Abstract
Nitric oxide (NO) production by inducible NO synthase (iNOS) during inflammation is an essential element of antimicrobial immunity but can also contribute to host-induced tissue damage. Under conditions of bacterial sepsis, large amounts of NO are produced, causing hypotension, a critical pathological feature of septic shock. In sepsis caused by gram-positive organisms, the bacterial factors contributing to host NO production are poorly characterized. We show that a soluble toxin of Streptococcus pneumoniae, pneumolysin (Pln), is a key component initiating NO production from macrophages. In contrast to wild-type bacteria, a mutant of S. pneumoniae lacking Pln failed to elicit NO production from murine macrophages. Purified recombinant Pln induced NO production at low concentrations and independently of exogenous gamma interferon (IFN-γ) priming of RAW 264.7 macrophages. However, IFN-γ was essential for Pln-induced NO production, since primary macrophages from mice lacking the IFN-γ receptor or interferon regulatory factor 1, a transcription factor essential for iNOS expression, failed to produce NO when stimulated with Pln. In addition, Pln acts as an agonist of tumor necrosis factor alpha and interleukin 6 production in macrophages. The properties of Pln, previously identified as a pore-forming hemolysin, also include a role as a general inflammatory agonist.
In addition to its function as an antimicrobial agent, neurotransmitter, and vasodilator, the reactive gas NO exerts both beneficial and deleterious effects during sepsis and acute inflammation, including circulatory and organ failure in septic shock (7, 36, 40). The short half-life of a few seconds is thought to limit the toxicity of NO for host tissues. NO is produced from the substrate l-arginine by three enzymes: inducible NO synthase (iNOS), characteristically found in macrophages, and two constitutive forms, endothelial cell NO synthase and neuronal cell NO synthase, found, respectively, in the two types of cells (24). During infection, host inflammatory mediators and bacterial products upregulate the expression of iNOS, whose expression and activity are normally tightly controlled (40). Both proinflammatory cytokines (e.g., tumor necrosis factor alpha [TNF-α] and interleukin 1 [IL-1]) and bacterial lipopolysaccharide (LPS) can activate iNOS synergistically with gamma interferon (IFN-γ). The mechanism of iNOS regulation is well characterized at the genetic and biochemical level. The major pathways depend upon IFN-γ-mediated upregulation of interferon regulatory factor 1 (IRF-1) expression; IRF-1 binds to the iNOS promoter and activates iNOS transcription synergistically with NF-κB, induced through TNF-α, IL-1, or LPS signaling (16, 41).
The prevalence of sepsis caused by gram-positive organisms is increasing (10). Streptococcus pneumoniae is the major pathogen causing invasive diseases, including sepsis, meningitis, and pneumonia (8, 33). Little is known about the components of gram-positive bacteria responsible for the host NO response. Two cell wall components of Staphylococcus aureus, lipoteichoic acid and peptidoglycan, have been reported to cause induction of NO, shock, and organ injury (6, 17). In S. pneumoniae, pneumococcal cell walls have been shown to stimulate NO production in vitro (4, 9, 18, 28); however, the specific component or components of pneumococci responsible for induction of iNOS or NO production are unknown. Here we analyze which factors of live pneumococci contribute to NO stimulation in murine macrophages. We found that pneumolysin (Pln), a pore-forming hemolysin, is the primary component of live pneumococci stimulating NO production in macrophages.
MATERIALS AND METHODS
Cell culture and reagents.
RAW 264.7 cells were purchased from the American Type Culture Collection (Rockville, Md.) and were cultured in Dulbecco minimal essential medium (DMEM) (BioWhittaker, Walkersville, Md.) supplemented with 10% fetal bovine serum (BioWhittaker) and 2 mM l-glutamine (Gibco BRL, Grand Island, N.Y.) without antibiotics or indicator dye in a 37°C incubator with 5% CO2. RAW cells were confirmed to be negative for Mycoplasma (Geneprobe; Fisher Scientific, Atlanta, Ga.) and endotoxin (Limulus amebocyte lysate, QCL-1000 kit; BioWhittaker). IRF-1−/− (22) and IFN-γR−/− (15) mice were obtained from Jackson Laboratory (Bar Harbor, Me.). IFN-γR−/− mice are on a 129/BL6 background. IRF-1−/− mice have been backcrossed onto the C57BL/6 background for six generations and one further generation by P.J.M. Mice were housed and bred under specific-pathogen-free conditions at St. Jude Children’s Research Hospital Animal Resource Center. Murine peritoneal macrophages were harvested 4 days after intraperitoneal injection of 3 ml of 3% thioglycolate broth. Erythrocytes were removed by hypotonic lysis, and a sample of cells was stained following cytospin. Preparations were >90% macrophages. Peritoneal macrophages were washed and resuspended in RPMI medium (Gibco BRL) containing 10% fetal calf serum and plated in 24-well plates at 2 × 106 cells per well. Adherent monolayers were treated with the agents described in the legends to Fig. 4 and 5 for 18 h.
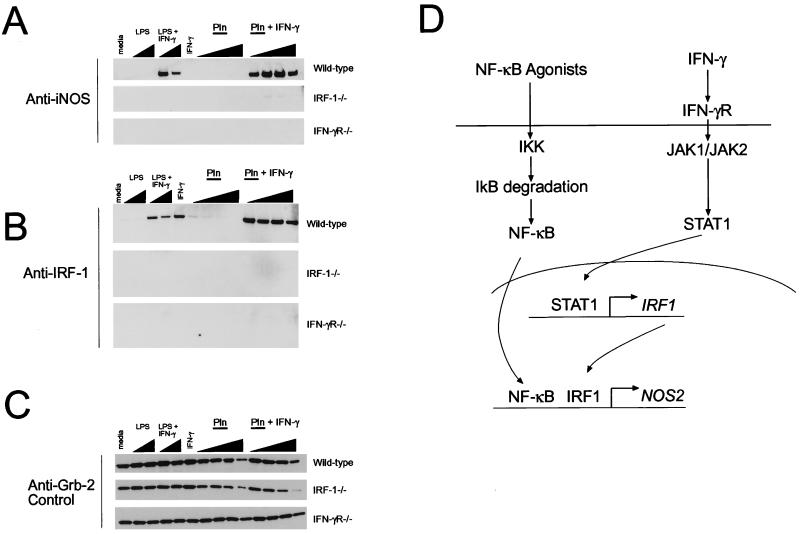
FIG. 4.
Stimulation of iNOS production by Pln is dependent on the IFN-γ signaling pathway. Peritoneally derived inflammatory macrophages were isolated from wild-type, IRF-1−/−, or IFN-γR−/− mice as described in Materials and Methods. Wild-type cells derived from C57BL/6 or 129/BL6 mice responded in similar fashions to the stimulants indicated. Wild-type results shown are from cells derived from C57BL/6 mice. Cells were stimulated with the different agents shown at the top of each panel for 18 h, and cell lysates were prepared. iNOS (A) and IRF-1 (B) were detected by immunoblotting. The adapter protein Grb-2, whose levels do not fluctuate in macrophages (25a), was used as a loading control and was detected by immunoblotting (C). Panel D shows a simplified diagram of some of the key components required for iNOS expression. Note that in the highest concentrations of Pln, there is less total protein loaded, which reflects Pln-induced toxicity to the cells. The molecular masses of the proteins are as follows: iNOS, ∼130 kDa; IRF-1, ∼43 kDa; and Grb-2, ∼26 kDa. Final concentrations of agonists used were as follows: LPS, 50 and 200 ng/ml; IFN-γ, 2 ng/ml in all cases; and recombinant Pln, 1.25, 2.5, 5, and 12.5 μg/ml. Data are representative of two independent experiments.
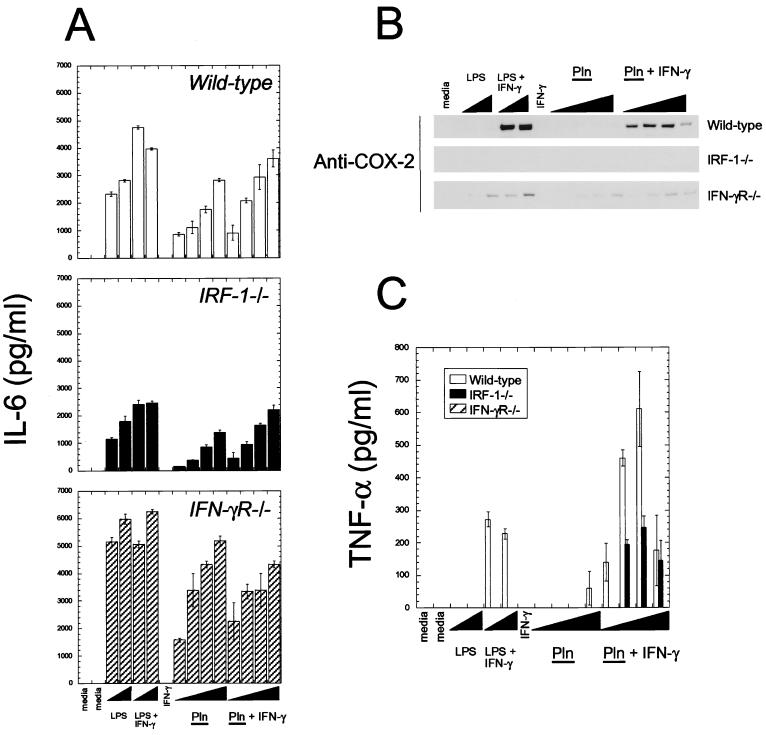
FIG. 5.
Pln is a general inflammatory agonist of macrophages. Peritoneally derived inflammatory macrophages were isolated from wild-type, IRF-1−/−, or IFN-γR−/− mice, as described in Materials and Methods, and stimulated with the different agents shown as described for Fig. 4. Concentrations of agonists were the same for Fig. 4. IL-6 (A) and TNF-α (C) were measured by ELISA and are reported as means from quadruplicate measurements. COX-2 was measured by immunoblotting as described in the legend to Fig. 4. Note that macrophages from IFN-γR−/− mice did not produce any detectable TNF-α under these conditions. Data are representative of two independent experiments.
The D39 capsular type 2 S. pneumoniae strain and its isogenic pneumolysin-negative mutant PLN-A (D. Briles, University of Alabama, Birmingham, Ala.) (3, 5) were grown in a liquid semisynthetic casein hydrolysate medium supplemented with yeast extract (C+Y medium) (20). The mutant strain PLN-A was grown in the presence of 1 μg of erythromycin per ml. The following reagents were obtained from Sigma (St. Louis, Mo.): DNase, erythromycin, isopropyl-β-d-thiogalactopyranoside (IPTG), leupeptin, LPS (from Escherichia coli serotype O111:B4), phenylmethylsulfonyl fluoride (PMSF), polymyxin B (PMB), RNase, sulfanilamide, naphthylethylenediamine dihydrochloride, and trypsin. Recombinant mouse IFN-γ was purchased from Genzyme (Cambridge, Mass.).
Measurement of nitrite.
Nitrite is the end product of NO in cell culture and is the substrate in the Griess reaction (12) for quantification of NO production (13). RAW 264.7 cells (105) were resuspended in 200 μl of DMEM and transferred into each well of a 96-well tissue culture plate (Costar, Cambridge, Mass.). Cells were incubated in a 5% CO2 atmosphere at 37°C for 2 h and then primed with 0.3 to 0.5 ng of IFN-γ per ml for 4 h. Bacteria were harvested by centrifugation upon reaching an optical density at 620 nm (OD620) of 0.5 and resuspended in DMEM. After incubation of RAW 264.7 cells with live pneumococci, pneumococcal cell wall, purified recombinant pneumolysin or hydrogen peroxide, the culture plates were centrifuged at 800 × g (10 min), 100-μl aliquots of each well were transferred into a new 96-well plate, and 100 μl of Griess reagent was added. The Griess reagent consisted of one part 1% sulfanilamide in 5% H3PO4 and one part 0.1% naphthylethylenediamine dihydrochloride in distilled water. The absorption of the purple azo dye resulting from the reaction of nitrite with the Griess reagent was measured at 546 nm by a multichannel spectrophotometer (Spectra Max 340; Molecular Devices, Sunnyvale, Calif.), and nitrite concentrations were determined with a nitrite standard curve.
Cell wall preparation.
S. pneumoniae cell wall was prepared from strain D39 and mutant strain PLN-A as described previously (37). Briefly, 1 liter of pneumococcal culture was grown in C+Y medium until the OD620 was 0.5 (1.5 × 108 CFU/ml), chilled in iced ethanol, centrifuged at 4°C (4,000 × g), and resuspended in 40 ml of ice-cold 50 mM Tris HCl (pH 7.0). This suspension was added into boiling 5% sodium dodecyl sulfate (SDS), kept boiling for 15 min, cooled, centrifuged (10 min, 12,000 × g), resuspended, washed twice in 1 M NaCl, and then washed six times in distilled water. Acid-washed glass beads equal to the volume of the pellet were added, vigorously vortexed, then removed by a sintered glass filter. The filtrate was centrifuged (15 min, 27,000 × g), and the pellet containing cell wall was resuspended in 100 mM Tris (pH 7.5) and incubated with 10 μg of DNase per ml, 50 μg of RNase per ml, and 100 μg of trypsin per ml to degrade DNA, RNA, and proteins, respectively (25, 37). Boiling and trypsin treatment destroyed any activity of pneumolysin in the cell wall preparation, since pneumolysin is heat-sensitive (14, 31) and not resistant to trypsin digestion (31). Samples were centrifuged, lyophilized, weighed, redissolved in 0.1 M Tris HCl (pH 7.5), and stored at −20°C.
Immunoblotting.
RAW 264.7 macrophages were primed with 0.3 ng of IFN-γ per ml for 4 h and incubated with D39 or PLN-A (106 CFU/ml) for 6 to 8 h. Cells were pelleted by centrifugation, and pellets were lysed on ice in extraction buffer (1% Triton X-100, 50 mM Tris HCl, 1 mM EDTA, 1 mM Na3VO4, 0.1 mg of PMSF per ml, 10 μg of leupeptin per ml). The protein extracts were boiled for 5 min, separated by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), and transferred to a polyvinylidene fluoride membrane. After being blocked in Tris-buffered saline with 0.1% Tween and 5% milk, blots were incubated with a murine monoclonal antibody specific for iNOS (1:2,000) (Transduction Labs, Lexington, Ky.). After being rinsed, the blots were incubated with a horseradish peroxidase-conjugated anti-mouse immunoglobulin G antibody (1:2,000) (Bio-Rad, Hercules, Calif.). After being washed, the blots were developed by using the ECL kit (Amersham, Little Chalfont, Buckinghamshire, England). Peritoneal macrophages were placed on ice after stimulation and washed with ice-cold phosphate-buffered saline. Cells were lysed directly in SDS sample buffer and vortexed vigorously to shear DNA. Samples were separated by gradient SDS-PAGE and transferred to nitrocellulose. Blots were blocked in 5% milk in phosphate-buffered saline and probed with polyclonal anti-iNOS (1:2,000) (Biomol, Plymouth Meeting, Pa.), anti-cyclooxygenase-2 (anti-COX-2) (1:250) (Transduction Labs), anti-IRF-1 (1:1,000) (Santa Cruz Biotechnology, Santa Cruz, Calif.), and anti-Grb-2 (1:1,000) (growth factor receptor-bound protein 2; Transduction Labs). Blots were developed with enhanced chemiluminescence (see Fig. 4).
ELISA.
Cytokine levels in the cell culture medium were determined by enzyme-linked immunosorbent assay (ELISA) with specific reagents for IL-6 (Pharmingen, San Diego, Calif.) and TNF-α (Endogen, Boston, Mass.) according to manufacturers’ instructions.
Production and purification of recombinant Pln.
DNA digestions, ligations, and gel electrophoresis were performed according to standard protocols (32). Cloning of the Pln gene was based on previously published protocols (23, 39). For plasmid preparation and purification, we used kits from Qiagen (Chatsworth, Calif.) and Promega (Wizard; Madison, Wis.). Chromosomal DNA was prepared from pneumococcus (serotype 4) as described previously (30). The full-length Pln gene was amplified by PCR with the primers lysin1 (5′-AAT CCA GGA TCC TAT TAG GAG GAG AAG ATG G-3′) and lysin2 (5′-TTT TGT CTC GAG CAT TCT CCT CTC CTA G-3′), with restriction sites identified by underlining. The pET-28c vector (Novagen, Cambridge, Mass.) carrying an N- and C-terminal His-tag configuration and a kanamycin cassette was used for cloning and expression of the recombinant gene encoding Pln in E. coli. Expression was induced by the addition of IPTG (1.5 mM). The Pln protein was subjected to denaturing conditions with a lysis buffer containing 6 M guanidine and a washing and eluting buffer containing 8 M urea and then purified by binding to Ni2+ immobilized on resin according to the manufacturer’s instructions (Qiagen, Valencia, Calif.). After dialysis to gradually remove urea (five times for 12 h at 4°C, in 20 mM Tris, 1 mM EDTA, 10 mM NaCl, 5 mM ditheothreitol), Pln was further purified with an ion-exchange column (HiTrapS; Pharmacia, Bridgewater, N.J.) and dialyzed again, and the levels of endotoxin were determined by the Limulus assay (Limulus amebocyte lysate, QCL-1000 kit; BioWhittaker). Hemolytic activity was verified qualitatively by dropping eluted Pln fractions on sheep blood agar plates.
RESULTS
Live D39 pneumococci induce dose- and time-dependent NO production by murine macrophages.
Live D39 pneumococci induced concentration-dependent (Fig. 1A) and time-dependent (Fig. 1B) NO production in IFN-γ-primed RAW 264.7 macrophages. Low concentrations of live pneumococci (initial concentration, ∼10 CFU/ml; grown to ∼107 to 108 CFU/ml after 18 h of incubation) were sufficient to induce detectable nitrite concentrations in the tissue culture supernatants during the incubation period. NO production began after 4 to 6 h and reached its maximum level after 18 h of standard incubation time as monitored by measurements at 3-h intervals (Fig. 1B). Immunoblotting analysis performed 6 h after incubation of the macrophages with D39 showed iNOS protein expression and confirmed the Griess experiments (Fig. 1C). An isogenic pneumococcal mutant strain, PLN-A, deficient in Pln, induced only low or undetectable nitrite activity in the Griess reaction (Fig. 1A), and markedly less iNOS activation was indicated by immunoblotting analysis (Fig. 1C). Untreated macrophages and macrophages primed with IFN-γ (0.3 ng/ml) were used as negative controls. This concentration of IFN-γ induced no or only minimal background activity in the Griess reaction (data not shown). Low levels of endotoxin, 0.035 ng/ml as determined by the Limulus amebocyte lysate assay, were detected in the ingredients of the C+Y medium. Incubation of RAW cells with pneumococci in the presence of PMB only marginally reduced nitrite production (11.7% ± 7.2%, mean ± standard deviation [SD]), indicating that endotoxin does not contribute significantly to pneumococcus-induced NO production.
FIG. 1.
Induction of NO and iNOS in RAW 264.7 macrophages by live pneumococci of the wild-type strain D39 but not those of its isogenic pneumolysin-negative mutant, PLN-A, in the presence of 0.3 ng of IFN-γ per ml. (A) NO induced by various concentrations of CFU of D39 or PLN-A, measured by assessing the levels of the end product nitrite in the Griess reaction after 18 h of incubation. Results are means + SD for three independent experiments. (B) Induction of NO production, as measured at various times of incubation with D39. Nitrite concentration was quantified with the Griess reaction (results are + SD for three independent experiments in triplicate wells). (C) Immunoblotting analysis for iNOS. RAW 264.7 cells were primed with 0.3 ng of IFN-γ (Ctrl) (negative control) per ml and treated with either 106 CFU of bacteria (D39 or PLN-A) per ml or 10 ng of LPS (positive control) per ml for 6 h. Cell lysates were subjected to SDS-PAGE and incubated with anti-iNOS-antibody (1:2,000) followed by horseradish peroxidase-labelled secondary antibody, and proteins were detected with the Amersham ECL kit.
Differences in NO stimulation by D39 and PLN-A were not due to differences in bacterial growth or lysis under the experimental conditions used, as measured by OD620. In addition, there was no difference in the pH values of the media used to analyze D39 and PLN-A. We also excluded nitrite production, degradation, and conversion to nitrate by D39 or PLN-A in DMEM (data not shown): pneumococci in the absence of macrophages did not produce any NO, incubation of a defined amount of nitrite with either pneumococci or macrophages for 18 h did not cause any decrease of the initial nitrite concentration, and no significant increase of nitrite in the presence of nitrate reductase was detected.
Purified Pln induces NO production in RAW macrophages.
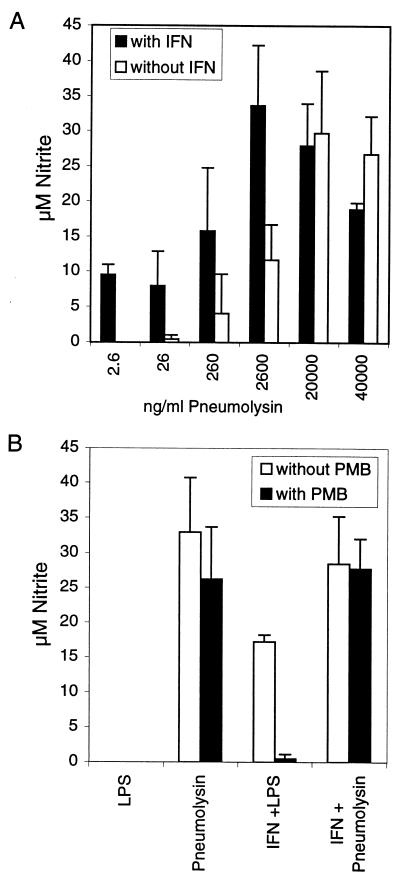
The results achieved with the PLN-A mutant suggested that Pln was critical for NO production. Therefore, we tested if purified recombinant Pln alone is able to induce NO production. Pln induced NO production in RAW macrophages after 18 h in the absence of exogenous mouse recombinant IFN-γ (Fig. 2). Purified Pln (20 μg/ml) without IFN-γ priming induced an average nitrite concentration of 32.9 μM (Fig. 2B). That is 50-fold more nitrite (on a weight basis) than the same concentration of pneumococcal cell wall induced with IFN-γ priming (Fig. 3). In the presence of low concentrations of IFN-γ (0.3 ng/ml), nitrite production was achieved by lower concentrations of Pln (Fig. 2A): 2.6 ng of Pln per ml induced ∼10 μM nitrite (Fig. 2A). This activity is comparable to the induction of ∼16 μM nitrite by 10 ng of LPS per ml (Fig. 2B). These results show that Pln itself can stimulate RAW macrophages to produce NO. The LPS in the recombinant Pln preparation (0.4 ng of endotoxin per ml in 200 μg of pneumolysin per ml) was unlikely to contribute to NO induction for the following reasons. Incubation of RAW macrophages with the same or higher concentrations of LPS in the absence of IFN-γ did not stimulate detectable NO production (Fig. 2B). Also RAW cells were coincubated with Pln and PMB, a strong inhibitor of endotoxin. In order to test the endotoxin-inhibitory effect of PMB, RAW cells were stimulated with IFN-γ (0.3 ng/ml) and LPS (10 ng/ml) in the presence and absence of PMB. PMB significantly reduced LPS-induced NO production under this condition (Fig. 2B). In contrast, PMB did not significantly reduce NO production induced by Pln independently of the priming of RAW cells with IFN-γ (0.3 ng/ml) (Fig. 2B). As a further control, Pln was heated at 95°C for 5 min. In contrast to LPS, pneumolysin is heat sensitive and its activity is destroyed at temperatures that are >60 to 70°C (14). After heating, the NO-inducing activity of Pln was reduced 91% at 20 μg/ml and 99% at 5 μg/ml.
FIG. 2.
(A) Dose curve for the NO production-inducing activity of purified recombinant Pln. Various concentrations of Pln were added to RAW 264.7 macrophages in the presence and absence of 0.3 ng of IFN-γ per ml, and production of NO was measured by the Griess reaction at 18 h (data from four independent experiments). (B) Induction of RAW 264.7 cell NO production by LPS or purified recombinant pneumolysin with and without IFN-γ in the presence and absence of PMB, as measured by the Griess assay at 18 h. LPS (5 ng/ml) in the absence of IFN-γ was not able to induce nitrite production in RAW 264.7 macrophages. Nitrite production induced by 20 μg of Pln per ml in the absence or presence of IFN-γ was not significantly inhibited by PMB, excluding an endotoxin effect. In the presence of 0.3 ng of IFN-γ per ml, LPS (10 ng/ml) induced nitrite production that was inhibited by PMB, thus confirming its efficacy. Results are means + SD for three independent experiments done with duplicate and quadruplicate wells.
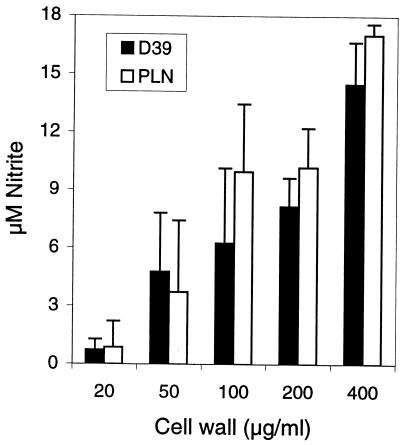
FIG. 3.
Dose curve for the NO production-inducing activity of purified pneumococcal cell wall. Various concentrations of cell wall fragments of D39 and PLN-A were added to RAW 264.7 macrophages in the presence of 0.3 ng of IFN-γ per ml, and production of NO was measured by the Griess reaction at 18 h (means + SD from quadruplicate wells).
We also tested the second pneumococcal cytotoxin, hydrogen peroxide, for its ability to produce NO (26). Only very high concentrations (millimolar range), which cannot be achieved by pneumococci in vitro (35), produced detectable NO, and they did so only if incubated for periods longer than 18 h (data not shown).
High concentrations of cell wall preparations derived from D39 and PLN-A stimulate NO production.
Having demonstrated that Pln stimulates NO production in RAW 264.7 macrophages, we compared the known NO production-inducing capacity of purified pneumococcal cell wall preparations (which do not contain pneumolysin) (4, 9, 18, 28) in order to exclude the possibility that the differences in levels of NO production induced by live D39 and PLN-A were due to differences in the NO production-inducing activity of liberated cell wall fragments. High concentrations of cell wall preparations of both D39 and PLN-A stimulated in a similar fashion dose-dependent NO production in macrophages in the presence of IFN-γ (Fig. 3). The endotoxin levels of the cell wall preparations were 0.031 to 0.132 ng/ml (in 1.4- to 1.7-mg/ml stock solution) due to contamination of trypsin and RNase used for the preparation. Incubation of RAW cells with pneumococcal cell wall in the presence of the endotoxin inhibitor PMB reduced nitrite production by 9.3% (±9.4%), indicating that endotoxin does not contribute significantly to cell wall-induced NO production.
Pneumolysin induces iNOS and NO through a pathway strictly dependent on IFN-γ signaling.
Addition of purified Pln to RAW cells induced iNOS expression and NO production without the addition of exogenous IFN-γ. In contrast, iNOS expression induced by other stimuli, for example, by LPS or TNF-α, is normally dependent on IFN-γ (16). To investigate this effect in more detail, we tested if macrophages lacking key elements of the IFN-γ signal transduction pathway were able to produce NO in response to Pln (Fig. 4). The signal transduction pathway that leads to iNOS expression is known in considerable detail at both the genetic and biochemical levels (27). Minimal requirements include NF-κB activation (via LPS or TNF-α) and an increase in IRF-1 expression mediated by IFN-γ activation of a signal transducer and activator of transcription (STAT1) (Fig. 4D). Inflammatory peritoneal macrophages were isolated from mice lacking either the IFN-γ receptor (IFN-γR) or IRF-1 and incubated with Pln (Fig. 4). The results showed that IFN-γ and an intact IFN-γ signal transduction pathway are essential for Pln-mediated iNOS expression. While wild-type macrophages (derived from C57BL/6 or 129/BL6 mice) made readily detectable iNOS in response to Pln and IFN-γ, neither IRF-1−/− nor IFN-γR−/− macrophages were able to make iNOS in response to Pln or control stimuli such as LPS and IFN-γ. The exception was that IRF-1−/− macrophages could make extremely small amounts of iNOS protein in response to Pln and IFN-γ. One possible reason for this is that STAT1 has been shown to also bind to the NOS2 (the gene encoding iNOS) promoter (11). In this scenario, STAT1 would bypass the requirement for IRF-1 for a minor effect on iNOS levels.
Pneumolysin is a strong agonist of multiple macrophage functions.
Pln appeared to provide a second signal similar to LPS or TNF-α when iNOS expression was induced together with IFN-γ (Fig. 4). To address the scope of Pln as a general agonist of macrophages, IL-6, TNF-α, and COX-2 expression was measured after stimulation of wild-type, IRF-1−/−, or IFN-γR−/− macrophages with Pln or Pln and IFN-γ. COX-2 is the induced, rate-limiting enzyme involved in prostaglandin synthesis. The results (Fig. 5) showed that Pln can induce IL-6, TNF-α, and COX-2 in a fashion similar to that of a general macrophage agonist such as LPS. Interestingly, COX-2 expression in response to Pln stimulation was dependent on IFN-γ signaling (Fig. 5B), as was TNF-α production (Fig. 5C), whereas Pln-induced IL-6 expression was independent of IFN-γ (Fig. 5A). Macrophages of each genotype made abundant amounts of IL-6 in response to Pln. This process was partly dependent on IRF-1. Thus, Pln can stimulate macrophages to produce multiple inflammatory agents.
DISCUSSION
Pln, a hemolysin belonging to the family of thiol-activated toxins (29), is an important pneumococcal virulence factor. We showed that in addition to its known activities, Pln can induce iNOS with resultant NO production. Pln was able to induce NO production in the absence of exogenous IFN-γ in the mouse macrophage cell line RAW 264.7. The amounts of Pln used in our in vitro assay are biologically relevant in the septic state: 2.6 ng of pneumolysin per ml, equivalent to ∼2.6 × 105 CFU of pneumococci per ml (14), induced 10 μM nitrite. During sepsis, concentrations of ≥105 CFU of bacteria per ml are reached in humans (21, 34) and up to 109 to 1010 CFU of pneumococci per ml in mice (3), equivalent to ∼10 to 100 μg of pneumolysin per ml (14) have been described. In contrast, the large amount of cell wall required to induce NO production is of doubtful relevance in human sepsis. A cell wall fragment concentration of 1 μg/ml is equivalent to ∼105 CFU of pneumococci per ml (37); therefore, the cell wall concentrations necessary to induce 10 μM nitrite translated to high concentrations of pneumococci (compare Fig. 3 and 1A): 100 to 400 μg of cell wall fragment per ml is equivalent to 1 × 107 to 4 × 107 CFU/ml.
Other studies have implicated pneumococcal cell wall components as capable of inducing NO production (4, 9, 18, 28). However, we have demonstrated that (in the case of live microorganisms) Pln is the main inducer of NO production in macrophages. A live Pln-deficient mutant induced low or undetectable iNOS and NO production compared to its isogenic parent, D39, indicating that the amounts of cell wall fragment released during bacterial growth were not sufficient to induce substantial NO production. In contrast, incubation of macrophages with <102 CFU/ml (initial concentration, growing to ∼108 CFU/ml after 18 h of incubation) of live pneumococcal strain D39 resulted in a measurable concentration of approximately 3 μM nitrite after 18 h of incubation.
We have begun to dissect the pathway(s) that Pln activates in macrophages. Since RAW cells appeared to produce NO and have iNOS expression that was independent of exogenous IFN-γ, we tested whether this cytokine was required by stimulating macrophages with a genetically inactivated IFN-γ signaling pathway. The results clearly showed that IFN-γ is an essential cofactor for Pln-induced iNOS expression. The data also suggest that RAW cells can make enough endogenous IFN-γ within the cultures to activate iNOS expression when exposed to Pln. Less likely, in our opinion, is the possibility that the mechanism of NOS2 activation may differ between RAW cells and primary macrophages.
In addition to our novel finding of Pln-mediated induction of iNOS, we confirmed and extended studies demonstrating induction of TNF-α by Pln (14). We showed that Pln also stimulated the production of other proinflammatory mediators such as IL-6 and COX-2, the rate-limiting enzyme responsible for induced prostanoid synthesis. COX-2 appears to have requirements similar to those of iNOS for induction in macrophages: IRF-1 (Fig. 5B), along with another signal that activates the NF-κB pathway, appears to be essential. TNF-α expression is also dependent on the activation of the NF-κB pathway. We speculate that Pln has the ability to act as a general activator of macrophages, possibly through activation of NF-κB. Whether this effect is direct or mediated via other agents or cytokines remains an open question. It seems reasonable to suggest that the proinflammatory effects of Pln may be physiologically relevant in streptococcal disease.
Pln is believed to act by binding to cholesterol in eucaryotic cell membranes and to form transmembrane pores (2). It is conceivable that pores result in the influx of a signal substance, leading to stimulation of NOS expression and production of NO. This pathway has been suggested as an explanation for pneumolysin-induced cochlear damage, wherein Ca2+ may induce NOS (1). However, macrophages have a Ca2+-independent NOS. Alternative signals, including proinflammatory cytokines that are induced by Pln, could act as possible secondary mediators stimulating NO production. Both IL-6 and TNF-α are upregulated in septic shock (19, 38).
In conclusion, Pln is a novel inducer of iNOS and NO production in macrophages that is strictly dependent on IFN-γ signaling. In addition, Pln stimulates the production of the inflammatory mediators TNF-α and IL-6 and the expression of COX-2.
ACKNOWLEDGMENTS
This work was supported by grant AI 27913, Cancer Center Support CORE grant P30 CA 21765, and the American Lebanese Syrian Associated Charities (ALSAC).
We thank Daimin Zhao for technical assistance and Elaine Tuomanen for many thoughtful discussions and suggestions and her critical reading of the manuscript.
REFERENCES
- 1.Amaee F R, Comis S D, Osborne M P. NG-Methyl-l-arginine protects the guinea pig cochlea from the cytotoxic effects of pneumolysin. Acta Oto-Laryngol. 1995;115:386–391. doi: 10.3109/00016489509139334. [DOI] [PubMed] [Google Scholar]
- 2.Andrew P W, Mitchell T J, Morgan P J. Relationship of structure to function in pneumolysin. Microb Drug Resist. 1997;3:11–17. doi: 10.1089/mdr.1997.3.11. [DOI] [PubMed] [Google Scholar]
- 3.Benton K A, Everson M P, Briles D E. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995;63:448–455. doi: 10.1128/iai.63.2.448-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernatowicz A, Ködel U, Frei K, Fontana A, Pfister H W. Production of nitrite by primary rat astrocytes in response to pneumococci. J Neuroimmunol. 1995;60:53–61. doi: 10.1016/0165-5728(95)00053-5. [DOI] [PubMed] [Google Scholar]
- 5.Berry A M, Yother J, Briles D E, Hansman D, Paton J C. Reduced virulence of a defined pneumolysin-negative mutant of Streptococcus pneumoniae. Infect Immun. 1989;57:2037–2042. doi: 10.1128/iai.57.7.2037-2042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Kimpe S J, Kengatharan M, Thiemermann C, Vane J R. The cell wall components peptidoglycan and lipoteichoic acid from Staphylococcus aureus act in synergy to cause shock and multiple organ failure. Proc Natl Acad Sci USA. 1995;92:10359–10363. doi: 10.1073/pnas.92.22.10359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fang F C. Mechanisms of nitric oxide-related antimicrobial activity. J Clin Investig. 1997;99:2818–2825. doi: 10.1172/JCI119473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.File T M, Jr, Tan J S. Incidence, etiologic pathogens, and diagnostic testing of community-acquired pneumonia. Curr Opin Pulm Med. 1997;3:89–97. doi: 10.1097/00063198-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Freyer D, Weih M, Weber J R, Bürger W, Scholz P, Manz R, Ziegenhorn A, Angestwurm K, Dirnagl U. Pneumococcal cell wall components induce nitric oxide synthase and TNF-alpha in astroglial-enriched cultures. Glia. 1996;16:1–6. doi: 10.1002/(SICI)1098-1136(199601)16:1<1::AID-GLIA1>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Friedman G, Silva E, Vincent J L. Has the mortality of septic shock changed with time? Crit Care Med. 1998;26:2078–2086. doi: 10.1097/00003246-199812000-00045. [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Morrison D C, Parmely T J, Russell S W, Murphy W J. An interferon-gamma-activated site (GAS) is necessary for full expression of the mouse iNOS gene in response to interferon-gamma and lipopolysaccharide. J Biol Chem. 1997;272:1226–1230. doi: 10.1074/jbc.272.2.1226. [DOI] [PubMed] [Google Scholar]
- 12.Green L C, Wagner D A, Glogowski J, Skipper P L, Wishnok J S, Tannenbaum S R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 13.Hevel J M, Marletta M A. Nitric-oxide synthase assays. Methods Enzymol. 1994;233:250–258. doi: 10.1016/s0076-6879(94)33028-x. [DOI] [PubMed] [Google Scholar]
- 14.Houldsworth S, Andrew P W, Mitchell T J. Pneumolysin stimulates production of tumor necrosis factor alpha and interleukin-1β by human mononuclear phagocytes. Infect Immun. 1994;62:1501–1503. doi: 10.1128/iai.62.4.1501-1503.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang S, Hendriks W, Althage A, Hemmi S, Bluethmann H, Kamijo R, Vilcek J, Zinkernagel R M, Aguet M. Immune response in mice that lack the interferon-gamma receptor. Science. 1993;259:1742–1745. doi: 10.1126/science.8456301. [DOI] [PubMed] [Google Scholar]
- 16.Kamijo R, Harada H, Matsuyama T, Bosland M, Gerecitano J, Shapiro D, Le J, Koh S I, Kimura T, Green S J, Mak T W, Taniguchi T, Vilcek J. Requirement for transcription factor IRF-1 in NO synthase induction in macrophages. Science. 1994;263:1612–1615. doi: 10.1126/science.7510419. [DOI] [PubMed] [Google Scholar]
- 17.Kengatharan K M, De Kimpe S, Robson C, Foster S J, Thiemermann C. Mechanism of gram-positive shock: identification of peptidoglycan and lipoteichoic acid moieties essential in the induction of nitric oxide synthase, shock, and multiple organ failure. J Exp Med. 1998;188:305–315. doi: 10.1084/jem.188.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim Y S, Täuber M G. Neurotoxicity of glia activated by gram-positive bacterial products depends on nitric oxide production. Infect Immun. 1996;64:3148–3153. doi: 10.1128/iai.64.8.3148-3153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krafte-Jacobs B, Bock G H. Circulating erythropoietin and interleukin-6 concentrations increase in critically ill children with sepsis and septic shock. Crit Care Med. 1996;24:1455–1459. doi: 10.1097/00003246-199609000-00005. [DOI] [PubMed] [Google Scholar]
- 20.Lacks S A, Hotchkiss R D. A study of the genetic material determining an enzyme activity in pneumococcus. Biochim Biophys Acta. 1960;39:508–517. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- 21.La Scolea L J, Jr, Dryja D. Quantitation of bacteria in cerebrospinal fluid and blood of children with meningitis and its diagnostic significance. J Clin Microbiol. 1984;19:187–190. doi: 10.1128/jcm.19.2.187-190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kündig T M, Amakawa R, Kishihara K, Wakeham A, Potter J, Furlonger C L, Narendran A, Suzuki H, Ohashi P S, Paige C J, Taniguchi T, Mak T W. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- 23.Mitchell T J, Walker J A, Saunders F K, Andrew P W, Boulnois G J. Expression of the pneumolysin gene in Escherichia coli: rapid purification and biological properties. Biochim Biophys Acta. 1989;1007:67–72. doi: 10.1016/0167-4781(89)90131-0. [DOI] [PubMed] [Google Scholar]
- 24.Moncada S, Palmer R M, Higgs E A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 25.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 25a.Murray, P. J. Unpublished data.
- 26.Nagase S, Takemura K, Ueda A, Hirayama A, Aoyagi K, Kondoh M, Koyama A. A novel nonenzymatic pathway for the generation of nitric oxide by the reaction of hydrogen peroxide and d- or l-arginine. Biochem Biophys Res Commun. 1997;233:150–153. doi: 10.1006/bbrc.1997.6428. [DOI] [PubMed] [Google Scholar]
- 27.Nathan C, Xie Q-W. Regulation of biosynthesis of nitric oxide. J Biol Chem. 1994;269:13725–13728. [PubMed] [Google Scholar]
- 28.Orman K L, Shenep J L, English B K. Pneumococci stimulate the production of the inducible nitric oxide synthase and nitric oxide by murine macrophages. J Infect Dis. 1998;178:1649–1657. doi: 10.1086/314526. [DOI] [PubMed] [Google Scholar]
- 29.Paton J C. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 30.Pearce B J, Yin Y B, Masure H R. Genetic identification of exported proteins in Streptococcus pneumoniae. Mol Microbiol. 1993;9:1037–1050. doi: 10.1111/j.1365-2958.1993.tb01233.x. [DOI] [PubMed] [Google Scholar]
- 31.Rubins J B, Duane P G, Charboneau D, Janoff E N. Toxicity of pneumolysin to pulmonary endothelial cells in vitro. Infect Immun. 1992;60:1740–1746. doi: 10.1128/iai.60.5.1740-1746.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Schuchat A, Robinson K, Wenger J D, Harrison L H, Farley M, Reingold A L, Lefkowitz L, Perkins B A. Bacterial meningitis in the United States in 1995. N Engl J Med. 1997;337:970–976. doi: 10.1056/NEJM199710023371404. [DOI] [PubMed] [Google Scholar]
- 34.Shenep J L, Flynn P M, Barrett F F, Stidham G L, Westenkirchner D F. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J Infect Dis. 1988;157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- 35.Spellerberg B, Cundell D R, Sandros J, Pearce B J, Idäpään-Heikkilä I, Rosenow C, Masure H R. Pyruvate oxidase as a determinant of virulence in Streptococcus pneumoniae. Mol Microbiol. 1996;19:803–813. doi: 10.1046/j.1365-2958.1996.425954.x. [DOI] [PubMed] [Google Scholar]
- 36.Thiemermann C. Nitric oxide and septic shock. Gen Pharmacol. 1997;29:159–166. doi: 10.1016/s0306-3623(96)00410-7. [DOI] [PubMed] [Google Scholar]
- 37.Tuomanen E, Liu H, Hengstler B, Zak O, Tomasz A. The induction of meningeal inflammation by components of the pneumococcal cell wall. J Infect Dis. 1985;151:859–868. doi: 10.1093/infdis/151.5.859. [DOI] [PubMed] [Google Scholar]
- 38.Waage A. Tumour necrosis factor and septic shock. Lancet. 1998;351:603. doi: 10.1016/S0140-6736(05)78598-6. [DOI] [PubMed] [Google Scholar]
- 39.Walker J A, Allen R L, Falmagne P, Johnson M K, Boulnois G J. Molecular cloning, characterization, and complete nucleotide sequence of the gene for pneumolysin, the sulfhydryl-activated toxin of Streptococcus pneumoniae. Infect Immun. 1987;55:1184–1189. doi: 10.1128/iai.55.5.1184-1189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong J M, Billiar T R. Regulation and function of inducible nitric oxide synthase during sepsis and acute inflammation. Adv Pharmacol. 1995;34:155–170. doi: 10.1016/s1054-3589(08)61084-4. [DOI] [PubMed] [Google Scholar]
- 41.Xie Q W, Kashiwabara Y, Nathan C. Role of transcription factor NF-kappa B/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–4708. [PubMed] [Google Scholar]