Abstract
Background
Severe complications may cause a fatal or disabling outcome in patients with Rickettsia japonica infection but are poorly understood.
Methods
We identified 11 patients with only Rickettsia japonica infection with metagenomics next generation sequencing (mNGS) during April to November 2021 at Yichang Central People’s Hospital, China. Clinical data were obtained through review of medical records.
Results
Most patients realized that they had symptoms about one or two days after being bitten. Fever (91%), pulmonary effusion (91%), rash or erythema (100%), abnormal urine (100%), neutropenia (100%), lymphopenia (100%), and thrombocytopenia (100%) were the most common clinical signs. Six severely ill patients were admitted to the intensive care unit and five had mild symptoms. Systemic manifestations such as vomiting (83%), neurological manifestations (100%), and disseminated intravascular coagulation (100%) were more frequently observed in the severe cases, 33.3% of whom developed purpura fulminans requiring amputation or skin graft, and 16.6% died two days after admission. Some patients experienced sequelae.
Conclusion
Our study found that patients with critical Rickettsia japonica infection complicating disseminated intravascular coagulation had high risk of poor outcome.
Keywords: Rickettsia japonica, Japanese spotted fever, infection, tick, disseminated intravascular coagulation, purpura fulminans
Introduction
Rickettsia japonica (R.japonica), first reported in Japan in 1984, causes severe tick-borne rickettsiosis named Japanese spotted fever (JSF).1 JSF usually manifests as high fever, erythema and eschar and leukocytosis; thrombocytopenia, increased levels of C-reactive protein and creatine kinase are observed.2,3 Clinical cases of R.japonica infection are mainly concentrated in Japan, and mostly reported in adjacent countries like South Korea, the Philippines and Thailand.4–6 However, cases of R.japonica infection in humans are scarce in China and its outbreak is sporadic and limited. Furthermore, little is known about its clinical course and data, especially for severe or fatal cases which may require prolonged hospitalization and intensive care support, are scarce. Therefore, in this presented case series, we analyzed 11 Chinese patients with JSF who were healthy before, focusing on the severe/fatal cases and differences between severe and mild patients. Investigating these cases would help us to have a better understanding of the risk factors on admission for JSF which may potentially cause poor outcomes.
Methods
Study Population
To determine the evolution and clinical characteristics of patients with confirmed infection with R.japonica, we conducted a single-center, retrospective observational study between April and November 2021, in a tertiary teaching hospital. The study population of 11 patients, without significant underlying disease, had only R.japonica infection.
Study Design
According to Sepsis-3,7 patients with septic shock were defined as severe cases, others were mild cases. Septic shock was defined as: vasopressors required to maintain MAP ≥ 65 mmHg and serum lactate level >2 mmol/L. Chest computed tomography (CT) scans were performed for each patient on admission. Laboratory findings were included only within 24 hours of admission. The patients’ Acute Physiology and Chronic Health Evaluation II (APACHE-2) and disseminated intravascular coagulation / the international society of thrombosis and hemostasis (DIC / ISTH) were calculated.8,9 Acute kidney injury (AKI) was defined according to Kidney Disease: Improving Global Outcomes (KDIGO) definition.10 Ethics review committee of our hospital certified that this retrospective observational study was exempt from ethical approval. The identifiable part of patients for figures was hidden and informed consent was obtained.
Metagenomic Next Generation Sequencing
Whole blood samples were collected at admission or during hospitalization with standard procedures and promptly stored in sterile containers and transported on dry ice to the laboratory for metagenomic next generation sequencing (mNGS). Information about all DNA and RNA present in the sample was recorded. All raw reads were quality filtered including removing low-quality reads, adapter contamination, duplicated reads, and reads shorter than 35 bp, and the human DNA was also filtered out. Finally, all reference genomes were from NCBI (ftp://ncbi.nlm.nih.gov/genomes/). The results of only R.japonica infection with no other evidence of any other pathogen, were included in the study.
Statistical Analysis
All data analyses were completed using Statistical Package for the Social Sciences (SPSS) for Windows (version 24; SPSS Inc, IBM, Chicago, IL, USA). The categorical demographic features, early clinical symptoms, and categorical variables are reported as frequencies and percentages. Continuous variables are presented as median and interquartile ranges (IQRs).
Results
Patient Characteristics
We identified 11 cases with R.japonica infection: six severe cases (Patient No.1–6) and five mild cases (Patient No.7–11). The six severe cases were all admitted to the intensive care unit (ICU) due to shock. All cases were from Yichang, Hubei province, China, which covers 21,227 square kilometers and occurred in April to November, 2021 which just coincides with active period of ticks. Most of the cases occurred after tick bite or mountain tourism and eschar was a specific sign. Ages ranged from 48 to 74 years with a median age of 58 years. Duration of symptoms before admission varied but mild patients had a significantly shorter median duration (5 days, 4–7) than severe patients (7 days, 6.25–7.75). Characteristics of these patients on admission are shown in Table 1.
Table 1.
Characteristics of the 11 Patients with R.japonica Infection on Admission, April 20, to November 20, 2021, Yichang, China
| Patient No. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Age/Gender | 68 F | 48 M | 54 M | 58 M | 69 F | 55 F | 70 F | 56 F | 58 M | 74 M | 71 F |
| Evidence of insect bites (site) | Eschar (Right waist) | History of mountain tourism | Eschar (Right inguinal region) | Eschar (Back) | Not found | Redness and swelling (Right hand) | Eschar (Right shoulder) | Eschar (Left lower abdomen) | Eschar (Left knee) | Eschar (Right thigh) | Eschar (Right lower abdomen) |
| Duration of symptoms before admission | 10 days | 6 days | 7 days | 8 days | 7 days | 6 days | 7 days | 7 days | 2 days | 4 days | 5 days |
| Constitutional symptom | Fatigue | Fever | Fever, myalgia | Fever | Fever, | Fever, myalgia | Fever | Fever | Fever | Fever | Fever |
| Dermatological manifestations (site) | Erythema (all-over body) | Rash (all-over body) | Erythema (all-over body) | Erythema (all-over body) | Rash (abdomen) | Rash (abdomen and limbs) | Rash (all-over body) | Rash (all-over body) | Rash (all-over body) | Rash (all-over body) | Rash (all-over body) |
| Circulatory condition (within 24 hours) | Shock | Shock | Shock | Shock | Shock | Shock | Stable | Stable | Stable | Stable | Stable |
| Respiratory manifestations | Shortness of breath, extensive pulmonary effusion | Shortness of breath, extensive pulmonary effusion | Shortness of breath, partial consolidation in lungs | Dyspnea, pulmonary effusion and consolidation | Shortness of breath, local pulmonary effusion | Shortness of breath, local pulmonary effusion | None | Little pulmonary effusion | Little pulmonary effusion | Little pulmonary effusion | Little pulmonary effusion |
| Neurological manifestations (within 24 hours) | Confusion | Coma, limb twitching | Deep coma | Limb twitching, deep coma | Incidental delirium | Delirium | None | Headache | None | Acute cerebral infarction | Headache |
| Gastrointestinal symptoms | Nausea, vomiting | Nausea, vomiting | None | Nausea, vomiting | Abdominal pain, vomiting | Anorexia, diarrhea | Anorexia, vomiting | None | None | None | Nausea |
| Renal manifestations (within 48 hours) | Anuria | Oliguria | Urine protein | Oliguria | Urine protein | Low urine output | Urine protein | Urine protein | Urine protein | Urine protein | Urine protein |
| Lymphadenopathya | NA | None | NA | NA | Yes | Yes | NA | Yes | Yes | Yes | Yes |
| Underlying conditions | Hypertension | None | None | Coronary heart disease | Chronic bronchitis | None | Old pulmonary tuberculosis | None | None | Hypertension | None |
Note: Lymphadenopathya included enlarged cervical, axillary and inguinal lymph nodes.
Abbreviation: NA, not available.
Most patients realized that they had symptoms about one or two days after being bitten. Regarding the initial symptoms and signs, fever (10/11, 91%) and rash or erythema (11/11,100%) were the most frequent complaints. Almost every case had pulmonary effusion (10/11, 91%) and abnormal urine (11/11, 100%). Meanwhile, systemic manifestations were observed in severe cases, namely vomiting (5/6, 83%), anuria or oliguria (3/6, 50%) and neurological manifestations (6/6100%). That was rarely observed as first symptom in other reports. Moreover, at the first contact, dark erythema could be observed in some critical patients and timely and effective treatment was started. However, these symptoms infrequently occurred in mild patients.
Laboratory Data
The most common laboratory abnormalities are detailed in Table 2. The data of laboratory tests were collected only within 24 hours of admission. Therefore, partial data were not available because some laboratory tests were not performed on admission.
Table 2.
Laboratory Tests of the 11 Patients with R.japonica Infection on Admission, April 20, to November 20, 2021, Yichang, China
| Reference Rangesa | Patient No. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| WBC(109/L) | 3.5–9.5 | 16.22 | 11.07 | 12.07 | 15.18 | 11.86 | 10.81 | 6.15 | 15.05 | 3.64 | 6.64 | 5.44 |
| RBC(1012/L) | 3.8–5.1 | 3.62 | 3.55 | 4.91 | 4.20 | 3.66 | 2.14 | 3.33 | 2.75 | 3.95 | 4.24 | 3.40 |
| PLT (109/L) | 125–350 | 26 | 23 | 27 | 33 | 34 | 24 | 48 | 68 | 76 | 102 | 87 |
| NEUT% | 40.0–75.0 | 95.4 | 96.7 | 95.5 | 91.3 | 88.8 | 91.3 | 80.6 | 90.7 | 90.4 | 90.7 | 85.1 |
| LY% | 20.0–50.0 | 3.6 | 2.2 | 3.5 | 5.7 | 7.4 | 5.7 | 13.8 | 7.4 | 6.9 | 5.9 | 7.9 |
| IL-6(pg/mL) | 0–7 | 1965 | >5000 | 198.8 | 203.6 | 208.6 | 571.9 | NA | 35.32 | 30.54 | 204.9 | 501.6 |
| CRP (mg/L) | 0–10 | 172.8 | 292.9 | 160.67 | 248.7 | 155.2 | 121.1 | 115.7 | 97.7 | 154.6 | 142.7 | 234.4 |
| PCT (ng/mL) | 0–0.05 | NA | 22.79 | 154.66 | 5.24 | 2.12 | 3.98 | 2.32 | 2.94 | 1.82 | 2.01 | 0.76 |
| SAA (mg/L) | 0–10 | NA | NA | NA | 625.0 | 588.2 | NA | NA | 501.1 | 884.0 | 837.2 | 670.7 |
| FER (ng/mL) | 25–350 | 1277.9 | >3000 | NA | >3000 | >3000 | 2596 | NA | >3000 | 2296 | NA | >3000 |
| D-Dimer(ug/mL) | 0–0.5 | NA | >20 | NA | >20 | 12.37 | 13.62 | >10 | NA | NA | 5.07 | NA |
| FDP (mg/L) | 0–5 | 81.26 | 53.78 | NA | NA | 11.98 | 59.23 | NA | NA | NA | NA | NA |
| LDH (IU/L) | 120–250 | 894 | 793 | 522 | 907 | 680 | 501 | 681 | 815 | 898 | 562 | 468 |
| α-HBDH(IU/L) | 95–250 | 561 | 543 | 266 | 571 | 468 | 377 | 479 | 565 | 455 | 400 | 321 |
| CK (IU/L) | 40–200 | 724 | NA | 1121 | 397 | 76 | NA | 39 | 160 | 844 | 315 | 243 |
| NT-proBNP (pg/mL) | 0–300 | NA | 9419 | NA | 11517 | 915 | 8041 | 1249 | NA | NA | NA | NA |
| AST (U/L) | 13–35 | 134 | 193 | 166 | 495 | 126 | 90 | 681 | 105 | 399 | 54 | 45 |
| ALT (U/L) | 7–40 | 36 | 117 | 93 | 220 | 60 | 42 | 106 | 49 | 189 | 37 | 13 |
| TBIL (umol/L) | 5.1–28.0 | 29.1 | 133 | 41.0 | 28.5 | 15.5 | 12.8 | 11.4 | 8.64 | 14.8 | 22.1 | 6.57 |
| DBIL (umol/L) | 0–10.0 | 22.9 | 85.79 | 29.1 | 22.2 | 9.1 | 7.2 | 4.18 | 3.85 | 7.6 | 7.8 | 3.71 |
| SCr(umol/L) | 237 | 739 | 111 | 239 | 93 | 85 | 60.6 | 65 | 104 | 121 | 86 | |
| CHOL(mmol/L) | 2.8–6.0 | NA | 1.94 | NA | 2.71 | 2.07 | 2.63 | 3.31 | NA | NA | NA | NA |
| TG (mmol/L) | 0.3–2.1 | NA | 3.46 | NA | 1.38 | 3.94 | 1.89 | 2.54 | NA | NA | 2.38 | NA |
| TP(g/L) | 65–85 | 29.26 | 50.58 | 44.83 | 50.57 | 43.70 | 46.6 | 52.02 | 44.42 | 60.0 | 61.62 | 53.44 |
| ALB(g/L) | 40–55 | 10.55 | 27.23 | 21.76 | 24.51 | 22.44 | 20.14 | 27.02 | 22.42 | 33.49 | 33.35 | 27.56 |
| PT(s) | 11.0–15.0 | 21.5 | 16.5 | 16.2 | 16.3 | 14.9 | 13.1 | 10.8 | 12.9 | 14.3 | 13.6 | 12.5 |
| PTA(%) | 70–150 | 42 | 64 | 66 | 66 | 78 | 102 | 120 | 103 | 83.0 | 94 | 111 |
| INR | 0.80–1.20 | 1.86 | 1.33 | 1.3 | 1.3 | 1.17 | 0.99 | 0.84 | 0.98 | 1.12 | 1.04 | 0.94 |
| Fib (g/L) | 2.00–4.00 | 1.07 | 3.76 | 3.77 | 5.22 | 1.91 | 2.30 | 3.08 | 2.46 | 4.56 | 5.18 | 3.95 |
| APTT(s) | 32.0–45.0 | 81.6 | 90 | 58.9 | 66.1 | 63.3 | 56.8 | 35.6 | 47.1 | 36.0 | 43.4 | 48.1 |
| TT(s) | 14.0–20.0 | 22.2 | 23.6 | 18.3 | 81.3 | 15.2 | 17.2 | 13.8 | 21.6 | 16.5 | 16.7 | 20.1 |
| FOBb | + | - | + | + | - | - | - | - | - | - | - | |
| 3P (+) | + | - | NA | - | - | + | NA | NA | NA | NA | NA | |
Notes: aReference ranges refer to those of Yichang Central People’s Hospital. bFOB measured by fecal occult blood cards. - showed negative result; + showed positive result.
Abbreviations: WBC, White blood cells; RBC, Red blood cell; PLT, Platelet; NEUT, Neutrophils; LY, Lymphocytes; IL-6, Interleukin-6; CRP, C-reactive protein; PCT, Procalcitonin; SAA, Serum amyloid a; FER, Serum ferritin; FDP, Fibrinogen and fibrin degradation products; LDH, Lactate dehydrogenase; α-HBDH, α-hydroxybutyrate dehydrogenase; CK, Creatine kinase; NT-proBNP, N-terminal pro-B-type natriuretic peptide; AST, Aspartic transaminase; ALT, Alanine transaminase; TBIL, Total bilirubin; DBLL, Direct bilirubin; SCr, Serum creatinine; CHOL, Cholesterol; TG, Triglyceride; TP, Total protein; ALB, Albumin; FOB, Fecal occult blood; 3P, Plasma protamine paracoagulation test.
For all patients, neutrophil percentage (NEUT %) was increased. Red blood cell (RBC) count, platelet (PLT) count, and lymphocytes percentage (LY %) were decreased. White blood cell (WBC) count was increased in all severe patients but normal in most mild patients (4/5 80%). Among them, PLT count, NEUT% and WBC count seemingly correlated with severity of disease and the median value of platelet count (26.5, 24.5–31.5) in severe patients was significantly lower than in mild patients (76, 68–87). In contrast, IL-6, CRP, PCT, SAA and FER, as indicators of inflammatory severity, suggested weak correlations with clinical severity. D-Dimer and fibrin degradation products (FDP) increased simultaneously with available data. In addition, fecal occult blood tested positive in three severe patients (3/6, 50%).
Therapies During Hospitalization
The main treatment regimen was Doxycycline (DO). Considering that we were in a highly endemic area of tick-borne disease, DO was empirically almost given every 12 hours from admission. In severe cases, five patients (5/6, 83%) were treated with DO and steroid hormones. Two severe patients (2/6, 33%) received extra quinolones. Five severe patients (5/6, 83%) were intubated and presented with concurrent hypotension requiring vasopressors. Continuous renal replacement therapy (CRRT), plasma exchange (PE) and transfusion were also employed. Regarding mild patients, they all recovered with oral DO alone. Additional adjunctive therapies are presented in Table 3.
Table 3.
Therapies of the 11 Patients with R.japonica Infection During Hospitalization, April 20, to November 20, 2021, Yichang, China
| Patient No. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Main medication* | DO 100mg po q12h | DO 100mg po q12h | DO 100mg IV q12h + LVX 0.5g IV qd | DO 100mg IV q12h + MXF 0.4g IV q12h | DO 100mg IV q12h | DO 100mg IV q12h | DO 100mg po q12h | DO 100mg po q12h | DO 100mg po q12h | DO 100mg po q12h | DO 100mg po q12h |
| Steroid hormones | MP 0.7mg/kg/d IV×11d | HC 2mg/kg/d IV×20 d | MP 0.7mg/kg/d IV×9d | MP 0.7mg/kg/d IV×4d | None | DEX 0.2mg/kg/d IV ×14d | None | None | None | None | None |
| Vasopressors | Yes | Yes | Yes | Yes | Yes | None | None | None | None | None | None |
| Respiratory support | MV | MV | ECMO | MV | NC | HFNC | NC | NC | None | None | NC |
| CRRT | Yes | Yes | Yes | Yes | None | Yes | None | None | None | None | None |
| PE | Yes | Yes | Yes | None | None | None | None | None | None | None | None |
| Transfusion* | Yes | Yes | Yes | None | None | Yes | None | None | None | None | None |
Note: *Transfusion referred to the transfusion of blood components.
Abbreviations: DO, Doxycycline; po, per os; IV, Intravenous drip; q12h, every 12 hours; qd, every day; LVX, Levofloxacin; MXF, Moxifloxacin; ECMO, Extracorporeal membrane oxygenation; MP, Methylprednisolone; HC, Hydrocortisone; DEX, Dexamethasone; MV, Mechanical ventilation; HFNC, High-flow nasal cannula oxygen therapy; NC, Nasal catheter oxygen inhalation; CRRT, Continuous renal replacement therapy; PE, Plasma exchange.
Clinical Outcome
The incidence of poor outcome was remarkably high for our severe JSF cases, reaching 50%. Besides, what was really troublesome was follow-up complications and organ failure. That significantly increased the length of hospital stay and treatment cost.
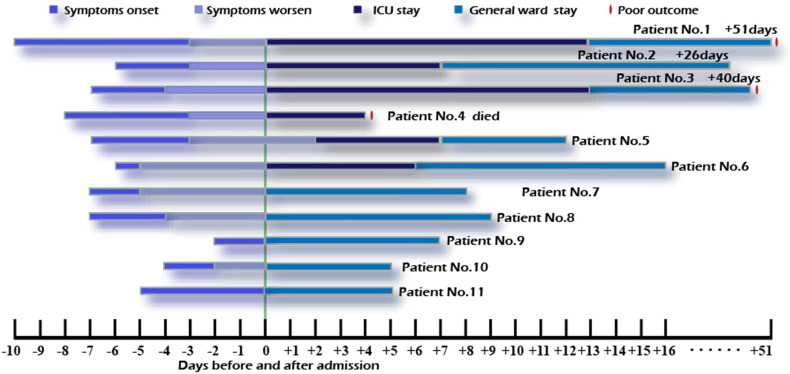
Patient No.1 and 3 were admitted directly to the ICU through the emergency department and later presented with purpura fulminans. Patient No.1 developed gangrene and underwent amputation. Patient No.3 avoided amputation, but got extensive cutaneous necrosis of extremities and required skin grafting. Patient No.4 died after four days. Other remaining patients were all cured and discharged. Schematic description of disease course and outcomes is shown in Figure 1.
Figure 1.
Schematic description for individual patients.
Disseminated intravascular coagulation (DIC) was a common complication for our severe cases, two of whom suffered from purpura fulminans. Deep venous thrombosis was demonstrated in Patients No.1–3 and 6. Intracranial hemorrhage was diagnosed in two severe cases and one mild case. And this mild patient was diagnosed with acute cerebral infarction the day before admission. In addition, we also detected arrhythmias in three severe patients (3/6, 50%) who never had it before (Table 4). Comparing with mild cases, multiple organ dysfunction was merely noted in severe patients. Without exception, they showed higher APACHE-2 score and longer hospital stay and length of ICU stay.
Table 4.
Clinical Outcome of the 11 Patients with R.japonica Infection, April 20, to November 20, 2021, Yichang, China*
| Patient No. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | |
| Duration from symptoms’ onset to medication | 10 days | 6 days | 7 days | 8 days | 7 days | 6 days | 7 days | 7 days | 2 days | 4 days |
5 days |
| Duration from admission to ICU | 0 hours | 5 hours | 0 hours | 10 hours | 2 days | 4 hours | None | None | None | None | None |
| Length of stay ICU | 16 days | 12 days | 13 days | 4 days | 5 days | 6 days | None | None | None | None | None |
| Hospital stay | 51 days | 26 days | 40 days | 4 days | 12 days | 16 days | 8 days | 9 days | 7 days | 5 days | 5 days |
| APACHE-2 score | 36 | 36 | 39 | 36 | 26 | 18 | NA | NA | NA | NA | NA |
| DIC/ISTH score | 7 | 6 | NA | 6 | 5 | 5 | 6 | NA | NA | 3 | NA |
| Organ failure | HF, RF, AKI | HF, RF, AKI | HF, RF, AKI | HF, RF, AKI | None | RF | None | None | None | None | None |
| Cerebral involvement | Intracranial hemorrhage | Arachnoid hemorrhage | None | None | None | None | None | NA | NA | Acute cerebral infarction | NA |
| Arrhythmia | Yes | None | None | Yes | Yes | None | None | None | None | None | None |
| Deep venous thrombosis | Yes | Yes | Yes | None | None | Yes | None | NA | None | None | NA |
| Other involvement | Limb gangrene | Hyperlipemia | Skin Infection | None | Hyperlipemia | None | Hyperlipemia | None | None | Hyperlipemia | None |
| Outcome | Amputation | Cured | Cutaneous necrosis | Died | Cured | Cured | Cured | Cured | Cured | Cured | Cured |
Abbreviations: HF, Heart failure; RF, Respiratory failure.
Discussion
We described homochronous combined mild and severe cases of JSF in China that reflect the largest number of serious R.japonica infections described by far. JSF is generally considered to cause mild symptoms. However, we found it continues to have potential for developing poor outcomes when neglected. Therefore, we summarized the clinical characteristics, laboratory findings, therapies and outcomes of 11 patients. Our study demonstrated that DIC carrying a risk of death and disability should cause concern in JSF patients.
In fact, it is first comprehensive case study in China region. Specifically, the first Chinese clinical case of R.japonica was discovered in Anhui Province, in 2013.11 Since then, 14 and 16 Japanese spotted fever cases occurred in Xinyang city and Zhejiang Province respectively during 2014–2017.12,13 Reportedly, R.japonica was broadly identified from H.longicornis, a tick from China, in Shandong Province, in 2015.12 Although, the presence and distribution of R.japonica are not very clear, previous studies suggested that R.japonica might be more frequent in China than is believed.13
Only individual cases with DIC and multiple organ dysfunction had been reported in JSF.14,15 In our case series, six severe cases rapidly developed DIC and multiple organ failure, including one fatal case and two cases which suffered from purpura fulminans.
Purpura fulminans is a disabling and even life-threatening disorder with cardinal manifestations presenting skin discoloration, DIC, fever, and septic shock.16 Several studies have documented that its mortality is extremely high and most survivors have poor outcomes and require amputation.17 Acute infectious purpura fulminans is the most common form of purpura fulminans. Rickettsia infection associated with purpura fulminans is recorded rarely, and seen only in R.rickettsii, R.australis, R.conorii, israelensis and probably indica.18–20 We described two cases of acute infectious purpura fulminans which, to the best of our knowledge, were the first discovered cases in R.japonica.
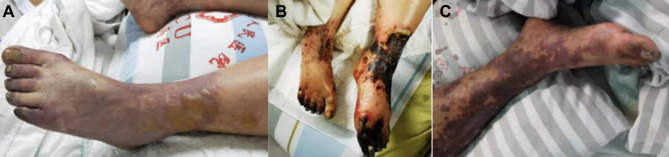
They initially manifested as cutaneous circumscribed ecchymosis and formation of bullae (Figure 2A), which was linked to the obstruction of small blood vessels in the superficial skin leading to telangiectasia and congestion.21 Later enhanced vascular permeability and extravasation of blood in the center of petechial lesions enlarged ecchymosis and extremities blackened (Figure 2B and C),22 which marked disease progression. In comparison with Patient No.1 who underwent amputation, blackened extremities gradually recovered owing to aggressive management in our Patient No.3. Consequently, full knowledge of the disease initiation and progression, prompt identification remain essential to prognosis.
Figure 2.
Cutaneous circumscribed ecchymosis and formation of bullae in Patient No.1 (A); gangrene in both lower extremities in Patient No.1 (B); extravasation of blood in the center of petechial lesions and enlargement of ecchymosis in Patient No.3 (C).
Systemic symptoms were found more frequently in severe than mild cases. These implied critical patients showed a propensity for multiple organ involvement. Even though pulmonary effusion secondary to microvascular leakage is always seen, the more extensive effusion may indicate more severe disease. Similarly, not only urine protein but also anuria and oliguria were present in severe cases. Consistent with a published study,23 we also found SCr had a strong correlation with serious infection.
The clinical manifestations seem to be consistent with the pathophysiology described in a murine model of rickettsiosis. Mice with R.australis infection acquired progressively severe vasculitis, multifocal hepatic necrosis, renal and pulmonary involvement.24 In another study, hematogenous dissemination, multifocal inflammatory lesions, interstitial pneumonitis and cerebral hemorrhages were exhibited in R.heilongjiangensis infected mice model.25 For severe patients, this endothelial inflammation leads to microvascular dysfunction increasing the likelihood of DIC and invasion of multiple organs including heart, brain, lungs and kidney, which suggests R.japonica may cause more severe rickettsiosis.
Noticeably, our observations were comparable to previous studies with some differences. Patient No.1 complained firstly of vomiting and did not have fever until the sixth day of admission. Therefore, we deem that lack of fever should not preclude the possibility of JSF. Nonspecific gastrointestinal symptoms may also be first and prominent in afebrile patients.
Comparing mild and severe cases, we found duration from symptoms’ onset to medication, PLT count, NEUT%, WBC count, D-Dimer, FDP, APACHE-2 score and multiple organ dysfunction might reveal severity of disease, which might be tested in future prospective studies. Unlike the most common sequelae of neurological involvement caused by Rocky Mountain spotted fever,26 JSF caused arrhythmias in three severe patients, which has not been previously reported. The combination therapy of DO and steroid hormones is workable and quinolones can be added if necessary.
Although our study has limitations, ie, the results were based on a small quantity of cases and the only methods depended on mNGS, a sample with relatively balanced population in mild and severe groups and a population with almost the same underlying condition enabled us to illustrate the different courses, and provide some relevant factors regarding the severity of disease.
We believe that these results will contribute to better understanding of severe R.japonica infection. Definitive treatment should be instituted on the basis of clinical and epidemiological clues as early as possible to avoid severe disease and poor outcome.
Funding Statement
This work was supported by Key Scientific Research Project of Department of Education, Hubei Province (D20201204).
Consent Statement
Written informed consent was provided by all patients to allow the case details and any accompanying images to be published.
Disclosure
Siyu Gao and Lingfeng Li are co-first authors for this study. The authors report no conflicts of interest in this work.
References
- 1.Mahara F, Koga K, Sawada S, et al. The first report of the rickettsial infections of spotted fever group in Japan: three clinical cases. Kansenshogaku zasshi. 1985;59(11):1165–1171. doi: 10.11150/kansenshogakuzasshi1970.59.1165 [DOI] [PubMed] [Google Scholar]
- 2.Kodama K, Senba T, Yamauchi H, Nomura T, Chikahira Y. Clinical study of Japanese spotted fever and its aggravating factors. J Infect Chemother. 2003;9(1):83–87. doi: 10.1007/s10156-002-0223-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Noguchi M, Oshita S, Yamazoe N, Miyazaki M, Takemura YC. Important clinical features of Japanese spotted fever. Am J Trop Med Hyg. 2018;99(2):466–469. doi: 10.4269/ajtmh.17-0576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amor CG, Marissa A, Miguel A, et al. Detection of antibodies against spotted fever group Rickettsia (SFGR), typhus group Rickettsia (TGR), and Coxiella burnetii in human febrile patients in the Philippines. Jpn J Infect Dis. 2003;56(1):26–28. [PubMed] [Google Scholar]
- 5.Moon-Hyun C, Seung-hyun L, Mi-Jeong K, et al. Japanese spotted fever, South Korea. Emerg Infect Dis. 2006;12(7):1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jariyanart G, Piyanate S, Wuttikon R, Toon R-A, Jeffries MC, Narongrid S. Human infection with Rickettsia sp. related to R. japonica, Thailand. Emerg Infect Dis. 2007;13(4):657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cecconi M, Evans L, Levy M, Rhodes A. Sepsis and septic shock. Lancet. 2018;392(10141):75–87. doi: 10.1016/S0140-6736(18)30696-2 [DOI] [PubMed] [Google Scholar]
- 8.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. doi: 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 9.Levi M, Toh CH, Thachil J, Watson HG. Guidelines for the diagnosis and management of disseminated intravascular coagulation. British Committee for Standards in Haematology. Br J Haematol. 2009;145(1):24–33. doi: 10.1111/j.1365-2141.2009.07600.x [DOI] [PubMed] [Google Scholar]
- 10.Levey AS, Eckardt KU, Dorman NM, Christiansen SL, Cheung M. Nomenclature for kidney function and disease: executive summary and glossary from a Kidney Disease: Improving Global Outcomes (KDIGO) consensus conference. Am J Transplant. 2021;21(2):901–902. doi: 10.1111/ajt.16114 [DOI] [PubMed] [Google Scholar]
- 11.Jiabin L, Wen H, Ting W, et al. Japanese spotted fever in Eastern China, 2013. Emerg Infect Dis. 2018;24(11):2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiangrong Q, Huiju H, Fujun H, et al. Rickettsia japonica and novel rickettsia species in ticks, China. Emerg Infect Dis. 2019;25(5):992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qunying L, Jianping Y, Liqun Y, et al. Rickettsia japonica infections in humans, Zhejiang Province, China, 2015. Emerg Infect Dis. 2018;24(11):2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kondo M, Nishii M, Gabazza EC, Kurokawa I, Akachi S. Nine cases of Japan spotted fever diagnosed at our hospital in 2008. Int J Dermatol. 2010;49(4):430–434. doi: 10.1111/j.1365-4632.2010.04359.x [DOI] [PubMed] [Google Scholar]
- 15.Nakata R, Motomura M, Tokuda M, et al. A case of Japanese spotted fever complicated with central nervous system involvement and multiple organ failure. Intern Med. 2012;51(7):783–786. doi: 10.2169/internalmedicine.51.6214 [DOI] [PubMed] [Google Scholar]
- 16.Childers BJ, Cobanov B. Acute infectious purpura fulminans: a 15-year retrospective review of 28 consecutive cases. Am Surg. 2003;69(1):86–90. [PubMed] [Google Scholar]
- 17.Endo A, Shiraishi A, Aiboshi J, Hayashi Y, Otomo Y. A case of purpura fulminans caused by Hemophilus influenzae complicated by reversible cardiomyopathy. J Intensive Care. 2014;2(1):13. doi: 10.1186/2052-0492-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen R, Babushkin F, Shapiro M, et al. Two cases of Israeli spotted fever with purpura fulminans, Sharon District, Israel. Emerg Infect Dis. 2018;24(5):835–840. doi: 10.3201/eid2405.171992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dalugama C, Gawarammana IB. Rare presentation of rickettsial infection as purpura fulminans: a case report. J Med Case Rep. 2018;12(1):145. doi: 10.1186/s13256-018-1672-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulmani M, Alekya P, Kumar VJ. Indian tick typhus presenting as purpura fulminans with review on rickettsial infections. Indian J Dermatol. 2017;62(1):1–6. doi: 10.4103/0019-5154.198030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatti UF, Williams AM, Raghavendran K, Georgoff PE. Four-extremity amputation following disseminated intravascular coagulation and purpura fulminans. BMJ Case Rep. 2019;12(3):e228028. doi: 10.1136/bcr-2018-228028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sahni A, Fang R, Sahni SK, Walker DH. Pathogenesis of rickettsial diseases: pathogenic and immune mechanisms of an endotheliotropic infection. Annu Rev Pathol. 2019;14:127–152. doi: 10.1146/annurev-pathmechdis-012418-012800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura T, Takagaki K, Matsubara Y, Kikuchi K. Predictive values of clinical parameters for severe Japanese spotted fever. J Infect Chemother. 2011;17(2):246–253. doi: 10.1007/s10156-010-0113-1 [DOI] [PubMed] [Google Scholar]
- 24.Feng HM, Wen J, Walker DH. Rickettsia australis infection: a murine model of a highly invasive vasculopathic rickettsiosis. Am J Pathol. 1993;142(5):1471–1482. [PMC free article] [PubMed] [Google Scholar]
- 25.Duan C, Meng Y, Wang X, Xiong X, Wen B. Exploratory study on pathogenesis of far-eastern spotted fever. Am J Trop Med Hyg. 2011;85(3):504–509. doi: 10.4269/ajtmh.2011.10-0660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jay R, Armstrong PA. Clinical characteristics of Rocky Mountain spotted fever in the United States: a literature review. J Vector Borne Dis. 2020;57(2):114–120. doi: 10.4103/0972-9062.310863 [DOI] [PubMed] [Google Scholar]