Abstract
The prevalence of active hepatitis B among asymptomatic persons remains unclear in Africa. Of 1206 newly diagnosed persons in Senegal, 12.3% had significant fibrosis and 31.3% had hepatitis B virus (HBV) DNA levels >2000 IU/mL. Overall, 128 (12.9%) were eligible for antiviral therapy. Generalized HBV screening allowed the identification of a large population requiring HBV care.
Keywords: hepatitis B, liver fibrosis, Senegal
In countries with generalized hepatitis B virus (HBV) infection epidemics, the World Health Organization (WHO) recommends HBV testing of the whole population [1]. In urban Senegal, we showed that hepatitis B surface antigen (HBsAg) testing uptake was <40% in routine human immunodeficiency virus (HIV) care [2]. Although improving the uptake of HBV screening is a crucial step toward the global viral hepatitis elimination strategy, few data on expected health benefits from large-scale testing in sub-Saharan Africa are available to guide its implementation. Meta-analyses focusing on HBV in Africa showed that 5% of individuals tested in primary care settings had liver cirrhosis and 18% were eligible for antiviral therapy [3, 4]. However, the study populations were often not representative of the general population and many did not report on participants’ symptoms at presentation. We assessed the proportion of asymptomatic persons living with HBV presenting with liver fibrosis in Senegal and evaluated their antiviral treatment needs.
METHODS
We considered all asymptomatic individuals >16 years of age presenting with a positive HBsAg test at 1 of 5 clinics in Dakar and Ziguinchor. We excluded individuals who were tested for HBV in the context of a suspected liver disease and/or those with clinical signs or symptoms of liver disease. Pregnant women, who generally have better access to early HBV testing in the context of antenatal care, were also excluded from our analysis. Local ethical committees of all participating study sites approved the study and written informed consent was obtained from all participants.
The presence of HBsAg was assessed using 1 of the following rapid tests: NOVATest (Atlas Link Biotech, CE2265), Determine HBsAg 2 (Abbott Diagnostics) and Rapid Signal HBsAg serum/plasma dipstrip (Orgenics). DNA was quantified using COBAS Ampliprep TaqMan 96 (version 2.0, Roche Diagnostics GmbH) and Generic HBV Charge Virale (Biocentric) with a viral load lower limit of detection of 20 IU/mL. Elevated alanine aminotransferase (ALT) was defined as values >40 IU/L [5]. We used transient elastography (Fibroscan, Echosens) to evaluate liver stiffness. Liver stiffness measurements (LSMs) were performed by trained and experienced investigators and considered reliable when they included >10 valid measurements with a success rate >60% and interquartile range (IQR)/median ratio ≤0.30. Significant fibrosis was defined as LSM >7.0 kPa and cirrhosis as LSM >11.0 kPa [1].
Individual characteristics were described using absolute numbers and proportions, or medians and IQR, and compared between sex using χ2 or Wilcoxon rank-sum tests. We used multivariable logistic regression to evaluate predictors of liver fibrosis. The proportion of participants eligible for antiviral therapy was evaluated according to European Association for the Study of the Liver (EASL) recommendations [5]. We performed sensitivity analyses of treatment eligibility proportions using (i) LSM cutoffs of 7.9 kPa for significant fibrosis and 11.7 kPa for cirrhosis, based on a meta-analysis [6] and (ii) WHO treatment recommendations (cirrhosis [LSM >12.5 kPa], or HBV DNA >20 000 IU/mL and ALT >19 IU/L [women]/>30 IU/L [men], and age >30 years) [1]. All statistical analyses were performed using Stata version 16.1 software (StataCorp).
Patient Consent Statement
Local ethical committees of all participating study sites approved the study and written informed consent was obtained from all participants.
RESULTS
Of 2675 HBsAg-positive individuals enrolled, we excluded 639 (23.9%) because they were either pregnant, on tenofovir, or had HIV coinfection. Of the remaining study population, 296 of 2036 (14.5%) individuals who were referred for HBV testing due to clinical signs of liver disease were not considered. We also excluded 555 individuals who failed to have an available LSM (Supplementary Figure 1). There was no difference in the distribution of sex (P = .17), age (P = .39), or ALT values (P = .37) between the final study population and excluded individuals. Of 1206 included asymptomatic persons with HBV, 453 (39.9%) were tested during a blood donation, 345 (30.4%) during a routine medical check, 141 (12.4%) during community/family screening, and 195 (17.2%) during a previous pregnancy. Median age and proportions followed in Dakar or Ziguinchor were similar between men and women (Table 1). Men were more likely to report alcohol consumption (10.7% vs 5.9%, P = .01) and to have elevated ALT (10.3% vs 6.4%, P = .02) than women, and their median HBV viral load was higher (648 vs 509 IU/mL, P = .04).
Table 1.
Characteristics of the Study Population by Sex (N = 1206)
| Characteristic | Women (n = 507) | Men (n = 698) |
P Value |
|---|---|---|---|
| Region of enrollment | .06 | ||
| Ziguinchor | 276 (54.4) | 419 (60.0) | |
| Dakar | 231 (45.6) | 279 (40.0) | |
| Age at enrollment, y, median (IQR) | 32 (26–39) | 32 (26–40) | .53 |
| Age >30 y | 273 (54.1) | 390 (56.2) | .46 |
| Screening reasons | <.001 | ||
| Community/family testing | 73 (15.4) | 68 (10.5) | |
| Antenatal care | 184 (38.8) | 0 (0.0) | |
| Blood donation | 98 (20.7) | 355 (54.7) | |
| Routine check-up | 119 (25.1) | 226 (34.8) | |
| Alcohol consumption | 28 (5.9) | 63 (10.7) | .01 |
| Family history of HCCa | 51 (19.2) | 44 (13.8) | .08 |
| HBeAg positiveb | 7 (3.0) | 3 (1.0) | .08 |
| ALT, IU/L, median (IQR) | 15 (10–22.45) | 19 (14–26) | <.001 |
| ALT >40 IU/L | 30 (6.4) | 69 (10.3) | .02 |
| HBV DNA, IU/mL, median (IQR)c | 509 (38–2906) | 648 (115–3380) | .04 |
| Category | .12 | ||
| <20 | 85 (20.0) | 86 (14.3) | |
| 20–2000 | 214 (50.4) | 320 (53.2) | |
| 2001–2000 | 93 (21.9) | 145 (24.1) | |
| >20 000 | 33 (7.8) | 50 (8.3) | |
| LSM, kPa, median (IQR) | 4.7 (4–5.6) | 5.5 (4.5–6.5) | <.001 |
| Category | <.001 | ||
| ≤7.0 | 478 (94.3) | 579 (83.1) | |
| 7.1–11.0 | 24 (4.7) | 91 (13.1) | |
| >11.0 | 5 (1.0) | 27 (3.9) |
Data are presented as No. (%) unless otherwise indicated. Some characteristics have different denominators because of missing data.
Abbreviations: ALT, alanine aminotransferase; HBeAg, hepatitis B envelope antigen; HBV, hepatitis B virus; HCC, hepatocellular carcinoma; IQR, interquartile range; LSM, liver stiffness measurement.
Data available for 584 individuals.
Data available for 540 individuals.
Data available for 1026 individuals. We excluded 1 individual without data on sex.
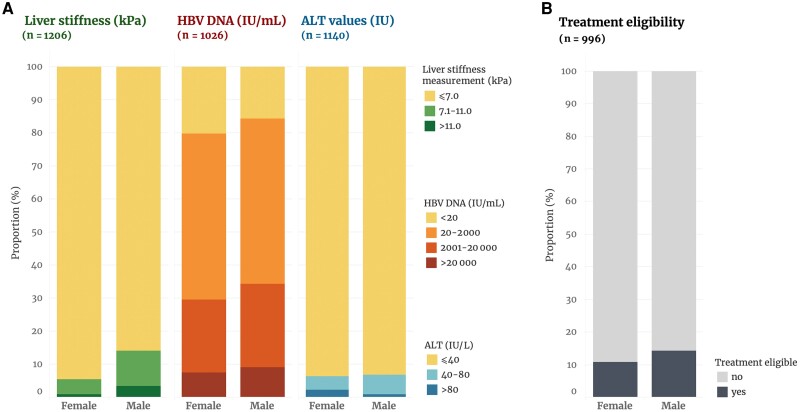
Of 1206 participants, 147 (12.3%) had significant liver fibrosis, including 32 of 1206 (2.7%) with cirrhosis (Table 1 and Figure 1). The proportion of participants with significant liver fibrosis (17.1% vs 5.7%, P < .001) and cirrhosis (4.0% vs 1.0%, P = .001) was higher in men than in women. In multivariable analyses adjusted for age, region, and alcohol consumption, participants with HBV DNA >2000 IU/mL (adjusted odds ratio [aOR] 1.60 [95% confidence interval {CI}, 1.01–2.52), those of male sex (aOR, 3.17 [95% CI, 1.86–5.38]), and those with elevated ALT (aOR, 2.73 [95% CI, 1.37–5.47]) were more likely to have significant fibrosis compared to others (Supplementary Table 1). The proportion of participants with an HBV viral load >2000 IU/mL was 32.5% (195/601) in men and 29.7% (126/425) in women, whereas 10.3% of men (69/671) and 6.4% of women (30/468) had ALT >40 IU/mL (Figure 1A).
Figure 1.
Chronic hepatitis B marker assessment (A) and antiviral treatment eligibility according to European Association for the Study of the Liver recommendations (B) among people with hepatitis B virus in Senegal, by sex. Abbreviations: ALT, alanine aminotransferase; HBV, hepatitis B virus.
Among 996 of 1206 (82.6%) participants with complete measurements of HBV viral load, ALT, and liver fibrosis, antiviral treatment initiation was indicated for 128 (12.9% [95% CI, 10.8%–15.1%]) of them (Figure 1B) based on EASL criteria. The proportion of treatment-eligible individuals was 14.2% (95% CI, 11.5%–17.3%) among men and 10.8% (95% CI, 8.0%–14.3%) among women (P = .12), and there was no difference by age (P = .3) or region (P = .73). In multivariable analysis, neither sex, age, or region was associated with treatment eligibility (Supplementary Table 2). When using the alternative liver fibrosis cutoffs [6], 10.7% (95% CI, 8.9%–12.8%) of participants were eligible for antiviral treatment, whereas 3.3% (95% CI, 2.3%–4.6%) were eligible based on the WHO guidelines. Of 103 (10.7%) individuals eligible according to EASL guidelines but not based on WHO criteria, 61 (59.2%) had HBV DNA >2000 IU/mL and 48 (46.6%) had significant fibrosis.
DISCUSSION
Among >1200 asymptomatic persons living with HBV in rural and urban areas of Senegal, 1 in 8 had significant liver fibrosis and one-third had elevated HBV DNA levels. Antiviral treatment was indicated in 13% of individuals at their first visit. These results support the need for generalized HBV testing, as well as liver disease assessment, among HBsAg-positive individuals to reach the elimination of HBV as a public health problem by 2030 in West Africa.
In our study, 12% of individuals with chronic HBV without clinical signs of liver disease had significant fibrosis, including 3% with liver cirrhosis. Our results align with estimates from a meta-analysis of African studies, in which the prevalence of significant fibrosis from primary care or general population cohorts was 10.4%, including 3.6% with cirrhosis [3]. With an estimated 2 million people living with HBV in Senegal, of whom <2% are diagnosed, our data provide compelling evidence for the need of urgent policies to identify individuals most at risk of liver complications and in need of antiviral treatment in the general population [7, 8].
In line with estimates from a meta-analysis including African studies, 14% of men and 11% of women with asymptomatic chronic HBV infection were eligible for antiviral treatment initiation in our study [4]. In a large study from The Gambia, only 4% of participants screened in the community setting were eligible for antiviral therapy, corresponding to our estimates when we used WHO-recommended criteria [9]. However, in a hospital-based cohort study in Ethiopia including nearly 1200 participants, >25% had a treatment indication based on EASL criteria and 15% using WHO criteria [10]. The presence of clinical signs of cirrhosis in nearly 10% of the included participants in the latter study may partially explain the differences with our study. These results highlight the importance of considering the context and reasons for HBV testing in the interpretation of treatment eligibility in African cohorts.
In Asian cohort studies, HBV replication was strongly associated with liver-related outcomes, including hepatocellular carcinoma (HCC) and liver-related mortality [11, 12]. HBV DNA replication >2000 IU/mL was observed in one-third of our participants and was associated with significant fibrosis, independent of age, sex, alcohol consumption, ALT values, and region. These findings suggest that HBV treatment eligibility criteria for HBV may have to be extended to include all patients with high HBV viral load, independent of liver fibrosis or inflammation, as increasingly discussed in the literature [13, 14].
Our findings highlight the burden of HBV-related liver disease and treatment needs in one of the largest study of asymptomatic individuals with HBV infection in Africa. The availability of detailed data on HBV virological markers and liver fibrosis allowed us to assess treatment eligibility in a large sample of the general population in West Africa, which will be crucial information for elimination strategies. Nevertheless, missing information on hepatitis C virus (HCV), hepatitis delta virus (HDV), or schistosomal infections from a significant proportion of participants precluded their consideration in our analyses. However, given the low prevalence of HCV and HDV infections found in our recent analysis in Dakar, these coinfections are unlikely to be relevant drivers of liver-related complications [15]. Although we focused on asymptomatic individuals, our results may have slightly under- or overestimated treatment eligibility among the general population because our participants were not randomly selected from the community. Finally, information on family history of HCC, an additional argument for initiating antiviral therapy, was not available for nearly one-half of participants.
In conclusion, a significant proportion of asymptomatic individuals living with HBV in Senegal present early signs of liver disease or HBV replication, and many of them require antiviral treatment. The implementation of large-scale HBV screening programs is needed to identify individuals most at risk of liver-related complications and reach the hepatitis elimination objectives in Africa.
Supplementary Material
Contributor Information
Adrià Ramírez Mena, Department of Infectious and Tropical Diseases, Fann University Hospital, Dakar, Senegal; Department of Infectious Diseases, Bern University Hospital, University of Bern, Bern, Switzerland; Graduate School of Health Sciences, University of Bern, Bern, Switzerland.
Mame Aissé Thioubou, Department of Gastroenterology, Hôpital Universitaire de la Paix, Ziguinchor, Senegal.
Kalilou Diallo, Department of Infectious Diseases, Hôpital Universitaire de la Paix, Ziguinchor, Senegal.
Judicaël Tine, Department of Infectious and Tropical Diseases, Fann University Hospital, Dakar, Senegal.
Ndeye Fatou Ngom, Centre de Traitement Ambulatoire, Fann University Hospital, Dakar, Senegal.
Louise Fortes, Department of Infectious and Tropical Diseases, Fann University Hospital, Dakar, Senegal.
Kiné Ndiaye, Centre de Traitement Ambulatoire, Fann University Hospital, Dakar, Senegal.
Jean-Claude Karasi, Department of Infectious Diseases, Centre Hospitalier du Luxembourg, Luxembourg, Luxembourg.
Carole Seguin-Devaux, Department of Infection and Immunity, Luxembourg Health Institute, Luxembourg, Luxembourg.
Henri Goedertz, Department of Infectious Diseases, Centre Hospitalier du Luxembourg, Luxembourg, Luxembourg.
Daouda Diouf, ENDA (Environnement, Développement et Action) Santé, Dakar, Senegal.
Moussa Seydi, Department of Infectious and Tropical Diseases, Fann University Hospital, Dakar, Senegal.
Benjamin Amaye Sambou, ENDA (Environnement, Développement et Action) Santé, Dakar, Senegal.
Vic Arendt, Department of Infectious Diseases, Centre Hospitalier du Luxembourg, Luxembourg, Luxembourg.
Gilles Wandeler, Department of Infectious and Tropical Diseases, Fann University Hospital, Dakar, Senegal; Department of Infectious Diseases, Bern University Hospital, University of Bern, Bern, Switzerland; Institute of Social and Preventive Medicine, University of Bern, Bern, Switzerland.
Noël Magloire Manga, Department of Infectious Diseases, Hôpital Universitaire de la Paix, Ziguinchor, Senegal.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. A. R. M. and G. W. conceived the study, conducted the statistical analyses, and wrote the first draft of the manuscript. A. R. M., A. T., J. T., K. D., N. F. M., K. N., and N. M. collected data. All authors read and critically reviewed the manuscript, and approved its final version.
Acknowledgments. We thank all of the participants, as well as the physicians and study nurses involved in patient care.
Disclaimer.The funders had no role in study design, data collection and analysis, preparation of the manuscript, or decision to submit the manuscript for publication.
Financial support. This work was supported by the Swiss National Science Foundation (PZ00P3_154730); the Ministère des Affaires étrangères et européennes de Luxembourg - Direction de la coopération au développement et de l'action humanitaire; and an investigator-initiated study from Roche Diagnostics.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. World Health Organization . Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection.2015. https://apps.who.int/iris/handle/10665/154590. Accessed 24 August 2022.
- 2. Ramírez Mena A, Tine JM, Fortes L, et al. Hepatitis B screening practices and viral control among persons living with HIV in urban Senegal. J Viral Hepat 2022; 29:60–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Surial B, Wyser D, Béguelin C, Ramírez-Mena A, Rauch A, Wandeler G. Prevalence of liver cirrhosis in individuals with hepatitis B virus infection in sub-Saharan Africa: systematic review and meta-analysis. Liver Int 2021; 41:710–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tan M, Bhadoria AS, Cui F, et al. Estimating the proportion of people with chronic hepatitis B virus infection eligible for hepatitis B antiviral treatment worldwide: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2021; 6:106–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lampertico P, Agarwal K, Berg T, et al. EASL 2017 Clinical practice guidelines on the management of hepatitis B virus infection. J Hepatol 2017; 67:370–98. [DOI] [PubMed] [Google Scholar]
- 6. Chon YE, Choi EH, Song KJ, et al. Performance of transient elastography for the staging of liver fibrosis in patients with chronic hepatitis B: a meta-analysis. PLoS One 2012; 7:e44930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McNaughton AL, Lourenço J, Bester PA, et al. Hepatitis B virus seroepidemiology data for Africa: modelling intervention strategies based on a systematic review and meta-analysis. PLoS Med 2020; 17:e1003068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization . Global hepatitis report, 2017. https://www.who.int/publications-detail-redirect/9789241565455. Accessed 30 May 2022.
- 9. Lemoine M, Shimakawa Y, Njie R, et al. Acceptability and feasibility of a screen-and-treat programme for hepatitis B virus infection in The Gambia: the Prevention of Liver Fibrosis and Cancer in Africa (PROLIFICA) study. Lancet Glob Health 2016; 4:e559–67. [DOI] [PubMed] [Google Scholar]
- 10. Aberra H, Desalegn H, Berhe N, et al. The WHO guidelines for chronic hepatitis B fail to detect half of the patients in need of treatment in Ethiopia. J Hepatol 2019; 70:1065–71. [DOI] [PubMed] [Google Scholar]
- 11. Chen C-J, Yang H-I, Su J, et al. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 2006; 295:65–73. [DOI] [PubMed] [Google Scholar]
- 12. Iloeje UH, Yang H-I, Su J, et al. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 2006; 130:678–86. [DOI] [PubMed] [Google Scholar]
- 13. Wong RJ, Kaufman HW, Niles JK, Kapoor H, Gish RG. Simplifying treatment criteria in chronic hepatitis B: reducing barriers to elimination [manuscript published online ahead of print 20 May 2022]. Clin Infect Dis 2022. doi: 10.1093/cid/ciac385 [DOI] [PubMed] [Google Scholar]
- 14. McNaughton AL, Lemoine M, van Rensburg C, Matthews PC. Extending treatment eligibility for chronic hepatitis B virus infection. Nat Rev Gastroenterol Hepatol 2021; 18:146–7. [DOI] [PubMed] [Google Scholar]
- 15. Ramirez-Mena A, Tine J, Fortes L, et al. Evaluation prospective de l’éligibilité au traitement antiviral dans une cohorte de personnes vivant avec l’hépatite B au Sénégal. [abstract PJ073]. In: Livre des résumés Alliance Francophone des Acteurs de Sante contre le VIH, Marseille, France; 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.