Abstract
Background/Objectives
The lip is a unique tissue type that acts as a “barrier” to the mouth and receives many external stimuli. It is also a common symptom in atopic dermatitis. Dupilumab was the first targeted biological drug approved for the treatment of moderate-to-severe atopic dermatitis (AD). There is no real-world clinical data on the use of dupilumab in patients with AD and cheilitis. This retrospective study compared the improvement in skin lesions in AD patients with cheilitis after dupilumab treatment and evaluated the improvement in cheilitis.
Methods
This is a retrospective case series. We investigated patients with AD treated with dupilumab in our department from September 2020 to May 2022, including those with cheilitis. Demographic information such as age, sex, AD or other atopy history, and the anatomical site of dermatitis was collected. Disease severity was assessed using the eczema area and severity index score (EASI), body surface area (BSA), and severity assessment of cheilitis (the cheilitis symptom score) at baseline and after 16 weeks.
Results
We reviewed 96 patients treated with dupilumab for AD, and including the 10 patients with cheilitis (10.4%). All patients demonstrated significant improvement in skin lesions, and lip symptoms improved in seven patients. Among AD patients with improved cheilitis, the average reduction in EASI was 35.0% for BSA (34.9%) and the cheilitis symptom score was 29.9% at week 8. At week 16, compared with the baseline score, the improvement in cheilitis symptom scores was 58.1%, EASI was 60.8%, and BSA was 56.2%, respectively.
Conclusion
Effective treatment of both the skin and cheilitis was achieved with dupilumab. The improvement in cheilitis involvement was slower than that in skin lesions. This case series confirms that dupilumab could be a valuable approach for treating patients with atopic dermatitis-associated lip involvement.
Keywords: atopic dermatitis, dupilumab, cheilitis, patch test
Introduction
Atopic dermatitis (AD) is a chronic, relapsing, type 2 inflammatory skin disease caused by genetic predisposition, skin barrier disruption, immune factors, and environmental exposures.1 Cheilitis refers to the superficial inflammatory conditions of the half-mucous membrane of the lip. This is also a secondary criterion for atopic dermatitis. Lip involvement is not only a cosmetic issue but also affects the lip’s structure and function of the lips and is frequently associated with significant pain. Uncomfortable, lesioned lips have a negative psychological effect, may interfere with working ability, and impairs quality of life (QoL).2
Dupilumab is the first targeted biological drug approved for the treatment of moderate-to-severe atopic dermatitis, which is not adequately controlled with other therapies. Dupilumab inhibits IL-4 and IL-13 receptors, which are linked to the T helper (Th)2 immune response.3,4 The effectiveness of dupilumab has been demonstrated in numerous clinical trials and practical applications.5 With the approval of dupilumab in 2017, recurrent cases have been reported to successfully treat skin lesions in certain areas, such as the perianal region, chronic hand eczema, and feet.6 Off-label use of dupilumab has been reported in other chronic inflammatory skin lesions,7,8 special populations,9 and pediatric patients. Data on the efficacy of dupilumab and other biological agents in the treatment of cheilitis are limited. This study describes the results obtained with dupilumab in patients eligible for the systemic treatment of atopic dermatitis with lip involvement.
Methods
From September 2020 to May 2022, 96 patients with AD were treated with dupilumab. Ten of these patients had cheilitis (8 males, 80%) and so they were included in the study. The patients were diagnosed with mild-to-severe AD with cheilitis according to the Hanifin and Rajka criteria and treated with dupilumab for at least 16 weeks. Dupilumab was administered at the labeled dosage (adolescents less than 60kg: 300mg for the first time, then 300mg every two weeks; adolescents >60 kg or all adults: 600 mg first (300 mg twice), then 300mg every two weeks). Written informed consent was obtained from the patients or their parents/guardians. For each subject, the following demographic and clinical data were recorded: age, sex, history of atopic dermatitis or another atopy, site of dermatitis, and adverse events (AEs). Laboratory data were analyzed, including serum IgE levels and patch test results were analyzed. The eczema area, severity index score (EASI), and body surface area (BSA) were used to assess AD severity. Based on the cheilitis symptom score, the skin lesion area, dryness, crusting, swelling, and pain were scored as (0–3) points according to no, mild, moderate, and severe, respectively. A drop of more than 50% in the total score was seen significantly. Evaluations were performed at baseline (W0), week 8(W8), and week 16 (W16) of the therapy. Patches were read on Day 2 (48 h). A positive response was graded as a (+, ++, or +++) reaction, corresponding to infiltration, papules, or vesicles. For safety evaluations, all new-onset clinical symptoms reported by the patient, or observed during physical examinations or laboratory monitoring, were recorded at each visit and evaluated as a possible AE. This study complies with the requirements of Declaration of Helsinki.
Statistical Analysis
Categorical variables were presented as frequencies and percentages, while the mean± standard deviation (SD) was used for continuous variables. The patients’ EASI, BSA, and cheilitis scoring indexes were analyzed. All statistical tests were performed using the SPSS 25.
Results
All ten patients had classical AD with cheilitis. At baseline, AD patients with involved cheilitis with an overall average EASI of 20.13 ± 8.32 (range:13.2–38.7), an average BSA of 15.0% (range:12–23%), a mean cheilitis symptom score was 10.29 ± 2.56, and a median of 10. A total of 57.1% of the patients had a cheilitis symptom score of > 10. No correspondence between the EASI and the cheilitis symptom scores was found. Six patients had an average IgE of 1182kIU/L, all higher than normal. The details are summarized in Tables 1 and 2.
Table 1.
Summary of Patients’ Characteristics
| Patient | Sex | Age | AD of Duration (Years) | Visible Areas | Comorbidities | EASI (W0/8/16) | BSA%(W0/8/16) | Serum IgE (IU/L) | Patch Test | Adverse Event |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 67 | 1 | Face, Hands, Torso, Leg | - | 37.4/22.6/16.7 | 23/18/10 | 1004 | - | - |
| 2 | M | 29 | 3 | Face, Hands, Torso | - | 23.8/15.7/11.7 | 18/13/9 | - | - | Head and Neck Dermatitis |
| 3 | F | 40 | 5 | Face, Hands, Torso, Leg | AR | 13.2/9.6/5.3 | 12/10/6 | - | - | - |
| 4 | M | 15 | 14 | Face, Hands, Torso, Leg | AR | 14.8/7.2/5.1 | 12/6/5 | >2000 | - | - |
| 5 | M | 11 | 4 | Face, Torso, Leg | - | 22.5/16.3/9.8 | 19/13/6 | - | + | - |
| 6 | M | 7 | 5 | Face, Torso, Leg | - | 18.3/12.6/4 | 15/9/5 | 436 | - | |
| 7 | M | 5 | 5 | Face, Hands, Torso, Leg | AR | 38.7/22.4/13.7 | 22/14/7 | >2000 | + | - |
| 8 | M | 7 | 5 | Face, Hands, Torso, Leg | AS, AR | 16.6/10.7/4.3 | 13/7/5 | 855 | - | - |
| 9 | M | 9 | 6 | Face, Hands, Torso, Leg | AK | 19/13.5/4.8 | 21/11/7 | 797 | - | - |
| 10 | F | 8 | 7 | Face, Torso | - | 16.8/12.4/9.8 | 12/7/6 | - | - | - |
Abbreviations: M, male; F, female; AR, allergic rhinitis; AK, allergic keratoconjunctivitis; AS, asthma; T0, baseline; T4, 16 weeks after induction; IGA, investigator’s global assessment; EASI, eczema area, and severity index score;-, none.
Table 2.
Assessment of the Lips (Baseline/8/16Weeks)
| Patient | Skin Lesion Area | Swelling | Dryness | Crusting | Pain | Total Score |
|---|---|---|---|---|---|---|
| 1 | 3/2/1 | 3/2/2 | 3/1/1 | 2/1/1 | 2/1/1 | 13/7/6 |
| 2 | 1/1/1 | 2/1/1 | 1/1/0 | 1/1/0 | 1/1/0 | 6/5/2 |
| 3 | 2/2/1 | 2/1/1 | 2/2/2 | 2/1/0 | 1/1/0 | 9/7/4 |
| 4 | 3/1 | 3/1 | 1/1 | 1/0 | 2/1 | 10/4 |
| 5 | 3/3 | 3/2 | 2/3 | 3/2 | 3/2 | 14/12 |
| 6 | 3/1 | 2/1 | 3/2 | 3/1 | 2/1 | 13/6 |
| 7 | 3/3/3 | 3/3/3 | 2/2/3 | 3/3/3 | 3/3/3 | 14/14/15 |
| 8 | 2/2/1 | 2/1/1 | 2/2/1 | 2/1/0 | 1/1/0 | 9/7/3 |
| 9 | 2/3/3 | 1/1/2 | 3/2/3 | 2/2/2 | 2/2/2 | 10/10/12 |
| 15 | 2/1/1 | 2/1/1 | 3/2/2 | 3/2/1 | 2/2/1 | 12/8/6 |
Abbreviations: 0, no; 1, mild; 2, moderate; 3, severe.
Clinical examination revealed dry, scaly lips in all patients. Swelling, erosion, and erythema of one or both lips and a vertical cleft lip with hemorrhagic tendency were also present in more severe cases (Figures 1 and 2). The lips were firm and soft on palpation. Among AD patients with cheilitis, three (30.0%) had allergic rhinitis (AR), one (10.0%) had asthma (AS), and one (10.0%) had allergic keratoconjunctivitis (AK). Seven patients with AD and cheilitis showed significant symptom improvement after receiving dupilumab. At week 8, the redness, itching, and partial peeling of the red border of the lips disappeared. The cheilitis symptom score was reduced by 29.9%, while the EASI and BSA scores were 35.0% and 34.9%, respectively. After 16 weeks of treatment with dupilumab, the overall clinical efficacy was demonstrated by a reduction in the skin and the cheilitis symptom scores. EASI 75 was achieved in 14.3% of patients, and EASI 50 was achieved in 85.7%. The mean BSA and cheilitis symptoms were reduced by 56.2% and 58.1%, respectively (Figure 1). Lichenification decreased significantly at week 16. The cheilitis symptom score reduction was by 58.1%, the EASI was 60.8%, and the BSA was 56.2%. Improvement of lip involvement was slower than improvement of skin lesions, but the cheilitis symptom score was reduced to almost the same level as the skin indexes after 16 weeks of treatment. In contrast, three (30.0%) patients showed improvement in AD symptoms with less significant improvement in cheilitis (Figure 2). The subjects who improved the least 3 (30.0%) in cheilitis had a mean±SD total score of 12.67±2.31 at baseline and 13.00±1.72 at W16. The EASI reduction was 62.1%, and that of BSA was 67.8% after 16 weeks of treatment. Three patients with less improvement in cheilitis underwent patch testing. Two (66.7%) of the patients had positive patch test results, including potassium dichromate, ethylenediamine, rosin, and rubber. All patients completed the treatment course with dupilumab through week 16, and one patient (patient. 3) had head and neck dermatitis as an AE event.
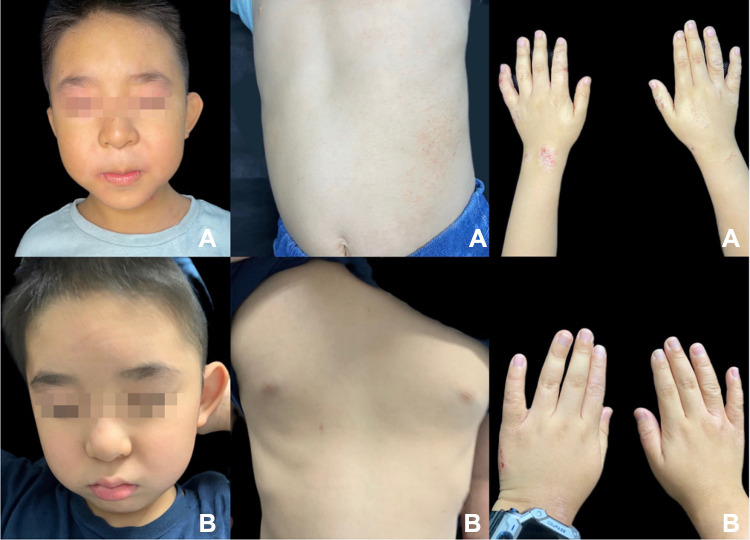
Figure 1.
Erythema, chapped, and lichenoid lesions in patient eight before dupilumab treatment (A). After the treatment, the body lesions were significantly improved, and the redness and dryness of the lips were reduced (B).
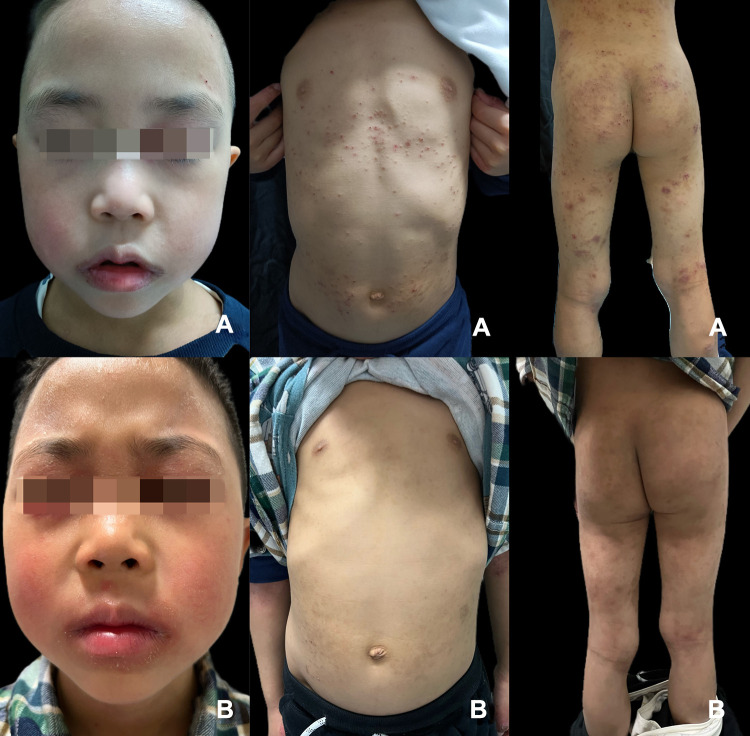
Figure 2.
Erythema, papules, and lichenoid lesions of patient seven before dupilumab (A). Remarkably improved lesions on the body after treatment; however, lips are still red, swollen, and chapped (B).
Discussion
Cheilitis in AD patients is characterized by scaling and wrinkling of the lips and often as lip-licking eczema in children, and even chapping and bleeding in severe cases.10 According to literature reports, cheilitis in AD patients affects up to 25% of children and 2–10% of adults, with a 2–3-fold increase in incidence in developed countries over the past 30 years. Cheilitis is a symptom of AD that significantly affects patients’ quality of life and family members.11 However, the pathogenesis of this disease has not been thoroughly studied. However, it has been suggested that cheilitis in AD patients is characterized by IgE, hypersecretion, violation of cytokine regulation of the Th1/Th2 lymphocyte ratio, deficiency of defined T-lymphocyte suppressors, and disruption of the apoptotic process.11
Furthermore, in a series of 202 patients with eczematous cheilitis from Singapore, endogenous etiology was the most common cause of cheilitis.12 Since cheilitis is a chronic relapsing disease, its clinical management requires comprehensive long-term therapy. The task is to reduce symptoms, restore broken epithelial barriers, and improve quality of life.11 The management of cheilitis is challenging due to its effects on the lips, such as topical irritation and lip-licking habits. However, limited data on the efficacy of biological agents in this specific AD localization have been reported, and consistent and high-quality evidence is lacking for guidelines on the treatment of AD with lip involvement.
The efficacy of dupilumab in AD has been demonstrated in several clinical trials,13,14 and we report here our experience with a series of AD patients with cheilitis who were treated with dupilumab. Dupilumab is approved for moderate to severe atopic dermatitis. It reduces type 2 inflammation by blocking the shared receptor subunits of IL-4 and IL-13, thereby inhibiting receptor signaling downstream of the JAK-STAT pathway. Dupilumab affects three important disease mechanisms in atopic dermatitis: the skin barrier, Th2 cell differentiation, and IgE class switching.15 These mechanisms may help to improve the symptoms of AD and cheilitis. Dupilumab was effective and well-tolerated in subjects with moderate-to-severe AD associated with lip involvement. The skin and lip lesions showed significant and rapid improvement after 16 weeks of subcutaneous treatment with dupilumab 300 mg. It is worth noting that AD symptoms improve faster than cheilitis, which may be related to factors such as external stimulation of the lips. Monotherapy is a new treatment option for patients with localized AD. This represents a new perspective on AD, as previous approaches have included different drugs targeting different anatomical regions. Using a single drug can improve treatment adherence, which is a severe problem for chronic diseases such as AD and reduce intolerance by avoiding polypharmacy.
In our case series, three patients had improved AD symptoms with poor improvement in lip symptoms, and two of them had positive patch test results, suggesting the presence of allergic contact dermatitis. There is some controversy regarding the relationship between contact allergies and atopic dermatitis. Patients often use topical medications and moisturizing emollients to repair the skin barrier and increase the skin moisture. Long-term use of these topical preparations may increase the risk of contact sensitization to their ingredients.16 In addition, an impaired skin barrier leads to increased skin absorption of irritants and allergens in AD patients, leading to further skin barrier disruption. Exposure to pollution, dust, pathogens, and chemicals can damage the epidermal barrier, thereby increasing the risk of contact allergies.17 In patients with AD who have a partial response to dupilumab, the presence of allergic exposure should be considered. Additionally, patch testing may yield essential and informative data on allergen exposure in patients with AD treated with dupilumab.
In conclusion, this case series results support that dupilumab treatment can be a useful optional approach for treating patients with atopic dermatitis-associated lip involvement. The limitations of the study were the small number of patients, the lack of patch test results in some patients, and the retrospective study design.
Conclusion
The efficacy of dupilumab in the treatment of cheilitis in patients with atopic dermatitis, mainly targeting the elements of the pathogenesis, can control the symptoms of the disease, prevent the development of complications, help restore the affected skin area and the red edge of the lips, and significantly improve the quality of life of these patients. Clinicians can consider a patch test approach to rule out ACD in refractory skin lesions after dupilumab treatment in patients with atopic dermatitis associated with cheilitis.
Acknowledgment
Patients/guardians in this manuscript provided written informed consent to publish their case details and photographs. Jinpeng Shan and Kamran Ali are co-first authors for this study.
Funding Statement
The study was conducted without any funding.
Data Sharing Statement
The statistically analyzed data is available in the tables, and the detailed data will be available on request from the corresponding author.
Institutional Board Review
The study was approved by the institutional ethical review board of Hangzhou First peoples’ hospital.
Informed Consent
The patients / one of the patient guardians in this manuscript provided informed written consent for the publication of their case details.
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Owen JL, Vakharia PP, Silverberg JI. The role and diagnosis of allergic contact dermatitis in patients with atopic dermatitis. Am J Clin Dermatol. 2018;19(3):293–302. doi: 10.1007/s40257-017-0340-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miniotti M, Lazzarin G, Ortoncelli M, Mastorino L, Ribero S, Leombruni P. Impact on health-related quality of life and symptoms of anxiety and depression after 32 weeks of Dupilumab treatment for moderate-to-severe atopic dermatitis. Dermatol Ther. 2022;35(5):e15407. doi: 10.1111/dth.15407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harb H, Chatila TA. Mechanisms of Dupilumab. Clin Exp Allergy. 2020;50(1):5–14. doi: 10.1111/cea.13491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Da J, Ali K, Lu K, et al. Off-label use of dupilumab for the treatment of moderate to severe atopic dermatitis in children aged below 6 years of age: a case series. Clin Exp Dermatol. 2022;47(2):423–425. doi: 10.1111/ced.14925 [DOI] [PubMed] [Google Scholar]
- 5.Mastorino L, Viola R, Panzone M, et al. Dupilumab induces a rapid decrease of pruritus in adolescents: a pilot real-life study. Dermatol Ther. 2021;34(6):e15115. doi: 10.1111/dth.15115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendricks AJ, Yosipovitch G, Shi VY. Dupilumab use in dermatologic conditions beyond atopic dermatitis - a systematic review. J Dermatolog Treat. 2021;32(1):19–28. doi: 10.1080/09546634.2019.1689227 [DOI] [PubMed] [Google Scholar]
- 7.Ali K, Wu L, Lou H, et al. Clearance of chronic actinic dermatitis with dupilumab therapy in Chinese patients: a case series. Front Med. 2022;9:803692. doi: 10.3389/fmed.2022.803692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Belmesk L, Muntyanu A, Cantin E, et al. Prominent role of type 2 immunity in skin diseases: beyond atopic dermatitis. J Cutan Med Surg. 2022;26(1):33–49. doi: 10.1177/12034754211027858 [DOI] [PubMed] [Google Scholar]
- 9.Qiu Y, Ali K, Lou H, Shan J, Wu L. Successful treatment of atopic dermatitis with dupilumab in a patient with non-Hodgkin’s lymphoma. Acta Derm Venereol. 2022;102:adv00625. doi: 10.2340/actadv.v101.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Georgakopoulou E, Loumou P, Grigoraki A, Panagiotopoulos A. Isolated lip dermatitis (atopic cheilitis), successfully treated with topical tacrolimus 0.03. Med Oral Patol Oral Cir Bucal. 2021;26(3):e357–e60. doi: 10.4317/medoral.24230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ilenko NM, Nikolishyna EV, Lytovchenko IY, Bar FA. Complex therapy of atopic cheilitis. Wiad Lek. 2021;74(2):310–312. doi: 10.36740/WLek202102125 [DOI] [PubMed] [Google Scholar]
- 12.Lim SW, Goh CL. Epidemiology of eczematous cheilitis at a tertiary dermatological referral centre in Singapore. Contact Dermatitis. 2000;43(6):322–326. doi: 10.1034/j.1600-0536.2000.043006322.x [DOI] [PubMed] [Google Scholar]
- 13.Simpson EL, Paller AS, Siegfried EC, et al. Efficacy and safety of dupilumab in adolescents with uncontrolled moderate to severe atopic dermatitis: a phase 3 randomized clinical trial. JAMA Dermatol. 2020;156(1):44–56. doi: 10.1001/jamadermatol.2019.3336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beck LA, Thaçi D, Deleuran M, et al. Dupilumab provides favorable safety and sustained efficacy for up to 3 years in an open-label study of adults with moderate-to-severe atopic dermatitis. Am J Clin Dermatol. 2020;21(4):567–577. doi: 10.1007/s40257-020-00527-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Senner S, Seegräber M, Frey S, Kendziora B, Eicher L, Wollenberg A. Dupilumab for the treatment of adolescents with atopic dermatitis. Expert Rev Clin Immunol. 2020;16(7):641–650. doi: 10.1080/1744666X.2020.1801420 [DOI] [PubMed] [Google Scholar]
- 16.Ozceker D, Haslak F, Dilek F, et al. Contact sensitization in children with atopic dermatitis. Allergologia et Immunopathologia. 2019;47(1):47–51. doi: 10.1016/j.aller.2018.06.002 [DOI] [PubMed] [Google Scholar]
- 17.Milam EC, Jacob SE, Cohen DE. Contact dermatitis in the patient with atopic dermatitis. J Allergy Clin Immunol Pract. 2019;7(1):18–26. doi: 10.1016/j.jaip.2018.11.003 [DOI] [PubMed] [Google Scholar]