Abstract
Purpose
Diopsys® NOVA fixed-luminance flicker full-field electroretinogram (ffERG) device is a potential adjunct to conventional flicker ffERG testing for assessing cone cell function. Magnitude of measured electrical response is known to vary with pupil size in conventional ffERG testing. The index study characterizes the relationship between magnitude of measured electrical activity and pupil size, both pupil diameter and pupil area, for this device.
Methods
Seventeen patients (34 eyes) with no known ocular diseases were enrolled in the study. Electrophysiologic function of cone cells was evaluated using fixed-luminance flicker ffERG before and after dilation. Linear regression models, with inter-eye correlations controlled as fixed-effects, were used to characterize the effect of pupil dilation on the magnitude of the measured responses.
Results
Mean age of study patients was 33.5 (standard deviation 7.4 years), and 35.3% of the subjects were female. Mean value of electrical response magnitude was 10.07±2.79µV before dilation and 15.30±4.08µV after dilation. The correlations of ERG magnitude with pupil diameter and with pupil area were not significant for either dilated or undilated eyes considered separately but were highly significant (p<0.001) for dilated and undilated eyes considered in aggregate. ERG magnitude tended to increase by 1.08 µV for every 1 mm increase in pupillary diameter.
Conclusion
An increase in pupil size, both pupil diameter and pupil area, is significantly associated with an increase in flicker ffERG magnitude recorded by the Diopsys device, suggesting that pupil size should be measured and considered when making clinical judgments based on the flicker ffERGs recorded by the device, and that pupil size-specific reference ranges could improve the clinical utility of the device.
Keywords: electroretinogram, ERG, flicker ERG, pupil, magnitude, Diopsys
Introduction
Electroretinography (ERG) is a diagnostic test that measures the electrophysiologic function of the retina.1 It measures the electrical response generated by different retinal cells when exposed to various visual stimuli.2 ERG is useful for assessing cumulative retinal function in diseases such as retinitis pigmentosa, cone-rod dystrophy, and retinal ischemia secondary to diabetic retinopathy, central retinal vein occlusion, or other retinal vascular disorders.3 In the well-established technique of full-field flicker (ffERG), a rapid (30 Hz) flicker of light over the entire retinal field is used to isolate the function of the cone cells pathway.3
Many factors can affect flicker ffERG measurements, including patient age, systemic circulation status, medications, and stimulus duration and interval.4 Another key factor that affects ERG response is pupil size (diameter and area).5 Numerous factors can result in variation in pupil size including gender, iris color, refractive error, age (pupil diameter tends to decrease with age), ophthalmic medications (eg, antiglaucoma drugs, topical corticosteroids),5 and certain acquired conditions, such as diabetes mellitus and pseudoexfoliation.6
To reduce the dependence on pupil size as well as to maximize the area of retina stimulated, the International Society of Clinical Electrophysiology of Vision (ISCEV) guidelines endorse that pupils be fully dilated prior to recording ERGs.7 However, some patients have medication allergies that preclude the use of mydriatics. Furthermore, it would be more convenient for both patients and clinicians to be able to perform the ERG testing without dilation since dilation often requires additional time, up to 30 minutes.3
Recently, a new office-based flicker ffERG device, the Diopsys NOVA fixed-luminance flicker ffERG device (Diopsys Inc., NJ), has been introduced. This device utilizes a small, hand-held Ganzfeld stimulator and skin electrodes to record responses.8 One of the device’s potential disadvantages is that, although normative data for non-dilated eyes is provided, the luminance of the stimulator does not adjust for pupil size, meaning that any alteration in ERG response due to pupil size could be misinterpreted as altered retinal function.9 Therefore, the objective of this study was to determine the relationship between pupil size and flicker ffERG magnitude recorded using the index device. This relationship has not yet been described for this device and could guide a more accurate interpretation of results.
Material and Methods
Subjects
The present study included 34 eyes from 17 healthy volunteers recruited by convenience sampling. All subjects were healthy and had neither known ocular disease nor known serious systemic diseases nor myopia of −6.0 diopters or more. All participants signed a written informed consent form after they were provided with information on the procedure. Institutional Review Board (IRB) approval was obtained from the Stanford IRB prior to the start of the study, and the study adhered to the tenants of the Declaration of Helsinki.
Data Collection Overview
For each of the subjects participating in the study, two sets of flicker ffERG recordings were made using the Diopsys NOVA device. The first set of recordings (Session 1) were obtained in non-dilated eyes. Then, the eyes were anesthetized with proparacaine hydrochloride 0.5% ophthalmic solution and subsequently dilated with tropicamide 1% and phenylephrine 2.5% ophthalmic solutions to a minimum of 5mm over approximately 20 minutes. The second set of flicker ffERG recordings (Session 2) were then obtained from the dilated eyes. Prior to each set of flicker ffERG recordings, pupil diameter was measured using slit-lamp biomicroscopy under the same photopic room illumination level (850 lx) that was present when obtaining the flicker ffERG recordings. Each pupil diameter measurement was made by a human examiner who recorded the minimum length required (estimated to the nearest 0.5 mm) for a slit beam passing through the pupil center to span the pupil. Pupil area was calculated from pupil diameter by approximating the pupil shapes of these healthy eyes as circles; specifically, pupil area was computed as 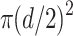 where d is pupil diameter. Details of the procedure used to obtain flicker ffERG recordings with the device are described below.
where d is pupil diameter. Details of the procedure used to obtain flicker ffERG recordings with the device are described below.
Flicker ERG Recordings by the Diopsys System
Flicker ERG was performed with an in-office ERG device (Diopsys Inc., NJ) in an illuminated room without visual and auditory distractions using standard procedures similar to those that have been detailed previously.9 Briefly, first the lower eyelids and forehead of the patient were thoroughly cleansed using alcohol swabs, and the forehead was additionally cleansed with skin prep gel (NuPrep®, Weaver and Company, Aurora, CO, USA). One hypoallergenic skin electrode was placed below each lower eyelid and a third electrode was attached to the forehead. Ten20 conductive paste (Weaver and Company) was used with the forehead electrode. The electrodes were then connected to the device. During recordings from either eye the electrode on the contralateral eyelid served as a reference. The subject was instructed to hold the hand-held Mini Ganzfeld Stimulator (LKC Technologies, Inc., Gaithersburg, MD, USA) over the eye being tested and to fixate on a center target with the other eye while keeping both eyes open. The full-field stimulus used consisted of white flashes flickering at 32Hz over a white background. Onset and offset times were 5 and 26.25 ms, respectively. White flash luminosity was 3 cd·s·m−2 over a white background of 28cd/m2. Voltages over a threshold of 50μV, suggestive of contamination by eye blinks or gross eye saccades, were rejected automatically.9 Both eyes were tested in each recording session.
Statistical Analyses
First, a box plot and summary statistics (mean and standard deviation) were used to perform an initial comparison of the ERG magnitude before dilation (Session 1) and after dilation (Session 2). Then, linear regression was used to characterize the association between pupil diameter and ERG magnitude for undilated eyes, for dilated eyes, and for undilated and dilated eyes considered together. Fixed-effect models were used to account for the correlation between eyes of the same subject, by including a fixed effect per subject for each model. For the case of undilated and dilated eyes considered together, an alternative fixed-effect linear regression model with a fixed-effect per eye rather than per subject was also evaluated (including fixed effects both per eye and per subject would result in an undetermined system). Subsequently, each of the above linear regressions was repeated with pupil area in place of pupil diameter. The results of each regression were assessed primarily by the coefficient relating pupil diameter or area to ERG magnitude, the p-value of this coefficient, and the overall adjusted R2 value for the model. All statistical analyses were completed with the use of RStudio statistical software (RStudio, PBC).
Results
Demographic Data of 34 Healthy Eyes
The present study included 34 eyes of 17 healthy subjects. The subjects were 11 men and 6 women, and the mean age was 33.5±7.4 years (Table 1).
Table 1.
Demographic Characteristics of Study Subjects
| Mean Age, Years (Standard Deviation) | 33.5 Years (7.4) |
|---|---|
| Gender | |
| Female | 6 (35.3%) |
| Male | 11 (64.7%) |
| Race/Ethnicity | |
| White | 22 (64.70%) |
| Hispanic/Latino | 2 (5.88%) |
| Asian | 8 (23.52%) |
| Other/Unknown | 2 (5.88%) |
Statistical Results
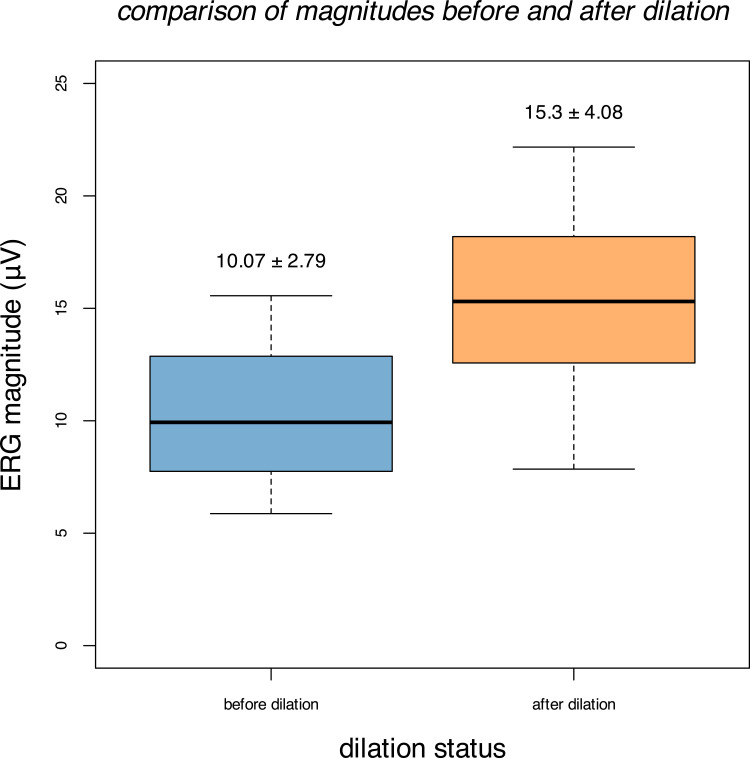
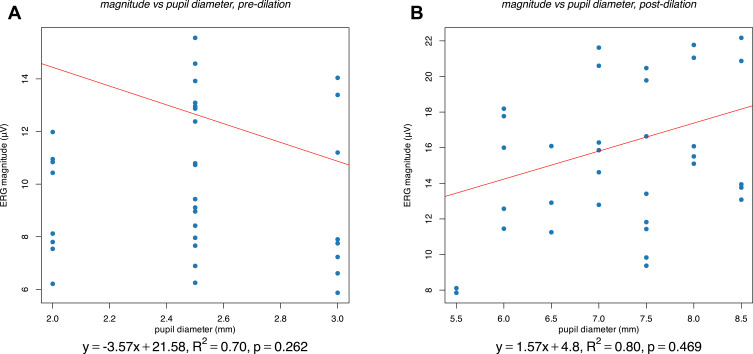
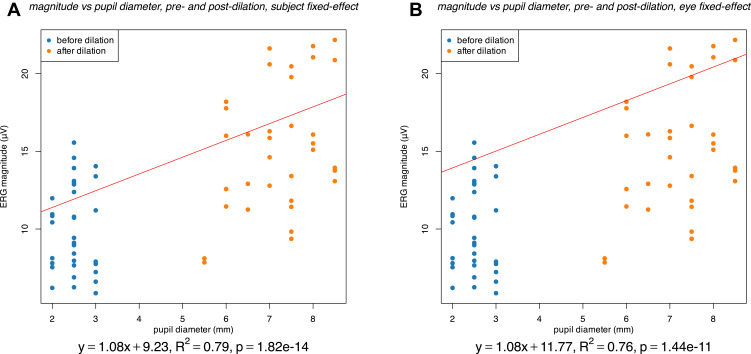
The mean ERG magnitude increased from 10.07±2.79 µV (mean ± standard deviation) before dilation to 15.30±4.08 µV after dilation (Figure 1). Controlling for inter-eye correlations using fixed-effect models, there was not a significant correlation between ERG magnitude and pupil diameter for either undilated eyes alone (Figure 2A, p = 0.262) or dilated eyes alone (Figure 2B, p = 0.469). However, considering eyes before and after dilation in aggregate, there was a highly significant (p < 0.001) correlation between ERG magnitude and pupil diameter, both when using a per subject fixed-effect (Figure 3A) and when considering a per eye fixed-effect (Figure 3B). Specifically, the ERG magnitude tended to increase by 1.08 µV for every 1 mm increase in pupillary diameter.
Figure 1.
Comparison of the ERG magnitudes recorded in eyes before (blue) and after (orange) dilation. Means (±SDs) are labeled for each group.
Figure 2.
Association between pupil diameter (mm) and ERG magnitude (µV) in eyes prior to dilation (A) and after dilation (B). The results of a fixed-effect linear regression model, with a per-subject fixed effect, are shown for both. These results include the pupil diameter coefficient and intercept (equation and red line), the p-value of the coefficient, and the adjusted R2 value of the model.
Figure 3.
Association between pupil diameter (mm) and ERG magnitude (µV) with eyes before (blue) and after (orange) dilation considered together. The results of a fixed-effect linear regression model, with either a per-subject fixed effect (A) or a per-eye fixed effect (B) are shown. These results include the pupil diameter coefficient and intercept (equation and red line), the p-value of the coefficient, and the adjusted R2 value of the model.
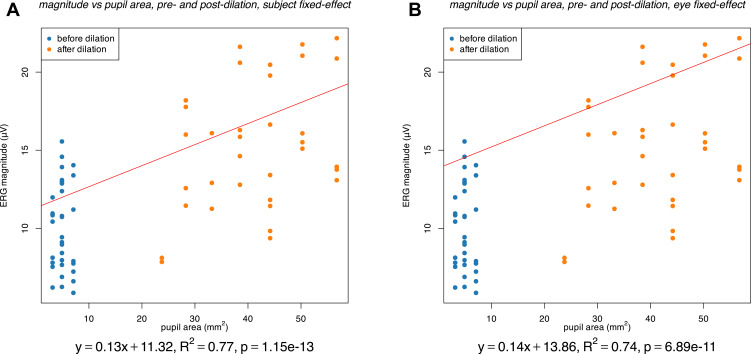
Repeating the above analysis with pupil area in place of pupil diameter resulted in highly similar results (Figure 4 and Table 2). Across the linear regression models considered, overall adjusted R2 ranged from 0.7 to 0.8, indicating moderately high goodness of fit.
Figure 4.
Association between pupil area (mm2) and ERG magnitude (µV) with eyes before (blue) and after (orange) dilation considered together. The results of a fixed-effect linear regression model, with either a per-subject fixed effect (A) or a per-eye fixed effect (B) are shown. These results include the pupil area coefficient and intercept (equation and red line), the p-value of the coefficient, and the adjusted R2 value of the model.
Table 2.
Summary of Regression Results – The Coefficient Associated with Pupil Size, Either Pupil Diameter or Pupil Area, the p-Value of This Coefficient, and the Adjusted R2 Value for the Overall Model for Various Fixed-Effect Regression Models of ffERG Magnitude Vs Pupil Size
| Pupil Diameter | Pupil Area | |||||
|---|---|---|---|---|---|---|
| Coefficient | p-value | Adjusted R2 | Coefficient | p-value | Adjusted R2 | |
| Pre-dilation, subject fixed-effect | −3.57 | 0.262 | 0.70 | −0.83 | 0.262 | 0.70 |
| Post-dilation, subject fixed-effect | 1.57 | 0.469 | 0.80 | 0.13 | 0.515 | 0.80 |
| Aggregated, subject fixed-effect | 1.08 | 1.82e-14 | 0.79 | 0.13 | 1.15e-13 | 0.77 |
| Aggregated, eye fixed-effect | 1.08 | 1.44e-11 | 0.76 | 0.14 | 6.89e-11 | 0.74 |
Discussion
The present results demonstrate the effect of pupil diameter on the magnitude of fixed-luminance flicker ffERG response recorded by the machine. Considering diameter before and after dilation in aggregate, there was a highly significant (p<0.001) positive correlation between pupil diameter and recorded flicker ffERG magnitude (Figure 3). Furthermore, this positive association was highly significant and approximately the same in magnitude (1.08 µV/mm) both when controlling for variation across individuals (Figure 3A) and when further controlling for variation across eyes (Figure 3B). Overall, this positive correlation between pupil diameter and the machine recorded fixed-luminance flicker ffERG magnitude is consistent with previously reported positive associations between pupil diameter and b-wave magnitude of conventional flash ffREG,10 between pupil diameter and conventional mfERG magnitude,11 and between pupil diameter and multifocal visual evoked potential (VEP).12
In contrast to the highly significant correlation between pupil diameter and ERG magnitude when undilated and dilated eyes were considered in aggregate, there was not a significant correlation when either undilated eyes (Figure 2A) or dilated eyes (Figure 2B) were considered separately. Multiple factors could have contributed to this discrepancy. First, because analyses of either dilated on undilated eyes alone included only half the number of data points as the aggregate analysis, lower statistical power would naturally be expected. However, this effect alone appears insufficient to explain the disparity given the vast difference in p-values as well as the difference in coefficient values. A likely much more significant effect was inaccuracy in pupil diameter measurements, which also represents a limitation to the present work more generally. Whereas automated pupillometery may be used for more reliable and precise measurements in future work, here manual slit-lamp examination was used and only precision up to 0.5 mm was attempted. Furthermore, although eyes were illuminated both during ERG testing and during pupil measurement, difference in illumination intensity could have caused pupil diameter at pupil measurement to differ from pupil diameter at ERG testing, even as the relative ordering of pupil diameters across eyes may be preserved between pupil measurement and ERG testing. Any fixed amount of noise in pupil size measurement (eg, 0.5 mm error per measurement) would be more significant when testing the effect of pupil diameter or ERG magnitude for a small range of pupil diameters, as with undilated or dilated eyes alone, than for a larger range of pupil diameters as with undilated and dilated eyes combined.
A further limitation of this study and challenge to detecting the association between pupil diameter and ERG magnitude through linear regression is potential inherent nonlinearity in the effect of pupil diameter on ERG magnitude. To account for one possible source of nonlinearity, regression against pupil area was considered alongside regression against pupil diameter, given that the extent of retinal illumination, and therefore the ERG magnitude, may be expected to be more directly proportional to pupil area than to pupil diameter, as reflected in the common practice of multiplying incident luminosity by pupil area to obtain Trolands of illumination.13 However, each regression with pupil area produced similar results as the corresponding regression against pupil diameter (Table 2), including similar adjusted R2 values, indicating that considering pupil area in place of pupil diameter did not significantly improve linearity in this case.
One likely contributor to nonlinearity is the Stiles–Crawford effect. This long-observed effect is the tendency of a light ray of a given intensity to produce the greatest retinal stimulation when entering the eye at the pupil center and increasingly less stimulation as its entry point moves towards the pupil periphery.14 In other words, the visual sensitivity of a unit of pupil area is inversely related to its distance from the center. While the physiologic origins of this effect are not completely understood, it is thought to follow from the directional sensitivity of photoreceptors, such that obliquely incident light produces less stimulation than light that is aligned with the photoreceptors, which tend to be aligned perpendicular to the retina; the orientation of photoreceptors is, in turn, thought to arise from a combination of development and phototropism.15 A corollary to the Stiles–Crawford effect is that an increase in pupil area by a fixed amount produces a smaller increase in retinal stimulation for a larger pupil than for a smaller pupil.3,16 Indeed, visual inspection of the ERG magnitude vs pupil area data (Figure 4) does appear to suggest a flattening of the curve at high pupil area values which would be consistent with the influence of the Stiles–Crawford effect.
In the future, the work presented here could be extended by gathering more data, specifically by including more study subjects, by recording at multiple time points while the pupils are undergoing dilation, and by repeating recording across multiple days. Repeating recordings of each subject across multiple days could help to account for confounders such as variation in electrode placement and electrode conductivity. Recording at intermediate levels of dilation (3–6 mm pupils, largely absent from the current data) would allow more precise characterization of the ERG magnitude and pupil size relationship. Utilizing such additional data, future analyses could include further investigation of potential nonlinear relationships between ERG magnitude and pupil diameter. Finally, extending this study of healthy subjects to subjects with retinal disease would further enhance the applicability of these results to clinical practice.
Conclusion
Consistent with prior studies on the effect of pupil size on measurements made by conventional ERG devices, the present study demonstrates that larger pupil size, both diameter and area, is positively associated with larger flicker ffERG magnitude as measured by the Diopsys NOVA device. This finding has important clinical implications. Currently, the manufacturer (Diopsys) only provides overall normative ERG data that is independent of pupil size. These results highlight the potential value of pupil size-specific normative data. Alternatively, the device could perhaps adjust illumination intensity according to pupil size, a strategy pursued by the competing RETeval® system with moderate success.3 Based on the results of the current study, clinicians and clinician-scientists should be careful to account for pupil size variation when using the NOVA device and analyzing its results.
Acknowledgments
The Diopsys ERG device at the Byers Eye Institute is on loan from Diopsys, Inc. for clinical and research usage. The abstract of this paper was presented at the Association for Research in Vision and Ophthalmology (ARVO) 2021 Conference as a poster presentation with interim findings. The poster’s abstract was published in “Poster Abstracts” in the Investigative Ophthalmology & Visual Science (IOVS) journal. URL: https://iovs.arvojournals.org/article.aspx?articleid=2773318. Azadeh Mobasserian and Moosa Zaidi are co-first authors in this work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.McCulloch DL, Marmor MF, Brigell MG, et al. ISCEV Standard for full-field clinical electroretinography (2015 update). Documenta Ophthalmologica. 2015;130(1):1–12. doi: 10.1007/s10633-014-9473-7 [DOI] [PubMed] [Google Scholar]
- 2.Degirmenci M, Demirel S, Batıoğlu F, Özmert E. Role of a mydriasis-free, full-field flicker ERG device in the detection of diabetic retinopathy. Documenta Ophthalmologica. 2018;137(3):131–141. doi: 10.1007/s10633-018-9656-8 [DOI] [PubMed] [Google Scholar]
- 3.Kato K, Kondo M, Sugimoto M, Ikesugi K, Matsubara H. Effect of pupil size on flicker ERGs recorded with RETeval system: new mydriasis-free full-field ERG system. Invest Ophthalmol Vis Sci. 2015;56(6):3684–3690. doi: 10.1167/iovs.14-16349 [DOI] [PubMed] [Google Scholar]
- 4.Guillon M, Dumbleton K, Theodoratos P, Gobbe M, Wooley CB, Moody K. The effects of age, refractive status, and luminance on pupil size. Optom Vis Sci. 2016;93(9):1093. doi: 10.1097/OPX.0000000000000893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park JW, Kang BH, Kwon JW, Cho KJ. Analysis of various factors affecting pupil size in patients with glaucoma. BMC Ophthalmol. 2017;17(1):1–8. doi: 10.1186/s12886-017-0564-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maa AY, Feuer WJ, Davis CQ, et al. A novel device for accurate and efficient testing for vision-threatening diabetic retinopathy. J Diabetes Complications. 2016;30(3):524–532. doi: 10.1016/j.jdiacomp.2015.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Robson AG, Nilsson J, Li S, et al. ISCEV guide to visual electrodiagnostic procedures. Documenta Ophthalmologica. 2018;136(1):1–26. doi: 10.1007/s10633-017-9621-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillmann K, Mansouri K, Rao HL, et al. A prospective evaluation of the repeatability and reliability of new steady-state pattern electroretinogram parameters. J Glaucoma. 2018;27(12):1079–1085. doi: 10.1097/IJG.0000000000001103 [DOI] [PubMed] [Google Scholar]
- 9.Wroblewski JJ, McChancy C, Pickel K, Buterbaugh H, Wieland T, Gonzalez A. Reproducibility of fixed-luminance and multi-luminance flicker electroretinography in patients with diabetic retinopathy using an office-based testing paradigm. J Diabetes Sci Technol. 2020;14(6):1095–1103. doi: 10.1177/1932296819882719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson MA, Jeffrey BG, Messias A, Robson AG. ISCEV extended protocol for the stimulus–response series for the dark-adapted full-field ERG b-wave. Documenta Ophthalmologica. 2019;138(3):217–227. doi: 10.1007/s10633-019-09687-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez P, Parks S, Dolan F, Keating D. The effects of pupil size on the multifocal electroretinogram. Documenta Ophthalmologica. 2004;109(1):67–72. doi: 10.1007/s10633-004-1545-7 [DOI] [PubMed] [Google Scholar]
- 12.Martins A, Balachandran C, Klistorner AI, Graham SL, Billson FA. Effect of pupil size on multifocal pattern visual evoked potentials. Clin Experiment Ophthalmol. 2003;31(4):354–356. doi: 10.1046/j.1442-9071.2003.00669.x [DOI] [PubMed] [Google Scholar]
- 13.Troland LT. On the measurement of visual stimulation intensities. J Exp Psychol. 1917;2(1):1. doi: 10.1037/h0071652 [DOI] [Google Scholar]
- 14.Stiles WS, Crawford BH, Parsons JH. The luminous efficiency of rays entering the eye pupil at different points. Proc R Soc Lond B Biol Sci. 1933;112(778):428–450. [Google Scholar]
- 15.Verschueren A, Boucherit L, Ferrari U, et al. Planar polarity in primate cone photoreceptors: a potential role in Stiles Crawford effect phototropism. Commun Biol. 2022;5(1):89. doi: 10.1038/s42003-021-02998-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Westheimer G. Directional sensitivity of the retina: 75 years of Stiles–Crawford effect. Proc R Soc B. 2008;275(1653):2777–2786. doi: 10.1098/rspb.2008.0712 [DOI] [PMC free article] [PubMed] [Google Scholar]