Abstract
Background
The relationship between remnant cholesterol (RC) and atherosclerotic cardiovascular risk has been given increasing attention in recent years. However, its association with verbal learning and memory performance has not been reported.
Methods
Data were extracted from the National Health and Nutrition Examination Survey (NHANES) 2011–2014 database. Participants aged ≥60 years with available fasting lipid data were included. Verbal learning and memory performance were evaluated using the Consortium to Establish a Registry for Alzheimer’s Disease Word List Memory Task (CERAD-WL) subtest. The CERAD total score was calculated as the mean of three immediate recalls and a delayed recall. RC was calculated as total cholesterol (TC) minus the sum of low-density lipoprotein cholesterol (LDL-C) and high-density lipoprotein cholesterol (HDL-C). Multivariate ordinal logistic regression was performed to evaluate the association between RC, as well as its derived marker, the TC/RC ratio, and age-stratified quartiles of the CERAD total score.
Results
A total of 1377 participants were analysed. On a continuous scale, per 1 mmol/L increase in RC and per 1 unit increase in the TC/RC ratio were associated with multivariable adjusted odds ratios (95% CI) of 0.74 (0.58–0.94) and 1.45 (1.13–1.87), respectively, for having a CERAD total score in a higher quartile. On a categorical scale, higher RC quartiles were associated with a CERAD total score in a lower quartile; in contrast, the higher TC/RC quartile was associated with a CERAD total score in a higher quartile (all P for trend < 0.05).
Conclusions
The current study suggests that lower RC levels and a higher TC/RC ratio are associated with better verbal learning and memory function, which indicates that lowering RC levels could be beneficial for preventing cognitive impairment in elderly individuals. Further research is needed to validate the causal roles of RC and the TC/RC ratio in cognition.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-022-01729-4.
Keywords: Remnant cholesterol, Total cholesterol/remnant cholesterol, Cognition, Verbal learning and memory function, Elderly, Cross-sectional study
Introduction
Dementia is a vital health problem in the global growing aging population. The estimated dementia population will increase from 57.4 (50.4–65.1) million cases globally in 2019 to 152.8 (130.8–175.9) million cases in 2050 [1]. Given the lack of effective pharmacological therapies for dementia thus far, the primary prevention of modifiable risk factors has become crucial in addressing the rising epidemic of cognitive impairment.
Dyslipidemia has been found to be independently associated with cognitive impairment. In observational studies, higher serum TC levels in mid-life were associated with a higher risk of developing dementia in late life [2], but the impact of serum TC levels in late life on the development of dementia remains unclear. A meta-analysis synthesizing 34 cohort studies found no association between serum TC levels and mild cognitive impairment, Alzheimer’s disease (AD), vascular dementia, any dementia, or cognitive decline [3]. Studies on serum LDL-C levels and dementia or cognitive decline also displayed differential results and seemed to vary by sex and the presence of cardiovascular risk factors [4]. However, a Mendelian randomization study showed that genetically low serum levels of LDL-C reduced the risk of AD [5]. Overall, inconsistent results have been found in the association between serum lipid profiles and cognitive impairment, limiting the clinical utility of lipid biomarkers. In addition to further research on traditional blood lipid parameters, the exploration of new lipid parameters to predict cognitive function is urgently needed, especially in the elderly population.
Remnant cholesterol (RC) represents the amount of cholesterol in the remnant lipoproteins transformed from triglyceride-rich lipoproteins in the blood [6]. Recently, RC has been shown to equally or even more precisely predict cardio-cerebrovascular outcomes compared to LDL-C or HDL-C [7–9]. Elevated RC levels are closely related to triglyceride metabolism disorders and insufficient APOE-mediated remnant lipoprotein clearance by the liver, such as type III hyperlipidemia [10]. Meanwhile, APOE variants are closely related to the risk of AD. Therefore, RC has the biological possibility of affecting cognitive function. However, no study has reported whether or how RC is associated with cognitive function.
In seeking a more plausible and reliable lipid marker of late-life cognitive impairment, the present study explored the relationship of RC with cognitive function utilizing data from a cohort of American elderly individuals in the National Health and Nutrition Examination Survey (NHANES) conducted between 2011 and 2014. Given the complexity of the metabolism of remnant lipoprotein in human plasma, the study also explored a new lipid marker derived from RC, the total cholesterol to remnant cholesterol (TC/RC) ratio, to further investigate the relationship of RC with cognitive function.
Methods
Study design and population
The NHANES database is a cross-sectional program with a 2-year-cycle design that aims to assess the health and nutritional status of civilian, noninstitutionalized resident populations in the United States. A complex, multistage sampling procedure was implemented to select eligible representative participants. The present study made use of the 2011–2012 and 2013–2014 cycles with 19,931 individuals, of which participants aged ≥60 years were eligible for cognitive function assessment and enrolled for analysis. Participants without fasting plasma lipid data or complete cognitive assessment results were excluded. Those without complete measurements of TC, LDL-C, and HDL-C values for the calculation of RC were also excluded.
The NHANES protocols were approved by the National Center for Health Statistics Ethics Review Board of the U.S. CDC. All participants received and completed written informed consent during the survey. All material for analysis can be accessed on the NHANES official website [11].
Measurement of blood lipids and the definition of exposure variables
TC and triglycerides (TGs) were measured using an enzymatic assay method. HDL-C was measured using the heparin-manganese precipitation method or a direct immunoassay technique. LDL-C was calculated from measured values of TC, TGs, and HDL-C according to the Friedewald formula as follows: [LDL-C] = [TC] – [HDL-C] – [TG/5]. The formula is valid for TG values less than or equal to 400 mg/dL. The exposure variables were remnant cholesterol (RC) and its derivative, the TC/RC ratio. RC was calculated as TC minus the sum of LDL-C and HDL-C [RC = TC-(LDL-C + HDL-C)], and the TC/RC ratio was calculated as TC divided by RC.
Assessment of verbal learning and memory function and the definition of the outcome variable
In NHANES 2011–2014, a series of evaluations of cognitive functioning were conducted during the MEC survey, including the word learning and recall modules from the Consortium to Establish a Registry for Alzheimer’s Disease (CERAD W-L). The CERAD W-L assesses the immediate and delayed learning ability for new verbal information (memory subdomain), consisting of three sequential learning trials and a delayed recall trial. For learning trials, participants were tasked with reading 10 unrelated words displayed on a screen aloud, one at a time, and were asked to recall as many words as possible immediately after their presentation. A delayed recall test was administered after the other two cognitive tests were completed. The outcome variable was the CERAD total score, which was calculated as the average of all three immediate recalls after each learning trial and the delayed recall. Given the significant effect of age on cognitive function, the age- stratified (≥60 to 70, ≥70 to 80, and ≥80 years) quartiles of the CERAD total test score were used [12, 13] to evaluate verbal learning and memory function. Specifically, the age-stratified quartiles of the CERAD total test score were calculated by first dividing the studypopulation into 3 age groups (≥60 to 70, ≥70 to 80, and ≥80 years) and then categorizing participants into 4 grades of verbal learning and memory function (1 to 4) by the weighted quartiles of CERAD total score separately in the 3 age groups. The lowest quartile indicated impaired verbal learning and memory function. The higher the quartile, the better the learning and memory function.
Covariates
Potential confounding factors were investigated, including age, sex (male and female), race, BMI status, educational level, smoking status, and drinking status. Race was defined as follows: 1) non-Hispanic white; 2) non-Hispanic black; and 3) other races, including Mexican American, other Hispanic, non-Hispanic Asian and multiracial. Educational level was defined as follows: 1) less than high school: less than 9th grade or no high school diploma; 2) high school graduate: high school graduate/GED or equivalent; and 3) college or above: some college or AA degree, college graduate or above. BMI was categorized into 1) normal, 2) overweight and 3) obese using 25 kg/m2 and 30 kg/m2 as the cutoffs. Smoking status was defined as follows: 1) current smokers: had smoked more than 100 cigarettes (including hand rolled cigarettes, cigars, and cigarillos) in their lifetime and had smoked in the last 28 days; 2) ex-smokers: had smoked more than 100 cigarettes in their lifetime but had not smoked in the last 28 days; and 3) never smokers: had not smoked more than 100 cigarettes in their lifetime and did not currently smoke [14]. Drinking status was defined as follows: 1) current drinker: had drunk at least 12 drinks in their lifetime and had drunk at least 1 drink in the past year; 2) former drinker: had drunk at least 12 drinks in their lifetime and had drunk no drinks in past year; and 3) lifetime abstainer: had drunk fewer than 12 drinks in their lifetime [15].
Other variables included in the baseline characteristics and subgroup analysis were marital status, statin use, diabetes, and hypertension. Marital status was defined as 1) married/cohabitating and 2) not married/never married. Diabetes and hypertension were based on self-report questionnaire data.
Statistical analyses
All statistical analyses were conducted by Stata SE 16.0 (Stata Corporation, College Station, TX). The complex survey design of the NHANES was taken into account by specifying primary sampling units (PSUs), strata, and sampling weights in the software’s svyset module. Sampling weights were constructed for the combined 2011–2012 and 2013–2014 4-year cycles by dividing the individual sampling weights by 2, according to the NHANES analytic tutorials [16].
Baseline characteristics were grouped by age-stratified quartiles of the CERAD total test score. Data are described as the mean (± standard deviation) for continuous variables, median (interquartile range) for skewed variables, and sample counts (weighted percentage) for categorical variables. A weighted trend test was performed across age-stratified quartile groups for each continuous and categorical variable.
In the statistical analysis of lipid-cognition associations, RC and the TC/RC ratio were treated as both continuous and categorical variables. Continuous RC and TC/RC variables were natural log-transformed to fit regression models due to their right-skewed distribution [7]. Categorical RC and TC/RC variables with four grades were created using quartiles as cutoffs. Multivariate ordinal logistic regression analysis was performed to evaluate the relationship among RC, the TC/RC ratio and grades of verbal learning and memory functioning. Models were further adjusted for potential risk factors or confounders based on prior studies. Model 1 included univariate analysis. Model 2 was adjusted for age, sex, race, and education level. Model 3 was further adjusted for BMI status, smoking status and drinking status plus the adjustments in Model 2. Subgroup analysis was performed to evaluate the robustness of the results in a diversity of demographic, disease-specific and lipid-stratified subgroups. A two-sided P < 0.05 was considered statistically significant.
Results
Characteristics of the study population
Finally, according to the study population selection process, 1377 participants aged ≥60 years were included in the study (Fig. 1). The mean age of the participants was 69.1 ± 6.5 years, and 55% were male. A total of 79% of the participants were non-Hispanic white, 61% had an educational level of college or above, and 32% were not married or never married. Thirty-eight percent of the participants were obese (BMI ≥28 kg/m2), 19%had a history of diabetes, and 59% had hypertension. For the baseline lipid profile, the mean TC level was 4.97 ± 1.07 mmol/L, and the median RC level was 0.54 (0.39, 0.78) mmol/L. For CERAD test performance, 26% of the participants had scores in the lowest age-stratified quartile. Details are depicted in Table 1.
Fig. 1.
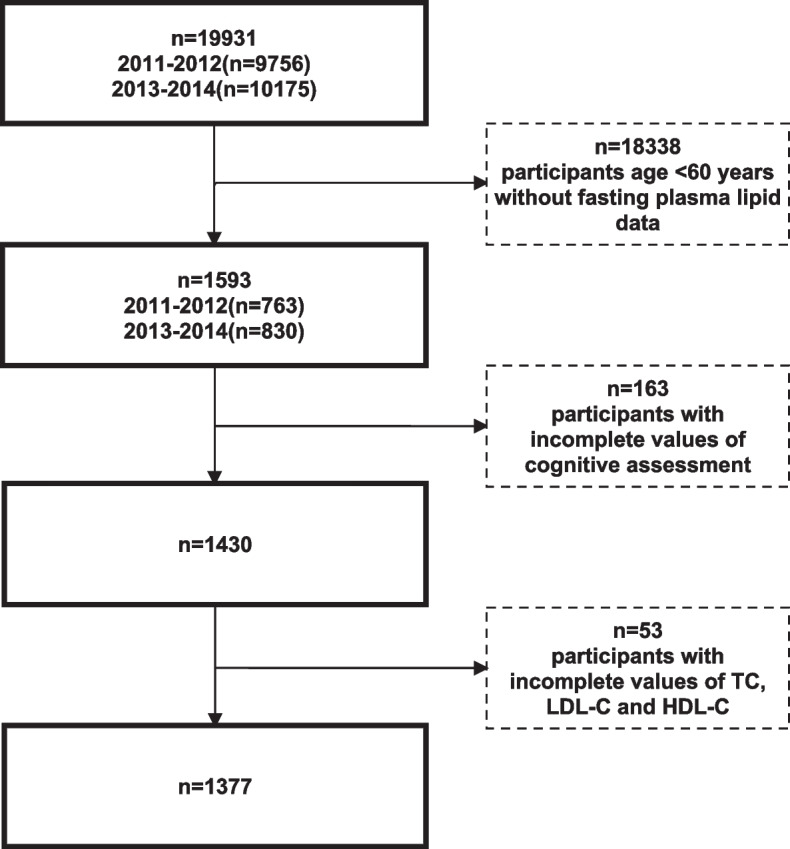
Flowchart of the sample selection process of the study population
Table 1.
Baseline characteristics of the study population grouped by age-stratified quartiles of the CERAD total score
| Groups by age-stratified quartiles of CERAD total score | ||||||
|---|---|---|---|---|---|---|
| Total (n = 1377) | 1st quartile (n = 458) |
2nd quartile (n = 381) |
3rd quartile (n = 305) |
4th quartile (n = 233) |
P for trend | |
| Age, yrsa | 69.1 ± 6.5 | 69.6 ± 7.4 | 69.3 ± 6.3 | 68.8 ± 6.3 | 68.6 ± 6.9 | < 0.001 |
| Gender, n(%)c | ||||||
| Female | 705(55) | 184(12) | 197(14) | 178(16) | 146(14) | < 0.001 |
| Male | 672(45) | 274(14) | 184(13) | 127(9.4) | 87(7.5) | |
| Race, n(%)c | ||||||
| White | 696(79) | 180(18) | 185(21) | 171(21) | 160(19) | < 0.001 |
| Black | 281(8.5) | 110(3) | 83(2.6) | 57(1.8) | 31(1) | |
| Other races | 400(12) | 168(4.8) | 113(3.6) | 77(2.4) | 42(1.2) | |
| Education level, n(%)c | ||||||
| Less than high school | 356(16) | 183(7.5) | 110(5.4) | 45(2.3) | 18(1.1) | < 0.001 |
| High school graduate | 329(23) | 113(6.8) | 94(6.8) | 72(5.5) | 50(3.9) | |
| College or above | 690(61) | 161(11) | 176(15) | 188(17) | 165(16) | |
| Marital status, n(%)c | ||||||
| Married/Cohabiting | 855(68) | 294(18) | 225(19) | 190(17) | 146(15) | < 0.001 |
| Not married/Never married | 522(32) | 164(8) | 156(9.2) | 115(8.1) | 87(6.3) | |
| BMI, n(%)c | ||||||
| Normal | 379(27) | 122(6.3) | 98(6.6) | 88(7.2) | 71(7.1) | < 0.001 |
| Overweight | 465(35) | 155(9.3) | 128(9.8) | 100(8.4) | 82(7.1) | |
| Obese | 533(38) | 181(10) | 155(11) | 117(9.4) | 80(7.4) | |
| Drinking status, n(%)c | ||||||
| Lifetime abstainer | 215(14) | 68(3.3) | 69(4) | 45(3.4) | 33(2.8) | < 0.001 |
| Former drinker | 368(23) | 136(7.4) | 109(6.8) | 77(5.2) | 46(3.7) | |
| Current drinker | 766(63) | 239(15) | 195(17) | 180(16) | 152(15) | |
| Smoking status, n(%)c | ||||||
| Never smoker | 682(49) | 222(12) | 179(13) | 149(12) | 132(12) | < 0.001 |
| Former smoker | 527(40) | 173(10) | 154(12) | 122(11) | 78(7.2) | |
| Current smoker | 166(11) | 63(3.7) | 46(2.7) | 34(2.5) | 23(1.9) | |
| Diabetes, n(%)c | 302(19) | 108(6.3) | 99(6.2) | 61(3.7) | 34(2.5) | < 0.001 |
| Hypertension, n(%)c | 859(59) | 290(16) | 241(17) | 189(14) | 139(12) | < 0.001 |
| Stroke, n(%)c | 99(6.7) | 35(2) | 34(2) | 15(1.3) | 15(1.4) | < 0.001 |
| Statin use, n(%)c | ||||||
| Statin user | 850(60) | 276(14) | 223(16) | 195(15) | 156(15) | < 0.001 |
| Non statin user | 527(40) | 182(12) | 158(12) | 110(9.6) | 77(6.5) | |
| TC, mmol/La | 4.97 ± 1.07 | 4.77 ± 1.12 | 4.91 ± 1.02 | 5.08 ± 1.07 | 5.17 ± 0.99 | < 0.001 |
| LDL-C, mmol/L a | 2.87 ± 0.93 | 2.73 ± 1.00 | 2.81 ± 0.89 | 2.95 ± 0.91 | 3.02 ± 0.85 | < 0.001 |
| HDL-C, mmol/L a | 1.47 ± 0.43 | 1.38 ± 0.45 | 1.46 ± 0.38 | 1.54 ± 0.41 | 1.54 ± 0.42 | < 0.001 |
| TG, mmol/L b | 1.19 (0.85,1.71) | 1.38 (0.90,1.75) | 1.21 (0.89,1.75) | 1.12 (0.78,1.64) | 1.10 (0.81,1.68) | < 0.001 |
| RC, mmol/L b | 0.54 (0.39,0.78) | 0.63 (0.41,0.81) | 0.55 (0.41,0.80) | 0.51 (0.36,0.75) | 0.50 (0.36,0.77) | < 0.001 |
* Data were shown as amean (± standard deviation), bmedian (interquartile range), cunweighted counts (weighted percentage)
Analysis of the association among RC, the TC/RC ratio and the CERAD total score
On a continuous scale, each 1 mmol/L increase in RC was associated with a multivariable adjusted odds ratio (95% CI) of 0.74 (0.58–0.94) for having a CERAD total score in a higher quartile. On a categorical scale, compared with that of the first quartile, the multivariable adjusted odds ratio (95% CI) was 0.66 (0.46–0.95) for the fourth quartile, 0.61 (0.44–0.86) for the third quartile, and 0.89 (0.67–1.17) for the second quartile. The P for trend test was 0.011 across the quartiles. Details are provided in Table 2.
Table 2.
Weighted multivariable ordinal logistic regression analysis between RC levels (log-transformed) and age-stratified quartiles of the CERAD total score
| RC, mmol/L | OR (95%CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Continuous, mmol/L | 0.72(0.56–0.93)* | 0.74(0.58–0.94)* | 0.77(0.61–0.96)* |
| Categorical (quartiles) | |||
| ≤0.39 mmol/L | Ref | Ref | Ref |
| 0.39 to ≤ 0.54 mmol/L | 0.87(0.67–1.13) | 0.91(0.68–1.21)* | 0.93(0.70–1.25) |
| 0.54 to ≤ 0.78 mmol/L | 0.56(0.40–0.80)** | 0.62(0.44–0.88)** | 0.64(0.47–0.88)** |
| > 0.78 mmol/L | 0.66(0.44–0.98)* | 0.67(0.46–0.98)* | 0.70(0.50–0.98)* |
| P for linear trend | 0.040 | 0.015 | 0.013 |
Model 1 univariate analysis
Model 2 adjusted for age, gender, education level, and race
Model 3 further adjusted for BMI status, smoking status, and drinking status plus model 2
*P < 0.05, **P < 0.01
On a continuous scale, each 1 unit increase in the TC/RC ratio was associated with a multivariable adjusted odds ratio (95% CI) of 1.45 (1.13–1.87) for having a CERAD total score in a higher quartile. On a categorical scale, compared with that of the first quartile, the multivariable adjusted odds ratio (95% CI) was 1.50 (1.03–2.18) for the fourth quartile, 1.38 (1.07–1.78) for the third quartile, and 1.12 (0.81–1.55) for the second quartile. The P for trend test was 0.020 across the quartiles. Details are provided in Table 3.
Table 3.
Weighted multivariable ordinal logistic regression analysis between the TC/RC ratio (log-transformed) and age-stratified quartiles of the CERAD total score
| TC/RC | OR (95% CI) | ||
|---|---|---|---|
| Model 1 | Model 2 | Model 3 | |
| Continuous | 1.68(1.29–2.19)** | 1.46(1.12–1.90)** | 1.40(1.10–1.78)** |
| Categorical (quartiles) | |||
| ≤6.08 | Ref | Ref | Ref |
| > 6.08 to ≤8.75 | 1.12(0.80–1.56) | 1.14(0.83–1.56) | 1.09(0.79–1.51) |
| > 8.75 to ≤12.94 | 1.38(1.09–1.76)** | 1.42(1.10–1.84)* | 1.33(1.03–1.71)* |
| > 12.94 | 1.87(1.25–2.80)** | 1.51(1.02–2.22)* | 1.41(1.00–1.97)* |
| P for linear trend | 0.002 | 0.020 | 0.028 |
Model 1 univariate analysis
Model 2 adjusted for age, gender, education level, and race
Model 3 further adjusted for BMI status, smoking status, and drinking status plus model 2
*P < 0.05, **P < 0.01
Subgroup analysis
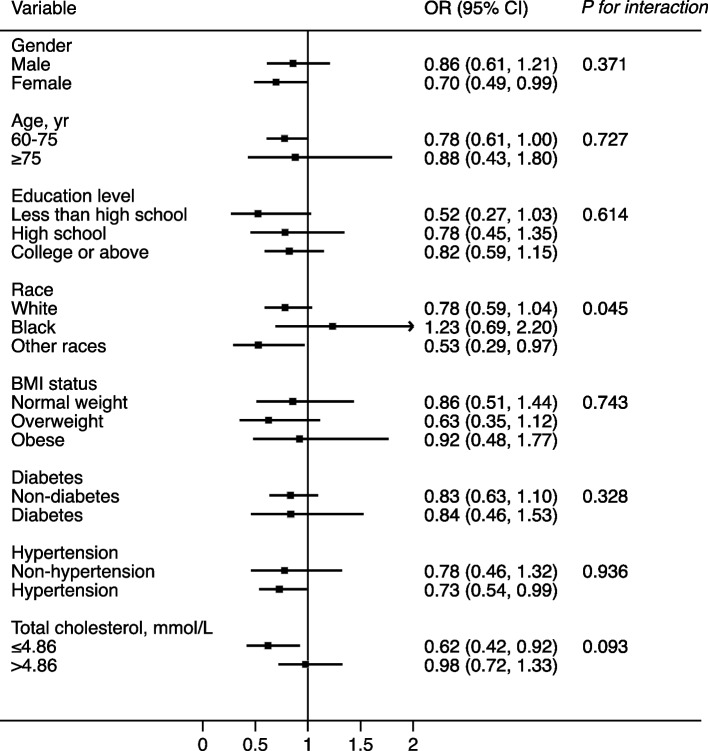
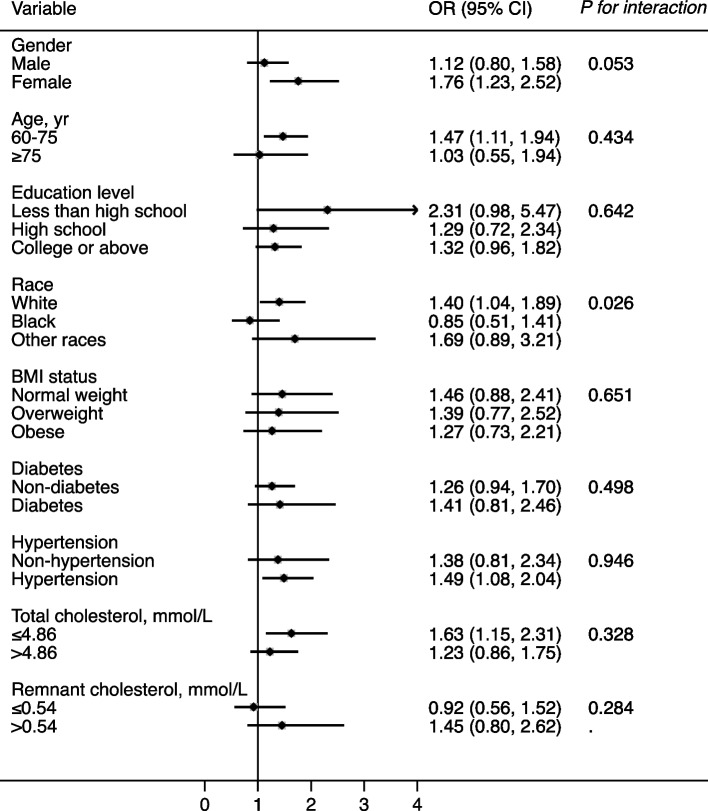
Stratified analyses were conducted for the relationship between RC levels, the TC/RC ratio, and age-stratified quartiles of the CERAD total score (Figs. 2 and 3). After adjusting for confounders, the effect size of the RC level as well as the TC/RC ratio on quartiles of the CERAD total score were almost all consistent across the subgroups, including sex, age, education level, BMI status, diabetes, hypertension, and TC level (P for interaction ≥0.05). Only the race of black was an exception, which deserves further study.
Fig. 2.
Effect size of RC levels on the age-stratified quartiles of the CERAD total score in subgroups. Notes: Model adjusted for age, sex, educational level, race, BMI status, smoking status, and drinking status
Fig. 3.
Effect size of the TC/RC ratio on the age-stratified quartiles of the CERAD total score in subgroups. Notes: Model adjusted for age, sex, educational level, race, BMI status, smoking status, and drinking status
Discussion
To our knowledge, this is the first study to explore the association between plasma RC levels, as well as the TC to RC ratio, and cognitive function. The present study had the following important findings. First, a higher level of RC was associated with a higher risk of verbal learning and memory function impairment. Second, a higher TC to RC ratio was associated with a lower risk of verbal learning and memory function impairment. Third, in comparison with RC levels, the TC/RC ratio showed a steadier relationship with verbal learning and memory function under multiple analytic approaches. Fourth, the effect sizes of RC levels as well as the TC/RC ratio on verbal learning and memory function were consistent across almost all subgroup analyses.
RC is defined as the cholesterol composition of remnants that are metabolized from TG-rich lipoproteins, including chylomicron and very low-density lipoprotein (VLDL) [17]. Quite a few observational studies have investigated the relationship between classical lipid components, such as TC, LDL-C, and HDL-C, and cognitive function. However, there is no current laboratory or epidemiological evidence available concerning the association between RC and cognitive function and the underlying mechanisms. Here, for the first time, this study provides a glimpse of the relationship between RC and cognitive function, suggesting that a lower RC level is associated with better verbal learning and memory function defined by the CERAD total score. The CERAD WL subtest is part of the CERAD Neuropsychology battery, which was originally designed to permit staging of AD. Utilizing a multiple analytic approach, the study demonstrated that a higher level of RC was correlated with worse verbal learning and memory function in American elderly individuals aged ≥60 years.
Although no relevant study is currently available, the study results have biologically plausible explanations. Remnants with diameters less than 70 nm can penetrate the intima of the arterial wall, leading to atherosclerosis [18]. The relationship between RC and atherosclerotic diseases [19], as well as its association with cardiovascular outcomes independent of LDL-C [20], has been established in previous studies. Due to its atherogenic properties, RC can contribute to atherosclerosis in both the carotid artery [21] and arterioles in the brain. A Mendelian randomization study confirmed the causal relationship of remnant lipoprotein-associated genes and ischemic stroke [22]. Prospective cohort studies on symptomatic intracranial atherosclerotic stenosis and ischemic stroke indicated that RC causes cerebral hypoperfusion [23]. In addition, population-based studies showed that a reduction in cerebral perfusion was associated with an increased risk of dementia [24, 25]. Previous studies also reported that RC enhanced oxidative stress and proinflammatory effects on vascular endothelial and smooth muscle cells [26], which might damage the blood‒brain barrier, subsequently altering amyloid degradation and cholesterol homeostasis [27] in the brain. A recent Mendelian randomization study on the risk factors for AD found that genetically elevated TC and LDL-C levels increased neurotic plaque burden, but the effects were driven by single nucleotide polymorphisms of APOE [28], whose genetic product is known to be the key ligand for remnant lipoprotein clearance by the liver. Combined with the results of the present study, disordered APOE-mediated clearance of remnant lipoprotein might partly participate in the development of cognitive impairment. Further research should be conducted to determine the underlying mechanisms and their clinical significance.
The present study proposed a new blood lipid index, the TC/RC ratio. The adverse effect of high TC levels on cognitive function has been abundantly investigated in previous studies. However, serum TC includes cholesterol molecules from a variety of subtypes of lipoprotein, and lowering cholesterol of different lipoprotein subtypes might produce differential outcomes. Therefore, the beneficial effect of cholesterol-lowering therapy on cognitive function is still controversial, especially in elderly individuals [29]. Based on previous studies and the relationship between RC levels and the CERAD total score found in the present study, it is supposed that TC, in combination with RC, might be a better bioindex for the prediction of cognitive function than TC or RC separately. In light of this, the present study examined and demonstrated that a higher TC/RC ratio is suggestive of better verbal learning and memory function assessed by CERAD tests.
In addition, the present study found that the TC/RC ratio showed a significant positive association not only with the CERAD total score but also with the CERAD delayed trial test score [see Additional file 1, Table S1], while RC levels showed no association with delayed trial test score [see Additional file 1, Table S2]. Previous studies have found that some of the measures in the CERAD WL subtest, particularly delayed recall of a word list, could more efficiently distinguish persons with dementia from those with normal cognition [30]. Therefore, the results of the present study indicated that the TC/RC ratio might have better value for predicting verbal learning and memory function than RC levels.
Comparisons with other studies and what the current work adds to the existing knowledge
Conclusively, the current study examined two available blood lipid indices for the assessment of verbal learning and memory function, which have not been reported by previous studies. For future clinical application, the present study provided evidence for the utilization of RC levels and the TC/RC ratio in the evaluation of verbal learning and memory function, which might assist in risk stratification for cognitive function impairment or AD susceptibility in elderly individuals. Considering both cardiovascular and cognitive benefits implied by the present study, the lower the RC level, the better. Regarding the benefit of a higher TC/RC ratio for verbal learning and memory function, there might be a potential atherogenic risk when the higher ratio is mainly attributed to a relatively high TC level rather than a low RC level. However, in the context of this study, the mean TC level was 4.97 ± 1.07 mmol/L, which was not a significant atherogenic level.
Study strengths and limitations
There are several strengths in the present study. First, the study observed a known cardiovascular risk factor, RC, and its negative relationship with verbal learning and memory function. Moreover, the study utilized data extracted from the NHANES database, which used complex, multistage sampling, and relatively convincing results could be drawn with a proper analytic approach. Additionally, the study proposed an RC derivative, the TC/RC ratio, and found its positive association with verbal learning and memory function, which had not been mentioned previously. Additionally, the results were estimated in several subpopulations, verifying its authenticity.
The study has several limitations. First, this was an observational study with a cross-sectional design, and the causal relationship could not be determined between RC levels or the TC/RC ratio and cognitive function. Second, in this study, LDL-C was calculated based on the Friedewald equation, which is not applicable when TG levels > 400 mg/dl. Therefore, the results of the study should not be interpreted in the situation of very high TG levels. Third, the present study used calculated fasting RC to represent the “remnant” cholesterol level; however, this calculated fasting RC includes not only cholesterol from remnants but also cholesterol from newly formed VLDL particles, which in fact overestimates the cholesterol levels of actual remnants. However, RC from the indirect formula has been widely used in numerous clinical studies and has been proven to be a convenient and reliable risk predictor. Finally, although the present study included many important potential covariates previously reported to affect cognition in the statistical models, the possibility that residual confounding factors remain could not be ruled out. Future large-scale, prespecified trials are needed to further explore this subject.
Conclusions
Utilizing a cohort of American elderly individuals aged over 60 years from the NHANES database, the present study found that in the context of a TG level below 400 mg/dl, a lower RC level and a higher TC/RC ratio were associated with better verbal learning and memory function. The present study indicated that lowering RC levels or increasing the TC/RC ratio when the TG level is below 400 mg/dl could possibly be beneficial for preventing cognitive impairment in elderly individuals. The study results, which need to be verified in future larger cohort studies, might help in guiding risk prediction and primary prevention of cognitive impairment in elderly individuals.
Supplementary Information
Additional file 1: Table S1. Weighted multivariable ordinal logistic regression analysis between RC levels (log-transformed) and age-stratified quartiles of the CERAD delayed score. Table S2. Weighted multivariable ordinal logistic regression analysis between the TC/RC ratio (log-transformed) and age-stratified quartiles of the CERAD delayed score.
Acknowledgements
Not applicable.
Abbreviations
- RC
Remnant cholesterol
- TC/RC
Total cholesterol/remnant cholesterol
- CERAD
Consortium to Establish a Registry for Alzheimer’s Disease Word List Memory Task
- NHANES
National Health and Nutrition Examination Survey
- TC
Total cholesterol
- LDL
Low-density lipoprotein
- HDL
High-density lipoprotein
- LDL-C
Low-density lipoprotein cholesterol
- HDL-C
High-density lipoprotein cholesterol
- TG
Triglycerides
- VLDL
Very low-density lipoprotein
- BMI
Body mass index
Authors’ contributions
Ying-Yi Xie: Methodology implementation, Formal analysis, Writing – original draft, Writing – review & editing. Liang Zhao: Validation. Li-Jian Gao: Validation. Rui-Xia Xu: Validation. Ying Gao: Validation. Ke-Fei Dou: Validation. Yuan-Lin Guo: Conceptualization, Methodology guidance, Project administration, Validation, Writing – review & editing. Yong-Ming He: Methodology guidance, Project administration, Validation, Writing – review & editing. The author(s) read and approved the final manuscript.
Funding
This study was supported by the Chinese Academy of Medical Science Innovation Fund for Medical Sciences (2021-I2M-1–009).
Availability of data and materials
The datasets analyzed during the current study are available on the NHANES official website, https://wwwn.cdc.gov/Nchs/Nhanes/.
Declarations
Ethics approval and consent to participate
The NHANES protocols were approved by the National Center for Health Statistics Ethics Review Board of the US CDC, and written informed consent from all the participants was provided during the survey.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yuan-Lin Guo, Email: guoyuanlin@fuwai.com.
Yong-Ming He, Email: heyongming@suda.edu.cn.
References
- 1.GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health. 2022;7(2):c105–25 [DOI] [PMC free article] [PubMed]
- 2.Liu Y, Zhong X, Shen J, Jiao L, Tong J, Zhao W, et al. Elevated serum TC and LDL-C levels in Alzheimer's disease and mild cognitive impairment: A meta-analysis study. Brain Res. 2020;1727:146554. doi: 10.1016/j.brainres.2019.146554. [DOI] [PubMed] [Google Scholar]
- 3.Anstey KJ, Ashby-Mitchell K, Peters R. Updating the Evidence on the Association between Serum Cholesterol and Risk of Late-Life Dementia: Review and Meta-Analysis. J Alzheimers Dis. 2017;56(1):215–228. doi: 10.3233/JAD-160826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ding D, Zhou F, Cao Y, Liang X, Wu W, Xiao Z, et al. Cholesterol profiles and incident cognitive decline among older adults: the Shanghai Aging Study. Age Ageing. 2021;50(2):472–479. doi: 10.1093/ageing/afaa140. [DOI] [PubMed] [Google Scholar]
- 5.Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjærg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer's disease and Parkinson's disease: Mendelian randomisation study. Bmj. 2017;357:1648. doi: 10.1136/bmj.j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruemmer D, Cho L. Remnant Cholesterol: The Leftovers and Their Contribution to Atherosclerotic Cardiovascular Disease. Circ Cardiovasc Imaging. 2021;14(4):e012615. doi: 10.1161/CIRCIMAGING.121.012615. [DOI] [PubMed] [Google Scholar]
- 7.Quispe R, Martin SS, Michos ED, Lamba I, Blumenthal RS, Saeed A, et al. Remnant cholesterol predicts cardiovascular disease beyond LDL and ApoB: a primary prevention study. Eur Heart J. 2021;42(42):4324–4332. doi: 10.1093/eurheartj/ehab432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Varbo A, Benn M, Tybjærg-Hansen A, Jørgensen AB, Frikke-Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61(4):427–436. doi: 10.1016/j.jacc.2012.08.1026. [DOI] [PubMed] [Google Scholar]
- 9.Burnett JR, Hooper AJ, Hegele RA. Remnant Cholesterol and Atherosclerotic Cardiovascular Disease Risk. J Am Coll Cardiol. 2020;76(23):2736–2739. doi: 10.1016/j.jacc.2020.10.029. [DOI] [PubMed] [Google Scholar]
- 10.Paquette M, Bernard S, Paré G, Baass A. Dysbetalipoproteinemia: Differentiating Multifactorial Remnant Cholesterol Disease From Genetic ApoE Deficiency. J Clin Endocrinol Metab. 2022;107(2):538–548. doi: 10.1210/clinem/dgab648. [DOI] [PubMed] [Google Scholar]
- 11.National Health and Nutrition Examination Survey. National Center for Health Statistics (NCHS), Hyattsville. 2011–2014. https://wwwn.cdc.gov/Nchs/Nhanes/. Accessed 12 Mar 2022.
- 12.Dong X, Li S, Sun J, Li Y, Zhang D. Association of Coffee, Decaffeinated Coffee and Caffeine Intake from Coffee with Cognitive Performance in Older Adults: National Health and Nutrition Examination Survey (NHANES) 2011–2014. Nutrients. 2020;12(3):840. doi: 10.3390/nu12030840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Sun W, Zhang D. Association of Zinc, Iron, Copper, and Selenium Intakes with Low Cognitive Performance in Older Adults: A Cross-Sectional Study from National Health and Nutrition Examination Survey (NHANES) J Alzheimers Dis. 2019;72(4):1145–1157. doi: 10.3233/JAD-190263. [DOI] [PubMed] [Google Scholar]
- 14.Cantini L, Mentrasti G, Russo GL, Signorelli D, Pasello G, Rijavec E, et al. Evaluation of COVID-19 impact on DELAYing diagnostic-therapeutic pathways of lung cancer patients in Italy (COVID-DELAY study): fewer cases and higher stages from a real-world scenario. ESMO Open. 2022;7(2):100471. doi: 10.1016/j.esmoop.2022.100471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Center for Health Statistics (NCHS). Adult Alcohol Use Information: Glossary - Alcohol. 2018. Available from: https://www.cdc.gov/nchs/nhis/alcohol/alcohol_glossary.htm. Accessed 12 Mar 2022.
- 16.National Center for Health Statistics (NCHS). Module 3: Weighting.National Health and Nutrition Examination Survey: Tutorials. 2021. Available from: https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx. Accessed 12 Mar 2022.
- 17.Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141(3):358–67. doi: 10.1016/j.pharmthera.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 18.Nordestgaard BG. Triglyceride-Rich Lipoproteins and Atherosclerotic Cardiovascular Disease: New Insights From Epidemiology, Genetics, and Biology. Circ Res. 2016;118(4):547–63. doi: 10.1161/CIRCRESAHA.115.306249. [DOI] [PubMed] [Google Scholar]
- 19.Lin A, Nerlekar N, Rajagopalan A, Yuvaraj J, Modi R, Mirzaee S, et al. Remnant cholesterol and coronary atherosclerotic plaque burden assessed by computed tomography coronary angiography. Atherosclerosis. 2019;284:24–30. doi: 10.1016/j.atherosclerosis.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 20.Castañer O, Pintó X, Subirana I, Amor AJ, Ros E, Hernáez Á, et al. Remnant Cholesterol, Not LDL Cholesterol, Is Associated With Incident Cardiovascular Disease. J Am Coll Cardiol. 2020;76(23):2725–2735. doi: 10.1016/j.jacc.2020.10.008. [DOI] [PubMed] [Google Scholar]
- 21.Qian S, You S, Sun Y, Wu Q, Wang X, Tang W, et al. Remnant Cholesterol and Common Carotid Artery Intima-Media Thickness in Patients With Ischemic Stroke. Circ Cardiovasc Imaging. 2021;14(4):e010953. doi: 10.1161/CIRCIMAGING.120.010953. [DOI] [PubMed] [Google Scholar]
- 22.Si S, Hou L, Chen X, Li W, Liu X, Liu C, et al. Exploring the Causal Roles of Circulating Remnant Lipid Profile on Cardiovascular and Cerebrovascular Diseases: Mendelian Randomization Study. J Epidemiol. 2022;32(5):205–214. doi: 10.2188/jea.JE20200305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim DE, Kim JY, Jeong SW, Cho YJ, Park JM, Lee JH, et al. Association between changes in lipid profiles and progression of symptomatic intracranial atherosclerotic stenosis: a prospective multicenter study. Stroke. 2012;43(7):1824–30. doi: 10.1161/STROKEAHA.112.653659. [DOI] [PubMed] [Google Scholar]
- 24.Wolters FJ, Zonneveld HI, Hofman A, van der Lugt A, Koudstaal PJ, Vernooij MW, et al. Cerebral Perfusion and the Risk of Dementia: A Population-Based Study. Circulation. 2017;136(8):719–728. doi: 10.1161/CIRCULATIONAHA.117.027448. [DOI] [PubMed] [Google Scholar]
- 25.Wolters FJ, de Bruijn RF, Hofman A, Koudstaal PJ, Ikram MA. Cerebral Vasoreactivity, Apolipoprotein E, and the Risk of Dementia: A Population-Based Study. Arterioscler Thromb Vasc Biol. 2016;36(1):204–10. doi: 10.1161/ATVBAHA.115.306768. [DOI] [PubMed] [Google Scholar]
- 26.Nakajima K, Tanaka A. Atherogenic postprandial remnant lipoproteins; VLDL remnants as a causal factor in atherosclerosis. Clin Chim Acta. 2018;478:200–215. doi: 10.1016/j.cca.2017.12.039. [DOI] [PubMed] [Google Scholar]
- 27.Saeed AA, Genové G, Li T, Lütjohann D, Olin M, Mast N, et al. Effects of a disrupted blood-brain barrier on cholesterol homeostasis in the brain. J Biol Chem. 2014;289(34):23712–22. doi: 10.1074/jbc.M114.556159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Andrews SJ, Fulton-Howard B, O'Reilly P, Marcora E, Goate AM. Causal Associations Between Modifiable Risk Factors and the Alzheimer's Phenome. Ann Neurol. 2021;89(1):54–65. doi: 10.1002/ana.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Power MC, Rawlings A, Sharrett AR, Bandeen-Roche K, Coresh J, Ballantyne CM, et al. Association of midlife lipids with 20-year cognitive change: A cohort study. Alzheimers Dement. 2018;14(2):167–177. doi: 10.1016/j.jalz.2017.07.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fillenbaum GG, van Belle G, Morris JC, Mohs RC, Mirra SS, Davis PC, et al. Consortium to Establish a Registry for Alzheimer's Disease (CERAD): the first twenty years. Alzheimers Dement. 2008;4(2):96–109. doi: 10.1016/j.jalz.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Weighted multivariable ordinal logistic regression analysis between RC levels (log-transformed) and age-stratified quartiles of the CERAD delayed score. Table S2. Weighted multivariable ordinal logistic regression analysis between the TC/RC ratio (log-transformed) and age-stratified quartiles of the CERAD delayed score.
Data Availability Statement
The datasets analyzed during the current study are available on the NHANES official website, https://wwwn.cdc.gov/Nchs/Nhanes/.