Abstract
Periodontal diseases are pathological processes resulting from infections and inflammation affecting the periodontium or the tissue surrounding and supporting the teeth. Pathogenic bacteria living in complex biofilms initiate and perpetuate this disease in susceptible hosts. In some cases, broad-spectrum antibiotic therapy has been a treatment of choice to control bacterial infection. However, increasing antibiotic resistance among periodontal pathogens has become a significant challenge when treating periodontal diseases. Thanks to the improved understanding of the pathogenesis of periodontal disease, which involves the host immune response, and the importance of the human microbiome, the primary goal of periodontal therapy has shifted, in recent years, to the restoration of homeostasis in oral microbiota and its harmonious balance with the host periodontal tissues. This shift in therapeutic goals and the drug resistance challenge call for alternative approaches to antibiotic therapy that indiscriminately eliminate harmful or beneficial bacteria. In this review, we summarize the recent advancement of alternative methods and new compounds that offer promising potential for the treatment and prevention of periodontal disease. Agents that target biofilm formation, bacterial quorum-sensing systems and other virulence factors have been reviewed. New and exciting microbiome approaches, such as oral microbiota replacement therapy and probiotic therapy for periodontal disease, are also discussed.
Keywords: Periodontal diseases, Biofilms, Antibiotic resistance, Quorum sensing inhibitors, Anti-virulence, Immune modulators, Novel therapeutic strategies
Background
Periodontal disease is one of the most common chronic inflammatory diseases in humans, affecting up to 90% of the global population [1, 2]. It is the pathologic process affecting the periodontium. According to the American Academy of Periodontology (AAP) and European Federation of Periodontology (EFP) guidelines, periodontal diseases and conditions are classified into the following categories: periodontal health, gingival diseases, and conditions; periodontitis; and other conditions affecting the periodontium. Based on the severity and complexity, periodontal disease can be further classified into stage I-IV [3, 4]. The most common forms of periodontal disease are gingivitis and periodontitis. These conditions involve a group of oral pathogens that can form biofilms on the tooth's surface and in the periodontal pocket and elicit a host inflammatory response. Dental biofilm-induced gingivitis involves the accumulation of dental biofilm and is characterized clinically by swelling, redness, and tissue bleeding [5]. In patients with gingivitis, the alveolar bone and the periodontal ligaments are usually not damaged [6]. In contrast, periodontitis is characterized by the destruction of alveolar bone and periodontal ligaments [5]. The clinical manifestations of periodontitis involve the periodontal pocket, an ideal place for the colonization of bacteria [7]. The primary oral pathogens that contribute to the initiation and progression of periodontitis include Porphyromonas gingivalis, Tannerella forsythia and Treponema denticola. These bacteria are known as the red complex as they are found together in periodontal pockets where extensive periodontal tissue damage is evident [8]. Other periodontitis-associated bacteria include Fusobacterium nucleatum, Aggregatibacter actinomycetemcomitans, and Prevotella intermedia [9]. In addition to pathogenic bacteria, the disease progression is influenced by host-specific and environmental risk factors (Fig. 1).
Fig. 1.
Overview of periodontal disease and factor that affects susceptibility to periodontal infections. Periodontal pathogens use an array of virulence factors to initiate periodontal disease. These virulence factors help the pathogens escape host cells' immune response. The progression of periodontal disease depends on the host’s response to microbial dysbiosis in biofilms. The severity and extent of periodontal disease are also influenced by host-specific, genetic, and environmental factors [7]
Physical removal of the biofilms and calculus by scaling has been the most widely used clinical option in periodontal disease treatment [10]. While dental biofilm-induced gingivitis can be reversed by improved oral hygiene and supragingival scaling, surgical periodontal procedures are performed with more advanced periodontal disease. Antimicrobial agents such as chlorhexidine and systemically administered antibiotics are sometimes used in conjunction with surgical treatments for periodontal diseases. The most commonly used systemic antibiotics include amoxicillin or metronidazole and extended-release doxycycline. Antibiotics are often given to patients for prophylactic purposes following invasive periodontal surgeries. However, in alignment with the current trend of increasing antimicrobial resistance in human pathogens, antibiotic resistance has increased among patients with periodontal disease in recent years [11]. The unique periodontal environment and biofilm formation make these bacteria less susceptible to antibiotics [12]. New therapeutic strategies are needed for periodontal diseases.
Several new therapeutic and preventive periodontal disease approaches have emerged in recent years due to the advancement in understanding bacterial pathogenesis, the human microbiome, and host-microbe interaction. Anti-virulence therapy combats against periodontal pathogens by neutralizing their virulence properties, representing a promising alternative to antibiotic treatment [13]. Host immune modulation by phytocompounds and microbiome-based approaches, such as oral microbiota replacement, are exciting new developments for periodontal disease treatment and prevention. This review discusses the challenge of antibiotic resistance and alternative strategies for periodontal disease treatment. Although this is not a systemic review, we have listed the literature search strategy in Fig. 2.
Fig. 2.
Flowchart of the literature search strategy and selection criteria. The literatures were retrieved from two databases: PubMed and Scopus. Search terms used in two separate searches includes: 1. (periodontal) AND (antibiotic) OR (virulence) OR (biofilm) OR (immunomodulatory AND therapy) OR (probiotic) OR (quorum AND sensing); 2. (periodontal* AND disease) AND (therapeutic*) AND (antibiotics OR antimicrobials) AND NOT (regenerative) AND NOT (rat or animal) AND NOT (case AND reports). The literatures were limited to articles published in English and within 15 years (2008–2022), while those of animal studies, case studies, or presented in the form of abstracts were excluded. 215 references relevant to the topics were cited in this review
Antibiotic therapy and drug resistance in periodontal bacteria
Antibiotics are used to treat infections in the oral cavity and are given in certain circumstances for prophylactic purposes during intraoral procedures where bacteremia is expected to occur [14]. The commonly used local and systemic antibiotics to treat periodontitis are listed in Tables 1 and 2. Local delivery means that the antibiotics are placed directly in the diseased periodontal pocket, and antibiotics for systemic delivery are usually administrated orally.
Table 1.
List of local antibiotics delivery systems used to treat periodontal patients
| Drugs | Polymer Type | Outcomes | References | |
|---|---|---|---|---|
| Metronidazole | Polycaprolactone (PCL) | Nanofiber | Effective local delivery system of metronidazole in combination with scaling and root planing showed betterment of clinical criteria, for example, plaque index (PI), pocket depth (PD), and gingival index (GI) | [158] |
| Doxycycline | PCL | Controlled delivery of drug was able to improve PD, PI, and GI effectively in periodontal disease | [159] | |
| Minocycline hydrochloride | Carbopol | Liposome | Liposomes containing minocycline hydrochloride showed biocompatibility along with improvement in rat periodontitis | [160] |
| Minocycline hydrochloride | Poly Ethylene Glycol (PEG) | 2% minocycline hydrochloride nanoliposomes strongly inhibited TNF-α secretion by LPS-stimulated macrophages up to 60 h | [161] | |
| Doxycycline | Carbopol | Slow release of drug from nanoliposome gel decreased MMP-8 in rat model of periodontitis | [162] | |
|
Metronidazole, Doxycycline |
Polymersomes | Nanoparticle | Antibiotics encapsulated in polymersomes decreased the number of P. gingivalis in monolayer cells as well as in organotypic cultures significantly | [163] |
| Metronidazole benzoate |
Thiolated Chitosan (TCS)- Poly(methacrylic acid) (PMAA) |
TCS-PMAA delivery system provided sustained and site-directed release of the drug. Also, the system led to an improvement in oral availability | [164] | |
| Doxycycline | Chitosan | Pre-clinical studies showed that nanoparticles loaded with doxycycline showed entrapment efficacy of 75% and showed antimicrobial activity against P. gingivalis | [165] | |
| Metronidazole | Chitosan | Microparticle | Hydrogel prepared from chitosan microparticles released metronidazole at an optimal pattern | [166] |
| hydroxyapatite (HA) and Ofloxacin | Poly Lactic-Co-glycolic acid (PDLGA) | PDLGA microspheres were shown to be biocompatible and porous in nature. This system delivered the drug optimally against E. coli and S. aureus | [167] | |
| Minocycline hydrochloride | Chitosan | Use of microsphere containing minocycline hydrochloride resulted in the decrease of PDs at 6 months. In addition, bleeding on probing was also decreased significantly in patients | [168] | |
| Doxycycline | Gelatin | Local delivery of antibiotics in the periodontal pocket along with scaling and root planing led to decrease in PDs. Moreover, the treatment method reduced the number of P. gingivalis significantly | [169] | |
| Azithromycin | PLGA | Gel | Site-directed delivery of 0.5% of azithromycin showed better clinical output in periodontitis patients | [170] |
| Metronidazole | Poly-gamma-glutamic acid | Hydrogel was prepared from a non-toxic and biodegradable material such as poly-gamma-glutamic acid. This hydrogel system could be polymerized by light and showed remarkable swelling ability along with a controlled manner of drug release | [171] | |
| Clarithromycin | Carboxy methylcellulose | In adjunction with scaling and root planing treatment, local delivery of 0.5% clarithromycin showed better clinical outputs at 6 months in smokers | [172] | |
| Moxifloxacin |
Pluronic Chitosan |
Reduction in PD was observed in patients who received moxifloxacin gels at 3 months. In addition, these gels were safe to use and significantly reduced the load of P. gingivalis among the periodontal pathogens Shows efficacy against A. actinomycetemcomitans |
[173] |
|
| Chlorhexidine | Chitosan, beta-glycerophosphate | Nontoxic hydrogel system carrying 0.1% chlorhexidine effectively reduced the number of oral pathogens | [162] | |
| Doxycycline | Hydroxyapatite | Scaffolds | Scaffolds loaded with doxycycline helps in the process of pre-osteoblasts of bone and tissue repairment in periodontal patients | [176, 177] |
Table 2.
List of Systemic antibiotics used to treat periodontal patients
| Antibiotic | Dose and Duration | Disease | Findings | Side Effects | References |
|---|---|---|---|---|---|
| Amoxicillin + Metronidazole |
Metronidazole: 250 mg Amoxicillin: 500 mg for 2 years |
Stage II periodontitis |
Antibiotic treatment decreased probing depth (PD) and gained clinical attachment Five randomized clinical trials showed improvement in PD and bleeding on probing |
One participant experienced nausea and vomiting and one developed candidiasis Few participants observed light pain or a burning sensation following laser treatment. No side-effects were observed due to administration of antimicrobial agents |
[178] |
|
Metronidazole: 500 mg Amoxicillin: 375 mg for 6 months |
Stage II periodontitis | Antibiotics reduced the count of sites containing PD as well as bleeding on probing. Antibiotics with SRP led to a decrease in the count of T. forsythia, F. nucleatum, and P. gingivalis | Majority of the side effects were related to gastrointestinal disturbances such as nausea, diarrhea, vomiting as well as stomach burning | [185, 186] | |
|
Metronidazole: 500 mg Amoxicillin: 500 mg for the time period of 6 months |
Stage III periodontitis | Treatment reduced the load of pathogens such as T. forsythia as well as T. denticola. It also decreased the number of PD sites | One patient reported mild intestinal disturbance and one experienced vertigo | [187] | |
| Amoxicillin + Metronidazole + Doxycycline |
Metronidazole: 250 mg Amoxicillin: 250 mg Doxycycline: 100 mg for 2 months |
Stage III periodontitis | This drug treatment decreased the number of sites with PD | No side effects were reported related to the antibiotic administration | [188] |
|
Amoxicillin + Clavulanic acid |
875 mg each 3 months | Stage I periodontitis | No difference was observed in PD between the control and test groups | / | [189] |
|
Doxycycline + Clindamycin + Metronidazole |
Doxycycline: 200 mg Metronidazole: 500 mg Clindamycin: 150 mg for 2 years |
Stage IV periodontitis |
Treatment decreased PD, gained clinical attachment, and reduced the load of P. gingivalis and Actinobacillus Actinomycetemcomitans |
/ | [190] |
| Azithromycin | 500 mg for 6 months | Stage II periodontitis | It helped to lessen PD and BANA scores | / | [191] |
| 500 mg for 6 months | Stage III periodontitis | No reduction in microbial load and PD was observed | Participants did not report any adverse effects from the use of antibiotic and placebo | [192] | |
| 500 mg for the time period of 1 year | Stage III periodontitis | Treatment regimen declined PD and periodontal pathogens significantly | Participants did not report any adverse effects from the use of antibiotic and placebo | [193] | |
| 500 mg once a day for 5 days | Stage III periodontitis | Administration of antibiotic failed to improve clinical parameters when compared to scaling and root planning (SRP) alone | One participant experienced nausea after taking antibiotic | [194] [195] | |
| Metronidazole | 400 mg thrice a day for 10 days | Moderate to severe periodontitis (Stage III or IV) with Type 2 Diabetes Mellitus | SRP alone improved the periodontitis and glycemic control significantly | / | [196] |
Antimicrobial resistance in periodontal bacteria is a growing concern worldwide. Bacterial pathogens become resistant to antibiotics via several central mechanisms, including degrading antibiotics, altering antibiotic targets, and actively effluxing the drugs from the cell [15].
Pathogens become resistant to antibiotics containing β-lactam rings by producing β-lactamases, which can degrade or modify β-lactam antibiotics [16]. Studies have reported β-lactamase production by periodontal pathogens, including Porphyromonas, Prevotella, and Fusobacterium species [17]. For P. gingivalis, the β-lactamase (amoxicillin-based) production rate in clinical isolates is around 7.6% [18]. Walker et al. reported that from 406 samples taken from the gingival crevicular fluid of patients with periodontal disease, β -lactamase property was detected in 64% of the periodontal patients [19]. β-lactamases can be found frequently in periodontal pockets of more than 3 mm deep [19]. Feres et al. demonstrated that bacterial species from subgingival plaque showed resistance against metronidazole and amoxicillin [20]. 21.6% of the P. gingivalis isolates from patients with periodontitis were reported to be resistant to metronidazole [21]. Different antibiotic-resistant levels in A. actinomycetemcomitans have also been reported depending on geographical distributions [22].
The tet gene is known to be responsible for tetracycline resistance. Most tet genes originated from periodontal pathogens such as P. gingivalis and F. nucleatum [23]. The percentage of tetracycline-resistant isolates in dental plaque-associated bacteria is high in children from South Asia and Japan [24]. Isolates of T. denticola that are resistant to tetracycline have also been reported [25]. Resistance to tetracycline is a frequent co-marker among penicillin-resistant species of oral origin [26]. Resistance to erythromycin usually occurs due to acquiring two significant genes, ermF and erm. Major periodontal bacteria such as T. forsythia and P. gingivalis can carry both emr and tet resistance genes [27]. Clindamycin resistance shows a high prevalence among periodontal isolates of F. nucleatum (36%), P. gingivalis (23%), and Prevotella melaninogenica (22%) in Columbia [22].
Bacteria associated with periodontal disease naturally form biofilms. Biofilm formation also contributes to the resistance of antibiotics to periodontal pathogens. Studies have demonstrated the increased exchange of antibiotic resistance encoding genes in biofilms [28], which causes the spreading of antibiotic resistance among periodontal bacterial species [29]. The biofilms matrix usually comprises extracellular polymeric substances (EPS), which hinders the dissemination of antibiotics into the bacterial biofilms [30]. The organized structure of biofilms and its matrix also confer diffusion–reaction inhibition to antibiotics, which leads to antibiotic tolerance. Sublethal concentrations of antibiotics in biofilms can also lead to the selection of drug resistance [31]. The slow growth of some bacterial cells in biofilms and the presence of persister cells are major contributing factors to the decreased antibiotic susceptibility in biofilms. It has been reported that bacteria cells in a biofilm can be 1000-fold more resistant to antibiotics than planktonic cells [32].
Novel treatment strategies for periodontal diseases
Coinciding with the increasing antibiotic resistance among periodontal pathogens, the primary goal of periodontal therapy has shifted in recent years to restoring homeostasis in oral microbiota and its harmonious balance with the host periodontal tissue. Such a shift in the treatment goal and the challenge of drug resistance calls for alternative approaches to traditional antibiotic therapy that indiscriminately eliminates resident commensal or pathogenic bacteria.
Periodontal pathogens use virulence factors to initiate periodontal disease and promote its progression. A promising alternative to antibiotic therapy is targeting pathogenic bacteria's virulence factors and their regulatory systems that control pathogenicity [13]. Such an approach aims to inhibit bacterial virulence factors and thus avert the harmful bacterial pathogens render to the host and reduce pathogen-induced host immune response, reducing the damage to the host tissues. Agents with anti-virulence properties could potentially stop the pathogenic activities of periodontal bacteria [33, 34], disarming its ability to cause periodontal diseases. Strategies can also be developed to target biofilms as forming biofilms by periodontal bacteria causes antibiotic resistance and enables them to evade host immune responses [35, 36]. Because host inflammatory response plays a vital role in periodontal disease, modulators of host innate immunity are another promising strategy to address periodontal disease. The novel oral microbiota replacement therapy resulting from recent advancements in human microbiome studies could potentially be an alternative measure for preventing and treating periodontal disease. Probiotics and oral microbiota replacement therapy are substituting resident periodontal bacteria with non-pathogenic bacteria and restoring healthy homeostasis of the oral microbiome [37].
Virulence factors in periodontal pathogens and anti-virulence agents
Virulence factors are either cellular components or secreted products of a pathogen that enable the pathogen to invade the host, evade the host's immune response or cause damage to the host. Several recent therapeutic approaches have been investigated that target critical virulence factors to disrupt the pathogenic processes of periodontal bacteria, which are summarized in Fig. 3.
Fig. 3.
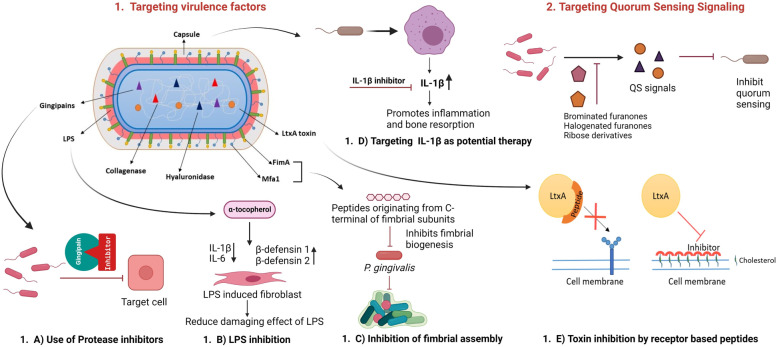
Schematic representation of anti-virulence strategies covered under this review. 1. Targeting virulence factor: (a) Protease Inhibitors: Microbial protease plays an essential role in the progression of periodontal diseases and thus could be a potential therapeutic target. The therapeutic approach focused on Gingipain inhibitor (Kgp specific inhibitor A71561) can significantly decrease virulence. (b) LPS inhibition: Cell surface components like LPS could be targeted for treating periodontal diseases. α-Tocopherol reduces inflammatory cytokines while increasing antimicrobial peptides, and β-defensins, thereby counteracting the damaging effects of LPS, which play a vital role in the pathogenesis of periodontal diseases. (c) Inhibition of fimbrial assembly: Fimbriae are a major structural component of periodontal bacteria. Studies have shown that peptides originating from the conserved C-terminal of FimA and Mfa1 subunits prevent the fimbrial assembly of P. gingivalis and interfere with biofilm formation. (d) Targeting IL-1β as a potential therapy: It has been shown that the capsular polysaccharide upregulates the expression of IL 1-β by activating the JNK pathway in macrophages. Increased IL 1- β levels lead to inflammation and bone resorption, indicating a possible therapeutic strategy to combat this periodontal pathogen. (e) Toxin inhibition by receptor-based peptide: A. actinomycetemcomitans, associated with periodontal disease, produces leukotoxin (LtxA) during colonization in the host to escape the host immune response. LtxA is the critical virulence factor of A. actinomycetemcomitans, which kills leukocytes by recognizing cholesterol and the β2 strands of lymphocyte function-associated antigen-1 (LFA-1) integrin. Molecular inhibitors mimic the target to compete for toxin binding, thus neutralizing toxin binding activity. Small synthetic receptor-specific peptides can hinder LtxA-mediated cytotoxicity by binding to the β domain of transmembrane protein LFA-1. 2. Targeting quorum sensing signalling: QS is the most distributed and studied bacterial communication, which helps periodontal bacteria communicate with each other through signalling molecules and behaviour coordination. Various compounds can block quorum-sensing signals produced by periodontal pathogens, thus inhibiting the disease progression pathway
A. Cell surface components as an anti-virulence target against initial infection
Capsules are required for microbial attachment to the host tissue surfaces, marking the first step in bacterial pathogenesis. Co-aggregation of P. gingivalis and F. nucleatum in the subgingival plaque is mediated by a capsular polysaccharide (CPS) and lipopolysaccharide (LPS) [38]. Increased encapsulation correlates well with increased evasion from phagocytosis and reduced host immune response [39]. The capsular polysaccharide of Actinobacillus actinomycetemcomitans upregulates the expression of Interleukin (IL) 1-β by activating the JNK pathway in macrophages. Increased levels of IL 1- β leads to inflammation and bone resorption. Such results support a possible therapeutic strategy to combat this periodontal pathogen by targeting its capsule [40]. IL 1-β could also be targeted for periodontal diseases as it contributes to periodontal pathology and the production of proteinase, which can contribute to the destruction of periodontal tissues. [41].
Natural compounds, alpha-mangostin and grape seed proanthocyanidin extracts, can inhibit the damaging effects of LPS [5]. Apart from that, the soluble film of alpha-mangostin showed antimicrobial and anti-inflammatory activity against periodontal pathogens, especially on P. gingivalis [42]. Grape seed contains proanthocyanidin, which helps to reduce the progression of periodontal disease by inhibiting the extracellular collagenase [43]. α-tocopherol reduces inflammatory cytokines while increasing antimicrobial peptides and β-defensins, which can modulate the immune response and counter the detrimental effects of LPS [44] [45]. Tormentic acid can be a potential therapeutic agent against periodontal diseases since it inhibits the production of IL-6 and IL-8 in gingival fibroblasts of humans [5, 46].
Fimbriae are proteinaceous appendages that protrude from the bacterial outer membrane that helps microbes attach to host cell surfaces and other microbial surfaces. P. gingivalis possess two antigenically distinct fimbriae subunits, FimA and Mfa1. FimA is found in short fimbriae, and Mfa1 is found in long fimbriae [5]. Immunization with a 43-kDa purified fimbrial protein offers protection against periodontal damage caused by P. gingivalis in a germ-free rat model [47]. In a recent study by Alaei et al., peptides originating from the conserved C-terminal of FimA and Mfa1 subunits prevent the fimbrial assembly of P. gingivalis and interfer with biofilm formation. The study also showed that treating these peptides reduces P. gingivalis adhesion to Streptococcus gordonii in a dual-species model [48].
B. LtxA disruption
Toxins play an essential role in bacterial pathogenesis. The Gram-negative bacteria A. actinomycetemcomitans, associated with periodontal disease, produces leukotoxin (LtxA) during colonization in the host to escape the host immune response [49]. Another toxin these bacteria produce is a cytolethal distending toxin (CDT). Both toxins play a role in compromising the host immune system by targeting different cells [50]. LtxA is the critical virulence factor of A. actinomycetemcomitans, which kills leukocytes by recognizing cholesterol and the β2 strands of lymphocyte function-associated antigen-1 (LFA-1) integrin [51]. Mechanical removal of plaque and antibiotic treatment, preferably tetracycline, was used. Antibiotics may be considered to treat molar/incisor pattern periodontitis, but only after scaling and root planing have been deemed ineffective. Increased resistance against antibiotics in A. actinomycetemcomitans calls for developing alternative treatment methods [52]. It has been reported that small synthetic receptor-specific peptides can hinder LtxA-mediated cytotoxicity by binding to the β domain of transmembrane protein LFA-1 [53]. Studies have found that targeting virulence factors like LtxA could effectively measure against periodontal diseases [54, 55]. Catechins, one class of flavonoids, can inhibit LtxA activity in its soluble form and outer membrane vesicle (OMVs) bound form [56]. At the same time, Chang et al. showed that catechins restructure the soluble form of LtxA, which prevents its interaction with cholesterol on the host cell surface [49].
C. Enzymatic virulence factors and their antagonists
Proteases, including gingipains, aminopeptidase IV, and collagenases, play a role in bacterial adhesion, degradation of host proteins (i.e. immunoglobulins), modulating inflammatory responses, and damaging host tissues [57]. Thus, restricting the functionality of these crucial virulence factors will collectively inhibit the microbes' pathogenicity.
Microbial proteases
Microbial proteases play a crucial role in the progression of periodontal disease, directly damaging host tissues or indirectly activating a myriad of host proteases and inactivating protease inhibitors in the host [37]. Extracellular proteases can be divided into the following groups: aspartic proteases, cysteine proteases, serine proteases, and metalloproteases [37]. Microbial proteases can serve as important therapeutic targets for treating periodontal diseases. Inhibitors of microbial proteases may prevent infection. These inhibitors can be inorganic or synthetic agents or modified “host products” [37]. Salivary histatin is a peptide with anti-protease activity against many proteases involved in periodontal diseases. Histatin has been delivered subgingivally to treat infections [58, 59]. Other inorganic or synthetic protease inhibitors, including chelators, such as EDTA that inhibit the metalloproteinases, and thiol-blocking agents that inhibit serine and cysteine proteases, are potential agents to inhibit the pathogenicity of periodontal pathogens [37].
P. gingivalis gingipains
Members of cysteine proteinases in P. gingivalis are called “trypsin-like enzymes” as they cut after the arginine or lysine residue at the C-terminal of polypeptides [5]. These enzymes are collectively known as gingipains, which can be one of two types: 1. Arginine specific that includes RgpA, RgpB, and 2. Lysine specific is known as KgpA [37]. Gingipains hydrolyze a wide range of extracellular and cell-bound proteins encoded by P. gingivalis [60].
In a mouse model, treatment of P. gingivalis before infection by a Kgp-specific inhibitor (A71561) designed by Curtis et al. decreased the P. gingivalis virulence significantly. In contrast, the Rgp-specific inhibitor leupeptin did not influence the pathogenicity [61]. It is stated that leupeptin can attenuate the degradation of an antimicrobial peptide (LL-37), suppress platelets' aggregation, and prevent the inhibition of chemoattractant proteins of monocyte caused by arginine-specific gingipains. The compound further precludes the destruction of collagens and recovers the IL-2 levels [62].
It has been reported that a compound in rhubarb roots named rhein can cause the downregulation of both genes, rgpA and kgp, in P. gingivalis when combined with polyphenols synergistically [63]. Another dual protease inhibitor that is synthetic in nature, KYT-41, can inhibit both rgpA and kgp proteases and show anti-inflammatory responses [64]. Recent studies have shown that synthetic molecule 2-deoxy-2,3-didehydro-N-acetylneuraminic acid, commonly known as DANA, helps inhibit the growth and biofilm of P. gingivalis by decreasing the activity of gingipains in vivo model [65].
The primary component of black tea, theaflavin, has dual effects on gene expression and the catalytic activity of these proteases. Theaflavin not only downregulates the expression of rgpA and kgp protease genes at sub-MICs but also inhibits the hydrolytic activity of gingipains in a dose-dependent manner [66, 67]. Fractions of cranberry polyphenols inhibited rgpA and kgp activities in P. gingivalis at a concentration of one µg/ml [68]. Macrocarpals found in the leaves of Eucalyptus globules inhibited both Arg- and Lys-specific gingipains in P. gingivalis in a dose-dependent manner [69].
Inhibition of Biofilms
Dental biofilm formation in the supragingival and subgingival areas is required to develop dental caries and periodontitis [70]. Bacteria in biofilms exhibit increased resistance to antimicrobial therapy compared to planktonic microbes. Biofilms are hard to treat with the use of antibiotics for several reasons, including decreased penetration of antibiotics to the microbes in the biofilms, increased expression of multiple efflux pumps, reduced growth as well as metabolism, induction of microbial stress response and changes in the expression of outer membrane proteins [70]. Recent randomized clinical trials showed that guided biofilm therapy, together with erythritol powder usage and ultrasonic piezo, is effective against biofilms in periodontal disease [71]. Besides that, several medicinal herbs and herbal compounds have been shown to inhibit biofilm formation and reduce inflammation; therefore, they have been explored as potential therapeutics for oral diseases, including periodontitis [72].
Oxyresveratol or trans-2,4,3′,5′-tetrahydroxystilbene, the main bioactive element of tropical tree Artocarpus lakoocha, has been shown to have antioxidant properties [73]. This compound interferes with bacterial cell wall integrity and prevents the growth and biofilm of periodontal pathogen A. actinomycetemcomitans [74].
Panduratin A, a natural compound found in Kaempferia pandurate, has antibiofilm and bactericidal activities [75]. Epigallocatechin gallate (EGCG), a polyphenol in green tea, has strong anti-oxidative and anti-inflammatory characteristics [76]. This compound is a catechin derivative with a galloyl group attached which interrupts the adhesion of periodontal bacteria to the infection site [77]. Studies showed that epigallocatechin gallate could significantly inhibit the biofilm formation of a significant periodontal pathogen P. gingivalis [78].
Cranberry extract containing bioactive compounds such as Licocalchone A and Proanthocyanidins also shows promising benefits against gingivitis by reducing inflammation, biofilm formation and inhibiting proteolytic activity of periodontal pathogens [79, 80]. Pomegranate extract interferes with quorum sensing signalling, which leads to biofilm disruption and impairment of bacteria motility [81]. In addition, pomegranate extract rich in flavonoids reduces inflammation via reducing oxidative stress and interference with NF-κB activity [82].
The effect of the bioactive compounds of plant origin on the growth and biofilm formation of periodontal pathogens and their potential mechanisms of action are summarized in Table 3.
Table 3.
Modulatory effects of plant-based compounds on biofilm formation property of periodontal pathogens
| Name of plant | Bioactive compounds | Mode of action | Inhibitory effect on the periodontal disease-causing strain | References |
|---|---|---|---|---|
|
Artocarpus lakoocha (Moraceae) |
Oxyresveratrol | SEM images showed oxyresveratrol might influence bacterial cell wall integrity | A. lakoocha extract prevented the growth and biofilm of periodontal pathogen namely A. actinomycetemcomitans, in a dose and time-dependent manner | [74] |
| Camellia sinensis | Epigallocatechin gallate (EGCG) | Galloyol moiety ester-linked to 3’-OH of catechin inhibited the adherence factors | Inhibits the growth and biofilm formation of P. gingivalis | [78] |
|
Kaempferia pandurate (Ginger family) |
Panduratin A | Destruction of the cell wall and cytoplasmic membrane |
Panduratin A showed MIC of 4 µg/ml against periodontal pathogens (P. gingivalis and P. intermedia) Panduratin A prevented dental biofilm composed of S. sanguis, and Actinomyces viscosus by more than 50% at 10 µg/ml following exposure of 15 min |
[197] [75] |
| Punica granatum (Pomegranate) |
Anthocyanins Caffeic acid Catechin Ellagic acid Epigallocatechin Gallic acid |
Flavonoids absorb free radicals to prevent oxidative damage of important biomolecules inhibits NF-κB activity to impart an anti-inflammatory effect |
Suppress the growth and biofilm formation of dental pathogens Mouthwash containing pomegranate extract lowered the plaque index of A. actinomycetemcomitans, P intermedia, and P. gingivalis |
[82] [198] |
|
Vaccinium macrocarpon (Ericaceae fruit), also known as cranberry extract |
Licocalchone A Proanthocyanidins |
Inhibition of the enzymes and proteases needed to form biofilms |
Cranberry extract can suppress the growth of F. nucleatum and P. gingivalis Adherence factors are inhibited in case of P. gingivalis as well as co-aggregation with other bacteria are suppressed Also inhibit the formation of biofilm by both bacteria at concentrations or greater than 62.5 μg/ml |
[80] |
Quorum sensing and quorum quenching in periodontal pathogens
Bacterial communication plays a vital role in establishing a host-infection process successfully [83]. Communication between oral biofilms has been demonstrated in numerous studies. Quorum sensing (QS) is the most distributed and studied bacterial communication, which helps periodontal bacteria communicate with each other through signalling molecules and behaviour coordination [84]. QS gene expression and signalling are mostly limited to periodontal pathogens, including P. gingivalis and A. actinomycetemcomitans isolated from patients with dental biofilm-associated diseases [85]. P. gingivalis uses signalling molecules for interspecies interaction to promote a microbial dysbiosis which leads to periodontal disease [86]. Thus, targeting those signalling pathways serves a new way for preventing periodontal diseases [86]. A study conducted by Azakami et al. reported that the homologous luxS gene (required for the synthesis of autoinducers-2), found in other periodontal pathogens, P. intermedia, and F. nucleatum, as well as in Eikenella corrodens, initiates the formation of biofilms in the oral cavity through LuxS-dependent pathway [87]. A. actinomycetemcomitans can also form mature biofilm through autoinducer-2 dependent quorum sensing manner [88]. AHL-mediated QS, like Aliivibrio fischeri, has not been reported in periodontal pathogens [85].
Developing a drug that could inhibit quorum sensing has been gaining attention in recent years. Quorum quenching, commonly known as inhibiting the signalling pathway of quorum sensing, can be an attractive solution for targeting periodontal bacteria by destroying bacterial communication, a critical aspect of biofilm formation and virulence factor expression [89, 90]. Plancak et al. have discovered naturally occurring inhibitory compounds and synthetic derivatives, which are classified based on their structural quorum sensing properties, [88]. Studies have shown that Delisea pulchra, a marine alga produces various components of vanilla extract, garlic, halogenated furanones and vanilla extract. These compounds have quorum quenching properties that help block the quorum sensing singling in periodontal pathogens [91–94]. It has been reported that adding ribose derivatives to the culture media was shown to negatively affect the formation of biofilms in A. actinomycetemcomitans [95].
Similar functional properties are shared by ribose binding protein and autoinducer-2; ribose binding protein acts as an antagonist on the receptors of autoinducer-2 [95]. Brominated furanones have a negative influence on biofilms of P. gingivalis, although they do not affect the growth of P. gingivalis [94]. Studies have shown that acylase, lactonase and oxyreductase are potential quorum quenching agents that inactivate the acyl-homoserine lactone, potentially affecting periodontal pathogens [96]. Thus, autoinducer-2 inhibition is an attractive therapeutic intervention target for treating periodontal diseases.
Immune modulation as a therapeutic approach for periodontal disease
Recently, immunomodulatory therapy that regulates the immune response has received much attention. Immunomodulatory therapy is a promising option for treating periodontal diseases [97]. Immunomodulators help to reduce bone loss by controlling the osteolytic and inflammatory process [98], preventing or intervening in the progression of periodontal disease.
The immune microenvironment of periodontitis-related tissue is critically essential for periodontal disease. Increased infiltration of leukocytes and the release of inflammatory molecules can resolve inflammation effectively. However, excessive amounts of this inflammatory response can cause serious harm to the periodontal tissues and the alveolar bone. Modulation of this microenvironment can not only supplement traditional treatments for periodontal disease but also may promote periodontal regeneration [97]. Different approaches have been taken to modulate leukocytes and the other inflammatory cytokines in the microenvironment, including immunomodulatory drug therapy, stem cell therapy, and gene therapy.
Among the leukocytes, macrophages play a significant role in various stages of periodontitis [99]. Neutrophils are key players in maintaining the homeostasis of periodontal health and are considered the first line of defence against periodontitis [98, 100, 101]. Monocytes also contribute to the defence system, and monocyte cells present in higher numbers in the intermediate stage of periodontal disease [102]. At the same time, T lymphocytes can play a vital role in gingival health and alveolar bone resorption [103–105].
Drug therapy targeting immune response is promising for treating periodontal diseases. Resveratrol is a natural polyphenol and can inhibit NF-kB activation in macrophages, and it shows immunomodulatory effects on F. nucleatum by upregulation of the antioxidant pathway [106, 107]. Metformin can inhibit the production of nitric oxide synthase in monocytes, affecting periodontitis [108]. Metformin reduces inflammation by the regulation of IL-1β [109]. Catechin is another polyphenol used as an immunomodulator. In the mice model, catechin is effective against gingivitis by reducing the lL-1β in the macrophage [110]. Gliclazide has antioxidant properties and can reduce the infiltration of neutrophils and macrophages, as shown in the rat model with periodontal disease [111]. Moreover, it can reduce inflammation by decreasing the level of TNF-α [111].
Recent studies have shown that curcumin can release pro-inflammatory molecules, which is effective against periodontal pathogens [112]. CMC2.24 is a modified version of curcumin that reduces phagocytic activity in macrophages, and its anti-inflammatory properties have been shown against periodontal disease [113, 114].
Trans-cinnamic aldehyde is also used as a drug for treating A. actinomycetemcomitans, which involves the downregulation of TNF-α and IL-1β cytokines [115]. Kava-205Me lowers the level of IFN-γ and IL-12 and is effective against P. gingivalis. Other drug therapy includes carnosic acid, glyburide, and bismuth drugs, which show their immunomodulation by infiltrating inflammatory cells and reducing pro-inflammatory cytokines such as IL-6, IL-1β and TNF-α [116, 117].
Immuno-related gene therapy is another novel approach targeting host immunity. It has been shown that downregulation of T-cell immune response cDNA7 (TIRC7) leads to a lower number of T-cells in gingival tissue, which affects the periodontal disease [118]. Plasmid DNA encoding miR-200c injected into gingiva has been shown to prevent gingival inflammation [119]. Cathepsin K (Ctsk) can reduce inflammation and osteoclast activity by lowering the level of TNF-α, INF-γ, IL-1α, IL-1β, and IL-12, thus regulating periodontal health [120]. P2X7 receptor (P2X7R) can modify the local microenvironment of periodontitis and improve the bone tissue regeneration [121]. The mode of action of TNF receptor and immunoglobulin Fc (TNFR: Fc) involves infiltration of leukocytes and reduction of cytokines, notably IL-1β, TNF-α, IL-6 and IL-10 [122].
Stem cell therapy has significant potential to impact periodontal health. Different mesenchymal stem cells can modulate the microenvironment coupled with the inflammatory response of leukocytes and cytokines associated with periodontal disease. These stem cells are being explored as a promising therapeutic intervention for periodontal disease. Periodontal ligament stem cells (PDLSCs) can interact with immune cells by modulating their activities and thus work as a potential therapeutic approach for treating periodontal diseases [123]. PDLSc can enhance tissue regeneration by converting macrophages to anti-inflammatory phenotypes and suppress the immune response by inactivating B-cells [124, 125]. PDLSc can be drastically elevated with resveratrol treatment. In periodontal patients, resveratrol treatment partially decreases bone loss and inhibits the infiltration of T-cells [126]. Gingiva-Derived Mesenchymal Stem cells (GMSCs) promote the polarization of M1 to M2 macrophages, lower the infiltration of neutrophils and decrease pro-inflammatory cytokines [127–129]. Mesenchymal stem cells derived from human exfoliated deciduous teeth showed reduced levels of TNF-α, IFN-γ and IL-2 and promoted anti-inflammatory response in macrophages [130, 131]. Dental Follicle Stem Cells (DFSCs) regulate peripheral blood mononuclear cells by enhancing the levels of IL-10 but lowering the levels of IFN-γ and IL-4 and can thus play an essential role as immunomodulators in the treatment of periodontal diseases [132, 133]. Bone marrow mesenchymal stem cells and dental pulp stem cells (DPSCs) decrease the level of TNF-α, IFN-γ and IL-17 in periodontal pathogens [131, 134, 135]. In addition, DPSCs can regenerate different tissue, which includes bone, and those cells are easy to store [136]. Thus, these approaches are getting much attention for treating periodontal diseases.
Low dose antibiotics are another group of agents that can modulate host immune response, potentially contributing to the periodontal disease progression or resolution. Doxycycline at a low dose, i.e. at sub-inhibitory concentrations, inhibits matrix metalloproteinase‐8 (MMP-8), which is present abundantly in periodontitis [137]. A pilot study by Ryan et al. investigated chemically modified tetracycline-3 in periodontal patients receiving scaling and root planing. Administration of this antimicrobial agent at a low dose (10 mg/d) reduced the levels of interleukin‐1-beta and matrix metalloproteinase‐8 in gingival crevicular fluid moderately [138]. Several other studies have shown reduce pocket depth (PD) and gain in clinical attachment level (CAL) in patients with periodontal disease when sub-antimicrobial dose of doxycycline was administrated [139, 140]. Also, studies reported significantly improved clinical parameters and reduced gingival inflammation in the host [139, 141, 142].
Table 4 lists the recent studies in the literature in which the immune microenvironment is targeted, together with the cytokines and leukocyte targets of these immune therapies.
Table 4.
A summary of immunotherapies and their targets
| Type of Therapy | Immunomodulatory activity on targets | References | |||
|---|---|---|---|---|---|
| Monocytes/Macrophage | Lymphocyte | Cytokines | |||
| Drug therapy | Resveratrol | Treatment of gingival keratinocytes with Resveratrol inhibited NF-kB activation and reduced the marker TREM-1 in monocytes | / | / | [107] |
| Metformin | In Monocytes, metformin hindered the production of nitric oxide synthase and nitric oxide induced with LPS. In addition, this drug decreased the expression of chemokine (C–C motif) ligand 2 (CCL-2) to improve inflammation | / | Delivery of metformin in diabetic mice with periodontitis effectively decreased NLRP3 and IL-1β levels reducing inflammation | [108, 109] | |
| Catechin | / | / | Catechin decreased IL-1β level from macrophages in mice model of gingivitis | [110] | |
| Gliclazide | Gliclazide treatment in a rat model of periodontitis reduced infiltration of neutrophils, macrophages and reduced activity of myeloperoxidase enzyme | / | This drug also reduced inflammation by declining the levels of IL-1β and TNF-α, as well as reduced the expression of COX-2 and cathepsin k | [111] | |
| Curcumin (CMC2.24) | Chemically-Modified version of Curcumin (CMC2.24) reduced phagocytic activity of macrophages | / | Also, curcumin reduced oxidative stress, bone resorption and inflammatory response by decreasing the levels of TNF-α, IL-1β, IL-10 | [113, 199] | |
| Trans-cinnamic aldehyde | / | / | A. actinomycetemcomitans infected cells were pre-treated with trans-cinnamic aldehyde which lowered the levels of TNF-α, IL-1β | [115] | |
| The Amyl-1–18 peptide | This peptide was derived from rice which can inhibit the secretion of IL-6 from macrophage | This peptide can also counteract LPS, suppress signal transduction in NF-kB as well as IL-1R signaling pathway | [200] | ||
| Kava-205Me | / | / |
Addition of methyl group to natural compound kavain is known as Kava-205Me (5,5-dimethyl-3-oxocyclohex-1-en-1-yl 4-methylbenzoate). Kavain is generally obtained from Piper methysticum Human cell lines were infected with P. gingivalis and treated with Kava-205Me at different doses. This compound could decrease the levels of IL-12, IL-10, IFN-γ and exotoxin. These cytokines indicate the early phase of inflammation |
[201] | |
| Carnosic Acid | Carnosic acid reduced infiltration of inflammatory cells | / | This phenolic diterpene which is a natural compound imparted anti-oxidative and anti-inflammatory properties in mice model challenged with LPS. Application of carnosic acid led to the decrease in expression of CXCL9, CXCL10, and CXCL11 | [202] | |
| Glyburide | Glyburide is a blood sugar lowering drug. Treatment with glyburide declines infiltration of inflammatory cells and number of osteoclast cells | / | THP-1 which is a macrophage-like cell line was stimulated with periodontal pathogenic bacteria and treated with glyburide, which resulted in decrease of IL-1β levels in culture media but increase in expression of endogenous IL-1β | [116] | |
| Bismuth drugs | / | / | Treatment of P. gingivalis challenged human cells with bismuth drugs decreased its iron acquisition ability and reduced the levels of proinflammatory cytokines such as IL-6, IL-1β and TNF-α | [117] | |
| Gene therapy |
T-cell immune response cDNA7 (TIRC7) Isoform of Atp6i |
/ | Downregulation of TIRC7 was achieved by shRNA and delivered locally by Adeno-Associated Virus (AAV). This modification of gene led to the lowering T cells in the gingival tissue | In addition, silencing of TIRC7 triggers the reduction in levels of IL-6, IL-17A and Cathepsin K (Ctsk) in periodontal tissue | [118] |
| MicroRNA-200c (miR-200c) | / | / | Plasmid DNA encoding miR-200c was directly injected into the gingiva which rescued the downregulation of miR-200c as well as prevented periodontal inflammation. In addition, miR-200c effectively decreased the levels of IL-6 and IL-8 at transcription level which is involved in sustaining systemic inflammation in periodontitis | [119] | |
| Ctsk | / | Ctsk was downregulated by shRNA and carried in AAV to deliver in periodontal ligament cells, which reduced the number of T cells and dendritic cells. This abolished inflammation and osteoclast activity | Silencing of Ctsk also decreased the levels of cytokines such as TNF-α, INF-γ, IL-1α, IL-1β, IL-12 and IL-17 while upregulates the levels of IL-6 in vivo | [120] | |
| P2X7R | / | / | P2X7 receptor (P2X7R) gene-modified stem cells help to reduce inflammation. Moreover, this genetically modified cells ameliorate the harsh local microenvironment of periodontitis through secreting molecules on neighboring cells and improve tissue regeneration | [121] | |
| TNFR: Fc | / | In rat model of periodontal disease, application of a fusion gene containing TNF receptor and immunoglobulin Fc (TNFR: Fc) by AAV increase the expression of TNFR protein in serum at therapeutic level. This protein also reduced infiltration of leukocytes at the site of infection | Moreover, TNFR protein decreased the levels of several cytokines such as IL-1β, TNF-α, IL-6 and IL-10 which reduced inflammation as well as prevented bone loss | [122] | |
| Stem cell therapy | Periodontal ligament stem cells (PDLSCs) |
Application of PDLSCs promote the conversion of macrophages to the anti-inflammatory (M2) phenotype which helps to enhanced regeneration of tissues |
PDLSCs can suppress immune response by performing anergy of T-cell and decreasing the proliferation, differentiation and activation of B cells | Moreover, a significant amount of decrease in the level of glycoprotein named CD1b was observed on dendritic cells due to the application of PDLSCs. This stem cell therapy inhibited the proliferation of T cell suggesting a promising a novel therapeutic approach | [124, 125, 203] |
| Gingiva-Derived Mesenchymal Stem cells (GMSCs) | Extracellular vesicles obtained from GMSC promote the polarization of M1 to M2 macrophages | GMSCs can reduce the infiltration of neutrophils, Th17 cells, as well as increase the migration of Tregs |
Application of GMSCs decreased pro-inflammatory cytokines such as TNF-α, IL-1β and IL-6 significantly while increases the anti-inflammatory cytokine IL-10 |
[108, 127, 128] | |
| Mesenchymal stem cells derived from human exfoliated deciduous teeth (SHEDs) | Enhance the differentiation of anti-inflammatory macrophages | Promote an immunomodulatory function in monocyte derived from dendritic cells | Application of SHED at different dose showed a decrease in levels of TNF-α, IFN-γ and IL-2, and an increase in IL-10 showed in rat model | [130, 131, 204] | |
| Dental Follicle Stem Cells (DFSCs) | / | Increased the number of Tregs |
When stimulated by IFN-γ, DFSCs can modulate peripheral blood mononuclear cells (PBMCs) obtained from healthy donor by enhancing the levels of IL-10 but decreasing the levels of IFN-γ and IL-4 DFSCs showed immune response to lipopolysaccharide (LPS) from P. gingivalis leading to decreased levels of TLR4 mRNA significantly. This was accompanied by an increased migration of the stem cells without altering the levels of IL-6 |
[132, 133] | |
| Bone Marrow Mesenchymal Stem Cells (BMMSCs) | Extracellular vesicles derived from BMMSCs decreased the levels of M1 macrophages and increased the levels of M2 macrophages | / |
In periodontitis model of rat, transplantation of Acetylsalicylic acid treated BMMSCs increased IL-10 and reduced tumor necrosis factor-α (TNF-α) and IL-17 reducing inflammation In another study of rat model with periodontal disease, application of BMMScs showed reduction in levels of IL-1β, TNF-α,IL-17 |
||
| Dental pulp stem cells (DPSCs) | Moreover, exosomes-incorporated in chitosan hydrogel obtained from DPSCs enhanced the conversion of proinflammatory state macrophages to an anti-inflammatory state | Increased the number of Tregs and decreased Th17 cells | LPS challenged co-culture of DPSCs and PBMCs showed decrease in TNF-α, IFN-γ, IL-2, IL-17 and an increase in IL-10 secretion without altering the levels of IL-6 and IL-1β | [131, 135, 209, 210] | |
| Other therapy |
Photodynamic therapy (PDT) |
/ | / |
Decreased the levels of TNF-α in asthmatic animals challenged with P. gingivalis Decreased the level of IL-6, IL-8, and CXCL10 in A. actinomycetemcomitans and promotes periodontal regeneration |
[211] [212] |
| Methylene blue-mediated photodynamic therapy (MBPDT) |
Induction of apoptosis in macrophage due to oxidative stress as well as mitochondria dependent apoptosis pathways were shown in vitro |
/ | Lower levels of IL-1β and TNF-α was observed | [213] | |
| Low-intensity pulsed ultrasound (LIPUS) | / | / | Treatment suppressed a range of cytokines at transcription level including IL-1a, IL-1b, IL-6, IL-8 as well as reduced the levels of IL-1ß and tumor necrosis factor a (TNFα) by periodontal ligament fibroblasts (PDLFs) | [214] | |
|
Singlet phototherapy |
Infiltration of macrophages in periodontitis and significant vascularization of periodontal tissue that contributed to tissue regeneration and ameliorate infection | / | / | [215] | |
|
Indocyanine green (ICG)-diode laser-based photothermal therapy (PTT) |
/ | / | Reduction of IL-1β and MMP-8 at 6 months after treatment in patients with gingival inflammation | [213] | |
Modification of periodontal microbiota by probiotic and microbiota replacement therapy
In contrast to the traditional view of individual pathogens being responsible for disease onset, the new perspective of periodontal disease deems that the transition from health to disease status is attributed to a shift in the global balance of the microbial flora (i.e. disruption of microbial homeostasis). Periodontal disease is considered a result of disrupted microbial homeostasis and microbe-mediated disruption of host homeostasis. Such a perspective has opened new paths for periodontal disease treatment, including restoring the homeostasis of the microbiota associated with periodontal health.
Recent studies have suggested probiotic therapy for treating periodontal diseases in the oral cavity [143, 144]. Probiotics help control diseases presumably through immune modulation and colonization resistance to pathogens. The selection of the microbial strain is crucial for the treatment outcome of the probiotic therapy. Most of the chosen probiotics originated from gut microbiota or fermented foods [145]. Recent clinical trials have shown that the administration of probiotics improves bacterial dysbiosis in periodontal patients [146]. Beneficial bacteria create a substantial barrier against colonization of endogenous and exogenous pathogens [8]. Antagonistic bacteria of probiotics have the potential to fight against periodontal bacteria [37]. The study by Van et al. has shown that clinical isolates collected from healthy patients significantly inhibited the growth of P. intermedia or P. gingivalis, which was high in the diseased patient sample [147]. These pathogen-inhibiting isolates include Bifidobacterium, Streptococcus, and Actinomyces. Commercially available dietary probiotics have been shown to have more substantial inhibitory properties against periodontal bacteria [8]. A successful periodontal therapeutic approach is characterized by the alleviation of periodontal inflammation, which relates to changes in microflora, and in that aspect, probiotic therapy may potentially plays a role [148].
A study comparing the prevalence of oral Lactobacilli in healthy subjects and patients with periodontitis (chronic) showed that Lactobacillus fermentum and Lactobacillus gasseri were the most common species in healthy subjects. In contrast, Lactobacillus plantarum was the most prevalent species in patients with periodontitis [149]. In addition, four species of Lactobacilli have shown to have the greatest antimicrobial properties, which is confirmed by international guidelines for probiotics evaluation. Those isolates include Lactobacillus salivarius, Lactobacillus plantarum, Lactobacillus rhamnosus and Lactobacillus paracasei, which can be used as a probiotics for maintaining oral health [150]. A report showed that Lactobacillus reuteri could be as efficacious as probiotics for reducing dental-biofilm induced by systemic or local risk factors [151]. Studies of fifty-one patients showed yogurt supplemented with Bifidobacterium animalis significantly impacted inflammatory parameters of gingiva and bacterial plaque [152].
Microbiota replacement therapy is a new disease treatment/prevention measure in which microbiota from healthy donors is transplanted to diseased patients. Replacement therapies have been successfully used to treat Clostridium difficile infections. Also, it has shown efficacy for treating one class of inflammatory bowel diseases known as Crohn’s disease. [153, 154]. Microbiota replacement therapies are currently being explored as an alternative approach to treating periodontal disease [37]. Naturally occurring and laboratory-derived oral bacteria can be used in replacement therapy of periodontal diseases. Protocols have been suggested for transferring microbiota from a healthy donor to a patient with periodontal diseases [155, 156]. Recent study protocol has shown that oral microbiota transplant therapy could effectively treat periodontal disease by modulating the oral microbiota. For oral microbiota transplant therapy, details of the donor's medical history and microbiota analysis are essential [157]. With the advancement of the human microbiome and a better understanding of the relationship between oral microbiota and oral diseases, it is expected that more practical and more effective microbiota replacement therapy approaches for periodontal diseases will be available in the future.
Conclusion
Although anti-virulence therapeutic strategies are relatively new in preventing and treating periodontal disease, novel drugs targeting the critical virulence factors of periodontal pathogens have already been identified, which serve as a promising alternative in the era of antibiotic resistance. A growing library of novel targets will open the door to rapidly identifying anti-virulence drugs.
Among the approaches that target specific virulence factors to treat periodontal diseases, protease inhibitors against gingipains have been tested in vivo. Although receptor-based peptide inhibitors against LtxA activity and peptides interfering with fimbrial biogenesis show significant results, animal models' potency must be tested to validate their effectiveness.
The formation of biofilm by oral pathogens makes these pathogens challenging to treat with antibiotics. Compounds with bioactive in quorum quenching and downregulating adherence factors in microbes can be used to prevent or clear up microbial biofilms. Further studies are required to test the quorum quenching strategies to combat biofilm formation by periodontal pathogens.
Plant-derived natural bioactive compounds impart general inhibitory effects on many oral pathogens. The growing emergence and spread of antimicrobial resistance call for renewed interests in plant-based compounds such as polyphenols, which have few side effects to the host. Antimicrobial activity and anti-inflammatory effects of these natural compounds make them an attractive remedy for periodontal diseases. However, the effective doses of these natural antimicrobial compounds need to be determined and their antimicrobial mechanisms assessed before they can be used in conjunction with traditional treatment approaches.
Immunomodulatory therapy and microbiome-based therapies show exciting promises in treating periodontal diseases. Still, further research is needed to improve their efficacy and to understand their long-term effects.
In summary, significant progress has been made in our understanding of periodontal disease and its treatment strategies in recent years. We can expect novel approaches will become available for periodontal disease prevention and treatment in the near future.
Acknowledgements
We thank our lab members for critically reviewing the manuscript, and we thank Ms. Caroline Monnin at the Neil John Maclean Health Sciences Library for her assistance with the literature search. The schematic illustration in Fig. 2 was prepared using biorender.com.
Abbreviations
- AAP
American Academy of Periodontology
- EFP
European Federation of Periodontology
- EPS
Extracellular polymeric substances
- CPS
Capsular polysaccharide
- LPS
Lipopolysaccharide
- IL
Interleukin
- LtxA
Leukotoxin
- CDT
Cytolethal distending toxin
- LFA-1
Lymphocyte function-associated antigen-1
- OMVs
Outer membrane vesicle
- DANA
2-Deoxy-2,3-didehydro-N-acetylneuraminic acid
- EGCG
Epigallocatechin gallate
- QS
Quorum sensing
- TIRC7
T-cell immune response cDNA7
- Ctsk
Cathepsin K
- TNFR: Fc
TNF receptor and immunoglobulin Fc
- PDLSCs
Periodontal ligament stem cells
- GMSCs
Gingiva-derived mesenchymal stem cells
- DFSCs
Dental follicle stem cells
- DPSCs
Dental pulp stem cells
- MMP-8
Matrix metalloproteinase‐8
- PD
Pocket depth
- CAL
Clinical attachment level
- PCL
Polycaprolactone
- PI
Plaque index
- GI
Gingival index
- PEG
Poly Ethylene Glycol
- TCS
Thiolated Chitosan
- PMAA
Poly (methacrylic acid)
- PDLGA
Poly Lactic-Co-glycolic acid
- PD
Probing depth
- AAV
Adeno-associated virus
- P2X7R
P2X7 receptor
- TNF-α
Tumor necrosis factor-α
- PDT
Photodynamic therapy
Author contributions
KD and MMH conceptualized and wrote the paper. AKC provided clinical information on periodontal disease therapies and contributed to the article's writing. KY contributed to the writing of the article. All authors have read and agreed to the submitted version of the manuscript.
Authors' information
MMH is a PhD student in the Department of Oral Biology. KD is a professor in the Department of Oral Biology with expertise in the areas of the oral microbiome, bacterial pathogenicity, and antibiotic resistance. KY is an assistant professor in the School of Dental Hygiene with expertise in root resorption and oral microbial disease. AKC is a periodontist with extensive clinical experience in periodontal disease; she is an associate professor in the Department of Dental Diagnostic and Surgical Sciences and the Dean of Dr. Gerald Niznick College of Dentistry.
Funding
The study was funded by a grant from the Natural Sciences and Engineering Research Council of Canada (RGPIN-05864–2019). The funding agency played no role in the study's design, the collection, analysis, and interpretation of data or the writing of the manuscript.
Availability of data and materials
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pihlstrom BL, Michalowicz BS, Johnson NW. Periodontal diseases. Lancet. 2005;366(9499):1809–1820. doi: 10.1016/S0140-6736(05)67728-8. [DOI] [PubMed] [Google Scholar]
- 2.Papapanou PN, Sanz M, Buduneli N, Dietrich T, Feres M, Fine DH, Flemmig TF, Garcia R, Giannobile WV, Graziani F, et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J Periodontol. 2018;89(Suppl 1):S173–S182. doi: 10.1002/JPER.17-0721. [DOI] [PubMed] [Google Scholar]
- 3.Caton JG, Armitage G, Berglundh T, Chapple ILC, Jepsen S, Kornman KS, Mealey BL, Papapanou PN, Sanz M, Tonetti MS. A new classification scheme for periodontal and peri-implant diseases and conditions - Introduction and key changes from the 1999 classification. J Periodontol. 2018;89(Suppl 1):S1–S8. doi: 10.1002/JPER.18-0157. [DOI] [PubMed] [Google Scholar]
- 4.Sanz M, Herrera D, Kebschull M, Chapple I, Jepsen S, Beglundh T, Sculean A, Tonetti MS, Participants EFPW, Methodological C. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J Clin Periodontol. 2020;47(Suppl 22):4–60. doi: 10.1111/jcpe.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.How KY, Song KP, Chan KG. Porphyromonas gingivalis: An Overview of Periodontopathic Pathogen below the Gum Line. Front Microbiol. 2016;7:53. doi: 10.3389/fmicb.2016.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinane DF, Stathopoulou PG, Papapanou PN. Periodontal diseases Nat Rev Dis Primers. 2017;3:17038. doi: 10.1038/nrdp.2017.38. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki N, Yoneda M, Hirofuji T. Mixed red-complex bacterial infection in periodontitis. Int J Dent. 2013;2013:587279. doi: 10.1155/2013/587279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genco RJ: Consensus report. Periodontal diseases: pathogenesis and microbial factors. Ann Periodontol 1996, 1(1):926–932. [DOI] [PubMed]
- 10.Herrera D, Sanz M, Jepsen S, Needleman I, Roldan S: A systematic review on the effect of systemic antimicrobials as an adjunct to scaling and root planing in periodontitis patients. J Clin Periodontol 2002, 29 Suppl 3:136–159; discussion 160–132. [DOI] [PubMed]
- 11.Rams TE, Degener JE, van Winkelhoff AJ. Antibiotic resistance in human chronic periodontitis microbiota. J Periodontol. 2014;85(1):160–169. doi: 10.1902/jop.2013.130142. [DOI] [PubMed] [Google Scholar]
- 12.ARF RF, & Brack, G. D. C.: Antibiotic resistance among patients with severe gum disease is increasing. In: BDJ Team. vol. 5: nature; 2018.
- 13.Fleitas Martinez O, Cardoso MH, Ribeiro SM, Franco OL. Recent Advances in Anti-virulence Therapeutic Strategies With a Focus on Dismantling Bacterial Membrane Microdomains, Toxin Neutralization, Quorum-Sensing Interference and Biofilm Inhibition. Front Cell Infect Microbiol. 2019;9:74. doi: 10.3389/fcimb.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Isola G: Antibiotics and Antimicrobials for Treatment of the Oral Microbiota: Myths and Facts in Research and Clinical Practice. Antibiotics (Basel) 2020, 9(2). [DOI] [PMC free article] [PubMed]
- 15.Reygaert WC. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018;4(3):482–501. doi: 10.3934/microbiol.2018.3.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilke MS, Lovering AL, Strynadka NC. Beta-lactam antibiotic resistance: a current structural perspective. Curr Opin Microbiol. 2005;8(5):525–533. doi: 10.1016/j.mib.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 17.Bernal LA, Guillot E, Paquet C, Mouton C. beta-Lactamase-producing strains in the species Prevotella intermedia and Prevotella nigrescens. Oral Microbiol Immunol. 1998;13(1):36–40. doi: 10.1111/j.1399-302x.1998.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 18.Eick S, Pfister W, Straube E. Antimicrobial susceptibility of anaerobic and capnophilic bacteria isolated from odontogenic abscesses and rapidly progressive periodontitis. Int J Antimicrob Agents. 1999;12(1):41–46. doi: 10.1016/s0924-8579(99)00056-4. [DOI] [PubMed] [Google Scholar]
- 19.Walker CB, Tyler KZ, Low SB, King CJ. Penicillin-degrading enzymes in sites associated with adult periodontitis. Oral Microbiol Immunol. 1987;2(3):129–131. doi: 10.1111/j.1399-302x.1987.tb00276.x. [DOI] [PubMed] [Google Scholar]
- 20.Feres M, Haffajee AD, Allard K, Som S, Goodson JM, Socransky SS. Antibiotic resistance of subgingival species during and after antibiotic therapy. J Clin Periodontol. 2002;29(8):724–735. doi: 10.1034/j.1600-051x.2002.290809.x. [DOI] [PubMed] [Google Scholar]
- 21.Ardila CM, Granada MI, Guzman IC. Antibiotic resistance of subgingival species in chronic periodontitis patients. J Periodontal Res. 2010;45(4):557–563. doi: 10.1111/j.1600-0765.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 22.Veloo AC, Seme K, Raangs E, Rurenga P, Singadji Z, Wekema-Mulder G, van Winkelhoff AJ. Antibiotic susceptibility profiles of oral pathogens. Int J Antimicrob Agents. 2012;40(5):450–454. doi: 10.1016/j.ijantimicag.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 23.van Winkelhoff AJ, Herrera Gonzales D, Winkel EG, Dellemijn-Kippuw N, Vandenbroucke-Grauls CM, Sanz M: Antimicrobial resistance in the subgingival microflora in patients with adult periodontitis. A comparison between The Netherlands and Spain. J Clin Periodontol 2000, 27(2):79–86. [DOI] [PubMed]
- 24.Ready D, Lancaster H, Mullany P, Bedi R, Wilson M. Antibiotic resistance in the cultivable plaque microbiota of children from different ethnic groups. Int J Antimicrob Agents. 2006;27(5):376–382. doi: 10.1016/j.ijantimicag.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 25.Roberts MC, Chung WO, Roe DE. Characterization of tetracycline and erythromycin resistance determinants in Treponema denticola. Antimicrob Agents Chemother. 1996;40(7):1690–1694. doi: 10.1128/aac.40.7.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bidault P, Chandad F, Grenier D. Risk of bacterial resistance associated with systemic antibiotic therapy in periodontology. J Can Dent Assoc. 2007;73(8):721–725. [PubMed] [Google Scholar]
- 27.Chung WO, Gabany J, Persson GR, Roberts MC. Distribution of erm(F) and tet(Q) genes in 4 oral bacterial species and genotypic variation between resistant and susceptible isolates. J Clin Periodontol. 2002;29(2):152–158. doi: 10.1034/j.1600-051x.2002.290210.x. [DOI] [PubMed] [Google Scholar]
- 28.Bowler P, Murphy C, Wolcott R. Biofilm exacerbates antibiotic resistance: Is this a current oversight in antimicrobial stewardship? Antimicrob Resist Infect Control. 2020;9(1):162. doi: 10.1186/s13756-020-00830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang BY, Chi B, Kuramitsu HK. Genetic exchange between Treponema denticola and Streptococcus gordonii in biofilms. Oral Microbiol Immunol. 2002;17(2):108–112. doi: 10.1046/j.0902-0055.2001.00001.x. [DOI] [PubMed] [Google Scholar]
- 30.Walsh LJ. Novel approaches to detect and treat biofilms within the root canals of teeth: a review. Antibiotics (Basel) 2020;9(3):129. doi: 10.3390/antibiotics9030129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flemming HC, Wingender J, Szewzyk U, Steinberg P, Rice SA, Kjelleberg S. Biofilms: an emergent form of bacterial life. Nat Rev Microbiol. 2016;14(9):563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 32.Donlan RM. Role of biofilms in antimicrobial resistance. ASAIO J. 2000;46(6):S47–52. doi: 10.1097/00002480-200011000-00037. [DOI] [PubMed] [Google Scholar]
- 33.Heras B, Scanlon MJ, Martin JL. Targeting virulence not viability in the search for future antibacterials. Br J Clin Pharmacol. 2015;79(2):208–215. doi: 10.1111/bcp.12356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudkin JK, McLoughlin RM, Preston A, Massey RC. Bacterial toxins: Offensive, defensive, or something else altogether? PLoS Pathog. 2017;13(9):e1006452. doi: 10.1371/journal.ppat.1006452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan J, Patil PC, Luzzio FA, Demuth DR: In Vitro and In Vivo Activity of Peptidomimetic Compounds That Target the Periodontal Pathogen Porphyromonas gingivalis. Antimicrob Agents Chemother 2018, 62(7). [DOI] [PMC free article] [PubMed]
- 36.Garcia-Fernandez E, Koch G, Wagner RM, Fekete A, Stengel ST, Schneider J, Mielich-Suss B, Geibel S, Markert SM, Stigloher C et al: Membrane Microdomain Disassembly Inhibits MRSA Antibiotic Resistance. Cell 2017, 171(6):1354–1367 e1320. [DOI] [PMC free article] [PubMed]
- 37.Allaker RP, Douglas CW. Novel anti-microbial therapies for dental plaque-related diseases. Int J Antimicrob Agents. 2009;33(1):8–13. doi: 10.1016/j.ijantimicag.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 38.Rosen G, Sela MN. Coaggregation of Porphyromonas gingivalis and Fusobacterium nucleatum PK 1594 is mediated by capsular polysaccharide and lipopolysaccharide. FEMS Microbiol Lett. 2006;256(2):304–310. doi: 10.1111/j.1574-6968.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 39.Singh A, Wyant T, Anaya-Bergman C, Aduse-Opoku J, Brunner J, Laine ML, Curtis MA, Lewis JP. The capsule of Porphyromonas gingivalis leads to a reduction in the host inflammatory response, evasion of phagocytosis, and increase in virulence. Infect Immun. 2011;79(11):4533–4542. doi: 10.1128/IAI.05016-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nishihara T, Ueda N, Amano K, Ishihara Y, Hayakawa H, Kuroyanagi T, Ohsaki Y, Nagata K, Noguchi T. Actinobacillus actinomycetemcomitans Y4 capsular-polysaccharide-like polysaccharide promotes osteoclast-like cell formation by interleukin-1 alpha production in mouse marrow cultures. Infect Immun. 1995;63(5):1893–1898. doi: 10.1128/iai.63.5.1893-1898.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng R, Wu Z, Li M, Shao M, Hu T. Interleukin-1beta is a potential therapeutic target for periodontitis: a narrative review. Int J Oral Sci. 2020;12(1):2. doi: 10.1038/s41368-019-0068-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tangsuksan P, Srichana T, Kettratad M, Nittayananta W. Antimicrobial and Anti-inflammatory Effects of alpha-Mangostin Soluble Film. J Int Soc Prev Community Dent. 2022;12(2):189–198. doi: 10.4103/jispcd.JISPCD_222_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koregol AC, Kalburgi NB, Puttarevanna T, Patil RS, Singh P, Sulakod K. Antimicrobial efficacy of grape seed extract in terminating the ramifications of plaque microorganisms: a randomized control study. Med Pharm Rep. 2022;95(2):185–190. doi: 10.15386/mpr-1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Izadi N, Keikha M, Ghazvini K, Karbalaei M. Oral antimicrobial peptides and new therapeutic strategies for plaquemediated diseases. Gene Reports. 2020;2020:21. [Google Scholar]
- 45.Derradjia A, Alanazi H, Park HJ, Djeribi R, Semlali A, Rouabhia M. alpha-tocopherol decreases interleukin-1beta and -6 and increases human beta-defensin-1 and -2 secretion in human gingival fibroblasts stimulated with Porphyromonas gingivalis lipopolysaccharide. J Periodontal Res. 2016;51(3):295–303. doi: 10.1111/jre.12308. [DOI] [PubMed] [Google Scholar]
- 46.Jian CX, Li MZ, Zheng WY, He Y, Ren Y, Wu ZM, Fan QS, Hu YH, Li CJ. Tormentic acid inhibits LPS-induced inflammatory response in human gingival fibroblasts via inhibition of TLR4-mediated NF-kappaB and MAPK signalling pathway. Arch Oral Biol. 2015;60(9):1327–1332. doi: 10.1016/j.archoralbio.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 47.Evans RT, Klausen B, Sojar HT, Bedi GS, Sfintescu C, Ramamurthy NS, Golub LM, Genco RJ. Immunization with Porphyromonas (Bacteroides) gingivalis fimbriae protects against periodontal destruction. Infect Immun. 1992;60(7):2926–2935. doi: 10.1128/iai.60.7.2926-2935.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alaei SR, Park JH, Walker SG, Thanassi DG. Peptide-based inhibitors of fimbrial biogenesis in Porphyromonas gingivalis. Infect Immun. 2019;87(3):e00750. doi: 10.1128/IAI.00750-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chang EH, Huang J, Lin Z, Brown AC. Catechin-mediated restructuring of a bacterial toxin inhibits activity. Biochim Biophys Acta Gen Subj. 2019;1863(1):191–198. doi: 10.1016/j.bbagen.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vega BA, Belinka Jr BA, Kachlany SC. Aggregatibacter actinomycetemcomitans Leukotoxin (LtxA; Leukothera((R))): Mechanisms of Action and Therapeutic Applications. Toxins (Basel) 2019;11(9):489. doi: 10.3390/toxins11090489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Balashova N, Dhingra A, Boesze-Battaglia K, Lally ET. Aggregatibacter actinomycetemcomitans leukotoxin induces cytosol acidification in LFA-1 expressing immune cells. Mol Oral Microbiol. 2016;31(1):106–114. doi: 10.1111/omi.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deas DE, Mealey BL. Response of chronic and aggressive periodontitis to treatment. Periodontol. 2000;2010(53):154–166. doi: 10.1111/j.1600-0757.2009.00334.x. [DOI] [PubMed] [Google Scholar]
- 53.Krueger E, Hayes S, Chang EH, Yutuc S, Brown AC. Receptor-Based Peptides for Inhibition of Leukotoxin Activity. ACS Infect Dis. 2018;4(7):1073–1081. doi: 10.1021/acsinfecdis.7b00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Isola G, Polizzi A, Santonocito S, Dalessandri D, Migliorati M, Indelicato F. New frontiers on adjuvants drug strategies and treatments in periodontitis. Scientia Pharmaceutica. 2021;89 (4):46. [Google Scholar]
- 55.Krueger E, Brown AC. Aggregatibacter actinomycetemcomitans leukotoxin: From mechanism to targeted anti-toxin therapeutics. Mol Oral Microbiol. 2020;35(3):85–105. doi: 10.1111/omi.12284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kato S, Kowashi Y, Demuth DR. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb Pathog. 2002;32(1):1–13. doi: 10.1006/mpat.2001.0474. [DOI] [PubMed] [Google Scholar]
- 57.Grenier D, La VD. Proteases of Porphyromonas gingivalis as important virulence factors in periodontal disease and potential targets for plant-derived compounds: a review article. Curr Drug Targets. 2011;12(3):322–331. doi: 10.2174/138945011794815310. [DOI] [PubMed] [Google Scholar]
- 58.Sztukowska MN, Roky M, Demuth DR. Peptide and non-peptide mimetics as potential therapeutics targeting oral bacteria and oral biofilms. Mol Oral Microbiol. 2019;34(5):169–182. doi: 10.1111/omi.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gusman H, Travis J, Helmerhorst EJ, Potempa J, Troxler RF, Oppenheim FG. Salivary histatin 5 is an inhibitor of both host and bacterial enzymes implicated in periodontal disease. Infect Immun. 2001;69(3):1402–1408. doi: 10.1128/IAI.69.3.1402-1408.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li N, Collyer CA. Gingipains from Porphyromonas gingivalis - Complex domain structures confer diverse functions. Eur J Microbiol Immunol (Bp) 2011;1(1):41–58. doi: 10.1556/EuJMI.1.2011.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Curtis MA, Aduse Opoku J, Rangarajan M, Gallagher A, Sterne JA, Reid CR, Evans HE, Samuelsson B. Attenuation of the virulence of Porphyromonas gingivalis by using a specific synthetic Kgp protease inhibitor. Infect Immun. 2002;70(12):6968–6975. doi: 10.1128/IAI.70.12.6968-6975.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jain H. Inhibition and attenuation of pathogenicity of Porphyromonas gingivalis by leupeptin: A review. Frontiers in Biology. 2017;12:192–198. [Google Scholar]
- 63.Azelmat J, Larente JF, Grenier D. The anthraquinone rhein exhibits synergistic antibacterial activity in association with metronidazole or natural compounds and attenuates virulence gene expression in Porphyromonas gingivalis. Arch Oral Biol. 2015;60(2):342–346. doi: 10.1016/j.archoralbio.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 64.Kataoka S, Baba A, Suda Y, Takii R, Hashimoto M, Kawakubo T, Asao T, Kadowaki T, Yamamoto K. A novel, potent dual inhibitor of Arg-gingipains and Lys-gingipain as a promising agent for periodontal disease therapy. FASEB J. 2014;28(8):3564–3578. doi: 10.1096/fj.14-252130. [DOI] [PubMed] [Google Scholar]
- 65.Yu S, Fan X, Zheng S, Lin L, Liu J, Pan Y, Li C. The sialidase inhibitor, DANA, reduces Porphyromonas gingivalis pathogenicity and exerts anti-inflammatory effects: An in vitro and in vivo experiment. J Periodontol. 2021;92(2):286–297. doi: 10.1002/JPER.19-0688. [DOI] [PubMed] [Google Scholar]
- 66.Ben Lagha A, Grenier D. Black tea theaflavins attenuate Porphyromonas gingivalis virulence properties, modulate gingival keratinocyte tight junction integrity and exert anti-inflammatory activity. J Periodontal Res. 2017;52(3):458–470. doi: 10.1111/jre.12411. [DOI] [PubMed] [Google Scholar]
- 67.Kong L, Qi X, Huang S, Chen S, Wu Y, Zhao L: Theaflavins inhibit pathogenic properties of P. gingivalis and MMPs production in P. gingivalis-stimulated human gingival fibroblasts. Arch Oral Biol 2015, 60(1):12–22. [DOI] [PubMed]
- 68.Yamanaka A, Kouchi T, Kasai K, Kato T, Ishihara K, Okuda K. Inhibitory effect of cranberry polyphenol on biofilm formation and cysteine proteases of Porphyromonas gingivalis. J Periodontal Res. 2007;42(6):589–592. doi: 10.1111/j.1600-0765.2007.00982.x. [DOI] [PubMed] [Google Scholar]
- 69.Nagata H, Inagaki Y, Yamamoto Y, Maeda K, Kataoka K, Osawa K, Shizukuishi S. Inhibitory effects of macrocarpals on the biological activity of Porphyromonas gingivalis and other periodontopathic bacteria. Oral Microbiol Immunol. 2006;21(3):159–163. doi: 10.1111/j.1399-302X.2006.00269.x. [DOI] [PubMed] [Google Scholar]
- 70.Kouidhi B, Al Qurashi YM, Chaieb K. Drug resistance of bacterial dental biofilm and the potential use of natural compounds as alternative for prevention and treatment. Microb Pathog. 2015;80:39–49. doi: 10.1016/j.micpath.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 71.Vouros I, Antonoglou GN, Anoixiadou S, Kalfas S. A novel biofilm removal approach (Guided Biofilm Therapy) utilizing erythritol air-polishing and ultrasonic piezo instrumentation: A randomized controlled trial. Int J Dent Hyg. 2022;20(2):381–390. doi: 10.1111/idh.12533. [DOI] [PubMed] [Google Scholar]
- 72.Isola G Current evidence of natural agents in oral and periodontal health. Nutrients. 2020;12(2):585. doi: 10.3390/nu12020585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kim YM, Yun J, Lee CK, Lee H, Min KR, Kim Y: Oxyresveratrol and hydroxystilbene compounds. Inhibitory effect on tyrosinase and mechanism of action. J Biol Chem 2002, 277(18):16340–16344. [DOI] [PubMed]
- 74.Teanpaisan R, Senapong S, Puripattanavong J. In vitro antimicrobial and antibiofilm activity of Artocarpus lakoocha (Moraceae) extract against some oral pathogens. Trop J Pharm Res. 2014;13(7):1149–1155. [Google Scholar]
- 75.Yanti, Rukayadi Y, Lee KH, Hwang JK: Activity of panduratin A isolated from Kaempferia pandurata Roxb. against multi-species oral biofilms in vitro. J Oral Sci 2009, 51(1):87–95. [DOI] [PubMed]
- 76.Melok AL, Lee LH, Mohamed Yussof SA, Chu T. Green tea polyphenol epigallocatechin-3-gallate-stearate inhibits the growth of Streptococcus mutans: a promising new approach in caries prevention. Dent J (Basel) 2018;6(3):38. doi: 10.3390/dj6030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sakanaka S, Okada Y. Inhibitory effects of green tea polyphenols on the production of a virulence factor of the periodontal-disease-causing anaerobic bacterium Porphyromonas gingivalis. J Agric Food Chem. 2004;52(6):1688–1692. doi: 10.1021/jf0302815. [DOI] [PubMed] [Google Scholar]
- 78.Asahi Y, Noiri Y, Miura J, Maezono H, Yamaguchi M, Yamamoto R, Azakami H, Hayashi M, Ebisu S. Effects of the tea catechin epigallocatechin gallate on Porphyromonas gingivalis biofilms. J Appl Microbiol. 2014;116(5):1164–1171. doi: 10.1111/jam.12458. [DOI] [PubMed] [Google Scholar]
- 79.Philip N, Walsh LJ Cranberry polyphenols: Natural weapons against dental caries. Dent J (Basel) 2019;7(1):20. doi: 10.3390/dj7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.de Medeiros AKB, de Melo LA, Alves RAH, Barbosa GAS, de Lima KC, Porto Carreiro ADF. Inhibitory effect of cranberry extract on periodontopathogenic biofilm: An integrative review. J Indian Soc Periodontol. 2016;20(5):503–508. doi: 10.4103/jisp.jisp_302_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Koh KH, Tham FY. Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J Microbiol Immunol Infect. 2011;44(2):144–148. doi: 10.1016/j.jmii.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Prasad D, Kunnaiah R. Punica granatum: A review on its potential role in treating periodontal disease. J Indian Soc Periodontol. 2014;18(4):428–432. doi: 10.4103/0972-124X.138678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Defoirdt T. Quorum-Sensing Systems as Targets for Antivirulence Therapy. Trends Microbiol. 2018;26(4):313–328. doi: 10.1016/j.tim.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 84.Basavaraju M, Sisnity VS, Palaparthy R, Addanki PK. Quorum quenching: Signal jamming in dental plaque biofilms. J Dent Sci. 2016;11(4):349–352. doi: 10.1016/j.jds.2016.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Frias J, Olle E, Alsina M. Periodontal pathogens produce quorum sensing signal molecules. Infect Immun. 2001;69(5):3431–3434. doi: 10.1128/IAI.69.5.3431-3434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hajishengallis G, Diaz PI. Porphyromonas gingivalis: Immune subversion activities and role in periodontal dysbiosis. Curr Oral Health Rep. 2020;7(1):12–21. doi: 10.1007/s40496-020-00249-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Azakami H, Teramura I, Matsunaga T, Akimichi H, Noiri Y, Ebisu S, Kato A. Characterization of autoinducer 2 signal in Eikenella corrodens and its role in biofilm formation. J Biosci Bioeng. 2006;102(2):110–117. doi: 10.1263/jbb.102.110. [DOI] [PubMed] [Google Scholar]
- 88.Plancak D, Music L, Puhar I. Quorum Sensing of Periodontal Pathogens. Acta Stomatol Croat. 2015;49(3):234–241. doi: 10.15644/asc49/3/6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lieberman JM. Appropriate antibiotic use and why it is important: the challenges of bacterial resistance. Pediatr Infect Dis J. 2003;22(12):1143–1151. doi: 10.1097/01.inf.0000101851.57263.63. [DOI] [PubMed] [Google Scholar]
- 90.Bhardwaj AK, Vinothkumar K, Rajpara N. Bacterial quorum sensing inhibitors: attractive alternatives for control of infectious pathogens showing multiple drug resistance. Recent Pat Antiinfect Drug Discov. 2013;8(1):68–83. doi: 10.2174/1574891x11308010012. [DOI] [PubMed] [Google Scholar]
- 91.Manefield M, Rasmussen TB, Henzter M, Andersen JB, Steinberg P, Kjelleberg S, Givskov M. Halogenated furanones inhibit quorum sensing through accelerated LuxR turnover. Microbiology (Reading) 2002;148(Pt 4):1119–1127. doi: 10.1099/00221287-148-4-1119. [DOI] [PubMed] [Google Scholar]
- 92.Bodini SF, Manfredini S, Epp M, Valentini S, Santori F. Quorum sensing inhibition activity of garlic extract and p-coumaric acid. Lett Appl Microbiol. 2009;49(5):551–555. doi: 10.1111/j.1472-765X.2009.02704.x. [DOI] [PubMed] [Google Scholar]
- 93.Choo JH, Rukayadi Y, Hwang JK. Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol. 2006;42(6):637–641. doi: 10.1111/j.1472-765X.2006.01928.x. [DOI] [PubMed] [Google Scholar]
- 94.Zhang L, Wang S, Zhou X, Xui Y. Quorum sensing inhibitor brominated furanone affects Porphyromonas gingivalis biofilm formation. Hua Xi Kou Qiang Yi Xue Za Zhi. 2011;29(5):469–472. [PubMed] [Google Scholar]
- 95.James D, Shao H, Lamont RJ, Demuth DR. The Actinobacillus actinomycetemcomitans ribose binding protein RbsB interacts with cognate and heterologous autoinducer 2 signals. Infect Immun. 2006;74(7):4021–4029. doi: 10.1128/IAI.01741-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim WJ, Soh Y, Heo SM. Recent Advances of Therapeutic Targets for the Treatment of Periodontal Disease. Biomol Ther (Seoul) 2021;29(3):263–267. doi: 10.4062/biomolther.2021.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yang B, Pang X, Li Z, Chen Z, Wang Y. Immunomodulation in the Treatment of Periodontitis: Progress and Perspectives. Front Immunol. 2021;12:781378. doi: 10.3389/fimmu.2021.781378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Sima C, Viniegra A, Glogauer M. Macrophage immunomodulation in chronic osteolytic diseases-the case of periodontitis. J Leukoc Biol. 2019;105(3):473–487. doi: 10.1002/JLB.1RU0818-310R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhou LN, Bi CS, Gao LN, An Y, Chen F, Chen FM. Macrophage polarization in human gingival tissue in response to periodontal disease. Oral Dis. 2019;25(1):265–273. doi: 10.1111/odi.12983. [DOI] [PubMed] [Google Scholar]
- 100.Hajishengallis G: New developments in neutrophil biology and periodontitis. Periodontol 2000 2020, 82(1):78–92. [DOI] [PubMed]
- 101.Armstrong CL, Klaes CK, Vashishta A, Lamont RJ, Uriarte SM. Filifactor alocis manipulates human neutrophils affecting their ability to release neutrophil extracellular traps induced by PMA. Innate Immun. 2018;24(4):210–220. doi: 10.1177/1753425918767507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagasawa T, Kobayashi H, Aramaki M, Kiji M, Oda S, Izumi Y. Expression of CD14, CD16 and CD45RA on monocytes from periodontitis patients. J Periodontal Res. 2004;39(1):72–78. doi: 10.1111/j.1600-0765.2004.00713.x. [DOI] [PubMed] [Google Scholar]
- 103.Figueredo CM, Lira-Junior R, Love RM: T and B Cells in Periodontal Disease: New Functions in A Complex Scenario. Int J Mol Sci 2019, 20(16). [DOI] [PMC free article] [PubMed]
- 104.Thorbert-Mros S, Larsson L, Kalm J, Berglundh T. Interleukin-17-producing T cells and interleukin-17 mRNA expression in periodontitis and long-standing gingivitis lesions. J Periodontol. 2019;90(5):516–521. doi: 10.1002/JPER.18-0326. [DOI] [PubMed] [Google Scholar]
- 105.Gonzales JR: T- and B-cell subsets in periodontitis. Periodontol 2000 2015, 69(1):181–200. [DOI] [PubMed]
- 106.Lim YRI, Preshaw PM, Lim LP, Ong MMA, Lin HS, Tan KS. Pterostilbene complexed with cyclodextrin exerts antimicrobial and anti-inflammatory effects. Sci Rep. 2020;10(1):9072. doi: 10.1038/s41598-020-66031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ben Lagha A, Andrian E, Grenier D. Resveratrol attenuates the pathogenic and inflammatory properties of Porphyromonas gingivalis. Mol Oral Microbiol. 2019;34(3):118–130. doi: 10.1111/omi.12260. [DOI] [PubMed] [Google Scholar]
- 108.Wang HW, Lai EH, Yang CN, Lin SK, Hong CY, Yang H, Chang JZ, Kok SH. Intracanal Metformin Promotes Healing of Apical Periodontitis via Suppressing Inducible Nitric Oxide Synthase Expression and Monocyte Recruitment. J Endod. 2020;46(1):65–73. doi: 10.1016/j.joen.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 109.Zhou X, Zhang P, Wang Q, Ji N, Xia S, Ding Y, Wang Q. Metformin ameliorates experimental diabetic periodontitis independently of mammalian target of rapamycin (mTOR) inhibition by reducing NIMA-related kinase 7 (Nek7) expression. J Periodontol. 2019;90(9):1032–1042. doi: 10.1002/JPER.18-0528. [DOI] [PubMed] [Google Scholar]
- 110.Lee HA, Song YR, Park MH, Chung HY, Na HS, Chung J. Catechin ameliorates Porphyromonas gingivalis-induced inflammation via the regulation of TLR2/4 and inflammasome signaling. J Periodontol. 2020;91(5):661–670. doi: 10.1002/JPER.18-0004. [DOI] [PubMed] [Google Scholar]
- 111.Araujo AA, Morais HB, Medeiros C, Brito GAC, Guedes PMM, Hiyari S, Pirih FQ, Araujo Junior RF. Gliclazide reduced oxidative stress, inflammation, and bone loss in an experimental periodontal disease model. J Appl Oral Sci. 2019;27:e20180211. doi: 10.1590/1678-7757-2018-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Solomon SM, Stafie CS, Sufaru IG, Teslaru S, Ghiciuc CM, Petrariu FD, Tanculescu O. Curcumin as a natural approach of periodontal adjunctive treatment and its immunological implications: a narrative review. Pharmaceutics. 2022;14(5):982. doi: 10.3390/pharmaceutics14050982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.de Almeida BD, Spolidorio LC, Johnson F, Golub LM, Guimaraes-Stabili MR, Rossa C., Jr Dose-response assessment of chemically modified curcumin in experimental periodontitis. J Periodontol. 2019;90(5):535–545. doi: 10.1002/JPER.18-0392. [DOI] [PubMed] [Google Scholar]
- 114.Forouzanfar F, Forouzanfar A, Sathyapalan T, Orafai HM, Sahebkar A. Curcumin for the Management of Periodontal Diseases: A Review. Curr Pharm Des. 2020;26(34):4277–4284. doi: 10.2174/1381612826666200513112607. [DOI] [PubMed] [Google Scholar]
- 115.Chung J, Kim S, Lee HA, Park MH, Kim S, Song YR, Na HS. Trans-cinnamic aldehyde inhibits Aggregatibacter actinomycetemcomitans-induced inflammation in THP-1-derived macrophages via autophagy activation. J Periodontol. 2018;89(10):1262–1271. doi: 10.1002/JPER.17-0727. [DOI] [PubMed] [Google Scholar]
- 116.Kawahara Y, Kaneko T, Yoshinaga Y, Arita Y, Nakamura K, Koga C, Yoshimura A, Sakagami R. Effects of Sulfonylureas on Periodontopathic Bacteria-Induced Inflammation. J Dent Res. 2020;99(7):830–838. doi: 10.1177/0022034520913250. [DOI] [PubMed] [Google Scholar]
- 117.Cheng T, Lai YT, Wang C, Wang Y, Jiang N, Li H, Sun H, Jin L. Bismuth drugs tackle Porphyromonas gingivalis and attune cytokine response in human cells. Metallomics. 2019;11(7):1207–1218. doi: 10.1039/c9mt00085b. [DOI] [PubMed] [Google Scholar]
- 118.Jiang H, Chen W, Zhu G, Zhang L, Tucker B, Hao L, Feng S, Ci H, Ma J, Wang L, et al. RNAi-mediated silencing of Atp6i and Atp6i haploinsufficiency prevents both bone loss and inflammation in a mouse model of periodontal disease. PLoS ONE. 2013;8(4):e58599. doi: 10.1371/journal.pone.0058599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Krongbaramee T, Zhu M, Qian Q, Zhang Z, Eliason S, Shu Y, Qian F, Akkouch A, Su D, Amendt BA, et al. Plasmid encoding microRNA-200c ameliorates periodontitis and systemic inflammation in obese mice. Mol Ther Nucleic Acids. 2021;23:1204–1216. doi: 10.1016/j.omtn.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen W, Gao B, Hao L, Zhu G, Jules J, MacDougall MJ, Wang J, Han X, Zhou X, Li YP. The silencing of cathepsin K used in gene therapy for periodontal disease reveals the role of cathepsin K in chronic infection and inflammation. J Periodontal Res. 2016;51(5):647–660. doi: 10.1111/jre.12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Xu XY, Tian BM, Xia Y, Xia YL, Li X, Zhou H, Tan YZ, Chen FM. Exosomes derived from P2X7 receptor gene-modified cells rescue inflammation-compromised periodontal ligament stem cells from dysfunction. Stem Cells Transl Med. 2020;9(11):1414–1430. doi: 10.1002/sctm.19-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Cirelli JA, Park CH, MacKool K, Taba M, Jr, Lustig KH, Burstein H, Giannobile WV. AAV2/1-TNFR: Fc gene delivery prevents periodontal disease progression. Gene Ther. 2009;16(3):426–436. doi: 10.1038/gt.2008.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Arora P, Li W, Huang X, Yu W, Huang R, Jiang Q, Chen C: Metabolic Reconfiguration Activates Stemness and Immunomodulation of PDLSCs. Int J Mol Sci 2022, 23(7). [DOI] [PMC free article] [PubMed]
- 124.Liu J, Chen B, Bao J, Zhang Y, Lei L, Yan F. Macrophage polarization in periodontal ligament stem cells enhanced periodontal regeneration. Stem Cell Res Ther. 2019;10(1):320. doi: 10.1186/s13287-019-1409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Liu O, Xu J, Ding G, Liu D, Fan Z, Zhang C, Chen W, Ding Y, Tang Z, Wang S. Periodontal ligament stem cells regulate B lymphocyte function via programmed cell death protein 1. Stem Cells. 2013;31(7):1371–1382. doi: 10.1002/stem.1387. [DOI] [PubMed] [Google Scholar]
- 126.Li W, Huang X, Yu W, Xu Y, Huang R, Park J, Moshaverinia A, Arora P, Chen C. Activation of Functional Somatic Stem Cells Promotes Endogenous Tissue Regeneration. J Dent Res. 2022;101(7):802–811. doi: 10.1177/00220345211070222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hong R, Wang Z, Sui A, Liu X, Fan C, Lipkind S, Xu Q. Gingival mesenchymal stem cells attenuate pro-inflammatory macrophages stimulated with oxidized low-density lipoprotein and modulate lipid metabolism. Arch Oral Biol. 2019;98:92–98. doi: 10.1016/j.archoralbio.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 128.Liu X, Wang Z, Song W, Sun W, Hong R, Pothukuchi A, Xu Q. Systematically transplanted human gingiva-derived mesenchymal stem cells regulate lipid metabolism and inflammation in hyperlipidemic mice with periodontitis. Exp Ther Med. 2020;19(1):672–682. doi: 10.3892/etm.2019.8256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang R, Ji Q, Meng C, Liu H, Fan C, Lipkind S, Wang Z, Xu Q. Role of gingival mesenchymal stem cell exosomes in macrophage polarization under inflammatory conditions. Int Immunopharmacol. 2020;81:106030. doi: 10.1016/j.intimp.2019.106030. [DOI] [PubMed] [Google Scholar]
- 130.Gao X, Shen Z, Guan M, Huang Q, Chen L, Qin W, Ge X, Chen H, Xiao Y, Lin Z. Immunomodulatory Role of Stem Cells from Human Exfoliated Deciduous Teeth on Periodontal Regeneration. Tissue Eng Part A. 2018;24(17–18):1341–1353. doi: 10.1089/ten.TEA.2018.0016. [DOI] [PubMed] [Google Scholar]
- 131.Zhou LL, Liu W, Wu YM, Sun WL, Dorfer CE, Fawzy El-Sayed KM. Oral Mesenchymal Stem/Progenitor Cells: The Immunomodulatory Masters. Stem Cells Int. 2020;2020:1327405. doi: 10.1155/2020/1327405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chatzivasileiou K, Lux CA, Steinhoff G, Lang H. Dental follicle progenitor cells responses to Porphyromonas gingivalis LPS. J Cell Mol Med. 2013;17(6):766–773. doi: 10.1111/jcmm.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yildirim S, Zibandeh N, Genc D, Ozcan EM, Goker K, Akkoc T. The Comparison of the Immunologic Properties of Stem Cells Isolated from Human Exfoliated Deciduous Teeth, Dental Pulp, and Dental Follicles. Stem Cells Int. 2016;2016:4682875. doi: 10.1155/2016/4682875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu L, Liu Y, Zhang X, Lin J. The therapeutic role of bone marrow stem cell local injection in rat experimental periodontitis. J Oral Rehabil. 2020;47(Suppl 1):73–82. doi: 10.1111/joor.12843. [DOI] [PubMed] [Google Scholar]
- 135.Shen Z, Kuang S, Zhang Y, Yang M, Qin W, Shi X, Lin Z. Chitosan hydrogel incorporated with dental pulp stem cell-derived exosomes alleviates periodontitis in mice via a macrophage-dependent mechanism. Bioact Mater. 2020;5(4):1113–1126. doi: 10.1016/j.bioactmat.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tatullo M, Gandolfi MG: Stem Cells from Dental Sources: Translational Applications in Medicine and Novel Approaches. Int J Mol Sci 2022, 23(8). [DOI] [PMC free article] [PubMed]
- 137.Bezerra B, Monajemzadeh, S., Silva, D., Pirih, F.Q: Modulating the Immune Response in Periodontitis. Frontiers in Dental Medicine (13 May 2022):33.
- 138.Golub LM, Lee HM: Periodontal therapeutics: Current host-modulation agents and future directions. Periodontol 2000 2020, 82(1):186–204. [DOI] [PMC free article] [PubMed]
- 139.Mohammad AR, Preshaw PM, Bradshaw MH, Hefti AF, Powala CV, Romanowicz M. Adjunctive subantimicrobial dose doxycycline in the management of institutionalised geriatric patients with chronic periodontitis. Gerodontology. 2005;22(1):37–43. doi: 10.1111/j.1741-2358.2004.00044.x. [DOI] [PubMed] [Google Scholar]
- 140.Corbella S, Calciolari E, Alberti A, Donos N, Francetti L. Systematic review and meta-analysis on the adjunctive use of host immune modulators in non-surgical periodontal treatment in healthy and systemically compromised patients. Sci Rep. 2021;11(1):12125. doi: 10.1038/s41598-021-91506-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Preshaw PM, Novak MJ, Mellonig J, Magnusson I, Polson A, Giannobile WV, Rowland RW, Thomas J, Walker C, Dawson DR, et al. Modified-release subantimicrobial dose doxycycline enhances scaling and root planing in subjects with periodontal disease. J Periodontol. 2008;79(3):440–452. doi: 10.1902/jop.2008.070375. [DOI] [PubMed] [Google Scholar]
- 142.Parvu AE, Alb SF, Craciun A, Taulescu MA. Efficacy of subantimicrobial-dose doxycycline against nitrosative stress in chronic periodontitis. Acta Pharmacol Sin. 2013;34(2):247–254. doi: 10.1038/aps.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Myneni SR, Brocavich K, H HW: Biological strategies for the prevention of periodontal disease: Probiotics and vaccines. Periodontol 2000 2020, 84(1):161–175. [DOI] [PubMed]
- 144.Meurman JH. Probiotics: do they have a role in oral medicine and dentistry? Eur J Oral Sci. 2005;113(3):188–196. doi: 10.1111/j.1600-0722.2005.00191.x. [DOI] [PubMed] [Google Scholar]
- 145.Zidar A, Kristl J, Kocbek P, Zupancic S. Treatment challenges and delivery systems in immunomodulation and probiotic therapies for periodontitis. Expert Opin Drug Deliv. 2021;18(9):1229–1244. doi: 10.1080/17425247.2021.1908260. [DOI] [PubMed] [Google Scholar]
- 146.Moraes RM, Schlagenhauf U, Anbinder AL. Outside the limits of bacterial viability: Postbiotics in the management of periodontitis. Biochem Pharmacol. 2022;201:115072. doi: 10.1016/j.bcp.2022.115072. [DOI] [PubMed] [Google Scholar]
- 147.van Essche M, Loozen G, Godts C, Boon N, Pauwels M, Quirynen M, Teughels W. Bacterial antagonism against periodontopathogens. J Periodontol. 2013;84(6):801–811. doi: 10.1902/jop.2012.120261. [DOI] [PubMed] [Google Scholar]
- 148.Furlaneto F, Ishikawa KH, Messora MR, Mayer MPA. Probiotics During the Therapeutic Management of Periodontitis. Adv Exp Med Biol. 2022;1373:353–375. doi: 10.1007/978-3-030-96881-6_19. [DOI] [PubMed] [Google Scholar]
- 149.Koll-Klais P, Mandar R, Leibur E, Marcotte H, Hammarstrom L, Mikelsaar M. Oral lactobacilli in chronic periodontitis and periodontal health: species composition and antimicrobial activity. Oral Microbiol Immunol. 2005;20(6):354–361. doi: 10.1111/j.1399-302X.2005.00239.x. [DOI] [PubMed] [Google Scholar]
- 150.Koll P, Mandar R, Marcotte H, Leibur E, Mikelsaar M, Hammarstrom L. Characterization of oral lactobacilli as potential probiotics for oral health. Oral Microbiol Immunol. 2008;23(2):139–147. doi: 10.1111/j.1399-302X.2007.00402.x. [DOI] [PubMed] [Google Scholar]
- 151.Krasse P, Carlsson B, Dahl C, Paulsson A, Nilsson A, Sinkiewicz G. Decreased gum bleeding and reduced gingivitis by the probiotic Lactobacillus reuteri. Swed Dent J. 2006;30(2):55–60. [PubMed] [Google Scholar]
- 152.Silva DR, Sardi JDCO, de Souza Pitangui N, Roque SM, da Silva ACB, Rosalen PL. Probiotics as an alternative antimicrobial therapy: Current reality and future directions. Journal of Functional Foods. 2020;73:104080. [Google Scholar]
- 153.Brandt LJ. Fecal transplantation for the treatment of Clostridium difficile infection. Gastroenterol Hepatol (N Y) 2012;8(3):191–194. [PMC free article] [PubMed] [Google Scholar]
- 154.Sultan S, El-Mowafy M, Elgaml A, Ahmed TAE, Hassan H, Mottawea W. Metabolic Influences of Gut Microbiota Dysbiosis on Inflammatory Bowel Disease. Front Physiol. 2021;12:715506. doi: 10.3389/fphys.2021.715506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Nascimento MM. Oral microbiota transplant: a potential new therapy for oral diseases. J Calif Dent Assoc. 2017;45(10):565–568. [PMC free article] [PubMed] [Google Scholar]
- 156.Pozhitkov AE, Leroux BG, Randolph TW, Beikler T, Flemmig TF, Noble PA. Towards microbiome transplant as a therapy for periodontitis: an exploratory study of periodontitis microbial signature contrasted by oral health, caries and edentulism. BMC Oral Health. 2015;15:125. doi: 10.1186/s12903-015-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nath S, Zilm P, Jamieson L, Kapellas K, Goswami N, Ketagoda K, Weyrich LS. Development and characterization of an oral microbiome transplant among Australians for the treatment of dental caries and periodontal disease: A study protocol. PLoS ONE. 2021;16(11):e0260433. doi: 10.1371/journal.pone.0260433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chaturvedi TP, Srivastava R, Srivastava AK, Gupta V, Verma PK. Evaluation of metronidazole nanofibers in patients with chronic periodontitis: A clinical study. Int J Pharm Investig. 2012;2(4):213–217. doi: 10.4103/2230-973X.107007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Chaturvedi TP, Srivastava R, Srivastava AK, Gupta V, Verma PK. Doxycycline poly e-caprolactone nanofibers in patients with chronic periodontitis - a clinical evaluation. J Clin Diagn Res. 2013;7(10):2339–2342. doi: 10.7860/JCDR/2013/5858.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Liu D, Yang P, Hu D, Liu F. Minocycline hydrochloride liposome controlled-release gel improves rat experimental periodontitis. Hua Xi Kou Qiang Yi Xue Za Zhi. 2013;31(6):592–596. [PubMed] [Google Scholar]
- 161.Liu D, Yang PS. Minocycline hydrochloride nanoliposomes inhibit the production of TNF-alpha in LPS-stimulated macrophages. Int J Nanomedicine. 2012;7:4769–4775. doi: 10.2147/IJN.S34036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jin HL, Wu W, Shu R. Doxycycline nano-liposome slow-release gel improves rat periodontitis. Shanghai Kou Qiang Yi Xue. 2010;19(5):508–511. [PubMed] [Google Scholar]
- 163.Wayakanon K, Thornhill MH, Douglas CW, Lewis AL, Warren NJ, Pinnock A, Armes SP, Battaglia G, Murdoch C. Polymersome-mediated intracellular delivery of antibiotics to treat Porphyromonas gingivalis-infected oral epithelial cells. FASEB J. 2013;27(11):4455–4465. doi: 10.1096/fj.12-225219. [DOI] [PubMed] [Google Scholar]
- 164.Saboktakin MR, Tabatabaie RM, Maharramov A, Ramazanov MA. Development and in vitro evaluation of thiolated chitosan–Poly(methacrylic acid) nanoparticles as a local mucoadhesive delivery system. Int J Biol Macromol. 2011;48(3):403–407. doi: 10.1016/j.ijbiomac.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 165.Xu S, Zhou Q, Jiang Z, Wang Y, Yang K, Qiu X, Ji Q. The effect of doxycycline-containing chitosan/carboxymethyl chitosan nanoparticles on NLRP3 inflammasome in periodontal disease. Carbohydr Polym. 2020;237:116163. doi: 10.1016/j.carbpol.2020.116163. [DOI] [PubMed] [Google Scholar]
- 166.Pichayakorn W, Boonme P. Evaluation of cross-linked chitosan microparticles containing metronidazole for periodontitis treatment. Mater Sci Eng C Mater Biol Appl. 2013;33(3):1197–1202. doi: 10.1016/j.msec.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 167.Jamal T, Rahman MA, Mirza MA, Panda AK, Talegaonkar S, Iqbal Z. Formulation, antimicrobial and toxicity evaluation of bioceramic based ofloxacin loaded biodegradable microspheres for periodontal infection. Curr Drug Deliv. 2012;9(5):515–526. doi: 10.2174/156720112802650644. [DOI] [PubMed] [Google Scholar]
- 168.Zingale J, Harpenau L, Bruce G, Chambers D, Lundergan W. The effectiveness of scaling and root planing with adjunctive time-release minocycline using an open and closed approach for the treatment of periodontitis. Gen Dent. 2012;60(4):300–305. [PubMed] [Google Scholar]
- 169.Rao SK, Setty S, Acharya AB, Thakur SL. Efficacy of locally-delivered doxycycline microspheres in chronic localized periodontitis and on Porphyromonas gingivalis. J Investig Clin Dent. 2012;3(2):128–134. doi: 10.1111/j.2041-1626.2011.00110.x. [DOI] [PubMed] [Google Scholar]
- 170.Pradeep AR, Bajaj P, Agarwal E, Rao NS, Naik SB, Kalra N, Priyanka N: Local drug delivery of 0.5% azithromycin in the treatment of chronic periodontitis among smokers. Aust Dent J 2013, 58(1):34–40. [DOI] [PubMed]
- 171.Bako J, Vecsernyes M, Ujhelyi Z, Kovacsne IB, Borbiro I, Biro T, Borbely J, Hegedus C. Composition and characterization of in situ usable light cured dental drug delivery hydrogel system. J Mater Sci Mater Med. 2013;24(3):659–666. doi: 10.1007/s10856-012-4825-x. [DOI] [PubMed] [Google Scholar]
- 172.Agarwal E, Pradeep AR, Bajaj P, Naik SB: Efficacy of local drug delivery of 0.5% clarithromycin gel as an adjunct to non-surgical periodontal therapy in the treatment of current smokers with chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2012, 83(9):1155–1163. [DOI] [PubMed]
- 173.Flemmig TF, Petersilka G, Volp A, Gravemeier M, Zilly M, Mross D, Prior K, Yamamoto J, Beikler T. Efficacy and safety of adjunctive local moxifloxacin delivery in the treatment of periodontitis. J Periodontol. 2011;82(1):96–105. doi: 10.1902/jop.2010.100124. [DOI] [PubMed] [Google Scholar]
- 174.Chi M, Qi M, A L, Wang P, Weir MD, Melo MA, Sun X, Dong B, Li C, Wu J et al: Novel Bioactive and Therapeutic Dental Polymeric Materials to Inhibit Periodontal Pathogens and Biofilms. Int J Mol Sci 2019, 20(2)278. [DOI] [PMC free article] [PubMed]
- 175.Ganguly A, Ian CK, Sheshala R, Sahu PS, Al-Waeli H, Meka VS. Application of diverse natural polymers in the design of oral gels for the treatment of periodontal diseases. J Mater Sci Mater Med. 2017;28(3):39. doi: 10.1007/s10856-017-5852-4. [DOI] [PubMed] [Google Scholar]
- 176.Iqbal H, Ali M, Zeeshan R, Mutahir Z, Iqbal F, Nawaz MAH, Shahzadi L, Chaudhry AA, Yar M, Luan S, et al. Chitosan/hydroxyapatite (HA)/hydroxypropylmethyl cellulose (HPMC) spongy scaffolds-synthesis and evaluation as potential alveolar bone substitutes. Colloids Surf B Biointerfaces. 2017;160:553–563. doi: 10.1016/j.colsurfb.2017.09.059. [DOI] [PubMed] [Google Scholar]
- 177.Alassy H, Pizarek JA, Kormas I, Pedercini A, Wolff LF. Antimicrobial adjuncts in the management of periodontal and peri-implant diseases and conditions: a narrative review. Front Oral Maxillofac. 2021;2021:3. [Google Scholar]
- 178.Goodson JM, Haffajee AD, Socransky SS, Kent R, Teles R, Hasturk H, Bogren A, Van Dyke T, Wennstrom J, Lindhe J: Control of periodontal infections: a randomized controlled trial I. The primary outcome attachment gain and pocket depth reduction at treated sites. J Clin Periodontol 2012, 39(6):526–536. [DOI] [PubMed]
- 179.Souza EQM, da Rocha TE, Toro LF, Guiati IZ, Ervolino E, Garcia VG, Wainwright M, Theodoro LH. Antimicrobial photodynamic therapy compared to systemic antibiotic therapy in non-surgical treatment of periodontitis: Systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2020;31:101808. doi: 10.1016/j.pdpdt.2020.101808. [DOI] [PubMed] [Google Scholar]
- 180.Al-Khureif AA, Mohamed BA, Siddiqui AZ, Khan AA, Divakar DD. Repeated application of photodynamic and antibiotic therapy as an adjunct to root surface debridement in patients with grade C and stage III or IV aggressive periodontitis. Photodiagnosis Photodyn Ther. 2020;29:101610. doi: 10.1016/j.pdpdt.2019.101610. [DOI] [PubMed] [Google Scholar]
- 181.Theodoro LH, Assem NZ, Longo M, Alves MLF, Duque C, Stipp RN, Vizoto NL, Garcia VG. Treatment of periodontitis in smokers with multiple sessions of antimicrobial photodynamic therapy or systemic antibiotics: A randomized clinical trial. Photodiagnosis Photodyn Ther. 2018;22:217–222. doi: 10.1016/j.pdpdt.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 182.Theodoro LH, Lopes AB, Nuernberg MAA, Claudio MM, Miessi DMJ, Alves MLF, Duque C, Mombelli A, Garcia VG. Comparison of repeated applications of aPDT with amoxicillin and metronidazole in the treatment of chronic periodontitis: A short-term study. J Photochem Photobiol B. 2017;174:364–369. doi: 10.1016/j.jphotobiol.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 183.Arweiler NB, Pietruska M, Pietruski J, Skurska A, Dolinska E, Heumann C, Auschill TM, Sculean A. Six-month results following treatment of aggressive periodontitis with antimicrobial photodynamic therapy or amoxicillin and metronidazole. Clin Oral Investig. 2014;18(9):2129–2135. doi: 10.1007/s00784-014-1193-6. [DOI] [PubMed] [Google Scholar]
- 184.Arweiler NB, Pietruska M, Skurska A, Dolinska E, Pietruski JK, Blas M, Auschill TM, Sculean A: Nonsurgical treatment of aggressive periodontitis with photodynamic therapy or systemic antibiotics. Three-month results of a randomized, prospective, controlled clinical study. Schweiz Monatsschr Zahnmed 2013, 123(6):532–544. [PubMed]
- 185.Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J Periodontol. 2009;80(3):364–371. doi: 10.1902/jop.2009.080540. [DOI] [PubMed] [Google Scholar]
- 186.Cionca N, Giannopoulou C, Ugolotti G, Mombelli A. Microbiologic testing and outcomes of full-mouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis. J Periodontol. 2010;81(1):15–23. doi: 10.1902/jop.2009.090390. [DOI] [PubMed] [Google Scholar]
- 187.Yek EC, Cintan S, Topcuoglu N, Kulekci G, Issever H, Kantarci A. Efficacy of amoxicillin and metronidazole combination for the management of generalized aggressive periodontitis. J Periodontol. 2010;81(7):964–974. doi: 10.1902/jop.2010.090522. [DOI] [PubMed] [Google Scholar]
- 188.Baltacioglu E, Aslan M, Sarac O, Saybak A, Yuva P. Analysis of clinical results of systemic antimicrobials combined with nonsurgical periodontal treatment for generalized aggressive periodontitis: a pilot study. J Can Dent Assoc. 2011;77:b97. [PubMed] [Google Scholar]
- 189.Rodrigues DC, Taba MJ, Novaes AB, Souza SL, Grisi MF. Effect of non-surgical periodontal therapy on glycemic control in patients with type 2 diabetes mellitus. J Periodontol. 2003;74(9):1361–1367. doi: 10.1902/jop.2003.74.9.1361. [DOI] [PubMed] [Google Scholar]
- 190.Sigusch B, Beier M, Klinger G, Pfister W, Glockmann E. A 2-step non-surgical procedure and systemic antibiotics in the treatment of rapidly progressive periodontitis. J Periodontol. 2001;72(3):275–283. doi: 10.1902/jop.2001.72.3.275. [DOI] [PubMed] [Google Scholar]
- 191.Dastoor SF, Travan S, Neiva RF, Rayburn LA, Giannobile WV, Wang HL. Effect of adjunctive systemic azithromycin with periodontal surgery in the treatment of chronic periodontitis in smokers: a pilot study. J Periodontol. 2007;78(10):1887–1896. doi: 10.1902/jop.2007.070072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Emingil G, Han B, Ozdemir G, Tervahartiala T, Vural C, Atilla G, Baylas H, Sorsa T. Effect of azithromycin, as an adjunct to nonsurgical periodontal treatment, on microbiological parameters and gingival crevicular fluid biomarkers in generalized aggressive periodontitis. J Periodontal Res. 2012;47(6):729–739. doi: 10.1111/j.1600-0765.2012.01488.x. [DOI] [PubMed] [Google Scholar]
- 193.Yashima A, Gomi K, Maeda N, Arai T. One-stage full-mouth versus partial-mouth scaling and root planing during the effective half-life of systemically administered azithromycin. J Periodontol. 2009;80(9):1406–1413. doi: 10.1902/jop.2009.090067. [DOI] [PubMed] [Google Scholar]
- 194.Morales A, Contador R, Bravo J, Carvajal P, Silva N, Strauss FJ, Gamonal J. Clinical effects of probiotic or azithromycin as an adjunct to scaling and root planning in the treatment of stage III periodontitis: a pilot randomized controlled clinical trial. BMC Oral Health. 2021;21(1):12. doi: 10.1186/s12903-020-01276-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Abdulkareem A, Abdulbaqi H, Gul S, Milward M, Chasib N, Alhashimi R. Classic vs. novel antibacterial approaches for eradicating dental biofilm as adjunct to periodontal debridement: an evidence-based overview. Antibiotics (Basel) 2021;11(1):9. doi: 10.3390/antibiotics11010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Qureshi A, Bokhari SAH, Haque Z, Baloch AA, Zaheer S. Clinical efficacy of scaling and root planing with and without metronidazole on glycemic control: three-arm randomized controlled trial. BMC Oral Health. 2021;21(1):253. doi: 10.1186/s12903-021-01620-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Park KM, Choo JH, Sohn JH, Lee SH, Hwang JK. Antibacterial activity of panduratin A isolated from Kaempferia pandurata against Porphyromonas gingivalis. Food Science and Biotechnology. 2005;14(2):286–289. [Google Scholar]
- 198.Bhadbhade SJ, Acharya AB, Rodrigues SV, Thakur SL. The antiplaque efficacy of pomegranate mouthrinse. Quintessence Int. 2011;42(1):29–36. [PubMed] [Google Scholar]
- 199.Deng J, Golub LM, Lee HM, Lin MC, Bhatt HD, Hong HL, Johnson F, Scaduto J, Zimmerman T, Gu Y: Chemically-Modified Curcumin 2.24: A Novel Systemic Therapy for Natural Periodontitis in Dogs. J Exp Pharmacol 2020, 12:47–60. [DOI] [PMC free article] [PubMed]
- 200.Aoki-Nonaka Y, Tabeta K, Yokoji M, Matsugishi A, Matsuda Y, Takahashi N, Sulijaya B, Domon H, Terao Y, Taniguchi M, et al. A peptide derived from rice inhibits alveolar bone resorption via suppression of inflammatory cytokine production. J Periodontol. 2019;90(10):1160–1169. doi: 10.1002/JPER.18-0630. [DOI] [PubMed] [Google Scholar]
- 201.Huck O, Han X, Mulhall H, Gumenchuk I, Cai B, Panek J, Iyer R, Amar S. Identification of a Kavain Analog with Efficient Anti-inflammatory Effects. Sci Rep. 2019;9(1):12940. doi: 10.1038/s41598-019-49383-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Hosokawa I, Hosokawa Y, Ozaki K, Matsuo T. Carnosic Acid Inhibits CXCR3 Ligands Production in IL-27-Stimulated Human Oral Epithelial Cells. Inflammation. 2019;42(4):1311–1316. doi: 10.1007/s10753-019-00991-6. [DOI] [PubMed] [Google Scholar]
- 203.Shin C, Kim M, Han JA, Choi B, Hwang D, Do Y, Yun JH. Human periodontal ligament stem cells suppress T-cell proliferation via down-regulation of non-classical major histocompatibility complex-like glycoprotein CD1b on dendritic cells. J Periodontal Res. 2017;52(1):135–146. doi: 10.1111/jre.12378. [DOI] [PubMed] [Google Scholar]
- 204.Silva Fde S, Ramos RN, de Almeida DC, Bassi EJ, Gonzales RP, Miyagi SP, Maranduba CP, Sant'Anna OA, Marques MM, Barbuto JA, et al. Mesenchymal stem cells derived from human exfoliated deciduous teeth (SHEDs) induce immune modulatory profile in monocyte-derived dendritic cells. PLoS ONE. 2014;9(5):e98050. doi: 10.1371/journal.pone.0098050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Zhang Y, Xiong Y, Chen X, Chen C, Zhu Z, Li L. Therapeutic effect of bone marrow mesenchymal stem cells pretreated with acetylsalicylic acid on experimental periodontitis in rats. Int Immunopharmacol. 2018;54:320–328. doi: 10.1016/j.intimp.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 206.Iguchi S, Suzuki D, Kawano E, Mashimo T, Kajiya M, Toriumi T, Kawai T, Kurihara H, Isokawa K, Sato S, et al. Effect of local bone marrow stromal cell administration on ligature-induced periodontitis in mice. J Oral Sci. 2017;59(4):629–637. doi: 10.2334/josnusd.16-0033. [DOI] [PubMed] [Google Scholar]
- 207.Ye QY, Li ZH, Wang YZ, Liu SY, Zhou J, Liu SY, Wang QT. Mesenchymal stem cells derived apoptotic extracellular vesicles attenuate pro-inflammatory macrophages induced by Porphyromonas gingivalis lipopolysaccharide. Zhonghua Kou Qiang Yi Xue Za Zhi. 2021;56(8):791–798. doi: 10.3760/cma.j.cn112144-20201027-00541. [DOI] [PubMed] [Google Scholar]
- 208.Du J, Shan Z, Ma P, Wang S, Fan Z. Allogeneic bone marrow mesenchymal stem cell transplantation for periodontal regeneration. J Dent Res. 2014;93(2):183–188. doi: 10.1177/0022034513513026. [DOI] [PubMed] [Google Scholar]
- 209.Lee S, Zhang QZ, Karabucak B, Le AD. DPSCs from Inflamed Pulp Modulate Macrophage Function via the TNF-alpha/IDO Axis. J Dent Res. 2016;95(11):1274–1281. doi: 10.1177/0022034516657817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ding G, Niu J, Liu Y. Dental pulp stem cells suppress the proliferation of lymphocytes via transforming growth factor-beta1. Hum Cell. 2015;28(2):81–90. doi: 10.1007/s13577-014-0106-y. [DOI] [PubMed] [Google Scholar]
- 211.Murakami-Malaquias-da-Silva F, Rosa EP, Oliveira JG, Avelar IS, Palma-Cruz M, Fernandes Silva JG, Rigonato-Oliveira NC, Bussadori SK, Negreiros RM, Ligeiro-de-Oliveira AP, et al. The role of periodontal treatment associated with photodynamic therapy on the modulation of systemic inflammation in the experimental model of asthma and periodontitis. Photodiagnosis Photodyn Ther. 2020;29:101619. doi: 10.1016/j.pdpdt.2019.101619. [DOI] [PubMed] [Google Scholar]
- 212.Peeridogaheh H, Pourhajibagher M, Barikani HR, Bahador A. The impact of Aggregatibacter actinomycetemcomitans biofilm-derived effectors following antimicrobial photodynamic therapy on cytokine production in human gingival fibroblasts. Photodiagnosis Photodyn Ther. 2019;27:1–6. doi: 10.1016/j.pdpdt.2019.05.025. [DOI] [PubMed] [Google Scholar]
- 213.Jiang C, Yang W, Wang C, Qin W, Ming J, Zhang M, Qian H, Jiao T. Methylene Blue-Mediated Photodynamic Therapy Induces Macrophage Apoptosis via ROS and Reduces Bone Resorption in Periodontitis. Oxid Med Cell Longev. 2019;2019:1529520. doi: 10.1155/2019/1529520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kusuyama J, Nakamura T, Ohnishi T, Albertson BG, Ebe Y, Eiraku N, Noguchi K, Matsuguchi T. Low-intensity pulsed ultrasound promotes bone morphogenic protein 9-induced osteogenesis and suppresses inhibitory effects of inflammatory cytokines on cellular responses via Rho-associated kinase 1 in human periodontal ligament fibroblasts. J Cell Biochem. 2019;120(9):14657–14669. doi: 10.1002/jcb.28727. [DOI] [PubMed] [Google Scholar]
- 215.Bazikyan EA, Syrnikova NV, Chunikhin AA, Zayratyants OV. Morphological evaluation of singlet phototherapy in the treatment of periodontal diseases in an experimental study. Stomatologiia (Mosk) 2018;97(1):22–26. doi: 10.17116/stomat201897122-26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing does not apply to this article as no datasets were generated or analyzed during the current study.