Abstract
Background
Pediatric tuberculosis (TB) remains a critical public health concern, yet bacteriologic confirmation of TB in children is challenging. Clinical, demographic, and radiological factors associated with a positive Mycobacterium tuberculosis specimen in young children (≤5 years) are poorly understood.
Methods
We conducted a prospective cohort study of young children with presumptive TB and examined clinical, demographic, and radiologic factors associated with invasive and noninvasive specimen collection techniques (gastric aspirate, induced sputum, nasopharyngeal aspirate, stool, and string test); up to 2 samples were taken per child, per technique. We estimated associations between these factors and a positive specimen for each technique using generalized estimating equations (GEEs) and logistic regression.
Results
A median (range) of 544 (507–566) samples were obtained for each specimen collection technique from 300 enrolled children; bacteriologic yield was low across all collection techniques (range, 1%–7% from Xpert MTB/RIF or culture), except for lymph node fine needle aspiration (29%) taken for children with cervical lymphadenopathy. Factors associated with positive M. tuberculosis samples across all techniques included prolonged lethargy (median [range] adjusted odds ratio [aOR], 8.1 [3.9–10.1]), history of exposure with a TB case (median [range] aOR, 6.1 [2.9–9.0]), immunologic evidence of M. tuberculosis infection (median [range] aOR, 4.6 [3.7–9.2]), large airway compression (median [range] aOR, 6.7 [4.7–9.5]), and hilar/mediastinal density (median [range] aOR, 2.9 [1.7–3.2]).
Conclusions
Identifying factors that lead to a positive M. tuberculosis specimen in very young children can inform clinical management and increase the efficiency of diagnostic testing in children being assessed for TB.
Keywords: tuberculosis, diagnosis, Mycobacterium tuberculosis, pediatric TB, Xpert MTB/RIF
We evaluated factors associated with positive Mycobacterium tuberculosis samples in children under 5 years old and found immunological evidence of M. tuberculosis infection, history of exposure with a tuberculosis patient, and prolonged lethargy were associated with a positive sample.
Tuberculosis (TB), an airborne infectious disease caused by Mycobacterium tuberculosis, remains a major cause of morbidity and mortality among children worldwide. Globally, an estimated 1 million children are diagnosed with TB, and over a quarter-million children die from TB disease each year [1]. Mortality among pediatric TB cases is most profound among infants and very young children (5 years of age and younger), who account for almost 80% of all TB deaths in children and adolescents age <15 years [1–3]. An estimated 96% of these deaths are among children not receiving TB treatment, widely attributed to the lack of available tools to properly detect and diagnose TB in very young patients [3, 4]. This underscores the potential for reducing TB-related mortality among infants and young children by improving diagnostic techniques and shortening time to treatment initiation.
Bacteriologic confirmation of M. tuberculosis in young children is one of the leading diagnostic challenges in pediatric laboratory medicine. Pediatric TB is paucibacillary, and the primary specimen used to confirm TB disease in adults (expectorated sputum) is not feasible to collect from young children. The reference standards for specimens in children with the highest sensitivity, gastric aspirate and induced sputum, are invasive procedures and require specialized equipment and training [5]. As a result, these procedures are often not performed in the resource-limited settings where the majority of TB exists; however, even in ideal settings equipped with advanced laboratory support, the diagnostic yield of these procedures remains suboptimal (25%–50%) [5, 6].
In the absence of bacteriologic confirmation, clinical diagnosis remains the de facto method to identify pediatric TB cases. Clinical diagnosis of pediatric TB lacks clinical standardization and is complicated both by nonspecific symptoms that often overlap with common childhood diseases (ie, pneumonia, malnutrition) and by the remarkable variability in immunologic response and radiologic disease manifestations among pediatric patients. As a consequence, it is widely recognized that the majority of pediatric TB remains mis- or undiagnosed, contributing to the unacceptably high levels of mortality observed among infants and young children [3, 4].
Strategies to improve bacteriologic confirmation of M. tuberculosis in very young children will require far less invasive sampling techniques that are tolerable and more feasible in resource-limited settings. Multiple studies have explored minimally or noninvasive collection techniques such as stool and urine collection and nasopharyngeal aspiration [7–10]. These approaches demonstrate that minimally invasive collection techniques are considerably more feasible, yet their bacteriologic yield generally remains inferior to invasive collection techniques [11]. Optimizing these techniques to improve bacteriologic yield will require a clear understanding of the clinical, demographic, and radiological factors associated with each specimen type.
Kenya is ranked as one of the top 20 high-burden TB and TB/HIV countries in the world by the World Health Organization (WHO) [12]. Here, we examine a large, prospective cohort of infants and young children presenting with symptoms consistent with TB disease in Kisumu, Kenya; we evaluated a comprehensive range of both invasive and minimally or noninvasive specimens to detect M. tuberculosis, including gastric aspirate, induced sputum, string test, stool, urine, nasopharyngeal aspirate, blood, and cervical lymph node fine needle aspirate. We report clinical and radiological factors associated with an M. tuberculosis sample with a positive result by Xpert MTB/RIF or culture among children with presumptive TB disease for each collection technique. The broader goal of this analysis is to inform targeted sampling collection strategies for both clinical care and future diagnostic studies seeking to improve bacteriologic yield among their cohort.
METHODS
Setting and Study Design
The TOTO TB study (Mtoto means “little child” in Swahili) was a prospective TB diagnostic cohort study conducted between October 2013 and August 2015 at pediatric clinics and hospitals in Kisumu County, Kenya. A detailed study protocol is described elsewhere [7]. Briefly, we recruited children ≤5 years old with presumptive TB disease from inpatient and outpatient clinics and as TB contacts and followed prospectively for up to 6 months. Children who presented with either parenchymal abnormality on chest x-ray (CXR) or cervical lymphadenopathy in addition to at least 1 persistent clinical symptom of TB (cough ≥14 days despite at least 5 days of antibiotics, fever ≥7 days despite 5 days of antibiotics/antimalarials, or malnutrition (weight-for-age z-score ≤−2.0 despite treatment for other causes) were recruited into the study. Children were excluded if they had received TB preventative therapy or had received anti-TB treatment in the past year [7].
Specimen Collection and M. tuberculosis Detection
We collected up to 2 samples of each specimen: gastric aspirate (GA), induced sputum (IS), nasopharyngeal aspirate (NPA), stool, string test (ST), urine, and blood. In addition, lymph node fine needle aspiration (FNA) was performed when indicated for children with cervical lymphadenopathy. For each specimen, up to 3 methods were used for detection of M. tuberculosis: (1) florescence microscopy (FM) for acid-fast bacilli, (2) liquid culture for mycobacteria growth indicator tube (MGIT), and (3) the rapid cartridge-based nucleic acid amplification test Xpert MTB/RIF (Xpert). Further details on collection, processing, and testing techniques are described elsewhere [7].
Chest Radiography
Analog or digital chest radiographs (CXRs) were reviewed by a study physician, and digital films were reviewed by a general radiologist for parenchymal abnormalities at enrollment; the determination of a CXR consistent with clinical TB and specific parenchymal and extraparenchymal abnormalities were reviewed by 3 independent, blinded expert reviewers with expertise in reading pediatric CXRs in high-burden settings. Final CXR determination was classified as “abnormal, likely TB,” “abnormal, equivocal,” and “normal.” Specific parenchymal findings included evidence of cavitation, airspace consolidation, calcification, or hyperinflation. Extraparenchymal findings included collapse, hilar/mediastinal density, large airway compression, hilar/mediastinal calcification, pleural fluid collection, pneumothorax, raised hemi-diaphragm, and enlarged cardiac silhouette. Final CXR determination for overall TB classification and the presence of specific findings was made if at least 2 reviewers concurred; if all 3 reviews were discordant, no final determination was made [13].
Clinical Factors and Definitions
HIV status was determined through prospective testing or history of HIV and/or use of antiretroviral therapy. Immunological staging for HIV-positive children was based on CD4% and age and was categorized as not significant, mild, moderate, or severe immunosuppression according to WHO guidelines [14]. Weight-for-age (WFA) was used as a proxy for nutritional status and standardized by calculating z-scores based on uniform growth standards for children developed by the WHO [15]. Per standardized guidelines, we defined malnutrition/failure to thrive as a WFA z-score ≤−2.0 [16]. Immunological evidence of M. tuberculosis infection was determined as either a positive tuberculin skin test (TST), defined as an induration of ≥10 mm for HIV-negative children or ≥5 mm for HIV-positive children (or children with malnutrition) or a positive interferon-gamma release assay (IGRA; QuantiFERON-TB Gold). History of exposure was defined as a child residing with someone with a bacteriologically confirmed case of TB disease within the past 24 months [17]. Prolonged lethargy was determined by study clinicians as unexplained lethargy (ie, decrease in playfulness, activity, or mental alertness) for ≥30 days despite antibiotics/antimalarials for ≥5 days [17].
Model Outcome, Covariates, and Development
Our primary outcome was detection of M. tuberculosis detected by MGIT or Xpert among all children enrolled with presumptive TB disease. Our primary covariates assessed included sex, HIV status, age, immunological evidence of TB infection (positive TST or IGRA), history of TB exposure, chest radiograph consistent with TB, and TB signs and symptoms (prolonged fever, cough, lethargy, and malnutrition). Given that TST is generally more widely available than IGRA, we also considered TST and IGRA results independently. We separately investigated associations between the outcomes and specific parenchymal and extraparenchymal findings on CXR. HIV status was categorized by immunological classification according to age and CD4%, as defined by the WHO [14].
The association between our primary outcome and covariates was assessed using 2 approaches: (1) a per-sample analysis and (2) a per-patient analysis. The per-sample analysis utilized results from individual samples for each specimen collection technique. In this approach, associations between covariates and the primary outcome were examined using logistic regression implemented through generalized estimating equations (GEEs) assuming an exchangeable correlation matrix to account for correlations that may arise due to repeated samples taken from the same child. The per-patient analysis considered any positive result from any sample from any specimen collection technique; a child was considered positive for M. tuberculosis if any sample from any test was positive for M. tuberculosis (“any positive result”). Associations in the per-patient analysis were examined using classical logistic regression. In both analyses, model parameter estimates and standard errors were transformed to compute odds ratios (ORs) and 95% CIs to determine if an association was statistically significant; CIs that did not contain the null value (1.0) were considered significant.
We developed a covariate-specific multivariable GEE and logistic model for individual demographic and clinical factors and calculated adjusted ORs (aORs). Model selection for each covariate was based on both quantitative and qualitative reasoning (ie, bivariate associations, a priori knowledge of plausible associations, causal diagrams, etc.) to identify minimally sufficient adjustment sets and reduce potentially spurious associations. We report all specific CXR findings as aORs adjusted for age, HIV status, and immunological evidence of TB infection. Details of the model selection process are presented in the Supplementary Data, along with results from all candidate and final selected models (Supplementary Table 1). We did not adjust for multiple comparisons given that models were based on pre-established, epidemiologically relevant comparisons rather than all possible comparisons; thus adjustment may increase type II error [18].
In a secondary analysis, we evaluated the robustness of our findings by analyzing only patients assigned a clinical case definition of TB disease (either laboratory-confirmed or clinically diagnosed intrathoracic TB disease) according to standardized research guidelines, likely providing a conservative estimate of associations [13, 16]. Detailed methods and results of these secondary analyses are provided in the Supplementary Data.
Patient Consent
Written informed consent was obtained from parents or legal guardians of participants. The study was approved by the institutional review boards (IRBs) of the US Centers for Disease Control and Prevention (CDC), the Kenya Medical Research Institute, and the Jaramogi Oginga Odinga Teaching and Referral Hospital. Children's Hospital Boston and Harvard Medical School relied on the review and oversight of the CDC IRB.
RESULTS
Participants and Specimen Collection
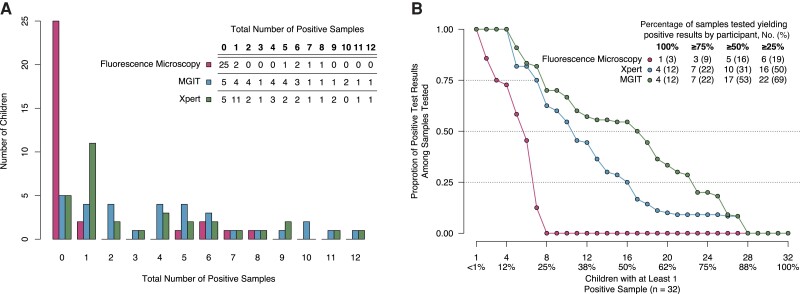
A total of 300 children with presumptive TB were enrolled in the study. After an intermediate assessment of bacteriologic yield, blood specimen collection was discontinued after 135 patients, and urine specimens were reduced to 1 sample per child after 138 patients. For each child, a median (interquartile range [IQR]) of 11 (10–12) samples for both FM and Xpert and 10 (9–11) samples for MGIT were successfully obtained and tested across all 8 sample collection techniques; total samples and results, stratified by test, are presented in Table 1. A total of 32 (11%) children had at least 1 M. tuberculosis–positive sample from any specimen from either Xpert or MGIT; 22 (69%) had a positive result on specimens from both invasive and minimally invasive techniques, 2 (6%) had a positive result on specimens from invasive-only techniques, 6 (19%) had a positive result from minimally invasive specimens only, and 2 (6%) were missing samples from invasive techniques but tested positive on samples from minimally invasive techniques. Among children with any positive specimen, the median (IQR) proportion of positive specimens among obtained samples was 0.0 (0.0–0.0) for FM, 0.2 (0.1–0.4) for Xpert, and 0.5 (0.2–0.7) for MGIT (Figure 1); the distributions of participant characteristics by sample collection technique are presented in Table 2. Detailed results on microbially confirmed patients, including patient-level bacteriologic yield and distribution of covariates by clinical case category, are available in Supplementary Tables 2 and 3.
Table 1.
Number of Samples Tested and Results From Each Collection Technique, by M. tuberculosis Detection Method
| Fluorescence Microscopy, No. (%) | Xpert Only, No. (%) | MGIT Only, No. (%) | Xpert or MGIT, No. (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | Mtb Negative | Mtb Positive | Total | Mtb Negative | Mtb Positive | Total | Mtb Negative | Mtb Positive | Total | Mtb Negative | Mtb Positive | ||
| Samples from invasive techniques | |||||||||||||
| Gastric aspirate | 561 | 553 (99) | 8 (1) | 562 | 538 (96) | 24 (4) | 530 | 495 (93) | 35 (7) | 562 | 523 (93) | 39 (7) | |
| Induced sputum | 544 | 538 (99) | 6 (1) | 543 | 524 (97) | 19 (3) | 517 | 489 (95) | 28 (5) | 543 | 511 (94) | 32 (6) | |
| Samples from non- or minimally invasive techniques | |||||||||||||
| Nasopharyngeal aspirate | 566 | 556 (98) | 10 (2) | 566 | 543 (96) | 23 (4) | 524 | 489 (93) | 35 (7) | 566 | 527 (93) | 39 (7) | |
| Stool | 507 | 501 (99) | 6 (1) | 506 | 487 (96) | 19 (4) | 438 | 427 (97) | 11 (3) | 510 | 487 (95) | 23 (5) | |
| String test | 542 | 539 (99) | 3 (1) | 540 | 526 (97) | 14 (3) | 524 | 504 (96) | 20 (4) | 542 | 519 (96) | 23 (4) | |
| Blooda | 0 | … | … | 0 | … | … | 96 | 95 (99) | 1 (1) | 96 | 95 (99) | 1 (1) | |
| FNAb | 2 | 2 (100) | 0 (0) | 14 | 11 (79) | 3 (21) | 13 | 10 (77) | 3 (23) | 14 | 10 (71) | 4 (29) | |
| Urinea | 377 | 376 (100) | 1 (0) | 374 | 368 (98) | 6 (2) | 327 | 324 (99) | 3 (1) | 377 | 371 (98) | 6 (2) | |
Abbreviations: FNA, cervical lymph node fine needle aspiration; MGIT, liquid culture for Mycobacteria Growth Indicator Tube; Mtb, Mycobacterium tuberculosis; Xpert, rapid cartridge-based nucleic acid amplification test (Xpert MTB/RIF).
Collection and testing of urine and blood specimens were changed based on interim review of assay yields; blood was discontinued after 135 children, and urine was reduced to 1 sample per child after the first 138 children.
FNA only collected when indicated (cervical lymphadenopathy).
Figure 1.
Exploring bacteriologic yield among children who tested positive for M. tuberculosis on at least 1 specimen (n = 32). A, Total number of positive samples per individual (absolute yield). B, Proportion of individual's total tests successfully obtained that yielded positive results (relative yield; ordered by decreasing value), as the full panel of samples was not obtained for each individual [7]. Patient-level details by testing technique can be found in Supplementary Table 2. Abbreviations: MGIT, liquid culture for Mycobacteria Growth Indicator Tube; Xpert, rapid cartridge-based nucleic acid amplification test (Xpert MTB/RIF).
Table 2.
Distribution of Study Participant Characteristics by Sample Collection Technique
| Gastric Aspirate, No. (%) | Induced Sputum, No. (%) | Nasopharyngeal Aspirate, No. (%) | Stool, No. (%) | String, No. (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mtb Positive | Mtb Negative | Mtb Positive | Mtb Negative | Mtb Positive | Mtb Negative | Mtb Positive | Mtb Negative | Mtb Positive | Mtb Negative | |
| Female | 12 (50) | 126 (50) | 11 (55) | 123 (50) | 14 (58) | 127 (50) | 7 (50) | 106 (49) | 7 (47) | 122 (49) |
| HIV status | … | … | … | … | … | … | … | … | … | … |
| HIV negative | 18 (78) | 189 (77) | 15 (83) | 185 (78) | 16 (73) | 192 (78) | 7 (58) | 160 (78) | 10 (71) | 188 (78) |
| No or mild immunosuppression | 1 (4) | 21 (9) | 0 (0) | 20 (8) | 2 (9) | 20 (8) | 1 (8) | 16 (8) | 1 (7) | 20 (8) |
| Moderate or high immunosuppression | 4 (17) | 34 (14) | 3 (17) | 32 (14) | 4 (18) | 33 (13) | 4 (33) | 30 (15) | 3 (21) | 32 (13) |
| Age | … | … | … | … | … | … | … | … | … | … |
| < 1 y | 7 (29) | 61 (24) | 5 (25) | 58 (24) | 7 (29) | 62 (25) | 4 (29) | 48 (22) | 3 (20) | 62 (25) |
| 1–<2 y | 7 (29) | 60 (24) | 4 (20) | 61 (25) | 4 (17) | 62 (25) | 2 (14) | 54 (25) | 4 (27) | 59 (24) |
| 2–5 y | 10 (42) | 130 (52) | 11 (55) | 125 (51) | 13 (54) | 128 (51) | 8 (57) | 113 (53) | 8 (53) | 126 (51) |
| Immunologic evidence of Mtb infection | … | … | … | … | … | … | … | … | … | … |
| Positive TST or IGRA | 18 (78) | 39 (16) | 15 (83) | 40 (17) | 18 (82) | 39 (16) | 10 (77) | 37 (18) | 13 (87) | 43 (18) |
| Positive IGRA only | 13 (62) | 33 (14) | 12 (71) | 34 (15) | 12 (60) | 34 (14) | 6 (50) | 30 (15) | 10 (71) | 35 (15) |
| Positive TST only | 15 (75) | 17 (10) | 12 (80) | 18 (11) | 15 (83) | 17 (10) | 9 (75) | 18 (13) | 11 (85) | 21 (12) |
| History of exposure | 21 (88) | 64 (25) | 15 (75) | 65 (27) | 20 (83) | 66 (26) | 10 (71) | 62 (29) | 12 (80) | 69 (28) |
| CXR consistent with TB | 11 (46) | 31 (14) | 9 (45) | 31 (14) | 12 (52) | 30 (13) | 7 (50) | 30 (16) | 8 (53) | 33 (15) |
| TB-like symptoms | … | … | … | … | … | … | … | … | … | … |
| Malnutrition | 11 (46) | 101 (40) | 11 (55) | 95 (39) | 11 (46) | 100 (40) | 9 (64) | 90 (42) | 9 (60) | 99 (40) |
| Prolonged cough | 21 (88) | 207 (82) | 15 (75) | 207 (85) | 19 (79) | 209 (83) | 11 (79) | 174 (81) | 14 (93) | 203 (82) |
| Prolonged fever | 13 (54) | 111 (44) | 11 (55) | 105 (43) | 15 (62) | 110 (44) | 8 (57) | 96 (45) | 8 (53) | 109 (44) |
| Prolonged lethargy | 6 (25) | 26 (10) | 7 (35) | 21 (9) | 7 (29) | 25 (10) | 6 (43) | 23 (11) | 5 (33) | 25 (10) |
Positive results from at least 1 of the 2 samples tested, per technique, as determined by either Xpert MTB/RIF or MGIT.
Abbreviations: IGRA, interferon-gamma release assay; MGIT, liquid culture for Mycobacteria Growth Indicator Tube; Mtb, Mycobacterium tuberculosis; TB, tuberculosis; TST, tuberculin skin test; Xpert, rapid cartridge-based nucleic acid amplification test (Xpert MTB/RIF).
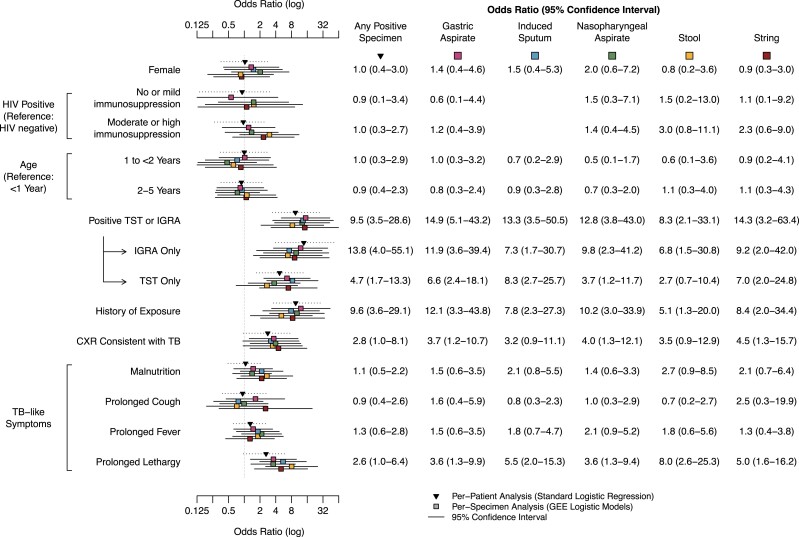
Odds ratios from per-specimen and per-patient analyses were calculated for GA, IS, NPA, stool, and string tests (Figures 2 and 3; Supplementary Figures 1–6); blood and urine were excluded due to insufficient bacteriological yield. Although FNA had a high yield among children in whom it was performed (4/13; 31%), it was also excluded due to limited samples and its irregular sampling protocol (performed only among children with suspected cervical lymphadenopathy). After adjusting for potential confounders, we found that the following covariates were associated with bacteriologic detection of M. tuberculosis across all 5 sample collection techniques: positive TST or IGRA (aOR range, 8.3–14.9), history of TB exposure (aOR range, 5.1–12.1), and prolonged lethargy (aOR range, 3.6–8.0) (Figure 2). The distributions of each factor for children included in the study are presented in Supplementary Table 2.
Figure 2.
Adjusted odds ratios of clinical and demographic characteristics for M. tuberculosis–positive vs –negative specimens detected by MGIT or Xpert among all children with symptoms concerning for TB disease (n = 300). Individual covariates were adjusted according to the methods. Only models with data sufficient for convergence are presented. Abbreviations: CXR, chest radiograph; GEE, generalized estimating equations; IGRA, interferon-gamma release assay; MGIT, liquid culture for Mycobacteria Growth Indicator Tube; TB, tuberculosis; TST, tuberculin skin test; Xpert, rapid cartridge based nucleic acid amplification test (Xpert MTB/RIF).
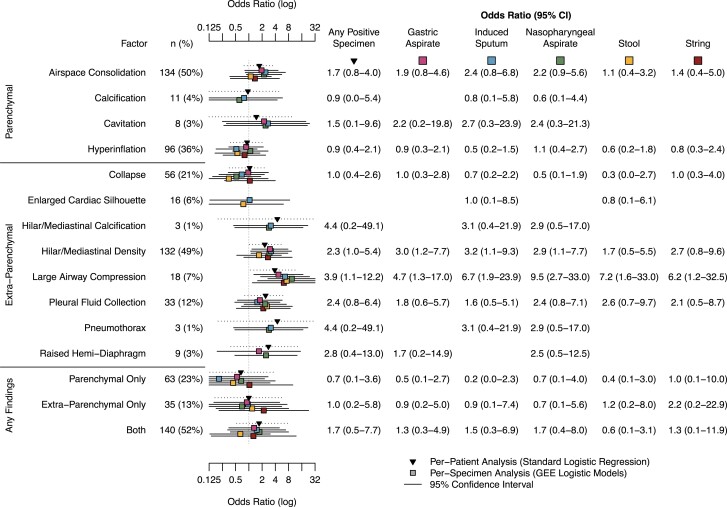
Figure 3.
Adjusted odds ratios of specific parenchymal and extraparenchymal findings on chest radiograph for M. tuberculosis–positive vs –negative specimens detected by MGIT or Xpert among all children with symptoms concerning for TB disease (n = 270). The absence of abnormal findings was the reference for each category. Only models with data sufficient for convergence are presented. Abbreviations: GEE, generalized estimating equations; MGIT, liquid culture for Mycobacteria Growth Indicator Tube; TB, tuberculosis; Xpert, rapid cartridge based nucleic acid amplification test (Xpert MTB/RIF).
A chest radiograph consistent with TB disease was associated with a positive result for gastric aspirate (aOR, 3.7; 95% CI, 1.2–10.7), NPA (aOR, 4.0; 95% CI, 1.3–12.1), and string test (aOR, 4.5; 95% CI, 1.3–15.7), but was not associated with a positive result for IS (aOR, 3.2; 95% CI, 0.9–11.1) or stool (aOR, 3.5; 95% CI, 0.9–12.9) samples (Figure 2). Observed patterns of associations remained when examining crude associations and associations among only those children ultimately classified as having TB disease (confirmed and unconfirmed TB); however, in general the strength of the association was slightly attenuated (Supplementary Figures 1–3).
Detailed results from chest radiographs were available for 270 (90%) children. Among these, 44 (16%) children were classified as “abnormal, likely TB,” 145 (54%) children were classified as “abnormal, equivocal,” and 17 (6%) were normal. The remaining 64 (24%) had discordant final reads, and no classification was made. The most common specific finding was parenchymal airspace consolidation (134/270, 50%), followed by extraparenchymal hilar/mediastinal density (132/270, 49%) and parenchymal hyperinflation (96/270, 36%) (Figure 3). Large airway compression was associated with an increased odds of a positive specimen across all collection techniques (aOR range, 4.7–9.5) (Figure 3). Hilar/mediastinal density was associated with positive gastric aspirate (aOR, 4.7; 95% CI, 1.3–17.0), induced sputum (aOR, 6.7; 95% CI, 1.9–23.9), and NPA (aOR, 9.5; 95% CI, 2.7–33.0) samples (Figure 3). These findings were consistent when analyzing crude associations (Supplementary Figure 4) or associations only among children classified as having TB disease (confirmed or unconfirmed), though in addition findings of extraparenchymal pneumothorax and hilar/mediastinal calcification were found to have increased odds of positive IS and NPA samples in the latter (Supplementary Figures 5 and 6).
DISCUSSION
We examined a range of invasive and noninvasive techniques for specimen collection in a large cohort of children under the age of 5 years with presumptive TB and found that children with immunological evidence of TB infection, history of TB exposure from a close contact, hilar/mediastinal density and large airway compression on chest radiograph, and prolonged lethargy were more likely to produce a positive bacteriologic result.
Our results extend and concur with previous work investigating bacteriologic yield in children with intrathoracic TB disease [19]. Importantly, we note that many clinical symptoms typically associated with pediatric TB disease and in TB diagnostic algorithms (prolonged cough, prolonged fever, malnutrition, age, and HIV status) were not associated with bacteriologic diagnosis. However, prolonged lethargy demonstrated the highest aOR among all TB-like symptoms, emphasizing the importance of prolonged lethargy to the evaluation of young children for TB. These trends persisted across specimen collection types, after carefully controlling for relevant covariates, and when evaluating a subset of children classified as having TB disease using standardized research case definitions.
These findings can both facilitate clinical decision-making and inform future diagnostic studies seeking to increase bacteriologic yield in their cohorts. We found strikingly similar trends in associations across specimen types, suggesting that factors associated with invasive procedures persist in noninvasive or minimally invasive procedures. While gastric aspiration and sputum induction are the most sensitive specimen collection techniques, they are highly invasive and often cause significant physical and mental distress to the child and family [5]. The use of noninvasive or minimally invasive specimen collection techniques may help minimize physical discomfort and psychological distress and encourage improved participation in sample collection among young children and their families. In addition, we found a relatively high yield of positive specimens from FNA taken among children with cervical lymphadenopathy (4/13 [31%] children, 29% of all FNA specimens tested on Xpert or MGIT). This finding complements other studies in highlighting the usefulness of FNA for microbial confirmation of TB disease, when indicated, in high-burden settings [20, 21].
Tests for immunologic evidence of TB infection (TST/IGRA) are incorporated into the standardized clinical case definitions but often not implemented in real-world clinical decision-making due to technical and logistical challenges associated with both IGRA and TST, and there are limited data on the use of IGRAs for children <5 years old. In this study, children with immunologic evidence of M. tuberculosis infection—either by IGRA or TST—demonstrated markedly higher odds of a positive M. tuberculosis specimen across all specimen collection techniques (median aOR, 13.3). Moreover, while chest x-ray is a key diagnostic tool used in the diagnosis of pediatric TB, few studies have reported specific parenchymal and extraparenchymal findings in very young children. We found that the specific extraparenchymal findings of large airway compression and hilar/mediastinal density may be associated with a positive specimen (median aORs, 6.7 and 2.9, respectively). Taken together, these results suggest that greater utilization of immunologic tests and scaling up of CXR read training and acuity may provide critical diagnostic support to clinicians.
We found notable differences in the strength of associations between specimen types. For example, although history of exposure was associated with increased M. tuberculosis detection among all sample collection techniques, the odds ratio for nasopharyngeal aspirate was twice that of stool (aORs, 10.2 and 5.1, respectively). However, a small number of children with bacteriologically confirmed TB contributed to wide confidence intervals and precluded our ability to determine intertechnique differences in associations. While this study benefitted from a large number of samples collected from multiple specimen collection techniques, the proportion of children with bacteriologic confirmation on any specimen was relatively small (11%); this contributed to increased uncertainty in our estimates and limited our ability to draw conclusions. Future work, particularly among cohorts with increased bacteriologic yield, may further explore these factors with more precision.
While the purpose of this study was to evaluate factors associated with a positive M. tuberculosis test result among all children with presumptive TB disease, only 32% of children had a final case definition of TB disease according to the standardized research guidelines (“confirmed” and “unconfirmed”) (Supplementary Methods). We likely included samples from children who did not have TB disease. This would bias our observed associations upward in relation to the true underlying association. We investigated this limitation by analyzing only those with a confirmed or unconfirmed TB diagnosis and found that the associations remained, though, as expected, they were slightly attenuated (Supplementary Figures 2 and 3). Therefore, the magnitude of associations should be interpreted with caution. Second, the extent to which enrolled cases are representative of all children with signs and symptoms consistent with TB disease is unknown. However, we enrolled children with presumptive TB according to current international guidelines, likely increasing the external validity of these results. Third, while we carefully considered possible biasing pathways among covariates, we cannot rule out external factors that may compromise the internal validity of this analysis. Observed associations, or lack thereof, may be a consequence of unknown and unmeasured confounders unaccounted for in this analysis. Developing individual, covariate-specific models also complicates comparisons across covariates. To facilitate more direct comparisons, we include results from full adjusted (all covariates) and full unadjusted (crude) ORs in Supplementary Table 1 and Supplementary Figure 1.
Identifying individual-level factors associated with bacteriologic confirmation of TB disease in very young children can inform approaches to improving TB diagnosis, hasten time to treatment, and prevent unnecessary mortality in this vulnerable population. Our findings highlight the importance of taking multiple data points into consideration when evaluating a child for TB disease and suggest that among young children with symptoms concerning for TB, those who have history of TB exposure, immunologic evidence of infection, prolonged lethargy, and hilar/mediastinal density and large airway compression on chest radiograph may be substantially more likely to produce an M. tuberculosis–positive specimen. When considered together, these factors may support diagnostic and clinical decision-making and present an opportunity to improve child treatment outcomes. Future studies, particularly in high-burden settings, may expand upon these findings and deepen our understanding of TB diagnosis in this population.
Supplementary Material
Acknowledgments
Financial support. This work was supported by the US Agency for International Development (USAID) and the US Centers for Disease Control and Prevention (CDC). A portion of this work was funded by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention and the Eunice Kennedy Shriver National Institute of Child Health & Human Development (K23HD072802 to R.S.). The findings and conclusions in this report are those of the author and do not necessarily represent the official position of the funding agencies. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability. All code and data needed to recreate results and figures are available at the following GitHub repository: https://github.com/jpsmithuga/peds_GEE_analysis.
Contributor Information
Jonathan P Smith, Division of Global HIV and Tuberculosis, Centers for Disease Control and Prevention, Atlanta, Georgia, USA; Department of Health Policy and Management, Yale University School of Public Health, New Haven, Connecticut, USA.
Rinn Song, Oxford Vaccine Group, Department of Paediatrics, University of Oxford, Oxford, UK.
Kimberly D McCarthy, Division of Global HIV and Tuberculosis, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Walter Mchembere, Center for Global Health Research, Kenya Medical Research Institute, Kisumu, Kenya.
Eleanor S Click, Division of Global HIV and Tuberculosis, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Kevin P Cain, Division of Global HIV and Tuberculosis, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
References
- 1. World Health Organization . Global Tuberculosis Report. World Health Organization; 2020. [Google Scholar]
- 2. Lewinsohn DA, Lewinsohn DM. Immunologic susceptibility of young children to Mycobacterium tuberculosis. Pediatr Res 2008; 63:115. [DOI] [PubMed] [Google Scholar]
- 3. Dodd PJ, Yuen CM, Sismanidis C, Seddon JA, Jenkins HE. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017; 5:e898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seddon JA, Shingadia D. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infect Drug Resist 2014; 7:153–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Guidance for National Tuberculosis Programmes on the Management of Tuberculosis in Children. 2nd ed. World Health Organization; 2014. [PubMed] [Google Scholar]
- 6. Winston CA, Menzies HJ. Pediatric and adolescent tuberculosis in the United States, 2008–2010. Pediatrics 2012; 130:e1425–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Song R, Click ES, McCarthy KD, et al. . Sensitive and feasible specimen collection and testing strategies for diagnosing tuberculosis in young children. JAMA Pediatr 2021; 175:e206069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nansumba M, Kumbakumba E, Orikiriza P, et al. . Detection yield and tolerability of string test for diagnosis of childhood intrathoracic tuberculosis. Pediatr Infect Dis J 2016; 35:146–51. [DOI] [PubMed] [Google Scholar]
- 9. Nicol MP, Wood RC, Workman L, et al. . Microbiological diagnosis of pulmonary tuberculosis in children by oral swab polymerase chain reaction. Sci Rep 2019; 9:10789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Marcy O, Ung V, Goyet S, et al. . Performance of Xpert MTB/RIF and alternative specimen collection methods for the diagnosis of tuberculosis in HIV-infected children. Clin Infect Dis 2016; 62:1161–8. [DOI] [PubMed] [Google Scholar]
- 11. Kay AW, González Fernández L, Takwoingi Y, et al. . Xpert MTB/RIF and Xpert MTB/RIF ultra assays for active tuberculosis and rifampicin resistance in children. Cochrane Database Syst Rev 2020; 8:CD013359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. World Health Organization . WHO Global Lists of High Burden Countries for Tuberculosis (TB), TB/HIV and Multidrug/Rifampicin-Resistant TB (MDR/RR-TB), 2021–2025. World Health Organization; 2021. [Google Scholar]
- 13. Click ES, Song R, Smith JP, et al. . Performance of Xpert MTB/RIF and mycobacterial culture on multiple specimen types for diagnosis of tuberculosis disease in young children and clinical characterization according to standardized research case definitions. Pediatr Infect Dis J 2022; 41:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization . WHO Case Definitions of HIV for Surveillance and Revised Clinical Staging and Immunological Classification of HIV-Related Disease in Adults and Children. World Health Organization; 2007. [Google Scholar]
- 15. World Health Organization, United Nations Children's Fund . Recommendations for Data Collection, Analysis and Reporting on Anthropometric Indicators in Children Under 5 Years Old. World Health Organization; 2019. [Google Scholar]
- 16. Graham SM, Cuevas LE, Jean-Philippe P, et al. . Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015; 61:S179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham SM, Ahmed T, Amanullah F, et al. . Evaluation of tuberculosis diagnostics in children: 1. Proposed clinical case definitions for classification of intrathoracic tuberculosis disease. Consensus from an expert panel. J Infect Dis 2012; 205:S199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology 1990; 1:43–6. [PubMed] [Google Scholar]
- 19. Marais BJ, Hesseling AC, Gie RP, Schaaf HS, Enarson DA, Beyers N. The bacteriologic yield in children with intrathoracic tuberculosis. Clin Infect Dis 2006; 42:e69–71. [DOI] [PubMed] [Google Scholar]
- 20. Fanny ML, Beyam N, Gody JC, et al. . Fine-needle aspiration for diagnosis of tuberculous lymphadenitis in children in Bangui, Central African Republic. BMC Pediatr 2012; 12:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wright CA, Warren RM, Marais BJ. Fine needle aspiration biopsy: an undervalued diagnostic modality in paediatric mycobacterial disease. Int J Tuberc Lung Dis 2009; 13:1467–75. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.