ABSTRACT
Previously, we showed that Enterococcus faecium clade B strains outcompeted health care-associated clade A1 strains in murine gastrointestinal colonization. Here, parenterally administered piperacillin-tazobactam and ceftriaxone significantly promoted colonization by clade A1 over clade B strains except that ceftriaxone, at the dose used, did not favor the least β-lactam-resistant A1 strain. The advantage that β-lactam administration gives to more highly ampicillin-resistant E. faecium over ampicillin-susceptible strains mirrors what occurs in hospitalized patients administered these antibiotics.
KEYWORDS: ceftriaxone, piperacillin-tazobactam, E. faecium, mouse GI colonization, ampicillin resistant
INTRODUCTION
Genome analyses have indicated a split of Enterococcus faecium into 2 clades (clades A and B), with branching of clade A (1, 2). While clade B is the predominant gut E. faecium clade in humans in the community and is generally antibiotic susceptible, gut colonization with clade A1 strains, which are more antibiotic resistant, including to vancomycin and ampicillin (AMP), is very common in hospitalized patients, replacing Enterococcus faecalis and clade B strains (1, 2). The different levels of AMP resistance are due, in large part, to differences in the pbp5 sequence (3, 4) and to different levels of expression of PBP5 (5).
Using a preconditioned gastrointestinal tract (GIT) mouse model, we previously showed that clade B strains significantly outcompeted clade A strains (6) in GIT colonization. This provided, experimentally, a basis for the observation that hospital-associated vancomycin-resistant Enterococcus (VRE) generally decreases and even disappears once patients are no longer on antibiotics (7–9). Using the same strain pairs, we report here results of GIT colonization and competition between clade A1 and clade B during systemic β-lactam administration.
E. faecium strains representing clade A1 (C68A1, TX82A1, and TX16A1) and clade B (COM15B and E980B), used previously (6), and MICs (per CLSI [10]) are in Table 1. Our established mouse GIT model and methods were used with preconditioning with gentamicin and clindamycin (6). Piperacillin-tazobactam (TZP) (3.37 mg/kg of body weight, every 12 h [Q12h]) and ceftriaxone (CRO) (0.5 mg/kg, Q12h) (both from Sigma-Aldrich Chemicals) were given subcutaneously (s.c.) as in reference 11 for 14 days starting 1 h postinoculation. Dosing was designed for proof of principle with minimal injection repeats (11). Approximately 109 CFU/mL (confirmed by subsequently plating) was given by oral gavage as previously described (6). Cages, animals, and fecal pellets were handled as in reference 6 under AWC-19-0139 of the University of Texas Health Science Center at Houston.
TABLE 1.
In vitro MICs for E. faecium strains
| Bacterial strain and description | MIC (μg/mL)a |
||
|---|---|---|---|
| AMP | CRO | TZP | |
| E. faecium TX82 AMPr, ERYr erm(B), TETr, VANr | 64 | 512 | 256 |
| E. faecium C68 AMPr, LZDns, TETr, VANr | 128 | >1,024 | 512 |
| E. faecium TX16 ERYr erm(B), TETr | 8–16 | 512 | 64 |
| E. faecium E980 | 0.75–1 | 256 | 16 |
| E. faecium COM15 | 0.12–0.19 | 128 | 4 |
CLSI breakpoints (10) are as follows: CLSI breakpoints for enterococci are ≤8 μg/mL (susceptible) and ≥16 μg/mL (resistant) with AMP results used to predict susceptibility to piperacillin-tazobactam. There are no CLSI breakpoints for E. faecium.
Statistical analyses were performed as previously described (6).
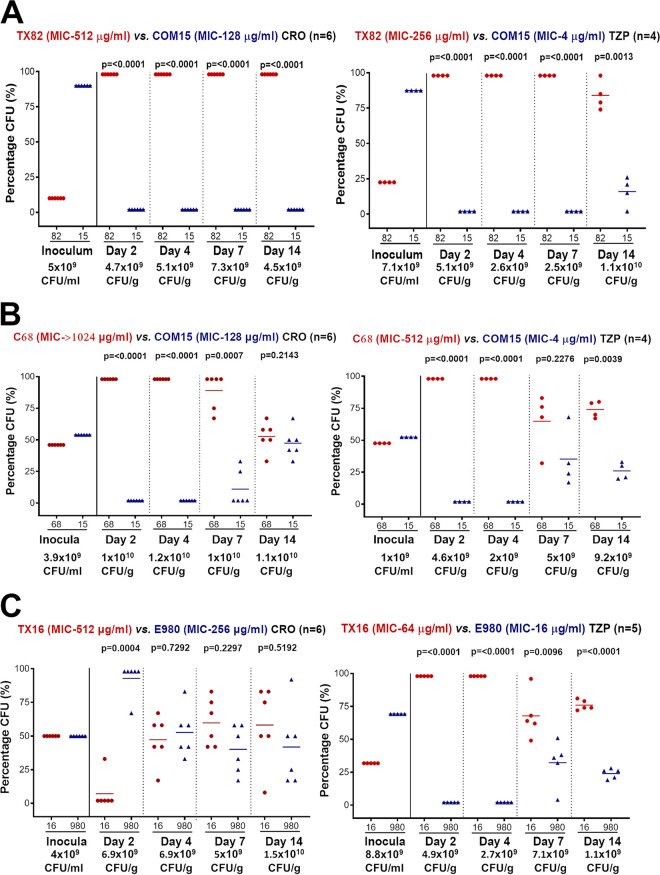
Figure 1A shows strain TX82A1 coinoculated with COM15B. Both s.c. CRO (top left) and s.c. TZP (top right) promoted a highly significant increase in the percentage of the TX82A1 versus COM15B at all time points despite less TX82A1 (10 to 22%) in the inoculated mixture. Most time points had no colonies of clade B recovered except for TZP at 14 days.
FIG 1.
Mouse gastrointestinal tract (GIT) colonization by E. faecium clade A1 versus clade B strains in a mixed inoculation (1:1) competition assay; shown are the percentages (%) of total E. faecium CFU of clade A1 versus that of clade B strains in the inoculum mix and from fecal pellets recovered 2, 4, 7, and 14 days after oral inoculation. The horizontal lines indicate the means. A paired t test was used to calculate P values for the percentage of bacteria recovered in the fecal pellets versus that of the inoculum mix. The total E. faecium CFU per milliliter (inoculum) or CFU per gram (pellets) is given below the day. (A) Top left panel shows the effect of s.c. CRO administration (n = 6) after inoculation of a mix of strains TX82A1 and Com15B. Top right panel shows the effect of s.c. TZP administration (n = 4) after oral inoculation of mice with the same mixture. Solid red circles represent TX82A1, and solid blue triangles represent Com15B. (B) Top left panel shows the effect of s.c. CRO administration (n = 6) after inoculation of a mix of strains C68A1 and Com15B. Top right panel shows the effect of s.c. TZP administration (n = 4) after oral inoculation of mice with the same mixture. Solid red circles represent C68A1, and solid blue triangles represent Com15B. (C) Top left panel shows results with s.c. CRO administration (n = 6) after inoculation of a mix of strains TX16A1 versus E980B. Top right panel shows the effect of s.c. TZP antibiotic treatment (n = 4) after inoculation of a mix of the same strains. Solid red circles represent TX16A1, and solid blue triangles represent E980B.
Similarly, for C68A1 coinoculated with COM15B, CRO (Fig. 1B, top left) resulted in highly significantly more of the clade A1 strain at 2 days, 4 days, and 7 days, despite having a lower percentage of C68A1 in the inoculum (46 to 48%). At 14 days, CRO resulted in a nonsignificant (NS) increase in C68A1. TZP (Fig. 1B, top right) also resulted in a highly significant increase in C68A1 versus COM15B at day 2, 4, and 14, while the difference at day 7 was NS.
Figure 1C shows strain TX16A1 (the least-β-lactam-resistant A1 strain) (Table 1) coinoculated with E980B (the more-β-lactam-resistant strain of the clade B strains used). Unlike the pairs above, CRO (top left) resulted in a significantly higher percentage of the commensal strain E980B on day 2. At 4 days, 7 days, and 14 days, the differences were nonsignificant. However, TZP (top right) resulted in highly significantly more TX16A1 than E980B at all time points, similar to results above with other strain pairs.
Many factors influence a bacterium’s ability to successfully colonize the GIT. These include various interactions with host components/host products, interactions with the cohabitating GI flora/its products, as well as the ability to utilize or withstand substances ingested, such as antibiotics (2, 7, 12).
Our previous study found that monoinoculation of E. faecium strains of clade A1, A2, or B into mice pretreated with antibiotics, but with no antibiotics after inoculation, resulted in the anticipated high density of each strain (109 CFU/g) on day 2, presumably related to a decrease/elimination of much of the flora by the preconditioning antibiotics. There was then a decrease to circa 104 CFU/g by day 14, reminiscent of reports that clade A1 VRE decrease or disappear once patients are off antibiotics (13), likely due to an effect of the return of other bacteria (7, 14). We also found that, when inoculated together without continuing antibiotics, clade B strains gradually outnumbered clade A strains after day 2 for most strain pairs, indicating that clade A1 strains were less “fit” than clade B E. faecium under the conditions used.
Here, we investigated the effect of the s.c. administration of two β-lactams on GIT colonization after coinoculation of strain pairs of E. faecium. As before, a high density (109 CFU/g) of E. faecium was achieved at day 2 by the antibiotic preconditioning. Unlike our previous study, however, under the continued presence of s.c. CRO or TZP, this high density was maintained through day 14, likely related to the continuing suppression of the normal GIT flora.
CRO lacks clinically relevant antienterococcal activity, but its primary excretion is into human bile, resulting in very high concentrations when given parenterally (15–17), and is known to support persistently high levels of stool VRE in mice. In the current study, we found that the more-β-lactam-resistant (Table 1) strains C68A1 and TX82A1 significantly outcompeted the more-susceptible community-associated E. faecium COM15B at 7/8 time points. The fold differences in CRO MICs between TX82A1 and COM15B and between C68A1 and COM15B are 4- and >8-fold, respectively. Although we do not know the CRO concentrations in the gut, our results indicate that there was sufficient CRO not only to promote a high density of E. faecium but also to favor the more-β-lactam-resistant A1 strains over the clade B strain, a result opposite to our earlier observations without β-lactam use.
In contrast, when the least β-lactam resistant (Table 1) of the A1 strains, TX16A1, was paired with E980B, a clade B strain with higher β-lactam MICs then COM15B used above, parenteral CRO favored E980B over TX16A1 at day 2 and at no time favored the A1 strain, unlike what was seen with other strain pairs. While MICs are an imprecise measure of susceptibility, the smaller difference in CRO MICs (2-fold) between these two strains versus those for the other strain pairs (4- and >8-fold) supports the concept that the degree of resistance would likely be important in determining a selective advantage.
For TZP, although some is secreted into the bile (1,125.3/13.9 μg/mL) (17–22), its primary excretion is via the kidneys; 68% and 80% of the unchanged piperacillin and tazobactam, respectively, of the administered dose gets excreted in the urine (23). Conversely, it is more potent than CRO against enterococci (Table 1). We noted that there was a bigger difference in TZP MICs (Table 1) between the A1 and B strain pairs (Fig. 1A to C, 64-fold, 128-fold, and 4-fold, respectively) than for CRO MICs. Here, with all 3 strain pairs, TZP conferred a highly significant selective advantage to the A1 versus the clade B strains (P < 0.005) at 11/12 time points. This indicates that, under these conditions, enough TZP gets into the GIT to sustain a high density of E. faecium as well as to select for the more-TZP-resistant E. faecium strain (the A1 strain) in each pair. Additionally, Fig. 1B data (day 14) show the narrowing of the colonization differences between clade A1 and clade B strains (left), which could be due to other transmissible genetic factors, e.g., hyaluronidaseEfm, that promote colonization of the mouse GI tract (24) and were not studied.
As was pointed out above, the GIT concentrations of TZP and CRO in this study are not known; however, published literature in mouse model has documented these levels (25, 26). Further, few if any recent hospital-associated clade A1 strains display the low β-lactam resistance (e.g., AMP MIC of 8 to 16 μg/mL) seen with TX16A1, a strain isolated in 1992. Thus, the difference in the effect of CRO and TZP on the outcome of competition between this clade A1 strain and normal flora clade B strains should not be extrapolated to humans or to current isolates.
In summary, we showed conditional predominance of clade A1 E. faecium strains over clade B strains in an in vivo competition model for mouse GIT colonization at 18/24 time points, with the “condition” being the parenteral administration of a β-lactam; predominance also appeared to correlate with the degree of β-lactam resistance. These results are consistent with the observation that AMP-resistant VRE (i.e., clade A1 E. faecium) can become the predominant flora in patients during hospitalization and receipt of antibiotics (27, 28), which typically includes a β-lactam, helping the more resistant E. faecium clade A1 strains overcome their relative lack of fitness when present with clade B strains (6) in the absence of antibiotics.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health from the Division of Microbiology and Infectious Diseases, National Institute of Allergy and Infectious Diseases (grant R21 AI103260-01 to B.E.M.). B.E.M. and K.V.S. were in part supported by R21AI133289-01A1 from NIAID.
We thank Karen Jacques-Palaz for technical assistance.
REFERENCES
- 1.Galloway-Pena J, Roh JH, Latorre M, Qin X, Murray BE. 2012. Genomic and SNP analyses demonstrate a distant separation of the hospital and community-associated clades of Enterococcus faecium. PLoS One 7:e30187. 10.1371/journal.pone.0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lebreton F, van Schaik W, McGuire AM, Godfrey P, Griggs A, Mazumdar V, Corander J, Cheng L, Saif S, Young S, Zeng Q, Wortman J, Birren B, Willems RJ, Earl AM, Gilmore MS. 2013. Emergence of epidemic multidrug-resistant Enterococcus faecium from animal and commensal strains. mBio 4:e00534-13. 10.1128/mBio.00534-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pietta E, Montealegre MC, Roh JH, Cocconcelli PS, Murray BE. 2014. Enterococcus faecium PBP5-S/R, the missing link between PBP5-S and PBP5-R. Antimicrob Agents Chemother 58:6978–6981. 10.1128/AAC.03648-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice LB, Bellais S, Carias LL, Hutton-Thomas R, Bonomo RA, Caspers P, Page MG, Gutmann L. 2004. Impact of specific pbp5 mutations on expression of beta-lactam resistance in Enterococcus faecium. Antimicrob Agents Chemother 48:3028–3032. 10.1128/AAC.48.8.3028-3032.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montealegre MC, Roh JH, Rae M, Davlieva MG, Singh KV, Shamoo Y, Murray BE. 2017. Differential penicillin-binding protein 5 (PBP5) levels in the Enterococcus faecium clades with different levels of ampicillin resistance. Antimicrob Agents Chemother 61:e02034-16. 10.1128/AAC.02034-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montealegre MC, Singh KV, Murray BE. 2016. Gastrointestinal tract colonization dynamics by different Enterococcus faecium clades. J Infect Dis 213:1914–1922. 10.1093/infdis/jiv597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim S, Covington A, Pamer EG. 2017. The intestinal microbiota: antibiotics, colonization resistance, and enteric pathogens. Immunol Rev 279:90–105. 10.1111/imr.12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donskey CJ, Hoyen CK, Das SM, Helfand MS, Hecker MT. 2002. Recurrence of vancomycin-resistant Enterococcus stool colonization during antibiotic therapy. Infect Control Hosp Epidemiol 23:436–440. 10.1086/502081. [DOI] [PubMed] [Google Scholar]
- 9.Shenoy ES, Paras ML, Noubary F, Walensky RP, Hooper DC. 2014. Natural history of colonization with methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Enterococcus (VRE): a systematic review. BMC Infect Dis 14:177. 10.1186/1471-2334-14-177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2021. Performance standards for antimicrobial susceptibility testing—31st ed. CLSI M100-ED 31. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 11.Rice LB, Hutton-Thomas R, Lakticova V, Helfand MS, Donskey CJ. 2004. Beta-lactam antibiotics and gastrointestinal colonization with vancomycin-resistant enterococci. J Infect Dis 189:1113–1118. 10.1086/382086. [DOI] [PubMed] [Google Scholar]
- 12.Palmer KL, Godfrey P, Griggs A, Kos VN, Zucker J, Desjardins C, Cerqueira G, Gevers D, Walker S, Wortman J, Feldgarden M, Haas B, Birren B, Gilmore MS. 2012. Comparative genomics of enterococci: variation in Enterococcus faecalis, clade structure in E. faecium, and defining characteristics of E. gallinarum and E. casseliflavus. mBio 3:e00318-11. 10.1128/mBio.00318-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donskey CJ, Chowdhry TK, Hecker MT, Hoyen CK, Hanrahan JA, Hujer AM, Hutton-Thomas RA, Whalen CC, Bonomo RA, Rice LB. 2000. Effect of antibiotic therapy on the density of vancomycin-resistant enterococci in the stool of colonized patients. N Engl J Med 343:1925–1932. 10.1056/NEJM200012283432604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wlodarska M, Willing B, Keeney KM, Menendez A, Bergstrom KS, Gill N, Russell SL, Vallance BA, Finlay BB. 2011. Antibiotic treatment alters the colonic mucus layer and predisposes the host to exacerbated Citrobacter rodentium-induced colitis. Infect Immun 79:1536–1545. 10.1128/IAI.01104-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hayton WL, Schandlik R, Stoeckel K. 1986. Biliary excretion and pharmacokinetics of ceftriaxone after cholecystectomy. Eur J Clin Pharmacol 30:445–451. 10.1007/BF00607958. [DOI] [PubMed] [Google Scholar]
- 16.Novick WJ, Jr. 1982. Levels of cefotaxime in body fluids and tissues: a review. Rev Infect Dis 4(Suppl):S346–S353. 10.1093/clinids/4.supplement_2.s346. [DOI] [PubMed] [Google Scholar]
- 17.Thabit AK. 2020. Antibiotics in the biliary tract: a review of the pharmacokinetics and clinical outcomes of antibiotics penetrating the bile and gallbladder wall. Pharmacotherapy 40:672–691. 10.1002/phar.2431. [DOI] [PubMed] [Google Scholar]
- 18.Sorgel F, Kinzig M. 1993. The chemistry, pharmacokinetics and tissue distribution of piperacillin/tazobactam. J Antimicrob Chemother 31(Suppl A):39–60. 10.1093/jac/31.suppl_a.39. [DOI] [PubMed] [Google Scholar]
- 19.Westphal JF, Brogard JM, Caro-Sampara F, Adloff M, Blickle JF, Monteil H, Jehl F. 1997. Assessment of biliary excretion of piperacillin-tazobactam in humans. Antimicrob Agents Chemother 41:1636–1640. 10.1128/AAC.41.8.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kundrapu S, Sunkesula VC, Jury LA, Cadnum JL, Nerandzic MM, Musuuza JS, Sethi AK, Donskey CJ. 2016. Do piperacillin/tazobactam and other antibiotics with inhibitory activity against Clostridium difficile reduce the risk for acquisition of C. difficile colonization? BMC Infect Dis 16:159. 10.1186/s12879-016-1514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taylor EW, Poxon V, Alexander-Williams J, Jackson D. 1983. Biliary excretion of piperacillin. J Int Med Res 11:28–31. 10.1177/030006058301100106. [DOI] [PubMed] [Google Scholar]
- 22.Wilcox MH, Brown A, Freeman J. 2001. Faecal concentrations of piperacillin and tazobactam in elderly patients. J Antimicrob Chemother 48:155–156. 10.1093/jac/48.1.155. [DOI] [PubMed] [Google Scholar]
- 23.Pfizer Inc. 2022. Zosyn (piperacillin/tazobactam). Pfizer Inc., New York, NY. https://www.pfizermedicalinformation.com/en-us/zosyn/clinical-pharmacology. [Google Scholar]
- 24.Rice LB, Lakticova V, Carias LL, Rudin S, Hutton R, Marshall SH. 2009. Transferable capacity for gastrointestinal colonization in Enterococcus faecium in a mouse model. J Infect Dis 199:342–349. 10.1086/595986. [DOI] [PubMed] [Google Scholar]
- 25.Perez F, Pultz MJ, Endimiani A, Bonomo RA, Donskey CJ. 2011. Effect of antibiotic treatment on establishment and elimination of intestinal colonization by KPC-producing Klebsiella pneumoniae in mice. Antimicrob Agents Chemother 55:2585–2589. 10.1128/AAC.00891-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stiefel U, Pultz NJ, Donskey CJ. 2007. Effect of carbapenem administration on establishment of intestinal colonization by vancomycin-resistant enterococci and Klebsiella pneumoniae in mice. Antimicrob Agents Chemother 51:372–375. 10.1128/AAC.00355-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taur Y, Xavier JB, Lipuma L, Ubeda C, Goldberg J, Gobourne A, Lee YJ, Dubin KA, Socci ND, Viale A, Perales MA, Jenq RR, van den Brink MR, Pamer EG. 2012. Intestinal domination and the risk of bacteremia in patients undergoing allogeneic hematopoietic stem cell transplantation. Clin Infect Dis 55:905–914. 10.1093/cid/cis580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ubeda C, Taur Y, Jenq RR, Equinda MJ, Son T, Samstein M, Viale A, Socci ND, van den Brink MR, Kamboj M, Pamer EG. 2010. Vancomycin-resistant Enterococcus domination of intestinal microbiota is enabled by antibiotic treatment in mice and precedes bloodstream invasion in humans. J Clin Invest 120:4332–4341. 10.1172/JCI43918. [DOI] [PMC free article] [PubMed] [Google Scholar]