Abstract
The suckling mouse has been used as a model to identify Vibrio cholerae intestinal colonization factors for over two decades, yet little is known about the location of recoverable organisms along the gastrointestinal (GI) tract following intragastric inoculation. In the present study, we determined the population dynamics of wild-type and avirulent mutant derivatives of both classical and El Tor biotype strains throughout the entire suckling mouse GI tract at various times after intragastric inoculation. Wild-type strains preferentially colonized the middle small bowel with a sharp demarcation between more proximal segments which had manyfold-fewer recoverable cells. Surprisingly, large and stable populations of viable cells were also recovered from the cecum and large bowel. Strains lacking toxin-coregulated pili (TCP−) were cleared from the small bowel; however, an El Tor TCP− strain colonized the cecum and large bowel almost as well as the wild-type strain. Strains lacking lipopolysaccharide O antigen (OA−) were efficiently cleared from the small bowel at early times but then showed net growth for the remainder of the infections. Moreover, large populations of the OA− strains were maintained in the large bowel. These results show that for the El Tor biotype neither TCP nor OA is required for colonization of the suckling mouse large bowel. Finally, similar percent recoveries of wild-type, TCP−, and OA− strains from the small bowel at an early time after infection suggest that TCP and OA are not required for strains of either biotype to resist bactericidal mechanisms in the suckling mouse GI tract.
The curved, highly motile gram-negative rod Vibrio cholerae is the causative agent of the severe and sometimes lethal diarrheal disease cholera. Human biopsy studies (17) as well as animal models (15, 16) indicate that V. cholerae is a noninvasive pathogen. During intestinal colonization, V. cholerae secretes cholera toxin, an A-B subunit type toxin which catalyzes the transfer of an ADP-ribose from NAD to a GTP-binding regulatory component of adenylate cyclase in enterocytes (18). The signs and symptoms of cholera can largely be reproduced by the administration of cholera toxin to human volunteers (25), suggesting that the activity of this enterotoxin primarily accounts for the clinical manifestations of V. cholerae infection. The bacterial properties which facilitate this organism’s capacity to survive and multiply in the small intestine, the principal locus of V. cholerae intestinal colonization, are incompletely understood.
A variety of animal models (31) and bacterial genetic screens have been used to study V. cholerae intestinal colonization factors. The most commonly used animal model is the suckling mouse. Unlike V. cholerae isolates in adult mice, isolates orally administered to 3- to 5-day-old mice colonize the intestine and cause fluid accumulation. Adult animals can be experimentally infected by V. cholerae either through the use of antibiotics to clear most of the normal flora prior to infection or through the use of surgical ties on the small bowel (e.g., the adult rabbit RITARD model) (30). Taylor et al. demonstrated that toxin-coregulated pili (TCP), a type 4 pilus whose expression is coregulated with cholera toxin, are required for colonization of the suckling mouse small bowel (33). Subsequent studies in human volunteers have established the requirement for TCP for V. cholerae human intestinal colonization (21). Although there may be substantive differences between the gastrointestinal (GI) tract of a suckling mouse and that of an adult human, the above-described finding lends validity to the use of the suckling mouse as a model for the study of V. cholerae intestinal colonization.
The suckling mouse model has been used to identify several other V. cholerae colonization factors. Prior to the recombinant DNA era, Baselski and colleagues determined that spontaneous lipopolysaccharide (LPS) rough V. cholerae strains are severely defective in colonization of the suckling mouse small intestine (4, 6). More recently, V. cholerae strains harboring mutations in wbf genes encoding the V. cholerae O1 O antigen (OA) (10, 23, 34) have been constructed and found to be severely defective in intestinal colonization, although the mechanism explaining this finding is unknown. Other V. cholerae gene products which have been shown to be important for colonization of the infant mouse include accessory colonization factors (13, 22, 29), a cell-associated mannose-fucose-resistant hemagglutinin (14), and metabolic factors, such as iron (20) and magnesium (10) transport proteins and arginine, purine, and biotin biosynthesis enzymes (6, 8, 10).
More than 20 years ago, Baselski and Parker studied the distribution of V. cholerae after oral infection of infant mice by using a radioactive tracer (5). They found that the capacity of different strains to colonize the infant mouse upper bowel differed significantly among strains and that the capacity to colonize the upper bowel is essential for the establishment of an infection. Although the suckling mouse model is currently commonly used to study V. cholerae pathogenesis, there has not been much attention paid to further characterization of the suckling mouse V. cholerae colonization model since the pioneering studies of Baselski and Parker. For example, the location of cells within different segments of the small bowel has not been addressed, nor has the intestinal distribution of El Tor biotype V. cholerae strains, the principal cause of cholera in the world at present, been studied. In this study, we have investigated the details of the population dynamics of a wild-type El Tor strain and a classical V. cholerae strain along the entire GI tract at multiple time points during the course of suckling mouse infection. In addition, the population dynamics of tcpA and wbf mutants of the El Tor and classical strains along the entire GI tract were determined.
MATERIALS AND METHODS
Bacterial strains.
The strains used in this study are listed in Table 1. The N16961 strain was a spontaneous streptomycin-resistant (Smr) mutant derived from the clinical isolate N16961 from Bangladesh (24). Competition experiments showed that the Smr N16961 strain was as fit for growth in vitro and as virulent in the suckling mouse model of cholera as the parental strain (data not shown). Strain NTCP was constructed by transduction of the tcpA::mTn5 allele from SC338 (10) into N16961 with a temperature-sensitive mutant of the generalized transducing phage CP-T1 (19, 27). The mTn5 insertion in tcpA in one transductant, designated NTCP, was confirmed by Southern blot analysis (data not shown). The N16961 wbfB::pSC95 strain, MA393, was made by plasmid integration of pSC95 into the wbfB locus. This plasmid contains an internal fragment of wbfB inserted into the suicide vector pGP704 (10, 26). To construct MA393, SM10λpir(pSC95) was mated on Luria-Bertani (LB) (12) agar plates with N16961. Exconjugants were then selected as streptomycin- and ampicillin-resistant colonies. Southern blot analysis was used to confirm the integration of pSC95 into wbfB in MA393 (data not shown).
TABLE 1.
V. cholerae strains used
| Designation | Description | Relevant phenotypea | Reference |
|---|---|---|---|
| N16961 | El Tor biotype | Wild type, Smr | This study |
| O395 | Classical biotype | Wild type, Smr | This study |
| TCP2 | O395N1 tcpA::Tn5 | TCP− Smr Kms | 21 |
| NTCP | N16961 tcpA::mTn5 | TCP− Smr Kmr | This study |
| O395R-1 | O395 wbfD::Tn5lac | LPS OA− Smr Kmr | 34 |
| MA393 | N16961 wbfB::pSC95 | LPS OA− Smr Apr | This study |
Kmr, kanamycin resistant; Apr, ampicillin resistant.
Intestinal colonization assay.
A modified version of the method of Baselski and Parker (5) was used for infection and recovery of V. cholerae from the suckling mouse intestine. Briefly, 4- to 5-day-old suckling CD-1 mice were separated from their mothers 1 h prior to inoculation with V. cholerae. Then, the mice were intragastrically inoculated with cultures of V. cholerae that had been grown overnight and then diluted in LB broth. The cells for the inocula were grown at 30°C in LB broth supplemented with 50 μg of streptomycin per ml (for all strains), 50 μg of ampicillin per ml (for MA393), and 30 μg of kanamycin per ml (for NTCP). Each overnight culture was diluted 1:1,000 in LB containing 8 μl of blue food-coloring dye per ml. Each mouse was then inoculated with 50 μl of the diluted culture as previously described (7). The bacterial titers in each inoculum were determined by plating serial dilutions of the inocula on the appropriate plates. Infected mice were kept at 26°C in the absence of their mothers. Mice were sacrificed at the designated time points, and the small and large bowels as well as the ceca were removed. The small bowel was cut into three segments of equal length, designated the proximal, middle, and distal small intestines. The cecum and large bowel, extending all the way to the rectum, were also separated. Each of these five segments was then mechanically homogenized in 4.5 ml of LB containing 20% (vol/vol) glycerol with a Tissue Tearor (Biospec Products, Bartlesville, Okla.), and serial dilutions were plated onto LB agar supplemented with 100 μg of streptomycin per ml to enumerate V. cholerae CFU per segment. The detection limit for this assay is 150 CFU/segment.
RESULTS AND DISCUSSION
Wild-type V. cholerae infection profiles.
Determination of the population dynamics of wild-type El Tor and classical V. cholerae strains along the entire length of the GI tracts of infected suckling mice describes an important parameter of the host-pathogen interaction and establishes a baseline for comparison of the colonization properties of mutant V. cholerae strains. For the studies of wild-type V. cholerae intestinal population dynamics, we chose El Tor strain N16961 because it is the subject of the ongoing V. cholerae genome project and classical strain O395 because it has been the subject of many previous investigations. Either N16961 or O395 was intragastrically inoculated into suckling mice. Then, at various times after inoculation, the GI tract was dissected and the total number of V. cholerae CFU in each segment was determined. At each time point tested, the total number of recoverable CFU represents the sum of the cells present in the input and their progeny minus the number of cells that were killed or excreted in the stool. Figures 1 and 2 depict the infection profiles for N16961 and O395, respectively. Panel A in each figure shows the percent recovery of input from the entire GI tract, excluding the stomach. In preliminary experiments, we found that the stomach contained no detectable CFU at the time points tested. Panel B in each figure shows the percent recovery of input from the small bowel only. Finally, panel C in each figure shows the number of V. cholerae CFU in various segments of the GI tract, including the proximal, middle, and distal small bowels, the cecum, and the large bowel. Panels A and B show the average percent recovery from multiple experiments. However, due to the variability in absolute numbers of CFU recovered from intestinal segments in different experiments, the data shown in panels C are from single experiments which are representative of multiple experiments performed.
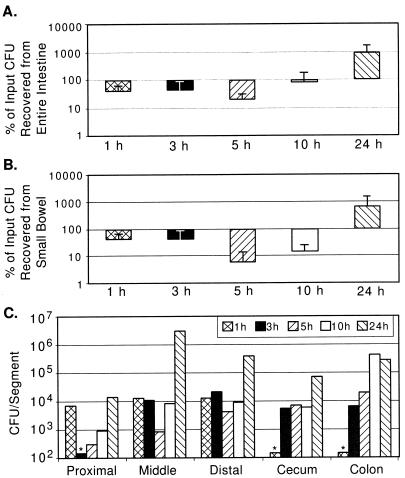
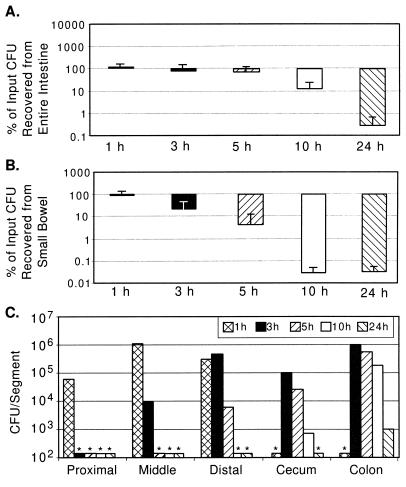
FIG. 1.
Wild-type V. cholerae N16961 (El Tor biotype) recovery from GI tracts of suckling mice over time. Five-day-old CD-1 mice were intragastrically inoculated with between 105 and 107 CFU as described in Material and Methods. Intestinal segments were aseptically removed and processed for bacterial quantification. (A) Percent CFU recovery from entire GI tract. For each time point, CFU from all segments were added and expressed as a percentage of the inoculum given to the mice at time zero. The averages and standard deviations of CFU recovered from four mice are shown. (B) Percent CFU recovery from small bowel. (C) Number of V. cholerae CFU recovered from indicated segments at various times postinoculation. The data are the averages from two mice from a single experiment in which the inoculum was 3 × 105 CFU. Columns with asterisks above them were below the limit of detection for this assay (150 CFU).
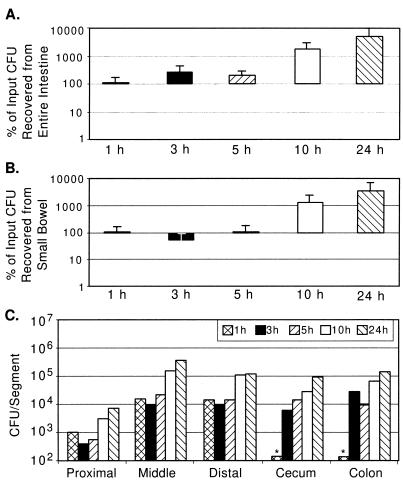
FIG. 2.
Wild-type V. cholerae O395 (classical biotype) recovery from GI tracts of suckling mice. (A) CFU recovery as a percentage of input (between 2 × 105 and 3 × 106) from entire GI tracts of four mice as described in the legend for Fig. 1A. (B) CFU recovery as a percentage of input from small bowel. (C) CFU per segment from a pair of mice inoculated with 2 × 105 CFU, as described in the legend for Fig. 1C.
During the 24 h of intestinal growth following intragastric inoculation, the total number of recoverable CFU of both N16961 and O395 increased 10-fold or more (Fig. 1A and 2A). However, the infection profiles for the classical and El Tor biotype strains differed significantly. With N16961 (El Tor), a rapid loss (∼50%) of total recoverable CFU by 1 h postinoculation was observed and the total recoverable number of N16961 CFU was not greater than the input number until after 10 h postinfection (Fig. 1A). Since this significant loss of CFU was apparent as early as 1 h after inoculation, a time point when few if any CFU had passed through to the cecum and large bowel, there must have been killing of the El Tor V. cholerae strain in the stomach and/or small bowel at the early stage of infection. In contrast, for O395 there was no detectable diminution in the number of recoverable CFU at the early time points following inoculation (Fig. 2A). At the 1-h time point, the percentage of recoverable CFU of N16961 was significantly lower than that for O395 (P < 0.08 by the Mann-Whitney U test). Since there may be cell loss as well as cell multiplication in the intestine, it is possible that there is significant loss of O395 CFU during the early time points after inoculation which is obscured by the intraintestinal growth of this classical strain.
For both V. cholerae biotypes, there were significant differences in the recovery of CFU from the three small bowel segments. For each time point tested, with the exception of the first, the proximal small bowel had lower numbers of both V. cholerae biotypes than the middle and distal small bowel segments (Fig. 1C and 2C). The numbers of recoverable N16961 and O395 CFU increased most dramatically in the middle small bowel segment, especially during the latter time points (Fig. 1C and 2C). For N16961, the largest increase in cell numbers occurred in the middle small bowel segment in the interval between 10 and 24 h after inoculation, and for O395, this increase occurred in the interval between 5 and 10 h after inoculation. Similarly, in the distal segment of the small bowel, the number of recoverable CFU of both biotypes, also increased during the latter time points after inoculation. Since the distal segment is downstream of the middle small bowel segment, the apparent principal site of V. cholerae multiplication, we cannot say to what extent these increased cell numbers are the product of in situ multiplication versus flowthrough from the middle segment.
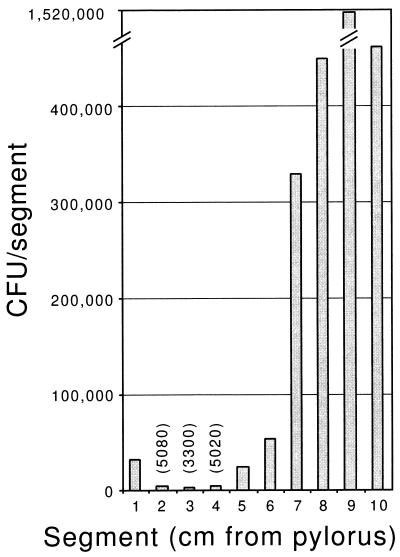
The finding that there are greater numbers of CFU recovered for each time point in the middle small bowel segment than in the proximal small bowel segment suggested the possibility that our division of the small intestine into three equal segments resulted in the combination of a region of the proximal small bowel that is not at all permissive for V. cholerae growth with a region similar to the middle segment that is permissive for V. cholerae growth. To investigate whether there is a region of the upper small bowel which is resistant to V. cholerae colonization, we dissected contiguous 1-cm segments beginning at the junction of the stomach and duodenum (pylorus) 10 h after intragastric inoculation of O395. All segments yielded recoverable O395, demonstrating that there is not a region of the proximal small bowel which is absolutely resistant to colonization; however, in each mouse there was a particular segment, 4 to 6 cm from the pylorus, which had at least 10-fold more recoverable O395 CFU than the more proximal segments. Data from a representative mouse are shown in Fig. 3. The mean numbers of CFU recovered from segments 1 to 6 were significantly less than those from segments 7 to 10 (P < 0.017 by Student’s two-tailed t test). Histologic studies attempting to identify differences in this region of the suckling CD-1 mouse small intestine are under way.
FIG. 3.
Wild-type V. cholerae O395 (classical biotype) recovery from segments of the small bowel of a suckling mouse. The mouse was intragastrically inoculated with 3 × 106 CFU, and CFU were recovered from 1-cm segments proceeding from the pylorus (x-axis origin) toward the cecum 10 h after infection. The numbers of CFU recovered from three of the segments are shown above their bars in parentheses.
Passage of V. cholerae through the ∼14-cm-long suckling mouse small bowel occurred fairly rapidly for both biotypes. There were no V. cholerae CFU in the cecum or large bowel at 1 h after inoculation, but there were significant numbers of CFU present in these organs by 3 h postinoculation (Fig. 1C and 2C). Although the large bowel is not believed to be the site of V. cholerae intestinal colonization, significant numbers of cells of both V. cholerae biotypes were recovered from the cecum and large bowel. Because of our experimental design, it is difficult to discern whether these CFU were the product of in situ multiplication or flowthrough of cells which colonized the small bowel. For the classical strain O395, Fig. 2A and B are nearly superimposable, with the exception of the 3-h and possibly the 5-h time points, indicating that most O395 replication is occurring within the small intestine. For the El Tor strain N16961, there is apparently some replication and/or survival in the large bowel, since Fig. 1A and B are not superimposable, especially at the 5- and 10-h time points. Thus, El Tor strains may be more proficient at colonizing the large bowel (see below). Despite the fact that the cecum is nearly 10 times shorter than each of the other intestinal segments we studied, comparable numbers of CFU were recovered from this segment. This may reflect the fact that in the mouse the cecum is a dead-end-like pouch extending off of the distal small bowel and may therefore accumulate cells exiting the small bowel. However, as for the large bowel, it is also possible that there is V. cholerae colonization of and multiplication within the cecum.
TcpA mutant strain infection profiles.
TcpA is the repeated polypeptide subunit of TCP and is an essential V. cholerae intestinal colonization factor. In competition experiments, when a tcpA strain is coinoculated with a wild-type strain, the competitive index observed is typically 10−3 to 10−4 in the suckling mouse small bowel; i.e., the tcpA strain is outcompeted approximately 1,000- to 10,000-fold by the wild type over a 24-h period (1, 30, 33). It is not known if tcpA strains survive and replicate within the small bowel when present as the sole inoculum or in the large intestine (which is usually not assayed in competition experiments). To begin to assess this, we infected suckling mice with tcpA derivatives of N16961 and O395 and observed the population dynamics of these cells at the time points we used to study the population dynamics of the wild-type strains. Figures 4 and 5 represent infection profiles of tcpA derivatives of N16961 and O395, respectively.
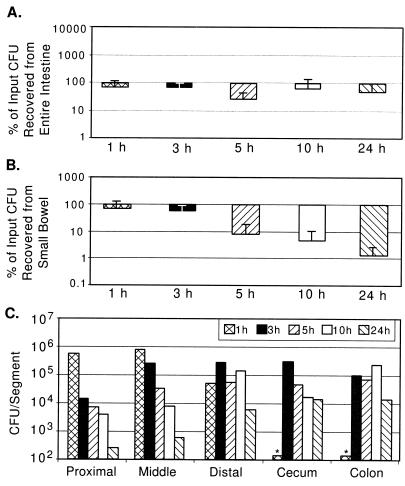
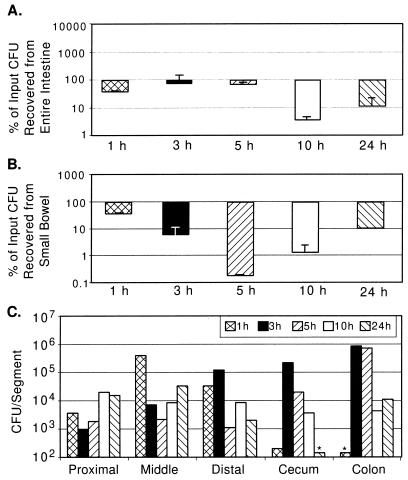
FIG. 4.
V. cholerae N16961 tcpA recovery from GI tracts of suckling mice. (A) CFU recovery as a percentage of input (between 3 × 105 and 4 × 106) from entire GI tracts of four mice as described in the legend for Fig. 1A. (B) CFU recovery as a percentage of input from small bowel. (C) CFU per segment from a pair of mice inoculated with 4 × 106 CFU, as described in the legend for Fig. 1C.
FIG. 5.
V. cholerae O395 tcpA recovery from GI tracts of suckling mice. (A) CFU recovery as a percentage of input (between 5 × 105 and 5 × 106) from entire GI tracts of five mice, as described in the legend for Fig. 1A. (B) CFU recovery as a percentage of input from small bowel. (C) CFU per segment from a pair of mice inoculated with 5 × 105 CFU, as described in the legend for Fig. 1C.
It was surprising to observe that for NTCP, the tcpA derivative of N16961, the percents recovery of CFU from the entire intestinal tract at each time point observed were not as dramatically reduced as predicted from the previously determined competitive index of this strain (1.6 × 10−3) (Fig. 4A). However, at 24 h, there is a large reduction (nearly 1,000-fold) in the numbers of NTCP CFU recovered from the small intestine compared with those of N16961 (Fig. 4B), demonstrating that TCP is a specific factor for small intestinal colonization. The survival (and potentially multiplication) of NTCP in the cecum and large bowel (Fig. 4C) indicates that TCP is not required for colonization of this niche and may suggest that El Tor V. cholerae possesses additional colonization factors for growth in the large bowel.
O395 exhibited progressive increases in recoverable CFU over the duration of the experiment (Fig. 2A and B), whereas the tcpA derivative of O395, TCP2, exhibited progressive decreases in the number of recoverable CFU during the experiment (Fig. 5A and B). In multiple experiments, TCP2 colonized the cecum and large bowel less efficiently than NTCP (e.g., Fig. 4C versus 5C), suggesting that classical strains may be less adapted for this host niche. To further explore this possibility, a competition assay was done between TCP2 and NTCP. Twenty-four hours after intragastric inoculation of equal numbers of both these tcpA strains, there were almost no recoverable CFU of either biotype from the small bowel, but there were more than 100 times more recoverable NTCP CFU than TCP2 CFU from the large bowel (data not shown). This result further suggests that El Tor strains may have colonization factors other than TCP that facilitate growth and multiplication in the large bowel. This finding might explain the clinical observation that El Tor strains of V. cholerae tend to colonize the human GI tract for longer periods than classical strains (3).
As with infections by N16961 and O395, recovery of NTCP CFU and TCP2 CFU in the cecum and large bowel occurred only 3 h postinoculation, suggesting that these tcpA strains pass through the small bowel at a rate similar to that of the parental strains. At 1 h postinoculation, a time point when all the recoverable NTCP CFU and TCP2 CFU are still in the small bowel (Fig. 4C and 5C), the percents recovery of NTCP and TCP2 are very similar to the percents recovery of N16961 and O395, respectively (Fig. 4B versus 1B and 5B versus 2B). This suggests that TCP does not function as a bacterial factor which imparts resistance to a host intestinal killing activity, as had been previously proposed (11, 28).
LPS mutant strain infection profiles.
The V. cholerae wbfB and wbfD (formerly rfbB and rfbD) genes are part of an operon that is required for biosynthesis of perosamine, the repeated carbohydrate moiety of the LPS O1 OA (32, 35). Strains harboring mutations within this operon lack OA and have been shown to be severely attenuated for colonization in suckling mice (23). Figures 6 and 7 depict the infection profiles for N16961 wbfB (strain MA393) and O395 wbfD (strain O395R-1), respectively. As with the tcpA strains, there is no net growth of either wbf strain after 24 h (Fig. 6A and 7A); however, during the 10- to 24-h interval, unlike the tcpA strains, these wbf strains did grow to a limited extent in the small intestine and perhaps in the large bowel (Fig. 6B and C and 7B and C). Since the data in Fig. 4 and 5 indicate that TCP is required for small intestinal colonization, the observation that there is some small bowel colonization by these two OA− strains suggests that there is at least some functional TCP production by the latter strains. This finding contradicts a recent report (23) suggesting that V. cholerae strains lacking OA are defective in assembling TCP on the cell surface.
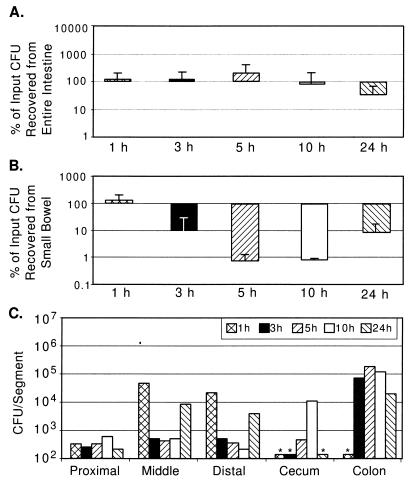
FIG. 6.
V. cholerae N16961 wbfB recovery from GI tracts of suckling mice. (A) CFU recovery as a percentage of input (between 2 × 105 and 6 × 106) from entire GI tracts of two mice, as described in the legend for Fig. 1A. (B) CFU recovery as a percentage of input from small bowel. (C) CFU per segment from a pair of mice inoculated with 3 × 106 CFU, as described in the legend for Fig. 1C.
FIG. 7.
V. cholerae O395 wbfD recovery from GI tracts of suckling mice. (A) CFU recovery as a percentage of input (between 2 × 105 and 1 × 106) from entire GI tracts of four mice, as described in the legend for Fig. 1A. (B) CFU recovery as a percentage of input from small bowel. (C) CFU per segment from a pair of mice inoculated with 2 × 105 CFU, as described in the legend for Fig. 1C.
For the two wbf strains, at times up to and including 5 h postinoculation, the percent recovery of CFU is similar to the percent recovery for wild-type parental strains (Fig. 1A versus 6A and 2A versus 7A), suggesting that these OA− strains are not more susceptible to host bactericidal factors in the GI tract than the wild-type parental strains. However, the percent recovery of CFU in the small bowel for the OA− strains was markedly reduced at later time points during infection compared to that for the wild-type parental strains (Fig. 1B versus 6B and 2B versus 7B), suggesting that OA is necessary for full colonization of this segment of the GI tract.
Conclusions.
Our studies characterizing the infection profiles of wild-type El Tor and classical biotype V. cholerae strains should prove useful as a baseline for future studies of V. cholerae strains harboring mutations in virulence genes, as well as for studies of host factors which influence colonization. For example, it is interesting that in suckling mice, like in humans, the middle small bowel is the main site of colonization by V. cholerae (2). Is this reflective of the distribution of the hypothesized but hitherto unidentified TCP receptor? An alternative explanation which warrants investigation is that maximal colonization of the middle small bowel may simply reflect the requirement for a short period of adaptation within the host GI tract, in this case occurring during passage through the stomach and proximal small bowel, for proper induction of V. cholerae colonization factors.
Although colonization of the large bowel by V. cholerae has largely been ignored by researchers, our finding that both El Tor and classical biotype strains can colonize this organ exceedingly well in suckling mice suggests the possibility that similar colonization may occur during human infections. Although we did not search for V. cholerae factors required for colonization of the cecum and large bowel, this study demonstrates that such colonization by an El Tor biotype strain occurred in the absence of TCP and that OA was not required for colonization of these organs by either biotype. The ability of an El Tor biotype strain to colonize the cecum and large bowel in a TCP-independent manner is interesting in light of recent findings that TCP expression as well as expression of other virulence factors in the ToxR-TcpP-ToxT regulon can phase-vary on and off via slipped-strand mispairing in tcpH(9). Colonization of the cecum and large bowel by TCP− V. cholerae cells might contribute to the shedding of V. cholerae by asymptomatic carriers and by some convalescing patients and could play a role in the dissemination of this organism.
ACKNOWLEDGMENTS
This research was supported by National Institutes of Health grants to M.K.W. (AI 42347) and A.C. (AI 40262), Pew Scholars awards in the Biomedical Sciences to M.K.W. and A.C., and the Center for Gastroenterology Research on Absorptive and Secretory Processes, NEMC (P30 DK34928).
M.J.A. and J.S. contributed equally to this work.
REFERENCES
- 1.Attridge S R, Manning P A, Holmgren J, Jonson G. Relative significance of mannose-sensitive hemagglutinin and toxin-coregulated pili in colonization of infant mice by Vibrio cholerae El Tor. Infect Immun. 1996;64:3369–3373. doi: 10.1128/iai.64.8.3369-3373.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Banwell J G, Pierce N F, Mitra R C, Brigham K L, Caranasos G J, Keimowitz R I, Fedson D S, Thomas J, Gorbach S L, Sack R B, Mondal A. Intestinal fluid and electrolyte transport in human cholera. J Clin Investig. 1970;49:183–195. doi: 10.1172/JCI106217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barua D, Greenough W B. Cholera. New York, N.Y: Plenum Medical Book Company; 1992. [Google Scholar]
- 4.Baselski V S, Medina R A, Parker C D. In vivo and in vitro characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1979;24:111–116. doi: 10.1128/iai.24.1.111-116.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baselski V S, Parker C D. Intestinal distribution of Vibrio cholerae in orally infected infant mice: kinetics of recovery of radiolabel and viable cells. Infect Immun. 1978;21:518–525. doi: 10.1128/iai.21.2.518-525.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baselski V S, Upchurch S, Parker C D. Isolation and phenotypic characterization of virulence-deficient mutants of Vibrio cholerae. Infect Immun. 1978;22:181–188. doi: 10.1128/iai.22.1.181-188.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camilli A, Beattie D, Mekalanos J. Use of genetic recombination as a reporter of gene expression. Proc Natl Acad Sci USA. 1994;91:2634–2638. doi: 10.1073/pnas.91.7.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilli A, Mekalanos J J. Use of recombinase gene fusions to identify Vibrio cholerae genes induced during infection. Mol Microbiol. 1995;18:671–683. doi: 10.1111/j.1365-2958.1995.mmi_18040671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll P A, Tashima K T, Rogers M B, DiRita V J, Calderwood S B. Phase variation in tcpH modulates expression of the ToxR regulon in Vibrio cholerae. Mol Microbiol. 1997;25:1099–1111. doi: 10.1046/j.1365-2958.1997.5371901.x. [DOI] [PubMed] [Google Scholar]
- 10.Chiang S L, Mekalanos J J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 11.Chiang S L, Taylor R K, Koomey M, Mekalanos J J. Single amino acid substitutions in the N-terminus of Vibrio cholerae TcpA affect colonization, autoagglutination, and serum resistance. Mol Microbiol. 1995;17:1133–1142. doi: 10.1111/j.1365-2958.1995.mmi_17061133.x. [DOI] [PubMed] [Google Scholar]
- 12.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 13.Everiss K D, Hughes K J, Kovach M E, Peterson K M. The Vibrio cholerae acfB colonization determinant encodes an inner membrane protein that is related to a family of signal-transducing proteins. Infect Immun. 1994;62:3289–3298. doi: 10.1128/iai.62.8.3289-3298.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franzon V L, Barker A, Manning P A. Nucleotide sequence encoding the mannose-fucose-resistant hemagglutinin of Vibrio cholerae O1 and construction of a mutant. Infect Immun. 1993;61:3032–3037. doi: 10.1128/iai.61.7.3032-3037.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freter R, O’Brien P C M. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: fitness and virulence of nonchemotactic Vibrio cholerae mutants in infant mice. Infect Immun. 1981;34:222–233. doi: 10.1128/iai.34.1.222-233.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freter R, O’Brien P C M, Macsai M S. Role of chemotaxis in the association of motile bacteria with intestinal mucosa: in vivo studies. Infect Immun. 1981;34:234–240. doi: 10.1128/iai.34.1.234-240.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gangarosa E J, Beisel W R, Benyajati C, Sprinz H, Piyaratn P. The nature of the gastrointestinal lesion in Asiatic cholera and its relationship to pathogenesis: a biopsy study. Am J Trop Med Hyg. 1960;9:125–135. doi: 10.4269/ajtmh.1960.9.125. [DOI] [PubMed] [Google Scholar]
- 18.Gill D M. Mechanism of action of cholera toxin. Adv Cyclic Nucleotide Res. 1977;8:85–118. [PubMed] [Google Scholar]
- 19.Hava, D. L., and A. Camilli. Submitted for publication.
- 20.Henderson D P, Payne S M. Vibrio cholerae iron transport systems: roles of heme and siderophore iron transport in virulence and identification of a gene associated with multiple iron transport systems. Infect Immun. 1994;62:5120–5125. doi: 10.1128/iai.62.11.5120-5125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrington D A, Hall R H, Losonsky G, Mekalanos J J, Taylor R K, Levine M M. Toxin, toxin-coregulated pili, and the toxR regulon are essential for Vibrio cholerae pathogenesis in humans. J Exp Med. 1988;168:1487–1492. doi: 10.1084/jem.168.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes K J, Everiss K D, Kovach M E, Peterson K M. Isolation and characterization of the Vibrio cholerae acfA gene, required for efficient intestinal colonization. Gene. 1995;156:59–61. doi: 10.1016/0378-1119(95)00054-a. [DOI] [PubMed] [Google Scholar]
- 23.Iredell J R, Stroeher U H, Ward H M, Manning P A. Lipopolysaccharide O-antigen expression and the effect of its absence on virulence in rfb mutants of Vibrio cholerae O1. FEMS Immunol Med Microbiol. 1998;20:45–54. doi: 10.1111/j.1574-695X.1998.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 24.Kaper J B, Lockman H, Baldini M M, Levine M M. Recombinant nontoxinogenic Vibrio cholerae strains as attenuated cholera vaccine candidates. Nature. 1984;308:655–658. doi: 10.1038/308655a0. [DOI] [PubMed] [Google Scholar]
- 25.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ogg J E, Timme T L, Alemohammad M M. General transduction in Vibrio cholerae. Infect Immun. 1981;31:737–741. doi: 10.1128/iai.31.2.737-741.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parsot C, Taxman E, Mekalanos J J. ToxR regulates the production of lipoproteins and the expression of serum resistance in Vibrio cholerae. Proc Natl Acad Sci USA. 1991;88:1641–1645. doi: 10.1073/pnas.88.5.1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterson K M, Mekalanos J J. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect Immun. 1988;56:2822–2829. doi: 10.1128/iai.56.11.2822-2829.1988. . (Erratum, 57:660, 1989.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rhine J A, Taylor R K. TcpA pilin sequences and colonization requirements for O1 and O139 Vibrio cholerae. Mol Microbiol. 1994;13:1013–1020. doi: 10.1111/j.1365-2958.1994.tb00492.x. [DOI] [PubMed] [Google Scholar]
- 31.Richardson S H. Animal models in cholera research. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C: ASM Press; 1994. pp. 203–226. [Google Scholar]
- 32.Stroeher U H, Karageorgos L E, Brown M H, Morona R, Manning P A. A putative pathway for perosamine biosynthesis is the first function encoded within the rfb region of Vibrio cholerae O1. Gene. 1995;166:33–42. doi: 10.1016/0378-1119(95)00589-0. [DOI] [PubMed] [Google Scholar]
- 33.Taylor R K, Miller V L, Furlong D B, Mekalanos J J. Use of phoA gene fusions to identify a pilus colonization factor coordinately regulated with cholera toxin. Proc Natl Acad Sci USA. 1987;84:2833–2837. doi: 10.1073/pnas.84.9.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ward H M, Manning P A. Mapping of chromosomal loci associated with lipopolysaccharide synthesis and serotype specificity in Vibrio cholerae O1 by transposon mutagenesis using Tn5 and Tn2680. Mol Gen Genet. 1989;218:367–370. doi: 10.1007/BF00331294. [DOI] [PubMed] [Google Scholar]