Abstract
Despite significant progress in breast cancer (BC) therapy, it is globally the most commonly diagnosed cancer and leads to the death of over 650,000 women annually. Androgen receptor (AR) is emerging as a potential new therapeutic target in BC. While the role of AR is well established in prostate cancer (PCa), its function in BC remains incompletely understood. Emerging data show that AR’s role in BC is dependent on several factors including, but not limited to, disease subtype, tumour microenvironment, and levels of circulating oestrogens and androgens. While targeting AR in PCa is becoming increasingly effective, these advances have yet to make any significant impact on the care of BC patients. However, this approach is increasingly being evaluated in BC and it is clear that improvements in our understanding of AR’s role in BC will increase the likelihood of success for AR-targeted therapies. This review summarizes our current understanding of the function of AR across BC subtypes. We highlight limitations in our current knowledge and demonstrate the importance of categorizing BC subtypes effectively, in relation to determining AR activity. Further, we describe the current state of the art regarding AR-targeted approaches for BC as monotherapy or in combination with radiotherapy.
Keywords: Androgen receptor, Breast cancer, Radiotherapy, DNA damage repair, Androgen deprivation therapy
Background
Breast cancer (BC) is the most common cancer in women with over 2.2 million estimated new cases and over 650,000 deaths each year worldwide [1]. While substantial improvements have been made over the years to reduce BC mortality, it remains a significant cause of death in women. BC is a heterogeneous disease with many subtypes with different molecular features. These subtypes are characterized by established biomarkers such as oestrogen receptor alpha (ER), progesterone receptor (PgR), and human epidermal growth factor receptor 2 (HER2) [2]. Molecular classification is important not only for patient prognosis but also to help predict therapy response and guide treatment strategies. To improve BC treatment, there remains a need to identify novel and alternative therapeutic targets for this disease, particularly in triple-negative breast cancer (TNBC) where systemic cytotoxic chemotherapy remains the primary pharmacological intervention. Although recent evidence has established a role for immunotherapy in metastatic TNBC, these benefits appear to be limited to patients with positive PD-L1 status [3]. In this respect, the androgen receptor (AR) is emerging as a new biomarker and a potential therapeutic target in BC.
AR is shown to be involved in all stages of BC development and is expressed in up to 30–80% of BC by immunohistochemistry (IHC), depending on subtype [4]. Notably, there is a wide range of reported AR+ samples in part due to a lack of standardization of cut-off levels to determine AR positivity. The exact mechanism of AR action in BC, however, remains elusive. The role of AR likely depends on the subtype of BC as well as levels of circulating hormones and tumour microenvironment. While data suggest that AR could be used as a potential biomarker, the degree of prognostic value remains unclear, making it difficult to identify which patients would benefit from therapeutics that exploit AR as a target. Furthermore, recent data suggest that AR is associated with radiation therapy (RT) resistance. Understanding the complex role of AR in BC subtypes would be useful in predicting which patients would benefit from targeted AR therapies.
AR biology
AR is a steroid hormone receptor that acts as a ligand-dependent transcription factor. Once the ligand binds, AR translocates to the nucleus of the cell where dimerized receptors bind to enhancer and promoter regions termed androgen response elements (AREs) of target genes. This leads to the initiation of transcription, cell proliferation and survival, and negative feedback to inactivate AR transcription [5]. Ligands that bind to AR principally include testosterone (T) and 5a-dihydrotestosterone (DHT). Other androgens such as androstenedione, androstenediol, and dehydroepiandrosterone (DHEA) have also been shown to bind AR, albeit with much less potency than T and DHT [6]. The levels of circulating androgens differ significantly between males and females. T and DHT are present at the highest concentrations in males while in females they are at the lowest compared to other androgens [7]. Given the differences in potency, the majority of AR activation in females will be likely due to T and DHT. AR also has the potential to be activated through ligand-independent mechanisms, including through interactions with PI3K/AKT, ERK, mTOR, and Wnt/β-catenin signalling pathways (reviewed in Anestis et al. [8]). AR activity is well established as a dependency of prostate cancer (PCa) throughout all stages of growth and progression, leading to the essential role of AR-directed therapies for PCa [9].
AR is expressed in various tissues in females as well, including breast tissue, and it is known to play a significant role in normal female biology, fertility, and breast development [10]. AR is also commonly expressed in BC, and while sex steroid signalling is very well established as being critical to the development of BC at all stages, the role of AR signalling remains unclear [11]. AR is broadly expressed across multiple types of BC, leading to its emergence as a target for BC therapeutics and the ongoing research exploring AR as a predictive and prognostic biomarker [12]. AR is expressed in 30–80% of BC, with more common co-expression with ER+ (70–90%) over ER– cancer (20–30%) [13–15]. The variation in expression across studies is due, in part, to differing definitions of AR positivity (Table 1). Recently, Ricciardelli et al. summarized the use of AR for BC prognosis and concluded that a higher median cut-off of AR positivity (≥ 78%) could more reliably predict BC survival compared to other commonly used cut-offs (1% or 10% nuclear positivity) [16]. Table 2 shows this variability in the definition of AR positivity across clinical trials, with a range of positive IHC staining from > 0% to 50%. The clinical significance of AR expression seems to differ based on the type of BC, which becomes more evident as we assess the prognostic value of AR by subtypes of BC.
Table 1.
Defining AR positivity across breast cancer samples
| References | ER status | HER2 status | Primary/metastatic | Antibody | AR+ definition | AR+ samples (%) | Total |
|---|---|---|---|---|---|---|---|
| Agrawal et al. [62] | ER+/ER− | ± | Primary Only | AR411 DAKO | Not Reported | 212 (43) | 488 |
| Gonzalez et al. [63] | ER+/ER− | Not reported | Primary & Lymph Node Metastases | AR411 DAKO | ≥ 1% | 83 (75) | 111 |
| Micello et al. [41] | ER− and PgR− | ± | Primary Only | AR27 Novocastra | ≥ 1% Nuclear | 128 (57) | 226 |
| Luo et al. [64] | ER− and PgR− | - | Not Explicitly Reported | Not Reported | ≥ 1% Nuclear | 38 (28) | 137 |
| Niemeier et al. [15] | ER+/ER− | ± | Not Explicitly Reported | AR441 DAKO | H score > 10 | 151(80) | 189 |
| Castellano et al. [65] | ER+ | ± | Primary Only | AR411 DAKO | ≥ 1% | 609 (71) | 859 |
| Hu et al. [28] | ER+/ER− | ± | Primary Only | AR411 DAKO | ≥ 10% Nuclear | 1155 (79) | 1467 |
| Loibl et al. [66] | ER+/ER− | ± | Primary Only | F39.4.1 BioGenex | ≥ 1% | 358 (53) | 673 |
| Park et al. [27] | ER+/ER− | ± | Not Explicitly Reported | AR441 Thermo Scientific | ≥ 10% Nuclear | 541 (58) | 931 |
| Yu et al. [67] | ER+/ER− | ± | Not Explicitly Reported | AR441 Lab Vision | ≥ 10% Cytoplasmic | 237 (72) | 327 |
| Gucalp et al. [49] | ER− and PgR− | ± | Primary and Metastases | AR411 DAKO | ≥ 10% Nuclear | 51 (12) | 424 |
| Honma et al. [68] | ER+/ER− | ± | Not Explicitly Reported | AR27 Novocastra | ≥ 10% Nuclear | 212 (53) | 403 |
| Tokunaga et al. [69] | ER+/ER− | ± | Primary Only | AR411 DAKO | ≥ 75% Nuclear | 155 (62) | 250 |
| Thike et al. [60] | ER− and PgR− | - | Not Explicitly Reported | AR27 NCL-AR-318 | ≥ 1% Nuclear | 267 (38) | 699 |
| Tsang et al. [70] | ER+/ER− | ± | Primary Only | AR441 DAKO | ≥ 1% Nuclear | 549 (48) | 1144 |
| Aleskandarany et al. [71] | ER+/ER− | ± | Not Explicitly Reported | Sc-816 Santa Cruz Biotech | H score ≥ 190 | 613 (54) | 1141 |
| Bronte et al. [72] | ER+/ER− | ± | Primary and Metastases | SP107 Cell Marque Ventana Medical Systems | ≥ 1% and ≥ 10% | 136 (83) and 131 (80) | 164 |
| Candelaria et al. [73] | ER− and PgR− | - | Not Explicitly Reported | AR441 DAKO | ≥ 10% | 45 (31) | 144 |
| Kensler et al. [13] | ER+ | ± | Not Explicitly Reported | AR441 DAKO | ≥ 1% Nuclear | 2475(82) | 3021 |
| Xiang et al. [74] | ER+/ER− | ± | Primary Only | ZA-0554 ZSGB | ≥ 10% Nuclear | 201 (67) | 298 |
| Zhao et al. [75] | ER− and PgR− | – | Not Explicitly Reported | Abcam ab113273 | Not Reported | 60 (29) | 210 |
Table 2.
Completed and ongoing trials assessing the safety and efficacy and targeting androgen receptor in breast cancer
| NCT number | Title | BC subtype | AR agent drug class | Therapeutic agent | AR eligibility criteria | Menopausal status | Phase |
|---|---|---|---|---|---|---|---|
| NCT02463032 | Efficacy and safety of GTx024 in patients with ER+/AR+ breast cancer | ER+/AR+ | SARM | Enobosarm | Not Defined | Postmenopausal | Phase II |
| NCT02955394 | Preoperative Fulvestrant with or without Enzalutamide in ER+/HER2− breast cancer | ER+/HER2−/AR+ | Antiandrogen | Enzalutamide + Fulvestrant | Not Defined | Postmenopausal | Phase II |
| NCT02910050 | Bicalutamide plus aromatase inhibitors in ER+/AR+/HER2− metastatic breast cancer (BETTER) | ER+/HER2−/AR+ | Antiandrogen + AI | Bicalutamide + AI | Not Defined | Postmenopausal | Phase II |
| NCT01616758 | Phase II study of GTx024 in women with metastatic breast cancer | ER+ (any AR status) | SARM | Enobosarm | Not Defined | Postmenopausal | Phase II |
| NCT00755885 | Abiraterone acetate in treating postmenopausal women with advanced or metastatic breast cancer | AR+/ER− or ER+ (any AR status) | CYP17-lyase inhibitor | Abiraterone acetate | Not Defined | Postmenopausal | Phase I/II |
| NCT03207529 | Alpelisib and Enzalutamide in treating patients with AR and PTEN+ metastatic breast cancer | HER2−/PTEN+/AR+ | Antiandrogen | Enzalutamide + Alpelisib | ≥ 1% nuclear staining | All | Phase I* |
| NCT02580448 | CYP17-Lyase and AR inhibitor treatment with Seviteronel trial (CLARITY-01) | TNBC/AR+ or ER+/AR+ | CYP17-lyase inhibitor | Seviteronel | Not Defined | Postmenopausal (if ER+) | Phase I/II |
| NCT02676986 | Short-term preoperative treatment with Enzalutamide, alone or in combination with Exemestane in primary breast cancer (ARB) | ER+ and TNBC/AR+ | Antiandrogen | Enzalutamide + Exemestane | > 0% nuclear staining | Postmenopausal (if ER+) | Phase II |
| NCT01990209 | Orteronel as monotherapy in patients with metastatic breast cancer (MBC) that expresses AR | ER/PgR+/AR+ or TNBC/AR+ | CYP17-lyase inhibitor | Orteronel | > 10% IHC staining | Postmenopausal or Pre with Ovarian Suppression (if ER+) | Phase II |
| NCT02067741 | CR1447 in endocrine responsive-HER2- and TN-AR+ breast cancer | ER/PgR+ (HER2−) or TNBC/AR+ | Antiandrogen | CR1447 (4-OH-testosterone) | > 0% IHC staining | Postmenopausal | Phase II |
| NCT02091960 | A study to assess the efficacy and safety of enzalutamide with trastuzumab in patients with HER2+, AR+ metastatic or locally advanced BC | HER2+/AR+ | Antiandrogen | Enzalutamide + Trastuzumab | Not Defined | All | Phase II |
| NCT00468715 | Bicalutamide in treating patients with metastatic breast cancer | ER−/PgR−/AR+ | Antiandrogen | Bicalutamide | > 10% nuclear staining | All | Phase II |
| NCT03055312 | Bicalutamide in treatment of AR+ metastatic triple-negative breast cancer | TNBC/AR+ | Antiandrogen | Bicalutamide | IHC ≥ 10% | All | Phase III |
| NCT01889238 | Safety and efficacy study of enzalutamide in patients with advanced, AR+, triple-negative breast cancer | TNBC/AR+ | Antiandrogen | Enzalutamide | Not Defined | All | Phase II |
| NCT02457910 | Taselisib and Enzalutamide in treating patients with AR+ triple-negative metastatic breast cancer | TNBC/AR+ | Antiandrogen | Enzalutamide + Taselisib | ≥ 10% nuclear staining | All | Phase Ib/II |
| NCT02605486 | Palbociclib in combination with Bicalutamide for the treatment of AR+ metastatic breast cancer | TNBC/AR+ | Antiandrogen | Bicalutamide + Palbociclib | ≥ 1% nuclear staining | All | Phase I/II |
| NCT02971761 | Pembrolizumab and Enobosarm in treating patients with AR+ metastatic TNBC | TNBC/AR+ | SARM | Enobosarm + Pembrolizumab | ≥ 50% nuclear staining | All | Phase II |
| NCT03090165 | Ribociclib and Bicalutamide in AR+ TNBC | TNBC/AR+ | Antiandrogen | Bicalutamide + Ribociclib | > 0% IHC staining | All | Phase I/II |
| NCT02353988 | AR-inhibitor Bicalutamide in treating patients with TNBC (Arbre) | TNBC/AR+ | Antiandrogen | Bicalutamide | > 10% nuclear staining | All | Phase II |
| NCT02750358 | Feasibility study of adjuvant Enzalutamide for the treatment of early-stage AR+ triple-negative breast cancer | TNBC/AR+ | Antiandrogen | Enzalutamide | ≥ 1% nuclear staining | All | Phase II |
| NCT03383679 | Study on AR- and triple-negative breast cancer (START) | TNBC/AR+ | Antiandrogen | Darolutamide + Capecitabine | ≥ 10% IHC staining | All | Phase II |
| NCT02689427 | Enzalutamide and paclitaxel before surgery in treating patients with stage I–III AR+ triple-negative breast cancer | TNBC/AR+ | Antiandrogen | Enzalutamide + Paclitaxel | ≥ 10% nuclear staining | All | Phase II* |
| NCT05095207 | Abemaciclib in combination with Bicalutamide for androgen receptor-positive, HER2-negative metastatic breast cancer | HER2−/AR+ | Antiandrogen | Abemaciclib + Bicalutamide | ≥ 1% IHC staining | All | Phase Ib/II* |
| NCT04869943 | Efficacy evaluation of Enobosarm monotherapy in treatment of AR+/ER+/HER2− metastatic breast cancer (ARTEST) | ER+/HER2−/AR+ | SARM | Enobosarm | ≥ 40% nuclear staining | Postmenopausal or Pre with Ovarian Suppression | Phase III* |
| NCT05065411 | Efficacy and safety evaluation of Enobosarm in combo with Abemaciclib in treatment of ER+/HER2− metastatic breast cancer | ER+/HER2−/AR+ | SARM | Enobosarm + Abemaciclib | ≥ 40% nuclear staining | All | Phase III* |
| NCT03650894 | Nivolumab, Ipilimumab, and Bicalutamide in HER2− breast cancer patients | HER2−/AR+ | Antiandrogen | Nivolumab + Ipilimumab + Bicalutamide | Not Defined | All | Phase II* |
*Study is Actively Recruiting
Androgens in breast cancer development
The role of androgens in BC development and progression has been contrasting. While it is well known that androgens can act to inhibit growth in BC, the androgen excess theory proposes that androgens are instrumental in the development of BC in both ER+ and ER− tumours (reviewed in Secreto et al. [17]). Studies have shown that androgens have anti-proliferative properties during puberty and oppose oestrogens, while oestrogen and progesterone promote breast development under physiologic conditions [18, 19]. The equilibrium between oestrogen and androgen allows for the control of mammary epithelial growth. While androgens are growth inhibitory, they are also precursors to oestrogens, so they can also act to stimulate breast cell growth and consequently overstimulate cell proliferation through conversion to oestrogen [17]. Under conditions of excess androgens, the balance between androgen and oestrogen is maintained at a new higher level; however, eventually the stimulatory effects of oestrogens predominate. Through this mechanism, the imbalance of androgen and oestrogen has been shown to lead to the development of ER+ BC. Prospective studies have repeatedly shown a link between high circulating androgens and the development of ER+ BC [20, 21]. Notably, this conversion from androgens to oestrogens is often inhibited through aromatase inhibitors (AIs) in ER+ BC, resulting in increased circulating androgens and decreased levels of oestrogen [22]. Circulating androgens are also associated with a roughly twofold increased BC risk in postmenopausal women. When adjusted for circulating oestrogen to account for the conversion of androgens to oestrogen the association is only partially diminished, confirming an effect of androgens on breast tissue independent of the effect of oestrogen [20, 21, 23].
Androgens and AR expression have also been implicated in the development of ER– BC. AR positivity is shown to be associated with overexpression of HER2 in apocrine tumours, such as tumours of the mammary gland, suggesting an interaction of AR and HER2 signalling pathways in these cells [24, 25]. When apocrine cells are stimulated by androgens, they produce epidermal growth factor (EGF), which results in cell growth and proliferation through stimulation of EGFR and HER2 [17]. Notably, EGFR is often overexpressed in BC, and it is possible that androgen stimulation would lead to the growth of cells under these conditions. Activation of both these pathways provides an opportunity for the development of ER– BC through androgen stimulation. Additionally, ER– BCs that retain AR expression are shown to have gene expression profiles that closely resemble that of ER+ BC.
AR prognostic value by subtype
Luminal breast cancer
PAM50 is a commonly used method of classifying intrinsic subtypes of BC using a minimal gene set [26]. Under the PAM50 classification, BC, which is both ER+ and PgR+, is subclassified as luminal A and B. The luminal A are low proliferating and luminal B are divided into HER2+ and HER2–. The majority of luminal BCs express AR by IHC, and this expression is associated with a favourable prognosis [27, 28]. One meta-analysis shows that in early BC, AR is more likely to be co-expressed in ER+ over ER– tumours (74.8% vs. 31.8%, respectively) [29]. AR may act through genomic signalling interference to reduce the proliferation of BC in the presence of oestrogen. One proposed mechanism for growth inhibition in the ER+ BC subtypes is by competitive binding of AR to the ER binding site on DNA [30]. A crosstalk between AR and ER has been proposed, and preclinical data suggest AR can antagonize ER signalling, dependent upon the relative levels of each hormone receptor [31]. Contrastingly, in BCs that do not express ER, AR is able to bind to oestrogen response elements (EREs) on the DNA and stimulate cell proliferation [32]. A large-scale clinical and gene expression meta-analysis from Bozovic-Spasojevic et al. confirmed the findings shown in previous studies where AR positivity conferred improved disease-free survival and overall survival in ER+ BC [33]. Further support for this observation was seen in a retrospective study where patients with tumours expressing both AR and ER had a better prognosis than those with either AR or ER positivity [32]. Additionally, the ratio of the hormone receptors has been suggested as being relevant for prognostic value, with high AR relative to ER proving to be predictive of hormone therapy resistance in early studies [34]. While several studies support the improved prognostic value of AR in ER+ BC, some propose the activation of signalling pathways that would lead to increased proliferation of BC cells. Steroid receptors including AR can have extranuclear functions involved in cell growth and survival. For instance, AR is able to activate cell proliferation through oestradiol stimulation of the formation of a complex between AR, ER and Src. This complex ultimately activates the PI3K/Akt and MAPK pathways [35, 36]. Disruption of the interaction between AR/Src weakens the formation of this complex and can inhibit proliferation [37, 38]. Similarly, EGF signalling was dependent on the formation of this complex, confirming that the AR/ER/Src association plays a role in cell cycle progression [39]. Further studies show that hormone therapy resistance occurs in ER+ models with AR overexpression through EGFR [40].
HER2-enriched breast cancer
AR is expressed in about 60% of HER2+ BC by IHC [4]. In women with HER2+ BC, findings consistently report a worse prognosis with AR positivity [41]. The mechanism proposed for this unfavourable prognosis is through AR signalling mediated transcriptional induction of ligands involved in Wnt/β catenin and HER2 signalling pathways. AR stimulated WNT7B activation leads to nuclear localization of β catenin and subsequent interaction of β catenin with AR to increase HER3 expression (42). Both Wnt and HER2 signalling pathways have the potential for positive feed-forward activation of AR activity, suggesting an androgen-independent activation of AR in these tumours. This has also been demonstrated in castrate-resistant prostate cancer (CRPC) [43].
The efficacy of treatment of HER2+ AR+ BC with enzalutamide and trastuzumab (HER2 mAb) is currently under investigation in a phase 2 clinical trial (NCT02091960). In contrast to these findings, however, a gene expression analysis has found that overall survival is in fact better for AR mRNA expression in HER2+ BC. These conflicting results could be attributed to there being only modest overlap between transcriptional and IHC profiles for AR [33, 44].
Triple-negative breast cancer (TNBC)
TNBC is defined as lacking expression of ER, PgR and HER2. This occurs in approximately 15–20% of BCs, but represents a disproportionate rate of mortality, as it is a more aggressive subtype [45]. Quadruple negative breast cancer (QNBC) is broadly TNBC that also does not express AR [46]. Patients with early TNBC suitable for surgery are frequently offered adjuvant chemotherapy, if deemed fit enough. However, these patients continue to have overall poorer survival and higher rates of distant metastasis following treatment [47]. Due to the lack of molecular targets, new therapeutics are needed for this subtype.
TNBC has recently been further categorized based on the gene expression profiles by the Lehmann molecular classification as follows: basal-like (BL1 and BL2), mesenchymal, mesenchymal stem-like, immunomodulatory, and luminal androgen receptor (LAR) [48]. AR positivity is reported between 12 and 50% of TNBC [4, 44, 49]. The existence of an AR-expressing subtype of TNBC along with somatic mutations identified in AR-responsive genes in some sequencing has sparked interest in AR as a target for therapy of this aggressive type [50, 51]. These Lehmann subtypes are shown to respond differently to therapies, with the LAR type being less proliferative and less sensitive to chemotherapy than the basal type [52, 53]. Many studies have shown that AR expression in TNBC is associated with lower histologic grade and lower clinical stage [54–56]. Other groups have shown that a lack of AR expression increases the risk of recurrence and metastasis in TNBC [57, 58]. A retrospective study found AR positivity in TNBC to be associated with improved disease-free survival, while another found LAR TNBC to have higher overall survival [59, 60]. Another recent study stratified patients into distinct TNBC risk groups, finding LAR (AR+, EGFR−) patients to be in a lower-risk group with a better prognosis and likely to benefit most from antiandrogen therapy. Alternatively, AR− and EGFR+ represent the high-risk group that has a worse prognosis and is more likely to benefit from chemotherapy [61]. The prognostic value of AR in TNBC is still uncertain, with some reports suggesting a positive prognosis with AR expression and others reporting no effect. This is likely variable based on the molecular profile of the cancer as well as the clinical context (Reviewed in Bozovic-Spasojevic et al. meta-analysis [33]). Understanding the prognostic value of AR under these variable molecular and clinical contexts will be instrumental in using AR-targeting therapies for the treatment of BC.
Apocrine breast cancer
Apocrine BCs are not classified as a distinct subtype within the PAM50 classification and they make up only around 1% of all BCs [76]. Apocrine BCs are ER/PgR– and generally, but not always, HER2−. Because of this and their uncommonness, apocrine BCs are generally grouped with TNBCs despite having distinct morphology. These tumours are characterized by having apocrine cells with oeosinophilic and granular cytoplasm and a low nuclear–cytoplasmic ratio. Additionally, activation of the AR signalling pathway is a prominent feature in apocrine BC [77]. Apocrine BCs are ER/PgR– and therefore may be either HER2-enriched or, more frequently triple-negative. Importantly not all AR+ but ER/PgR– BCs are apocrine.
Triple-negative apocrine BC frequently carry actionable genomic alterations including alterations in PIK3CA and PTEN [78]. Approximately 80% of invasive apocrine tumours occur in postmenopausal women [79]. Due to its relatively low prevalence, it remains unclear whether the clinical features and outcomes of apocrine BC differ significantly from non-apocrine BC (either AR+ or AR–). Recently, an AR+ apocrine cell line model was shown to have an AR-dependent proliferative response to androgens and expression of genes that are normally expressed in ER+ luminal tumours [44]. Further work showed that in this model AR binds and regulates ER cis-regulatory elements through a FoxA1-dependent mechanism leading to the gene expression profile overlap with ER+ BC [80]. Apocrine BC’s strong association with AR highlights their importance as a model to understand AR signalling in BC, and these tumours should be enriched for clinical trials investigating AR-targeted agents.
Androgen-targeting therapy
Targeting androgens in PCa has been a goal of treatment since the 1940s, with new classes of antiandrogens developed as knowledge of androgen biosynthesis and signalling has increased (Fig. 1). Nonsteroidal antiandrogens were developed to target AR without the nonspecific effects of their steroidal predecessors. While these drugs are safer, they have a disadvantage of lower affinity for AR, leaving about 5–10% of DHT uninhibited and able to bind and activate AR. Newer generations of antiandrogens were developed to address this issue. These next-generation agents include abiraterone, an inhibitor of cytochrome P17 (CYP17), and enzalutamide. CYP17 is required in the androgen biosynthesis pathway, and its inhibition leads to decreased levels of DHT. The history and development of antiandrogen therapies are described more extensively in other reviews [81].
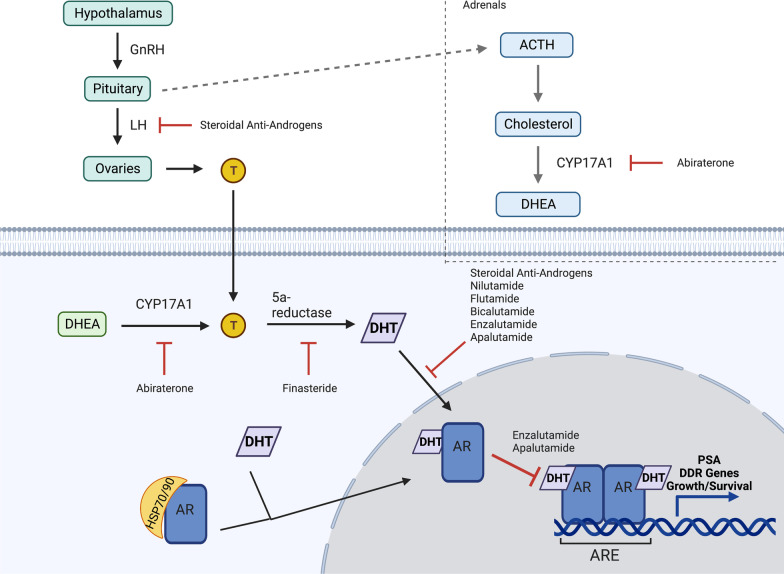
Fig. 1.
AR signalling pathways and therapeutic targets of AR. Androgens such as T are produced from cholesterol. CYP17A1 is the enzyme responsible for converting precursors to DHEA, and T is converted to DHT through 5a-reductase. DHT activates AR, resulting in its release from HSP70/90. AR then dimerizes and translocates to the nucleus where it can bind to AREs and modulate the transcription of target genes. Abiraterone irreversibly inhibits CYP17A1 activity. Bicalutamide and enzalutamide are AR antagonists that block the binding of DHT to AR. Enzalutamide also inhibits the nuclear translocation of AR. Abbreviations: GnRH—gonadotropin-releasing hormone, LH—luteinizing hormone, T—testosterone, ACTH—adrenocorticotropic hormone, DHEA—dehydroepiandrosterone, ARE—androgen response element, and PSA—prostate-specific antigen
Evidence of a role for androgens and AR in BC development and progression has led to considerable interest in AR as a potential therapeutic target. Antiandrogens such as bicalutamide and enzalutamide have shown early success in preclinical and clinical trials of antitumour response, and further trials have enrolled both ER– and ER+ anti-oestrogen-resistant patients [24, 82, 83]. The goal of using ADT as a therapy in these patients is to block the activation of AR and associated pathways such as ErbB that are involved in BC progression. However, anti-AR strategies in BC have yet to be established due to a lack of consistent positive trial data and, as detailed below, this can partly be explained by the biological complexity of AR signalling in BC.
In contrast to strongly AR+ TNBC, low-level AR expression has been associated with more aggressive forms of TNBC, suggesting that in at least some subtypes of BC, ADT may have a tumour-promoting effect [84]. Cochrane et al. presented clinical data that a high nuclear ratio of AR relative to ER in patients treated with tamoxifen (an anti-oestrogen therapy) predicted failure in therapy. They then went on to show that enzalutamide treatment decreased growth in both ER+ and ER−/AR+ tumours, suggesting a role for antiandrogens in hormone resistant cancers [85]. They were the first to show that androgen-targeted therapies could have clinical benefit in ER– BC, either alone or in combination with tamoxifen and/or AIs. They also suggest a role for targeting AR in recurrent ER+ BC, where selective targeting of the ER pathway could lead to the tumour cells switching to androgen dependence.
Clinically, however, data do not support AR as a biomarker for selecting anti-oestrogen therapy. Results from NCT00004205 show that in postmenopausal ER+ BC patients, AR expression did not predict treatment efficacy of anti-oestrogen therapy monotherapy. Alternatively, AR expression has been shown to be useful in predicting the efficacy of antiandrogen therapy, including AR positivity in TNBC predicting response to enzalutamide [86, 87]. While much attention has been focused on drugs that block androgens, research is also looking into the efficacy of 17α hydroxylase/17–20 lyase (CYP17) inhibitors (i.e. abiraterone) that block androgen, oestrogen and glucocorticoid synthesis. Early results from these trials show mixed responses and the studies are ongoing. These studies are covered in more detail by other reviews [24, 82, 83].
There are currently multiple clinical trials evaluating the use of AR antagonists in BC. In one study of AR+ TNBC, patients were treated with bicalutamide 150 mg daily. The results showed clinical benefit (defined as a complete or partial response or stable disease) of 19%, with a median progression-free survival of 12 weeks [49]. This study was the first proof that AR antagonist treatment is beneficial in AR+ TNBC. Additional phase II trials are ongoing testing bicalutamide and enzalutamide in AR+ TNBC, showing a clinical benefit of 20% for bicalutamide (NCT00468715) and 28–33% for enzalutamide [86]. Further studies are evaluating enzalutamide in combination with other therapeutics such as trastuzumab in AR+ /HER2+ BC (NCT02091960), and paclitaxel in early-stage AR+ TNBC (NCT02689427). Many of these trials show promise of alternate therapeutic options for TNBC beyond cytotoxic chemotherapy alone.
While most AR-targeted therapies are aimed at inhibiting signalling, there is evidence of AR activation producing growth repression. This has been well demonstrated in some PCas, especially those that have adapted to low androgen environment growth. In the case of PCas, there is typically a biphasic growth where either ADT or supraphysiologic androgens produce growth suppression [88, 89]. This has been replicated in BC exposed to high concentrations of oestrogen [90, 91]. Several mechanisms have been proposed for the observation of growth repression at high concentrations of androgens including cMyc activation and activation of negative regulators of the cell cycle [92]. High doses of androgens have been shown to induce DNA damage. This is consistent with previous reports that AR activation results in transient double-strand DNA breaks to release DNA topologies that inhibit the function of RNA polymerase during transcription [93]. Because of its ability to promote DNA damage, AR is also a promising target for combined therapeutics with radiation (discussed later). Due to the ability of AR to act as either a positive or negative regulator of cell growth and proliferation depending on the molecular features of the BC and the presence of oestrogens, both AR agonists and antagonists are being actively tested as potential therapies. While synthetic androgens have proven to have undesired side effects, selective AR modulators (SARMs) have promising preclinical effects on reducing tumour burden in ER+ BC and have fewer side effects [94]. Recently it was shown in a patient-derived xenograft (PDX) model of BC that treatment with a SARM, but not an AR antagonist, inhibited cell proliferation. This was further shown to occur through a reprogramming of the ER cistrome to an AR cistrome, likely through a FOXA1-dependent mechanism [95]. This was further corroborated by Hickey et al. findings that AR agonist treatment could be combined with standard of care in ER+ BC to enhance antitumour response. They showed that activation of AR in this context alters the genomic distribution of ER as well as other co-activators leading to decreased expression of ER-regulated cell cycle genes and upregulation of AR-regulated tumour suppressor genes [96]. Evaluation of SARMs in ER+ and AR+ BC is currently under further investigation in a phase II trial (NCT02463032).
AR and DNA damage repair
Steroid hormone receptors such as ER in BC are known to be involved in regulating the DNA damage response (DDR), and suppression of ER signalling with tamoxifen has been shown to augment RT response [97–99]. Goodwin et al. presented a mechanism by which this association between steroid hormones and DNA repair extends to AR activity in a PCa model [100]. In men with localized PCa, the standard of care is a combination of ADT with radiation, as the combination has been shown to be more effective than either treatment on its own [101–103]. AR activity allows for DNA DSB resolution, independent of cell cycling effects, by regulating some of the accessory factors necessary for DDR. AR acts as a transcriptional regulator of DDR factors, including DNA-PKcs, a kinase important in non-homologous end joining (NHEJ), to increase DNA repair and cell survival following DNA damage induction. Furthermore, DNA-PKcs then create a positive feedback circuit wherein AR is further activated to increase its transcription-activating potential [100]. Polkinghorn et al. confirmed this role of AR as a transcriptional regulator of DNA repair genes, and they showed that treatment with ADT decreases the expression of these genes [104]. Additional studies have also implicated a role for an AR-PARP1 signalling axis in breast cancer and have shown that combined inhibition of AR and PARP leads to enhanced antitumour activity by modulating the DDR [105, 106]. The implications for a role of AR in DDR make it an interesting target for combination therapeutics with RT, as AR inhibition should potentiate radiation-induced damage in a tumour-selective manner. It should also be noted, however, that while AR signalling allows for damage repair, supraphysiological androgens are associated with the induction of DSBs [107]. For this reason, supraphysiological androgens are also under evaluation as a method of augmenting radiation response. This is actively being studied in PCa, and while it may be useful in BC, its use may be limited by side effects seen in previous studies with androgen agonists in BC.
Radiation therapy and AR
RT along with pharmacotherapy and surgery continues to play an important role in the treatment of BC. RT following breast-conserving surgery halves the rate at which the disease recurs and reduces the BC death rate by approximately a sixth [108]. While this multimodal treatment approach has proven to be effective for many women, some will still go on to develop recurrent disease, including many women diagnosed with TNBC. Speers et al. showed that patients with TNBC who have AR expression above the median were more likely to have locoregional recurrence following RT; however, there was no difference in locoregional recurrence in the TNBC patients who were not treated with RT. Importantly, this was shown in retrospective data sets and therefore it cannot be assumed that the clinicopathological status of the patients in the RT-treated and non-RT-treated groups are comparable [109]. A retrospective study of BC found that following RT, relapsed tumours were more likely to express AR than non-relapsed tumours [110]. Additionally, Yard et al. found that within a subgroup of 28 BC cell lines from an RT resistance screen AR mRNA levels were correlated to relative RT resistance. They went on to show that AR+ BC cells that were treated with DHT before RT had less DNA damage than cells treated with enzalutamide before RT. They attributed this to increased activation of DNA-PKcs [111].
As previously discussed, TNBC is generally associated with poor survival despite RT and chemotherapy and these patients also suffer increased rates of locoregional recurrence [112–114]. Consequently, strategies that can improve responses to RT in these patients would be of significant clinical value. A subgroup of TNBC is known to express AR, and this group has been shown to be susceptible to targeting of the AR [48]. Clinical trials are currently underway to assess this blockade in patients with metastatic BC expressing AR (NCT00468715, NCT03055312, NCT00755885, NCT01889238, NCT02580448—clinicaltrials.gov). Further studies have demonstrated that targeting AR may be useful not only as a monotherapy, but as a means of radiosensitizing TNBC [100, 104, 109, 115]. It is suggested that inhibiting AR with enzalutamide in AR+ TNBC (as well as in PCa) can decrease the potential for DNA repair, leading to amplified damage from RT and ultimately cell death. This has been hypothesized to occur, in part, due to an abrogation of the expression of DNA-PKcs [100, 104, 109]. This radiosensitization has also been observed using seviteronel in AR+ TNBC, where radiosensitization was induced due to delayed DSB repair [116]. The potential for AR-targeting- therapies as a means of radiosensitizing TNBC is under active investigation and remains a promising therapeutic strategy for this subtype.
Conclusions
While AR continues to show promise as a biomarker and a therapeutic target in BC, its role in the modern-day management of patients remains uncertain. The previous sections illustrated that the prognostic value of AR may differ based on the clinical BC subtype (primarily based on PAM50 criteria). However, the precise association of AR positivity with prognosis remains unclear. One possible explanation for this may be that AR positivity, for instance as characterized by AR gene expression, does not accurately predict AR activity. Given the differences in biology and prognosis across BC subtypes, in order to accurately test the association between AR expression and activity, the method by which the BC subtypes are classified is of critical importance. To date, BC subtypes have primarily been characterized using PAM50 criteria which are molecularly stratified based on gene expression profiling and relate to oestrogen and progesterone hormone receptor status. While this classification system is useful, it has its limitations, namely substantial variation within groups and lack of representation of rarer subtypes [117, 118]. Based on the understanding that much of the gene expression landscape is driven by copy number alterations (CNAs), a new classification system for BC was developed [119].
The Molecular Taxonomy of Breast Cancer International Consortium (METABRIC, www.cbioportal.org) used a combination of gene expression and CNAs to stratify BC into 10 integrative clusters (IntClust/s). These IntClusts cover the subtypes defined using other approaches; however, they also include novel tumour subtypes [120, 121]. The IntClusts represent distinct clinical characteristics and response to therapeutics, and one may hypothesize that assessing AR positivity across clusters may help to resolve some of the conflicting prognostic significance seen when stratifying by PAM50 criteria. While more detailed in silico analysis, followed by validation in preclinical and clinical data sets is required to fully evaluate AR in BC IntClusts, we propose that the IntClust classification system may provide additional value in understanding the biological and clinical significance of AR expression in BC. This deeper understanding will support the characterization of BC subtypes in which AR status is more prognostic of outcome and help identify subtypes where therapeutically targeting AR may be most effective. Recent work has given more clarity on the clinical role of AR in ER+ BC; however, uncertainties remain in the other disease subtypes. It can be argued that understanding AR as it relates to the genomic landscape of BC, as classified by integrative clusters, would be a more nuanced way of stratifying patients for AR therapy. Alternatively, biomarkers that capture active AR signalling driving tumorigenesis/tumour maintenance may be necessary to faithfully identify patients most likely to benefit from AR-directed therapy. However, identifying and validating these biomarkers is unlikely to be trivial and may require interrogation of both transcriptional regulation and gene expression through methods such as chromatin immunoprecipitation (ChIP) and RNA sequencing. Targeting AR has shown promising results in multiple clinical trials, along with androgen-targeting therapies in combination with other therapies, such as tamoxifen in ER+ BC or RT in TNBC. Harnessing the full potential of targeting AR will require a more refined understanding of the role of AR in each subtype of BC.
Abbreviations
- ADT
Androgen deprivation therapy
- AI
Aromatase inhibitor
- AR
Androgen receptor
- ARE
Androgen response element
- BC
Breast cancer
- ChIP
Chromatin immunoprecipitation
- CNA
Copy number alteration
- CRPC
Castration-resistant prostate cancer
- CYP17
Cytochrome P17
- DDR
DNA damage repair
- DHEA
Dehydroepiandrosterone
- DHT
5A-dihydrotestosterone
- DSB
Double-strand break
- EGF
Epidermal growth factor
- ER
Oestrogen receptor alpha
- HER2
Human epidermal growth factor receptor 2
- IHC
Immunohistochemistry
- IntClust
Integrative cluster
- NHEJ
Non-homologous end joining
- PARP
Poly(ADP-ribose) polymerase
- PCa
Prostate cancer
- PgR
Progesterone receptor
- QNBC
Quadruple negative breast cancer
- RT
Radiation therapy
- SARM
Selective AR modulator
- T
Testosterone
- TNBC
Triple-negative breast cancer
Author contributions
All authors contributed equally to conception and revision of the manuscript. The manuscript was drafted by EK and SA. All authors approved the final version and agree to be accountable for all aspects of the work.
Funding
Not applicable.
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
All samples used in the METABRIC study were obtained with the consent of patients and appropriate approval from ethical committees (REC ref 07/H0308/161). The METABRIC study was performed in accordance with the Declaration of Helsinki. Detailed information about tissue collection can be found in the METABRIC publication [119].
Consent for publication
Not applicable.
Competing interests
CC is a member of AstraZeneca’s iMED External Science Panel, of Illumina’s Scientific Advisory Board, and is a recipient of research grants (administered by the University of Cambridge) from AstraZeneca, Genentech, Roche, and Servier. The other authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021. [DOI] [PubMed]
- 2.Koboldt DC, Fulton RS, McLellan MD, Schmidt H, Kalicki-Veizer J, McMichael JF, et al. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8. [DOI] [PubMed] [Google Scholar]
- 4.Collins LC, Cole KS, Marotti JD, Hu R, Schnitt SJ, Tamimi RM. Androgen receptor expression in breast cancer in relation to molecular phenotype: results from the Nurses’ Health Study. Mod Pathol. 2011;24(7):924–931. doi: 10.1038/modpathol.2011.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shang Y, Myers M, Brown M. Formation of the androgen receptor transcription complex. Mol Cell. 2002;9(3):601–610. doi: 10.1016/s1097-2765(02)00471-9. [DOI] [PubMed] [Google Scholar]
- 6.Jasuja R, Ramaraj P, Mac RP, Singh AB, Storer TW, Artaza J, et al. Delta-4-androstene-3,17-dione binds androgen receptor, promotes myogenesis in vitro, and increases serum testosterone levels, fat-free mass, and muscle strength in hypogonadal men. J Clin Endocrinol Metab. 2005;90(2):855–863. doi: 10.1210/jc.2004-1577. [DOI] [PubMed] [Google Scholar]
- 7.Handelsman DJ, Hirschberg AL, Bermon S. Circulating testosterone as the hormonal basis of sex differences in athletic performance. Endocr Rev. 2018;39(5):803–829. doi: 10.1210/er.2018-00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anestis A, Zoi I, Papavassiliou AG, Karamouzis MV. Androgen receptor in breast cancer-clinical and preclinical research insights. Molecules. 2020;25(2):358. doi: 10.3390/molecules25020358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Sawyers CL, Scher HI. Targeting the androgen receptor pathway in prostate cancer. Curr Opin Pharmacol. 2008;8(4):440–448. doi: 10.1016/j.coph.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou X. Roles of androgen receptor in male and female reproduction: lessons from global and cell-specific Androgen Receptor Knockout (ARKO) mice. J Androl. 2010;31(3):235–243. doi: 10.2164/jandrol.109.009266. [DOI] [PubMed] [Google Scholar]
- 11.Hickey TE, Robinson JLL, Carroll JS, Tilley WD. Minireview: the androgen receptor in breast tissues: growth inhibitor, tumor suppressor, oncogene? Mol Endocrinol. 2012;26(8):1252–1267. doi: 10.1210/me.2012-1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kono M, Fujii T, Lim B, Sri Karuturi M, Tripathy D, Ueno NT, et al. Androgen receptor function and androgen receptor–targeted therapies in breast cancer: a review. JAMA Oncol. 2017;3(9):1266–1273. doi: 10.1001/jamaoncol.2016.4975. [DOI] [PubMed] [Google Scholar]
- 13.Kensler KH, Regan MM, Heng YJ, Baker GM, Pyle ME, Schnitt SJ, et al. Prognostic and predictive value of androgen receptor expression in postmenopausal women with estrogen receptor-positive breast cancer: results from the Breast International Group Trial 1–98. Breast Cancer Res. 2019;21(1):1–11. doi: 10.1186/s13058-019-1118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumder A, Singh M, Tyagi SC. Post-menopausal breast cancer: from estrogen to androgen receptor. Oncotarget. 2017;8(60):102739. doi: 10.18632/oncotarget.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niemeier LA, Dabbs DJ, Beriwal S, Striebel JM, Bhargava R. Androgen receptor in breast cancer: expression in estrogen receptor-positive tumors and in estrogen receptor-negative tumors with apocrine differentiation. Mod Pathol. 2010;23(2):205–212. doi: 10.1038/modpathol.2009.159. [DOI] [PubMed] [Google Scholar]
- 16.Ricciardelli C, Bianco-Miotto T, Jindal S, Butler LM, Leung S, McNeil CM, et al. The magnitude of androgen receptor positivity in breast cancer is critical for reliable prediction of disease outcome. Clin Cancer Res. 2018;24(10):2328–2341. doi: 10.1158/1078-0432.CCR-17-1199. [DOI] [PubMed] [Google Scholar]
- 17.Secreto G, Girombelli A, Krogh V. Androgen excess in breast cancer development: implications for prevention and treatment. Endocr Relat Cancer. 2019;26(2):R81–R94. doi: 10.1530/ERC-18-0429. [DOI] [PubMed] [Google Scholar]
- 18.Casey RW, Wilson JD. Antiestrogenic action of dihydrotestosterone in mouse breast. Competition with estradiol for binding to the estrogen receptor. J Clin Investig. 1984;74(6):2272–2278. doi: 10.1172/JCI111654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peters AA, Ingman WV, Tilley WD, Butler LM. Differential effects of exogenous androgen and an androgen receptor antagonist in the peri- and postpubertal murine mammary gland. Endocrinology. 2011;152(10):3728–3737. doi: 10.1210/en.2011-1133. [DOI] [PubMed] [Google Scholar]
- 20.Missmer SA, Eliassen AH, Barbieri RL, Hankinson SE. Endogenous estrogen, androgen, and progesterone concentrations and breast cancer risk among postmenopausal women. J Natl Cancer Inst. 2004;96(24):1856–1865. doi: 10.1093/jnci/djh336. [DOI] [PubMed] [Google Scholar]
- 21.Key TJ, Appleby P, Barnes I, Reeves G, Dorgan JF, Longcope C, et al. Endogenous sex hormones and breast cancer in postmenopausal women: reanalysis of nine prospective studies. J Natl Cancer Inst. 2002;94(8):606–616. doi: 10.1093/jnci/94.8.606. [DOI] [PubMed] [Google Scholar]
- 22.Dimitrakakis C, Bondy C. Androgens and the breast. Breast Cancer Res. 2009;11(5):1–9. doi: 10.1186/bcr2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaaks R, Rinaldi S, Key TJ, Berrinno F, Peeters PHM, Biessy C, et al. Postmenopausal serum androgens, oestrogens and breast cancer risk: the European prospective investigation into cancer and nutrition. Endocr Relat Cancer. 2005;12(4):1071–1082. doi: 10.1677/erc.1.01038. [DOI] [PubMed] [Google Scholar]
- 24.Chia KM, O’Brien M, Brown M, Lim E. Targeting the androgen receptor in breast cancer. Curr Oncol Rep. 2015;17(2):1–6. doi: 10.1007/s11912-014-0427-8. [DOI] [PubMed] [Google Scholar]
- 25.Vranic S, Tawfik O, Palazzo J, Bilalovic N, Eyzaguirre E, Lee LM, et al. EGFR and HER-2/neu expression in invasive apocrine carcinoma of the breast. Mod Pathol. 2010;23(5):644–653. doi: 10.1038/modpathol.2010.50. [DOI] [PubMed] [Google Scholar]
- 26.Bernard PS, Parker JS, Mullins M, Cheung MCU, Leung S, Voduc D, et al. Supervised risk predictor of breast cancer based on intrinsic subtypes. J Clin Oncol. 2009;27(8):1160. doi: 10.1200/JCO.2008.18.1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park S, Koo JS, Kim MS, Park HS, Lee JS, Lee JS, et al. Androgen receptor expression is significantly associated with better outcomes in estrogen receptor-positive breast cancers. Ann Oncol. 2011;22(8):1755–1762. doi: 10.1093/annonc/mdq678. [DOI] [PubMed] [Google Scholar]
- 28.Hu R, Dawood S, Holmes MD, Collins LC, Schnitt SJ, Cole K, et al. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin Cancer Res. 2011;17(7):1867–1874. doi: 10.1158/1078-0432.CCR-10-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vera-Badillo FE, Templeton AJ, De Gouveia P, Diaz-Padilla I, Bedard PL, Al-Mubarak M, et al. Androgen receptor expression and outcomes in early breast cancer: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(1):319. doi: 10.1093/jnci/djt319. [DOI] [PubMed] [Google Scholar]
- 30.Peters AA, Buchanan G, Ricciardelli C, Bianco-Miotto T, Centenera MM, Harris JM, et al. Androgen receptor inhibits estrogen receptor-α activity and is prognostic in breast cancer. Cancer Res. 2009;69(15):6131–6140. doi: 10.1158/0008-5472.CAN-09-0452. [DOI] [PubMed] [Google Scholar]
- 31.Panet-Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000;167(1–2):139–150. doi: 10.1016/s0303-7207(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 32.Elebro K, Borgquist S, Simonsson M, Markkula A, Jirström K, Ingvar C, et al. Combined androgen and estrogen receptor status in breast cancer: treatment prediction and prognosis in a population-based prospective cohort. Clin Cancer Res. 2015;21(16):3640–3650. doi: 10.1158/1078-0432.CCR-14-2564. [DOI] [PubMed] [Google Scholar]
- 33.Bozovic-Spasojevic I, Zardavas D, Brohee S, Ameye L, Fumagalli D, Ades F, et al. The prognostic role of androgen receptor in patients with early-stage breast cancer: a metaanalysis of clinical and gene expression data. Clin Cancer Res. 2017;23(11):2702–2712. doi: 10.1158/1078-0432.CCR-16-0979. [DOI] [PubMed] [Google Scholar]
- 34.Harvell DME, Spoelstra NS, Singh M, McManaman JL, Finlayson C, Phang T, et al. Molecular signatures of neoadjuvant endocrine therapy for breast cancer: characteristics of response or intrinsic resistance. Breast Cancer Res Treat. 2008;112(3):475–488. doi: 10.1007/s10549-008-9897-4. [DOI] [PubMed] [Google Scholar]
- 35.Castoria G, Auricchio F, Migliaccio A. Extranuclear partners of androgen receptor: at the crossroads of proliferation, migration, and neuritogenesis. FASEB J. 2017;31(4):1289–1300. doi: 10.1096/fj.201601047R. [DOI] [PubMed] [Google Scholar]
- 36.Giovannelli P, Di Donato M, Giraldi T, Migliaccio A, Castoria G, Auricchio F. Targeting rapid action of sex steroid receptors in breast and prostate cancers. Front Biosci (Landmark Ed). 2011;16:453–461. doi: 10.2741/3849. [DOI] [PubMed] [Google Scholar]
- 37.Migliaccio A, Castoria G, De Falco A, Bilancio A, Giovannelli P, Di Donato M, et al. Polyproline and Tat transduction peptides in the study of the rapid actions of steroid receptors. Steroids. 2012;77(10):974–978. doi: 10.1016/j.steroids.2012.01.014. [DOI] [PubMed] [Google Scholar]
- 38.Migliaccio A, Varricchio L, De Falco A, Castoria G, Arra C, Yamaguchi H, et al. Inhibition of the SH3 domain-mediated binding of Src to the androgen receptor and its effect on tumor growth. Oncogene. 2007;26(46):6619–6629. doi: 10.1038/sj.onc.1210487. [DOI] [PubMed] [Google Scholar]
- 39.Migliaccio A, Di Domenico M, Castoria G, Nanayakkara M, Lombardi M, De Falco A, et al. Steroid receptor regulation of epidermal growth factor signaling through Src in breast and prostate cancer cells: Steroid antagonist action. Cancer Res. 2005;65(22):10585–10593. doi: 10.1158/0008-5472.CAN-05-0912. [DOI] [PubMed] [Google Scholar]
- 40.Ciupek A, Rechoum Y, Gu G, Gelsomino L, Beyer AR, Brusco L, et al. Androgen receptor promotes tamoxifen agonist activity by activation of EGFR in ERα-positive breast cancer. Breast Cancer Res Treat. 2015;154(2):225–237. doi: 10.1007/s10549-015-3609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Micello D, Marando A, Sahnane N, Riva C, Capella C, Sessa F. Androgen receptor is frequently expressed in HER2-positive, ER/PR-negative breast cancers. Virchows Arch. 2010;457(4):467–476. doi: 10.1007/s00428-010-0964-y. [DOI] [PubMed] [Google Scholar]
- 42.Ni M, Chen Y, Lim E, Wimberly H, Bailey STT, Imai Y, et al. Targeting androgen receptor in estrogen receptor-negative breast cancer. Cancer Cell. 2011;20(1):119–131. doi: 10.1016/j.ccr.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Li W, Zhang Y, Yuan X, Xu K, Yu J, et al. Androgen receptor regulates a distinct transcription program in androgen-independent prostate cancer. Cell. 2009;138(2):245–256. doi: 10.1016/j.cell.2009.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doane AS, Danso M, Lal P, Donaton M, Zhang L, Hudis C, et al. An estrogen receptor-negative breast cancer subset characterized by a hormonally regulated transcriptional program and response to androgen. Oncogene. 2006;25(28):3994–4008. doi: 10.1038/sj.onc.1209415. [DOI] [PubMed] [Google Scholar]
- 45.Garrido-Castro AC, Lin NU, Polyak K. Insights into molecular classifications of triple-negative breast cancer: improving patient selection for treatment. Cancer Discov. 2019;9(2):176–198. doi: 10.1158/2159-8290.CD-18-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang C, Pan B, Zhu H, Zhou Y, Mao F, Lin Y, et al. Prognostic value of androgen receptor in triple negative breast cancer: a meta-analysis. Oncotarget. 2016;7(29):46482. doi: 10.18632/oncotarget.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anders CK, Abramson V, Tan T, Dent R. The evolution of triple-negative breast cancer: from biology to novel therapeutics. Am Soc Clin Oncol Educ B. 2016;36:34–42. doi: 10.1200/EDBK_159135. [DOI] [PubMed] [Google Scholar]
- 48.Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Investig. 2011;121(7):2750–2767. doi: 10.1172/JCI45014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin Cancer Res. 2013;19(19):5505–5512. doi: 10.1158/1078-0432.CCR-12-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486(7403):395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Safarpour D, Pakneshan S, Tavassoli FA. Androgen receptor (AR) expression in 400 breast carcinomas: is routine AR assessment justified? Am J Cancer Res. 2014;4(4):353. [PMC free article] [PubMed] [Google Scholar]
- 52.Santonja A, Sánchez-Muñoz A, Lluch A, Chica-Parrado MR, Albanell J, Chacón JI, et al. Triple negative breast cancer subtypes and pathologic complete response rate to neoadjuvant chemotherapy. Oncotarget. 2018;9(41):26406. doi: 10.18632/oncotarget.25413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang HS, Kuang XY, Sun WL, Xu Y, Zheng YZ, Liu YR, et al. Androgen receptor expression predicts different clinical outcomes for breast cancer patients stratified by hormone receptor status. Oncotarget. 2016;7(27):41285. doi: 10.18632/oncotarget.9778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ogawa Y, Hai E, Matsumoto K, Ikeda K, Tokunaga S, Nagahara H, et al. Androgen receptor expression in breast cancer: relationship with clinicopathological factors and biomarkers. Int J Clin Oncol. 2008;13(5):431–435. doi: 10.1007/s10147-008-0770-6. [DOI] [PubMed] [Google Scholar]
- 55.Park S, Koo J, Park HS, Kim JH, Choi SY, Lee JH, et al. Expression of androgen receptors in primary breast cancer. Ann Oncol. 2009;21(3):488–492. doi: 10.1093/annonc/mdp510. [DOI] [PubMed] [Google Scholar]
- 56.Mrklić I, Pogorelić Z, Ćapkun V, Tomić SŽ. Expression of androgen receptors in triple negative breast carcinomas. Acta Histochem. 2013;115(4):344–348. doi: 10.1016/j.acthis.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Rakha EA, El-Sayed ME, Green AR, Lee AHS, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109(1):25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 58.Sutton LM, Cao D, Sarode V, Molberg KH, Torgbe K, Haley B, et al. Decreased androgen receptor expression is associated with distant metastases in patients with androgen receptor-expressing triple-negative breast carcinoma. Am J Clin Pathol. 2012;138(4):511–516. doi: 10.1309/AJCP8AVF8FDPTZLH. [DOI] [PubMed] [Google Scholar]
- 59.Masuda H, Baggerly KA, Wang Y, Zhang Y, Gonzalez-Angulo AM, Meric-Bernstam F, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin Cancer Res. 2013;19(19):5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Thike AA, Chong LYZ, Cheok PY, Li HH, Yip GWC, Bay BH, et al. Loss of androgen receptor expression predicts early recurrence in triple-negative and basal-like breast cancer. Mod Pathol. 2014;27(3):352–360. doi: 10.1038/modpathol.2013.145. [DOI] [PubMed] [Google Scholar]
- 61.Astvatsaturyan K, Yue Y, Walts AE, Bose S. Androgen receptor positive triple negative breast cancer: clinicopathologic, prognostic, and predictive features. PLoS ONE. 2018;13(6):e0197827. doi: 10.1371/journal.pone.0197827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Agrawal AK, Jeleń M, Grzebieniak Z, Zukrowski P, Rudnicki J, Nienartowicz E. Androgen receptors as a prognostic and predictive factor in breast cancer. Folia Histochem Cytobiol. 2008;46(3):269–276. doi: 10.2478/v10042-008-0039-y. [DOI] [PubMed] [Google Scholar]
- 63.Gonzalez LO, Corte MD, Vazquez J, Junquera S, Sanchez R, Alvarez AC, et al. Androgen receptor expresion in breast cancer: relationship with clinicopathological characteristics of the tumors, prognosis, and expression of metalloproteases and their inhibitors. BMC Cancer. 2008;8:1–10. doi: 10.1186/1471-2407-8-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Luo X, Shi YX, Li ZM, Jiang WQ. Expression and clinical significance of androgen receptor in triple negative breast cancer. Chin J Cancer. 2010;29(6):585–590. doi: 10.5732/cjc.009.10673. [DOI] [PubMed] [Google Scholar]
- 65.Castellano I, Allia E, Accortanzo V, Vandone AM, Chiusa L, Arisio R, et al. Androgen receptor expression is a significant prognostic factor in estrogen receptor positive breast cancers. Breast Cancer Res Treat. 2010;124(3):607–617. doi: 10.1007/s10549-010-0761-y. [DOI] [PubMed] [Google Scholar]
- 66.Loibl S, Müller BM, Von Minckwitz G, Schwabe M, Roller M, Darb-Esfahani S, et al. Androgen receptor expression in primary breast cancer and its predictive and prognostic value in patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat. 2011;130(2):477–487. doi: 10.1007/s10549-011-1715-8. [DOI] [PubMed] [Google Scholar]
- 67.Yu Q, Niu Y, Liu N, Zhang JZ, Liu TJ, Zhang RJ, et al. Expression of androgen receptor in breast cancer and its significance as a prognostic factor. Ann Oncol. 2011;22(6):1288–1294. doi: 10.1093/annonc/mdq586. [DOI] [PubMed] [Google Scholar]
- 68.Honma N, Horii R, Iwase T, Saji S, Younes M, Ito Y, et al. Clinical importance of androgen receptor in breast cancer patients treated with adjuvant tamoxifen monotherapy. Breast Cancer. 2013;20(4):323–330. doi: 10.1007/s12282-012-0337-2. [DOI] [PubMed] [Google Scholar]
- 69.Tokunaga E, Hisamatsu Y, Taketani K, Yamashita N, Akiyoshi S, Okada S, et al. Differential impact of the expression of the androgen receptor by age in estrogen receptor-positive breast cancer. Cancer Med. 2013;2(6):763–773. doi: 10.1002/cam4.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tsang JYS, Ni YB, Chan SK, Shao MM, Law BKB, Tan PH, et al. Androgen receptor expression shows distinctive significance in ER positive and negative breast cancers. Ann Surg Oncol. 2014;21(7):2218–2228. doi: 10.1245/s10434-014-3629-2. [DOI] [PubMed] [Google Scholar]
- 71.Aleskandarany MA, Abduljabbar R, Ashankyty I, Elmouna A, Jerjees D, Ali S, et al. Prognostic significance of androgen receptor expression in invasive breast cancer: transcriptomic and protein expression analysis. Breast Cancer Res Treat. 2016;159(2):215–227. doi: 10.1007/s10549-016-3934-5. [DOI] [PubMed] [Google Scholar]
- 72.Bronte G, Bravaccini S, Ravaioli S, Puccetti M, Scarpi E, Andreis D, et al. Androgen receptor expression in breast cancer: what differences between primary tumor and metastases? Transl Oncol. 2018;11(4):950–956. doi: 10.1016/j.tranon.2018.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Candelaria RP, Adrada BE, Wei W, Thompson AM, Santiago L, Lane DL, et al. Imaging features of triple-negative breast cancers according to androgen receptor status. Eur J Radiol. 2019;114:167–174. doi: 10.1016/j.ejrad.2019.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xiang G, Liu F, Liu J, Meng Q, Li N, Niu Y. Prognostic role of Amphiregulin and the correlation with androgen receptor in invasive breast cancer. Pathol Res Pract. 2019;215(6):152414. doi: 10.1016/j.prp.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 75.Zhao S, Ma D, Xiao Y, Li X, Ma J, Zhang H, et al. Molecular subtyping of triple-negative breast cancers by immunohistochemistry: molecular basis and clinical relevance. Oncologist. 2020;25(10):e1481–e1491. doi: 10.1634/theoncologist.2019-0982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mills AM, Gottlieb CE, Wendroth SM, Brenin CM, Atkins KA. Pure apocrine carcinomas represent a clinicopathologically distinct androgen receptor-positive subset of triple-negative breast cancers. Am J Surg Pathol. 2016;40(8):1109–1116. doi: 10.1097/PAS.0000000000000671. [DOI] [PubMed] [Google Scholar]
- 77.Gatalica Z. Immunohistochemical analysis of apocrine breast lesions. Pathol Res Pract. 1997;193(11–12):753–758. doi: 10.1016/S0344-0338(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 78.Sun X, Zuo K, Yao Q, Zhou S, Shui R, Xu X, et al. Invasive apocrine carcinoma of the breast: clinicopathologic features and comprehensive genomic profiling of 18 pure triple-negative apocrine carcinomas. Mod Pathol. 2020;33(12):2473–2482. doi: 10.1038/s41379-020-0589-x. [DOI] [PubMed] [Google Scholar]
- 79.Zhang N, Zhang H, Chen T, Yang Q. Dose invasive apocrine adenocarcinoma has worse prognosis than invasive ductal carcinoma of breast: evidence from SEER database. Oncotarget. 2017;8(15):24579. doi: 10.18632/oncotarget.15597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Robinson JLL, MacArthur S, Ross-Innes CS, Tilley WD, Neal DE, Mills IG, et al. Androgen receptor driven transcription in molecular apocrine breast cancer is mediated by FoxA1. EMBO J. 2011;30(15):3019–3027. doi: 10.1038/emboj.2011.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Student S, Hejmo T, Poterała-Hejmo A, Leśniak A, Bułdak R. Anti-androgen hormonal therapy for cancer and other diseases. Eur J Pharmacol. 2020;866:172783. doi: 10.1016/j.ejphar.2019.172783. [DOI] [PubMed] [Google Scholar]
- 82.Proverbs-Singh T, Feldman JL, Morris MJ, Autio KA, Traina TA. Targeting the androgen receptor in prostate and breast cancer: several new agents in development. Endocr Relat Cancer. 2015;22(3):R87–R106. doi: 10.1530/ERC-14-0543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rahim B, O’Regan R. AR signaling in breast cancer. Cancers (Basel). 2017;9(3):21. doi: 10.3390/cancers9030021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McNamara KM, Yoda T, Takagi K, Miki Y, Suzuki T, Sasano H. Androgen receptor in triple negative breast cancer. J Steroid Biochem Mol Biol. 2013;133(1):66–76. doi: 10.1016/j.jsbmb.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 85.Cochrane DR, Bernales S, Jacobsen BM, Cittelly DM, Howe EN, D’Amato NC, et al. Role of the androgen receptor in breast cancer and preclinical analysis of enzalutamide. Breast Cancer Res. 2014;16(1):1–19. doi: 10.1186/bcr3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J, et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J Clin Oncol. 2018;36(9):884. doi: 10.1200/JCO.2016.71.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anestis A, Sarantis P, Theocharis S, Zoi I, Tryfonopoulos D, Korogiannos A, et al. Estrogen receptor beta increases sensitivity to enzalutamide in androgen receptor-positive triple-negative breast cancer. J Cancer Res Clin Oncol. 2019;145(5):1221–1233. doi: 10.1007/s00432-019-02872-9. [DOI] [PubMed] [Google Scholar]
- 88.Kokontis JM, Hay N, Liao S. Progression of LNCaP prostate tumor cells during androgen deprivation: hormone-independent growth, repression of proliferation by androgen, and role for p27Kip1 in androgen-induced cell cycle arrest. Mol Endocrinol. 1998;12(7):941–953. doi: 10.1210/mend.12.7.0136. [DOI] [PubMed] [Google Scholar]
- 89.Schweizer MT, Antonarakis ES, Wang H, Ajiboye AS, Spitz A, Cao H, et al. Effect of bipolar androgen therapy for asymptomatic men with castration-resistant prostate cancer: results from a pilot clinical study. Sci Transl Med. 2015;7(269):269ra2. doi: 10.1126/scitranslmed.3010563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Reddel RR, Sutherland RL. Effects of pharmacological concentrations of estrogens on proliferation and cell cycle kinetics of human breast cancer cell lines in vitro. Cancer Res. 1987;47(20):5323–5329. [PubMed] [Google Scholar]
- 91.Song RXD, Mor G, Naftolin F, McPherson RA, Song J, Zhang Z, et al. Effect of long-term estrogen deprivation on apoptotic responses of breast cancer cells to 17β-estradiol. J Natl Cancer Inst. 2001;93(22):1714–1723. doi: 10.1093/jnci/93.22.1714. [DOI] [PubMed] [Google Scholar]
- 92.Mohammad OS, Nyquist MD, Schweizer MT, Balk SP, Corey E, Plymate S, et al. Supraphysiologic testosterone therapy in the treatment of prostate cancer: models, mechanisms and questions. Cancers (Basel). 2017;9(12):166. doi: 10.3390/cancers9120166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Haffner MC, Aryee MJ, Toubaji A, Esopi DM, Albadine R, Gurel B, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42(8):668–675. doi: 10.1038/ng.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Narayanan R, Ahn S, Cheney MD, Yepuru M, Miller DD, Steiner MS, et al. Selective androgen receptor modulators (SARMs) negatively regulate triple-negative breast cancer growth and epithelial: mesenchymal stem cell signaling. PLoS ONE. 2014;9(7):e103202. doi: 10.1371/journal.pone.0103202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ponnusamy S, Asemota S, Schwartzberg LS, Guestini F, McNamara KM, Pierobon M, et al. Androgen receptor is a non-canonical inhibitor of wild-type and mutant estrogen receptors in hormone receptor-positive breast cancers. iScience. 2019;21:341–358. doi: 10.1016/j.isci.2019.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hickey TE, Selth LA, Chia KM, Laven-Law G, Milioli HH, Roden D, et al. The androgen receptor is a tumor suppressor in estrogen receptor–positive breast cancer. Nat Med. 2021;27(2):310–320. doi: 10.1038/s41591-020-01168-7. [DOI] [PubMed] [Google Scholar]
- 97.Allred DC, Anderson SJ, Paik S, Wickerham DL, Nagtegaal ID, Swain SM, et al. Adjuvant tamoxifen reduces subsequent breast cancer in women with estrogen receptor-positive ductal carcinoma in situ: a study based on NSABP protocol B-24. J Clin Oncol. 2012;30(12):1268. doi: 10.1200/JCO.2010.34.0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Medunjanin S, Weinert S, Schmeisser A, Mayer D, Braun-Dullaeus RC. Interaction of the double-strand break repair kinase DNA-PK and estrogen receptor-alpha. Mol Biol Cell. 2010;21(9):1620–1628. doi: 10.1091/mbc.E09-08-0724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Medunjanin S, Weinert S, Poitz D, Schmeisser A, Strasser RH, Braun-Dullaeus RC. Transcriptional activation of DNA-dependent protein kinase catalytic subunit gene expression by oestrogen receptor-alpha. EMBO Rep. 2010;11(3):208–213. doi: 10.1038/embor.2009.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Goodwin JF, Schiewer MJ, Dean JL, Schrecengost RS, de Leeuw R, Han S, et al. A hormone-DNA repair circuit governs the response to genotoxic insult. Cancer Discov. 2013;3(11):1254–1271. doi: 10.1158/2159-8290.CD-13-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bolla M, Gonzalez D, Warde P, Dubois JB, Mirimanoff RO, Storme G, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337(5):295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 102.Widmark A, Klepp O, Solberg A, Damber JE, Angelsen A, Fransson P, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 103.Warde P, Mason M, Ding K, Kirkbride P, Brundage M, Cowan R, et al. Combined androgen deprivation therapy and radiation therapy for locally advanced prostate cancer: a randomised, phase 3 trial. Lancet. 2011;378(9809):2104–2111. doi: 10.1016/S0140-6736(11)61095-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Polkinghorn WR, Parker JS, Lee MX, Kass EM, Spratt DE, Iaquinta PJ, et al. Androgen receptor signaling regulates DNA repair in prostate cancers. Cancer Discov. 2013;3(11):1245–1253. doi: 10.1158/2159-8290.CD-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Min A, Jang H, Kim S, Lee KH, Kim DK, Suh KJ, et al. Androgen receptor inhibitor enhances the antitumor effect of PARP inhibitor in breast cancer cells by modulating DNA damage response. Mol Cancer Ther. 2018;17(12):2507–2518. doi: 10.1158/1535-7163.MCT-18-0234. [DOI] [PubMed] [Google Scholar]
- 106.Luo J, Jin J, Yang F, Sun Z, Zhang W, Shi Y, et al. The correlation between PARP1 and BRCA1 in AR positive triple-negative breast cancer. Int J Biol Sci. 2016;12(12):1500. doi: 10.7150/ijbs.16176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ju B-G, Lunyak VV, Perissi V, Garcia-Bassets I, Rose DW, Glass CK, et al. A topoisomerase IIbeta-mediated dsDNA break required for regulated transcription. Science. 2006;312(5781):1798–1802. doi: 10.1126/science.1127196. [DOI] [PubMed] [Google Scholar]
- 108.Darby S, McGale P, Correa C, Taylor C, Arriagada R, Clarke M, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. Lancet. 2011;378(9804):1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Speers C, Zhao SG, Chandler B, Liu M, Wilder-Romans K, Olsen E, et al. Androgen receptor as a mediator and biomarker of radioresistance in triple-negative breast cancer. NPJ Breast Cancer. 2017;3(1):1–10. doi: 10.1038/s41523-017-0038-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ravaioli S, Tumedei MM, Foca F, Maltoni R, Rocca A, Massa I, et al. Androgen and oestrogen receptors as potential prognostic markers for patients with ductal carcinoma in situ treated with surgery and radiotherapy. Int J Exp Pathol. 2017;98(5):289–295. doi: 10.1111/iep.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yard BD, Adams DJ, Chie EK, Tamayo P, Battaglia JS, Gopal P, et al. A genetic basis for the variation in the vulnerability of cancer to DNA damage. Nat Commun. 2016;7:1–14. doi: 10.1038/ncomms11428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lowery AJ, Kell MR, Glynn RW, Kerin MJ, Sweeney KJ. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133(3):831–841. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 113.Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 114.Kyndi M, Sørensen FB, Knudsen H, Overgaard M, Nielsen HM, Overgaard J. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26(9):1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 115.Spratt DE, Evans MJ, Davis BJ, Doran MG, Lee MX, Shah N, et al. Androgen receptor upregulation mediates radioresistance after ionizing radiation. Cancer Res. 2015;75(22):4688–4696. doi: 10.1158/0008-5472.CAN-15-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Michmerhuizen AR, Chandler B, Olsen E, Wilder-Romans K, Moubadder L, Liu M, et al. Seviteronel, a novel CYP17 lyase inhibitor and androgen receptor antagonist, radiosensitizes AR-positive triple negative breast cancer cells. Front Endocrinol (Lausanne). 2020;11:35. doi: 10.3389/fendo.2020.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Badve S, Dabbs DJ, Schnitt SJ, Baehner FL, Decker T, Eusebi V, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24(2):157–167. doi: 10.1038/modpathol.2010.200. [DOI] [PubMed] [Google Scholar]
- 118.Weigelt B, Geyer FC, Reis-Filho JS. Histological types of breast cancer: how special are they? Mol Oncol. 2010;4(3):192–208. doi: 10.1016/j.molonc.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Curtis C, Shah SP, Chin SF, Turashvili G, Rueda OM, Dunning MJ, et al. The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature. 2012;486(7403):346–352. doi: 10.1038/nature10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rueda OM, Sammut SJ, Seoane JA, Chin SF, Caswell-Jin JL, Callari M, et al. Dynamics of breast-cancer relapse reveal late-recurring ER-positive genomic subgroups. Nature. 2019;567(7748):399–404. doi: 10.1038/s41586-019-1007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pereira B, Chin SF, Rueda OM, Vollan HKM, Provenzano E, Bardwell HA, et al. The somatic mutation profiles of 2,433 breast cancers refines their genomic and transcriptomic landscapes. Nat Commun. 2016;7:1–16. doi: 10.1038/ncomms11479. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.