Abstract
Between March 2021 and February 2022, SARS-CoV-2 neutralizing antibodies dynamics was investigated in a prospective observational study in 903 healthcare workers of a hospital in Switzerland. A surrogate neutralization assay measuring the competitive inhibition of the angiotensin converting enzyme 2 (ACE2) binding to the spike protein (S) of the SARS-CoV-2 wild type virus and to five variants of concern (Alpha, Beta, Gamma, Delta, Omicron) was used. We observed a broad distribution of neutralization activity among participants and substantial differences in neutralizing titers against variants. Participants were grouped based on combinations of vaccination status (1, 2 or 3 doses) and/or prior or subsequent SARS-CoV-2 infection/reinfection. Triple vaccination resulted in the highest neutralization response, as did double vaccination with prior or subsequent infection. Double vaccination without infection showed an intermediate neutralization response while SARS-CoV-2 infection in non-vaccinated participants resulted in poor neutralization response. After triple vaccination or double vaccination plus infection, additional vaccination and/or reinfection had no impact on neutralizing antibody titers over the observed period. These results strongly support the booster dose strategy, while additional booster doses within short time intervals might not improve immunization. However, dynamics of neutralizing antibodies titers needs to be monitored individually, over time and include newly emerging variants.
Keywords: Epidemiology, Neutralization test, SARS-CoV-2, Vaccination
End of 2019, a new virus named Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) was reported in Wuhan City (China) which is responsible of the still ongoing pandemic of Coronavirus Disease 2019 (COVID-19). In March 2022, half a billion individuals have been infected worldwide and 2.5 billions have been vaccinated (WHO, 2022). In Switzerland, 2.5 millions infections were recorded and 70% of the population was vaccinated with two doses of mRNA vaccine (Pfizer-BioNTech or Moderna) while 42% received a third booster dose [1]. In addition, a vaccine dose was recommended to individuals who had been infected before vaccination. Re-infections post-vaccination were reported with a frequency of 1%–15% depending on the type of study and the viral variant under investigation [[2], [3], [4]]. Individuals with a positive SARS-CoV-2 serology can be categorized into three major groups: vaccinated without infection, convalescent after infection, vaccinated plus infection. These groups can be subdivided depending on the number of vaccination doses received and the timing of infection before or after vaccination.
Serological investigations look for the presence of virus-specific antibodies as a marker of previous infection or vaccination [5]. However, these analyses do not assess whether the detected antibodies display a protective antiviral activity [6]. In SARS-CoV-2, the viral spike protein (S) is the primary target for neutralizing antibodies, which inhibit its binding to the host angiotensin-converting enzyme 2 (ACE2) receptor, the trigger of cell membrane fusion between the virus and the human cell [[7], [8], [9], [10]]. As neutralizing antibodies play a key role for viral clearance, the quantification of their activity provides a good estimate of immune protection [[11], [12], [13]]. The gold standard for measuring SARS-CoV-2 neutralizing antibodies activity relies on quantification of the reduction of virus-induced cytopathic effects after infection of ACE2-expressing cells with live virus but simpler cell-free neutralization assays have been developed [14]. In the present study, we used a surrogate neutralization assay measuring the competitive inhibition of ACE2 binding to a trimeric S protein loaded on beads [15]. This method is quantitative, high throughput and allows the simultaneous evaluation of the neutralization activity targeting spike protein encoded by different SARS-CoV-2 variants of concern [16,17].
Here, we investigated at four time points, between March 2021 and February 2022, the dynamics of SARS-CoV-2 neutralizing antibodies against the original Wuhan wild type virus and five major variants of concern (Alpha, Beta, Gamma, Delta and Omicron BA.1). This prospective observational study was conducted in health-care workers at the “Ensemble Hospitalier de la Côte” (EHC), a public hospital in Morges, Western Switzerland with 1′800 employees, 240 acute beds and 85 post-acute beds. The objective of the investigation was to quantify the neutralization activity of anti-SARS-CoV-2 antibodies in seropositive participants according to their vaccination and convalescent status.
2. Materials and methods
2.1. Study design
A prospective observational study was proposed to all EHC employees, Morges, Switzerland (n = 1′800). Participants over 18 years old were included on a voluntary basis after written informed consent at one of the following study visits: March 2021, June 2021, September 2021, and February 2022. Volunteers had the opportunity to be recruited or drop out at any of the four visits.
2.2. Questionnaire
All participants filled in a questionnaire with demographic characteristics, history and date of positive SARS-CoV-2 RT-PCR or antigen (AG) tests as well as date of first, second and/or third vaccination (Supplementary File 1). Questionnaires were manually digitalized.
2.3. Serum sampling
Blood was obtained at the inclusion and follow-up visits (10 ml Monovette® without anticoagulant) and processed as previously described [18].
2.4. Serological method
The samples were analyzed with a standard serological test for IgG anti-Spike (anti-S) and IgG anti-Nucleocapsid (anti-N) SARS-CoV-2 antibodies using the Luminex® system [19] as previously described [18]. Samples with a positive serology were further investigated using a surrogate neutralization test [15]. Dilutions of serum samples in PBS were added to plate wells containing S proteins-coupled beads. Variant investigated include the sequence of the wild type Wuhan, Alpha, Beta, Gamma, Delta and Omicron BA.1. The positive control for 100% neutralization consisted of a cocktail of two neutralizing antibodies binding distinct epitopes on the SARS-CoV-2 Spike protein. In absence of neutralizing antibodies, a tagged ACE2-Fc can freely bind to the S protein and induce maximal fluorescence intensity (MFIs). Neutralizing antibodies bind to the S protein and compete with its binding to ACE2: this inhibition effect can be quantified by reduced fluorescence intensities. Results are presented as IC50 of the calculated inhibition curve. Neutralization responses were classified in four categories: <50: undetectable neutralizing activity, 50–100: low neutralizing activity, >100–150: moderate neutralizing activity, >150: strong neutralizing activity. All sera were processed at the Laboratory of Immunology and Allergy, Lausanne University Hospital (CHUV), Switzerland.
2.5. Group definition
Participants were grouped according to data extracted from questionnaires and serological results. In absence of a history of documented infection (positive RT-PCR or AG tests) volunteers with a positive anti-S SARS-CoV-2 serology prior to the first vaccination (n = 32) or with a positive anti-N serology (n = 58) were excluded from the group vaccinated only. As the date of infection was unknown they were not included in the group vaccination/infection. Time course representation, in convalescent subjects and in those vaccinated with two doses or three doses, t = 0 was defined as the date of the first positive RT-PCR or AG test, of the second or the third vaccination dose, respectively. The time interval in days elapsed between t = 0 and the date of the study visit is represented. For vaccinated individuals (two or three doses), the status of infection before or after vaccination was determined using respectively, the second or third vaccination date as reference. For participants vaccinated with a single dose, the date of the first dose was used. For participants with two reported SARS-CoV-2 infection episodes, the first date was used unless otherwise specified in the text.
2.6. Statistical analysis
All analyses were performed with R version 4.0.2. Local polynomial regression fitting was performed using stat_smooth method loess. Graphs were drawn with ggplot2. Median and interquartile ranges were used to describe continuous variables. Kruskal–Wallis test by rank was used to compare the different groups (pairwise.wilcox.test). The significance level was set with two-sided p < 0.05.
2.7. Ethics
The Cantonal Ethical Review Board for Human Research (CER-VD, Commission cantonale d’éthique de la recherche sur l’être humain) approved the study protocol and the participants' informed consent form (Authorization Nr 2020–02300).
3. Results
3.1. Demographics of study volunteers
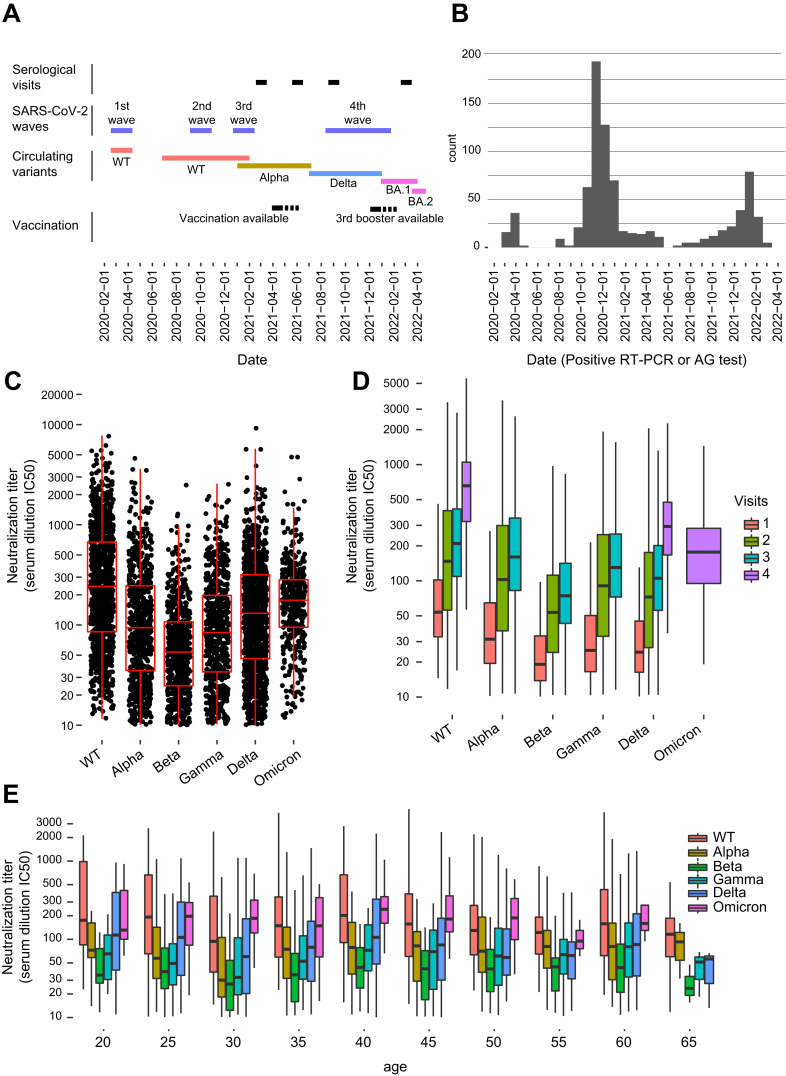
A total of 903 volunteers participated to this prospective observational study, representing half of the 1′800 hospital employees. 191 participated to all four visits, 147 to three visits, 207 to two visits and 358 to one visit for a total of 1977 sera. The majority of study participants were women (84%) and the median age was 43 years (IQR 33–52) (Fig. 1 A and Fig. S1A). 74.5% of participants were vaccinated with two doses (or one dose if they had a previously documented infection). Half of them received a third (respectively second) booster dose. 39.6% of participants reported a history of SARS-CoV-2 infection documented by a positive RT-PCR or AG test. The majority of these infections occurred during the epidemic waves preceding the first vaccination campaign in March 2021 (Fig. 1A and B). At the first visit, 45.3% of volunteers had a positive SARS-CoV-2 serology, 75% at the second visit, 85% at the third and 94.8% at the last visit (Table S1). A positive anti-S serology was observed in both convalescent and vaccinated individuals, while the anti-N serology was only positive after a natural infection. Re-infections post-vaccination occurred in 15.9% of volunteers, almost exclusively with the Omicron BA.1 strain in December 2021–January 2022, prior to the last visit (Fig. S1B).
Fig. 1.
A. Schematic representation of the study period: study visits, circulating SARS-CoV-2 variants, key epidemiological and immunization events. BA.1 and BA.2 are from the Omicron lineage. B. Positive SARS-CoV-2 RT-PCR and AG tests in volunteers throughout the study. C. Aggregate data of neutralizing antibodies titers against the Wuhan SARS-CoV-2 wild type and different variants (Alpha, Beta, Gamma, Delta, Omicron) throughout the study. D. Dynamics of neutralization response against the different variants at each of the four visits. Omicron was only investigated at the fourth visit, because it emerged after the third visit. Alpha, Beta and Gamma were only investigated at the first, second and third visit, as they disappeared at the time of the fourth visit. E. Age of volunteers versus neutralization response to the different variants.
3.2. Neutralization activity across variants
The neutralization activity was investigated against the S protein of the Wuhan SARS-CoV-2 wild type and of the variants of concern Alpha, Beta, Gamma, Delta and Omicron. The neutralization assay was available at the time of the third study visit when variants Alpha, Beta and Gamma were circulating worldwide while Delta was emerging and Omicron was absent. Therefore, for the first, second and third visits, neutralization tests were performed on the SARS-CoV-2 wild type plus Alpha, Beta, and Gamma. At the fourth visit, Alpha, Beta and Gamma variants had disappeared while the Delta was being progressively replaced by Omicron (BA.1). Hence, neutralization tests were performed on the SARS-CoV-2 wild type plus Delta and Omicron. Overall, we observed a broad distribution of neutralization activity among participants indicating an important variability in inter-individual humoral immune responses and among viral variants (Fig. 1C). The Beta and Gamma variants escaped significantly the neutralizing activities of anti-S antibodies while the SARS-CoV-2 wild type showed the highest response to neutralizing antibodies (Fig. 1C). A progressive increase of neutralization titers was observed across the four visits mirroring the increasing number of volunteers who were vaccinated and/or reported an infection during the study period (Fig. 1D). The relative low increase in neutralization response between the second (June 2021) and third (September 2021) visit is likely linked to the absence of vaccination campaign and low prevalence of infections during the summer 2021 (Fig. 1B and D). We observed no significant difference in neutralizing activity among age groups (Fig. 1E).
3.3. Neutralization antibody titers in convalescent and vaccinated individuals
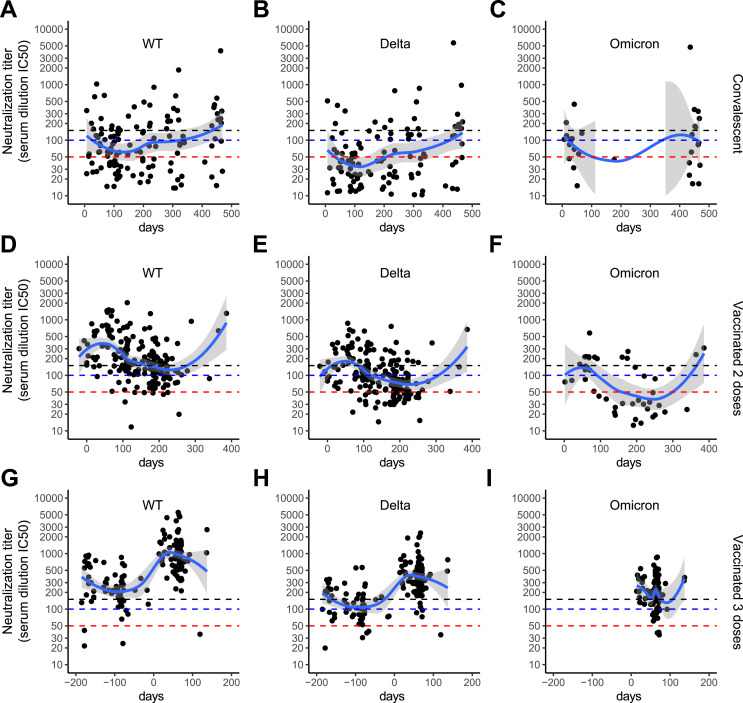
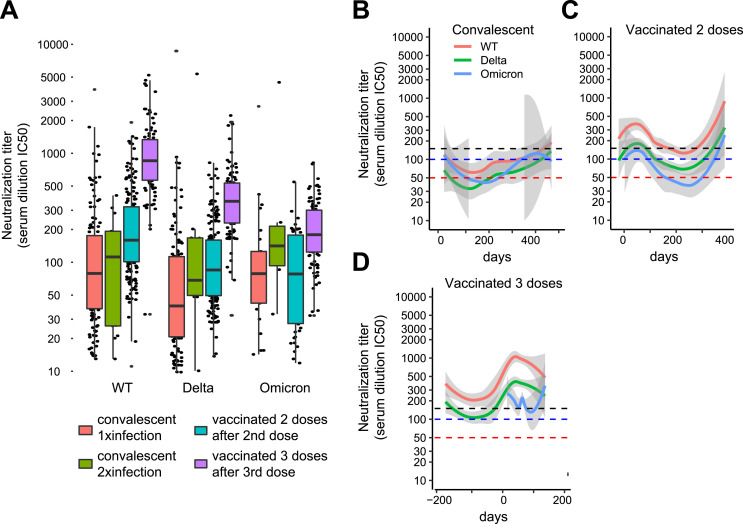
Participants with a positive serology (n = 773) were classified according to the vaccination status (one dose, two doses, or three doses) and/or the convalescent status (history of SARS-CoV-2 infection and reinfection documented by a positive RT-PCR or AG test) (Table 1 ). We first investigated the dynamics of the serological response in volunteers vaccinated with two or three doses without history of infection and in convalescent individuals without history of vaccination. These three groups displayed a simple immunization event (second vaccination, third vaccination or infection) that was used as reference time point to follow the dynamics of the neutralizing antibodies response. The date of the second or third vaccination and of the first positive RT-PCR or AG test was set as t = 0 for vaccinated and convalescent volunteers, respectively. The group of participants who were vaccinated with one single dose is not shown as most volunteers registered only for the second visit and then dropped out of the study. A local polynomial regression-fitting model was used to display an average neutralization response curve across variants and for each group. Convalescent participants showed the lowest neutralization activity after a three to four-month time interval following the reported infection (Fig. 2 A–C). Vaccination with two doses without a history of infection resulted in a robust neutralization response that slowly decreased over a three to six-month time interval (Fig. 2D–F). The third booster dose of SARS-CoV-2 mRNA vaccine was followed by a rapid and significant increase in neutralization titers (Fig. 2G–I). As the booster dose was made available shortly before the last visit, the dynamic of antibody titers after the vaccination was not recorded. The increases in neutralization titers observed beyond 250 days after a documented infection or a second vaccination likely represent reinfections, that were in part asymptomatic (Fig. 2A–F). The highest neutralization response was observed after triple vaccination and an intermediate response after two vaccinations. An increase of neutralization titers was observed after a second infection in convalescent only individuals in whom the response was comparable to that observed after double vaccination (Fig. 3 A). Although we observed substantial differences comparing the neutralization activities against the variants, the overall trends remained similar: convalescent individuals had the lowest neutralization response and a progressive increase of the neutralization activity was observed after double and triple vaccination (Fig. 3B–D and Fig. S2A-F).
Table 1.
Groups of volunteers according to their vaccination status (no, one, two or three vaccine doses) and/or convalescent status (no, one or two reported positive SARS-CoV-2 PCR or AG tests as a documentation of infection/reinfection). Number of volunteers/number of serological data throughout the study visits are reported. Of importance, volunteers could be included and drop out at any of the four study visits.
| Immune status | One reported positive test | Two reported positive tests | No reported positive RT-PCR or AG tests | Total |
|---|---|---|---|---|
| Convalescent | 89/139 | 6/15 | 96/118 | |
| Vaccination 1 dose | 30/75 | 10/22 | 57/75 | |
| Vaccination 2 doses | 68/172 | 17/48 | 183/296 | |
| Vaccination 3 doses | 82/227 | 6/19 | 129/255 | |
| Total | 269 | 39 | 465 | 773/1461 |
Fig. 2.
Dynamics of neutralizing antibodies titers over time against the Wuhan SARS-CoV-2 wild type, Delta and Omicron variants. As Omicron was only investigated at the last visit, fewer points are represented. Dashed lines: < 50 (red): no neutralizing activity; 50–100 (blue): low neutralizing activity; 100–150 (black): moderate neutralizing activity; > 150: high neutralizing activity [24]. A-C. Convalescent non-vaccinated volunteers. Time 0 corresponds to the reported date of the first positive SARS-CoV-2 RT-PCR or AG test as documentation of infection. D-F. Volunteers vaccinated with two doses and without history of COVID-19. Time 0 corresponds to second vaccination date. G-I. Volunteers vaccinated with three doses and without history of COVID-19. Time 0 corresponds to the third vaccination date.
Fig. 3.
A. Neutralizing antibodies titers against the Wuhan SARS-CoV-2 wild type, Delta and Omicron variants in convalescent individuals after the first or second infection and in vaccinated individuals after the second or third vaccination. Only the serological data after either the first or second infection and the second or third vaccination are shown in the graph. B-D. Neutralization response against the Wuhan SARS-CoV-2 wild type, Delta and Omicron variants in convalescent individuals and in individuals vaccinated with two or three doses.
3.4. Neutralization antibody titers in vaccinated individuals with documented COVID-19 infections
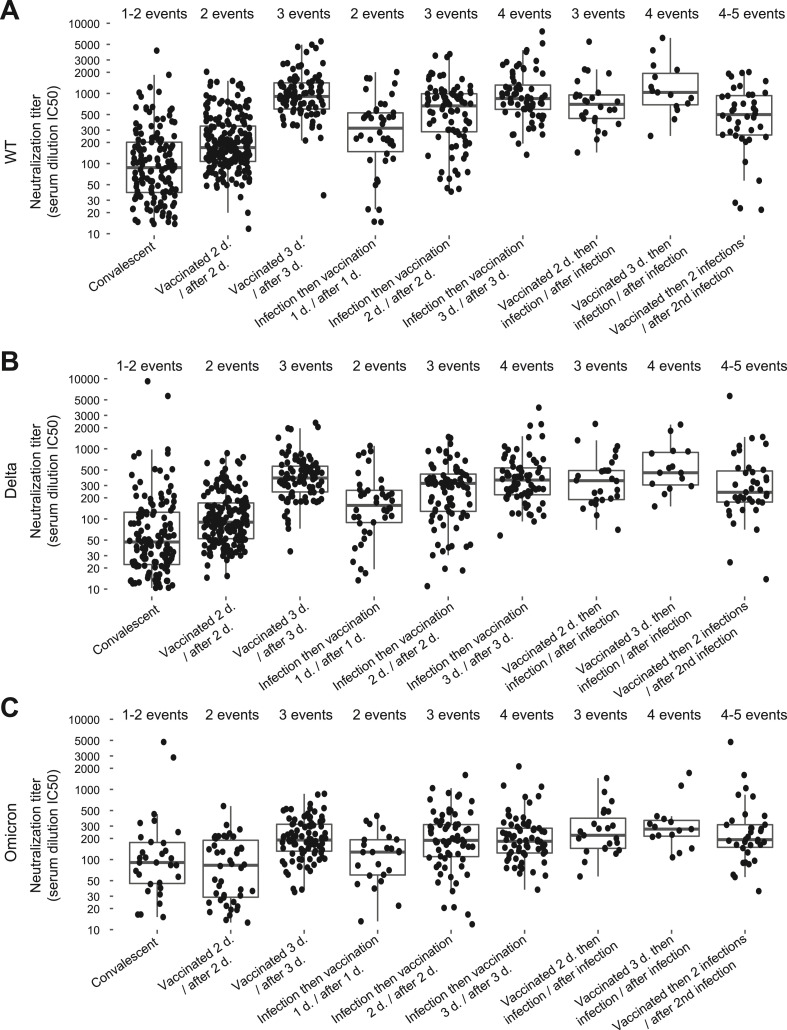
We investigated the different combinations of vaccinations (one, two, or three doses) with a documented SARS-CoV-2 infection occurring prior or post vaccination. A two-dose vaccination administered prior or after a natural infection (three immunization events) resulted in a neutralization response comparable to that obtained after a triple vaccination (three immunization events) (Fig. 4 A–C). The same neutralization dynamics was observed against the SARS-CoV-2 wild type, Delta and Omicron variant, although with Omicron differences were less pronounced likely due to its ability to escape humoral response. Among the volunteers, 39 reported two COVID-19 episodes confirmed by positive RT-PCR or AG test more than 60 days apart. All were vaccinated with either two or three doses and were re-infected recently with the Omicron BA.1 variant. Four (2 vaccinations and 2 infections) or five (3 vaccinations and 2 infections) immunization events did not further boost the neutralization titers compared to those observed after three immunization events (3 vaccinations or 2 vaccinations and 1 infection) (Fig. 4A–C, Table S2). Of note, volunteers with two infections showed the lowest neutralization titers at the study visit preceding the second infection, which was in agreement with a higher probability of getting re-infected (Fig. S3A-B). The same variation trends were observed against all variants.
Fig. 4.
Neutralization responses after different numbers of immunization events including infections and/or vaccinations. A. Wuhan SARS-CoV-2 wild type. B. Delta variant. C. Omicron variant.
4. Discussion
In this one-year prospective observational study in 903 hospital employees, we observed important variations in neutralizing anti-SARS-CoV-2 antibodies activities in seropositive individual [13]. At the group level, overall trends in viral neutralization could be predicted based on the history of vaccination and/or infection. However, large inter-individual differences highlight the difficulty to accurately predict the level of protection and illustrate the value of individual assessments of neutralizing antibodies.
As reported in other studies [20,21], neutralization titers differed significantly against the tested SARS-CoV-2 variants. Antibodies showed the highest neutralizing activity against the Wuhan wild type virus related to its spike protein being used for the development of mRNA vaccines. In convalescent non-vaccinated individuals, mostly exposed to the Alpha, Delta and Omicron variants, the neutralization activity was highest against the Wuhan wild type virus; although after natural infection, a variant-specific increase in neutralization titers would be expected. This discrepant observation could suggest that immunity to variants is not solely due to neutralization antibodies against a mutated S-protein but also to a more complex interplay with the immune system.
Significant differences in neutralizing antibodies activity were also observed among the different groups based on history of vaccination (one dose, two doses, or three doses) and SARS-CoV-2 infection. We showed that individuals with a convalescent status had a significantly lower neutralization response compared to vaccinated individuals [22,23]. SARS-CoV-2 antibody response after infection was previously shown to correlate with the severity of the disease [[24], [25], [26]]. In the present study, only volunteers with low to mild COVID-19 infections (only one volunteer reported a hospitalization) were investigated, which represents a good estimate of the immunization profile in the general population.
In individuals vaccinated with two doses, we observed significant neutralization titers followed by a progressive decrease beyond three months after the second dose. A third booster dose resulted in a significant rebound of neutralization activity. A similar boosted neutralization response was observed in individuals with SARS-CoV-2 breakthrough infection after two vaccine doses [27] and in convalescent individuals who received two vaccine doses after infection. These observations suggest that the sequential order of different immunological stimulations (vaccination followed by infection or viceversa) does not significantly impact the level of neutralization antibodies [28,29]. A maximal neutralization response was observed after three immunological stimuli (triple vaccination or double vaccination preceded or followed by infection) while double vaccinated or convalescent individuals showed significantly lower neutralization titers. After three immunization events, additional vaccination or reinfection had limited impact on the neutralization activity. This suggests that additional boosters (four or five immunization events) after reaching a neutralization antibodies titers plateau might be of limited value in the following three to six months period while the persistence of neutralization activity beyond this period remains to be investigated. Indeed, as a significant decrease in neutralizing antibody titers was observed over time in double vaccinated individuals, a similar decline might occur after three immunization events. In addition, significant differences might occur over time among triple vaccinated individuals and those with hybrid immunity (vaccination and natural infection). These heterologous immunization regimens can result in different long-term neutralization responses [28,30], as shown in a recent study showing that booster durability was longer in participants who had breakthrough infection [31]. Interestingly, we only observed reinfections in vaccinated individuals, but with more than 75% of volunteers being vaccinated the significance of this observation is unclear.
Among limitations of the present study, the used neutralization test is a proxy of the immune response measuring solely the antibodies activity on the interaction between the S protein and its ACE2 receptor in vitro. The complex interplay of humoral and cellular immune response to infection and/or vaccination was not investigated. While we observed no significant correlation between neutralizing antibody titers and age, other studies showed a consistent decrease of immunity in older individuals [26,32,33]. As the present investigation was restricted to working individuals younger than 65 years, we are unable to draw any conclusions on the duration of immunity in the elderly.
In conclusion, a triple vaccination or a natural infection prior or after a double vaccination result in a robust neutralization response strongly supporting a booster dose strategy. Although additional booster doses within three to six months after a third immunization event (infection or vaccination) might not boost immunity, the decline of neutralizing antibodies titers beyond this time window needs to be monitored on an individual basis by including new variants in neutralization assays. Yet the protection conferred by neutralizing antibodies against emerging variants is unpredictable: for example, immunity was shown to be strongly reduced against the most recent Omicron BA.4 and BA.5 variants [4] and appears to more rapidly decline [34]. A booster vaccine dose integrating newly appearing variants might contribute to meet the complex challenge of maintaining an effective immunity over time.
Funding sources
The study was supported by unrestricted research grants in the field of diagnosis of SARS-CoV-2 infection and epidemiology of COVID-19 pandemic from Ferring International Center, Saint-Prex, Switzerland. The project was partially supported by the patients’ safety program, General Direction, EHC, Morges, Switzerland and the R&D programs of the Institute of Microbiology and the Service of Immunology and Allergy Service, Department of Laboratories, CHUV, Lausanne, Switzerland.
Declaration of competing interest
G. Greub is medical advisor of Resistell, a startup active in the development of a new instrument to faster antibiotic susceptibility results. G. Greub has a research agreement with Becton Dickinson (USA) and Resistell (Switzerland), both unrelated to the present work. The rest of the authors have no conflict of interest to declare.
Acknowledgments
We would like to warmly thank the following persons for their outstanding contributions to study logistics: Emilie Alves, Nathalie Divorne Formenton, Anne Durrer, Valérie Klein, Giulia Marchetti, Karen Masnada, Dominique Peschoud, Benjamin Suatton, and Coralie Verdelet for the organization of volunteer's recruitment, collection of informed consent and questionnaires at EHC. Yvana Codija, Amandine Lauper, Mégane Singer, Melissa Teste for blood sampling from volunteers at EHC. Staff of the Reception desk at CHUV for management of study questionnaires and blood samples. Cyril André, Department of laboratories, CHUV for study coordination. Laboratory of Immunology and Allergy, CHUV for processing and analyzing blood samples. Carla Maceda Marques, Institute of Microbiology, CHUV for digitalization of study questionnaires. Frédéric André, Noah Boegli, and Eric Maurin, Department of Information systems, EHC, and Franck Hottin and Fabien Faverjon, Department of Information systems, CHUV for programming recruitment schedules, anonymization and distribution of serological results.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.micinf.2022.105077.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Foph FOoPH. 2022. COVID-19 Switzerland. [Google Scholar]
- 2.Levin-Rector A., Firestein L., McGibbon E., Sell J., Lim S., Lee E.H., et al. Reduced odds of SARS-CoV-2 reinfection after vaccination among New York city adults, June–August 2021. medRxiv. 2021:2021. doi: 10.1093/cid/ciac380. 12.09.21267203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhotra S., Mani K., Lodha R., Bakhshi S., Mathur V.P., Gupta P., et al. SARS-CoV-2 reinfection rate and estimated effectiveness of the inactivated whole virion vaccine BBV152 against reinfection among health care workers in New Delhi, India. JAMA Netw Open. 2022;5 doi: 10.1001/jamanetworkopen.2021.42210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hachmann N.P., Miller J., Collier A.Y., Ventura J.D., Yu J., Rowe M., et al. Neutralization escape by SARS-CoV-2 Omicron subvariants BA.2.12.1, BA.4, and BA.5. N Engl J Med. 2022;387:86–88. doi: 10.1056/NEJMc2206576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caruana G., Croxatto A., Coste A.T., Opota O., Lamoth F., Jaton K., et al. Diagnostic strategies for SARS-CoV-2 infection and interpretation of microbiological results. Clin Microbiol Infect. 2020;26:1178–1182. doi: 10.1016/j.cmi.2020.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okba N.M.A., Muller M.A., Li W., Wang C., GeurtsvanKessel C.H., Corman V.M., et al. Severe acute respiratory Syndrome Coronavirus 2-specific antibody responses in Coronavirus disease patients. Emerg Infect Dis. 2020;26:1478–1488. doi: 10.3201/eid2607.200841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu L.H., Wang P.F., Nair M.S., Yu J., Rapp M., Wang Q., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 8.Cerutti G., Guo Y.C., Zhou T.Q., Gorman J., Lee M., Rapp M., et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe. 2021;29:819. doi: 10.1016/j.chom.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barnes C.O., Jette C.A., Abernathy M.E., Dam K.M.A., Esswein S.R., Gristick H.B., et al. SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature. 2020;588:682. doi: 10.1038/s41586-020-2852-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benton D.J., Wrobel A.G., Xu P.Q., Roustan C., Martin S.R., Rosenthal P.B., et al. Receptor binding and priming of the spike protein of SARS-CoV-2 for membrane fusion. Nature. 2020;588 doi: 10.1038/s41586-020-2772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang N.Y.L., Pang A.S.R., Chow V.T., Wang D.Y. Understanding neutralising antibodies against SARS-CoV-2 and their implications in clinical practice. Military Med Res. 2021;8 doi: 10.1186/s40779-021-00342-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Khoury D.S., Cromer D., Reynaldi A., Schlub T.E., Wheatley A.K., Juno J.A., et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 13.Chia W.N., Zhu F., Ong S.W.X., Young B.E., Fong S.W., Le Bert N., et al. Dynamics of SARS-CoV-2 neutralising antibody responses and duration of immunity: a longitudinal study. Lancet Microbe. 2021;2:E240–E249. doi: 10.1016/S2666-5247(21)00025-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y.Y., Wang J., Li Q.L., Hu H., Lu J.H., Chen Z.L. Advances in neutralization assays for SARS-CoV-2. Scand J Immunol. 2021:94. [Google Scholar]
- 15.Fenwick C., Turelli P., Pellaton C., Farina A., Campos J., Raclot C., et al. Vol. 2021. 2021. (A multiplexed high-throughput neutralization assay reveals a lack of activity against multiple variants after SARS-CoV-2 infection). 04.08.21255150. [Google Scholar]
- 16.Malik J.A., Ahmed S., Mir A., Shinde M., Bender O., Alshammari F., et al. The SARS-CoV-2 mutations versus vaccine effectiveness: new opportunities to new challenges. J Infect Public Health. 2022;15:228–240. doi: 10.1016/j.jiph.2021.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harvey W.T., Carabelli A.M., Jackson B., Gupta R.K., Thomson E.C., Harrison E.M., et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol. 2021;19:409–424. doi: 10.1038/s41579-021-00573-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacot D., von Rotz U., Blondet F., Aebischer O., Matthieu P., De Rham M., et al. SARS-CoV-2 seroprevalence in hospital healthcare workers in Western Switzerland at the end of the second pandemic wave. J Med Microbiol. 2022:71. doi: 10.1099/jmm.0.001558. [DOI] [PubMed] [Google Scholar]
- 19.Fenwick C., Croxatto A., Coste A.T., Pojer F., Andre C., Pellaton C., et al. Changes in SARS-CoV-2 spike versus nucleoprotein antibody responses impact the estimates of infections in population-based seroprevalence studies. J Virol. 2021 Jan 13;95(3) doi: 10.1128/JVI.01828-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bates T.A., Leier H.C., Lyski Z.L., McBride S.K., Coulter F.J., Weinstein J.B., et al. Neutralization of SARS-CoV-2 variants by convalescent and BNT162b2 vaccinated serum. Nat Commun. 2021;12:5135. doi: 10.1038/s41467-021-25479-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stamatatos L., Czartoski J., Wan Y.H., Homad L.J., Rubin V., Glantz H., et al. mRNA vaccination boosts cross-variant neutralizing antibodies elicited by SARS-CoV-2 infection. Science. 2021 Jun 25;372(6549):1413–1418. doi: 10.1126/science.abg9175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuzmina A., Khalaila Y., Voloshin O., Keren-Naus A., Boehm-Cohen L., Raviv Y., et al. SARS-CoV-2 spike variants exhibit differential infectivity and neutralization resistance to convalescent or post-vaccination sera. Cell Host Microbe. 2021;29:522–528 e2. doi: 10.1016/j.chom.2021.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavanaugh A.M., Spicer K.B., Thoroughman D., Glick C., Winter K. Reduced risk of reinfection with SARS-CoV-2 after COVID-19 vaccination - Kentucky, may-june 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1081–1083. doi: 10.15585/mmwr.mm7032e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenwick C., Turelli P., Pellaton C., Farina A., Campos J., Raclot C., et al. A high-throughput cell- and virus-free assay shows reduced neutralization of SARS-CoV-2 variants by COVID-19 convalescent plasma. Sci Transl Med. 2021:13. doi: 10.1126/scitranslmed.abi8452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shrock E., Fujimura E., Kula T., Timms R.T., Lee I.H., Leng Y., et al. Viral epitope profiling of COVID-19 patients reveals cross-reactivity and correlates of severity. Science. 2020:370. doi: 10.1126/science.abd4250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Beltran W.F., Lam E.C., Astudillo M.G., Yang D.N., Miller T.E., Feldman J., et al. COVID-19-neutralizing antibodies predict disease severity and survival. Cell. 2021;184:476. doi: 10.1016/j.cell.2020.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Evans J.P., Zeng C., Carlin C., Lozanski G., Saif L.J., Oltz E.M., et al. Neutralizing antibody responses elicited by SARS-CoV-2 mRNA vaccination wane over time and are boosted by breakthrough infection. Sci Transl Med. 2022;14 doi: 10.1126/scitranslmed.abn8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bates T.A., McBride S.K., Leier H.C., Guzman G., Lyski Z.L., Schoen D., et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci Immunol. 2022 doi: 10.1126/sciimmunol.abn8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walls A.C., Sprouse K.R., Bowen J.E., Joshi A., Franko N., Navarro M.J., et al. SARS-CoV-2 breakthrough infections elicit potent, broad, and durable neutralizing antibody responses. Cell. 2022;185:872–880 e3. doi: 10.1016/j.cell.2022.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crotty S. Hybrid immunity. Science. 2021;372:1392–1393. [Google Scholar]
- 31.Qu P., Faraone J.N., Evans J.P., Zheng Y.M., Yu L., Ma Q., et al. N Engl J Med; 2022. Durability of booster mRNA vaccine against SARS-CoV-2 BA.2.12.1, BA.4, and BA.5 subvariants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bates T.A., Leier H.C., Lyski Z.L., Goodman J.R., Curlin M.E., Messer W.B., et al. Age-dependent neutralization of SARS-CoV-2 and P.1 variant by vaccine immune serum samples. JAMA, J Am Med Assoc. 2021;326:868. doi: 10.1001/jama.2021.11656. [-+] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang H.S., Costa V., Racine-Brzostek S.E., Acker K.P., Yee J., Chen Z.M., et al. Association of age with SARS-CoV-2 antibody response. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyke K.E., Atmar R.L., Islas C.D., Posavad C.M., Szydlo D., Paul Chourdhury R., et al. Rapid decline in vaccine-boosted neutralizing antibodies against SARS-CoV-2 Omicron variant. Cell Rep Med. 2022;3 doi: 10.1016/j.xcrm.2022.100679. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.