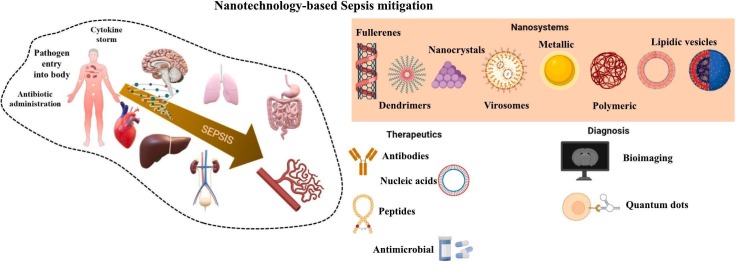
Abstract
COVID-19 acquired symptoms have affected the worldwide population and increased the load of Intensive care unit (ICU) patient admissions. A large number of patients admitted to ICU end with a deadly fate of mortality. A high mortality rate of patients was reported with hospital-acquired septic shock that leads to multiple organ failures and ultimately ends with death. The patients who overcome this septic shock suffer from morbidity that also affects their caretakers. To overcome these situations, scientists are exploring progressive theragnostic techniques with advanced techniques based on biosensors, biomarkers, biozymes, vesicles, and others. These advanced techniques pave the novel way for early detection of sepsis-associated symptoms and timely treatment with appropriate antibiotics and immunomodulators and prevent the undue effect on other parts of the body. There are other techniques like externally modulated electric-based devices working on the principle of piezoelectric mechanism that not only sense the endotoxin levels but also target them with a loaded antibiotic to neutralize the onset of inflammatory response. Recently researchers have developed a lipopolysaccharide (LPS) neutralizing cartridge that not only senses the LPS but also appropriately neutralizes with dual mechanistic insights of antibiotic and anti-inflammatory effects. This review will highlight recent developments in the new nanotechnology-based approaches for the diagnosis and therapeutics of sepsis that is responsible for the high number of deaths of patients suffering from this critical disease.
Keywords: Sepsis, Infection, Antimicrobial, Anti-inflammatory, Nanomedicine, Combination therapy
Graphical abstract
1. Introduction
Sepsis is understood as the dysregulated immune answer by endogenous factors towards the organ dysfunction caused by bacterial, viral, fungal, and parasitic infections. The status of sepsis can be defined in one sentence, explaining critical life taking disease. As time has progressed researchers, and health practitioners from different corners of the world just revised the protocols related to sepsis and settled in the terms of severity, organ damage, healing time, drug-disease interaction, pathology, drug resistance and many more [1]. Sepsis is a complex and crucial biochemical and pathophysiological dysfunction triggered by an infection. There is no such truthful definition in the scientific world to define sepsis. Its definition has been updated and modified from time to time with the advancement and research in this area. The latest definition states, sepsis is “organ dysfunction that is lethal to life due to improper host defence response to contagion” [2]. Presently, the World Health Organisation's (WHO) conclusion-making authority World Health Assembly (WHA) identifies sepsis as a crucial danger to patient safety and public health. This leads them to intensify their approaches to diagnosis, prevention, treatment, and management of sepsis. This crucial illness is becoming a major cause of death in intensive care unit (ICU) admitted patients globally. This is proven by the analysis called ‘Global Burden of Diseases, Injuries, and Risk Factors Study’ which testified that around 11 million deaths occur from 50 million cases that happen every year and account for around 20% of total deaths worldwide [3]. On a contemporary note, about 2 million septic sufferers and 300 thousand associated deaths are reported annually. This condition gets worsened in the case of the paediatric age group. Presently, sepsis is persistent in around 3 million children's cases worldwide [4]. Significantly, the major admission in ICUs is reported from middle- and low-income nations. This reporting can be attributed due to improper cleanliness in terms of fumigation and neutralization of pathogen load or high patient load that limits the time devoted to this process [5].
The fundamental steps to improvise clinical results in terms of death rate are early detection of sepsis using advanced molecular techniques and periodic medical prophylaxis [6]. The diagnosis of sepsis is very much crucial because of its fewer specific symptoms and signs. Sepsis has been challenging because of the unavailability of a proven standard test that is used for its diagnosis [7].
Apart from diagnosis, sepsis management guidelines majorly concentrated on three windows i.e., hemodynamic steadiness, contagion control and intonation of the septic responses [8]. Some of the other intrusions include fewer specific parameters of organ support like ventilation on a mechanical basis, oxygen therapy, renal replacement therapy, corticosteroids, and hemodynamic support [9]. For the management of sepsis, multi-model medicinal tactics are needed which are typically based on the severity of the disease [10]. A case with mild severity with one organ involvement can be treated by average therapeutic support while on the other hand, multiple organ dysfunction with a high level of severity needs invasive therapies. Moreover, for the management of the infection in sepsis, integral medicines are broad-spectrum antibiotics but the crucial challenge that make some hurdles in the treatment is the development of resistance against drugs by pathogens that unfavourably affect the septic condition and enhance the death rate two-fold [11]. Around 215,000 deaths are because of neonatal sepsis which is believed to be due to drug resistance to pathogens [12].
Furthermore, apart from the antimicrobial treatment, new adjunctive treatments like artificial antimicrobial peptides (AMPs), antioxidants, blood purification, anti-inflammatory mediators and immunomodulators enhance the life expectancy of septic patients. But these alternatives yield minimal efficacy in most cases. This inequality may be elaborated by sepsis-derived pharmacokinetically associated biological differences, leading to compromised biological distribution and deprived efficacy of the conventionally used therapeutic agent. This compromised treatment fiasco might be due to the development of drug resistance. The compromised pharmacokinetic parameters might be due to the initiation of host response that triggers a variety of cytokines, endogenous biomolecules and reactive oxygen species that hinder cellular functions and hampers the pharmacokinetic profile of the chemotherapeutics [13].
Nanotechnology is the master key that will help in unlocking various positive areas in the pharmaceutical and medical sciences to overcome the above-mentioned challenges. A variety of nano-based sensors like immune, magnetic, optical, and electrochemical sensors have been identified. These new alternatives with better specificity and sensitivity offer fast and consistent outcomes in the comparison to traditional tactics. Many physiological attributes of nanoparticles such as shape, size, and enhanced surface area help in their longer half-life, and tailored biodistribution in accordance with target specificity, in comparison to the conventional dosage forms. Thus, nanoengineered modalities will help in improvising the activities of drugs like antioxidants, antimicrobials, anti-inflammatory agents [14].
To the best of the authors' knowledge, a variety of reviews on the application of nanotechnology to sepsis with respect to diagnostic purpose, biosensors, and nanomedicines utilize promising relevance of toll-like receptors (TLR) inhibitors. Moreover, a summary regarding surfaces invigorated with metal and metal oxide nanoparticles is reported by Khan et al. which is potentially useful in sepsis management. Along with that Yuk et al. have reported relevance of nanotechnology regarding the identification and prophylaxis of sepsis [15]. However, a lot of research has been done on the advancement in earlier diagnosis and management of sepsis with improvised sensitivity. On a further note, extensive research in nanoscale particles and associated formulations-based treatments have revealed improved outcomes in vitro and in vivo. So, at this point, it becomes necessary to systematically compile the current developments on new nanotechnology-based diagnosis and treatment answers for sepsis. This review eyes on a wide-ranging overview of nano-level diagnosis application, new and ancillary nanotherapeutics and preventive measures based on nanotechnology including present assessment for septic detection and its supervision [16]. At the start, an apprised definition to describe sepsis, pathological and physiological points, and issues associated with the diagnosis and treatment of sepsis are discussed. This is followed by new progressions in the world of nanomedicine for the growth of new diagnostic tools and new nanoscale formulations to treat this crucial life-taking disease sepsis. Some future approaches and the marketing potential of such nano-based products for both diagnosis and therapy for beneficial patient outcomes are discussed [17].
2. Current aspects of sepsis from definition to current challenges
To date, the definition of sepsis is not well explained and there is no golden standard as this disease is inclusive of more than one pathological pathway. This section includes the presently modified definition of sepsis and its pathophysiology herein. The very first definition was reported in 1991 by the American College of Chest Physicians and the Society of Critical Care Medicine in terms of Systemic Inflammatory Response Syndrome (SIRS), severe sepsis, sepsis, and septic shock [18].
SIRS is identified if two or more points of the following symptoms are present: Temperature > 100.4°F (38 °C) or < 96.8°F (36 °C); WBC > 12,000/mm3 or < 4000/mm3; Respiratory rate (RR) > 20/min or PaCO2 < 32 mmHg; Pulse rate > 90 beats/min. Formerly sepsis is defined as the function of SIRS i.e., inflammation produced in the body due to infection showing mild signs like elevated temperature, tachycardia, tachypnoea, and raised WBC count [19]. Severe sepsis was simplified as the septic condition assigned with some degree of organ dysregulation. This includes renal failure assigned as decreased urine output and elevated creatinine levels, neuro dysfunction assigned as confusion and delirium, pulmonary failure assigned as hypoxemic respiratory failure, gastrointestinal dysfunction assigned as liver failure, cardiac dysfunction assigned as sepsis-induced cardiomyopathy, hypotension, or hypoperfusion. The septic shock was stated as the subsection of the severe sepsis linked with elevated serum lactate level (> 2 mmol/L) arterial hypotension despite adequate fluid resuscitation. SIRS criteria were into consideration from 1991 to 2016. After this Sequential Organ Failure Assessment (SOFA) Score comes into consideration which was created by a group of scientists of the European Society of Intensive Care Medicine working on sepsis management and related problems [20].
2.1. Defining the sepsis
In the current scenario, “Sepsis-3”; in which sepsis is elaborated as a “life-endangering organ dysfunction caused by an uncontrolled or dysregulated host response to infection”, that results in a characteristic constellation of physiologic and biochemical abnormalities. The present definition describes the severity level and latent deadliness of a pathogen that initiates a biological progression in which the host immune system starts a defence mechanism that makes a lethal effect on the host's body. The level of organ dysfunction is represented by the SOFA score in terms of the degree of oxygenation, coagulation, liver function, blood pressure, level of consciousness and renal function. For clinical application quick SOFA (qSOFA) is considered in terms of respiratory status of >22, hemodynamics; systolic blood pressure (SBP) < 100 mm of Hg and any degree of altered mental state (see Table 1 ). Sepsis is likely to present if the patient has 2 or more qSOFA points. The critical care guidelines are pulled out for implementation because of the COVID-19 breakout [21].
Table 1.
Scoring based on SOFA and quick-SOFA and various physical parameters.
| Sequential Organ Failure Assessment (SOFA) Score | Mark | ||
| 1. | Oxygenation | PaO2/Fio2 > 400 PaO2/Fio2 = 301–400 PaO2/Fio2 < 300 PaO2/Fio2 = 101–200 with ventilation support PaO2/Fio2 < 100 with ventilation support |
0 1 2 3 4 |
| 2. | Coagulation | Platelets >150 k/mm3 Platelets 101-150 k/mm3 Platelets 51-100 k/mm3 Platelets 21-50 k/mm3 Platelets <20 k/mm3 |
0 1 2 3 4 |
| 3. | Blood pressure | MAP >70 MAP <70 ON dopa <5 μg/kg/min or any dobutamine On dopa >5 μg/kg/min, epi < 0.1 μg/kg/min Or NE < 0.1 μg/kg/min On dopa >15 μg/kg/min, epi >0.1 μg/kg/min, or NE >0.1 μg/kg/min |
0 1 2 3 4 |
| 4. | Liver function | Total bilirubin <1.2 mg/dL Total bilirubin 1.2–1.9 mg/dL Total bilirubin 2.0–5.9 mg/dL Total bilirubin 6–11.9 mg/dL Total bilirubin >12.0 mg/dL |
0 1 2 3 4 |
| 5. | Renal function | Cr < 1.2 mg/dL Cr 1.2–1.9 mg/dL Cr 2–3.4 mg/dL Cr 3.5–4.9 mg/dL or urine output <500 mL/d Cr > 5 mg/dL or urine output <200 mL/d |
0 1 2 3 4 |
| 6. | Level of consciousness | GCS 15 GCS 13–14 CGS 10–12 CGS 6–9 CGS < 6 |
0 1 2 3 4 |
| Quick SOFA (qSOFA) Score | |||
| 1. | Respiratory status | Respiratory rate > 22 | 1 |
| 2. | Hemodynamics | SBP < 100 mmHg | 1 |
| 3. | Mentation | Altered mentation (Any degree) | 1 |
As discussed above, sepsis has different severity levels and initiates by an invading pathogen. This results in an inappropriate response from host's defence system that results in multiple organ failures and ultimately death. Generally, cardiovascular, cellular, endothelial dysfunction and coagulation are septic apocalypse pronounced as the four horsemen. The complicated and multiple pathophysiological pathways of sepsis are responsible for hampering the diagnosis and treatment of all related septic complications [22]. It is clearly identified that a timely diagnosis is very important to take over the severity of sepsis and produce a favourable outcome that benefits the patient. The etiology of sepsis is significant for both diagnosis and delivery of medicaments [23].
2.2. Etiology of sepsis
The pathophysiology of sepsis is typically studied as a primary hyperinflammatory stage and a stage of immunosuppression. In the early stages, the hyperinflammatory stage of sepsis generates a cytokine storm. Primarily both adaptive and innate immune is activated that are mainly involved in the pathophysiological process. The pathogen attacks the host and initiates an inflammation response. Host immune cell-based expression was observed on receptors like pattern recognition receptors (PRP), TLRs (Toll-like receptors) and NODs (Nod-like receptors) both in the extracellular matrix and cytosol. TLRs play a vital role in the recognition of pathogens, detecting pathogen-assisted molecular patterns (PAMPS) and damage-assisted molecular patterns (DAMPS) through the injured endogenous cells [24]. The stimulation of DAMPS initiates the TLRs to generate an inflammatory response. Similarly, NODs also play a vital role in the detection of pathogens that penetrate cytosol and lead to the genesis of inflammasomes that aid in cytokine production to produce inflammation. This overall program generates an inflammatory scenario with the activation of leukocytes, coagulation and complement roadways that fortify the cellular, cardiovascular, and endothelial dysfunction that show characteristics of sepsis. Other parameters show imbalance in the general homeostatic mechanics of the neuroendocrine and immune system at the time of sepsis with variable cellular energetics mechanisms, dysregulated epithelial and endothelial features, and irreversible organ dysfunction [25].
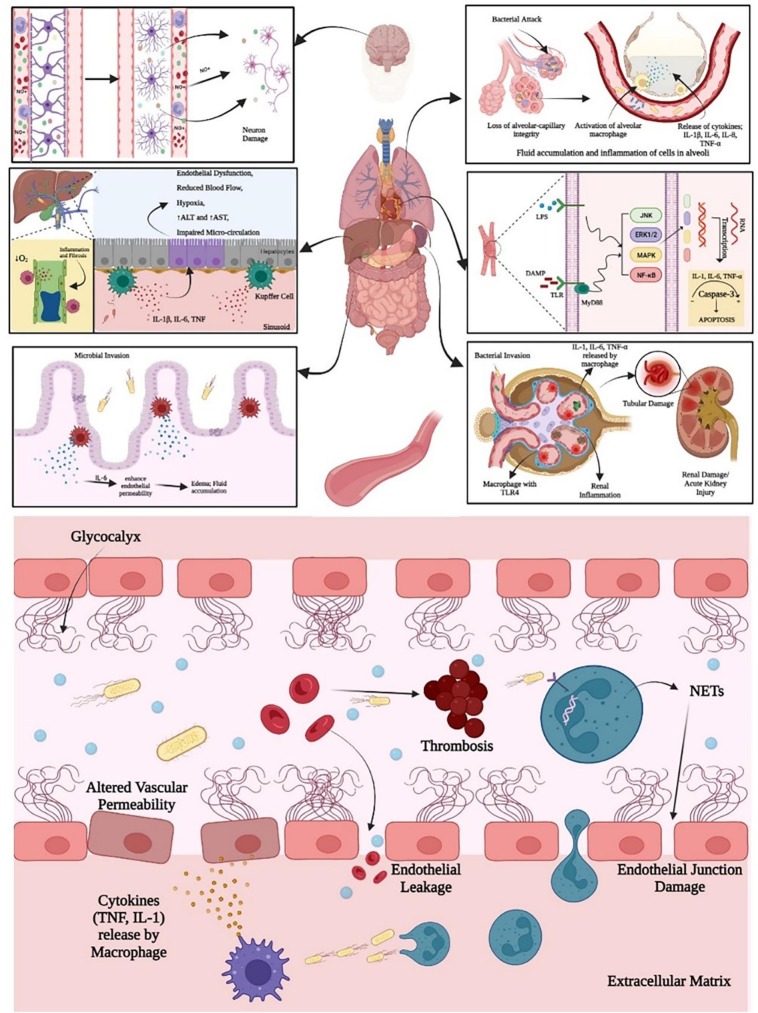
The extended immunosuppressive stage is a crucial, multifactorial procedure growing from defence cell exhaustion. This is because apoptotic scenario initiate the mechanism of immune suppression by sepsis. Knowingly if the chief players in the natural and innate inflammatory response are tangled, the patient is likely to enter the second phase of infection [26]. Post-mortem studies of sepsis patients confirmed the induction of apoptosis from an immune cell. Undeniably, sepsis quickly stimulates profound programmed cell death showing nonspecific immunological responses. This includes dendritic cells, macrophages/monocytes, γδ T-cells, NK cells and specific immunological responses e.g., T-cells and B-cells +CD4 (Fig. 1 ). Nevertheless, cell death of neutrophils is hindered and along with that Treg cells are majorly resisted from programmed cell death viz. apoptosis. Specifically, therapeutics targeting apoptosis via diverse pathways can enhance life expectancy in sepsis. Thus, it is important to know what parameters are expressed in patients, particularly for overcoming the primary and secondary infectious states [27].
Fig. 1.
Molecular pathways of sepsis in brain, lungs, heart, liver, intestine, kidney and blood.
The complicated pathophysiology of sepsis obstructs the effective diagnosis and prophylaxis of the disorder. Moreover, the current understanding of sepsis enables the exploration of novel therapeutics and diagnostic tools at different levels for a heterogeneous population. Biomarkers are the best tools that assist in stratifying patients into different heterogeneous sub-groups. On a contemporary note, it will be beneficial to examine whether the patient falls in the early hyperinflammatory stage or has reached the highly complicated immunosuppressive stage [28].
Both stages work collectively and are associated with an enhanced mortality rate. These observations have been made from the case studies of dead patients in which an early peak had shown a low intensity and another peak was observed after 3–4 months and lasted for 3 years. These observations have been made in sepsis cases of diabetic foot ulcer patients [29]. The highest death count in the initial duration is ascribed to an irresistible inflammatory response. This immune response generates a cytokine storm that causes hyperthermia, inadequate resuscitation, cardiac or pulmonary arrest, and refractory shock. This immunosuppression along with secondary infection, tissue, or organ failure reports mortality after some time. The timely diagnosis and improvised intensive care provides less mortality rate, but patients may still face death after a period due to continuous immunosuppression, chronic metabolism, and immune failure.
It is interesting to know about the pathophysiological scenarios that lead to cytokine storm and the factors that initiate the immunosuppressive phase are the ones which results in a high mortality rate. Therefore, due to the novel understandings, the immune response generated in sepsis is the chief area to focus on for better outcome [30].
2.3. Therapies available and under clinical trial for sepsis management
Sepsis is a dysregulated systemic inflammatory response syndrome towards an infection and the main cause of death in the ICUs. There are nanoformulations which are undergoing clinical trials for the treatment of infection, like amikacin loaded lipid nanocrystals, trade name MAT2501® by Matinas Biopharm. Amikacin is an aminoglycoside antibiotic that acts by killing the bacteria and prevent their growth and also helps to overcome the multi-drug resistance trouble [31].
MAT2501 is designed to provide the safe and targeted delivery of this potent drug and would avoid toxicity levels by delivering the drug directly to the cells infected with bacteria. Another investigative nanoformulation is Resatorvid emulsion under the trade name of TAK-242® manufactured by Takeda Global Research & Development Center, Inc. for the management of sepsis. Resatorvid is a therapeutic agent which acts through antagonising the TLR4 receptor selectively to offer the anti-inflammatory and neuroprotective effect in brain sepsis for use in the conditions of severe sepsis [32]. Commercially available Neulasta® is Pegylated filgratism and comprises of granulocyte colony-stimulating factor (G-CSF) which helps to increase production of white blood cells and recommended for use in neutropenic sepsis. Many other products like Spi-Argent®, Arikayce® etc. are compiled in Table 2 .
Table 2.
List of nanoformulations marketed and in clinical trials for management of infection and sepsis.
| Name | Manufacturer | Nanocomposite | Application | Clinical phase | Reference |
|---|---|---|---|---|---|
| TAK-242® | Takeda Global Research & Development Center, Inc. | Resatorvid emulsion | Sepsis | Phase IIa | [32] |
| Spi-Argent® | Spire Biomedical Corporation | Silver containing polymer | Antimicrobial Coating device | Phase IV | [33] |
| Neulasta® | Amgen Inc. | Filgrastim-bound polymeric NPs | Febrile neutropenia | Marketed | [34] |
| AmBiosome® | Gilead Sciences Ltd. | Liposomal Amphotericin B | Fungal infection | Marketed | [35] |
| PerOssal® | OSARTIS GmbH | Calcium sulfate and nanocystalline hydroxyapatite | Antibiotic delivery | Marketed | [36] |
| Arikayce® | Insmed Limited | Amikacin Liposomal nebuliser dispersion | Antibiotic delivery to lungs | Marketed | [37] |
2.4. Clinical challenges and failure of therapeutics
Currently, sepsis cure needs advanced approaches for management and treatment of the disorder. Regrettably, it looks nearly dreadful to choose a single model or a single drug as antisepsis. Controlling sepsis is a crucial process and requires initial resuscitation, ventilation assistance, sugar control, steroids, anti-coagulants, vasopressors, and anti-inflammatory agents. Rather than such designed approaches, other hurdles include regulation of hemodynamic areas and selection of appropriate fluid [38].
In the sepsis initiation phase or the starting phase, hypovolemia (condition in which the liquid portion of the blood plasma is too low) occurs because of vasodilation, and capillary leakage shoots up and requires a large amount of infusion fluids to maintain volume of distribution (VD) [39]. Consequently, this results in endothelial dysfunction and enhancement in the volume of distribution lowers therapeutic concentration of antimicrobial and prophylaxis in plasma. Further challenges that encumber efficacy are poor cellular and tissue targeting, short half-life (t1/2), poor aqueous solubility and bioavailability of diverse anti-microbial and anti-inflammatory representatives. Mediators such as peptides that show anti-inflammatory activities in in-vitro conditions failed to show similar activities in the in-vivo conditions because of the biotransformation due to cellular metabolic enzymes. Additionally, the intricate pathophysiology is inclusive of multiple pathways along with cytokines flush. This requires a multilevel approach because only one drug may not be that effective in severe conditions. The remnants of toxins produced by bacteria can be encountered by antitoxin drugs to neutralize any immune response [40].
As suggested fluid administration is the best way to control hemodynamic dysregulation but maintaining the administration balance is a herculean task. However, the bolus administration of fluid decreases arterial elasticity but might lead to major vasodilation and hyperdynamic condition [41]. On the other hand, excess fluid administration is linked with tissue and organ failure and ultimately death. Generally, vasopressors are administered for hemodynamic control, and proved to be effective and safe.
However, antimicrobial therapy is the major therapeutic for sepsis. But mismanaged therapy in the initial hours may lead to an eight times higher death rate in hospitals. On the opposite face, a 70% earlier inflammatory response is seen in improper pragmatic antibiotic prophylaxis, which was compared to proper prophylaxis as reported in past [42].
The administration of antimicrobials and supportive therapy is not so effective and impactful in reducing deaths due to septic conditions. The immune seize and multidrug-resistant bacteria influence patients suffering from secondary infections. Thus, to counteract this, redesign of unidirectional strategies that modulate functionalities of the immune system with optimized supportive care are required. So, the above strategies can prevent immune-seizing or weakening of inflammatory actions. Recent research explores treatment that ranges from extracorporeal blood cleaning tactics to diverse pharmaceutical and pharmacological methods that involves blocking the proinflammatory cytokines, antioxidant roles and immunomodulation. These current new sepsis studies and preclinical blueprints are safe and effective in preclinical studies [43].
2.5. Diagnostic techniques and challenges in sepsis
Sepsis is not localized microbial contamination with respect to the host immune system. In this, the immune system becomes dysregulated along with several nonspecific signs and symptoms as discussed in the above section. For instance, increased body temperature is a major body biological response whereas hyperthermia is reported in highly ill patients where no response to an infection. Additionally, due to invading pathogens, tachycardia, and leukopenia can be seen in critical patients. In many cases, sepsis might be undetected in subjects facing hypoxia and thrombocytopenia without any stage of infection or over-detection might be seen in the postoperative subjects who are on antimicrobial therapy. This observation provides that SIRS criteria were inapplicable and not taken much into consideration. Furthermore, the application of such standards is not recommended as the diagnostic tool to check the severity of sepsis and set the medical treatment [44].
Along with symptom-based detection, plasma tests, are a confirmatory and reliable method as a diagnostic tool. However, the plasma test encounters a major drawback as it consumes 24 h to 48 h assay time. Apart from this, nearly 35% of pre-assumed cases of septic subjects were identified to be negative in the culture test. This might be because of previously running antibiotic treatment or very low colony-forming units (CFU) below the detection level [45].
On a traditional basis, techniques based on molecular principles and serum analysis are the primary techniques used for the diagnosis of sepsis. A generally used way to detect the pathological infection in sepsis in the systemic circulation is through blood culture examination. Traditional detection tactics are basically grounded on some superior molecular technologies like polymerase chain reaction (PCR) etc. and the examination of the blood culture which is majorly laboratory oriented [46]. These methods are greatly handled by trained personnel as the method includes a lot of steps and resources with a certain specificity and confined limit of detection (LOD). Rather than that, there are various molecular technologies for instance polymerase chain reaction (PCR), microarray, hybridization approaches and isothermal amplification techniques. All such methods with different magnitude of sensitivities and specificities are utilized for the diagnosis of the pathogen which causes infection. Presently, mankind needs sepsis monitoring through the biological markers that can produce a better and more effective outcome in the detection of sepsis. But still, biomarkers that are specific for this crucial disease are not in the clinical application except in the reputed medical centres. However, there are 170+ biomarkers reported in the detection of sepsis. Of all of these, only a few of them can produce the desired results with various complications, few merits, and drawbacks [47].
Due to above stated diagnostic challenges, tracing via the rightly selected biomarkers provide early detection of sepsis and provides the early better treatment options for a patient in septic condition. Biomarkers detect the presence or absence of an infection and can be used as the confirmatory test for sepsis and to identify the severity of the illness. From the available biomarkers, acute-phase protein i.e., C-reactive protein (CRP) is studied widely [48,49]. It is highly sensitive and specific to the infection rate and inflammation. In another study, it is identified that procalcitonin (PCT) is a better, more accurate, and precise marker than CRP. PCT is secreted at the time of systemic inflammation triggered by a microbial infection. Nevertheless, the above-discussed biomarkers tend to rise in diseases like pancreatitis, traumas and burns which are non-inflammatory diseases. Additionally, powerful medical encounters are allied with the diverse sufferer groups and dissimilarities in the period of advancement of singular indicators. Therefore, a novel diagnostic and treatment approach favourable to the accessibility of on-bed diagnosis is urgently required. At first, the sophisticated validated method is important in the planning of personalized medical management protocol for positive favourable results. For example, hourly mismanagement in the initiation of antimicrobial prophylaxis enhances 8–11% mortality jeopardy [50].
The above literature provides a critical definition that the complication of single biomarker expression as the identifier for sepsis diagnosis is inappropriate specifically in patients suffering from critical sepsis. Incorporation of the combination or fusion of useful biomarkers for detection is an emerging approach. It shows a better application in this field than individual biomarkers. However, prior to clinical application, more studies are required to calibrate the fusion or combination of the biomarkers. This disorder is usually linked with a cascade of inflammatory (pro and anti) cytokines at different phases of sepsis [51].
3. Nanomedicine-based advancement in sepsis
Nanomedicine is seen as a powerful and attractive tool for targeted delivery in the treatment of sepsis as well as diagnosis. Nano delivery systems have the capability to tailor the kinetic profile of therapeutic agents and enhance the biological distribution to the desired organs. This effective targeting offers sufficient delivery to the injured or inflamed part, with minimum exposure to other peripheral organs and drug toxicity. A sophisticated nano-scale universe is used for medicinal persistence to pick the active ingredient to the target in a very controlled manner from the point of delivery. Many targets are capable of effective therapies for sepsis but are limited due to problems with targeted delivery.
Presently, solutions based on nanotechnology are under evaluation for the identification and severity of infection, and organ dysfunction and for offering answers to the detection of immune dysregulation. In the past few years, NPs have already been suggested as ways to diagnose and treat sepsis. Conventional dosage forms tend to go all over the body, cause unwanted side effects, and are quickly cleared by the kidneys, so they don't stay in the body for long. When it comes to circulation, NPs with the best surface, composition, and size has a longer half-life and a different biodistribution profile than the free drug [52]. NPs also help spread out drugs that don't dissolve well in water, which makes them more bioavailable. With a large ratio of surface area to volume, NPs can also have multiple copies of ligands on their surfaces to interact with a certain type of cell [53]. Also, active targeting of an antibiotic to bacteria in an infected tissue is another way to improve the therapeutic index of antibiotics. As vaccine adjuvants or delivery vehicles, they have been shown to cause more effective immune responses. Aside from this, many nanomaterials have stronger antimicrobial properties than their bulk form. This helps fight antibiotic resistance. Some antimicrobial nanotherapeutics have the potential to stop biofilms from forming and kill pathogens that live inside cells [54]. Prominently, nanoparticle surface chemistry is helpful in functionalizing more than one therapeutic agent to target specifically one kind of cell. The diversity of the organic and inorganic pharmaceutical carriers and polymers being used in the nano systemic delivery have revealed antimicrobial and anti-inflammatory activities that will be helpful in the treatment and the management of infection. Such an additive and synergistic approach in the treatment will be helpful to address the issues like antibiotic drug resistance and may assist in antibiotic potency and mechanical strategy. Many scientists are utilizing such a phenomenon for producing fusion of nanoformulations in which active ingredient profits can be enhanced by focusing on different physiological processes involved in the progression of sepsis. This kind of fused or hybrid formulation can be formulated by combining the properties like antimicrobial and anti-inflammatory of various compounds which will combinedly offer adjuvant, additive or synergistic effect [55]. There are a lot of benefits of hybrid nanoformulations like quicker and sensitive detection whereas photodynamic effects tempted by the nano-level universe can be utilized instantaneously for extracorporeal blood fumigation. The nanotechnology-based universe offers a stage for invention in the path like targeted and focused drug delivery, improvised drug actions, and minimization of the adverse drug reactions (ADR) for outstanding diagnosis and cure of sepsis.
Hereby some novel nanotherapeutic are planned and studied in past decades to effectively target sepsis (see Table 3 ) [56]. The below section highlights the nanotechnology-based approaches explored to date in various sepsis-induced organs [57].
Table 3.
Nanoparticles formulated for sepsis management and its preclinical outcomes.
| Organ | Nanotherapeutic system | Composition | In-vitro/In-vivo study | Inference | Reference |
|---|---|---|---|---|---|
| Brain | Micellar nanoparticles | CG3R6-TAT (Antimicrobial peptide) | In-vitro MICs study/In-vivo S. aureus-induced meningitis rabbit model | Nanosystem able to cross BBB and suppressed the growth of Staphylococcus aureus in the brain. | [58] |
| Nanoparticles | Amphotericin B, Polybutylcyanoacrylate, Tween-80 | Cryptococcal meningitis mouse model | Nanosystems offered great potential in drug delivery across BBB | [76] | |
| Lungs | Nanovesicles | 25-hydroxycholesterol and didodecyldimethlammonium bromide | In-vivo biodistribution in male ICR mice model | Nanosystems can accumulate in the lung tissues and significantly lower the NF-KB and SREBP2 pathways reducing the inflammatory cytokine levels | [59] |
| Bio-responsive nanoparticles | Ciprofloxacin and ((2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide) | Acute lung bacterial infection mouse model | Nanoparticle targeting to IMEs and local cues as triggers to deliver therapeutics in on-demand manners is demonstrated | [60] | |
| Intestine | Gold nanoparticles | 4,6-diamino-2-pyrimidinethiol and gold | Microflora distribution of Au in mice | Developed nanosystem showed great potential as alternatives to oral antibiotics | [61] |
| Polymeric nanoparticles | Ceria, triphenylphosphine and ROS-responsive organic polymer (mPEG-TK-PLGA) | Sepsis-induced AKI mice model | Nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin has favourable potentials in the sepsis-induced AKI therapy | [62] | |
| Lipidic nanoparticles | Cathepsin-B, antimicrobial peptide and vitamin C | Immune-compromised septic mice model | Nanosystem offered elimination of MDR bacteria | [63] | |
| Skin | Nanoemulsion | Alpha-tocopherol and chitosan oleate | Ex-vivo punch tests on human skin biopsies | Nanosystem enhanced cell production in fibroblast and keratinocytes cell medium and found to help treat topical skin infection | [64] |
| Nano-scaffolds | Silk fibroin, chitin and silver nanoparticles | In-vitro antibacterial and antifungal activity evaluation | Fabricated nano-scaffolds offered prominent activity against S. aureus, E. coli and C. albicans | [65] | |
| Kidney | Metallic nanoparticles | Iron oxide | In-vivo biodistribution in mice model | Nanoparticles found to be effective against urinary microbes like E. coli | [66] |
| Nitric oxide releasing nanoparticles | Chitosan and silane hydrogel | In-vivo mouse infection model | NO-np lead to a reduction in angiogenesis preventing bacterial dissemination from abscesses. NO-np may be useful therapeutics for microbial abscesses | [67] |
3.1. Nanotechnology as therapy against CNS sepsis
The blood–brain barrier (BBB) is the prime lining that prohibits the entry of most drugs into the brain. BBB block the admission of toxins, foreign agents, and xenobiotics in the brain due to the tight junctions in endothelial cells. BBB offers good protection, but this protection is observed as a hindrance to therapeutics intended for brain delivery. The intracellular or extracellular pathogens are closely correlated with the breakdown of the BBB barrier and majorly mark the CNS infection. Sepsis-induced in CNS is coined as sepsis-associated encephalopathy (SAE) or acute brain dysfunction or sepsis-associated delirium with marked cerebral dysfunction. SAE accounts for 17.7% of the total septic cases. SAE is reversible and life threatening that deteriorates the patient's mental status due to sepsis, antimicrobial therapy, sedation, neurological disorder and pre-existing psychological state [68]. Recent reports have claimed that these infections are produced due to the change in the cytoskeleton proteins. By prohibiting the interferon-γ signalling with antibodies, injuries in blood vessels and the endothelial cells might be reversed.
Most glial cells like astrocytes and microglia in CNS significantly contribute to inflammatory responses within the brain. In septic conditions, the shielding layers that cover the spinal cord and brain are inflamed because of infection in cerebrospinal fluid (CSF) [69]. Past studies illustrated that nucleotide-binding oligomerization domain-2 plays an important role in the glial cell-mediated CNS inflammation. Toll-like receptors (TLRs) arbitrate innate protection and initiate stimulation of pro-inflammatory agents and cause CNS neurotoxicity. CNS-induced sepsis spreads at a very fast pace and should be detected very quickly. Some novel nano techniques work on antigen detection or molecular amplification that works as modern diagnosis and timely prophylaxis of CNS sepsis.
Generally, studies report that multi-drug resistant pathogens exhibit CNS-related sepsis. Demerits with BBB is that it not only limits the foreign particle entry but also the same fate observed with targeted drug delivery. Recent studies recommended that nanoformulations like peptides, liposomes, nanostructured lipid carriers or nanoparticles are effective for the targeted delivery into the brain and have the potential to cross BBB [[70], [71]]. Lipidic vesicles encapsulate small peptides and therapeutics that are reported to diminish septic conditions in preclinical studies. Moreover, some of the key nanotherapeutics are discussed below which have the potential to cross BBB and benefit the patient in recover from CNS sepsis [72].
3.1.1. Antimicrobial peptides
Antimicrobial peptides (AMPs) are potent in inhibiting microorganism growth using the host system's innate immunity and offer magnificent antimicrobial activity. Hence, AMPs offer a potent nanotool and an excellent alternative to antibiotics. Some peptides which are explored for brain-targeted drug delivery include nanofibers of chitosan-layered self-assembled peptide palmitoyl-GGGAAAKRK used for gene silencing in the brain administered via oral route or nasal route to minimize their liver localization [58]. Micellar form of CG3R6-TAT peptide in the form of nanodots has been reported to cure sepsis-induced meningitis in CNS [73].
In another research, an emission probe (HBT, 2-(2-hydroxyphenyl)-benzothiazole) was synthesized via an aggregation process and conjugated with peptides (HHC36 with KRWWKWWRR sequence). This identified peptide selectively destroys the cell lining of both Gram (+) and Gram (−) bacteria by the fluorescence rays-up procedure. Poly-prodrug antimicrobials (triclosan and methacrylate) have been synthesized and show great healing efficiency against methicillin-resistant Staphylococcus aureus [74].
3.1.2. Polymeric and inorganic nanoparticles
Nanoparticles i.e., inorganic, and organic act as carriers for targeting the active pharmaceutical ingredient (API) to overcome the blood–brain barrier and as an antimicrobial against MDR (multi-drug resistant) induced infections from last decades. Modified nanocarriers formed with active ligand (proteins, peptides, and antibodies) conjugated on the surface to target receptors that aid in the blood–brain barrier. More accurate and enhanced targeted delivery can be achieved using this active targeting that offers specificity and selectivity. Ceria and ceria-zirconia (inorganic nanoparticles) shows neuronal protection in the sepsis infection [75]. Amphotericin B, an antifungal drug combined with a delivery carrier like polybutylcyanoacrylate NPs coated with tween 80 enables better brain delivery and bypass the blood–brain barrier (BBB) [76].
From the diagnostic point of view, nanoparticle-based assay, SERS (surface enhanced Raman scattering), electrochemical sensors and many more can be used for detection of the sepsis pathogens. Silver nanoparticles (AgNPs) and magnetic beads are employed with DNA-based SERS assay for the concurrent diagnosis of the most general pathogens which are responsible for sepsis. By this method, quantitative analysis for each pathogen can be done in the multiplex form i.e., in the picomolar strength detection with accuracy [77].
3.2. Nanotechnology against lung sepsis
ICU patients are more susceptible to lung sepsis due to ventilator-induced pulmonary injury, intrapulmonary ischemia, and blunt thoracic injury. As the pathogen load increases the inflammation response increases due to increased vascular permeation, endothelial activation generates proinflammatory mediators, neutrophils activation as well as accumulation and modulation of immune response by lung. This leads to exaggeration of the condition that causes respiratory distress syndrome due to compromised respiration and multiple organ failure. In chronic lung diseases (CLDs) or pulmonary disorders mainly inhalation therapy based on metered-dose inhalers is employed. CLDs include diseases like bronchiectasis, lung abscess, empyema. CLDs account for 27.4% of total sepsis cases. As the progression in science and technology paves the way, nanomedicines are considered during the last decade with scalability and prominent design of drug targeting to lungs. The formulation scientist has explored both localized and systemic delivery to the respiratory system of proteins, peptides, small molecules, or siRNA gene targeting. Major reports are based on nanoengineered mediated local delivery of therapeutics for CLDs like sepsis and COPD. Currently, an aerosol-based delivery system is convenient and widely accepted for the pulmonary cure. It covers the large surface area of the lungs and greatly vascularised fine epithelial lining which covers the airways. This helps in quick absorption of the drug and a better degree of retention in the respiratory site [78]. Furthermore, bioavailability is maximum through the airway route because of minimal enzymatic activity in comparison with another administration route. Moreover, this nanotechnological way of treatment improvises patient compliance [79]. Amikacin liposome inhalation suspension (ALIS; Arikayce®), a liposomal formulation of the aminoglycoside antibacterial drug amikacin administered via inhalation following nebulization, is designed to facilitate targeted and localized drug delivery to the lungs while minimizing systemic exposure [80].
Apart from this nanomedicine mediated therapy cures lung sepsis due to breakthrough modulation in physicochemical properties that widely affect the pharmacokinetic and pharmacodynamic characteristics. These pharmaceutical engineered conditions allow nanotherapeutic to adhere to the targeted site for a long duration and treat the septic infection. Furthermore, with a predesigned absorption profile of the drug, nominal side effects, phenomenal retention time, and better bio-distribution. Presently, a wide variety of nano-carriers are there in the pharmaceutical world such as liposomal nano-carriers, polymeric nanoparticles, solid lipid nanoparticles which are being explored for local lung delivery.
Even though nano-carrier-based targeted drug delivery has been optimized. The major challenge in nano-drug delivery is the rapid uptake of the nanomaterials in the mononuclear phagocytic system. The optimization of this concept can control the local as well as systemic toxicity that leads to toxicity in the extra-pulmonary organs. Various scientists have reported that when nanoparticles are administered into the lungs through inhalation delivery, some portion of nanotherapeutics is moved to extra-pulmonary organs like heart, blood, liver, brain etc. Translocation of nanoparticles portion to the extra-pulmonary organ is through systemic route and organ distribution after crossing the blood/air barrier, this makes them plausible therapeutic carriers for other deadly diseases such as diabetes. This approach can be harnessed as the combinatorial approach in pharmaceutical science.
Recently, Kim et al. fabricated lung-selective nanotherapeutics that supress cytokine storm due to any viral and bacterial infection. They reported that 25-hydroxycholesterol and didodecyldimethlammonium bromide (DDAB) nanovesicles are fantastic candidatures for restoring intracellular cholesterol levels and cytokine storm. They demonstrated that 25HC@DDAB are able to accumulate in the lung tissues and significantly lower down the NF-KB and SREBP2 pathways reducing the inflammatory cytokine levels, even in cases of COVID-19 [59]. Moreover, Zhang et al. strategized a report on targeting the infectious micro-environments (IMEs) through bioresponsive nanoparticles. The nanoparticles are composed of pH sensitive copolymers able to self-assemble into a micelle loaded with ciprofloxacin and ((2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide) as prominent antibiotic and anti-inflammatory. Fabricated nanotherapeutic was demonstrated by an acute lung bacterial infection mouse model [60].
3.3. Nanotherapeutics against intestinal sepsis
Intestinal infections account for 10.8% of total sepsis. Several hypotheses have been postulated related to the role of gastrointestinal tract in sepsis pathophysiology. The gut infection induces intestinal inflammation and initiates hyperpermeation that translocates bacteria from the lumen to the intestine and drives the inflammatory mediated process. The above brief evidence is clearly proved in the clinical setup in patients with the leaky gut barrier.
To treat intestinal sepsis effectively different nanosystems have been studied for the capability to deliver the therapeutic agent to the targeted site with good efficiency. In the intestinal region, major infections are bacterial and resistant to the drug due to sufficient barriers in the way of drug action. Another hurdle in sepsis infection is the reoccurrence of the infection after suitable treatment. So, the challenge is to develop a kind of nano system that can deliver the effective amount of the therapeutic agent to the desired site and overcome the drug resistance and inhibits the reoccurrence protocol [81]. Till now, various nanosystems have been developed as potential antiseptic therapeutics. The unique characteristics of these nanosystems are small size, better functionalization, better pharmacokinetic profile etc. Different nanosystems developed for the management of intestinal sepsis are discussed below.
3.3.1. Metallic nanoparticles
Recently, metallic nanoparticles are explored for various medical applications. Metals such as gold, silver, titanium, zinc, iron are considered best due to better biocompatibility and antimicrobial activity. Metallic nanoparticles (MNPs) can be prepared by various chemical, photochemical, and electrochemical techniques. Majorly, gold and silver are widely known for their antimicrobial effects. In a study, it was found that gold nanoparticles are efficient to restore gut-intestinal flora. The antimicrobial effect of gold nanoparticles against E. coli was very well reported along with enhancement in M2 macrophages with interleukin-10 (IL-10) and reduced levels of inflammatory cytokines such as TNF-α, IL-6. IL-6β in intestinal in vivo sepsis model [61]. Gold nanoparticles boost the relative occurrence of probiotics and offer protection to the intestinal environment. Additionally, silver nanoparticles were found effective against both Gram-negative and Gram-positive bacterial contagion.
Another kind of metallic NPs is superparamagnetic iron oxide nanoparticles (SPIONPs) offer a small size of <20 nm. These can be used for theragnostic purposes using magnetic resonance imaging and for therapeutics [66]. Apart from this SPIONPs act as biosensors and cell labelling. SPIONPs become the center of attraction as nanomedicine in past years as they act on IL-10 in macrophages resulting in the inhibition of inflammation in intestinal sepsis induced by lipopolysaccharides [82].
3.3.2. Polymer-based nanoparticles
As discussed earlier, polymeric nanoparticles are highly biocompatible, biodegradable, target-specific and encapsulate the drug molecule. PEGylation is a technique used to enhance circulation time by preventing non-specific protein absorption, opsonization and subsequent clearance. In past, polymeric nanoparticles were grafted with ROS-responsive linking mitochondrial targeting of atorvastatin for acute kidney injury (AKI) developed due to sepsis. mPEG-TK-PLGA (PTP) magnificently improvises the biological compatibility, dispersity and enhancing the half-life in blood circulation. Additionally, NPs will be able to hold and release API in sustained manner. NPs of Atv/PTP-TCeria accumulate in kidneys and are able to ROS-dependent release drug. NPs of TCeria target mitochondria and eliminate excess of ROS. The ROS-based nano-drug delivery system combines atorvastatin and nanoparticles of Ceria targeting mitochondria that favours potential therapy in the sepsis-induced AKI therapy [62].
3.3.3. Lipidic nanoparticles
Lipidic NPs and vesicles include liposomes (LS), solid-lipid NPs (SLNs) and nanoemulsions (NEs) which are majorly utilized to deliver anti-microbial in sepsis. Advantages of lipid NPs are effortless endocytosis and effective targeting. Hou et al. formulated lipidic nanoparticles with an aim to target cathepsin-B (CatB) and an antimicrobial peptide (AMP) to macrophages for the management of lethal sepsis. AMP-CatB mRNA-loaded vitamin C lipid nanoparticles were delivered to macrophages to overcome multidrug-resistant bacterial sepsis in mice [63].
3.4. Nanotechnology-based approaches for skin related sepsis
As per clinical reports of skin sepsis, it accounts for <10% of total sepsis in patients with bacteraemia, diabetes mellitus, endocarditis, or necrotic infections. However, data available on skin sepsis is not precise. But the risk for invasive infections increases when pathogens are embedded into the subcutaneous tissue. For overcoming this infection-related sepsis, antibiotic therapy is employed. But these therapies tend to fail due to resistance and are incompetent to target the infection in deeper layers due to compromised physicochemical properties.
To overcome these hurdles, various novel drug delivery protocols have been designed which allow the administration of the antibiotic to the targeted site in a very short amount but in an effective manner. Various researchers have developed nanotechnological therapies inclusive of immune-based antimicrobial molecules or photothermal in metallic nanoparticles, polymeric nanoparticles, nano-colloidal carriers for effective delivery to the skin. The ideal properties of nanocarriers are biodegradable, non-immunogenic, non-toxic, offering appreciable drug release, delivered to the targeted site only [83]. There are various carriers for effective delivery of antimicrobial agents to treat skin sepsis which are discussed below.
3.4.1. Nanoemulsions
These are the heterogeneous universe of two immiscible fluids which is controlled and stabilized by appropriate surfactant. Nanoemulsion size usually varies in the range of 15–400 nm. Furthermore, such systems are isotropic and transparent dispersion in nature which can be divided mainly into three classes i.e., water in oil (w/o) system, oil in water (o/w) system, and multiple or bicontinuous emulsion system. Nanoemulsions have a lot of merits like non-toxic nature, non-irritant, and merged into different pharmaceutical systems (i.e., liquids, sprays, creams, foams), high stability profile, and solubilizes both lipophilic and hydrophilic drugs.
Bonferoni et al. prepared α-tocopherol loaded nanoemulsion constituted of chitosan oleate. The authors reported that chitosan oleate and α-tocopherol enhance cell production in fibroblast and keratinocytes cell mediums. This study recommends that a combination of chitosan oleate and α-tocopherol in a nano system is helpful in treating topical skin sepsis [64].
Song et al. prepared an antibacterial film of a chlorhexidine acetate nanoemulsion in order to treat MRSA (methicillin-resistant Staphylococcus aureus) infection. In the outcomes, it is seen that chlorhexidine nanoemulsion-loaded film offers greater and quicker action against MRSA when compared to the aqueous solution of the API. Additionally, chlorhexidine nanoemulsion destroy the cellular walls and membranes of MRSA [84].
Razdan et al. developed nanoscale emulsion loaded with antibiotic levofloxacin (LFX) and antibiofilm agent clove oil for biofilm wound infections. LFX-NE inhibited quorum sensing activity responsible for biofilm formation and was able to eradicate pre-formed biofilms of P. aeruginosa as demonstrated by laser scanning microscopy and field emission scanning electron microscopy. They revealed high antibacterial activity with 16-fold and 8-fold reduction in minimum inhibitory concentration against P. aeruginosa and Escherichia coli and Klebsiella pneumoniae, respectively [85].
3.4.2. Metallic nanoparticles
Metallic nanoparticles are extensively explored for diagnosis purposes or medical imagining, biosensors, antimicrobial coating, and targeted drug delivery. As discussed, earlier silver and gold nanoparticles are studied for the therapeutic perspective as they possess antimicrobial properties and can recognize the targeted site more efficiently. The antibacterial ability of the silver nanoparticles destroys the plasma membrane of a bacterial cell resulting in the lysis of the pathogen. Such nanoparticles can also conjugate with DNA to stop its replication process or the ribosomes to stop protein production.
Mehrabani et al. developed nano scaffolds of silk fibroin, chitin, and silver nanoparticles using freeze-drying. The prepared nano scaffolds exhibit prominent activity against Escherichia coli, Staphylococcus aureus, Candida albicans and show excellent biological compatibility, biodegradability with blood clotting aptitude, water-uptake ability and magnificent antimicrobial activity when compared with silver nanoparticles alone [65].
3.4.3. Polymeric nanoparticles
Polymeric nanoparticles are formulated by natural, synthetic, and semi-synthetic polymers within a size range of 10–1000 nm. API can be incorporated depending upon the type of polymer used based on the principle of dissolution and dispersion. The benefit of polymeric nanoparticles is the permeation ability respective to their chemical nature, size, and viscosity. However, natural polymers offer biocompatibility, site targeting and control release properties but are also associated with some limitations like batch manufacturing variability and can be immunogenic whereas synthetic polymers are easy to manufacture and easy to predict their properties.
Nguyen et al. formulated polymeric nanoparticles composed of fibroblast growth factor-2 in a combination of carboxymethyl chitosan and calcium chloride (1:0.8) with 95% encapsulation efficiency. The prepared formulation was incubated at pH 5.8 and 7.4 for 2 days which shows 36.36 and 58.47% release of fibroblast growth factor-2. Observation revealed that in vitro deprivation of fibroblast growth factor-2 due to trypsin enzyme can be prevented in terms of protein-loaded carboxymethyl chitosan-loaded nanoparticles [86]. In another study, El-Feky et al. formulate chitosan-coated silver sulfadiazine nanoparticles. The formulated particles offer controlled release of the silver sulfadiazine to stop the growth of the Gram-positive and Gram-negative bacteria to cure skin sepsis [87].
3.4.4. Nanogels
These systems are composed of hydrogels of particles in the nanometre range. These are the 3D crossed-linked polymer net with the ability to uptake a considerable quantity of biological liquids or fluids. Drug loading is a natural process in the formulation of nanogels and it does not require organic solvents or high-energy methods. These systems are biologically compatible and avoid the deprivation of the API due to enzymes. Silver sulfadiazine incorporated nanogels of chitosan and alginate was developed by El-Feky et al. The formulated system reported a good burst release of the API in phosphate buffer of pH 7.4 while performing in vitro studies tailed by a long-term release for more than a duration of 3 h. Nanogel offers great therapeutic efficacy in contrast to conventional marketed products of respective API.
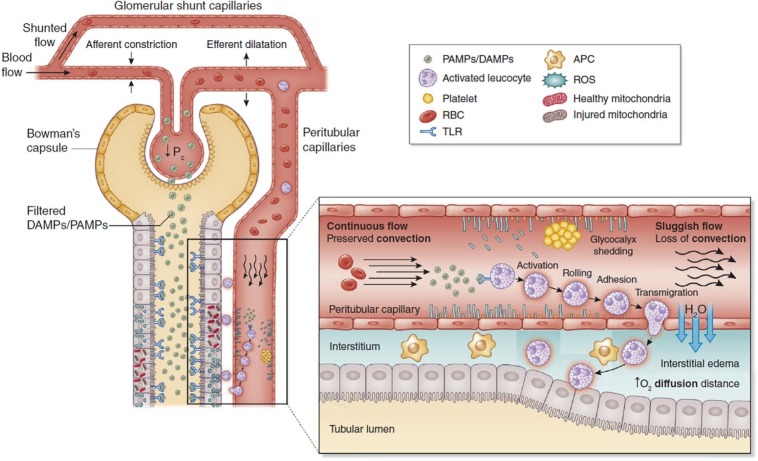
3.5. Nanotherapeutics against urinary or renal sepsis
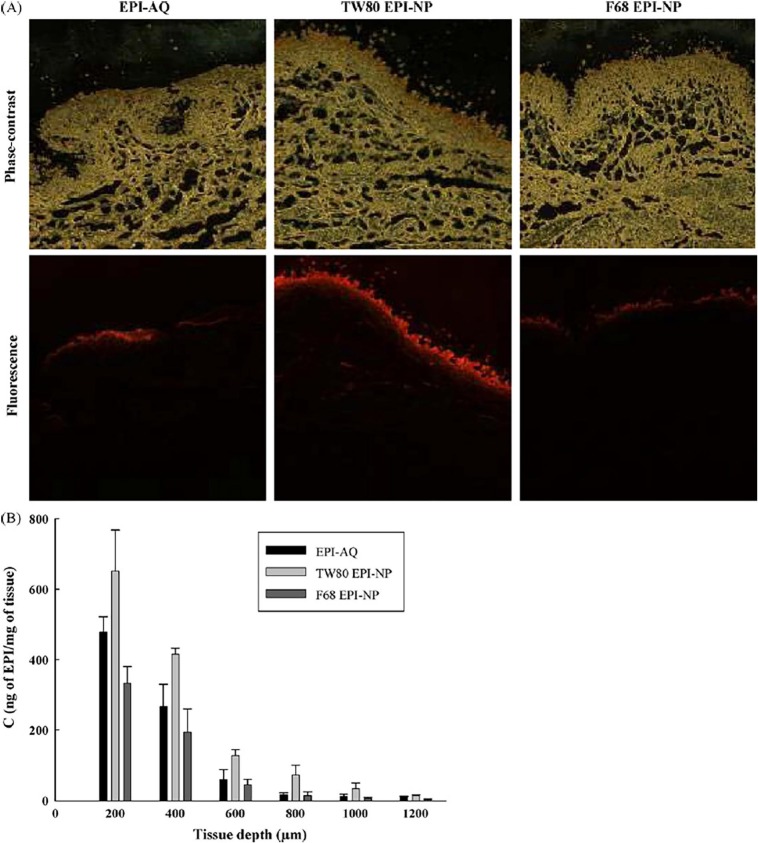
Urinary sepsis cases account for 27.6% of the total sepsis cases. In the context of urinary or renal sepsis, pathogens are present in the urinary bladder and build dormant intracellular reservoirs in the epithelial cell layer of the bladder that may cause reinfection (see Fig. 2 ) [88]. So, to overcome this, therapeutic must be targeted very specifically to the bladder. However, traditional therapies are not so target-specific. On the other hand, nanomedicine has the ability to target specifically and also offer controlled release. Furthermore, the release rate and drug loading are dependent on the chemical nature of the nanoparticles. Chang and co-workers compared epirubicin as a free drug and epirubicin-loaded poly(ethyl-2-cyanoacrylate) (PECA) nanoparticles. These nanoparticles exhibit a high penetration ability, greater specificity, and no toxicity to the urothelial tissues. It can be concluded that drug loaded PECA nanoparticles may be effective in curing renal sepsis (see Fig. 3 ) [89].
Fig. 2.
Inflammatory and microcirculatory alterations in acute kidney sepsis. PAMPs: Pathogen-associated molecular patterns; DAMPs: Damage-associated molecular patterns; TLRs: Toll-like receptors; TECs: Tubular epithelial cells; APC: Antigen presenting cell; ROS: reactive oxygen species; RBC: Red blood cells Reproduced with permission from Ref. [88]. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Representative figure of epirubicin permeation using A) fluorescent and phase contrast of pig bladder after 2 h permeation of epirubicin loaded solution, epirubicin loaded tween 80 nanoparticles and pluronic F68 nanoparticles; B) Epirubicin amount permeated in tissue. Reproduced with permission from Ref. [89].
Nanomaterials that possess antimicrobial characteristics or increase the effectiveness of the loaded antimicrobials are called nano-antibiotics. Nano-antibiotics kill the microbes by various mechanisms like (1) by producing reactive oxygen species that destroy the bacterial cell components, (2) by interfering in the energy transduction mechanism, (3) by destroying the cell wall of an infectious agent, (4) by blocking enzymatic activity and DNA synthesis [90]. The best advantage of using nanomedicine in the treatment of urinary sepsis is that it could target intracellular reservoirs to avoid the rehappening of infection in the renal tract. There is a diverse range of nanoparticles that are employed for the cure of renal sepsis and some of them are discussed hereon.
3.5.1. Nitric oxide-releasing nanoparticles
Like reactive oxygen species, reactive nitrogen species, and nitric oxide show similar antimicrobial action. However, nanoparticles that can deliver nitric oxide are still not found. There is certain hypothesis presently available on the formulation and effectiveness of the nanoparticles that could be efficient as carriers for nitric oxide. Silica nanoparticles were formulated that have the ability to release nitric oxide which degrades the biological cell layer of Gram-positive and Gram-negative microbes like S. aureus, E. coli, P. aeruginosa, and S. epidermidis. These nanoparticles are found to be non-toxic for mammalian tissues. Silane hydrogen nanoparticles were studied to be efficient bactericidal material against MRSA and outstandingly helpful in the treatment of renal sepsis [67].
3.5.2. Metallic nanoparticles
Huang et al. reported bactericidal efficiency and cell internalization effect of zinc oxide (ZnO) nanoparticles for S. aureus and enhanced membrane permeability. The major benefit of using zinc nanoparticles is the manufacturing cost and it blocks ultraviolet rays. The mechanism of the action of ZnO-NPs is the lysis of proteins and lipids of the cellular membrane layer of bacteria [91].
Gold nanoparticles conjugated with the antimicrobial drug can be utilized in the local administration to target microbes. A study revealed that gold nanoparticles (AuNPs) linked with vancomycin kill vancomycin-resistant E. faecalis and E. faecium, which are responsible for urinary infection. Gold conjugated NPs offer 50 times increased activity in the performance of vancomycin. On the other hand, AuNPs in double amounts can be used to produce a bactericidal effect against E. coli which is involved in causing renal sepsis. Saha et al. developed the conjugated combination of AuNPs with antibiotics, streptomycin, kanamycin and ampicillin respectively to enhance bactericidal action and stability [92].
Staphylococcus aureus in septic conditions was targeted through monoclonal antibodies (MAbs) in contradiction with a particular surface protein. AuNPs unification with secondary antibodies was selected to link to primary antibodies. AuNPs were seen bounded superficially on bacterial nanoclusters when analysed by transmission electron microscope (TEM). Later, when laser energy was introduced to bacterial cells showed that superficially attached nanoparticles penetrate the cell wall. This gave a justification that the laser energy produces a thermal effect locally in the surrounding nanoparticles, called photothermolysis. This high thermal property of AuNPs produces physical destruction of the microbe which is confirmed by TEM studies [93]. Ferrosoferric oxide (Fe3O4) nanoparticles were crafted in the shape of nanoeggs and coated with gold superficially and vancomycin was encapsulated in it. These nanoparticles were effective against urinary microbes such as E. coli, S. saprophyticus, and MRSA [94].
Nanoparticles formulated of titanium dioxide (TiO2) can be used in the degradation of microbial cells. Photocatalytic character titanium dioxide nanoparticles (TNPs) are responsible for the killing of infectious agents. When TNPs are exposed to ultraviolet rays show better bactericidal properties, verified against E. coli and P. aeruginosa. Kuhn et al. studied the potency of these nanoparticles against several urinary infection-causing microbes and found that the highest activity was found in E. coli followed by E. faecium, P. aeruginosa and S. aureus respectively and was related to their membrane thickness [95].
Nanosized silver particles show better efficacy against S. aureus and E. coli when compared with silver particles. This approach of nanotechnology can be helpful in the management of renal sepsis. However, longer exposure to soluble silver particles in the renal area may cause some side effects too like acute kidney injury (AKI) and intestinal injury whereas silver nanoparticles (AgNPs) were found to be non-toxic and safer in use. Furthermore, some past reports stated that highly concentrated delivery of the AgNPs cause a bad effect on the mitochondria and reduces their activity. Therefore, more investigation should be conducted on the concentration of silver nanoparticles used in clinical practice [96].
3.5.3. Chitosan nanoparticles
Previously, it was thought that chitosan-based nanoparticles were only effective against fungi and viruses, but further studies evaluated that it is also effective against bacteria. High molecular weight chitosan (MWC) was found effective in Gram-positive bacteria. While low MWC was found effectual towards Gram-negative bacteria. Conjugation of chitosan with sulfamethoxazole offers a great bactericidal response against P. aeruginosa. There is a variety of mechanism proposed on which chitosan work for destruction of microbes. Chitosan nanoparticles bind to the bacterial cell membrane which is negatively charged and enhance the permeability of the membrane which causes leakage in the cellular membrane. This allows the cellular component to outflow and ultimately causes bacterial cell death. Another mechanism proposed for chitosan is that it prevents bacterial development through chelating metal ions that are essential for bacteria for their enzymatic activity. Water-soluble chitosan offers greater antibacterial action in the treatment of renal sepsis. Oleoyl-chitosan nanoparticles were found effective in inhibiting the growth of S. aureus and E. coli by destroying the cellular membrane of bacteria that will benefit the patient suffering from urinary sepsis [97].
4. Next generation-based alternatives for sepsis treatment
The above sections highlight the nanomedicine-mediated systems studied so far for different type of sepsis. Currently, conventional drug delivery systems have failed to manage sepsis. However, nano-drug delivery systems are limited to preclinical studies for the diagnosis and management of sepsis. For a futuristic approach new system should be developed for better management as current systems have a lot of drawbacks like challenges of drug resistance, toxicity, less specificity, less targeting, etc. For effective targeting of sepsis in a particular organ, future approaches like combinational or combinatorial therapy in the nano range, nano-derived sensors and anti-microbial peptides with surface modification could be a next-generation therapeutic approach.
4.1. Nano combinatorial-based therapeutics
The complex pathophysiology of sepsis with various signs and symptoms like microbial infection and inflammation in the infected part results in organ dysfunction or even worse. So, it becomes necessary to overcome these issues for the effective management of sepsis. At this point, single drug delivery is not worth it because it will manage some symptoms and a higher dose will cause toxicity. Many researchers have explored dual targeting approaches. Handa and co-workers developed combinatorial treatment by co-administrating moxifloxacin (MOX) and rutin loaded in nano-carriers of polycaprolactone (PCL). PCL NPs containing MOX as an antibiotic is used to kill bacteria and rutin is an antioxidant that downregulates the production of cytokines and decreases inflammation. The lyophilized formulation was subjected to in vitro evaluation in the J774 cell line. Results from the in-vitro cell line experiment confirmed the phagocytic action of developed PCL NPs and concluded that MOX and rutin are safe in use and can be used as a combinatorial therapy [98]. In another study, Hou et al. formulated AMPs with cathepsin B in the lysosomes incorporated in vitamin C nanoparticles for adoptive translocation in macrophages. In their experiment, AMP was used to kill bacteria whereas cathepsin B was used as a component to transport the AMPs into lysosomes. The whole nano-combinatorial system was designed to overcome the MDR bacteria tempted sepsis. Obtained results via in-vivo studies stated that adoptive transfer of AMP (antimicrobial peptide) conjugated with cathepsin B (CatB) in the lysosomes leads to removal of MDR bacteria resulting in complete recovery of septic mice [99].
4.2. Nano-derived sensors
Nano-derived sensors such as electrochemical sensors and immuno-sensors can be helpful as diagnostic tool for sepsis detection at different severity levels. Nano-sensors can be abbreviated as biosensors as they are biocompatible. The biosensor is composed of an analyte, bio-identifier, signal transducer and display panel. They can measure minuscule signals from the body fluid, for example, whole blood, serum, plasma, tissue fluid or others within small aliquots. Nano-derived sensors are a novel approach to diagnosis with more accurate sensitive detection. A brief discussion is made on next-generation nanotechnology in the development of nano-derived sensors as follows.
4.2.1. Immunosensors
These are the analytical system that use antibody-antigen reactions and generate sensitive and selective data for the identification of immunological-based reagents that may apply to sepsis biological markers. Immunosensors in the detection of disease are a fine and attractive option because of their low dissociation constant, simple fabrication, reproducibility, low cost, high affinity towards antibodies and reliability. This next-generation technology is promising in the identification of biomarkers through immunological analysis with improvised sensitivity of the electrochemical signals. For a thought, nanobodies or single-domain antigen-binding strains provide benefits of better solubility, smaller size, stability, antigen specificity and monomeric behaviour for manufacturing nano-derived sensors as the next generation of innovative therapeutics for the management of sepsis [100].
Li et al. discovered specific and sensitive PCT immunosensing through linking appropriate nanobodies which are recognized and extracted from a camel along with beneficial silica-covered Cadmium telluride quantum dots nanoparticles (CdTe QD NPs) to amplify signals associated with nanoparticles to produce an extremely sensitive diagnostic method. This sandwich immunoassay yields better signal strength about four times with nanobodies. The capturing component is chitosan-graphene nanocomposite adapted on glassy carbon electrodes. The nanobodies-II as a diagnosis component coupled with silica-coated CdTe QD NPs for PCT identification. The above-said studies were well verified from clinical and preclinical studies [101].
4.2.2. Electrochemical sensors
These are the portable type of nano-derived sensors consisting of a molecule identification system and a transducer which converts electrochemical signals to analytical data. These are more frequently used than other biosensors because they are sensitive, selective in data generation, and provide reproducibility. It is already reported that C-reactive protein (CRP) is a general biomarker of sepsis which is released against the infection response or cytokine storm, specifically IL-6 at the time of inflammation. Still in biomedical and pharmaceutical science role of CRP is not known but it might conjugate with microbial components and leads to removal by macrophages. In a non-septic individual, CRP content is 10 mg/L but a considerable rise is seen within 4–5 h after cell damage which leads to several hundred times within 24–48 h. Thus, CRP is a prominent tool to determine the rate of infection or injury. For the very first time in 2012, Ibupoto et al. developed nanotubes of zinc oxide (ZnO) combined with monoclonal anti-C-reactive protein clone CRP-8 by a basic physical adsorption mechanism utilized to diagnose CRP. The isoelectric point of ZnO (9.5) played an important role as biomolecule like CRP with low isoelectric point bind ZnO superficially and along with that ZnO possesses distinctive characteristics like high polarity and good resistance to dissolution. The use of ZnO nanosystem in electrochemical nano-sensor is facilitated by isoelectric point, potential rigid linking with immobilized antibody and piezoelectric character assists to produce the current in the CRP tempted charged surroundings. This facilitates sensors evident to be additionally sensitive, quick, selective, and reproducible compared to bulk ZnO tools. Nano-derived sensors based on antibody immobilized ZnO nanotubes offer a straight range of diagnosis from 1.0 × 105 to 1.0 × 100 mg/L [102].
4.3. Nano-formulations modification with antimicrobial peptides
Antimicrobial peptides (AMPs) as host defence peptides are a subset of small peptides which are part of the innate immune system that offers an inhibitory effect against viruses, fungi, bacteria and parasites. AMPs nanoformulations are novel systems that overcome multidrug-resistant microbial infection. They offer extremely quick killing activity of microbes. In comparison with traditional therapeutics, AMPs work by different mechanisms to kill bacteria. The multimodal killing mechanism diminishes the chances of the development of resistance [103].
Furthermore, AMPs can increase the permeation ability of conjugated antibiotics resulting in a high accumulation of antibiotics in cells. Fan et al. formulated liposomes conjugated with AMPs; levofloxacin (LEV); S-thanatin (Ts), an antibiotic agent. To improve the stability of the formulation and to avoid self-contact, cholesterol was added along with aminopropyl-polyethyleneglycol-(2000)-carbamyldistearoyl phosphatidylethanolamine (NHS-PEG2000-DSPE) and hydrogenated soybean phosphatidylcholine (HSPC) to soothe liposomes and facilitate fusion of liposomes with bacterial membrane. PEGylation technique helps in the long systemic circulation. The prepared liposomes were then loaded with LEV through the (NH4)2SO4 gradient technique. The amalgamation of LEV and Ts expresses synergism in the treatment of sepsis with considerable bacterial clearance when studied in a preclinical sepsis-induced mouse model in comparison with normal LEV liposomes. MICs were found to be twice to 16 folds lesser with Ts-LEV liposomes than free API in experimented 17 clinical studies of Klebsiella pneumoniae. The mechanism was planned in the report as (1) enhanced drug uptake through liposomes than free drug formulation, (2) specifically targeted delivery through antimicrobial peptide, (3) due to structural integrity loss to microbial cell membrane results in more drug uptake, and (4) increased API entry due to lipophilicity of liposomes. Hence, the study showed that combinatorial AMP-antibiotic nanotechnology led to a synergistic effect and can be seen as the smooth option in targeted delivery scenarios [104].
5. Futuristic perspective and conclusion
The recent COVID-19 pandemic represents a critical relation between sepsis and severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This relationship could be understood by a weak immune system which opens the door for coronavirus and inflammatory response by the host's immune system. So, in simplified words, sepsis is a deadly disease with the impeachment of morbid and mortality to patients and needs much-awaited focus with translational impact on early diagnostic areas and effective pharmaceutical supervision.
Sepsis is a disorder inclusive of complex pathophysiology with a moderate to high risk of life-endangering organ failure. Remarkably, the major challenge is the resistance to the treatment of antibiotics and in a few cases, patients had no therapeutics in hand. However, nanotechnological approaches are seen as attractive medicinal prophylaxis useful in overcoming most challenges in comparison with conventional therapy. The information discussed in this review states recent advancements in the diagnosis and therapeutics explored so far for the management of sepsis in terms of uniqueness, feasibility, and comparative analysis. Early sepsis detection is a crucial part of treatment due to a deficiency of signs and symptoms, majorly grounded on the patient's medical background and clinical experiences. Nano-derived biosensors play a significant role in the identification of different severity levels of the disease. Moreover, from a futuristic point of view, more sensitive, cost-effective, and reliable biosensors can be developed to produce more accurate analytical data which can help design perfect therapeutic prophylaxis in the management of sepsis. As reported in various experiments, nano-derived sensors offer a fast, sensitive, and specific outcome in the identification of diversity of pathogen detectors and biomarkers. Dependency on a signal biomarker is not worthy as generally PCT and CRP biomarkers show altered expression in many diseases like heat stroke, surgery, and trauma rather than sepsis. So further improvement in the nano-derived sensors is necessary. Rather than this, some specific validated sepsis biomarkers have shown few diagnostic and prognostic values listed as soluble urokinase-type plasminogen receptor (suPAR), soluble formula of triggering receptor expressed on myeloid cells-1 (TREM-1), pro-adrenomedullin (pro-ADM). On this note, nanotechnology-based detection of new biomarkers can be seen at fractional limits of diagnosis.
Antibacterial sensitivity is also a major factor with increasing dependency on time-eating traditional methods. In septic conditions, hemodynamic alterations were seen in the patient which build a challenge in achieving required antimicrobial pharmacokinetic targets resulting in treatment failure, drug resistance and suboptimal dosing. MDR infections in sepsis results in the use of combinatorial therapies based on susceptibility. Furthermore, many nanoformulations loaded with surface-modified antimicrobial peptides are used as a targeted system with enhanced therapeutic potency. Most importantly, as per current knowledge dysregulated inflammatory and immune responses in sepsis enhanced the evaluation of nano-therapeutics of associated treatments. Anti-inflammatory therapy by nano-formulations is under investigation in various preclinical and clinical studies.
In conclusion, the septic condition is denoted by uncontrolled inflammatory and immune responses which give birth to many challenges in terms of diagnosis and management. A vast number of nano-based detection tools and medicinal alternatives have been screened in different in vitro and in vivo sepsis experiments and considerable fruitful outcomes have been obtained. Rather than the antibiotic nanoformulations, ancillary remedies and their site-specific transfer with nanosystem have been evaluated as well in sepsis management. However more efforts need to be directed for these nanotechnological therapeutic facilities to became available for clinical use in future.
Declaration of Competing Interest
The authors declare no conflict of interest among themselves.
Acknowledgments
The authors acknowledge the Department of Pharmaceuticals, Ministry of Chemical and Fertilizers, Govt. of India for support. The NIPER-R communication number for the review article is NIPER-R/Communication/354. Corresponding Author RS acknowledges the DST-SERB for providing SIRE fellowship with Award No. SIR/2022/000678. The support provided by University of Central Lancashire to host the SIRE fellowship is gratefully acknowledged.
Data availability
Data will be made available on request.
References
- 1.Remick D.G. Pathophysiology of sepsis. Am. J. Pathol. 2007;170(5):1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sagy M., Al-Qaqaa Y., Kim P. Definitions and pathophysiology of sepsis. Curr. Probl. Pediatr. Adolesc. Health Care. 2013;43(10):260–263. doi: 10.1016/j.cppeds.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Jacobi J. Pathophysiology of sepsis. Am. J. Health Syst. Pharm. 2002;59(suppl_1):S3–S8. doi: 10.1093/ajhp/59.suppl_1.S3. [DOI] [PubMed] [Google Scholar]
- 4.Walker O., Kenny C.B., Goel N. Paediatrics and Child Health (United Kingdom) Vol. 29. 2019. Neonatal sepsis. [Google Scholar]
- 5.Hotchkiss R.S., Karl I.E. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348(2):138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
- 6.Mendoza D., Ascuntar J., Rosero O., Jaimes F. Improving the diagnosis and prognosis of sepsis according to the sources of infection. Emerg. Med. J. 2022;39(4) doi: 10.1136/emermed-2021-211910. [DOI] [PubMed] [Google Scholar]
- 7.Gotts J.E., Matthay M.A. Sepsis: pathophysiology and clinical management. Bmj. 2016:353. doi: 10.1136/bmj.i1585. [DOI] [PubMed] [Google Scholar]
- 8.Ramos Corrêa Pinto L., Azzolin K. de O., Lucena A. de F., Moretti M.M.S., Haas J.S., Moraes R.B., et al. Septic shock: clinical indicators and implications to critical patient care. J. Clin. Nurs. 2021;30(11–12) doi: 10.1111/jocn.15713. [DOI] [PubMed] [Google Scholar]
- 9.Öztürk Birge A., Karabag Aydin A., Köroğlu Çamdeviren E. Intensive care nurses’ awareness of identification of early sepsis findings. J. Clin. Nurs. 2021;31:2886–2899. doi: 10.1111/jocn.16116. [DOI] [PubMed] [Google Scholar]
- 10.Baudouin S.v. Springer Science & Business Media; 2009. Sepsis. [Google Scholar]
- 11.Irvan I., Febryan F., Suparto S. Sepsis and treatment based on the newest guideline. J. Anestesiol. Indonesia (online). 2018;10(1) [Google Scholar]
- 12.Baudouin S.v. Sepsis. Springer; 2008. Sepsis: introduction and epidemiology; pp. 1–4. [Google Scholar]
- 13.Sutton C.D., Garcea G., Pollard C., Berry D.P., Dennison A.R. The introduction of a nutrition clinical nurse specialist results in a reduction in the rate of catheter sepsis. Clin. Nutr. 2005;24(2):220–223. doi: 10.1016/j.clnu.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 14.Wheeler D.S. Introduction to pediatric sepsis. Open Inflamm. J. 2011;4(Suppl 1-M):1. doi: 10.2174/1875041901104010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poeze M., Ramsay G., Gerlach H., Rubulotta F., Levy M. An international sepsis survey: a study of doctors’ knowledge and perception about sepsis. Crit. Care. 2004;8(6):1–5. doi: 10.1186/cc2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain N.K., Jain V.M., Maheshwari S. Clinical profile of neonatal sepsis. Kathmandu Univ. Med. J. (KUMJ). 2003;1(2):117–120. [PubMed] [Google Scholar]
- 17.Hecker A., Padberg W., Hecker M. Sepsis: current clinical practices and new perspectives: introduction to the special issue. Vol. 10. J. Clin. Med. MDPI. 2021:443. doi: 10.3390/jcm10030443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatia M., He M., Zhang H., Moochhala S. Sepsis as a model of SIRS. Front. Biosci. Landmark. 2009;14(12):4703–4711. doi: 10.2741/3561. [DOI] [PubMed] [Google Scholar]
- 19.Simpson S.Q. SIRS in the time of sepsis-3. Chest. 2018;153(1):34–38. doi: 10.1016/j.chest.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 20.Nathens A.B., Marshall J.C. Sepsis, SIRS, and MODS: what’s in a name? World J. Surg. 1996;20(4):386–391. doi: 10.1007/s002689900061. [DOI] [PubMed] [Google Scholar]
- 21.Marik P.E., Taeb A.M. SIRS, qSOFA and new sepsis definition. J. Thorac. Dis. 2017;9(4):943. doi: 10.21037/jtd.2017.03.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doganyigit Z., Eroglu E., Akyuz E. Inflammatory mediators of cytokines and chemokines in sepsis: from bench to bedside. Hum. Exp. Toxicol. 2022;41 doi: 10.1177/09603271221078871. [DOI] [PubMed] [Google Scholar]
- 23.Toker A.K., Kose S., Turken M. Comparison of SOFA score, SIRS, qSOFA, and qSOFA+ L criteria in the diagnosis and prognosis of sepsis. Eur. J. Med. 2021;53(1):40. doi: 10.5152/eurasianjmed.2021.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Poll T., van de Veerdonk F.L., Scicluna B.P., Netea M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017;17 doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 25.Brunn G.J., Platt J.L. The etiology of sepsis: turned inside out. Trends Mol. Med. 2006;12(1):10–16. doi: 10.1016/j.molmed.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Grondman I., Pirvu A., Riza A., Ioana M., Netea M.G. Biomarkers of inflammation and the etiology of sepsis. Biochem. Soc. Trans. 2020;48(1):1–14. doi: 10.1042/BST20190029. [DOI] [PubMed] [Google Scholar]
- 27.van Dillen J., Zwart J., Schutte J., van Roosmalen J. Maternal sepsis: epidemiology, etiology and outcome. Curr. Opin. Infect. Dis. 2010;23(3):249–254. doi: 10.1097/QCO.0b013e328339257c. [DOI] [PubMed] [Google Scholar]
- 28.Jones A.E., Heffner A.C., Horton J.M., Marchick M.R. Etiology of illness in patients with severe sepsis admitted to the hospital from the emergency department. Clin. Infect. Dis. 2010;50(6):814–820. doi: 10.1086/650580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stassi C., Mondello C., Baldino G., Spagnolo E.V. Diagnostics. Vol. 10. 2020. Post-mortem investigations for the diagnosis of sepsis: A review of literature. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raju T.N.K. Ignac Semmelweis and the etiology of fetal and neonatal sepsis. J. Perinatol. 1999;19(4):307–310. doi: 10.1038/sj.jp.7200155. [DOI] [PubMed] [Google Scholar]
- 31.Caster J.M., Patel A.N., Zhang T., Wang A. Investigational nanomedicines in 2016: a review of nanotherapeutics currently undergoing clinical trials. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017;9(1) doi: 10.1002/wnan.1416. [DOI] [PubMed] [Google Scholar]
- 32.Sha T., Sunamoto M., Kitazaki T., Sato J., Ii M., Iizawa Y. Therapeutic effects of TAK-242, a novel selective Toll-like receptor 4 signal transduction inhibitor, in mouse endotoxin shock model. Eur. J. Pharmacol. 2007;571(2–3):231–239. doi: 10.1016/j.ejphar.2007.06.027. [DOI] [PubMed] [Google Scholar]
- 33.Kampf G., Dietz B., Grosse-Siestru C., Wendt C, Martiny H. Microbicidal Activity of a New Silver-Containing Polymer, SPI-ARGENT II. Antimicrob. Agents Chemother. 1998;49(9):2440–2442. doi: 10.1128/AAC.42.9.2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Etheridge M.L., Campbell S.A., Erdman A.G., Haynes C.L., Wolf S.M., McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9(1):1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.AmBisome Liposomal 50 mg Powder for dispersion for infusion Summary of Product Characteristics (SmPC)-(emc), Gilead Sciences Ltd. Updated 2019 [Google Scholar]
- 36.Rauschmann M.A., Wichelhaus T.A., Stirnal V., Dingeldein E., Zichner L., Schnettler R., et al. Nanocrystalline hydroxyapatite and calcium sulphate as biodegradable composite carrier material for local delivery of antibiotics in bone infections. Biomaterials. 2005;26(15):2677–2684. doi: 10.1016/j.biomaterials.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 37.Arikayce liposomal 590mg nebuliser dispersion . Insmed Limited; Updated 2022. Summary of Product Characteristics (SmPC) - (emc) [Google Scholar]
- 38.Ulloa L., Brunner M., Ramos L., Deitch E.A. Scientific and clinical challenges in sepsis. Curr. Pharm. Des. 2009;15(16):1918–1935. doi: 10.2174/138161209788453248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perner A., Cecconi M., Cronhjort M., Darmon M., Jakob S.M., Pettilä V., et al. Expert statement for the management of hypovolemia in sepsis. Intensive Care Med. 2018;44 doi: 10.1007/s00134-018-5177-x. [DOI] [PubMed] [Google Scholar]
- 40.Chiesa C., Panero A., Osborn J.F., Simonetti A.F., Pacifico L. Diagnosis of neonatal sepsis: a clinical and laboratory challenge. Clin. Chem. 2004;50(2):279–287. doi: 10.1373/clinchem.2003.025171. [DOI] [PubMed] [Google Scholar]
- 41.Brown R.M., Semler M.W. Fluid management in sepsis. J. Intensive Care Med. 2019;34 doi: 10.1177/0885066618784861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weledji E.P., Ngowe M.N. The challenge of intra-abdominal sepsis. Int. J. Surg. 2013;11(4):290–295. doi: 10.1016/j.ijsu.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 43.Salomão R., Ferreira B.L., Salomão M.C., Santos S.S., Azevedo L.C.P., Brunialti M.K.C. Sepsis: evolving concepts and challenges. Braz. J. Med. Biol. Res. 2019;52 doi: 10.1590/1414-431X20198595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Carrigan S.D., Scott G., Tabrizian M. Toward resolving the challenges of sepsis diagnosis. Clin. Chem. 2004;50(8):1301–1314. doi: 10.1373/clinchem.2004.032144. [DOI] [PubMed] [Google Scholar]
- 45.Salomão R., Ferreira B.L., Salomão M.C., Santos S.S., Azevedo L.C.P., Brunialti M.K.C. Sepsis: evolving concepts and challenges. Braz. J. Med. Biol. Res. 2019;52(4) doi: 10.1590/1414-431X20198595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Merino I., de la Fuente A., Domínguez-Gil M., Eiros J.M., Tedim A.P., Bermejo-Martín J.F. Digital PCR applications for the diagnosis and management of infection in critical care medicine. Crit. Care. 2022;26 doi: 10.1186/s13054-022-03948-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papafilippou L., Claxton A., Dark P., Kostarelos K., Hadjidemetriou M. Nanotools for sepsis diagnosis and treatment. Adv. Healthc. Mater. 2021;10(1):2001378. doi: 10.1002/adhm.202001378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo A. la, Oh D.K., Park C.J., Hong S.B. Monocyte distribution width compared with C-reactive protein and procalcitonin for early sepsis detection in the emergency department. PLoS One. 2021;16(4 April) doi: 10.1371/journal.pone.0250101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H.E., Shapiro N.I., Safford M.M., Griffin R., Judd S., Rodgers J.B., et al. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Konrad R., Michael B., C RN, S HC. New approaches to sepsis: molecular diagnostics and biomarkers. Clin. Microbiol. Rev. [Internet]. 2012 Oct 1;25(4):609–634. doi: 10.1128/CMR.00016-12. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nesseler N., Launey Y., Aninat C., Morel F., Mallédant Y., Seguin P. Clinical review: the liver in sepsis. Crit. Care [Internet]. 2012;16(5):235. doi: 10.1186/cc11381. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kale T., Bendale K., Singh K.K., Chaudhari P. Albumin based iohexol nanoparticles for computed tomography: an in vivo study. J. Biomed. Nanotechnol. 2019;15(2) doi: 10.1166/jbn.2019.2690. [DOI] [PubMed] [Google Scholar]
- 53.Shegokar R., Singh K.K. Surface modified nevirapine nanosuspensions for viral reservoir targeting: in vitro and in vivo evaluation. Int. J. Pharm. 2011;421(2):341–352. doi: 10.1016/j.ijpharm.2011.09.041. [DOI] [PubMed] [Google Scholar]
- 54.Razdan K., Garcia-Lara J., Sinha V.R., Singh K.K. Pharmaceutical strategies for the treatment of bacterial biofilms in chronic wounds. Drug Discovery Today. 2022;27(8):2137–2150. doi: 10.1016/j.drudis.2022.04.020. [DOI] [PubMed] [Google Scholar]
- 55.Handa M., Sharma A., Verma R.K., Shukla R. Polycaprolactone based nano-carrier for co-administration of moxifloxacin and rutin and its in-vitro evaluation for sepsis. J. Drug Deliv. Sci. Technol. 2019;54(June) [Google Scholar]
- 56.Nichols R.L. Intraabdominal sepsis: characterization and treatment. J. Infect. Dis. 1977;135(Supplement):S54–S57. doi: 10.1093/infdis/135.supplement.s54. [DOI] [PubMed] [Google Scholar]
- 57.Yuk S.A., Sanchez-Rodriguez D.A., Tsifansky M.D., Yeo Y. Recent advances in nanomedicine for sepsis treatment. Ther. Deliv. [Internet]. 2018 May 1;9(6):435–450. doi: 10.4155/tde-2018-0009. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mazza M., Patel A., Pons R., Bussy C., Kostarelos K. Peptide nanofibres as molecular transporters: from self-assembly to in vivo degradation. Faraday Discuss. 2013;166 doi: 10.1039/c3fd00100h. [DOI] [PubMed] [Google Scholar]
- 59.Kim H., Lee H.S., Ahn J.H., Hong K.S., Jang J.G., An J., et al. Lung-selective 25-hydroxycholesterol nanotherapeutics as a suppressor of COVID-19-associated cytokine storm. Nano Today. 2021;38 doi: 10.1016/j.nantod.2021.101149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang C.Y., Gao J., Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv. Mater. 2018;30(43):1803618. doi: 10.1002/adma.201803618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li J., Cha R., Zhao X., Guo H., Luo H., Wang M., et al. Gold nanoparticles cure bacterial infection with benefit to intestinal microflora. ACS Nano. 2019 May 28;13(5):5002–5014. doi: 10.1021/acsnano.9b01002. [DOI] [PubMed] [Google Scholar]
- 62.Yu H., Jin F., Liu D., Shu G., Wang X., Qi J., et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10(5):2342. doi: 10.7150/thno.40395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hou X., Zhang X., Zhou W., et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 2020;15:41–46. doi: 10.1038/s41565-019-0600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bonferoni M.C., Riva F., Invernizzi A., Dellera E., Sandri G., Rossi S., et al. Alpha tocopherol loaded chitosan oleate nanoemulsions for wound healing. Evaluation on cell lines and ex vivo human biopsies, and stabilization in spray dried Trojan microparticles. Eur. J. Pharm. Biopharm. 2018;123:31–41. doi: 10.1016/j.ejpb.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 65.Mehrabani M.G., Karimian R., Rakhshaei R., Pakdel F., Eslami H., Fakhrzadeh V., et al. Chitin/silk fibroin/TiO2 bio-nanocomposite as a biocompatible wound dressing bandage with strong antimicrobial activity. Int. J. Biol. Macromol. 2018;116:966–976. doi: 10.1016/j.ijbiomac.2018.05.102. [DOI] [PubMed] [Google Scholar]
- 66.Lamichhane N., Sharifabad M.E., Hodgson B., Mercer T., Sen T. In: Nanoparticle Therapeutics. Keshewani P., Singh K.K, editors. Elsevier; 2022. Superparamagnetic iron oxide nanoparticles (SPIONs) as therapeutic and diagnostic agents. [Google Scholar]
- 67.Han G., Martinez L.R., Mihu M.R., Friedman A.J., Friedman J.M., Nosanchuk J.D. Nitric oxide releasing nanoparticles are therapeutic for Staphylococcus aureus abscesses in a murine model of infection. PLoS One. 2009;4(11) doi: 10.1371/journal.pone.0007804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dinan K., Dinan T. Antibiotics and mental health: the good, the bad and the ugly. J. Intern. Med. John Wiley and Sons Inc. 2022:1–12. doi: 10.1111/joim.13543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bowton D.L. CNS effects of sepsis. Crit. Care Clin. 1989;5(4):785–792. [PubMed] [Google Scholar]
- 70.Handa M., Tiwari S., Yadav A.K., Almalki W.H., Alghamdi S., Alharbi K.S., et al. Therapeutic potential of nanoemulsions as feasible wagons for targeting Alzheimer’s disease. Drug Discov. Today [Internet] 2021 Jul;26(12):2881–2888. doi: 10.1016/j.drudis.2021.07.020. https://linkinghub.elsevier.com/retrieve/pii/S1359644621003251 Available from: [DOI] [PubMed] [Google Scholar]
- 71.Zwain T., Alder J.E., Sabagh B., Shaw A., Burrow A.J., Singh K.K. Tailoring functional nanostructured lipid carriers for glioblastoma treatment with enhanced permeability through in-vitro 3D BBB/BBTB models. Mater. Sci. Eng. C. 2021;121 doi: 10.1016/j.msec.2020.111774. [DOI] [PubMed] [Google Scholar]
- 72.Fang F., Wirdefeldt K., Jacks A., Kamel F., Ye W., Chen H. CNS infections, sepsis and risk of Parkinson’s disease. Int. J. Epidemiol. 2012;41(4):1042–1049. doi: 10.1093/ije/dys052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martin L., van Meegern A., Doemming S., Schuerholz T. Antimicrobial peptides in human sepsis. Front. Immunol. 2015;6:404. doi: 10.3389/fimmu.2015.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schuerholz T., Brandenburg K., Marx G. Annual Update in Intensive Care and Emergency Medicine 2012. 2012. Antimicrobial peptides and their potential application in inflammation and sepsis; pp. 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Soh M., Kang D., Jeong H., Kim D., Kim D.Y., Yang W., et al. Ceria–Zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew. Chem. 2017;129(38):11557–11561. doi: 10.1002/anie.201704904. [DOI] [PubMed] [Google Scholar]
- 76.Xu Nan, Gu Julin, Zhu Yuanjie, Wen Hai, Ren Qiushi, Chen Jianghan. Efficacy of intravenous amphotericin B-polybutylcyanoacrylate nanoparticles against cryptococcal meningitis in mice International. Journal of Nanomedicine. 2011;6:905–913. doi: 10.2147/IJN.S17503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khan S.T., Musarrat J., Al-Khedhairy A.A. Countering drug resistance, infectious diseases, and sepsis using metal and metal oxides nanoparticles: current status. Colloids Surf. B Biointerfaces. 2016;146:70–83. doi: 10.1016/j.colsurfb.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 78.Kaur R., Dennison S.R., Burrow A.J., Rudramurthy S.M., Swami R., Gorki V., et al. Nebulised surface-active hybrid nanoparticles of voriconazole for pulmonary Aspergillosis demonstrate clathrin-mediated cellular uptake, improved antifungal efficacy and lung retention. J. Nanobiotechnol. 2021;19(1):19. doi: 10.1186/s12951-020-00731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hou X., Zhang X., Zhao W., Zeng C., Deng B., McComb D.W., et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 2020;15(1):41–46. doi: 10.1038/s41565-019-0600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirley M. Amikacin liposome inhalation suspension: a review in Mycobacterium avium complex lung disease. Drugs. 2019 Apr 1;79(5):555–562. doi: 10.1007/s40265-019-01095-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fay K.T., Ford M.L., Coopersmith C.M. The intestinal microenvironment in sepsis. Biochim. Biophys. Acta (BBA)-Molecular Basis of Disease. 2017;1863(10):2574–2583. doi: 10.1016/j.bbadis.2017.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pan Y., Li J., Xia X., Wang J., Jiang Q., Yang J., et al. β-Glucan-coupled superparamagnetic iron oxide nanoparticles induce trained immunity to protect mice against sepsis. Theranostics. 2022;12(2):675. doi: 10.7150/thno.64874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Horseman M.A., Surani S. A comprehensive review of Vibrio vulnificus: an important cause of severe sepsis and skin and soft-tissue infection. Int. J. Infect. Dis. 2011;15(3):e157–e166. doi: 10.1016/j.ijid.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 84.Song Z., Sun H., Yang Y., Jing H., Yang L., Tong Y., et al. Enhanced efficacy and anti-biofilm activity of novel nanoemulsions against skin burn wound multi-drug resistant MRSA infections. Nanomedicine. 2016;12(6):1543–1555. doi: 10.1016/j.nano.2016.01.015. [DOI] [PubMed] [Google Scholar]
- 85.Razdan K., Gondil V.S., Chhibber S., Singh K.K., Sinha V.R. Levofloxacin loaded clove essential oil nanoscale emulsion as an efficient system against Pseudomonas aeruginosa biofilm. J. Drug Deliv. Sci. Technol. 2022 Feb;68 [Google Scholar]
- 86.Nguyen M., Watanabe H., Budson A.E., Richie J.P., Hayes D.F., Folkman J. Elevated levels of an angiogenic peptide, basic fibroblast growth factor, in the urine of patients with a wide spectrum of cancers. JNCI: J. Natl. Cancer Inst. 1994;86(5):356–361. doi: 10.1093/jnci/86.5.356. [DOI] [PubMed] [Google Scholar]
- 87.El-Feky G.S., Sharaf S.S., el Shafei A., Hegazy A.A. Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydr. Polym. 2017;158:11–19. doi: 10.1016/j.carbpol.2016.11.054. [DOI] [PubMed] [Google Scholar]
- 88.Peerapornratana S., Manrique-Caballero C.L., Gómez H., Kellum J.A. Acute kidney injury from sepsis: current concepts, epidemiology, pathophysiology, prevention and treatment. Kidney Int. 2019;96 doi: 10.1016/j.kint.2019.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Chang L.C., Wu S.C., Tsai J.W., Yu T.J., Tsai T.R. Optimization of epirubicin nanoparticles using experimental design for enhanced intravesical drug delivery. Int. J. Pharm. 2009;376(1–2):195–203. doi: 10.1016/j.ijpharm.2009.04.045. [DOI] [PubMed] [Google Scholar]
- 90.He J., Hong M., Xie W., Chen Z., Chen D., Xie S. Progress and prospects of nanomaterials against resistant bacteria. J. Control. Rel. Elsevier B.V. 2022:301–323. doi: 10.1016/j.jconrel.2022.09.030. [DOI] [PubMed] [Google Scholar]
- 91.Yan G., Huang Y., Bu Q., Lv L., Deng P., Zhou J., et al. Zinc oxide nanoparticles cause nephrotoxicity and kidney metabolism alterations in rats. J. Environ. Sci. Health A. 2012;47(4):577–588. doi: 10.1080/10934529.2012.650576. [DOI] [PubMed] [Google Scholar]
- 92.Saha K., Agasti S.S., Kim C., Li X., Rotello V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012;112(5):2739–2779. doi: 10.1021/cr2001178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zharov V.P., Mercer K.E., Galitovskaya E.N., Smeltzer M.S. Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles. Biophys J. 2006;90(2):619–627. doi: 10.1529/biophysj.105.061895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xie J., Huang J., Li X., Sun S., Chen X. Iron oxide nanoparticle platform for biomedical applications. Curr. Med. Chem. 2009;16(10):1278–1294. doi: 10.2174/092986709787846604. [DOI] [PubMed] [Google Scholar]
- 95.Kühn K.P., Chaberny I.F., Massholder K., Stickler M., Benz V.W., Sonntag H.G., et al. Disinfection of surfaces by photocatalytic oxidation with titanium dioxide and UVA light. Chemosphere. 2003;53(1) doi: 10.1016/S0045-6535(03)00362-X. [DOI] [PubMed] [Google Scholar]
- 96.Kühn M., Ivleva N.P., Klitzke S., Niessner R., Baumann T. Investigation of coatings of natural organic matter on silver nanoparticles under environmentally relevant conditions by surface-enhanced Raman scattering. Sci. Total Environ. 2015;535:122–130. doi: 10.1016/j.scitotenv.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 97.Xing K., Shen X., Zhu X., Ju X., Miao X., Tian J., et al. Synthesis and in vitro antifungal efficacy of oleoyl-chitosan nanoparticles against plant pathogenic fungi. Int. J. Biol. Macromol. 2016;82:830–836. doi: 10.1016/j.ijbiomac.2015.09.074. [DOI] [PubMed] [Google Scholar]
- 98.Handa M., Sharma A., Verma R.K., Shukla R. Polycaprolactone based nano-carrier for co-administration of moxifloxacin and rutin and its in-vitro evaluation for sepsis. J. Drug Deliv. Sci. Technol. 2019;54 [Google Scholar]
- 99.Hou X., Zhang X., Zhao W., Zeng C., Deng B., McComb D.W., et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat. Nanotechnol. 2020;15(1):41–46. doi: 10.1038/s41565-019-0600-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Park K.S., Lee J., Lee C., Park H.T., Kim J.W., Kim O.Y., et al. Sepsis-like systemic inflammation induced by nano-sized extracellular vesicles from feces. Front. Microbiol. 2018;9:1735. doi: 10.3389/fmicb.2018.01735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li H., Qu F. Synthesis of CdTe quantum dots in sol−gel-derived composite silica spheres coated with calix [4] arene as luminescent probes for pesticides. Chem. Mater. 2007;19(17):4148–4154. [Google Scholar]
- 102.Ibupoto Z.H., Jamal N., Khun K., Willander M. Development of a disposable potentiometric antibody immobilized ZnO nanotubes based sensor for the detection of C-reactive protein. Sens. Actuators B Chem. 2012;166:809–814. [Google Scholar]
- 103.Boullet H., Bentot F., Hequet A., Ganem-Elbaz C., Bechara C., Pacreau E., et al. Small antimicrobial peptide with in vivo activity against sepsis. Molecules. 2019;24(9):1702. doi: 10.3390/molecules24091702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Fan X., Fan J., Wang X., Wu P., Wu G. S-thanatin functionalized liposome potentially targeting on Klebsiella pneumoniae and its application in sepsis mouse model. Front. Pharmacol. 2015;6:249. doi: 10.3389/fphar.2015.00249. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.