Abstract
Haemophilus ducreyi, the causative agent of chancroid, produces a hemolysin, whose role in virulence is not well defined. To assess the possible role of hemolysin in pathogenesis, we evaluated its target cell range by using wild-type H. ducreyi 35000, nonhemolytic mutants with the hemolysin structural gene deleted, and isogenic strains expressing different amounts of hemolytic activity. The cytotoxicity of the various cell types was assessed by quantitating the release of lactate dehydrogenase into culture supernatants as a measure of cell lysis. In these experiments, human foreskin fibroblasts, human foreskin epithelial cells, and, to a lesser extent, HEp-2 cells were lysed by H. ducreyi hemolysin. Hemolysin also lysed human blood mononuclear cells and immune system cell lines including U937 macrophage-like cells, T lymphocytes, and B lymphocytes. In contrast, human polymorphonuclear leukocytes were not sensitive to hemolysin under the conditions tested. We also analyzed the effect of hemolysin on invasion of human epithelial cells and found that H. ducreyi strains expressing cloned hemolysin genes showed a 10-fold increase in invasion compared to the control strain. These data support the hypothesis that the H. ducreyi hemolysin is important in the pathogenesis of chancroid and may contribute to ulcer formation, invasion of epithelial cells, and evasion of the immune response.
Haemophilus ducreyi is a gram-negative bacterium that causes the sexually transmitted genital ulcer disease chancroid. Chancroid ulcers are painful and can persist for several months if untreated, exposing the patient to secondary infections (20). Inguinal lymphoadenopathy occurs in up to 50% of chancroid patients (20), and chancroid ulcers have been associated with increased heterosexual transmission of human immunodeficiency virus (6, 25, 45). Chancroid is most common in developing countries, although outbreaks occur in the United States, especially among individuals of lower socioeconomic status (43). Diagnosis of chancroid can be difficult since ulcers often resemble those of syphilis or herpes and isolation of H. ducreyi from lesions is frequently unsuccessful (43). For these reasons, the incidence of chancroid is probably higher than is currently recognized.
The initiation and progression of chancroid lesions has been studied by using human and animal models of chancroid (28, 34, 35, 40, 43). Three to ten days after inoculation with H. ducreyi, a papule develops, which either resolves or progresses to form a pustule. The pustule eventually ulcerates and involves cells of both the epidermis and the dermis (20). Histopathological analysis demonstrates that typical chancroid lesions consist of a deep necrotic ulcer containing disintegrating epithelial cells (20) and an infiltrate of polymorphonuclear leukocytes (PMNs), Langerhans’ cells, macrophages, and CD4+ T cells (34, 35). Despite the presence of these inflammatory cells, the lesions persist if untreated and viable H. ducreyi can be recovered. Several potential virulence factors, including lipooligosaccharide (7), cytolethal distending toxin (9, 29), fine tangled pili (5), and a cell-associated hemolysin (22, 41), have been identified, although their contribution to the pathogenesis of chancroid is not well understood.
Expression of the cell-associated hemolysin of H. ducreyi requires two adjacent genes, hhdB and hhdA (23), which are similar to the hemolysins of Serratia marcescens, Edwardsiella tarda, and Proteus mirabilis (23, 27, 44). The S. marcescens hemolysin, encoded by shlB and shlA, is the most thoroughly characterized of this group of hemolysins. ShlB is an outer membrane protein, which is required for secretion and activation of the hemolysin structural protein, ShlA (4, 32). Once secreted, ShlA interacts with target cell membranes, oligomerizes, and forms pores 2.5 to 3.0 nm in diameter, which lyse the target cell (33).
The effect of the H. ducreyi hemolysin on some human cell types has been previously examined (2, 21). These studies demonstrated the cytotoxic effect of the H. ducreyi hemolysin on human foreskin fibroblasts (HFFs) by using H. ducreyi 35000 and isogenic transposon mutants with insertions in hhdB (2, 11, 21). In the present study, we examined the range of cell types with which the hemolysin can interact by using a mutant with a deletion in hhdA and cloned hemolysin genes expressing different amounts of hemolysin. We found that in addition to its action on erythrocytes (RBCs) and HFFs, the hemolysin can lyse other cell types relevant to chancroid including human foreskin epithelial cells (HFEs) and immune system cells such as macrophages, T cells and B cells. In addition, we found that hemolysin enhances the invasion of HEp-2 epithelial cells. These results are consistent with a role for hemolysin in tissue destruction and/or immune system avoidance in chancroid ulcers.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are described in Table 1 and Fig. 1. Human cell lines were obtained from the American Type Culture Collection (Manassas, Va.).
TABLE 1.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli HB101 | Noninvasive strain, negative control | 18 |
| S. typhimurium SL1344 | Highly invasive strain, positive control | 16 |
| H. influenzae Rd | Nonhemolytic, noncytotoxic, negative control, ATCC 33391 | M. Roberts, University of Washington |
| H. ducreyi | ||
| 35000 | Wild type, hemolytic | 41 |
| 35000ΔAPC | 35000 hhdA::cat, nonhemolytic, Cmr | This study |
| Plasmids | ||
| pPT376 | pUC19 with hhdA, Apr | 41 |
| pBluescript KS(−) | Cloning vector, Apr | Stratagene |
| pBluescript SK(−) | Cloning vector, Apr | Stratagene |
| pPT376KS | hhdA on pBluescript, Apr | This study |
| pPT376ΔPst | pPT376KS with internal hhdA PstI fragment replaced with BamHI linkers, Apr | This study |
| pUC-ΔEcat | Source of cat cassette, Cmr | B. Green, Lederle-Praxis, Rochester, N.Y. (13) |
| pPT376ΔPstCm | pPT376ΔPst with hhdA::cat, Apr Cmr | This study |
| pLS88 | H. ducreyi shuttle vector, Kmr Smr | 48 |
| pBFD | Deletion derivative of pLS88, Smr | This study |
| pCR2.1 | TA cloning vector, Apr Kmr | Invitrogen, San Diego, Calif. |
| pLSSK | H. ducreyi shuttle vector, Smr | This study |
| pLSKS | H. ducreyi shuttle vector, Smr | This study |
| pBABN | hhdBA on pUC19 | 11 |
| pLSBA+ | hhdBA on pLSSK, Smr | This study |
| pLSBA+2 | hhdBA on pLSSK in same orientation as lac promoter, Smr | This study |
| pLSBAHis | hhdBA fused to His tag on pLSSK | 11 |
| pLSB+ | pLSSK with hhdB in same orientation as lac promoter, Smr | This study |
| pPT384-TZ18 | pTZ18R with hhdBA in same orientation as lac promoter, Apr | 41 |
| pLS384− | hhdBA insert of pPT384-TZ18 in pLSKS opposite the lac promoter, Smr | This study |
| pLS384-2 | hhdBA on pLSSK in opposite orientation of lac promoter, Smr | This study |
| pLS376− | hhdA on pLSSK in opposite orientation of lac promoter, Smr | This study |
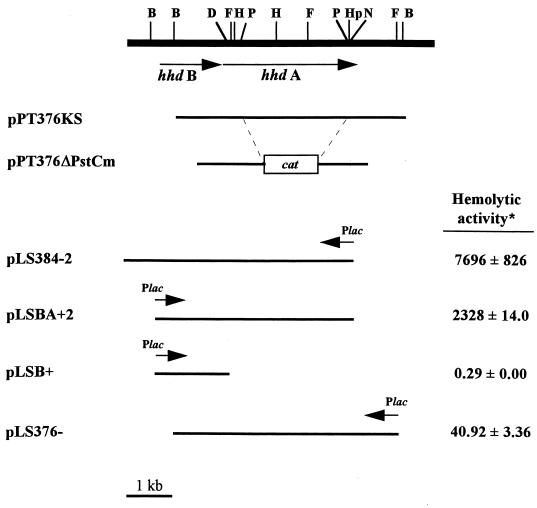
FIG. 1.
Plasmids used in this study and their hemolytic activities in 35000ΔAPC. ∗, data is expressed as a percentage of the hemolytic activity of strain 35000; the mean of a typical experiment performed in duplicate is shown with standard error. The experiment was repeated at least three times with similar results. The hemolytic activities of 35000ΔAPC and 35000ΔAPC(pLSSK) were 0.13% ± 0.00% and 0.66% ± 0.52%, respectively. B, BglII; D, DraIII; F, FspI; H, HindIII; Hp, HpaI; N, NcoI; P, PstI.
Media and growth conditions.
H. ducreyi strains were maintained as frozen suspensions in 50% glycerol at −70°C and cultured on chocolate agar with enrichment (PML Microbiologicals, Wilsonville, Oreg.) at 35°C in candle jars for 48 h. Liquid cultures were grown aerobically in Hd broth (42) at 35°C with shaking for 16 to 20 h. Mid-logarithmic-phase cultures were prepared by diluting overnight cultures 1:5 in fresh Hd broth and incubating them for 4 to 5 h with shaking at 35°C. Antibiotic concentrations for H. ducreyi were 50 μg of streptomycin per ml, 1 to 2 μg of chloramphenicol per ml, and 10 μg of ampicillin per ml. Escherichia coli and Salmonella typhimurium cultures were grown in Luria-Bertani (LB) broth or on LB agar plates (31) supplemented, when appropriate, with 100 μg of streptomycin per ml, 30 μg of chloramphenicol per ml, or 100 μg of ampicillin per ml.
Manipulation of DNA.
Standard techniques were used for the isolation and manipulation of plasmid and chromosomal DNA (31). Restriction enzymes, T4 DNA ligase, T4 DNA polymerase, and oligonucleotide primers were purchased from Gibco BRL Life Technologies (Gaithersburg, Md.); deoxynucleoside triphosphates (dNTPs) were purchased from Promega (Madison, Wis.).
Construction of an isogenic, hemolysin-deficient H. ducreyi mutant.
Strain 35000ΔAPC, a hemolysin-deficient mutant of strain 35000, was constructed as follows. First, the 5.2-kb SstI-SalI insert of pPT376 (41) containing part of hhdB and all of hhdA was cloned into pBluescript KS(−) (Stratagene). The resulting plasmid, pPT376KS, was digested with PstI to delete a 2.6-kb internal fragment of hhdA, treated with T4 DNA polymerase to produce blunt ends, and then ligated to BamHI linkers (Promega), creating pPT376ΔPst. The 1.2-kb BamHI fragment of pUC-ΔEcat containing the chloramphenicol acetyltransferase (cat) gene was then cloned into the BamHI site of pPT376ΔPst to produce pPT376ΔPstCm. Thus, the hhdA::cat construct on pPT376ΔPstCm expresses only the first 182 amino acids of HhdA. Plasmid pPT376ΔPstCm was introduced into wild-type H. ducreyi 35000 by electroporation, and transformants were selected on charcoal agar plates containing 1 μg of chloramphenicol per ml. A total of 23 transformants were obtained, of which 10 were nonhemolytic on bilayer horse blood agar plates (41). Of the 10 nonhemolytic transformants, 7 were subjected to analysis by PCR with primers A start and rev A end (Table 2) under the following conditions: 2 min at 92°C, followed by 30 cycles of 45 s at 92°C, 1 min at 63°C, and 5 min at 68°C, and ending with 7 min at 68°C, in a reaction mixture containing 50 mM Tris (pH 9.2), 16 mM (NH4)2SO4, 5 mM MgCl2, 0.5 mM total dNTPs, 0.3 μM (each) primer, and 2.5 U of Long Extend Polymerase (Boehringer Mannheim, Indianapolis, Ind.). A 3.6-kb PCR product was amplified from wild-type hhdA, while a product of 2.2 kb was obtained for the hhdA::cat allele. PCR products of 3.6 and 2.2 kb were amplified from six of the seven clones tested, indicating that they resulted from single crossovers and contained both wild-type hhdA and hhdA::cat; these were discarded. A single product of 2.2 kb was amplified from the seventh clone, indicating replacement of wild-type hhdA with the hhdA::cat allele. This clone was named 35000ΔAPC and was subjected to Southern blot analysis, which confirmed deletion of the wild-type hhdA allele (data not shown). 35000ΔAPC is nonhemolytic both on bilayer horse blood agar plates and in liquid hemolysin assays and can be complemented with hhdA (data not shown).
TABLE 2.
Primers used in this study
| Primer name | Sequencea | Nucleotide recognition site (accession no.) |
|---|---|---|
| A start | 5′-gga tcc aaG GAG ATA CAT ATA TGA AAA AAT GGA-3′ | 1885–1909 (U32175) |
| rev A end | 5′-gtc gac TCG AAT GGC CAT CTT AGC ATC GAC-3′ | 5398–5421 (U32175) |
| 88R | 5′-aga tca aga tcT GCA ATG CAA GGT CGC CAA CAC-3′ | 2991–3010 (L23118) |
| 88BFD | 5′-aga tca aga tct CTG CCG CAC AGC TCC ATA GG-3′ | 4711–4730 (L23118) |
| 5′BlueMCS | 5′-gat gat cAG CGG ATA ACA ATT TCA CAC AGG-3′ | 827–846 (X52324) |
| 3′BlueMCS | 5′-gat gat cAC GCT TAC AAT TTC CAT TCG C-3′ | 451–470 (X52324) |
| NcoF | 5′-ATG CAA ATG GTA TAA AAG TTA G-3′ | 5077–5099 (U32175) |
| A end Sal | 5′-gtc gac CCA CTT ACA TCA ATC-3′ | 5576–5591 (U32175) |
Primer sequences homologous to the template are shown in capital letters, non-homologous sequences are in lowercase letters, and restriction sites incorporated into primers to facilitate the cloning of PCR products are underlined.
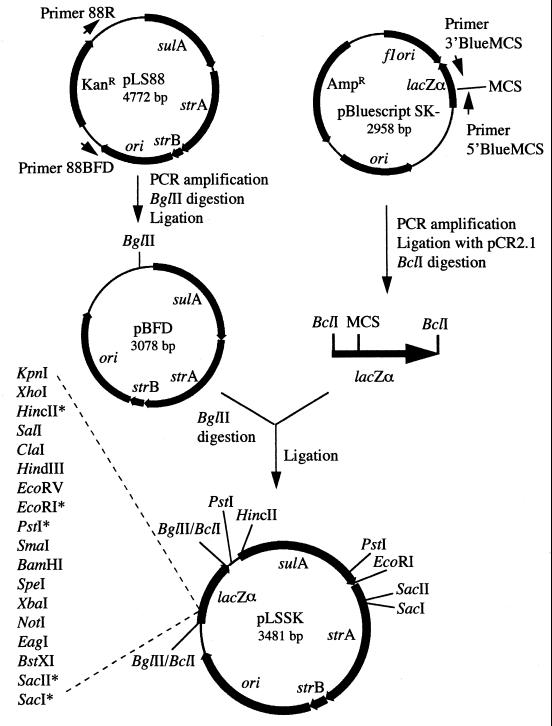
Construction of pLSSK and pLSKS.
Plasmids pLSSK and pLSKS, derivatives of pLS88 (48), were constructed as described below and in Fig. 2. First, a 3-kb PCR product was amplified from pLS88 with primers 88R and 88BFD (Table 2) under the following conditions: 3 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at 55°C, and 1 min at 72°C, and ending with 10 min at 72°C, in a reaction mixture containing 2.5 mM MgCl2, 10 mM Tris (pH 8.3), 50 mM KCl, 0.5 mM (total) dNTPs, 0.36 μM (each) primer, and 0.5 U of Taq polymerase (Sigma, St. Louis, Mo.). The PCR product was digested with BglII and self-ligated to form plasmid pBFD. pBFD thus contains the origin of replication and genes for streptomycin and sulfonamide resistance. Next, the lacZα gene and the multiple-cloning site of pBluescript SK(−) (Stratagene) were PCR amplified with primers 5′BlueMCS and 3′BlueMCS (Table 2) under the conditions described above, except that 5 mM MgCl2 and 0.10 μM primers were used. This 441-bp PCR product was cloned into pCR2.1 with the TA cloning kit as specified by the manufacturer (Invitrogen). The resulting plasmid was digested with BclI and ligated into the BglII site of pBFD, generating pLSSK. Plasmid pLSKS was constructed similarly, except that the lacZα gene and multiple-cloning site was amplified from pBluescript KS(−). Therefore, pLSKS is identical to pLSSK, except that the multiple-cloning site is reversed. Both pLSSK and pLSKS are similar to pLS88 in that they encode streptomycin and sulfonamide resistance and replicate in both E. coli and H. ducreyi; however, they have the additional advantages that they are smaller, contain a multiple-cloning site, and allow blue-white color selection in E. coli.
FIG. 2.
Construction of pLSSK and pLSKS. pLSSK carries streptomycin resistance, sulfonamide resistance, lacZα, and the multiple-cloning site of pBluescript SK(−). Plasmid pLSKS contains the multiple-cloning site of pBluescript KS(−) and is therefore identical to pLSSK, except that the multiple-cloning site is reversed in lacZα. Details of the construction are described in Materials and Methods. Asterisks mark restriction sites in the multiple-cloning site (MCS) that are not unique.
Construction of hemolysin-encoding plasmids.
The plasmids used in this study are shown in Table 1 and Fig. 1, and their construction is described below. Plasmid pLSBA+2 was derived from pBABN, whose construction is described elsewhere (11). Briefly, pBABN carries hhdBA, wherein hhdB has been altered so that it has the E. coli consensus ribosome binding site and hhdA lacks the native stop codon. The entire 5.2-kb fragment was cloned into the BamHI-SalI sites of pLSSK so that transcription proceeds from the vector-encoded lac promoter. The resulting plasmid was named pLSBA+. To restore the native stop codon to hhdA on pLSBA+, a 334-bp PCR product was amplified from H. ducreyi 35000 chromosomal DNA with primers NcoF and A end Sal (Table 2) under the following conditions: 2 min at 94°C, followed by 30 cycles of 1 min at 94°C, 1 min at 55°C, and 2 min at 72°C, and ending with 7 min at 72°C, in a reaction mixture containing 50 mM Tris (pH 9.2), 16 mM (NH4)2SO4, 5 mM MgCl2, 0.5 mM (total) dNTPs, 0.3 μM (each) primer, and 2.5 U of Taq DNA polymerase (Promega). This 334-bp product was sequenced to confirm that no mutations had been introduced during amplification (data not shown), digested with HpaI and SalI, and used to replace the corresponding fragment of pLSBA+ (the HpaI site is within the PCR fragment). The resulting plasmid, named pLSBA+2 (Fig. 1), contains hhdBA and 167 bp of DNA downstream of the hhdA stop codon. Since pLSBA+2 lacks sequence upstream of hhdBA, expression proceeds from the vector-encoded lac promoter.
Plasmid pLSB+ (Fig. 1) was derived from pLSBAHis (11). Plasmid pLSBAHis was digested with HindIII and then religated to form pLSB+. Thus, pLSB+ contains hhdB expressed from the vector-encoded lac promoter and an E. coli consensus ribosome binding site, and the first 290 bp of hhdA on pLSSK.
pLS384-2 (Fig. 1) is identical to pLSBA+2, except that it contains the region 5′ to hhdB, which is believed to contain the hhdB promoter and has the native ribosome binding site (23). pLS384-2 was constructed from pLS384− (not shown), which contains the 7.0-kb SmaI-SalI fragment of pPT384-TZ18 (41) in pLSKS. pLS384− thus carries ∼1.0 kb of DNA downstream of hhdA. To delete this downstream DNA, the 3′ HpaI-SalI fragment of pLS384− was replaced with a smaller, PCR-amplified fragment containing only 167 bp of DNA downstream of hhdA in the same manner as described above for pLSBA+2. The resulting plasmid was named pLS384-2.
pLS376− was constructed by cloning the 5.8-kb SalI-SmaI fragment of pPT376 (41) into pLSKS. pLS376− thus contains the C-terminal 470 bp of hhdB and the full-length hhdA genes transcribed opposite the vector-encoded lac promoter.
Liquid hemolysin assays.
Quantitative hemolysin assays were performed on logarithmic-phase cultures (1.5 ml), which were microcentrifuged for 1 min and then resuspended in 1 ml of 0.85% NaCl–10 mM CaCl2. Serial twofold dilutions were made in 96-well round-bottom plates in the same buffer, and washed horse RBCs (ca. 1% [wt/vol]) were added to each well. After a 1-h incubation at 37°C, the plate was centrifuged for 10 min at 500 × g, 100 μl of the supernatant was removed, and the absorbance at 540 nm was determined. All hemolytic values are the average of duplicate samples and represent the dilution of the test culture (at OD540 = 1.0) that lyses 50% of the RBCs. This hemolytic activity was determined by using the following equation: hemolytic activity = percent lysis/(50 × OD540 × D). Percent lysis was determined by comparison to RBCs lysed with distilled water (total lysis), OD540 is the optical density of the test culture at 540 nm, and D is the dilution of the test culture. The hemolytic activity of the strains varied from experiment to experiment (up to threefold), but the relative amounts of hemolytic activity were consistent between strains within experiments.
Cell culture.
HEp-2 (ATCC CCL-23), U937 (ATCC CRL-1593), Daudi (ATCC CCL-213), Jurkat (ATCC TIB-152), and Raji (ATCC CCL-86) cells were cultured, at 37°C in a humidified atmosphere of 5% CO2 and 95% air, in RPMI 1640 medium supplemented with glutamine (ICN Biomedicals, Inc., Aurora, Ohio), sodium bicarbonate (Sigma), and 10% fetal bovine serum (Gibco BRL Life Technologies). Primary cultures of HFEs and HFFs were obtained as previously described (3) and maintained in keratinocyte serum-free medium (KSFM; Gibco BRL Life Technologies) or RPMI 1640 medium, respectively. Twice weekly, HEp-2, HFF, or HFE monolayers were treated with a solution of trypsin-EDTA (Gibco BRL Life Technologies) to produce a single-cell suspension and then plated at a dilution of 1:20 in fresh medium. U937, Daudi, Jurkat, and Raji cells were also split twice weekly by being diluted 1:20 in fresh medium. To induce adherence, U937 cells were treated for 24 h with 250 ng of phorbol myristate acetate (Sigma) per ml of culture medium. Adherent U937 cells were washed once with balanced salt solution, scraped into culture medium with a glass rod, washed once in fresh culture medium, and then used in the cytotoxicity assay as described below.
Isolation of monocytes and PMNs from human blood.
Fresh venous blood (30 ml) from healthy volunteers was collected in EDTA-treated tubes and immediately processed for isolation of mononuclear cells and PMNs in using MonoPoly Resolving Medium (ICN Biomedicals, Inc.) as specified by the manufacturer. Purified cells were stained to confirm morphology by using the Diff-Quik stain set (Dade Diagnostics, Aguada, P.R.). PMN preparations consisted of >99% PMNs; mononuclear cell preparations were mixtures of monocytes, lymphocytes, and platelets. Cells were used in cytotoxicity assays immediately after isolation.
Cytotoxicity assays.
Approximately 4 h before each assay, 2 × 104 (HEp-2, HFF, and HFE) or 5 × 104 (U937, Daudi, Raji, Jurkat, mononuclear cells, and PMNs) cells were plated in 96-well flat-bottom plates in RPMI 1640 medium–1% fetal bovine serum or KSFM as appropriate. Overnight cultures of H. ducreyi strains were diluted 1:5 in Hd broth (42) and incubated at 35°C for 5 h with shaking. These bacterial samples (1.5 ml) were microcentrifuged for 1 min and then resuspended in RPMI 1640 medium–1% fetal bovine serum or KSFM (1 ml); the culture densities were similar between experiments. Dilutions (1:5 to 1:500) of each bacterial sample were made, and 100 μl was added to quadruplicate wells. The plate was centrifuged for 10 min at 150 × g and then incubated at 37°C in humidified 5% CO2–95% air for 3 h. A 50-μl volume of the supernatant was tested for lactate dehydrogenase (LDH) activity by the CytoTox 96 nonradioactive cytotoxicity assay (Promega) as specified by the manufacturer. The normalized LDH activity was calculated in the same manner as the hemolytic activities described above. Complete lysis of target cells for controls was achieved by freezing and thawing (HEp-2 cells) or treatment with Triton X-100 (all other cell types). Liquid hemolysin assays were performed on test bacterial cultures at the same time as the cytotoxicity assays.
Invasion and adherence assays.
HEp-2 cells were seeded into 24-well plates at a density of 5 × 105 to 1 × 106 cells per well and incubated for 16 to 20 h. Overnight cultures of H. ducreyi were diluted 1:5 in Hd broth and incubated with shaking for 4 to 5 h at 35°C. The cultures were then microcentrifuged for 1 min and resuspended in cell culture medium to an approximate OD540 of 1.0. Overnight cultures of E. coli HB101 were diluted 1:200 in LB broth and grown with shaking at 35°C. S. typhimurium SL1344 was grown overnight in LB broth at 37°C without shaking and then diluted 1:25 in LB broth and incubated for 4 to 5 h without shaking. SL1344 and HB101 were microcentrifuged for 1 min and then resuspended in culture medium to an approximate OD540 of 0.1. Each bacterial suspension was then diluted 1:10 in culture medium, and 100 μl of this dilution was added to triplicate wells of HEp-2 cells. The plate was centrifuged for 10 min at 150 × g and then incubated at 37°C in humidified 5% CO2–95% air for 1 h. The wells were washed three times with warm phosphate-buffered saline and then incubated for 1 h with 1 ml of culture medium containing 30 μg of gentamicin. Three washes with phosphate-buffered saline were performed, and then HEp-2 cells were lysed with trypsin-EDTA. Released bacteria were diluted in LB broth and plated on chocolate agar (H. ducreyi) or LB agar (HB101 and SL1344). All strains were similar in their sensitivity to gentamicin (data not shown).
Adherence of H. ducreyi strains to HEp-2 cells was measured as previously described (39), except that adherence was measured after a 90-min incubation.
Statistical methods.
Student’s t distribution was used to determine P values for differences between sample means (37).
RESULTS
Development of the cytotoxicity assay.
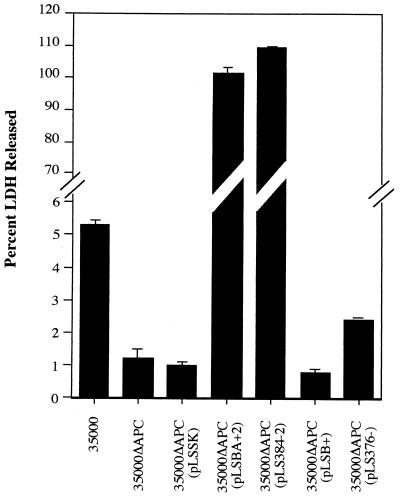
The goal of this study was to determine the target cell range of the H. ducreyi cell-associated hemolysin. However, previous studies have suggested that the target cell range of the secreted cytolethal distending toxin might overlap that of the hemolysin, making it difficult to determine the contribution of each toxin separately (21). Since hemolysin is poorly expressed in E. coli (11), we developed an assay to measure cytotoxicity in H. ducreyi, which would minimize the possible effects of the cytolethal distending toxin or other unidentified toxins. In this assay, H. ducreyi cultures were grown to the mid-logarithmic phase to induce hemolysin expression, the bacterial cells were washed to reduce the amount of secreted cytolethal distending toxin introduced into the assay mixture, and LDH activity was measured after only 3 h of coincubation with target cells. Initial experiments to evaluate this assay were performed with HFFs since previous studies have shown that the hemolysin is cytotoxic to these cells (2, 21). To confirm these observations, we constructed a mutant of H. ducreyi 35000 by replacing hhdA with hhdA::cat via allelic exchange (see Materials and Methods and Fig. 1). The resulting mutant was named 35000ΔAPC and is nonhemolytic both on bilayer horse blood agar plates and in liquid hemolysin assays (data not shown). Cytotoxicity assays with these strains demonstrated that strain 35000 caused the release of approximately 5% of the total cellular LDH from HFFs while 35000ΔAPC caused the release of only 1% of the LDH (Fig. 3), confirming previous observations that hemolysin is a fibroblast contact cytotoxin (2, 21). While this difference was statistically significant (P < 0.001), the low levels of LDH released suggested that this assay may be insufficiently sensitive to detect the activity of hemolysin on other cell types.
FIG. 3.
Hemolysin is cytotoxic to HFFs. H. ducreyi strains (ca. 5 × 107 CFU) were incubated with HFFs (2 × 104) for 3 h, and the LDH activity present in culture supernatants was measured. For each strain, the mean of a typical experiment performed in triplicate is shown with standard errors. The experiment was repeated at least three times with similar results.
To improve the sensitivity of the cytotoxicity assay, we sought ways to increase hemolysin expression by H. ducreyi. Hemolysin expression in other organisms (e.g., E. tarda [15] and S. marcescens [26]) is increased when the iron concentration in the culture medium is reduced. We had previously shown that hemolysin expression was optimal in the logarithmic phase in Hd broth (41), but the complex nature of this and other media for growth of H. ducreyi limits our ability to control the amount of iron and other nutrients which might affect hemolysin expression. As an alternative, we constructed plasmids from which cloned hemolysin genes were expressed. This was facilitated by the construction of a pair of H. ducreyi shuttle vectors (pLSSK and pLSKS) that were derived from pLS88 (Fig. 2). These vectors are smaller than pLS88, contain a multiple-cloning site, and allow blue-white screening in E. coli (Fig. 2). Using these cloning vectors, we constructed pLSBA+2 and pLS384-2, which express ca. 20- to 80-fold more hemolytic activity than strain 35000 does (Fig. 1). Both plasmids carry full-length hhdBA but differ in their upstream sequences: pLS384-2 extends 704 bp upstream of hhdB, a region believed to contain the promoter (23, 41), while pLSBA+2 expresses hhdBA from the vector-encoded lac promoter (Fig. 1).
Strain 35000ΔAPC with the hemolysin-expressing plasmids pLSBA+2 or pLS384-2 caused the release of large amounts of LDH (≥100% compared to lysis by Triton X-100) from HFFs, substantially more than that released by the control consisting of vector alone [35000ΔAPC(pLSSK)] (Fig. 3). Thus, a short (3-h) coincubation of H. ducreyi strains expressing cloned hemolysin genes with HFFs was cytotoxic, similar to results obtained with wild-type hemolysin expression and long incubation times (24 h) (2, 21). To rule out the possibility that HhdB caused the observed cytotoxicity, we constructed a plasmid (pLSB+, Fig. 1) which carries only hhdB. As expected, 35000ΔAPC(pLSB+) was neither hemolytic (Fig. 1) nor cytotoxic, causing the release of <1% of the LDH, comparable to the control 35000ΔAPC(pLSSK) (Fig. 3). In contrast, pLS376−, which carries only hhdA, expressed ca. 40% of the hemolytic activity of the wild-type strain 35000 (Fig. 1) and restored a corresponding level of cytotoxicity to 35000ΔAPC (Fig. 3). Because the levels of hemolytic activity and cytotoxicity were very low for this strain, 35000ΔAPC(pLS376−) was excluded from further cytotoxicity assays.
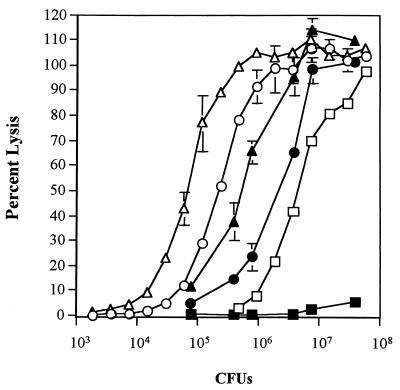
To determine if LDH release correlated with hemolytic activity, dose-response experiments were performed with 35000ΔAPC(pLSBA+2) and 35000ΔAPC(pLS384-2) compared to the wild-type strain 35000 (Fig. 4). In these experiments, lysis of HFFs paralleled lysis of RBCs for 35000ΔAPC(pLSBA+2) and 35000ΔAPC(pLS384-2) at all bacterial cell concentrations, demonstrating a correlation between hemolytic activity and cytotoxicity. These experiments also demonstrated that horse RBCs are more sensitive than HFFs to lysis by hemolysin-producing H. ducreyi strains even though hemolysis was measured after only a 1-h incubation whereas LDH release was measured after a 3-h incubation. This difference was not due to the different buffers used for the cytotoxicity and hemolysin assays, since the hemolytic activity of the strains was similar in both media (data not shown). Strain 35000 was not as cytotoxic as expected from its hemolytic activity, perhaps suggesting that (i) a threshold number of hemolysin molecules are needed to lyse HFFs, (ii) HFFs are able to repair damage caused by the lower levels of hemolysin, or (iii) inhibition or degradation of hemolysin by other cellular factors can be overcome with higher levels of hemolysin.
FIG. 4.
Dose-dependent lysis of RBCs and HFFs by hemolysin-producing H. ducreyi. Symbols: ▵, 35000ΔAPC(pLS384-2) versus RBCs; ▴, 35000ΔAPC(pLS384-2) versus HFFs; ○, 35000ΔAPC(pLSBA+2) versus RBCs; ●, 35000ΔAPC(pLSBA+2) versus HFFs; □, 35000 versus RBCs; ■, 35000 versus HFFs. Dilutions of the strains indicated were made and incubated with washed horse RBCs or HFFs for 1 or 3 h, respectively. The percent lysis of RBCs and HFFs was determined as described in Materials and Methods. 35000ΔAPC(pLSSK) was not cytotoxic or hemolytic at any of the dilutions tested (data not shown). For each combination, the mean of a typical experiment is shown with standard errors. Hemolysis was measured in duplicate; LDH release was measured in triplicate. Three repetitions yielded similar results.
H. ducreyi hemolysin is cytotoxic for human epithelial cells.
Our cytotoxicity assay with strains expressing cloned hemolysin genes and short incubation times was consistent with previous results showing that wild-type levels of hemolysin are cytotoxic to HFFs after a 24-h cocultivation (2, 21). We therefore expanded our analysis of the target cell range of hemolysin to other epidermal cells including a human epithelial cell line (HEp-2) and primary cultures of HFEs (Table 3). To compare the relative cytotoxicity between experiments and cell types, LDH release was adjusted to 50% and corrected for slight differences in the turbidity of test cultures (normalized LDH release [see Materials and Methods]). For comparison, the data demonstrating cytotoxicity of hemolysin for HFFs (Fig. 3) are recalculated and presented in Table 3 as normalized LDH release. In these experiments, HFEs and HEp-2 cells were lysed by 35000ΔAPC(pLSBA+2) or 35000ΔAPC(pLS384-2) but not by 35000ΔAPC(pLSSK), similar to the results with HFFs. Strain 35000 did not cause significant cytotoxicity for HFEs or HEp-2 cells in this assay with short incubation times. Significantly, 35000ΔAPC and 35000ΔAPC(pLSSK) were not cytotoxic in these assays, indicating that LDH release due to other H. ducreyi products including cytolethal distending toxin was not significant in our assay, even though the cytolethal distending toxin acts on HEp-2 cells during extended incubations (9, 29). As expected, 35000ΔAPC complemented with hhdB alone (pLSB+) was not cytotoxic.
TABLE 3.
H. ducreyi hemolysin is cytotoxic to fibroblasts and human epithelial cells
| Strain | Normalized LDH releasea from:
|
||
|---|---|---|---|
| HFF | HFE | HEp-2 cells | |
| 35000 | 0.08 ± 0.00 | 0.20 ± 0.01 | 0.10 ± 0.01 |
| 35000ΔAPC | 0.02 ± 0.01 | 0.18 ± 0.03 | 0.05 ± 0.01 |
| 35000ΔAPC(pLSSK) | 0.02 ± 0.00 | 0.17 ± 0.01 | 0.05 ± 0.01 |
| 35000ΔAPC(pLSBA+2) | 19.25 ± 3.49 | 28.37 ± 8.69 | 2.74 ± 0.20 |
| 35000ΔAPC(pLS384-2) | 59.78 ± 2.85 | 88.70 ± 13.46 | 3.98 ± 0.25 |
| 35000ΔAPC(pLSB+) | 0.01 ± 0.00 | 0.14 ± 0.04 | 0.08 ± 0.04 |
| 35000ΔAPC(pLS376−) | 0.04 ± 0.00 | NDb | ND |
Values represent normalized LDH release (see Materials and Methods) and are the average of quadruplicate samples (HFFs were assayed in triplicate) ± standard error. The results of a typical experiment are shown; the experiment was repeated at least three times with similar results.
N.D., not determined.
H. ducreyi hemolysin lyses human immune system cells.
We next assessed the ability of the H. ducreyi hemolysin to lyse human immune system cells by using both cells freshly isolated from human blood and several immune system cell lines. Human PMNs and mononuclear cells were separated by density gradient centrifugation and analyzed for their susceptibility to hemolysin (Table 4). In these experiments, cytotoxicity attributable to hemolysin was observed in the mononuclear cell fraction, which contained monocytes, lymphocytes, and platelets. Both 35000ΔAPC(pLSBA+2) and 35000ΔAPC(pLS384-2) were cytotoxic with normalized LDH values of 1.52 and 44.53, respectively, consistent with their relative hemolytic activities. As expected, 35000ΔAPC, 35000ΔAPC(pLSB+), and 35000ΔAPC(pLSSK) were not cytotoxic and the amount of hemolysin produced by strain 35000 in these experiments was not sufficient to cause cytotoxicity to mononuclear cells (Table 4). In contrast to the results seen with mononuclear cells, the low levels of LDH released from PMNs incubated with 35000ΔAPC(pLSBA+2) and 35000ΔAPC(pLS384-2) suggested that this cell type is relatively insensitive to lysis by hemolysin under these experimental conditions (Table 4).
TABLE 4.
H. ducreyi hemolysin is cytotoxic to mononuclear cells but not PMNs
| Strain | Normalized LDH releasea from:
|
|
|---|---|---|
| Mononuclear cells | PMNs | |
| 35000 | 0.00 ± 0.02 | 0.26 ± 0.22 |
| 35000ΔAPC | 0.12 ± 0.03 | 0.41 ± 0.06 |
| 35000ΔAPC(pLSSK) | 0.00 ± 0.11 | 0.32 ± 0.25 |
| 35000ΔAPC(pLSBA+2) | 1.52 ± 0.11 | 0.18 ± 0.20 |
| 35000ΔAPC(pLS384-2) | 44.53 ± 4.46 | 0.85 ± 0.14 |
| 35000ΔAPC(pLSB+) | 0.00 ± 0.25 | 0.15 ± 0.22 |
Values represent normalized LDH release and are the average of quadruplicate samples ± standard error. The results of a typical experiment are shown; the experiment was repeated twice with mononuclear cells and three times with PMNs with similar results.
To expand the observation that H. ducreyi hemolysin lyses blood mononuclear cells, cytotoxicity assays were also performed with immune system cell lines including human macrophage-like cells (U937), T lymphocytes (Jurkat cells), and B lymphocytes (Daudi and Raji cells) (Table 5). In these experiments, U937 macrophage-like cells were lysed by 35000ΔAPC(pLSBA+2) and 35000ΔAPC(pLS384-2) but not by 35000ΔAPC with vector alone (pLSSK) or hhdB alone (pLSB+). The normalized LDH values for 35000ΔAPC(pLSBA+2) and 35000ΔAPC(pLS384-2) were 5.67 and 69.62, respectively, compared to 0.18 for 35000ΔAPC(pLSSK) (P < 0.001). The wild-type strain 35000 was only slightly cytotoxic (0.35 ± 0.05) for this cell line. Similar results were obtained with T lymphocytes (Jurkat cells), with hemolysin-expressing clones causing significantly more LDH release than the control with vector alone. Among the B-lymphocyte lines, Daudi cells were more sensitive to lysis by hemolysin than were Raji cells. Both cell lines released similar amounts of LDH after incubation with 35000ΔAPC(pLSBA+2). However, Daudi cells were ca. sevenfold more sensitive to lysis by 35000ΔAPC(pLS384-2) than Raji cells were. Both cell lines were minimally affected by the hemolysin levels produced by strain 35000.
TABLE 5.
H. ducreyi hemolysin is cytotoxic to human immune system cell lines
| Strain | Normalized LDH releasea from:
|
|||
|---|---|---|---|---|
| U937 cells | Jurkat cells | Daudi cells | Raji cells | |
| 35000 | 0.35 ± 0.05 | 1.08 ± 0.07 | 0.21 ± 0.01 | 0.12 ± 0.01 |
| 35000ΔAPC | 0.08 ± 0.02 | 0.07 ± 0.00 | 0.09 ± 0.02 | 0.08 ± 0.01 |
| 35000ΔAPC(pLSSK) | 0.18 ± 0.07 | 0.04 ± 0.01 | 0.06 ± 0.03 | 0.03 ± 0.02 |
| 35000ΔAPC(pLSBA+2) | 5.67 ± 0.30 | 73.63 ± 2.83 | 1.91 ± 0.18 | 1.16 ± 0.19 |
| 35000ΔAPC(pLS384-2) | 69.62 ± 10.42 | 225.95 ± 17.22 | 30.09 ± 0.12 | 4.23 ± 0.23 |
| 35000ΔAPC(pLSB+) | 0.17 ± 0.04 | 0.02 ± 0.05 | 0.06 ± 0.02 | 0.06 ± 0.02 |
Values represent normalized LDH release and are the average of quadruplicate samples ± standard error. The results of a typical experiment are shown; the experiment was repeated at least three times with similar results.
Since hemolysin expression of the strains varied slightly between experiments, we determined the relative sensitivity of the cell types to hemolysin by calculating the ratio of hemolytic activity (data not shown) to normalized LDH (Table 6). For simplicity, this analysis was restricted to a single hemolysin-producing strain, 35000ΔAPC(pLS384-2). In this analysis, we found that T cells were the most sensitive to hemolysin-mediated lysis (i.e., had the lowest ratio of hemolytic activity to normalized LDH) followed by U937 macrophage-like cells, HFEs, HFFs, and Daudi cells. Raji and HEp-2 cells were only modestly affected by hemolysin, while PMNs were not lysed by any of the strains under these conditions.
TABLE 6.
Ratios of hemolytic activity to normalized LDH release indicate relative sensitivity to lysis by hemolysin
| Cell type | Ratio (hemolytic activity/nLDH)a |
|---|---|
| Jurkat | 2.21 |
| U937 | 4.85 |
| HFE | 5.63 |
| HFF | 13.40 |
| Daudi | 16.60 |
| HEp-2 | 92.52 |
| Raji | 118.06 |
Ratios for 35000ΔAPC(pLS384-2) are shown for representative experiments. Hemolytic activities were determined in duplicate; normalized LDH release (nLDH) was determined in quadruplicate. Small numbers indicate relatively high sensitivity to lysis by hemolysin.
H. ducreyi hemolysin enhances invasion of human epithelial cells.
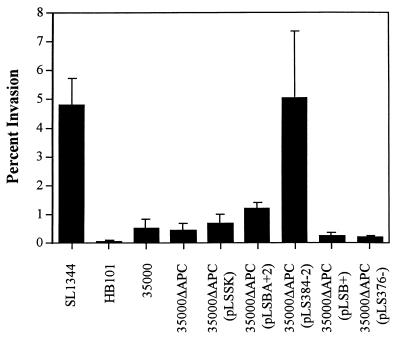
Since H. ducreyi has previously been found to invade HEp-2 cells and HFEs (39), we examined the role of hemolysin in invasion of HEp-2 cells by the hemolysin-deficient mutant, strains expressing cloned hemolysin genes, and strain 35000 (Fig. 5). Low multiplicities of infection (1 to 10 CFU per epithelial cell) and short incubation times (1 h) were used to minimize cytotoxicity to HEp-2 cells by the hemolysin. The highly invasive S. typhimurium SL1344 (16) and the noninvasive E. coli HB101 served as positive and negative controls, respectively. Invasion of HEp-2 cells by 35000 ranged from 0.25 to 0.95% of the inoculum, consistent with previous results (39). Invasion by 35000ΔAPC was not significantly different from invasion by 35000, 35000ΔAPC(pLSSK), or 35000ΔAPC(pLS376−). Similarly, invasion by strain 35000ΔAPC(pLSBA+2) was not significantly different from invasion by 35000 or 35000ΔAPC(pLSSK) (P < 0.10); however, 35000ΔAPC(pLS384-2) invaded significantly more than 35000(pLSSK) (P = 0.05), at levels similar to those of SL1344. Coinfection experiments showed that 35000ΔAPC(pLS384-2) did not enhance uptake of HB101, suggesting that increases in invasion were specific to the bacterial cell producing hemolysin (data not shown). Furthermore, plasmid pLSB+ did not affect invasion by 35000ΔAPC, indicating that HhdB alone plays no direct role in the invasion of these cells (Fig. 5).
FIG. 5.
H. ducreyi hemolysin enhances invasion of human epithelial cells. The strains indicated were tested for invasion by using a gentamicin protection assay at a multiplicity of infection of approximately 1 bacterium per epithelial cell. The data shown is from a single experiment performed in triplicate; bars indicate standard error. The experiment was repeated at least three times with similar results.
Hemolysin may enhance invasion simply by increasing the adherence of strains to cells and allowing other virulence factors better access to cell membranes. To examine this possibility, we measured the adherence of 35000, 35000ΔAPC, 35000ΔAPC(pLSSK), and 35000ΔAPC(pLS384-2) to HEp-2 cells and found that all four strains adhered at similar levels (data not shown). These results suggest that the hemolysin is not acting as an adhesin in these experiments but instead acts at a different step in the invasion process.
DISCUSSION
In this study, we found that the target cell range of the H. ducreyi cell-associated hemolysin includes T cells, macrophages, HFEs, HFFs, and B cells. T cells and macrophages were the cell types most affected by hemolysin, followed by HFEs, HFFs, B cells, and HEp-2 cells, while PMNs were relatively insensitive to lysis by hemolysin in our experiments. In addition, we showed that hemolysin expression enhances the invasion of H. ducreyi into HEp-2 cells and that this invasion is specific for H. ducreyi, since hemolysin-producing strains did not allow the invasion of E. coli in coinfection experiments.
In addition to expanding the cell range of hemolysin on clinically relevant cell types, our experiments differed in several ways from previous studies showing the cytotoxicity of hemolysin for HFFs and HaCaT cells (2, 21). First, previous studies (2, 11, 21) used mutants with mutations in hhdB rather than hhdA to demonstrate differences in wild-type and hemolysin mutant activity. The hhdA gene encodes the hemolysin structural protein based on the hemolytic activity of purified HhdA (11) and the homology of hhdA to the S. marcescens shlA hemolysin gene (23, 27, 41). HhdB is probably involved in secretion and activation of HhdA, similar to its homologue, the S. marcescens shlB gene (4, 32). While HhdB activity may be specific to HhdA, it is also possible that this protein affects the secretion and/or activation of other, as yet unidentified, proteins of H. ducreyi. Second, we used quantitative LDH release assays, cloned hemolysin genes, and modified incubation conditions to analyze the effect of this cell-associated hemolysin and minimize the effect of the secreted cytolethal distending toxin (9, 29). We showed that the results of these short-term cytotoxicity assays, using strains expressing cloned hemolysin genes, mimic results obtained for HFFs with wild-type-hemolysin-expressing strains and long exposure times (2, 21). These modifications allowed us to determine the specificity of the hemolysin for many cell types important in chancroidal disease while avoiding the contaminating effects of other H. ducreyi toxins. We hypothesize that since macrophages, T cells, and HFEs were more sensitive than HFFs to overexpressed hemolysin, they are also likely targets for wild-type levels of hemolysin in the previously described cocultivation assay (2, 21). The interaction of hemolysin with B cells and HEp-2 cells needs to be studied further to determine if this level of sensitivity is relevant to wild-type hemolysin expression levels.
The target cell specificity of hemolysins (cytolysins) from other organisms is thought to partially define the host range and disease manifestation in the host. For example, the RTX leukotoxins LktA of Pasteurella haemolytica and AaltA of Actinobacillus actinomycetemcomitans lyse only ruminant or primate leukocytes, respectively, suggesting a role in attenuating the immune response to these organisms (47). However, other RTX hemolysins, including HlyA of E. coli, AppA of Actinobacillus pleuropneumoniae, and CyaA of Bordetella pertussis, have a broad target cell range including RBCs and nucleated cells from different species, suggesting a broader role in pathogenesis (47). The Proteus mirabilis hemolysin (hpmBA), which is homologous to the H. ducreyi hemolysin, has a broad cell target range which includes human bladder and renal epithelial cells, B cells, monocytes, and African green monkey kidney cells (8, 38). Similarly, the H. ducreyi hemolysin appears to have a broad target cell range, as demonstrated here and by previous work (22, 41), suggesting that the hemolysin is important in the interactions of H. ducreyi with many cell types in chancroidal lesions. For example, hemolysin may contribute to ulcer formation by lysing keratinocytes or fibroblasts. Chancroid ulcers contain large numbers of T cells and macrophages, and yet viable H. ducreyi persists in these lesions (20). Our observation that T cells and macrophages are sensitive to hemolysin may suggest a role for hemolysin in inhibition of an effective cell-mediated immune response that clears the H. ducreyi infection. While the data presented here defines the target cell range of hemolysin and the relative sensitivity of the various cell types to hemolysin, further experiments are needed before we can determine the in vivo relevance of this target cell range, including the effect of hemolysin on virulence and survival of H. ducreyi in animal models and levels of hemolysin expression in chancroidal lesions. In addition, the nature of the different susceptibilities of the target cells, possibly based on differences in (i) membrane composition, (ii) hemolysin receptor expression, or (iii) the ability of the affected cell to repair membrane damage induced by the hemolysin, will also be interesting subjects of future research.
Although our experiments measured target cell lysis, the H. ducreyi hemolysin may also have other, more subtle effects on cells. Lysis may occur only where there are many hemolysin-producing bacteria per target cell or where hemolysin expression is high. H. ducreyi grows as microcolonies in the rabbit model of chancroid, suggesting that high bacterium-to-target-cell ratios can occur in vivo (28) and that hemolysin-mediated lysis of cells may occur in these areas of the lesion. In areas where there are few bacteria per target cell or where hemolysin expression is low, the hemolysin may alter cellular function rather than lyse the cell outright. Effects of sublytic concentrations of hemolysin have been documented with other bacteria, including the closely related S. marcescens hemolysin, which induces chemiluminescence in PMNs and stimulates the release of leukotriene B4 (17). Streptolysin O of Streptococcus pyogenes induces interleukin-1β (IL-1β), IL-6, IL-8, and prostaglandin E2 expression by keratinocytes (30), while the E. coli hemolysin increases the release of leukotrienes from PMNs and of IL-1β from monocytes and inhibits antigen processing and presentation by murine macrophages (46). Further exploration of the effects of sublytic doses of hemolysin on cellular functions is needed to understand the contribution of the H. ducreyi hemolysin to pathogenesis.
Our experiments showing that strains with cloned hemolysin genes were clearly more invasive than strains containing vector alone suggests a role for hemolysin in invasion of epithelial cells. Other organisms including Edwardsiella tarda, Shigella flexneri, and Listeria monocytogenes produce hemolysins that enhance invasion by allowing cell entry into or escape from the phagocytic vacuole (10, 14, 36). We saw no significant difference in invasion by the wild-type and isogenic hemolysin mutant strains, perhaps reflecting the difficulty in demonstrating small losses in invasiveness for an organism in which only ca. 1% of the inoculum is internalized. Alternatively, the effect of deleting only one of many factors required for invasiveness may be difficult to measure. Other researchers have demonstrated that mutants defective in lipooligosaccharide biosynthesis are impaired in invasion of HFEs; however, this may simply be a consequence of their decreased adherence to these cells (12). This is not the case for hemolysin, since wild-type, mutant, and overexpressing strains adhered at similar levels to HEp-2 cells. Experiments on the regulation of hemolysin expression, expression levels of hemolysin in vivo, and the mechanism of invasion of H. ducreyi are needed to clarify the role of hemolysin in invasion of this organism.
While several researchers have confirmed invasion of epithelial cells by H. ducreyi (12, 39), invasion of fibroblasts is more controversial. Lammel et al. reported that H. ducreyi could be found within HFFs by transmission electron microscopy (19). However, Alfa et al. were unable to confirm this observation and further demonstrated lack of invasion of HFFs by H. ducreyi in a gentamicin protection assay (1). The fact that HFFs are sensitive targets for hemolysin may suggest that H. ducreyi lyses these cells, confounding identification of intracellular bacteria by microscopy and allowing an influx of gentamicin that kills intracellular bacteria. This is apparently the case with wild-type P. mirabilis, which expresses a hemolysin that lyses human renal epithelial cells, resulting in an apparent decrease in invasion compared to hemolysin-deficient mutants in gentamicin protection assays (8).
The in vivo role of the hemolysin in chancroid pathogenesis is still unclear in human and animal models of chancroid. Palmer et al. evaluated a hemolysin-deficient mutant in a human model of H. ducreyi infection and found that the mutant produced erythema and pustules similar to the wild-type parent; they concluded that hemolysin plays a minor role in early lesion development (24). However, this model does not fully mimic chancroidal disease, since later stages of infection (ulceration, immune system avoidance, and transmission) cannot be evaluated and inoculations are made on the upper arm, not on genital skin. In contrast, a role for hemolysin in survival in its only known niche, the human host, is suggested by the observations that (i) all strains of H. ducreyi express hemolysin in vitro; (ii) hemolysin is produced in vivo, since both humans and animals produce antibodies to HhdA after infection with H. ducreyi (11); and (iii) the target cell range of hemolysin includes clinically relevant cell types. Further studies evaluating the levels of hemolysin expression in vivo, the sublytic effects of hemolysin on target cells, and the effect of hemolysin on different animal models of chancroid are needed to clarify the contribution of hemolysin to the pathogenesis of human disease.
ACKNOWLEDGMENTS
This work was supported by grant AI33522 from the National Institutes of Health to P.A.T. G.E.W. is supported by NIH STD/AIDS Research Training Grant T32 AI07140.
We thank Steve Moseley, Stephen Lory, Derek Wood, and Rodney Welch for helpful discussions and critical review of the manuscript.
REFERENCES
- 1.Alfa M J, DeGagne P, Hollyer T. Haemophilus ducreyi adheres to but does not invade cultured human foreskin cells. Infect Immun. 1993;61:1735–1742. doi: 10.1128/iai.61.5.1735-1742.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alfa M J, DeGagne P, Totten P A. Haemophilus ducreyi hemolysin acts as a contact cytotoxin and damages human foreskin fibroblasts in cell culture. Infect Immun. 1996;64:2349–2352. doi: 10.1128/iai.64.6.2349-2352.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blanton R A, Kupper T S, McDougall J K, Dower S. Regulation of interleukin 1 and its receptor in human keratinocytes. Proc Natl Acad Sci USA. 1989;86:1273–1277. doi: 10.1073/pnas.86.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braun V, Ondraczek R, Hobbie S. Activation and secretion of Serratia hemolysin. Int J Med Microbiol Virol Parasitol Infect Dis. 1993;278:306–315. doi: 10.1016/s0934-8840(11)80847-9. [DOI] [PubMed] [Google Scholar]
- 5.Brentjens R J, Ketterer M, Apicella M A, Spinola S M. Fine tangled pili expressed by Haemophilus ducreyi are a novel class of pili. J Bacteriol. 1996;178:808–816. doi: 10.1128/jb.178.3.808-816.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron D W, Simonsen J N, D’Costa L J, Ronald A R, Maitha G M, Gakinya M N, Cheang M, Nydinya-Achola J O, Piot P, Brunham R C, Plummer F A. Female to male transmission of human immunodeficiency virus type 1: risk factors for seroconversion in men. Lancet. 1989;ii:403–407. doi: 10.1016/s0140-6736(89)90589-8. [DOI] [PubMed] [Google Scholar]
- 7.Campagnari A A, Wild L M, Griffiths G E, Karalus R J, Wirth M A, Spinola S M. Role of lipooligosaccharides in experimental dermal lesions caused by Haemophilus ducreyi. Infect Immun. 1991;59:2601–2608. doi: 10.1128/iai.59.8.2601-2608.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chippendale G R, Warren J W, Trifillis A L, Mobley H L T. Internalization of Proteus mirabilis by human renal epithelial cells. Infect Immun. 1994;62:3115–3121. doi: 10.1128/iai.62.8.3115-3121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cope L D, Lumbley S, Latimer J L, Klesney-Tait J, Stevens M K, Johnson L S, Purven M, Munson J R S, Lagergard T, Radolf J D, Hansen E J. A diffusible cytotoxin of Haemophilus ducreyi. Proc Natl Acad Sci USA. 1997;94:4056–4061. doi: 10.1073/pnas.94.8.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cossart P. Listeria monocytogenes: strategies for entry and survival in cells and tissues. In: Tindall B, editor. Bailliere’s clinical infectious diseases. Vol. 1. Philadelphia, Pa: Bailliere; 1994. pp. 285–304. [Google Scholar]
- 11.Dutro S M, Wood G E, Totten P A. Prevalence of, antibody response to, and protective immunity induced by Haemophilus ducreyi hemolysin. Infect Immun. 1999;67:3317–3328. doi: 10.1128/iai.67.7.3317-3328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibson B W, Campagnari A A, Melaugh W, Phillips N J, Apicella M A, Grass S, Wang J, Palmer K L, Munson J R S. Characterization of a transposon Tn916-generated mutant of Haemophilus ducreyi 35000 defective in lipooligosaccharide biosynthesis. J Bacteriol. 1997;179:5062–5071. doi: 10.1128/jb.179.16.5062-5071.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hansen E J, Latimer J L, Thomas S E, Helminen M, Albritton W L, Radolf J D. Use of electroporation to construct isogenic mutants of Haemophilus ducreyi. J Bacteriol. 1992;16:5442–5449. doi: 10.1128/jb.174.16.5442-5449.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.High N, Mounier J, Prevost M, Sansonetti P. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirono I, Tange N, Aoki T. Iron-regulated haemolysin gene from Edwardsiella tarda. Mol Microbiol. 1997;24:851–856. doi: 10.1046/j.1365-2958.1997.3971760.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoiseth S K, Stocker B A D. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981;291:238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- 17.Konig W, Faltin Y, Scheffer J, Schoffler H, Braun V. Role of cell-bound hemolysin as a pathogenicity factor for Serratia infections. Infect Immun. 1987;55:2554–2561. doi: 10.1128/iai.55.11.2554-2561.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Korth M J, Schneider R A, Mosely S L. An F41-K88-related genetic determinant of bovine septicemic Escherichia coli mediates expression of CS31A fimbriae and adherence to epithelial cells. Infect Immun. 1991;59:2333–2340. doi: 10.1128/iai.59.7.2333-2340.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lammel C J, Dekker N P, Palefsky J, Brooks G F. In vitro model of Haemophilus ducreyi adherence to and entry into eukaryotic cells of genital origin. J Infect Dis. 1993;167:642–650. doi: 10.1093/infdis/167.3.642. [DOI] [PubMed] [Google Scholar]
- 20.Morse S A. Chancroid and Haemophilus ducreyi. Clin Microbiol Rev. 1989;2:137–157. doi: 10.1128/cmr.2.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palmer K L, Goldman W E, Munson J R S. An isogenic haemolysin-deficient mutant of Haemophilus ducreyi lacks the ability to produce cytopathic effects on human foreskin fibroblasts. Mol Microbiol. 1996;21:13–19. doi: 10.1046/j.1365-2958.1996.00615.x. [DOI] [PubMed] [Google Scholar]
- 22.Palmer K L, Grass S, Munson J R S. Identification of a hemolytic activity elaborated by Haemophilus ducreyi. Infect Immun. 1994;62:3041–3042. doi: 10.1128/iai.62.7.3041-3043.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Palmer K L, Munson J R S. Cloning and characterization of the genes encoding the haemolysin of Haemophilus ducreyi. Mol Microbiol. 1995;18:821–830. doi: 10.1111/j.1365-2958.1995.18050821.x. [DOI] [PubMed] [Google Scholar]
- 24.Palmer K L, Thornton A C, Fortney K R, Hood A F, Munson J R S, Spinola S M. Evaluation of an isogenic hemolysin-deficient mutant in the human model of Haemophilus ducreyi infection. J Infect Dis. 1998;178:191–199. doi: 10.1086/515617. [DOI] [PubMed] [Google Scholar]
- 25.Plummer F A, Simonsen J N, Cameron D W, Ndinya-Achola J O, Kreiss J K, Gakinya M N, Waiyaki P, Cheang M, Piot P, Ronald A R, Ngugi E N. Cofactors in male-female sexual transmission of human immunodeficiency virus type 1. J Infect Dis. 1991;163:233–239. doi: 10.1093/infdis/163.2.233. [DOI] [PubMed] [Google Scholar]
- 26.Poole K, Braun V. Iron regulation of Serratia marcescens hemolysin gene expression. Infect Immun. 1988;56:2967–2971. doi: 10.1128/iai.56.11.2967-2971.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Poole K, Schiebel E, Braun V. Molecular characterization of the hemolysin determinant of Serratia marcescens. J Bacteriol. 1988;170:3177–3188. doi: 10.1128/jb.170.7.3177-3188.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Purcell B K, Richardson J A, Radolf J D, Hansen E J. A temperature-dependent rabbit model for production of dermal lesions by Haemophilus ducreyi. J Infect Dis. 1991;164:359–367. doi: 10.1093/infdis/164.2.359. [DOI] [PubMed] [Google Scholar]
- 29.Purven M, Lagergard T. Haemophilus ducreyi, a cytotoxin-producing bacterium. Infect Immun. 1992;60:1156–1162. doi: 10.1128/iai.60.3.1156-1162.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruiz N, Want B, Pentland A, Caparon M. Streptolysin O and adherence synergistically modulate proinflammatory responses of keratinocytes to group A streptococci. Mol Microbiol. 1998;27:337–346. doi: 10.1046/j.1365-2958.1998.00681.x. [DOI] [PubMed] [Google Scholar]
- 31.Sambrook J, Frisch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 32.Schiebel E, Schwarz H, Braun V. Subcellular location and unique secretion of the hemolysin of Serratia marcescens. J Biol Chem. 1989;264:16311–16320. [PubMed] [Google Scholar]
- 33.Schonherr R, Hilger M, Broer S, Benz R, Braun V. Interaction of Serratia marcescens hemolysin (ShlA) with artificial and erythrocyte membranes. Eur J Biochem. 1994;223:655–663. doi: 10.1111/j.1432-1033.1994.tb19038.x. [DOI] [PubMed] [Google Scholar]
- 34.Spinola S M, Orazi A, Arno J N, Fortney K, Kotylo O, Chen C, Campagnari A A, Hood A F. Haemophilus ducreyi elicits a cutaneous infiltrate of CD4 cells during experimental human infection. J Infect Dis. 1996;173:394–402. doi: 10.1093/infdis/173.2.394. [DOI] [PubMed] [Google Scholar]
- 35.Spinola S M, Wild L M, Apicella M A, Gaspari A A, Campagnari A A. Experimental human infection with Haemophilus ducreyi. J Infect Dis. 1994;169:1146–1150. doi: 10.1093/infdis/169.5.1146. [DOI] [PubMed] [Google Scholar]
- 36.Strauss E J, Ghori N, Falkow S. An Edwardsiella tarda strain containing a mutation in a gene with homology to shlB and hpmB is defective for entry into epithelial cells in culture. Infect Immun. 1997;65:3924–3932. doi: 10.1128/iai.65.9.3924-3932.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strickberger M W. Genetics. 3rd ed. New York, N.Y: Macmillan Publishing Co.; 1985. [Google Scholar]
- 38.Swihart K G, Welch R A. Cytotoxic activity of the Proteus hemolysin HpmA. Infect Immun. 1991;58:1861–1869. doi: 10.1128/iai.58.6.1861-1869.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Totten P A, Lara J C, Norn D V, Stamm W E. Haemophilus ducreyi attaches to and invades cultured human foreskin epithelial cells in vitro. Infect Immun. 1994;62:5632–5640. doi: 10.1128/iai.62.12.5632-5640.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Totten P A, Morton W R, Knitter G H, Clark A M, Kiviat N B, Stamm W E. A primate model for chancroid. J Infect Dis. 1994;169:1284–1290. doi: 10.1093/infdis/169.6.1284. [DOI] [PubMed] [Google Scholar]
- 41.Totten P A, Norn D V, Stamm W E. Characterization of the hemolytic activity of Haemophilus ducreyi. Infect Immun. 1995;63:4409–4416. doi: 10.1128/iai.63.11.4409-4416.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Totten P A, Stamm W E. Clear broth and plate media for culture of Haemophilus ducreyi. J Clin Microbiol. 1994;32:2019–2023. doi: 10.1128/jcm.32.8.2019-2023.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trees D L, Morse S A. Chancroid and Haemophilus ducreyi: an update. Clin Microbiol Rev. 1995;8:357–375. doi: 10.1128/cmr.8.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Uphoff T S, Welch R A. Nucleotide sequencing of the Proteus mirabilis calcium-independent hemolysin genes (hpmA and hpmB) reveals sequence similarity to the Serratia marcescens hemolysin genes (shlA and shlB) J Bacteriol. 1990;172:1206–1216. doi: 10.1128/jb.172.3.1206-1216.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wasserheit J N. Epidemiological synergy. Interrelationships between human immunodeficiency virus infection and other sexually transmitted diseases. Sex Transm Dis. 1992;19:61–77. [PubMed] [Google Scholar]
- 46.Welch R A. Pore-forming cytolysins of Gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 47.Welch R A, Forestier C, Lobo A, Pellett S, Thomas J W, Rowe G. The synthesis and function of the Escherichia coli hemolysin and related RTX exotoxins. FEMS Microbiol Immunol. 1992;105:29–36. doi: 10.1111/j.1574-6968.1992.tb05883.x. [DOI] [PubMed] [Google Scholar]
- 48.Willson P J, Albritton W L, Slaney L, Setlow J K. Characterization of a multiple antibiotic resistance plasmid from Haemophilus ducreyi. Antimicrob Agents Chemother. 1989;33:1627–1630. doi: 10.1128/aac.33.9.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]