have demonstrated that diet or lifestyle interventions may increase health-related quality of life and eGFR and lower mean BP and serum cholesterol in people with CKD [15, 16]. Public health strategies should consider screening for CKD and the associated risk factors in order to target individuals for more intensive treatment to reduce the associated risk factors for CKD.
This study had several limitations. First, the laboratory tests were conducted at local laboratories of >700 health examination centres across the country. Hence we cannot exclude the possibility of variations between the participating laboratories, despite the fact that all laboratories completed a standardized program. Second, dietary intake and work-related physical activity were not assessed in the present study and hence we were unavailable to determine their associations with the prevalence of CKD. In addition, regional distribution of traditional medicine using Aristolochia sp. and air pollution and water quality were not evaluated in our study, which may influence the risk of CKD and related risk factors. Third, to ensure comparability across regions, we used the World Health Organization criteria to define CKD. However, the relationship of creatinine or CKD and the risk of microvascular and macrovascular complications and premature death has not been extensively examined in Chinese adults. Fourth, due to the cross-sectional design of the present study, self-reported hypertension and diabetes and potential reverse causation bias, causal associations between risk factors and CKD cannot be assessed (Supplementary Figures 4 and 5). Fifth, given only a small proportion of participants had measured albumin, we could not define albuminuria in this study. Further studies with detailed data on albumin are warranted. Finally, the study participants for preventive health examination involved an employed cohort rather than the general population, which may have resulted in selection bias due to the healthy worker effect. In addition, the age pyramid of the studied population did not exactly match the adult China population (Supplementary Figure 6). However, there were 575 million visits for such common health practices in China in 2018, accounting for 42% of the total Chinese population. Health screening affords a valuable opportunity to investigate the prevalence and determinants of CKD in this population.
Overall, the results of the present study demonstrated that CKD was common among Chinese adults and is an important public health problem. The strong associations of major risk factors with CKD highlight the need for public health strategies for detection and treatment of modifiable risk factors for the prevention of CKD in the general population. Given its aging population, China is likely to experience a higher CKD-related burden than any other country. Consequently, CKD should be included as an additional target in any public health strategy to reduce the burden of non-communicable diseases in low- and middle-income populations such as China.
Supplementary Material
Acknowledgements
The most important acknowledgment is to the participants in the study and the members of the survey teams in the Meinian Research Institute. This study has been approved by the Institutional Review Board of Peking University Health Science Center (IRB00001052-19077). Informed consent was obtained from all individual participants included in the study.
Contributor Information
Zhenhuang Zhuang, Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China.
Mingkun Tong, Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China.
Robert Clarke, Clinical Trial Service Unit & Epidemiological Studies Unit (CTSU), Nuffield Department of Population Health, University of Oxford, United Kingdom.
Bo Wang, Department of Epidemiology, Meinian Institute of Health, Beijing, China; Peking University Health Science Center, Meinian Public Health Institute, Beijing, China.
Tao Huang, Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China; Key Laboratory of Molecular Cardiovascular Sciences (Peking University), Ministry of Education, Beijing, China; Center for Intelligent Public Health, Academy for Artificial Intelligence, Peking University, Beijing, China.
Liming Li, Department of Epidemiology and Biostatistics, School of Public Health, Peking University Health Science Center, Beijing, China; Peking University Center for Public Health and Epidemic Preparedness & Response, Beijing, China.
Funding
The study was supported by grants from the National Key R&D Program of China (2020YFC2003401).
Authors’ contributions
T.H. and L.L. designed the research. Z.Z., M.T. and T.H. wrote the first draft of the article and performed the data analysis. Z.Z., M.T. and T.H. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors critically revised the manuscript and approved the final version prior to publication. T.H. and L.L. are the guarantors for the study.
Data Availability Statement
The de-identified datasets used in the study will be available from the corresponding author upon reasonable request. We will make the data without identifiers available to users only under a data-sharing agreement that provides for a commitment to using the data only for research purposes and not to attempt to identify any individual participant. Individuals who would like to access the data should contact the corresponding author.
Conflict of Interest Statement
We declare no competing interests.
REFERENCES
- 1. Zhang L, Wang F, Wang Let al. . Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet 2012;379:815–22. 10.1016/S0140-6736(12)60033-6. [DOI] [PubMed] [Google Scholar]
- 2. Camara NOS, Iseki K, Kramer Het al. . Kidney disease and obesity: epidemiology, mechanisms and treatment. Nat Rev Nephrol 2017;13:181–90. 10.1038/nrneph.2016.191. [DOI] [PubMed] [Google Scholar]
- 3. Zhang L, Zhao MH, Zuo Let al. . China Kidney Disease Network (CK-NET) 2015 annual data report. Kidney Int Suppl 2019;1:E1–81. 10.1016/j.kisu.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen W, Chen W, Wang Het al. . Prevalence and risk factors associated with chronic kidney disease in an adult population from southern China. Nephrol Dial Transplant 2009;24:1205–12. 10.1093/ndt/gfn604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen W, Liu Q, Wang Het al. . Prevalence and risk factors of chronic kidney disease: a population study in the Tibetan population. Nephrol Dial Transplant 2011;26:1592–9. 10.1093/ndt/gfq608. [DOI] [PubMed] [Google Scholar]
- 6. Zhang L, Zhang P, Wang Fet al. . Prevalence and factors associated with CKD: a population study from Beijing. Am J Kidney Dis 2008;51:373–84. 10.1053/j.ajkd.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 7. Wang F, Yang C, Long Jet al. . Executive summary for the 2015 annual data report of the China Kidney Disease Network (CK-NET). Kidney Int 2019;95:501–5. 10.1016/j.kint.2018.11.011. [DOI] [PubMed] [Google Scholar]
- 8. Lin B, Shao L, Luo Qet al. . Prevalence of chronic kidney disease and its association with metabolic diseases: a cross-sectional survey in Zhejiang province, Eastern China. BMC Nephrol 2014;15:36. 10.1186/1471-2369-15-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Viswanathan G, Upadhyay A. Assessment of proteinuria. Adv Chronic Kidney Dis 2011;18:243–8. 10.1053/j.ackd.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 10. Levey AS, Stevens LA, Schmid CHet al. . A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–12. 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zhang M, Chen Y, Tang Let al. . Applicability of chronic kidney disease epidemiology collaboration equations in a Chinese population. Nephrol Dial Transplant 2014;29:580–6. 10.1093/ndt/gft374. [DOI] [PubMed] [Google Scholar]
- 12. The Tenth Report of the Chinese National Health and Nutrition Examination Survey—Nutrition and Health Status, 1st ed. Beijing: People's Medical Publishing House, 2008. [Google Scholar]
- 13. Lew QJ, Jafar TH, Koh HWet al. . Red meat intake and risk of ESRD. J Am Soc Nephrol 2017;28:304–12. 10.1681/ASN.2016030248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhu M, Lyu J, Yu CQet al. [Study on genetic structure differences and adjustment strategies in different areas of China]. Zhonghua Liu Xing Bing Xue Za Zhi 2019;40:20–5. 10.3760/cma.j.issn.0254-6450.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 15. Palmer SC, Maggo JK, Campbell KLet al. . Dietary interventions for adults with chronic kidney disease. Cochrane Database Syst Rev 2017;2017:CD011998. 10.1002/14651858.CD011998.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Galbraith L, Jacobs C, Hemmelgarn BRet al. . Chronic disease management interventions for people with chronic kidney disease in primary care: a systematic review and meta-analysis. Nephrol Dial Transplant 2018;33:112–21. 10.1093/ndt/gfw359. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The de-identified datasets used in the study will be available from the corresponding author upon reasonable request. We will make the data without identifiers available to users only under a data-sharing agreement that provides for a commitment to using the data only for research purposes and not to attempt to identify any individual participant. Individuals who would like to access the data should contact the corresponding author.