Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of coronavirus disease 2019 (COVID-19), has rapidly spread worldwide. The monitoring of animals has shown that certain species may be susceptible to be infected with the virus. The present study aimed to evaluate the presence of SARS-CoV-2 antibodies by ELISA and virus neutralization (VN) in pets from owners previously confirmed as COVID-19-positive in Argentina. Serum samples of 38 pets (seven cats and 31 dogs) were obtained for SARS-CoV-2 antibody detection. Three out of the seven cats and 14 out of the 31 dogs were positive for SARS-CoV-2 by ELISA, and one cat and six dogs showed the presence of neutralizing antibodies in which the cat and two of the six dogs showed high titers. Another dog from which three serum samples had been obtained within eight months from the diagnosis of its owner showed the presence of antibodies at different times by both ELISA and VN. However, the results showed that the antibodies decreased slightly from the first to the third sample. Our results provide evidence that SARS-CoV-2 infection in pets living with COVID-19-positive humans from Argentina during the outbreak of SARS-CoV-2 can be detected by serology assay.
Keywords: SARS-CoV-2, COVID-19, Dogs, Cats, Antibodies, Serological detection
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the etiological agent of coronavirus disease 2019 (COVID-19), was first detected in Wuhan, China, and then rapidly spread worldwide (Zhou et al., 2020a). Although the available data suggest that SARS-CoV-2 emerged from an animal source, there is currently not enough evidence to corroborate either the source or route of transmission (Zhou et al., 2020b). Thus, several studies are being carried out to gain insights into the susceptibility of different animal species, including domestic animals to SARS-CoV-2. Genome analysis has shown that SARS-CoV-2 has 96.2 % overall genome sequence identity with Bat CoV RaTG13, indicating that this virus could have also originated from bats (Zhou et al., 2020b), similarly to that occurred with SARS-CoV and MERS-CoV, two other zoonotic coronaviruses reported in 2003 and 2012, respectively (Gautam et al., 2020). Molecular detection of SARS-CoV-2 has been reported in various animals (dogs, cats, zoo tigers, zoo lions, ferrets, mink and white-tailed deer) worldwide, including Argentina (Fuentealba et al., 2021; Hale et al., 2022; McAloose et al., 2020; Oreshkova et al., 2020; Segalés et al., 2020; Sit et al., 2020). In addition, several species, including cats (Shi et al., 2020), ferrets (Kim et al., 2020, Kim et al., 2020), fruit bats (Schlottau et al., 2020), deer mice (Fagre et al., 2021) and white-tailed deer (Hale et al., 2022), are capable of intraspecies transmission. A high prevalence of SARS-CoV-2 antibodies has been reported in pets from households with at least one COVID-19-positive human (Fritz et al., 2020, Zhang et al., 2020). However, the extent of natural infections of animals with SARS-CoV-2 is still largely unknown. As many other CoVs, SARS-CoV-2 uses its surface spike (S) glycoprotein to gain entry into host cells via binding to the ACE2 receptor (Abdel-Moneim and Abdelwhab, 2020; Munir et al., 2020). Therefore, this glycoprotein is closely associated with and targeted by neutralizing antibody responses and protective immunity (Robbiani et al., 2020). In Argentina, in the period 2020–2021, 75 cases of SARS-CoV-2 infection in pets, all diagnosed by molecular assays, were reported to the OIE. However, no analysis of anti-SARS-CoV-2 antibody detection has yet been reported. The Enzyme-Linked Immunosorbent Assay (ELISA) is a useful assay to analyze the presence of antibodies in an animal or population, but does not provide information on the functionality of the antibodies detected. In contrast, assays such as the virus neutralization (VN) test are able to detect neutralizing antibodies, which are considered to be an important mechanism for immunity and, potentially, for the clearance of the virus infection. Thus, the VN assay is important to determine the functionality of the immune system against infection. Based on the above, the present study aimed to evaluate the presence of SARS-CoV-2 antibodies by ELISA and the VN test in pets from COVID-19-positive owners in Argentina.
2. Materials and methods
A total of 38 pets (7 cats and 31 dogs) were sampled during the first wave and beginning of the second wave of COVID-19 in Argentina. Blood samples (3–5 ml) were then collected without anticoagulant for serum extraction at least 15 days after the positive diagnosis of the pet owners, and were stored at − 20 ºC, until further analysis. The samples were taken at their homes, and all the animals were in close contact with their COVID-19-positive owners. Most of the animals selected in this study had no clinical signs; however, some of them presented some signs such as anorexia, lethargy, diarrhea, cough and sneezing. Each owner gave their written consent to allow the collection of samples from their pets, and all the protocols were approved by the Institutional Animal Care and Use Committee (CICUAL) from the FCV-UNLP under the protocol code 105–4–20P.
SARS-CoV-2 antibody detection by ELISA in cats and dogs was carried out at the Facultad de Farmacia y Bioquímica, Universidad Nacional de Buenos Aires, Buenos Aires, Argentina, under the service modality using a bridge multi-species ELISA. The kit detects the specific antibodies against the S protein of SARSCoV-2 in serum samples from different. Results were calculated as specific absorbance (A = the mean of each sample minus the mean of the blank control) and expressed as Positivity Index: PI = A sample/AC+ . The cut-off value of the assay was set at PI = 10.0 through receiver operating characteristic (ROC) curves analysis.
For the VN assay, Vero E6 cells were seeded in 96-well plates at a density of 1.5 × 104 cells per well in DMEM 10 % FBS and incubated for 24 h at 37 ºC and 5 % CO2. SARS-CoV-2 D6124G variant (B.1 lineage) used in the assays were isolated from nasopharyngeal specimens at the Instituto de Investigaciones Biotecnológicas, Universidad Nacional de San Martín (IIB, UNSAM), Buenos Aires, Argentina. Three hundred of 50 % tissue culture infectious doses (TCID50) were preincubated with serially diluted sera for 1 h at 37 ºC starting at a serum dilution of 1/8. Each serum dilution was tested in duplicate. Then, a virus-serum mixture was added onto Vero E6 cells in a final volume of 100 µl in DMEM 2 % FBS. After 72 h incubation at 37ºC and 5 % CO2, cultures were fixed with formaldehyde 3 % at 4ºC for 24 h and stained with crystal violet solution in methanol. The cytopathic effect (CPE) of the virus on the cell monolayer was assessed visually, and the neutralization titer (NT) was defined as the inverse of the highest serum dilution without any CPE.
3. Results and discussion
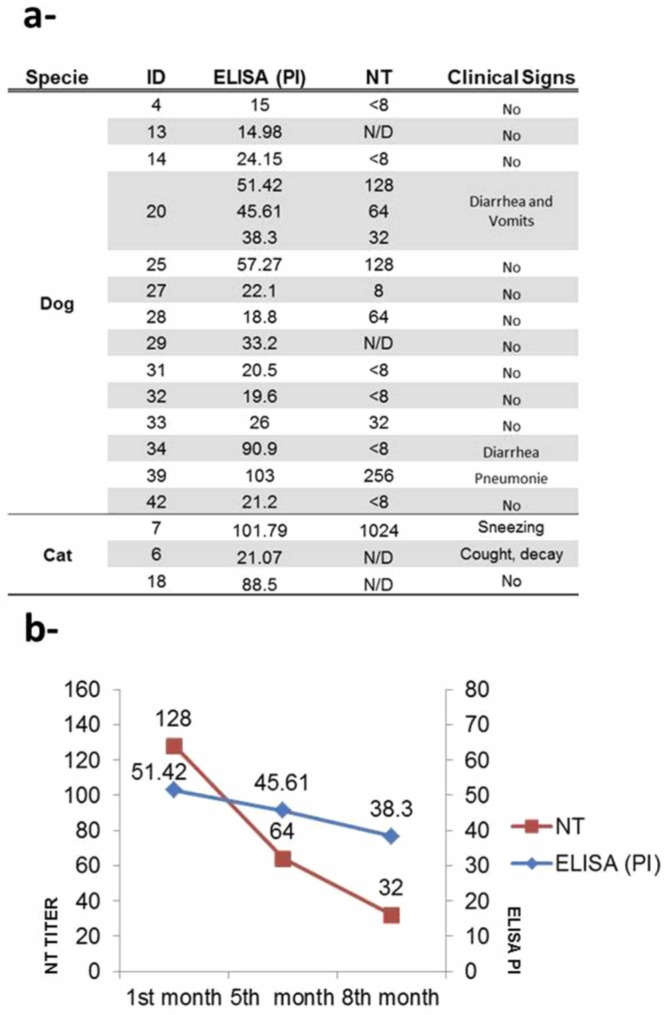
Multiple animal species, including domestic animals such as dogs and cats, are susceptible to SARS-CoV-2 infection under experimental conditions and under natural virus exposure (Mahdy et al., 2020, Bosco-Lauth et al., 2021). In this study, the serum samples from dogs analyzed by ELISA showed that 14 out of the 31 dogs were positive to SARS-CoV-2, with different positivity index (PI) values above the standard cut-off criterion (PI ≥ 10) revealing the presence of specific antibodies against SARS-CoV-2. In addition, six of the 14 dogs positive by ELISA were also reactive by the VN assay, two of which (dogs ID 25 and 39) showed high titers ( Fig. 1a). However, two out of the dogs positive by ELISA could not be evaluated by VN because the serum volume was insufficient. Besides, one dog, from which three samples had been obtained, on december 23rd 2020, april 24th 2021 and july 9th 2021 (ID 20–1, 20–2 and 20–3), showed antibodies detected by both ELISA and VN. Although the PI decreased around 13 points from the first to the third sample, after 8 months, the antibodies remained detectable (Fig. 1b). These results are similar to those reported in humans by Messiah et al. (2022), who found that most of the children showed detectable SARS-CoV-2 antibodies in three successive samples for more than 6 months. Hamer et al. (2021) observed that, in the 15 dogs and cats from which sequential serum samples had been collected, the VN titer values were relatively stable or increased in the 2–3 months of follow-up. However, these authors did not evaluate the VN titers in a longer time. In addition, the dog ID 20 had vomiting and diarrhea at the time its owner was undergoing COVID-19. Another dog, ID 34, had diarrhea and showed the presence of antibodies detectable by ELISA with a PI of 90.9. However, no neutralizing antibodies were detected by VN. The ELISA 2.0 kit used in this study detects antibodies against the S protein of SARS-CoV-2, and it is possible that there is some cross reaction with antibodies of other coronaviruses of dogs. Previous studies in humans have determined immune responses to the viral proteins spike (S1, S2 and RBD) and nucleoprotein (N) by ELISA, showing a strong correlation between levels of RBD binding antibodies and SARS-CoV-2 neutralizing antibodies (Premkumar et al., 2020). On the other hand, it has been observed that the high response to N or S2 and pre-existing cross-reactive antibodies to respiratory viruses in animals are associated with SARS-CoV-2 N or S2-specific antibodies, although those antibodies were not able to neutralize the virus (Kim et al., 2020, Kim et al., 2020). Thus, it is necessary to emphasize the importance of the VN assay of not overestimating the seroepidemiological results of SARS-CoV-2.
Fig. 1.
Positivity index (PI); neutralization titer (NT); virus neutralization (VN); no data (N/D). a- Data showing the PI and NT values obtained by ELISA and VN for the dog and cat serum samples analyzed. Clinical signs of each animal are shown in the last column; b- Line graphs indicating the PI and NT values obtained by ELISA and VN for the dog (ID 20) sampled at different times (ID 20–1, 20–2 and 20–3) (1, 5 and 8 months post-infection of owners).
Regarding the cats sampled, three out of the seven resulted positive by ELISA. The serological analysis by ELISA of the serum sample from cat ID 7 showed the presence of antibodies against the protein S of SARS-CoV-2, PI value of 101.79. In addition, a higher NT of 1024 was observed. This cat has been previously reactive to SARS-CoV-2 by real time RT-PCR (Fuentealba et al., 2021), and the serum sample was taken 35 days after this positive testing. The animal presented only sneezing for about 3 days after close contact with its COVID-19-positive owners. These results strongly suggest that SARS-CoV-2 actively replicated in this animal, inducing a robust antibody response. The serum sample from other two cats, ID 6 and 18 also showed antibodies against SARS-CoV-2 by ELISA, indicating that the animals were also infected with the virus. One of them (ID 6), showed respiratory signs (intermittent cough/bronchospasm), moderate decay and some sporadic neurological symptoms, at the same time as its owners had COVID-19. In these animals, it was not possible to perform the VN test since the volume of sample obtained was insufficient (Fig. 1a). The other four cats analyzed were negative by ELISA.
The results obtained in this study showed that some SARS-CoV-2-seropositive cats had respiratory signs such as sneezing and cough while some dogs had digestive signs such as vomiting and diarrhea. Similar results were obtained by Colitti et al. (2021), who found that, in 5 out of 14 seropositive animals, the owners reported that the animals experienced clinical signs concurrent with the owner’s COVID-19. However, most research has shown that dogs exposed to SARS-CoV-2 could produce anti-SARS-CoV-2 antibodies without exhibiting signs (Shi et al., 2020, Hamer et al., 2021) and that cats can develop asymptomatic infection or mild symptoms and virus shedding (Barrs et al., 2020; Abdel-Moneim and Abdelwhab, 2020).
The susceptibility of cats and dogs has been reported in other studies (Fritz et al., 2020, Sit et al., 2020, Zhang et al., 2020, Barrs et al., 2020). However, the clinical and pathological consequences of SARS-CoV-2 infection in these species are not entirely clear and warrant further research.
In this study, a total of 40 samples from 38 animals that had recently been in close contact with their COVID-19-positive owners were tested by ELISA. Three out of seven cats (43 %) and 14 out of 31 dogs (45 %) were positive to antibodies against the glycoprotein S of SARS-CoV-2. This situation very likely explains the high percentages of SARS-CoV-2 antibody detection. High antibody titers detected by VN have been previously found in cats living with COVID-19 patients, while sera collected from cats in veterinary hospitals have shown minor neutralizing activity, indicating that the high neutralization titers could be due to the close contact between cats and COVID-19 patients (Zhang et al., 2020). In other studies, the prevalence observed was much lower than that observed in this study, but the samples had been obtained at a companion animal veterinary medical center at the time of admission, and thus there was no known association with COVID-19 (Dileepan et al., 2021, Zhang et al., 2020). Finally, although previous studies have reported that cats are more susceptible to SARS-CoV-2 than dogs (Abdel-Moneim and Abdelwhab, 2020), in the present study, we found similar prevalence in dogs and cats. Probably, a larger number of samples, especially of cats, should be analyzed to confirm these data.
In summary, our study provides the first results of serological assays (ELISA and VN) in dogs and cats living with COVID-19-positive humans from Argentina during the SARS-CoV-2 outbreak.
Conflict of Interest
The authors report no declarations of interest.
Acknowledgments
This study was funded by the Agencia Nacional de Promoción a la Investigación, Desarrollo Tecnológico e Innovación Productiva (I+D+i), Argentina, Project 444–468.
References
- Abdel-Moneim A.S., Abdelwhab E.M. Evidence for SARS-CoV-2 infection of animal. Hosts. Pathog. 2020;9:529. doi: 10.3390/pathogens9070529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrs V.R., Peiris M., Tam K.W.S., Law P.Y.T., Brackman C.J., To E.M.W., Yu V.Y.T., Chu D.K.W., Perera R.A.P.M., Sit T.H.C. SARS-CoV-2 in quarantined domestic cats from COVID-19 households or close contacts, Hong Kong, China. Emerg. Infect. Dis. 2020;26(12):3071–3074. doi: 10.3201/eid2612.202786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosco-Lauth A.M., Root J.J., Porter S.M., Walker A.E., Guilbert L., Hawvermale D., Pepper A., Maison R.M., Hartwig A.E., Gordy P., Bielefeldt-Ohmann H., Bowen R.A. Peridomestic mammal susceptibility to severe acute respiratory syndrome coronavirus 2 infection. Emerg. Infect. Dis. 2021;27(8):2073–2080. doi: 10.3201/eid2708.210180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colitti B., Bertolotti L., Mannelli A., Ferrara G., Vercelli A., Grassi A., Rosati S. Cross-sectional serosurvey of companion animals housed with SARS-CoV-2–infected owners, Italy. Emerg. Infect. Dis. 2021;27(7):1919–1922. doi: 10.3201/eid2707.203314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dileepan M., Di D., Huang Q., Ahmed S., Heinrich D., Ly H., Liang Y. Seroprevalence of SARS-CoV-2 (COVID-19) exposure in pet cats and dogs in Minnesota, USA. Virulence. 2021;12(1):1597–1609. doi: 10.1080/21505594.2021.1936433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagre A., Lewis J., Eckley M., Zhan S., Rocha S.M., Sexton N.R., Burke B., Geiss B., Peersen O., Bass T., Kading R., Rovnak J., Ebel G.D., Tjalkens R.B., Aboellail T., Schountz T. SARS-CoV-2 infection, neuropathogenesis and transmission among deer mice: Implications for spillback to New World rodents. PLoS Pathog. 2021;17(5) doi: 10.1371/journal.ppat.1009585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M., Rosolen B., Krafft E., Becquart P., Elguero E., Vratskikh O., Denolly S., Boson B., Vanhomwegen J., Gouilh M., Kodjo A., Chirouze C., Rosolen S.G., Legros V., Leroy E.M. High prevalence of SARS-CoV-2 antibodies in pets from COVID-19+ households. One Health. 2020;11 doi: 10.1016/j.onehlt.2020.100192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentealba N.A., Moré G., Bravi M.E., Unzaga J.M., De Felice L., Salina M., Viegas M., Nabaes Jodar M.S., Valinotto L.E., Rivero F.D., Di Lullo D., Pecoraro M., Panei C.J. First detection and molecular analysis of SARS-CoV-2 from a naturally infected cat from Argentina. Vet. Microbiol. 2021;260 doi: 10.1016/j.vetmic.2021.109179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A., Kaphle K., Shrestha B., Phuyal S. Susceptibility to SARS, MERS, and COVID-19 from animal health perspective. Open Vet. J. 2020;10(2):164–177. doi: 10.4314/ovj.v10i2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale V.L., Dennis P.M., McBride D.S., Nolting J.M., Madden C., Huey D., Ehrlich M., Grieser J., Winston J., Lombardi D., Gibson S., Saif L., Killian M.L., Lantz K., Tell R.M., Torchetti M., Robbe-Austerman S., Nelson M.I., Faith S.A., Bowman A.S. SARS-CoV-2 infection in free-ranging white-tailed deer. Nature. 2022;602(7897):481–486. doi: 10.1038/s41586-021-04353-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer S.A., Pauvolid-Corrêa A., Zecca I.B., Davila E., Auckland L.D., Roundy C.M., Tang W., Torchetti M.K., Killian M.L., Jenkins-Moore M., Mozingo K., Akpalu Y., Ghai R.R., Spengler J.R., Barton Behravesh C., Fischer R.S.B., Hamer G.L. SARS-CoV-2 infections and viral isolations among serially tested cats and dogs in households with infected owners in Texas, USA. Viruses. 2021;13(5):938. doi: 10.3390/v13050938. (https://doi) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Seiler P., Jones J.C., Ridout G., Camp K.P., Fabrizio T.P., Jeevan T., Miller L.A., Throm R.E., Ferrara F., Fredrickson R.L., Lowe J.F., Wang L., Odemuyiwa S.O., Wan X.F., Webby R.J. Antibody responses to SARS-CoV-2 antigens in humans and animals. Vaccin. (Basel) 2020;8(4):684. doi: 10.3390/vaccines8040684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.I., Kim S.G., Kim S.M., Kim E.H., Park S.J., Yu K.M., Chang J.H., Kim E.J., Lee S., Casel M.A.B., Um J., Song M.S., Jeong H.W., Lai V.D., Kim Y., Chin B.S., Park J.S., Chung K.H., Foo S.S., Poo H., Mo I.P., Lee O.J., Webby R.J., Jung J.U., Choi Y.K. Infection and rapid transmission of SARS-CoV-2 in ferrets. Cell Host Microbe. 2020;27(5):704–709. doi: 10.1016/j.chom.2020.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahdy M.A.A., Younis W., Ewaida Z. An overview of SARS-CoV-2 and animal infection. Front Vet. Sci. 2020;7 doi: 10.3389/fvets.2020.596391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAloose D., Laverack M., Wang L., Killian M.L., Caserta L.C., Yuan F., Mitchell P.K., Queen K., Mauldin M.R., Cronk B.D., Bartlett S.L., Sykes J.M., Zec S., Stokol T., Ingerman K., Delaney M.A., Fredrickson R., Ivancic M., Jenkins-Moore M., Mozingo K., Franzen K., Bergeson N.H., Goodman L., Wang H., Fang Y., Olmstead C., McCann C., Thomas P., Goodrich E., Elvinger F., Smith D.C., Tong S., Slavinski S., Calle P.P., Terio K., Torchetti M.K., Diel D.G. From people to panthera: natural SARS-CoV-2 infection in tigers and lions at the Bronx Zoo. ASM J. mBio. 2020;11(5):10–13. doi: 10.1128/mBio.02220-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messiah S.E., DeSantis S.M., Leon-Novelo L.G., Talebi Y., Brito F.A., Kohl H.W., Valerio-Shewmaker M.A., Ross J.A., Swartz M.D., Yaseen A., Kelder S.H., Zhang S., Omega-Njemnobi O.S., Gonzalez M.O., Wu L., Boerwinkle E., Lakey D.L., Shuford J.A., Pont S.J. Durability of SARS-CoV-2 antibodies from natural infection in children and adolescents. Pediatrics. 2022;149(6) doi: 10.1542/peds.2021-055505. e2021055505. [DOI] [PubMed] [Google Scholar]
- Munir K., Ashraf S., Munir I., Khalid H., Muneer M.A., Mukhtar N., Amin S., Ashraf S., Imran M.A., Chaudhry U., Zaheer M.U., Arshad M., Munir R., Ahmad A., Zhao X. Zoonotic and reverse zoonotic events of SARS-CoV-2 and their impact on global health. Emerg. Microbes Infect. 2020;9:2222–2235. doi: 10.1080/22221751.2020.1827984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oreshkova N., Molenaar R.J., Vreman S., Harders F., Oude Munnink B.B., Hakze-van der Honing R.W., Gerhards N., Tolsma P., Bouwstra R., Sikkema R.S., Tacken M.G., de Rooij M.M., Weesendorp E., Engelsma M.Y., Bruschke C.J., Smit L.A., Koopmans M., van der Poel W.H., Stegeman A. SARS-CoV-2 infection in farmed minks, the Netherlands, April and May 2020. Eur. Surveill. 2020;25(23):2001005. doi: 10.2807/1560-7917.ES.2020.25.23.2001005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premkumar L., Segovia-Chumbez B., Jadi R., Martinez D.R., Raut R., Markmann A., Cornaby C., Bartelt L., Weiss S., Park Y., Edwards C.E., Weimer E., Scherer E.M., Rouphael N., Edupuganti S., Weiskopf D., Tse L.V., Hou Y.J., Margolis D., Sette A., Collins M.H., Schmitz J., Baric R.S., de Silva A.M. The receptor binding domain of the viral spike protein is an immunodominant and highly specific target of antibodies in SARS-CoV-2 patients. Sci. Immunol. 2020;5(48):eabc8413. doi: 10.1126/sciimmunol.abc8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbiani D.F., Gaebler C., Muecksch F., Lorenzi J.C.C., Wang Z., Cho A., Agudelo M., Barnes C.O., Gazumyan A., Finkin S., Hägglöf T., Oliveira T.Y., Viant C., Hurley A., Hoffmann H.H., Millard K.G., Kost R.G., Cipolla M., Gordon K., Bianchini F., Chen S.T., Ramos V., Patel R., Dizon J., Shimeliovich I., Mendoza P., Hartweger H., Nogueira L., Pack M., Horowitz J., Schmidt F., Weisblum Y., Michailidis E., Ashbrook A.W., Waltari E., Pak J.E., Huey-Tubman K.E., Koranda N., Hoffman P.R., West A.P., Jr, Rice C.M., Hatziioannou T., Bjorkman P.J., Bieniasz P.D., Caskey M., Nussenzweig M.C. Convergent antibody responses to SARS-CoV-2 in convalescent individuals. Nature. 2020;584(7821):437–442. doi: 10.1038/s41586-020-2456-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlottau K., Rissmann M., Graaf A., Schön J., Sehl J., Wylezich C., Höper D., Mettenleiter T.C., Balkema-Buschmann A., Harder T., Grund C., Hoffmann D., Breithaupt A., Beer M. SARS-CoV-2 in fruit bats, ferrets, pigs, and chickens: an experimental transmission study. Lancet Microbe. 2020 doi: 10.1016/S2666-5247(20)30089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segalés J., Puig M., Rodon J., Avila-Nieto C., Carrillo J., Cantero G., Terrón M.T., Cruz S., Parera M., Noguera-Julián M., Izquierdo-Useros N., Guallar V., Vidal E., Valencia A., Blanco I., Blanco J., Clotet B., Vergara-Alert J. Detection of SARS-CoV-2 in a cat owned by a COVID-19-affected patient in Spain. Proc. Natl. Acad. Sci. U. S. A. 6. 2020;117(40):24790–24793. doi: 10.1073/pnas.2010817117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit T.H.C., Brackman C.J., Ip S.M., Tam K.W.S., Law P.Y.T., To E.M.W., Yu V.Y.T., Sims L.D., Tsang D.N.C., Chu D.K.W., Perera R.A.P.M., Poon L.L.M., Peiris M. Infection of dogs with SARS-CoV-2. Nature. 2020;586:776–778. doi: 10.1038/s41586-020-2334-5PMC7292505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Wen Z., Zhong G., Yang H., Wang C., Huang B., Liu R., He X., Shuai L., Sun Z., Zhao Y., Liu P., Liang L., Cui P., Wang J., Zhang X., Guan Y., Tan W., Wu G., Chen H., Bu Z. Susceptibility of ferrets, cats, dogs, and other domesticated animals to SARS–coronavirus 2. Science. 2020;368:1016–1020. doi: 10.1126/science.abb7015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Zhang H., Huang K., Yang Y., Hui X., Gao J., He X., Li Ch, Gong W., Zhang Y., Peng Ch, Gao X., Chen H., Zou Z., Shi Z., Jin M. SARS-CoV-2 neutralizing serum antibodies in cats: a serological investigation. bioRxiv. 2020 doi: 10.1101/2020.04.01.021196. 2020.04.01.021196. [DOI] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P., Yang X.L., Wang X.G., Hu B., Zhang L., Zhang W., Si H.R., Zhu Y., Li B., Huang C.L., Chen H.D., Chen J., Luo Y., Guo H., Jiang R.D., Liu M.Q., Chen Y., Shen X.R., Wang X., Zheng X.S., Zhao K., Chen Q.J., Deng F., Liu L.L., Yan B., Zhan F.X., Wang Y.Y., Xiao G.F., Shi Z.L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2951-z. 〈https://doi〉 [DOI] [PMC free article] [PubMed] [Google Scholar]