Abstract
Background
Despite national guideline recommendations for universal biomarker testing (KRAS, NRAS, BRAF, and mismatch repair and microsatellite instability [MMR/MSI]) in all patients with metastatic colorectal cancer (mCRC), little is known regarding adherence to these recommendations in routine practice.
Methods
We retrospectively reviewed patients with mCRC diagnosed between January 1, 2013, and December 27, 2018, from a de-identified electronic health record–derived database. We analyzed disparities in KRAS, NRAS, BRAF, and MMR/MSI testing by race, age, sex, and insurance status using χ2 tests and t tests. We evaluated changes in biomarker testing over time with attention to changes around dates of landmark publications and guideline updates using χ2 tests and Cochran-Armitage tests.
Results
A total of 20 333 patients were identified of which 66.6% had test results for any biomarker. Rates of test results for all 4 biomarkers statistically significantly increased over time (P < .001). However, as of June 30, 2018, the rate of test results was only 46% for NRAS, 56% for KRAS, and 46% for BRAF. As of December 31, 2017, the rate of MMR/MSI testing was 59%. Higher documented testing rates were associated with younger age, lower Eastern Cooperative Oncology Group performance status, and commercial insurance. There were no clinically meaningful and/or statistically significant differences in documented testing rates by tumor sidedness, race, sex, or initial stage.
Conclusions
Increased rates of documented testing for NRAS, BRAF, and MMR/MSI in mCRC was seen between 2013 and 2018 reflecting adoption of guideline recommendations. However, the rate of documented testing remains lower than expected and warrants additional research to understand the extent to which this may represent a clinical practice quality concern.
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer death (1). Approximately 50%-60% of patients who are diagnosed with CRC will eventually develop metastatic CRC (mCRC) and the standard of care in the majority of these cases is systemic therapy (2,3). Recent data suggest that mCRC is a molecularly heterogenous disease, and this heterogeneity results in variable prognoses and responses to treatment. Therefore, testing for RAS, BRAF (V600E), mismatch repair (MMR) and Microsatelite Instability (MSI), and HER-2 amplification are standard of care for patients with mCRC based on landmark trials and clinical practice guideline endorsement by the National Comprehensive Cancer Network (NCCN) (3).
The RAS genes (KRAS, NRAS, and HRAS) are mutated in approximately 50% of CRCs (4,5). Post hoc analyses of large prospective trials have suggested that treatment with anti–epidermal growth factor receptor (EGFR) antibodies do not confer any benefit to patients with RAS-mutant mCRC (6-8). Based on this data, in 2009 the NCCN recommended testing all patients diagnosed with mCRC for KRAS exon 2, codons 12 and 13 (9). Guidelines have since expanded to include testing for KRAS exons 3 and 4 and NRAS exons 2, 3, and 4 (3). Further studies have suggested that even among patients with RAS wild-type (WT) tumors, those with right-sided tumors do not benefit from the addition of an anti-EGFR antibody in the first-line setting (6,8,10,11). Therefore, the NCCN guidelines currently recommend anti-EGFR therapy only for patients with RAS WT, left-sided tumors in the front-line treatment, although some providers still use anti–vascular endothelial growth factor (VEGF) inhibitors with chemotherapy in this setting (12).
BRAF is downstream of RAS in the mitogen-activated protein kinase pathway, and the presence of BRAF V600E mutation, found in approximately 10% of CRC, is associated with a poor prognosis (13,14). Accumulating evidence has suggested that BRAF V600E mutations confer decreased response to anti-EGFR antibody therapy (15–17). Based on this data, in 2010 the NCCN initially recommended testing BRAF for mCRC patients who had RAS WT tumors, and these guidelines were expanded in 2015 to recommend testing BRAF in all mCRC patients (18,19). Currently, targeted agents against the mitogen-activated protein kinase pathway exist with the recent approval of the BEACON regimen combining the anti-EGFR inhibitor—cetuximab and anti BRAF inhibitor encorafenib supporting widespread testing for this biomarker (19).
Microsatellite-instability high (MSI-H) or mismatch repair deficinet (dMMR) tumors represent 4%-5% of mCRC (20). Since 2003, MMR and MSI status have been known to be prognostic and predictive of response to fluorouracil-based chemotherapy in early stage disease (21), and universal testing for MMR/MSI for the detection of Lynch syndrome was recommended for all newly diagnosed CRC by the Evaluation of Genomic Applications in Practice and Prevention working group in 2009 (22). More recently, dMMR/MSI-H mCRC tumors have demonstrated a clinically significant response to immune checkpoint inhibitor therapy in advanced stage disease (23,24). The NCCN guidelines recommended universal testing for MMR/MSI in patients with mCRC in 2015 and, in 2018, recommended the treatment of dMMR/MSI-H mCRC with pembrolizumab after progression on front-line systemic therapy (25,26). Recently, pembrolizumab was approved in the front-line setting for dMMR/MSI-H mCRC, and the NCCN guidelines were updated accordingly (24).
Although guidelines recommend testing for RAS, BRAF, and MMR/MSI, rates of testing are thought to be low. One retrospective review of patients between 2010 and 2012 found that only 28% of patients had MMR/MSI testing, although testing was not universally recommended during that time period (27). More recently, a retrospective review of 1497 patients by Gutierrez et al. (28) found that guideline-aligned biomarker testing was completed in only 40% of patients between 2013 and 2017. With important treatment decisions hinging on the results of biomarker testing, the widespread adoption of testing has substantial implications for public health (29).
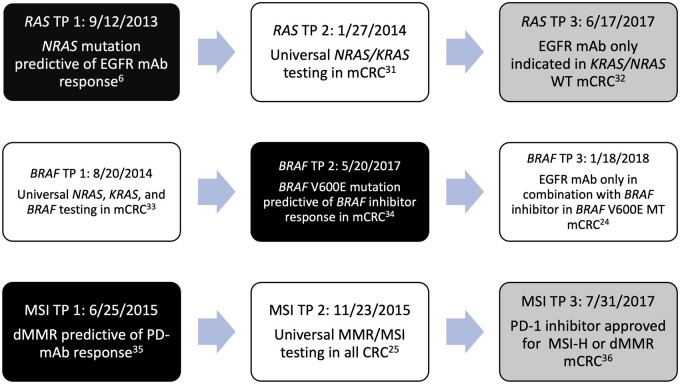
The purpose of our study was to analyze changes in the rates and adoption of biomarker testing over time and to evaluate adherence to clinical guideline recommendations in mCRC. We assessed the rates of testing for MMR/MSI, KRAS, NRAS, and BRAF mutations by key historical time points including presentation of landmark data at national meetings, key journal publications, and updates to NCCN guidelines (see Figure 1). In addition, we evaluated the therapeutic implications of biomarker testing by analyzing the use of anti-EGFR therapy over time.
Figure 1.
Landmark time points in RAS, BRAF, and MMR/MSI testing since 2012. The key time points used during the analysis are depicted in this figure for each biomarker evaluated (RAS, BRAF, and MMR/MSI). White boxes indicate NCCN guideline update, black boxes indicate landmark publications or presentations, and gray boxes indicated US FDA label change or approval. dMMR = deficient mismatch repair; EGFR = epidermal growth factor receptor; FDA = Food and Drug Administration; mAb = monoclonal antibody; mCRC = metastatic colorectal cancer; MMR = mismatch repair; MSI = microsatellite instability; MSI-H = microsatellite instability high; MT = mutated; NCCN = National Comprehensive Cancer Network; PD-1 = programmed death ligand 1; TP = time point; WT = wild type.
Methods
Database
The patient data in this retrospective cohort study originated from the nationwide Flatiron Health database—a longitudinal, de-identified database derived from electronic health records (EHR). As of the time of this study, data originated from approximately 280 US cancer clinics (approximately 800 sites of care) and contains patient-level structured and unstructured data curated via technology-enabled chart abstraction (30,31). Institutional review board approval of the study protocol was obtained prior to study conduct and included a waiver of informed consent.
Study Design and Patient Population
We selected patients who were diagnosed with mCRC between January 1, 2013, and December 31, 2018. Eligibility criteria included individuals aged 18 years or older and with either de novo metastatic disease or a metastatic recurrence on or after January 1, 2013. Diagnosis of mCRC was determined by chart-documented International Classification of Disease (ICD) codes (ICD-9 153.x or 154.x or ICD-10 C18x, C19x, C20x, or C21x).
Patient data were collected from the date of metastatic diagnosis until the end of the study period. We allowed 6 months from the date of metastatic disease diagnosis for the patient to undergo testing to be counted as “tested” to avoid biases from differential length of follow-up. We then grouped patients into 6-month intervals according to date of metastatic diagnosis and compared rates of testing by each 6-month cohort. The end of our diagnosis cutoff was June 31, 2018, and the end of our analysis was December 31, 2018, to allow for patients diagnosed close to June 2018 to have 6 months of lead time for testing. Finally, we analyzed the rate of front-line anti-EGFR treatment in the cohort of patients with documented RAS WT disease.
Study Measures
Baseline data collected from the Flatiron Health Database included demographic information and initial Eastern Cooperative Oncology Group performance status (ECOG PS) at time of metastatic diagnosis. We recorded every insurance type a patient held during the study period. Tumor sidedness was determined through chart extraction and characterized as either right-sided (cecum, ascending colon, hepatic flexure) or left-sided (descending colon and rectum) based on diagnosis code (C18x). Those who were not categorized as left- or right-sided as above were classified as unspecified or unknown. Transverse colon tumors were excluded from this analysis, because of challenges in defining them as left- or right-sided within the available data.
Testing data for KRAS, NRAS, BRAF, and MMR/MSI with either immunohistochemistry, polymerase chain reaction, or next-generation sequencing were derived from chart documentation. Chart abstraction evaluated testing of specific biomarkers following the Flatiron Health Database protocol. Technology was used to assist in surfacing documents that could contain biomarker testing information, and all identified data were confirmed by database abstractors. Biomarkers were marked as missing or undetermined if results were not available. The patients could have been tested at any laboratory and could have received full panel testing or partial testing. Information regarding the testing methods used was not available for all patients and therefore not included in the analysis. Data were gathered in de novo metastatic and metastatic recurrence. Data for MMR/MSI testing were only analyzed in de novo mCRC patients as those with recurrent disease may have been tested earlier in their diagnosis.
We aimed to examine changes in rates of testing for NRAS, BRAF, and MMR/MSI by landmark time points, which are outlined in Figure 1 (6,32-37). We did not analyze change in rates of testing by landmark time periods for KRAS as there were no major guideline changes for KRAS testing during the time periods we examined.
Statistical Analysis
Patient demographic and tumor characteristics were summarized and tabulated based on mutation testing (KRAS, NRAS, or BRAF for the full cohort; MMR/MSI testing for those with metastatic disease at diagnosis). χ2 and t tests were used to assess whether there were relationships between molecular testing at any point and patient and/or tumor characteristics. Cochran-Armitage tests for trend were used to assess whether the rates of testing change over time. We fit multivariable logistic regression models to simultaneously assess the effect of covariates of interest on testing. Models investigated the association between patient demographic/tumor characteristics and mutation testing. Additional models included an interaction between age and ECOG performance status score. Rates of front-line use of anti-EGFR targeted therapy for left- or right-side tumor were also assessed by 6-month intervals (based on first-line therapy start date) for RAS WT patients, with Cochran-Armitage test for trend to assess changes over time. Statistical significance was identified with a 2-sided P value less than .05. All analyses were done using SAS software (version 9.4).
Results
Patient Characteristics
A total of 20 333 patients were included in our analysis; their demographics are outlined in Table 1. The median age of the patients was 65 years. The majority (93.1%) of patients was treated at community centers, and 6.9% of patients were treated at academic centers. The majority of patients was diagnosed with stage IV disease (57.7%) at initial presentation. The most common type of insurance was commercial insurance (38.5%).
Table 1.
Patient demographics and rate of mCRC recommended molecular marker testing by patients’ characteristics
| Patient characteristic | Total, No. (%) |
KRAS, NRAS, or BRAF testinga |
MMR/MSI testing for de novo mCRC patients |
||||
|---|---|---|---|---|---|---|---|
| Not tested, No. (%) | Tested, No. (%) | P | Not tested, No. (%) | Tested, No. (%) | P | ||
| Total | 20 333 | 6798 (33.4) | 13 535 (66.6) | 5302 (45.22) | 6423 (54.78) | ||
| Median age at metastatic diagnosis, y (range) | 65 (18-85) | 69 (18-85) | 64 (18-85) | <.001 | 67 (23-85) | 61 (18-85) | <.001 |
| Age group, y | <.001 | <.001 | |||||
| 40 and younger | 788 (3.88) | 199 (25.25) | 589 (74.75) | 159 (28.55) | 398 (71.45) | ||
| 41-65 | 9422 (46.34) | 2562 (27.19) | 6860 (72.81) | 2325 (39.61) | 3544 (60.39) | ||
| 66-75 | 5488 (26.99) | 1798 (32.76) | 3690 (67.24) | 1454 (48.87) | 1521 (51.13) | ||
| Older than 75 | 4635 (22.80) | 2239 (48.31) | 2396 (51.69) | 1364 (58.69) | 960 (41.31) | ||
| Gender | .0487 | .28 | |||||
| Female | 11 201 (55.09) | 3117 (34.15) | 6011 (65.85) | 2375 (44.66) | 2943 (55.34) | ||
| Male | 9128 (44.89) | 3678 (32.84) | 7523 (67.16) | 2925 (45.67) | 3480 (54.33) | ||
| Stage at initial diagnosis | <.001 | ||||||
| I | 548 (2.70) | 195 (35.58) | 353 (64.42) | 0 | 0 | ||
| II | 2228 (10.96) | 814 (36.54) | 1414 (63.46) | 0 | 0 | ||
| III | 4902 (24.11) | 1549 (31.58) | 3354 (68.42) | 0 | 0 | ||
| IV | 11 725 (57.66) | 3777 (32.21) | 7948 (67.79) | 5302 | 6423 | ||
| Unknown | 930 (4.57) | — | — | — | — | ||
| Race | .24 | .42 | |||||
| African American | 2123 (10.44) | 656 (30.90) | 1467 (69.10) | 561 (44.24) | 707 (55.76) | ||
| Asian | 74 (0.36) | 172 (31.73) | 370 (68.27) | 120 (40.82) | 174 (59.18) | ||
| Hispanic or Latino | 542 (2.67) | 21 (30.90) | 53 (69.10) | 23 (50) | 23 (50) | ||
| White | 13 245 (65.14) | 4360 (32.92) | —8885 (67.08) | 3397 (45.09) | 4136 (54.91) | ||
| Other or unknownb | 4349 (21.39) | — | — | ||||
| ECOG performance status | <.001 | <.001 | |||||
| 0 | 6003 (29.52) | 1640 (27.32) | 4363 (72.68) | 1218 (36.38) | 2130 (63.62) | ||
| 1 | 5102 (25.09) | 1469 (28.79) | 3633 (71.21) | 1216 (41.09) | 1743 (58.91) | ||
| 2 | 1688 (8.30) | 651 (38.57) | 1037 (61.43) | 495 (48.43) | 527 (51.57) | ||
| 3 | 485 (2.39) | 243 (50.10) | 242 (49.90) | 170 (58.22) | 122 (41.78) | ||
| 4 | 50 (0.25) | 27 (54.00) | 23 (46.00 | 14 (48.28) | 15 (51.72) | ||
| Unknownc | 7005 (34.45) | — | — | — | — | ||
| Type of insurance | <.001 | <.001 | |||||
| Commercial | 7874 (38.53) | 2324 (29.67) | 5510 (70.33) | 1883 (39.75) | 2854 (60.25) | ||
| Commercial and Medicare | 3566 (17.54) | 1243 (34.86) | 2323 (65.14) | 890 (48.85) | 932 (51.15) | ||
| Medicare | 3238 (15.92) | 1262 (32.22) | 1976 (67.78) | 905 (50.53) | 886 (49.47) | ||
| Medicaid | 987 (4.85) | 318 (38.97) | 669 (61.03) | 288 (45.28) | 348 (54.72) | ||
| Medicare and Medicaid | 832 (4.09) | 291 (34.98) | 541 (65.02) | 205 (48.46) | 218 (51.54) | ||
| Other or unknown | 3876 (19.06) | 1360 (35.09) | 2516 (64.91) | 1231 (48.95) | 1285 (51.05) | ||
| Tumor location | .10 | .21 | |||||
| Left | 8702 (42.80) | 2804 (32.22) | 5898 (67.78) | 2025 (41.26) | 2883 (58.74) | ||
| Right | 4045 (19.89) | 1244 (30.75) | 2801 (69.25) | 1014 (42.80) | 1355 (57.20) | ||
| Unknown or unspecified | 7586 (35.93) | ||||||
| Practice type | N/Ac | N/A | — | N/A | N/A | — | |
| Academic | 1403 (6.90) | — | — | — | — | ||
| Community | 18 930 (93.10) | — | — | — | — | ||
Patients could have been tested for either BRAF, KRAS, or NRAS. “—” signifies no data; ECOG = Eastern Cooperative Oncology Group; mCRC = metastatic colorectal cancer; MMR = mismatch repair; MSI = microsatellite instability.
Patient without documented data in this category in the database.
Practice type information was only available for the full cohort for this analysis.
Rates of Testing by Demographic
KRAS, NRAS, or BRAF
Over the study period, 66.6% of patients were tested for either KRAS, NRAS, or BRAF, and 33.4% of patients were not tested for any these biomarkers. Of the patients, 30.5% were tested for all of the biomarkers, and 23.7% of patients were tested for KRAS but not NRAS or BRAF. Patients aged younger than 40 years had higher rates of testing (75%), and those aged older than 75 years had lower rates of testing (52%; P < .001). Rate of testing was higher among patients with lower ECOG PS (0 or 1) (P < .001). Patients with commercial insurance had highest rates of testing (70%), and patients with Medicaid had lowest rates of testing (61%) (P < .001). There were no statistically significant differences in testing by sex, race, or tumor-sidedness.
Microsatellite Instability
Demographic associations with MMR/MSI testing were similar to those found with KRAS, NRAS, and BRAF as patients with younger age (P < .001), lower ECOG PS (<.001), and commercial insurance (P < .001) had highest rates of testing (Table 1).
Multivariate Analysis
We found similar results on multivariable analysis as reported in the univariable analyses (Table 1). Regression results are presented in Supplementary Figures (available online). Our analysis demonstrated a statistically significant interaction between age and ECOG PS 2 or higher for RAS and BRAF testing, so that the negative effect of age (ie, older patients are less likely to be tested) was even more pronounced among those who were ECOG PS 2 or higher (results not shown).
The Adoption of Biomarker Testing Over Time
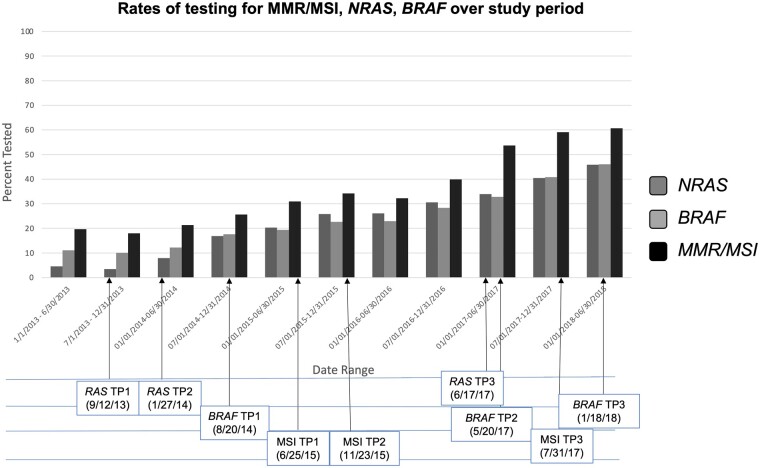
Rates of testing for KRAS (P < .001), NRAS (P < .001), BRAF (P < .001), and MMR/MSI (P < .001) increased statistically significantly over time for patients diagnosed with mCRC (Figure 2). The rates of biomarker testing by landmark time point are outlined in Table 2. Although testing of NRAS, BRAF, and MMR/MSI increased across almost all landmark time points analyzed, rates of testing remained below 60% (below 50% for NRAS and BRAF) by the final time period analyzed.
Figure 2.
Rates of testing RAS,BRAF, and MMR/MSI testing over time. Changes in rates of biomarker testing are depicted in this figure for RAS, BRAF, and MSI with arrows depicting the relevant time point during the analysis (refer to Figure 1 for time point description). MMR = mismatch repair; MSI = microsatellite instability; TP = time point.
Table 2.
Analysis of biomarker testing by landmark time point (online only)
| Time periods | % tested | Comparison | P |
|---|---|---|---|
| NRAS | |||
| Period 1: January 1, 2013, to TP 1 | 4.10 | Referent | |
| [September 12, 2013, landmark publication—NRAS MT predictive of EGFR inhibitor response (6)] | |||
| Period 2: TP 1 to TP 2 | 3.82 | Period 1 vs period 2 | .69 |
| [January 1, 27, 2014, NCCN guideline update recommends universal NRAS and KRAS testing in mCRC (32)] | |||
| Period 3: TP 2 to TP 3 | 23.45 | Period 2 vs period 3 | <.001 |
| [June 17, 2017, FDA label change—EGFR inhibitor only indicated in RAS WT mCRC (33,54)] | |||
| Period 4: TP 3 to June 30, 2018a | 42.78 | Period 3 vs period 4 | <.001 |
| BRAF | |||
| Period 1: January 1, 2013, to TP 1 | 11.54 | Referent | |
| [August 20, 2014, NCCN guideline update recommends universal KRAS, NRAS, and BRAF testing in mCRC (34)] | |||
| Period 2: TP 1 to TP 2 | 23.96 | Period 1 vs period 2 | <.001 |
| [May 20, 2017, landmark publication—BRAF v600E MT predictive of BRAF inhibitor response (35)] | |||
| Period 3: TP 2 to TP 3 | 40.14 | Period 2 vs period 3 | <.001 |
| [January 18, 2018, NCCN guideline update recommends EGFR inhibitor only in combination with BRAF inhibitor in BRAF v600E mutated mCRC (25)] | |||
| Period 4: TP 3 to June 30, 2018 | 45.85 | Period 3 vs period 4 | <.001 |
| MMR/MSI | |||
| Period 1: January 1, 2013, to TP 1 | 23.29 | Referent | |
| [June 25, 2015, landmark publication—MMR predictive of PD-1 mAb response (36)] | |||
| Period 2: TP 1 to TP 2 | 32.01 | Period 1 vs period 2 | <.001 |
| [November 23, 2015, NCCN guideline update recommends MMR/MSI testing in all CRC (55)] | |||
| Period 3: TP 2 to TP 3 | 42.92 | Period 2 vs period 3 | <.001 |
| [July 31, 2017, FDA label change—nivolumab approved for dMMR/MSI-H mCRC (56)] | |||
| Period 4: TP 3 to June 30, 2018 | 59.31 | Period 3 vs period 4 | <.001 |
June 30, 2018, marks the end of available data. CRC = colorectal cancer; EGFR = epidermal growth factor receptor; FDA = Food and Drug Administration; mAb = monoclonal antibody; mCRC = metastatic colorectal cancer; MMR = mismatch repair; MSI = microsatellite instability; MSI-H = microsatellite instability high; dMMR- deficient mismatch repair; MT = mutated; NCCN = National Comprehensive Cancer Network; PD-1 = programmed death ligand 1; TP = time point; WT = wild type.
Use of Anti-EGFR Therapy in Front-Line Over Time
Patients with left-sided, RAS WT tumors were increasingly treated with anti-EGFR therapy in the front-line setting (P = .002) though rates were only 19.12% by the final time period analyzed. Patients with right-sided, RAS WT tumors trended toward decreasing rates of front-line anti-EGFR therapy (P = .21).
Discussion
Over the last decade, international guidelines have endorsed testing recommendations for KRAS, NRAS, BRAF, and MMR/MSI in mCRC. Our study demonstrates an increase in testing rates over time for these biomarkers. These results suggest that oncologists are receptive to landmark publications, US Food and Drug Administration labeling changes, and updates in NCCN guidelines and adapt their practice accordingly. Nevertheless, as of 2018 the rates of testing remained quite low with only 56% of patients receiving KRAS testing, 46% of patients receiving NRAS testing, and 46% of patients receiving BRAF testing (35). Rate of MMR/MSI testing remained high at 82% tested as of 2018, possibly because of earlier understanding of its predictive and prognostic importance, earlier testing recommendations by international guidelines, and the growing use of immunotherapy in the dMMR/MSI-H mCRC patients (3,24,38).
On average, patients who were tested tended to be younger and have a better ECOG performance status. Younger patients may receive more aggressive workup and treatment; for example, increased consideration of anti-EGFR therapy may lead to increased rates of RAS and BRAF testing (39). Conversely, patients with a poorer performance status may receive less testing as clinicians try to avoid aggressive workup in patients who may not be eligible to receive treatment.
Prior studies have shown that older patients are less likely to be referred to a medical oncologist and to receive therapy for mCRC compared with their younger counterparts (40,41). The reasons for this disparity are likely multifactorial, including a paucity of prospective data in the elderly population because of underrepresentation in clinical trials, increased rate of medical comorbidities, and challenges with social support leading to more fragmented care (42,43). These same challenges may contribute to the decreased testing rates in the elderly. Notably, however, anti-EGFR therapy is commonly used and may be well tolerated in older adults, making biomarker testing important in this cohort (29,44). Immunotherapy may also be well tolerated by patients with a poor performance status, and therefore MMR/MSI testing remains imperative in the elderly patient population especially as data show that older patients may have higher rates of MSI-H tumors compared with younger cohorts (24,45–47).
Patients with commercial insurance had the highest rates of RAS, BRAF, and MMR/MSI testing, whereas patients with Medicaid had the lowest rates. It is well established that patients with Medicaid or lack of insurance suffer from statistically significantly worse outcomes. Large, retrospective database analyses have shown that uninsured and Medicaid-insured patients present at more advanced stages of colorectal cancer due in part to lack of early screening (48). Additionally, CRC patients with non-Medicaid insurance enjoy a statistically significantly longer survival compared with patients with Medicaid insurance (49–51). The same barriers to care that lead to reduced screening and poorer outcomes in the uninsured or Medicaid-insured population likely contribute to a reduced rate of biomarker testing in this cohort. In addition, because older individuals (or those who would be eligible for Medicare) were less likely to be tested, age and performance status may be affecting the interaction between insurance status and biomarker testing.
We found that rates of KRAS, NRAS, and BRAF testing increased over time, and the rates of testing appeared to increase across almost all time periods examined. Even with this increase in testing over time, however, many patients remain untested as of our final data period. In fact, only a minority of patients were tested for the 4 biomarkers demonstrating a significant need for improvement in our management of these patients. Understanding the factors that contribute to suboptimal testing rates, as well as the reasons for disparity in testing between subpopulations, is beyond the scope of this analysis and would be difficult to evaluate through this database alone. However, additional research and educational initiatives may help uncover and dismantle these barriers for testing.
Finally, we saw a statistically significant increase in the use of anti-EGFR therapy in the front-line setting for left-sided tumors and a decreased use for right-sided tumors, which was not statistically significant. This aligns with the NCCN guidelines recommendation for the use of anti-EGFR therapy plus chemotherapy for left-sided, RAS WT tumors in this setting, based on data demonstrating superiority over the combination of chemotherapy and anti-VEGF (3,11). However, overall use of anti-EGFR therapy remains quite low in the front-line setting with less than 20% of patients with left-sided, RAS WT tumors receiving such treatment by the final time point analyzed. The gradual adoption of these guidelines may be because of the short time interval between the publication of the above data in June 2017 and the end of our data collection in 2018 (52). Furthermore, the exclusion of transverse colon tumors from this analysis, because of challenges in defining them as left- or right-sided within the database may have also affected these results.
There are several limitations to our study. These data are contingent on clinical information as documented in the EHR and the manual abstraction of that information, which may introduce variability, errors, or subjectivity. The use of trained professional abstractors, following consistent abstraction guidance, policies, and procedures, may mitigate these risks (30). Missing data in the EHR are also a source of potential bias. For example, although we did not find an association with race, multiple studies have found an independent association between race and poor outcomes (48–50). This may be explained by 21% of patients being characterized as other or unknown race in our cohort. Chart documentation of patient variables may have impacted indication of biomarker testing as well. In addition, it is possible that providers used outside laboratories for biomarker testing, which was not included in the electronic medical record, or testing was performed by providers outside of the Flatiron Health network. This may have resulted in a lower rate of documented testing in our analysis. Finally, errors in recording the correct ICD code or failure to include appropriate tumor sidedness may have affected our analyses (53). However, these factors are unlikely to affect the overall observed trends over time.
The NCCN guidelines do not specify a recommended method of testing these biomarkers. Over the time period analyzed, adoption of comprehensive next-generation sequencing testing has increased, which may have led to higher rates of testing across all biomarkers (28). With this trend, we would expect to find a significant increase in the percentage of patients having their tumor samples tested. However, by the final time point analyzed, 59% of patients had MMR/MSI testing, whereas only 43% had NRAS testing, suggesting a large percentage of patients had either no testing or had only limited biomarker testing. Additional research is needed to understand barriers to universal testing, and ongoing monitoring is needed to ensure testing rates are improved.
This type of big data analysis fails to capture the thought process behind the decision regarding testing of patients; for example, older patients may be tested at a lower rate because more of them may be unfit for chemotherapy, and their management may have been focused on supportive care. Moreover, some providers may have only tested for RAS and BRAF on progression after front-line therapy in mCRC, which our data may have missed given the 6-month window after diagnosis to determine compliance to testing. Data supporting the benefit of anti-EGFR therapy in the front-line setting were not available for most of the timeline of this study (2013-2018). Therefore, during first-line treatment, testing may have not been considered necessary by some providers. Finally, the large sample size of this database results in statistical significance even with minimal difference between groups. As such, the small differences in rates of testing between males and females or stages of diagnosis (although statistically significant) are unlikely to represent a clear underlying signal.
In conclusion, testing of biomarkers KRAS, NRAS, BRAF, and MMR/MSI have increased over time in accordance with landmark publications and guideline changes. However, the rate of documented testing remains low and can be improved on substantially. Increased testing rates could result in improved diagnoses of hereditary CRC as in Lynch syndrome, optimization of treatment, and ultimately improved patient outcomes. Additional research is warranted to confirm these findings, characterize the reasons for nontesting, and understanding barriers for adoption of testing among providers, which may ultimately lead to educational initiatives to improve testing trends.
Funding
This work was supported by Fox Chase Cancer Center grant (3 P30 CA006927).
Notes
Role of the funder: The funder had no role in the design of the study; collection, analysis and interpretation of the data; the preparation of the manuscript or decision to submit it for publication.
Disclosures: All authors have no conflicts of interest to disclose.
Author contributions: Conceptualization—PI, MD, EH, SN, ED. Data Curation—MD, EH. Formal analysis—PI, MD, EH, SN, ED. Writing original draft—PI, MD, EH, SN, ED. Writing review and editing—PI, MD, EH, SN, ED.
Prior presentations: Presented at the 2020 American Society of Clinical Oncology Gastrointestinal symposium on January 24, 2020, San Francisco, California, USA.
Supplementary Material
Contributor Information
Pritish Iyer, Department of Medical Oncology, Fox Chase Cancer, Philadelphia, PA, USA.
Mengying Deng, Department of Biostatistics, Fox Chase Cancer Center, Philadelphia, PA, USA.
Elizabeth A Handorf, Department of Biostatistics, Fox Chase Cancer Center, Philadelphia, PA, USA.
Shazia Nakhoda, Department of Medical Oncology, Fox Chase Cancer, Philadelphia, PA, USA.
Efrat Dotan, Department of Medical Oncology, Fox Chase Cancer, Philadelphia, PA, USA.
Data Availability
The data that support the findings of this study are openly available in electronic form at Fox Chase Cancer Center biostatistical department including the database obtained from Flatiron and the analysis conducted at our site.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A.. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. [DOI] [PubMed] [Google Scholar]
- 2.Surveillance, Epidemiology, and End Results (SEER) Program Populations (1975-2018) (www.seer.cancer.gov/popdata), National Cancer Institute, DCCPS, Surveillance Research Program. https://seer.cancer.gov/data-software/documentation/seerstat/nov2018/.
- 3. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology colon cancer, version 1.2020. 2020. https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- 4. Cox AD, Fesik SW, Kimmelman AC, Luo J, Der CJ.. Drugging the undruggable RAS: mission possible? Nat Rev Drug Discov. 2014;13(11):828-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henry JT, Johnson B.. Current and evolving biomarkers for precision oncology in the management of metastatic colorectal cancer. Chin Clin Oncol. 2019;8(5):49. [DOI] [PubMed] [Google Scholar]
- 6. Douillard J-Y, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023-1034. [DOI] [PubMed] [Google Scholar]
- 7. Van Cutsem E, Köhne CH, Hitre E, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360(14):1408-1417. [DOI] [PubMed] [Google Scholar]
- 8. Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065-1075. [DOI] [PubMed] [Google Scholar]
- 9. Engstrom PF, Arnoletti JP, Benson AB, et al. ; for the National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: colon cancer. J Natl Compr Canc Netw. 2009;7(8):778-831. [DOI] [PubMed] [Google Scholar]
- 10. Tejpar S, Stintzing S, Ciardiello F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. 2017;3(2):194-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Venook AP, Niedzwiecki D, Innocenti F, et al. Impact of primary (1º) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). J Clin Oncol. 2016;34(suppl 15):3504-3504. [Google Scholar]
- 12. Benson AB, Venook AP, Cederquist L, et al. Colon cancer, version 1.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(3):370-398. [DOI] [PubMed] [Google Scholar]
- 13. Roth AD, Tejpar S, Delorenzi M, et al. Prognostic role of KRAS and BRAF in stage II and III resected colon cancer: results of the translational study on the PETACC-3, EORTC 40993, SAKK 60-00 trial. J Clin Oncol. 2010;28(3):466-474. [DOI] [PubMed] [Google Scholar]
- 14. Stintzing S, Miller-Phillips L, Modest DP, et al. ; for the FIRE-3 Investigators. Impact of BRAF and RAS mutations on first-line efficacy of FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab: analysis of the FIRE-3 (AIO KRK-0306) study. Eur J Cancer. 2017;79:50-60. [DOI] [PubMed] [Google Scholar]
- 15. Loupakis F, Ruzzo A, Cremolini C, et al. KRAS codon 61, 146 and BRAF mutations predict resistance to cetuximab plus irinotecan in KRAS codon 12 and 13 wild-type metastatic colorectal cancer. Br J Cancer. 2009;101(4):715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Artale S, Sartore-Bianchi A, Veronese SM, et al. Mutations of KRAS and BRAF in primary and matched metastatic sites of colorectal cancer. J Clin Oncol. 2008;26(25):4217-4219. [DOI] [PubMed] [Google Scholar]
- 17. Di Nicolantonio F, Martini M, Molinari F, et al. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26(35):5705-5712. [DOI] [PubMed] [Google Scholar]
- 18. Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306-1315. [DOI] [PubMed] [Google Scholar]
- 19. Kopetz S, Grothey A, Yaeger R, et al. Encorafenib, binimetinib, and cetuximab in BRAF V600E-mutated colorectal cancer. N Engl J Med. 2019;381(17):1632-1643. [DOI] [PubMed] [Google Scholar]
- 20. Lynch HT, de la Chapelle A.. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919-932. [DOI] [PubMed] [Google Scholar]
- 21. Ribic CM, Sargent DJ, Moore MJ, et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349(3):247-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berg A, Armstrong K, Botkin J, et al. Recommendations from the EGAPP working group: genetic testing strategies in newly diagnosed individuals with colorectal cancer aimed at reducing morbidity and mortality from Lynch syndrome in relatives. Genet Med. 2009;11(1):35-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diaz LA, Le DT, Yoshino T, et al. KEYNOTE-177: phase 3, open-label, randomized study of first-line pembrolizumab (Pembro) versus investigator-choice chemotherapy for mismatch repair-deficient (dMMR) or microsatellite instability-high (MSI-H) metastatic colorectal carcinoma (mCRC). J Clin Oncol. 2018;36(suppl 4):TPS877. [Google Scholar]
- 25. Benson AB, Venook AP, Al-Hawary MM, et al. NCCN guidelines insights: colon cancer, version 2.2018. J Natl Compr Canc Netw. 2018;16(4):359-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. National Comprehensive Cancer Network. NCCN clinical practice guidelines in colon cancer, version 2.2015. 2015.
- 27. Shaikh T, Handorf EA, Meyer JE, Hall MJ, Esnaola NF.. Mismatch repair deficiency testing in patients with colorectal cancer and nonadherence to testing guidelines in young adults. JAMA Oncol. 2018;4(2):e173580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gutierrez ME, Price KS, Lanman RB, et al. Genomic profiling for KRAS, NRAS, BRAF, microsatellite instability, and mismatch repair deficiency among patients with metastatic colon cancer. J Clin Oncol Precis Oncol. 2019;(3):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dotan E, Devarajan K, D’Silva AJ, et al. Patterns of use and tolerance of anti-epidermal growth factor receptor antibodies in older adults with metastatic colorectal cancer. Clin Colorectal Cancer. 2014;13(3):192-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma X, Long L, Moon S, Adamson BJS, Baxi SS.. Comparison of population characteristics in real-world clinical oncology databases in the US: flatiron health, SEER, and NPC. medRxiv 2020.03.16.20037143; doi:10.1101/2020.03.16.20037143. [Google Scholar]
- 31. Birnbaum B, Nussbaum N, Seidl-Rathkopf K, et al. Model-assisted cohort selection with bias analysis for generating large-scale cohorts from the EHR for oncology research. arXiv preprint arXiv:200109765.2020.
- 32. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology, version 1.2014. 2014. [DOI] [PubMed]
- 33. Vectibix (Panitumumab) [package insert]. Thousand Oaks, CA: Amgen; 2017. [Google Scholar]
- 34. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology colon cancer, version 8.2014. 2014. [DOI] [PubMed]
- 35. Kopetz S, McDonough SL, Morris VK, et al. Randomized trial of irinotecan and cetuximab with or without vemurafenib in BRAF-mutant metastatic colorectal cancer (SWOG 1406). J Clin Oncol. 2017;35(suppl 4):520-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Le DT, Uram JN, Wang H, et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509-2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Opdivo (Nivolumab) [package insert]. Princeton, NJ: Bristol Myers-Squibb; 2017. [Google Scholar]
- 38. Benson AB, Arnoletti JP, Bekaii-Saab T, et al. ; for the National Comprehensive Cancer Network. Colon cancer. J Natl Compr Canc Netw. 2011;9(11):1238-1290. [DOI] [PubMed] [Google Scholar]
- 39. Thiebault Q, Defossez G, Karayan-Tapon L, Ingrand P, Silvain C, Tougeron D.. Analysis of factors influencing molecular testing at diagnostic of colorectal cancer. BMC Cancer. 2017;17(1):765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vijayvergia N, Li T, Wong YN, Hall MJ, Cohen SJ, Dotan E.. Chemotherapy use and adoption of new agents is affected by age and comorbidities in patients with metastatic colorectal cancer. Cancer. 2016;122(20):3191-3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Luo R, Giordano SH, Freeman JL, Zhang D, Goodwin JS.. Referral to medical oncology: a crucial step in the treatment of older patients with stage III colon cancer. Oncologist. 2006;11(9):1025-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. McCleary NJ, Dotan E, Browner I.. Refining the chemotherapy approach for older patients with colon cancer. J Clin Oncol. 2014;32(24):2570-2580. [DOI] [PubMed] [Google Scholar]
- 43. Dotan E, Browner I, Hurria A, Denlinger C.. Challenges in the management of older patients with colon cancer. J Natl Compr Canc Netw. 2012;10(2):213-224; quiz 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lonardi S, Schirripa M, Buggin F, et al. ; for the Gono Group. First-line FOLFOX plus panitumumab versus 5FU plus panitumumab in RAS-BRAF wild-type metastatic colorectal cancer elderly patients: The PANDA study. J Clin Oncol. 2020;38(suppl 15):4002-4002. [Google Scholar]
- 45. Poynter JN, Siegmund KD, Weisenberger DJ, et al. ; for the Colon Cancer Family Registry Investigators. Molecular characterization of MSI-H colorectal cancer by MLHI promoter methylation, immunohistochemistry, and mismatch repair germline mutation screening. Cancer Epidemiol Biomarkers Prev. 2008;17(11):3208-3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kakar S, Burgart LJ, Thibodeau SN, et al. Frequency of loss of hMLH1 expression in colorectal carcinoma increases with advancing age. Cancer. 2003;97(6):1421-1427. [DOI] [PubMed] [Google Scholar]
- 47. Aasebø KØ, Dragomir A, Sundström M, et al. Consequences of a high incidence of microsatellite instability and BRAF-mutated tumors: a population-based cohort of metastatic colorectal cancer patients. Cancer Med. 2019;8(7):3623-3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY.. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222-231. [DOI] [PubMed] [Google Scholar]
- 49. Pulte D, Jansen L, Brenner H.. Social disparities in survival after diagnosis with colorectal cancer: contribution of race and insurance status. Cancer Epidemiol. 2017;48:41-47. [DOI] [PubMed] [Google Scholar]
- 50. Tawk R, Abner A, Ashford A, Brown CP.. Differences in colorectal cancer outcomes by race and insurance. Int J Environ Res Public Health. 2015;13(1):48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Walker GV, Grant SR, Guadagnolo BA, et al. Disparities in stage at diagnosis, treatment, and survival in nonelderly adult patients with cancer according to insurance status. J Clin Oncol. 2014;32(28):3118-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Venook AP, Niedzwiecki D, Lenz H-J, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. 2017;317(23):2392-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Luhn P, Kuk D, Carrigan G, et al. Validation of diagnosis codes to identify side of colon in an electronic health record registry. BMC Med Res Methodol. 2019;19(1):177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Erbitux (Cetuximb) [package insert]. Indianapolis, IN: Eli Lilly and Company; 2017. [Google Scholar]
- 55. National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology in colon cancer, version 2.2016. 2016. [DOI] [PubMed]
- 56. Keytruda (Pembrolizumab) [package insert]. Whitehouse Station, NJ: Merck; 2017. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are openly available in electronic form at Fox Chase Cancer Center biostatistical department including the database obtained from Flatiron and the analysis conducted at our site.