Abstract
Background:
Given recent changes in the legal status of cannabis, the risks and benefits associated with its use have become an important public health topic. A growing body of research has demonstrated that posttraumatic stress disorder (PTSD) and recreational cannabis use (RCU) frequently co-occur, yet findings are inconsistent (e.g., direction of effect) and methodological variability makes comparison across studies difficult.
Methods:
We conducted a comprehensive systematic review of all studies (N = 45) published before May 2020 regarding etiologic models of co-occurring RCU and PTSD, as well as provided a methodological critique to inform suggestions for future research initiatives.
Results:
Findings indicate that a majority of studies (n = 37) demonstrated a significant association between RCU and PTSD. Findings provide evidence for the self-medication and high-risk models posited to explain co-occurring RCU and PTSD despite variability in assessment of RCU, which includes commonly used non-standardized self-report questions.
Conclusion:
The association between RCU and PTSD is likely bidirectional. Results inform clinicians and researchers working in the mental health and cannabis use fields how the variability in findings on the association between RCU and PTSD may be attributable, in part, to methodological issues that permeate the extant literature pertaining to RCU and PTSD.
Keywords: cannabis, posttraumatic stress disorder, comorbidity, etiology
1. Introduction
In the United States (U.S.), the National Comorbidity Survey – Replication (NCS-R) from 2001–03 found that 42.5% of the general adult population had engaged in lifetime recreational cannabis use (RCU; cannabis used without medical justification) and 6.8% met the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) diagnostic criteria for posttraumatic stress disorder (PTSD; Cougle et al., 2011). The prevalence rates for current (i.e., past 12-months) RCU and PTSD were 9.5% and 3.6%, respectively. Additionally, the NCS-R found significant associations between lifetime and current PTSD and lifetime, current, and daily RCU (Cougle et al., 2011). Specifically, the prevalence of lifetime, current, and daily RCU among those meeting criteria for current PTSD was 65.1%, 14.0%, and 3.1%, respectively (Cougle et al., 2011). Generally, individuals with PTSD and co-occurring substance use may have a poorer quality of life compared to those without co-occurring substance use (Watson et al., 2011). Further, co-occurring substance use might negatively interfere with the treatment and management of PTSD (McCauley et al., 2012).
To best address treatment and management challenges of co-occurring RCU and PTSD (e.g., higher PTSD treatment dropout, poorer PTSD treatment adherence, worse PTSD treatment outcome; Bedard-Gilligan et al., 2018), we must understand the etiological and maintenance factors contributing to their high co-occurrence. There are several models that have been proposed to explain co-occurring substance use disorders (SUDs) and PTSD broadly (Berenz et al., 2019), but have been applied to co-occurring RCU and PTSD. The shared liability model suggests that RCU and PTSD frequently co-occur due to common familial risk (i.e., genetic factors, shared environmental influences; Krueger & Markon, 2006). The self-medication model suggests that individuals with PTSD are more likely to use cannabis because it serves as a means to cope with trauma-related symptoms (Khantzian, 1997). It has been hypothesized that PTSD may increase risk of RCU through using cannabis to cope with distressing PTSD symptoms (Sarvet et al., 2018). The high-risk model suggests that RCU increases risk for trauma exposure and, consequently, PTSD (Brady et al., 2004). RCU may increase risk of trauma exposure and PTSD by impairing one’s judgment and ability to discern danger cues (Karila et al., 2014), which could increase participation in risky behaviors and/or unsafe situations. Additionally, substance using individuals are more likely to associate with peers who engage in risk-taking behaviors (Fergusson et al., 2002), which may increase risk for trauma exposure and PTSD. Previous research utilizing these three models as a framework to examine the relationship between RCU and PTSD has yielded mixed findings (Cougle et al., 2011; Ehlers et al., 2016; Holliday & Pedersen, 2017; Welsh et al., 2017). Specifically, Holliday and Pedersen (2017) did not find a significant bivariate correlation between PTSD and cannabis misuse; Ehlers and colleagues (2016) did not find support for the high-risk model; Cougle and colleagues (2011) found support for the self-medication model, but Welsh and colleagues (2017) did not. A number of factors likely contribute to discordant results including methodological variability.
1.1. Methodological concerns
The term “cannabis use” has evolved over the past few decades with the literature using “use,” “misuse,” and “abuse” interchangeably as it progressed to the current terms of “RCU,” which includes all use without a prescription (and is the construct of interest in this review), and “medicinal cannabis use,” or prescribed cannabis use. Reasons for specifically engaging in RCU rather than another substance may be associated with differing means of legal status, access, misperceptions of safety, as well as social (e.g., to be more sociable), enhancement (e.g., to get high), and coping motives (Buckner et al., 2019).
Given the perceived safety and social acceptability of RCU (Berg et al., 2015), combined with the increasing number of states with medicinal cannabis, individuals are more likely to have greater access to RCU as a means of coping with PTSD. Turna and colleagues (2017) noted that anxiety and related disorders (i.e., PTSD) are the most common mental conditions affecting U.S. citizens. Notably, given the mixed findings regarding the developmental timeline of co-occurring RCU and PTSD, it is just as likely that due to the perceived safety and social acceptability of RCU, as well as the availability of medical cannabis, the prevalence of RCU may be higher which may put individuals at increased risk for experiencing traumatic events and developing PTSD.
Researchers have noted a number of methodological concerns within the growing RCU literature, including variability in what investigators consider problematic cannabis use or “misuse” (Asbridge et al., 2014). The definition provided by the NSDUH states that any non-medicinal use (i.e., recreational use) within an individual’s lifetime is considered cannabis “misuse” (Substance Abuse and Mental Health Service Administration, 2019). Not only does the focus on recreational use with the NSDUH definition complicate our understanding of temporal relationships between RCU and other variables (e.g., cannot determine whether PTSD precedes RCU per the self-medication model, or whether RCU precedes trauma exposure/PTSD per the high-risk model), any use may hardly capture problematic use worthy of clinical attention. On the opposite end of the RCU severity spectrum, DSM-5 criteria for cannabis use disorder (CUD) are thought to more accurately identify those with problematic use. Unlike RCU, changes from DSM-IV to DSM-5 PTSD did not lead to as much variability in measurement of PTSD (American Psychiatric Association, 2013). Despite these major revisions, results from the National Stressful Events Survey documented similar estimates of DSM-IV lifetime PTSD (10.6%) and DSM-5 lifetime PTSD (9.4%) among a representative group of U.S. adults (Kilpatrick et al., 2013).
Lack of comprehensive assessment of PTSD could contribute to the variability identified within the PTSD and RCU literature. Specifically, inconsistencies could be attributed to restricted consideration of time of onset. When assessing PTSD, it is important to assess time of onset of symptoms and/or the course of the diagnosis in order to help establish a timeline of how co-occurring PTSD and RCU developed. The bidirectional association between PTSD and RCU calls for a comprehensive PTSD assessment, which includes time of onset of symptoms/diagnosis, in order to establish direction of effect.
Given the predominant models of RCU and PTSD co-occurrence are phenotypic predictive models (i.e., PTSD → RCU, RCU → PTSD), longitudinal studies are preferred over cross-sectional studies. Specifically, the goal of this line of research is estimating a direction of effect of one phenotype onto the other. Despite the limitation that no causal inferences can be determined from cross-sectional and observational data, a majority of the literature examining RCU and PTSD is cross-sectional and observational due to ethical dilemmas surrounding investigating the development of trauma exposure/PTSD (Newman et al., 2006).
1.2. Aims
Given the increasing public attention and empirical examination of RCU, focus has been placed on identifying predictors of use, including PTSD. To date, no systematic review of the literature has been initiated examining the intersection of RCU and PTSD. The present systematic review aims to evaluate and summarize the empirical evidence regarding the association between RCU and PTSD, as well as examine the support for each model posited to explain the association as a means of determining what gaps exist in the literature. A secondary aim is to provide a critique of the methodological approaches used to examine co-occuring RCU and PTSD. It is hoped that the present review and methodological critique will aid researchers and clinicians alike as they attempt to create and implement research protocols and interpret research findings regarding the association between RCU and PTSD.
2. Methods
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were enlisted during the review process (Moher et al., 2009).
2.1. Databases & search methodology
Two databases (PubMed and PsycINFO) were searched in May 2020 using the following search terms aimed at capturing literature within the intersection of RCU and PTSD: (([cannabis] OR [cannabis use disorder]) OR (“cannabis” OR “cannabis use” OR “cannabis use disorder” OR “marijuana” OR “marijuana use” OR “marijuana use disorder” OR “cannabis abuse” OR “cannabis dependence” OR “marijuana abuse” OR “marijuana dependence”)) AND ([Posttraumatic Stress Disorder] OR (“posttraumatic stress disorder” OR “PTSD”)).
2.2. Inclusion and exclusion criteria
Given the early stage of the literature regarding RCU and PTSD and the secondary aim of evaluating research methodology, attempts were made to balance capturing as much of the literature as possible while maintaining strict inclusion criteria to improve comparability across studies. Thus, prior to beginning the initial search, the following inclusion and exclusion criteria were determined: (1) PTSD (i.e., diagnosis and/or symptoms) must be included in primary analyses related to RCU, (2) RCU must be differentiated from other forms of substance use (i.e., no polysubstance use including cannabis) or describe attempts made to identify RCU specifically, (3) the study cannot evaluate a form of cannabis without THC (e.g., CBD oil), (4) the study cannot be focused on medicinal cannabis use (i.e., prescribed cannabis use), (5) the study cannot be a conceptual article, a review, or qualitative in nature, (6) the study cannot evaluate the effects of an intervention, (7) must be peer reviewed, (8) must use human subjects, and (9) must be in English.
2.3. Identification
During the first stage of the study selection process, searches conducted in PubMed and PsycINFO resulted in 330 and 179 articles being identified, respectively. This resulted in 365 potentially relevant studies after excluding duplicates.
The second stage of the study selection process involved examining each of the 365 articles and excluding those that did not meet the inclusion and exclusion criteria. 320 studies met one or more of these criteria and were excluded from further consideration. This process resulted in a total of 45 relevant studies for inclusion in the present review. Figure 1 presents the flow chart for the selection of the included studies. Two authors (T.A.H and A.J.Z) independently coded each article based on the specific inclusion and exclusion criteria previously mentioned. The authors found no discrepancies during the independent coding process (i.e., 100% agreement).
Figure 1:
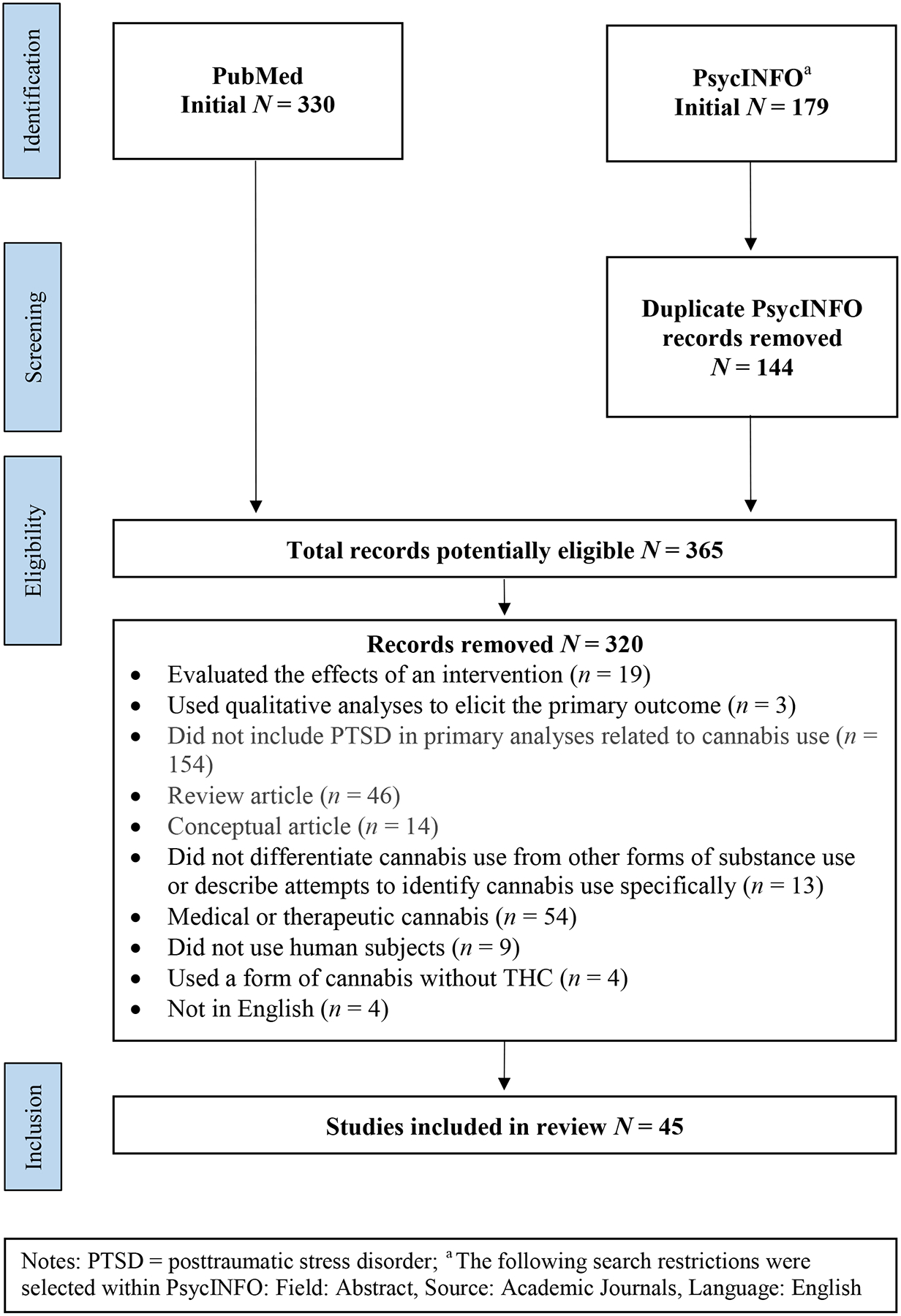
Flowchart of systematic review
2.4. Self-medication, high-risk, and shared liability models categorization
Study categorization was based on predictor and outcome variables since the self-medication and high-risk models assume temporal order. If the predictor was PTSD symptoms or diagnosis and the outcome was RCU or CUD symptoms or diagnosis, then the study was classified as testing the self-medication model. If the predictor was RCU or CUD symptoms or diagnosis and the outcome was PTSD symptoms or diagnosis, then the study was classified as testing the high-risk model. If the study was a twin/family study or included genetically-informed shared risk, then it was classified as testing the shared liability model.
3. Results
The results section is separated thematically, and key results are summarized in Figure 2. Basic relations are briefly reviewed, and more weight is applied to the sections pertaining to the methodological quality of the current literature at the intersection of RCU and PTSD, which will provide the basis for further discussion and empirical recommendations in the discussion.
Figure 2:

Infographic displaying results of systematic review of the association between recreational cannabis use (RCU) and posttraumatic stress disorder (PTSD)
3.1. Relationship between RCU and PTSD
Table 1 presents information regarding articles extracted for the present review, specifically, article title, authors, sample information, study design, RCU assessment, PTSD assessment, etiologic model of co-occurrence tested, key findings, and standardized effect size. Thirty-seven out of 45 (82.2%) studies demonstrated significant associations between RCU and PTSD. Eight out of the 45 (17.8%) studies did not demonstrate significant associations between RCU and PTSD.
Table 1.
Study information from the articles included in the present systematic review.
| Study Title | Author | Sample | Population | Study Design | RCU Measurement | PTSD Measurement | Model of Comorbidity | Results | Cohen’s d or β |
|---|---|---|---|---|---|---|---|---|---|
| Trends in documented cannabis use disorder among hospitalized adult epilepsy patients in the U.S. | Lekoubou et al., 2020 | 1,980,707 hospitalized adults with epilepsy | Clinical (adults) | Cross-sectional | ICD-9-CM diagnostic codes for CUDs (lifetime diagnosis excluding those “in remission”) | ICD-9-CM diagnostic codes for PTSD (lifetime diagnosis) | Self-Medication Model | PTSD did not have a significant association with CUD (OR = 1.13; CI: .99–1.31; p = .08) | Cohen’s d = .07 |
| Post-traumatic stress and marijuana outcomes: The mediating role of marijuana protective behavioral strategies | Jordan et al., 2020 | 7,307 U.S. college students | College students | Cross-sectional | Self-report: MUG; CUDIT-R (past month cannabis use frequency, quantity; past six-month CUD symptoms) | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | There was not a significant effect between PTSD symptoms and cannabis use frequency (β = .02) or quantity (β = .04). There was a significant positive direct effect between PTSD symptoms and CUD symptoms (β = .17) | β = .02, .04, .17 |
| PTSD and associated factors among drivers surviving road traffic crashes in SW Ethiopia | Alenko et al., 2019 | 398 public transportation workers | Ethiopian community | Cross-sectional | Structured or semi-structured interview: WHO’s ASSIST (past 3-month risk problems from current cannabis use) | Self-report: TSQ (past week PTSD symptoms) | High-Risk Model | Individuals with severe-risk cannabis use were at greater risk for developing PTSD (AOR = 2.51, CI: 1.96–7.52, p = .03) | Cohen’s d = .51 |
| Substance use disorders in refugee and migrant groups in Sweden: A nationwide cohort study of 1.2 million people | Harris et al., 2019 | 1,241,901 refugees, migrants, & Swedish-born adults | Swedish adults | Cross-sectional | ICD-10 diagnostic codes for CUDs (lifetime diagnosis) | ICD-10 diagnostic codes for PTSD (lifetime diagnosis) | Self-Medication Model | PTSD was associated with CUDs (LRT χ2 (2) = 11.7; p = .003). PTSD was associated with CUDs in the Swedish-born population (HR = 11.50; CI: 9.30–14.23; p < .001) than non-refugee (HR: 5.92; CI: 3.58–9.81; p < .001) or refugee migrants (HR: 2.60; CI: 0.63–10.69; p = .19) | Cohen’s d = .01, 1.35, .98, .53 |
| Longitudinal associations between sleep, intrusive thoughts, and alcohol problems among veterans | Miller et al, 2019 | 361 veterans | Veterans / military personnel | Longitudinal | Self-report: TLFB (past 6-month cannabis use quantity and frequency) | Self-report: IDAS (traumatic intrusions subscale) [past two-weeks PTSD symptoms] | High-Risk Model | Correlations for cannabis use at baseline with PTSD symptoms at baseline, 6 months, and 12 months were correlated with r = .23, .18, and .14, respectively (p < .05) | Cohen’s d = .47, .37, .28 |
| Interactive effects of PTSD and substance use on suicidal ideation and behavior in military personnel: Increased risk from marijuana use | Allan et al., 2019 | 545 military personnel | Veterans / military personnel | Longitudinal | Structured or semi-structured interview: ASI (past month cannabis use) | Self-report: PCL (past month PTSD symptoms) | High-Risk Model | At high (β = .02; p = .01), but not low (β = .00; p = .96) levels of PTSD symptoms, more days using cannabis predicted increased PTSD symptoms over time | β = .02, .00 |
| Associations of PTSD, chronic pain, and their comorbidity on CUD: Results from an American nationally representative study | Bilevicius et al., 2019 | 36,309 U.S. adults | U.S. adults | Cross-sectional | Structured or semi-structured interview: AUDADIS (CUD module) [past year CUD] | Structured or semi-structured interview: AUDADIS (PTSD module) [past year PTSD] | Self-Medication Model | PTSD+digestive pain (AOR = 6.13, CI: 3.79–9.92; p < .001), PTSD+nerve pain (AOR = 4.21, CI: 2.51–7.07; p < .001), and PTSD+any chronic pain (AOR = 5.08, CI: 3.30–7.83; p < .001) were associated with greater odds of CUD compared to pain alone | Cohen’s d = 1.00, .79, .90 |
| PTSD is a risk factor for multiple addictions in police officers hospitalized for alcohol | Brunault et al., 2019 | 133 police officers in rehab | First responders | Cross-sectional | Self-report: CAST (past year cannabis use) | Self-report: PCL (past month provisional PTSD diagnosis) | Self-Medication Model | PTSD diagnosis was not a significant predictor of CUD severity (p = 0.82) | Cohen’s d = .12 |
| PTSD symptom severity, cannabis, and gender: A zero-inflated negative binomial regression model | Rehder & Bowen, 2019 | 536 U.S. college students | College students | Cross-sectional | Self-report: Frequency of cannabis use was assessed via report of the number of days used over the past 60 days | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | The probability of using cannabis was moderated by gender (OR = 0.96, CI: 0.93–0.99, p = .026), such that for males, as PTSD symptom severity increased, likelihood of using cannabis increased | Cohen’s d = −.02 |
| An EMA study examining PTSD symptoms, prenatal bonding, and substance use among pregnant women | Sanjuan et al., 2019 | 33 trauma-exposed pregnant women | Trauma-exposed adult women | Longitudinal | Self-report: Any cannabis use was assessed via participant report of any use since the previous EMA session (i.e., 24 hours) | Structured or semi-structured interview: CAPS; Self-report: PCL (lifetime and current PTSD; daily PTSD symptoms) | Self-Medication Model | There were moderate associations between greater daily peak PTSD symptoms and cannabis use (β = 0.35, CI: .10–.57) within-subjects | β = .35 |
| Sexual minority women and cannabis use: The serial impact of PTSD symptom severity and coping motives | Walukevich-Dienst et al., 2019 | 439 Louisiana college students | College students | Cross-sectional | Self-report: MUF (lifetime cannabis use frequency) | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | PTSD symptoms did not significantly predict cannabis use frequency (β = −.01; p = .06) | β = −.01 |
| Comorbidity in illness-induced PTSD versus PTSD due to external events in a nationally representative study | Sommer et al., 2018 | 36,309 U.S. adults | U.S. adults | Cross-sectional | Structured or semi-structured interview: AUDADIS (CUD module) [past year CUD] | Structured or semi-structured interview: AUDADIS (PTSD module) [past year PTSD] | Self-Medication Model | Individuals with illness-induced PTSD had significantly greater odds of CUD compared to those with other trauma-induced PTSD (AOR = 1.98, CI: 1.01–3.89, p < .05) | Cohen’s d = .38 |
| Use of protective behavioral strategies among young adult veteran marijuana users | Pedersen et al., 2018 | 180 veterans | Veterans / military personnel | Cross-sectional | Self-report: Frequency of cannabis use was assessed via report of the number of days used over the past six months; MACQ | Self-report: PCL (past month provisional PTSD) | Self-Medication Model | Participants who screened positive for PTSD reported more cannabis use problems (M = 2.54, SD = 3.13) than participants not screening positive for PTSD (M = 1.54, SD = 2.56), t (176) = 2.20, p = .03) | Cohen’s d = .35 |
| Regular past year cannabis use in women veterans and associations with sexual trauma | Browne et al., 2018 | 647 women veterans | Veterans / military personnel | Cross-sectional | Self-report: Frequency of cannabis use was assessed via “how often in the past year did you use cannabis” with 7 response options from “not at all” to “every day” | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | Current PTSD symptoms was a significant risk factor for cannabis use above and beyond covariates (OR = 1.02, CI: 1.00–1.04, p < .05) | Cohen’s d = .01 |
| The impact of PTSD clusters on cannabis use in a racially diverse trauma-exposed sample: An analysis from EMA | Buckner et al., 2018 | 87 trauma-exposed U.S. adults | Trauma-exposed adults | Cross-sectional | Self-report: MPS; TLFB (past 90-days cannabis problems and use frequency) | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | Participants with PTSD symptoms reported more cannabis-related problems (χ2 = 27.15, p < .001) than participants without PTSD symptom | Cohen’s d = 1.22 |
| Self- and other-directed forms of violence and their relationship with lifetime DSM-5 psychiatric disorders: Results from the NESARC− III | Harford et al., 2018 | 36,309 U.S. adults | U.S. adults | Cross-sectional | Structured or semi-structured interview: AUDADIS (CUD module) [lifetime CUD] | Structured or semi-structured interview: AUDADIS (PTSD module) [past year PTSD] | Self-Medication Model | Individuals with PTSD had increased risk of also having CUD (OR = 3.6, p < .01) | Cohen’s d = .71 |
| Trajectories of cannabis use beginning in adolescence associated with symptoms of PTSD in the mid-thirties | Lee et al., 2018 | 674 racial and ethnic minority adults | Racial and ethnic minority adults | Longitudinal | Self-report: Frequency of cannabis use was assessed via “how often have you used cannabis” and “how often have you used cannabis in the past 5 years” with 5 response options from “never” to “once a week or more” | Self-report: PCL (past month PTSD symptoms) | High-Risk Model | The chronic cannabis use group (AOR = 4.68, p < .01), the late quitting group (AOR = 6.18, p < .01), and the moderate cannabis use group (AOR = 3.97, p < .01) were all associated with an increased likelihood of having PTSD symptoms at age 36 compared with the no cannabis use group | Cohen’s d = .85, 1.00, .76 |
| DSM-5 CUD in the National Epidemiologic Survey on Alcohol and Related Conditions-III: gender-specific profiles | Kerridge et al., 2018 | 36,309 U.S. adults | U.S. adults | Cross-sectional | Structured or semi-structured interview: AUDADIS (CUD module) [past year CUD] | Structured or semi-structured interview: AUDADIS (PTSD module) [past year PTSD] | High-Risk Model | Any (AOR = 1.7, CI: 1.12 – 2.57, p < .05), moderate (AOR = 2.0, CI: 1.08 – 3.81, p < .05), and severe CUD (AOR = 3.7, CI: 1.98 – 7.02, p < .05) among men was associated with PTSD. Any (AOR = 1.6, CI: 1.01 – 2.48, p < .05) and moderate CUD (AOR = 3.4, CI: 1.69 – 6.98, p < .05) was associated with PTSD among women | Cohen’s d = .29, .38, .72, .26, .68 |
| Daily-level associations between PTSD and cannabis use among young sexual minority women | Dworkin et al., 2017 | 114 sexual minority women | Sexual minority adult women | Longitudinal | Self-report: Any cannabis use was assessed via participant report of any use during the past 24 hours | Self-report: PCL (past 24-hours PTSD symptoms) | Self-Medication Model | Women with higher overall daily PTSD scores across time had a higher likelihood of using cannabis on any given day (OR = 2.67, CI: 1.71–4.41, p < .001) | Cohen’s d = .54 |
| Association between substance use diagnoses and psychiatric disorders in an adolescent and young adult clinic-based population | Welsh et al., 2017 | 483 adolescents and young adults | Adolescents and young adults | Cross-sectional | DSM-IV-TR diagnostic codes for CUDs (lifetime diagnosis) | DSM-IV-TR diagnostic codes for PTSD (lifetime diagnosis) | Self-Medication Model | PTSD and CUD were not significantly associated (AOR = 1.52, CI: .49 – 4.69, p = .47) | Cohen’s d = .23 |
| The mediating roles of coping, sleep, and anxiety motives in cannabis use and problems among returning veterans with PTSD and MDD | Metrik et al., 2016 | 301 veterans | Veterans / military personnel | Cross-sectional | Self-report: MPS; TLFB; Structured or semi-structured interview: SCID (lifetime and past year CUD; past 90-days cannabis use problems and past 6 months cannabis use frequency) | Structured or semi-structured interview: CAPS (lifetime and past month PTSD) | Self-Medication Model | PTSD was associated with greater cannabis use (OR = 1.75, CI: 1.22, 2.52, p < .01). PTSD predicted the likelihood of being diagnosed with a CUD (OR = 1.71, CI: 1.14, 2.59, p ≤ .01). Individuals with PTSD had more cannabis use problems compared to individuals without PTSD (χ2 = 2.14, p < .05) | Cohen’s d = .31, .30, .32 |
| Lifetime history of traumatic events in a young adult Mexican American sample: Relation to substance dependence, affective disorder, acculturation stress, and PTSD | Ehlers et al., 2016 | 614 Mexican-American adults | U.S. Mexican-American adults | Cross-sectional | Structured or semi-structured interview: SSAGA (CUDs module) (lifetime diagnosis) | Structured or semi-structured interview: SSAGA (PTSD module) [lifetime diagnosis] | High-Risk Model | Cannabis dependence did not significantly co-occur with PTSD (OR = 2.02, CI: 1.12–3.62, p = .02; p = .01 significance level) | Cohen’s d = .39 |
| Prevalence and correlates of cannabis use in an outpatient VA PTSD clinic | Gentes et al., 2016 | 719 veterans | Veterans / military personnel | Cross-sectional | Self-report: Frequency of cannabis use was assessed via “how often have you used cannabis in the past 6 months” with 7 response options from “no use” to “daily use” | Structured or semi-structured interview: CAPS (past month PTSD symptoms) | Self-Medication Model | Past 6-month cannabis use was associated with PTSD severity (AOR = 1.30, CI: 1.01–1.66, p < .05) | Cohen’s d = .15 |
| Associations of PTSD symptoms with marijuana and synthetic cannabis use among young adult US Veterans: a pilot investigation | Grant et al., 2016 | 790 veterans | Veterans / military personnel | Cross-sectional | Self-report: Any and frequency of cannabis use was assessed via report of any lifetime cannabis use and number of days used during the past month, respectively | Self-report: PC-PTSD (past month PTSD symptoms) | High-Risk Model | Cannabis use in the past month was associated with a positive screen for PTSD (OR = 4.19, CI: 2.44, 7.18) | Cohen’s d = .79 |
| Prevalence and mental health correlates of illegal cannabis use among bisexual women | Robinson et al., 2016 | 262 Canadian bisexual women | Sexual minority adult women | Cross-sectional | Self-report: DUDIT-E (past year cannabis use) | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | There was no significant association between cannabis use and PTSD | Cohen’s d = .02, .33 |
| Cannabis use among Navy personnel in Sri Lanka: a cross sectional study | de Silva et al., 2016 | 671 Navy personnel | Veterans / military personnel | Cross-sectional | Self-report: Any cannabis use was assessed via report of any use during the past 12 months | Self-report: PCL (past month PTSD symptoms) | High-Risk Model | There was a significant association between cannabis use and PTSD (AOR = 4.20, CI: 1.08–16.38) | Cohen’s d = .79 |
| Mental health symptom severity in cannabis using and non-using Veterans with probable PTSD | Johnson et al., 2016 | 700 veterans | Veterans / military personnel | Cross-sectional | Structured or semi-structured interview: WHO’s ASSIST (past 3-month risk of problems from current cannabis use) | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | There was no association between PTSD scores and frequency of cannabis use (OR = 0.99, CI: 0.97–1.01, p = 0.39) | Cohen’s d = −.01 |
| Associations among trauma, PTSD, cannabis use, and CUD in a nationally representative epidemiologic sample | Kevorkian et al., 2015 | 34,396 U.S. adults | U.S. adults | Cross-sectional | Structured or semi-structured interview: AUDADIS (lifetime cannabis use and CUD modules) [past year CUD] | Structured or semi-structured interview: AUDADIS (PTSD module) [past year PTSD] | Self-Medication Model | PTSD showed a significant association with CUD (OR = 1.22, CI: 1.21–1.22, p < .001), but was only marginally associated with lifetime cannabis use (OR = 0.99, CI: .99–.99, p < .001) | Cohen’s d = .11, −.01 |
| The moderating role of experiential avoidance in the relationship between PTSD symptom severity and cannabis dependence | Bordieri et al., 2014 | 123 adults with substance use disorders | U.S. adults | Cross-sectional | Structured or semi-structured interview: SCID (current diagnosis) | Structured or semi-structured interview: CAPS (past month PTSD symptoms) | Self-Medication Model | There was a conditional relationship between PTSD symptom severity and cannabis dependence when experiential avoidance scores were one SD above the mean (β = 0.05, z = 3.07, p = .002), and at the mean (β = 0.03, z = 2.50, p = .01) | β = .05, .03 |
| Marijuana, expectancies, and post-traumatic stress symptoms: A preliminary investigation | Earleywine & Bolles, 2014 | 653 veterans | Veterans / military personnel | Cross-sectional | Self-report: Quantity of cannabis use was assessed via report of “how much marijuana do you use in a month” with 14 response options from “less than ¼ ounce” to “more than 3 ounces” | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | There was a significant indirect effect of PTSD symptoms on cannabis use via PTSD expectancies (β = 0.04, p < .01) | β = .04 |
| Negative cognitions as a moderator in the relationship between PTSD and substance use in a psychiatrically hospitalized adolescent sample | Allwood et al., 2014 | 188 U.S. adolescents | Clinical (adolescents) | Cross-sectional | Structured or semi-structured interview: K-SADS-PL (SUD module) [lifetime SUD symptoms including age of onset] | Structured or semi-structured interview: K-SADS-PL (PTSD module) [lifetime PTSD including age of onset] | Self-Medication Model | PTSD was significantly associated with CUD symptoms (β = .73, p < .001) at high (+1 SD) negative view of the future. Average age of onset of PTSD was 10.65 years (SD = 3.47). Average of onset of SUD was 14.67 years (SD = 1.43) |
β = .73 |
| Patterns of drug and alcohol use associated with lifetime sexual revictimization and current PTSD among three national samples of adolescent, college, and household-residing women | Walsh et al., 2014 | 1,763 girls; 2,000 college women; and 3,001 household-residing women | Adolescents and young adults | Cross-sectional | Self-report: Non-experimental cannabis use was assessed via participant report of cannabis use at least four times during the past year | Structured diagnostic interview with yes/no responses indicating the presence of DSM-IV PTSD symptoms during the past 6 months | Self-Medication Model | For adolescents, past 6-month PTSD was associated with increased odds for cannabis use (OR = 2.50, CI: 1.20–4.80). For household women, past 6-month PTSD was associated with increased odds for cannabis use (OR = 3.50, CI: 2.24–5.45) | Cohen’s d = .51, .69 |
| Trauma exposure, PTSD and risk for alcohol, nicotine, and marijuana dependence in Israel | Walsh et al., 2014 | 1,317 Jewish household residents | Jewish community | Cross-sectional | Structured or semi-structured interview: AUDADIS (CUD module) [past year CUD] | Structured or semi-structured interview: AUDADIS (PTSD module) [past year PTSD symptoms and diagnosis] | Self-Medication Model | PTSD symptoms were associated with increased odds of cannabis dependence (OR =1.1, CI: 1.04–1.2). A PTSD diagnosis was associated with increased odds of cannabis dependence (OR = 2.6, CI: 1.2–5.9) | Cohen’s d = .05, .53 |
| High school students’ posttraumatic symptoms, substance abuse and involvement in violence in the aftermath of war | Schiff et al., 2012 | 4,151 U.S. high school students | U.S. adolescents | Cross-sectional | Self-report: Any cannabis use was assessed via report of any lifetime cannabis use | Self-report: UCLA PTSD-RI (past month PTSD symptoms) | Self-Medication Model | PTSD symptoms increased odds of lifetime cannabis use (OR = 1.04, CI: 1.03–1.05, p < .001) | Cohen’s d = .02 |
| PTSD and cannabis use in a nationally representative sample | Cougle et al., 2011 | 5,672 U.S. adults | U.S. adults | Cross-sectional | Self-report: Any and frequency of cannabis use was assessed via report of lifetime cannabis use and past 12 months use with 6 response options from “none” to “nearly every day”, respectively | Structured or semi-structured interview: WHO’s WMH-CIDI (lifetime and past month PTSD) | Self-Medication Model | Lifetime PTSD was associated with lifetime (OR = 2.4, CI: 1.7 – 3.5, p < .01), current (OR = 1.4, CI: 1.0 – 2.1, p < .01), and daily cannabis use (OR = 1.9, CI: 1.1 – 3.2, p < .05). Current PTSD was related to lifetime (OR = 2.4, CI: 1.6 – 3.5, p < .01), current (OR = 2.0, CI: 1.4 – 3.1, p < .01), and daily cannabis use (OR = 2.1, CI: 1.1 – 3.9, p < .05). | Cohen’s d = .48, .19, .35, .48, .38, .41 |
| Interpersonal victimization, PTSD, and change in adolescent substance use prevalence over a ten-year period | McCart et al., 2011 | 7,329 U.S. adolescents | U.S. adolescents | Longitudinal | Self-report: Non-experimental cannabis use was assessed via participant report of cannabis use at least four times during their lifetime | Structured or semi-structured interview: National Women’s Study PTSD module (lifetime PTSD) | Self-Medication Model | PTSD was significantly associated with both lifetime cannabis use (OR = 3.06, CI: 1.62 – 5.81, p < .001) and lifetime non-experimental cannabis use (OR = 3.53, CI: 1.72 – 7.22, p < .001) over the 10-year period | Cohen’s d = .62, .70 |
| The relationship between substance use and PTSD in a methadone maintenance treatment program | Villagonzalo et al., 2011 | 80 individuals in a methadone maintenance treatment program | Clinical (adults) | Cross-sectional | Self-report: Frequency and quantity of cannabis use was assessed via report of how often used with 5 response options from “only used it once” to “every day”, and how much used per session with 5 response options from “less than .1 g” to “more than 4.0 g” (lifetime cannabis use) | Self-report: PCL (past month PTSD symptoms) | Self-Medication Model | Severity of cannabis use was associated with total PTSD symptoms (F2,65 = 3.71, p < .05) | Cohen’s d = .44 |
| Association between psychopathology and substance use among school-going adolescents in Cape Town, South Africa | Saban et al., 2010 | 939 South African adolescents | South African adolescents | Cross-sectional | Self-report: Any cannabis use was assessed via report of any lifetime cannabis use | Self-report: HTQ (lifetime PTSD) | Self-Medication Model | Grade 11 students had a 2.3% increased risk of cannabis use with every one-point increase in the PTSD total score | Cohen’s d = .01 |
| Substance use, childhood traumatic experience, and PTSD in an urban civilian population | Khoury et al., 2010 | 587 urban primary care patients | Urban community | Cross-sectional | Self-report: KMSK (lifetime CUD) | Self-report: PSS (past 2-week PTSD symptoms) | Self-Medication Model | Lifetime cannabis dependence was associated with PTSD symptoms (F = 10.12, p <.01) and all PTSD symptoms (Intrusive: F = 4.16, p <.05; Avoidance: F = 11.25, p <.01; and Hyperarousal: F = 7.72, p <.01). | Cohen’s d = .26, .17, .28, .23 |
| Identifying risk factors for marijuana use among veterans affairs patients | Goldman et al., 2010 | 5,512 veterans | Veterans / military personnel | Cross-sectional | Self-report: Any cannabis use was assessed via report of any use during the past year | Structured or semi-structured interview: MINI (current PTSD) | Self-Medication Model | PTSD increased odds of past year cannabis use (OR = 1.64, CI: 1.4–2.0) | Cohen’s d = .27 |
| Impact of Hurricane Rita on adolescent substance use | Rohrbach et al., 2009 | 280 U.S. adolescents | U.S. adolescents | Longitudinal | Self-report: Frequency of cannabis use was assessed via report of how often used over past 30 days with 8 response options from “zero” to “more than 100 times” | Self-report: UCLA PTSD-RI (past month PTSD symptoms) | Self-Medication Model | The PTSD severity score was positively associated with increases in cannabis use at 7-month follow up (β = .11, SE = .06, p < .05) | β = .11 |
| PTSD contributes to teen and young adult CUDs | Cornelius et al., 2010 | 693 U.S. teens and young adults | U.S. adolescents and young adults | Cross-sectional | Structured or semi-structured interview: SCID (lifetime CUD) | Structured or semi-structured interview: SCID (lifetime PTSD) | Self-Medication Model | PTSD is directly associated with CUD (β = 0.19, z = 4.40, p < 0.001) | β = .19 |
| Exposure to the tsunami disaster, PTSD symptoms and increased substance use–an Internet based survey of male and female residents of Switzerland | Vetter et al., 2008 | 2,921 Switzerland residents after a natural disaster | Switzerland community | Cross-sectional | Self-report: Any increase in cannabis use was assessed via report of any increase in cannabis use since the tsunami | Self-report: PTSS (past few days PTSD symptoms) | Self-Medication Model | PTSD symptoms were significantly associated with increases in cannabis use following the tsunami for both men (OR = 1.15, CI: 1.06 – 1.25) and women (OR = 1.10, CI: 1.07 – 1.14) | Cohen’s d = .08, .05 |
| Consumption of cigarettes, alcohol, and marijuana among New York City residents six months after the September 11 terrorist attacks | Vlahov et al., 2004 | 1,570 New York residents after 9/11 terrorist attacks | New York community | Longitudinal | Self-report: Frequency of cannabis use was assessed via participant report of the number of times they used cannabis during the past week | Structured or semi-structured interview: National Women’s Study PTSD Module (lifetime PTSD) | Self-Medication Model | Rates of PTSD were statistically similar for individuals who increased cannabis use vs. those who did not (4.0% vs. 1.4%, p = 0.25) | Cohen’s d = .06 |
| PTSD and substance use in inner-city adolescent girls | Lipschitz et al., 2003 | 54 inner-city adolescent girls | U.S. Urban adolescents | Cross-sectional | Structured or semi-structured interview: K-SADS-PL (CUD module) [lifetime CUD symptoms including age of onset] | Self-report: PCL (past month provisional PTSD including age of onset) | Self-Medication Model | PTSD increased likelihood of using cannabis regularly (χ2 = 9.33, df = 1, p = 0.002). Age of onset of PTSD symptoms ranged from 5–17 years (M = 13.5, SD = 3.2). Age of onset of cannabis abuse ranged from 13–18 years (M = 15.0, SD = 1.5) | Cohen’s d = .71 |
3.2. Support for etiologic models of co-occurring RCU and PTSD
Thirty-seven of the 45 (82.2%) studies tested the self-medication model, with 30 (81.1%) reporting significant associations of PTSD with RCU (Table 1). Eight of the 45 (17.8%) studies tested the high-risk model, and seven (87.5%) were supportive, finding associations between RCU with PTSD. None of the 45 (0%) studies tested the shared liability model, highlighting a critical gap to fill in this developing literature.
3.3. RCU assessment
As shown in Table 1, the most common assessment technique for RCU was self-report without the use of a questionnaire (i.e., RCU frequency [number of times used over x amount of time], dichotomous RCU [any use, lifetime use, non-experimental use]) and 90% and 100% of the studies that used continuous RCU frequency and dichotomous RCU were supportive based on significant findings, respectively.
The second most common assessment technique for RCU was a structured or semi-structured interview (i.e., Alcohol Use Disorder and Associated Disabilities Interview Schedule [AUDADIS], Structured Clinical Interview for DSM-IV [SCID-IV], World Health Organization’s Alcohol, Smoking, and Substance Involvement Screening Test [ASSIST], Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version [K-SADS-PL], Semi-Structured Assessment for the Genetics of Alcoholism [SSAGA], Addiction Severity Index [ASI]) and 86.7% of the studies that used structured or semi-structured interviews were supportive based on significant findings.
The third most common assessment technique for RCU was a self-report questionnaire (i.e., Timeline Followback [TLFB], Marijuana Problems Scale [MPS], Cannabis Use Disorders Identification Test – Revised [CUDIT-R], Marijuana Use Grid [MUG], Marijuana Use Form [MUF], Marijuana Consequences Questionnaire [MACQ], Kreek-McHugh-Schluger-Kellogg Scale [KMSK], Cannabis Abuse Screening Test [CAST], Drug Use Disorders Identification Test – Extended [DUDIT-E]) and 66.7% of the studies that used self-report questionnaires were supportive based on significant findings.
The least common assessment technique for RCU was diagnostic codes from medical records (i.e., ICD and DSM diagnostic codes), and 33.3% of the studies that used ICD and DSM diagnostic codes were supportive based on significant findings, respectively.
Twenty-three of 27 (85.2%) studies that used only self-report measures were supportive based on significant findings (Table 1). Twelve of 14 (85.7%) studies that enlisted the use of only structured or semi-structured interviews were supportive based on significant findings. One of three (33.3%) studies that used only DSM or ICD diagnostic codes was supportive based on significant findings. The one study that used both self-report and a structured or semi-structured interview was supportive based on significant findings (Metrik et al., 2016).
3.4. PTSD assessment
As shown in Table 1, the most common assessment technique for PTSD was a self-report questionnaire (i.e., PTSD Checklist [PCL], Primary Care PTSD Screen [PC-PTSD], PTSD Symptom Scale [PSS], University of California, Los Angeles PTSD Reaction Index [UCLA PTSD-RI], Trauma Screening Questionnaire [TSQ], Inventory of Depression and Anxiety Symptoms Scale [IDAS], Harvard Trauma Questionnaire [HTQ], Posttraumatic Stress Scale [PTSS]) and all were 100% supportive except studies that used the PCL, which were 76.5% supportive based on significant findings.
The second most common assessment technique for PTSD was a structured or semi-structured interview (i.e., AUDADIS, Clinician-Administered PTSD Scale [CAPS], National Women’s Study PTSD module, SSAGA, K-SADS-PL, World Health Organization’s World Mental Health Composite International Diagnistic Interview [WHO-WMH-CIDI], Mini International Neuropsychiatric Interview [MINI], SCID-IV, unspecified structured diagnostic interview) and 88.9% of studies that used structured or semi-structured interviews were supportive based on significant findings.
The least common assessment technique for PTSD was diagnostic codes from medical records (i.e., ICD and DSM diagnostic codes), and 33.3% of the studies that used ICD and DSM diagnostic codes were supportive based on significant findings, respectively.
Twenty of the 24 (83.3%) studies that used only self-report measures were supportive based on significant findings (Table 1). Fifteen of the 17 (88.2%) studies that enlisted the use of only structured or semi-structured interviews were supportive based on significant findings. One of the three (33.3%) studies that used only DSM or ICD diagnostic codes was supportive based on significant findings. The one study that used both self-report and a structured or semi-structured interview was supportive based on significant findings (Sanjuan et al., 2019).
3.5. Cross-sectional versus longitudinal studies
Thirty-seven of 45 (82.2%) studies used a cross-sectional design to test the self-medication and high-risk models. The remaining eight of 45 (17.8%) studies utilized a longitudinal study design to test the self-medication and high-risk models. Seven of eight (87.5%) longitudinal studies demonstrated significant associations between RCU and PTSD. Both the self-medication and high-risk models assume that causes precede their effects across time, which is difficult to capture with a cross-sectional study design. However, six of 37 (16.2%) cross-sectional studies tried to address the limitation of time by assessing age of onset of RCU and PTSD, or by using lifetime RCU to predict current PTSD or lifetime PTSD to predict current RCU (Alenko et al., 2019; Allwood et al., 2014; Cougle et al., 2011; de Silva et al., 2016; Lipschitz et al., 2003; Metrik et al., 2016). Specifically, 2 studies assessed age of onset of RCU or CUD and PTSD (Allwood et al., 2014; Lipschitz et al., 2003), 2 studies used lifetime PTSD to predict current RCU (Cougle et al., 2011; Metrik et al., 2016), and 2 studies used lifetime RCU or CUD to predict current PTSD (Alenko et al., 2019; de Silva et al., 2016). All six of these cross-sectional studies (100%) demonstrated significant associations between RCU and PTSD. Out of the remaining 31 cross-sectional studies that did not try to establish a temporal relationship between RCU and PTSD, 23 studies (74.2%) demonstrated significant associations between RCU and PTSD.
3.5.1. Self-medication model
Thirty-two of 45 (71.1%) studies used a cross-sectional design to test the self-medication model. Twenty-six of those 32 (81.3%) cross-sectional studies demonstrated significant associations between RCU and PTSD. Five of 45 (11.1%) studies used a longitudinal design to test the self-medication model. Four of those five (80.0%) longitudinal studies demonstrated significant associations between RCU and PTSD.
3.5.2. High-risk model
Five of 45 (11.1%) studies used a cross-sectional design to test the high-risk model. Four of those five (80.0%) cross-sectional studies demonstrated significant associations between RCU and PTSD. Three of 45 (6.7%) studies used a longitudinal design to test the high-risk model, and all 3 demonstrated significant associations between RCU and PTSD.
3.6. Study samples
Significant associations between RCU and PTSD supporting the self-medication and high-risk developmental models were found across multiple study samples, with the exception of first responders. Of the two clinical adult studies, only one reported significant associations between RCU and PTSD (50%). The only study of a clinical adolescent sample found significant associations between RCU and PTSD (100%). There were three studies of college samples, and two found significant associations between RCU and PTSD, while one did not (66.7%). The studies with only adolescents (n=5) all found significant associations between RCU and PTSD (100%). One study of adolescents and young adults combined did not find significant associations between RCU and PTSD, while two studies did find that they were significantly associated (66.7%). All five studies with community samples found significant associations between RCU and PTSD (100%). The one study using a nationwide sample found significant associations between RCU and PTSD (100%). All but one of the 10 of studies with veterans and military personnel found significant associations between RCU and PTSD (90%), as did the one study of women veterans (100%). Seven United States adult survey samples found significant associations between RCU and PTSD, while one study did not (87.5%). Both studies of trauma-exposed individuals found significant associations between RCU and PTSD (100%). The one study investigating a sample of racial and ethnic minority adults found significant associations between RCU and PTSD (100%). One study with women who identified as sexual minoriies found significant associations between RCU and PTSD, and one did not (50%). No studies with first responders found significant associations between RCU and PTSD (0%).
4. Discussion
This review aimed to synthesize the limited RCU and PTSD literature, which is an emerging area of empirical interest given the potential health-related consequences that have been associated with RCU (Cohen et al., 2019). Following review of the studies fitting inclusion criteria (N = 45), the majority of studies (n = 37) demonstrated a significant association between RCU and PTSD. The variability in findings may be attributable, in part, to methodological issues that permeate the extant literature pertaining to RCU and PTSD. Thus, the second aim of this review was to critically examine the literature to identify such concerns. Discussion of these concerns is separated thematically and recommendations regarding how to approach methodological issues are provided within each section.
4.1. RCU and PTSD co-occurrence
Although the majority of studies identified a significant association between RCU and PTSD, eight studies did not. Each study included in the review had at least one methodological limitation that may have created bias, including the eight studies demonstrating non-significant results. Notably, two of the eight studies that did not find a significant association between RCU and PTSD used a measure assessing PTSD symptoms on a continuum (i.e., PCL, PC-PTSD Screen) and created a dichotomous PTSD diagnosis variable (Brunault et al., 2019; Walukevich-Dienst et al., 2019). Dichotomizing continuous variables can lead to several problems, such as losing variability and reducing statistical power to detect a relation between variables (Altman & Royston, 2006). Two studies that did not find a significant association between RCU and PTSD used diagnostic codes from medical records to assess lifetime CUD and PTSD (Lekoubou et al., 2020; Welsh et al., 2017). Both studies tested the self-medication model and it is possible that the self-medicating relationship between PTSD and CUD is more immediate and thus needs to be assessed closer in time opposed to within a lifetime. One study that did not demonstrate associations between RCU and PTSD used a more stringent significance threshold (i.e., p = .01) to control for multiple comparisons, but the association between cannabis dependence and PTSD would have been considered significant under different circumstances with a p-value equal to .02 (Ehlers et al., 2016). One study that did not demonstrate associations between RCU and PTSD excluded cannabis using individuals from their sample for other drug use or alcohol dependence (Johnson et al., 2016). Cannabis users who were excluded from the study were psychiatrically more complex (Johnson et al., 2016), and it is possible that other drug and alcohol use should be taken into account as confounding variables because research shows that nicotine and alcohol use are strongly associated with RCU (Fite et al., 2019; Yurasek et al., 2017). One study that did not demonstrate associations between RCU and PTSD used a measure that has shown to perform poorly with their bisexual sample (MacLeod et al., 2015; Robinson et al., 2016). The other study that did not demonstrate significant associations between RCU and PTSD curated a question with free response to assess RCU frequency during the past week (Vlahov et al., 2004). The unknown psychometric properties of this item may have contributed to not finding an effect.
Although biases were present in the studies that did not find a significant association between RCU and PTSD, it is critical to note that many of these same limitations were also present in the studies that did identify a significant association. For example, Harris and colleagues (2019) used diagnostic codes from medical records to assess lifetime CUD and PTSD when investigating the self-medication model. However, Harris and colleagues (2019) used ICD-10 codes as opposed to the ICD-9-CM codes Lekoubou and colleagues used (2020). In transitioning from ICD-9 to ICD-10, it is unknown why there were decreases in rates of substance use disorders or whether the decrease is related to coding errors (Yoon & Chow, 2017). Additionally, five studies created a dichotomous PTSD diagnosis variable from a measure assessing PTSD symptoms on a continuum and found a significant association between RCU and PTSD despite losing variability (Alenko et al., 2019; de Silva et al., 2016; B. F. Grant et al., 2016; Metrik et al., 2016; Pedersen et al., 2018). Thus, it is likely that there is a relationship between RCU and PTSD that explains their greater-than-chance co-occurrence, but the eight studies that did not find significant associations between RCU and PTSD were likely influenced by another source of error in addition to measurement error. Effect sizes in these eight studies did not vary substantially, ranging from Cohen’s d of −.01 to .39 and β of −.01. Comparitively, effect sizes in the studies that did find significant associations between RCU and PTSD were higher, ranging from Cohen’s d of −.01 to 1.35 and β of .02 to .73, but many were modest with 13 of the 37 studies finding an effect that was considered less than small (i.e., effect size < .20).
4.2. Support for etiologic models of co-occurring RCU and PTSD
The self-medication model presumes that individuals with PTSD use cannabis to cope with distressing symptoms (Khantzian, 1997). The self-medication model is attractive in that it is intuitive to clients with mood and anxiety disorders who report using cannabis without a prescription to self-medicate (Sarvet et al., 2018). Furthermore, there has been an increase in the number of states with medicinal cannabis (35 as of November 2021) that include PTSD as an approved condition for medicinal cannabis. Medicinal cannabis laws may be associated with greater acceptance of the therapeutic value of cannabis, leading individuals to self-medicate. Therefore, it is unsurprising that the majority of studies in the present review tested only the self-medication model and that it has received the most theoretical attention and empirical support (Allwood et al., 2014; Bilevicius et al., 2019; Bordieri et al., 2014; Browne et al., 2018; Buckner et al., 2018; Cornelius et al., 2010; Cougle et al., 2011; Dworkin et al., 2017; Earleywine & Bolles, 2014; Gentes et al., 2016; Goldman et al., 2010; Harford et al., 2018; Harris et al., 2019; Jordan et al., 2020; Kevorkian et al., 2015; Khoury et al., 2010; Lipschitz et al., 2003; McCart et al., 2011; Metrik et al., 2016; Pedersen et al., 2018; Rehder & Bowen, 2019; Rohrbach et al., 2009; Saban et al., 2010; Sanjuan et al., 2019; Schiff et al., 2012; Sommer et al., 2018; Vetter et al., 2008; Villagonzalo et al., 2011; Walsh, Elliott, et al., 2014; Walsh, Resnick, et al., 2014). Notably, the high-risk model was supported by seven of the eight studies in the present review (Alenko et al., 2019; Allan et al., 2019; de Silva et al., 2016; S. Grant et al., 2016; Kerridge et al., 2018; Lee et al., 2018; Miller et al., 2019). Surprisingly, there were no twin/family studies or genetically-informed shared risk studies included in the present review that tested the shared liability model. It is impossible to fully understand the association between RCU and PTSD from both the self-medication and high-risk models’ perspective without first understanding their shared underlying risk. Future research should continue to investigate potentially predictive associations between RCU and PTSD since seven of the eight longitudinal studies in the present review found support for a potentially predictive relationship (4 of 7 self-medication model studies and 3 of 7 high-risk model studies) between the two phenotypes (Allan et al., 2019; Dworkin et al., 2017; Lee et al., 2018; McCart et al., 2011; Miller et al., 2019; Rohrbach et al., 2009; Sanjuan et al., 2019). However, if a longitudinal study found support for the self-medication model or high-risk models, then they did not simultaneously test the opposing direction of effect. Therefore, future research should simultaneously test both the self-medication and high-risk models with longitudinal studies and more sophisticated modeling, such as cross-lagged panel analyses, since they are not mutually exclusive. It may be the case that individuals experience risk from both phenotypes, and it may be that different models explaining the development of co-occurring RCU and PTSD are more relevant for certain individuals. Genetically informed studies can be expensive, but a more feasible genetically-informed option using the shared liability model framework would be to test how shared genetic risk for RCU and PTSD influences these phenotypes in individuals, which could be done both cross-sectionally and longitudinally.
4.3. RCU assessment
Studies that utilized self-report and semi-structured or structured interviews to assess RCU were more likely to find significant results compared to studies that used ICD and DSM diagnostic codes from medical records, which is likely due to increased variability in responses and the limitation that electronic health record data may lead to underestimates of cannabis use (Palamar & Le, 2022). A majority of studies found small to small-medium inter-relations of RCU and PTSD. Notably, studies that used the TLFB and K-SADS-PL to assess RCU were more likely to find medium and large effects compared to other assessment tools.
Cannabis used to be seen primarily as a recreational drug, but medicinal cannabis has rapidly increased over the past couple decades (Carliner et al., 2017). As the motives behind RCU evolve (e.g., enhancement, coping, social, medicinal; Buckner et al., 2019), assessment methods should also evolve. This evolution of RCU assessment is reflected in most of the studies included in the present review.
Despite the concern regarding the variability in assessment present within the RCU literature (e.g., experimental use, regular/frequent use, problematic/disordered use), the majority of studies (6 of 8; 75%) included in the present review that were conducted from 2000–2010 assessed any RCU or frequency of RCU without the use of a standardized self-report assessment tool (Goldman et al., 2010; Lipschitz et al., 2003; Rohrbach et al., 2009; Saban et al., 2010; Vetter et al., 2008; Vlahov et al., 2004), which does not attempt to capture motives behind RCU or assess problematic or disordered RCU. The majority of studies (19 of 37; 51.2%) conducted from 2011–2020 assessed problematic and/or disordered RCU. Although more studies between 2011–2020 compared to 2000–2010 (51.2% vs. 25%) assessed RCU in a way that attempted to capture problematic and/or disordered use, the use of non-standardized self-report assessment tools did not decrease a study’s chance of finding significant associations between RCU and PTSD in either decade.
The majority of studies from 2011–2020 included in the present review used some form of structured or semi-structured interview or standardized self-report measurement tool. While structured or semi-structured interviews may be more ideal for assessment of problematic and/or disordered behaviors compared to self-report measures, they are not always the most feasible option. Therefore, it is equally as important for psychometrically valid self-report measures assessing problematic and/or disordered RCU to be developed. A recent review of screening tools for CUDs and their adaptation for DSM-5 revealed that 5 self-report questionnaires (i.e., Problematic Use of Marijuana [PUM], CAGE – cannabis, Severity of Dependence Scale [SDS], CAST, CUDIT-R) were the only ones that had good characteristics for a short screening tool (Artigaud et al., 2020). Additionally, the Screen of Drug Use (SoDU) was found to be a promising screening tool for CUD in primary care settings (Tiet et al., 2019). Two studies in the present review utilized measures from their list (Brunault et al., 2019; Jordan et al., 2020). Surprisingly, the increased quality of information gathered from assessment tools, such as the CUDIT-R and CAST which attempt to capture problematic and/or disordered recreational use, did not increase the chance of finding significant results or a larger effect compared to non-standardized and other self-report assessment techniques. The standardized assessment tool that was more likely to find a larger effect was the TLFB, which does not capture problematic and/or disordered recreational use and instead asks individuals to retrospectively estimate the number of days of RCU anywhere from 7 days to 2 years prior (Robinson et al., 2014). Lifetime or frequent RCU may not prove clinically meaningful; however, problematic or disordered recreational use that does not meet the threshold of CUD may still be relevant. Future research should focus on CUD opposed to RCU in general and include continuous CUD symptom scores to further explicate these associations. Additionally, future research investigating the self-medicating association between RCU and PTSD should strongly consider assessing trauma-specific, coping-related use.
4.4. PTSD assessment
Studies that utilized self-report and semi-structured or structured interviews to assess PTSD were more likely to find significant results compared to studies that used ICD and DSM diagnostic codes from medical records, which is likely due to increased variability in responses. A majority of studies found small to small-medium inter-relations of PTSD and RCU. However, the study that used the K-SADS-PL was more likely to find a medium effect between PTSD and RCU. Additionally, the K-SADS-PL assesses the onset of PTSD symptoms, which was wisely used in two cross-sectional studies included in this review to help establish the onset of PTSD prior to RCU (Allwood et al., 2014; Lipschitz et al., 2003).
The use of semi-structured or structured interviews are beneficial in the assessment of PTSD and about 40% of the studies in the present review utilized such methods. Moreover, the remaining studies implemented standardized self-report measures of PTSD with the exception of the 3 studies that used ICD or DSM diagnostic codes (Harris et al., 2019; Lekoubou et al., 2020; Welsh et al., 2017). One study used the PC-PTSD, which is a brief screening tool used in primary care settings to identify individuals likely meeting diagnostic criteria for PTSD (Cameron & Gusman, 2003). However, the tool only has four items and although psychometrically sound, provides little detail regarding symptom severity beyond presence of at least one symptom within each PTSD symptom cluster. More continuous measures of PTSD would likely add more detailed insight into the relations between PTSD and RCU. Twenty-four studies took this approach and measured PTSD symptoms using a standardized self-report measure, semi-structured, or structured interview without dichotomizing into a PTSD diagnosis. Four studies used the CAPS (Weathers et al., 2018), which is the gold standard in PTSD assessment, and all found significant associations between greater PTSD symptoms and RCU (Bordieri et al., 2014; Gentes et al., 2016; Metrik et al., 2016; Sanjuan et al., 2019). Sanjuan and colleagues (2019) utilized both the PCL and the CAPS in their longitudinal study investigating the self-medication model and found significant associations between daily peak PTSD symptoms and RCU. The PCL and the CAPS are the most widely used, psychometrically sound PTSD assessment tools and future research should continue to utilize them when investing the association between PTSD and RCU.
The PCL is a self-report assessment tool that can be used to capture PTSD symptoms, as well as make a provisional PTSD diagnosis, more accurately than other self-report assessment tools since its questions correspond directly to the DSM-IV (Weathers et al., 1993) or DSM-5 (Weathers et al., 2013) symptom criteria for PTSD. The PCL was utilized by 17 studies included in this review and 13 of which found significant results. Therefore, studies that used the PCL were more likely to find significant results when investigating the association between PTSD and RCU in adult samples. In comparison, the next most used self-report assessment tool was the UCLA PTSD-RI, which was used by two studies included in this review and both found significant results. However, the UCLA PTSD-RI is only validated for children and adolescents (Kaplow et al., 2020), and should continue to be used when investigating the association between PTSD and RCU in children and adolescent samples.
The CAPS is a structured interview that can be used to capture PTSD symptoms over the past week, as well as make a current (past month) or lifetime diagnosis (Weathers et al., 2018). The CAPS was utilized by four studies included in this review and all found significant results. However, three of those studies were cross-sectional and did not fully utilize the quality of information gathered by the gold standard in PTSD assessment. Similar to the K-SADS-PL, the CAPS also assesses time of onset of symptoms, which could be used to help establish if PTSD developed before or after RCU when investigating the association between the two. However, the K-SADS-PL is only validated for children and adolescents (Kaufman et al., 1997). A version of the CAPS for children and adolescents ages 7 and above is available (Pynoos et al., 2015). Thus, the CAPS should continue to be used when investigating the association between PTSD and RCU and future studies should utilize the time of onset of symptoms information to help establish the direction of effect or predictability.
4.5. Cross-sectional versus longitudinal studies
A majority of studies in this review utilized a cross-sectional opposed to a longitudinal study design to investigate the association between RCU and PTSD. The self-medication and high-risk models presume that RCU or PTSD comes before the other, thus a longitudinal study design is more desirable to investigate the direction of effect. All except one of the longitudinal studies included in this review found support for a potentially predictive relationship between RCU and PTSD regarding the self-medication model, which should be given more weight than the results of cross-sectional studies included in this review. Most cross-sectional study results are just an association rather than predictive. However, all of the cross-sectional studies included in this review that tried to establish PTSD or RCU prior to the other found support for a potentially predictive relationship between the two phenotypes. A majority of the remaining cross-sectional studies included in this review found support for an association between RCU and PTSD regardless of the various ways RCU and PTSD were assessed regarding time. For example, a study testing the self-medication model used past month PTSD symptoms to predict past 6-month CUD symptoms and found a significant association. Similarly, another study testing the self-medication model used past 2-weeks PTSD symptoms to predict a lifetime CUD diagnosis and found a significant association. Regardless of study design, RCU and PTSD frequently co-occur, and the self-medication and high-risk models posited to explain the association appear to be well-supported (Allan et al., 2019; Dworkin et al., 2017; Lee et al., 2018; McCart et al., 2011; Miller et al., 2019; Rohrbach et al., 2009; Sanjuan et al., 2019).
4.6. Conclusions and future directions
The intersection of RCU and PTSD is a growing field of interest; however, the existing literature has several methodological concerns. As RCU increases in prevalence and potentially contributes to negative physical and mental health outcomes, it is critical to take a more hierarchical approach to the investigation of RCU, particularly as it relates to PTSD. Overall, the results of this systematic review suggest that the literature to date does provide evidence for the self-medication and high-risk models posited to explain the development of co-occurring RCU and PTSD across multiple populations (e.g., clinical, community, veterans, adolescents, adults), but that empirically rigorous investigation of the self-medication, high-risk, and shared liability model is lacking due to numerous methodological limitations. First, future studies should use longitudinal data thoroughly, which includes the use of time of onset of RCU and PTSD in order to further establish how co-occurring RCU and PTSD develops in reference to the self-medication and high-risk models. Additionally, using longitudinal data thoroughly involves simultaneously testing both the self-medication and high-risk models because they are not mutually exclusive and it is likely that RCU and PTSD interact to maintain each other once developed. Second, future studies should continue using conceptually and psychometrically valid self-report and structured or semi-structured interviews in the assessment of RCU and PTSD to capture the full RCU spectrum (i.e., any use through CUD symptom severity) and PTSD symptom severity, respectively. Additionally, future research that seeks to provide sound evidence for the self-medication model should focus on incorporating an assessment tool to measure the motivation of someone’s RCU that includes coping with symptoms as a separate indicator. Assessment of polysubstance use (i.e., alcohol, tobacco/nicotine) is also warranted as alcohol, tobacco, and cannabis consumption is associated with increased odds of same-day substance co- and tri-use (Roche et al., 2019). Third, although the current evidence supports a bidirectional developmental relationship between RCU and PTSD, the current literature is not definitive since samples have not been free of RCU and PTSD at the start of the studies included in this review. Future studies may benefit from using samples that have not been trauma exposed or initiated cannabis use prior to the study in order to investigate how RCU and trauma/PTSD develop when one has truly not been experienced before the other. Fourth, future studies should include genetically informed studies and genetically-informed shared risk studies testing the non-mutually exclusive shared liability model, which suggests that RCU and PTSD co-occur due to common familial risk factors (i.e., shared genes, common environment, or both). Taken together, future research will be able to obtain a more comprehensive understanding of how RCU and PTSD may be associated with one another.
Acknowledgements
Funding for this research and manuscript preparation was supported by the National Institute on Drug Abuse grant F31 DA048559 to Mr. Hicks. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the the National Institutes of Health. The authors gratefully acknowledge Drs. Michael Southam-Gerow and Sergio Chaparro for their contribution to the project.
References
- Alenko A, Berhanu H, Abera Tareke A, Reta W, Bariso M, Mulat E, Kenenisa C, Debebe W, Tolesa K, & Girma S (2019). Posttraumatic Stress Disorder and Associated Factors Among Drivers Surviving Road Traffic Crashes in Southwest Ethiopia. Neuropsychiatr Dis Treat, 15, 3501–3509. 10.2147/NDT.S233976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan NP, Ashrafioun L, Kolnogorova K, Raines AM, Hoge CW, & Stecker T (2019, Nov). Interactive effects of PTSD and substance use on suicidal ideation and behavior in military personnel: Increased risk from marijuana use. Depress Anxiety, 36(11), 1072–1079. 10.1002/da.22954 [DOI] [PubMed] [Google Scholar]
- Allwood MA, Esposito-Smythers C, Swenson LP, & Spirito A (2014). Negative cognitions as a moderator in the relationship between PTSD and substance use in a psychiatrically hospitalized adolescent sample. J Trauma Stress, 27(2), 208–216. 10.1002/jts.21907 [DOI] [PubMed] [Google Scholar]
- Allwood MA, Esposito-Smythers C, Swenson LP, & Spirito A (2014, Apr). Negative cognitions as a moderator in the relationship between PTSD and substance use in a psychiatrically hospitalized adolescent sample. J Trauma Stress, 27(2), 208–216. 10.1002/jts.21907 [DOI] [PubMed] [Google Scholar]
- Altman DG, & Royston P (2006, May 6). The cost of dichotomising continuous variables. BMJ, 332(7549), 1080. 10.1136/bmj.332.7549.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). American Psychiatric Pub. [Google Scholar]
- Artigaud L, Fener C, Bisch M, Schwan R, Schwitzer T, De Ternay J, Adamson SJ, Rolland B, & Laprevote V (2020, May 28). [Screening tools for cannabis use disorders and their adaptation to DSM-5: A literature review]. Encephale. 10.1016/j.encep.2020.02.010 (Les outils de reperage pour les troubles de l’usage du cannabis et leur adaptation au DSM-5 : une revue de litterature.) [DOI] [PubMed] [Google Scholar]
- Asbridge M, Duff C, Marsh DC, & Erickson PG (2014). Problems with the identification of ‘problematic’ cannabis use: examining the issues of frequency, quantity, and drug use environment. Eur Addict Res, 20(5), 254–267. 10.1159/000360697 [DOI] [PubMed] [Google Scholar]
- Bedard-Gilligan M, Garcia N, Zoellner LA, & Feeny NC (2018). Alcohol, cannabis, and other drug use: Engagement and outcome in PTSD treatment. Psychology of Addictive Behaviors, 32(3), 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenz EC, McNett S, & Paltell K (2019). Development of comorbid PTSD and substance use disorders. In Vujanovic AA & Back SE (Eds.), Posttraumatic stress and substance use disorders: A comprehensive clinical handbook. Routledge. [Google Scholar]
- Berg CJ, Stratton E, Schauer GL, Lewis M, Wang Y, Windle M, & Kegler M (2015, Jan). Perceived harm, addictiveness, and social acceptability of tobacco products and marijuana among young adults: marijuana, hookah, and electronic cigarettes win. Subst Use Misuse, 50(1), 79–89. 10.3109/10826084.2014.958857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilevicius E, Sommer JL, Asmundson GJG, & El-Gabalawy R (2019, Nov). Associations of PTSD, chronic pain, and their comorbidity on cannabis use disorder: Results from an American nationally representative study. Depress Anxiety, 36(11), 1036–1046. 10.1002/da.22947 [DOI] [PubMed] [Google Scholar]
- Bordieri MJ, Tull MT, McDermott MJ, & Gratz KL (2014, Oct 1). The Moderating Role of Experiential Avoidance in the Relationship between Posttraumatic Stress Disorder Symptom Severity and Cannabis Dependence. J Contextual Behav Sci, 3(4), 273–278. 10.1016/j.jcbs.2014.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Back SE, & Coffey SF (2004). Substance abuse and posttraumatic stress disorder. Current directions in psychological science, 13(5), 206–209. [Google Scholar]
- Browne KC, Dolan M, Simpson TL, Fortney JC, & Lehavot K (2018, Sep). Regular past year cannabis use in women veterans and associations with sexual trauma. Addict Behav, 84, 144–150. 10.1016/j.addbeh.2018.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunault P, Lebigre K, Idbrik F, Mauge D, Adam P, El Ayoubi H, Hingray C, Barrault S, Grall-Bronnec M, Ballon N, & El-Hage W (2019). Posttraumatic Stress Disorder Is a Risk Factor for Multiple Addictions in Police Officers Hospitalized for Alcohol. Eur Addict Res, 25(4), 198–206. 10.1159/000499936 [DOI] [PubMed] [Google Scholar]
- Buckner JD, Jeffries ER, Crosby RD, Zvolensky MJ, Cavanaugh CE, & Wonderlich SA (2018). The impact of PTSD clusters on cannabis use in a racially diverse trauma-exposed sample: An analysis from ecological momentary assessment. Am J Drug Alcohol Abuse, 44(5), 532–542. 10.1080/00952990.2018.1430149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Walukevich KA, & Lewis EM (2019, Jan). Cannabis use motives on weekends versus weekdays: Direct and indirect relations with cannabis use and related problems. Addict Behav, 88, 56–60. 10.1016/j.addbeh.2018.08.012 [DOI] [PubMed] [Google Scholar]
- Cameron RP, & Gusman D (2003). The primary care PTSD screen (PC-PTSD): development and operating characteristics. Primary care psychiatry, 9(1), 9–14. [Google Scholar]
- Carliner H, Brown QL, Sarvet AL, & Hasin DS (2017, Nov). Cannabis use, attitudes, and legal status in the U.S.: A review. Prev Med, 104, 13–23. 10.1016/j.ypmed.2017.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen K, Weizman A, & Weinstein A (2019, May). Positive and Negative Effects of Cannabis and Cannabinoids on Health. Clin Pharmacol Ther, 105(5), 1139–1147. 10.1002/cpt.1381 [DOI] [PubMed] [Google Scholar]
- Cornelius JR, Kirisci L, Reynolds M, Clark DB, Hayes J, & Tarter R (2010, Feb). PTSD contributes to teen and young adult cannabis use disorders. Addict Behav, 35(2), 91–94. 10.1016/j.addbeh.2009.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, & Hawkins KA (2011). Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav, 25(3), 554–558. 10.1037/a0023076 [DOI] [PubMed] [Google Scholar]
- Cougle JR, Bonn-Miller MO, Vujanovic AA, Zvolensky MJ, & Hawkins KA (2011, Sep). Posttraumatic stress disorder and cannabis use in a nationally representative sample. Psychol Addict Behav, 25(3), 554–558. 10.1037/a0023076 [DOI] [PubMed] [Google Scholar]
- de Silva VA, Jayasekera N, & Hanwella R (2016). Cannabis use among Navy personnel in Sri Lanka: a cross sectional study. BMC Res Notes, 9, 174. 10.1186/s13104-016-1988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva VA, Jayasekera N, & Hanwella R (2016, Mar 17). Cannabis use among Navy personnel in Sri Lanka: a cross sectional study. BMC Res Notes, 9, 174. 10.1186/s13104-016-1988-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin ER, Kaysen D, Bedard-Gilligan M, Rhew IC, & Lee CM (2017, Nov). Daily-level associations between PTSD and cannabis use among young sexual minority women. Addict Behav, 74, 118–121. 10.1016/j.addbeh.2017.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earleywine M, & Bolles JR (2014, Jul-Aug). Marijuana, expectancies, and post-traumatic stress symptoms: a preliminary investigation. J Psychoactive Drugs, 46(3), 171–177. 10.1080/02791072.2014.920118 [DOI] [PubMed] [Google Scholar]
- Ehlers CL, Kim C, Gilder DA, Stouffer GM, Caetano R, & Yehuda R (2016, Dec). Lifetime history of traumatic events in a young adult Mexican American sample: Relation to substance dependence, affective disorder, acculturation stress, and PTSD. J Psychiatr Res, 83, 79–85. 10.1016/j.jpsychires.2016.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergusson DM, Swain-Campbell NR, & Horwood LJ (2002, Aug). Deviant peer affiliations, crime and substance use: a fixed effects regression analysis. J Abnorm Child Psychol, 30(4), 419–430. 10.1023/a:1015774125952 [DOI] [PubMed] [Google Scholar]
- Fite PJ, Brown S, Hossain W, Manzardo A, Butler MG, & Bortolato M (2019, Jan). Tobacco and cannabis use in college students are predicted by sex-dimorphic interactions between MAOA genotype and child abuse. CNS Neurosci Ther, 25(1), 101–111. 10.1111/cns.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentes EL, Schry AR, Hicks TA, Clancy CP, Collie CF, Kirby AC, Dennis MF, Hertzberg MA, Beckham JC, & Calhoun PS (2016, May). Prevalence and correlates of cannabis use in an outpatient VA posttraumatic stress disorder clinic. Psychol Addict Behav, 30(3), 415–421. 10.1037/adb0000154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman M, Suh JJ, Lynch KG, Szucs R, Ross J, Xie H, O’Brien CP, & Oslin DW (2010, Mar). Identifying risk factors for marijuana use among veterans affairs patients. J Addict Med, 4(1), 47–51. 10.1097/ADM.0b013e3181b18782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, & Hasin DS (2016, Jan). Epidemiology of DSM-5 Drug Use Disorder: Results From the National Epidemiologic Survey on Alcohol and Related Conditions-III. JAMA Psychiatry, 73(1), 39–47. 10.1001/jamapsychiatry.2015.2132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Pedersen ER, & Neighbors C (2016, May). Associations of Posttraumatic Stress Disorder Symptoms With Marijuana and Synthetic Cannabis Use Among Young Adult U.S. Veterans: A Pilot Investigation. J Stud Alcohol Drugs, 77(3), 509–514. 10.15288/jsad.2016.77.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford TC, Chen CM, Kerridge BT, & Grant BF (2018, Apr). Self- and other-directed forms of violence and their relationship with lifetime DSM-5 psychiatric disorders: Results from the National Epidemiologic Survey on Alcohol Related Conditions-III (NESARC-III). Psychiatry Res, 262, 384–392. 10.1016/j.psychres.2017.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S, Dykxhoorn J, Hollander AC, Dalman C, & Kirkbride JB (2019, Nov). Substance use disorders in refugee and migrant groups in Sweden: A nationwide cohort study of 1.2 million people. PLoS Med, 16(11), e1002944. 10.1371/journal.pmed.1002944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday SB, & Pedersen ER (2017, Oct). The association between discharge status, mental health, and substance misuse among young adult veterans. Psychiatry Res, 256, 428–434. 10.1016/j.psychres.2017.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MJ, Pierce JD, Mavandadi S, Klaus J, Defelice D, Ingram E, & Oslin DW (2016, Jan 15). Mental health symptom severity in cannabis using and non-using Veterans with probable PTSD. J Affect Disord, 190, 439–442. 10.1016/j.jad.2015.10.048 [DOI] [PubMed] [Google Scholar]
- Jordan HR, Madson MB, Bravo AJ, Pearson MR, & Protective Strategies Study T (2020). Post-traumatic stress and marijuana outcomes: The mediating role of marijuana protective behavioral strategies. Subst Abus, 41(3), 375–381. 10.1080/08897077.2019.1635965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplow JB, Rolon-Arroyo B, Layne CM, Rooney E, Oosterhoff B, Hill R, Steinberg AM, Lotterman J, Gallagher KAS, & Pynoos RS (2020, Jan). Validation of the UCLA PTSD Reaction Index for DSM-5: A Developmentally Informed Assessment Tool for Youth. J Am Acad Child Adolesc Psychiatry, 59(1), 186–194. 10.1016/j.jaac.2018.10.019 [DOI] [PubMed] [Google Scholar]
- Karila L, Roux P, Rolland B, Benyamina A, Reynaud M, Aubin HJ, & Lancon C (2014). Acute and long-term effects of cannabis use: a review. Curr Pharm Des, 20(25), 4112–4118. 10.2174/13816128113199990620 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, & Ryan N (1997, Jul). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry, 36(7), 980–988. 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Kerridge BT, Pickering R, Chou P, Saha TD, & Hasin DS (2018). DSM-5 cannabis use disorder in the national epidemiologic survey on alcohol and related conditions-III: gender-specific profiles. Addictive Behaviors, 76, 52–60. [DOI] [PubMed] [Google Scholar]
- Kevorkian S, Bonn-Miller MO, Belendiuk K, Carney DM, Roberson-Nay R, & Berenz EC (2015, Sep). Associations among trauma, posttraumatic stress disorder, cannabis use, and cannabis use disorder in a nationally representative epidemiologic sample. Psychol Addict Behav, 29(3), 633–638. 10.1037/adb0000110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khantzian EJ (1997, Jan-Feb). The self-medication hypothesis of substance use disorders: a reconsideration and recent applications. Harv Rev Psychiatry, 4(5), 231–244. 10.3109/10673229709030550 [DOI] [PubMed] [Google Scholar]
- Khoury L, Tang YL, Bradley B, Cubells JF, & Ressler KJ (2010, Dec). Substance use, childhood traumatic experience, and Posttraumatic Stress Disorder in an urban civilian population. Depress Anxiety, 27(12), 1077–1086. 10.1002/da.20751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, & Friedman MJ (2013, Oct). National estimates of exposure to traumatic events and PTSD prevalence using DSM-IV and DSM-5 criteria. J Trauma Stress, 26(5), 537–547. 10.1002/jts.21848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, & Markon KE (2006). Reinterpreting comorbidity: a model-based approach to understanding and classifying psychopathology. Annu Rev Clin Psychol, 2, 111–133. 10.1146/annurev.clinpsy.2.022305.095213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Brook JS, Finch SJ, & Brook DW (2018, Jan 2). Trajectories of cannabis use beginning in adolescence associated with symptoms of posttraumatic stress disorder in the mid-thirties. Subst Abus, 39(1), 39–45. 10.1080/08897077.2017.1363121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekoubou A, Fox J, Bishu KG, & Ovbiagele B (2020, Jul). Trends in documented cannabis use disorder among hospitalized adult epilepsy patients in the United States. Epilepsy Res, 163, 106341. 10.1016/j.eplepsyres.2020.106341 [DOI] [PubMed] [Google Scholar]
- Lipschitz DS, Rasmusson AM, Anyan W, Gueorguieva R, Billingslea EM, Cromwell PF, & Southwick SM (2003). Posttraumatic stress disorder and substance use in inner-city adolescent girls. J Nerv Ment Dis, 191(11), 714–721. 10.1097/01.nmd.0000095123.68088.da [DOI] [PubMed] [Google Scholar]
- Lipschitz DS, Rasmusson AM, Anyan W, Gueorguieva R, Billingslea EM, Cromwell PF, & Southwick SM (2003, Nov). Posttraumatic stress disorder and substance use in inner-city adolescent girls. J Nerv Ment Dis, 191(11), 714–721. 10.1097/01.nmd.0000095123.68088.da [DOI] [PubMed] [Google Scholar]
- MacLeod MA, Bauer GR, MacKay J, Robinson M, & Ross LE (2015). Notes on measuring posttraumatic stress disorder in bisexuals using the PTSD Checklist–Civilian Version (PCL-C). Journal of Bisexuality, 15(1), 69–81. [Google Scholar]
- McCart MR, Zajac K, Danielson CK, Strachan M, Ruggiero KJ, Smith DW, Saunders BE, & Kilpatrick DG (2011). Interpersonal victimization, posttraumatic stress disorder, and change in adolescent substance use prevalence over a ten-year period. J Clin Child Adolesc Psychol, 40(1), 136–143. 10.1080/15374416.2011.533411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCauley JL, Killeen T, Gros DF, Brady KT, & Back SE (2012, Sep 1). Posttraumatic Stress Disorder and Co-Occurring Substance Use Disorders: Advances in Assessment and Treatment. Clin Psychol (New York), 19(3). 10.1111/cpsp.12006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Jackson K, Bassett SS, Zvolensky MJ, Seal K, & Borsari B (2016). The mediating roles of coping, sleep, and anxiety motives in cannabis use and problems among returning veterans with PTSD and MDD. Psychol Addict Behav, 30(7), 743–754. 10.1037/adb0000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metrik J, Jackson K, Bassett SS, Zvolensky MJ, Seal K, & Borsari B (2016, Nov). The mediating roles of coping, sleep, and anxiety motives in cannabis use and problems among returning veterans with PTSD and MDD. Psychol Addict Behav, 30(7), 743–754. 10.1037/adb0000210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MB, Metrik J, Borsari B, & Jackson KM (2019, Nov). Longitudinal Associations between Sleep, Intrusive Thoughts, and Alcohol Problems Among Veterans. Alcohol Clin Exp Res, 43(11), 2438–2445. 10.1111/acer.14191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & The Prisma Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med, 6(7), e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman E, Risch E, & Kassam-Adams N (2006). Ethical issues in trauma-related research: A review. Journal of Empirical Research on Human Research Ethics, 1(3), 29–46. [DOI] [PubMed] [Google Scholar]
- Palamar JJ, & Le A (2022). Electronic health record data may lead to underestimates of cannabis use-especially among older populations. Journal of the American Geriatrics Society. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen ER, Villarosa-Hurlocker MC, & Prince MA (2018, Jan). Use of Protective Behavioral Strategies among Young Adult Veteran Marijuana Users. Cannabis, 1(1), 14–27. https://www.ncbi.nlm.nih.gov/pubmed/29757318 [PMC free article] [PubMed] [Google Scholar]
- Pynoos RS, Weathers FW, Steinberg AM, Marx BP, Layne CM, Kaloupek DG, Schnurr PP, Keane TM, Blake DD, Newman E, Nader KO, & Kriegler JA (2015). Clinician-Administered PTSD Scale for DSM-5 - Child/Adolescent Version. Scale available from the National Center for PTSD at HYPERLINK “file:///Users/hicksta/Google Drive/VCU/Prelims/For Publication/Drug and Alcohol Dependence/R&R/www.ptsd.va.gov”www.ptsd.va.gov.
- Rehder K, & Bowen S (2019). PTSD Symptom Severity, Cannabis, and Gender: A Zero-Inflated Negative Binomial Regression Model. Subst Use Misuse, 54(8), 1309–1318. 10.1080/10826084.2019.1575421 [DOI] [PubMed] [Google Scholar]
- Robinson M, Sanches M, & MacLeod MA (2016). Prevalence and mental health correlates of illegal cannabis use among bisexual women. Journal of Bisexuality, 16(2), 181–202. [Google Scholar]
- Robinson SM, Sobell LC, Sobell MB, & Leo GI (2014, Mar). Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav, 28(1), 154–162. 10.1037/a0030992 [DOI] [PubMed] [Google Scholar]
- Roche DJO, Bujarski S, Green R, Hartwell EE, Leventhal AM, & Ray LA (2019, Jul 1). Alcohol, tobacco, and marijuana consumption is associated with increased odds of same-day substance co- and tri-use. Drug Alcohol Depend, 200, 40–49. 10.1016/j.drugalcdep.2019.02.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrbach LA, Grana R, Vernberg E, Sussman S, & Sun P (2009, Fall). Impact of hurricane Rita on adolescent substance use. Psychiatry, 72(3), 222–237. 10.1521/psyc.2009.72.3.222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saban A, Flisher AJ, & Distiller G (2010, Dec). Association between psychopathology and substance use among school-going adolescents in Cape Town, South Africa. J Psychoactive Drugs, 42(4), 467–476. 10.1080/02791072.2010.10400709 [DOI] [PubMed] [Google Scholar]
- Sanjuan PM, Pearson MR, Poremba C, Amaro HLA, & Leeman L (2019, Feb 1). An ecological momentary assessment study examining posttraumatic stress disorder symptoms, prenatal bonding, and substance use among pregnant women. Drug Alcohol Depend, 195, 33–39. 10.1016/j.drugalcdep.2018.11.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarvet AL, Wall MM, Keyes KM, Olfson M, Cerda M, & Hasin DS (2018, May 1). Self-medication of mood and anxiety disorders with marijuana: Higher in states with medical marijuana laws. Drug Alcohol Depend, 186, 10–15. 10.1016/j.drugalcdep.2018.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff M, Pat-Horenczyk R, Benbenishty R, Brom D, Baum N, & Astor RA (2012, Oct). High school students’ posttraumatic symptoms, substance abuse and involvement in violence in the aftermath of war. Soc Sci Med, 75(7), 1321–1328. 10.1016/j.socscimed.2012.05.010 [DOI] [PubMed] [Google Scholar]
- Sommer JL, Mota N, Edmondson D, & El-Gabalawy R (2018, Jul - Aug). Comorbidity in illness-induced posttraumatic stress disorder versus posttraumatic stress disorder due to external events in a nationally representative study. Gen Hosp Psychiatry, 53, 88–94. 10.1016/j.genhosppsych.2018.02.004 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Service Administration. (2019). Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health.
- Tiet QQ, Leyva YE, Browne K, & Moos RH (2019). Screen of drug use: Diagnostic accuracy for cannabis use disorder. Addictive Behaviors, 95, 184–188. [DOI] [PubMed] [Google Scholar]
- Turna J, Patterson B, & Van Ameringen M (2017, Nov). Is cannabis treatment for anxiety, mood, and related disorders ready for prime time? Depress Anxiety, 34(11), 1006–1017. 10.1002/da.22664 [DOI] [PubMed] [Google Scholar]
- Vetter S, Rossegger A, Rossler W, Bisson JI, & Endrass J (2008, Mar 19). Exposure to the tsunami disaster, PTSD symptoms and increased substance use - an Internet based survey of male and female residents of Switzerland. BMC Public Health, 8, 92. 10.1186/1471-2458-8-92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villagonzalo KA, Dodd S, Ng F, Mihaly S, Langbein A, & Berk M (2011, Sep-Oct). The relationship between substance use and posttraumatic stress disorder in a methadone maintenance treatment program. Compr Psychiatry, 52(5), 562–566. 10.1016/j.comppsych.2010.10.001 [DOI] [PubMed] [Google Scholar]
- Vlahov D, Galea S, Ahern J, Resnick H, Boscarino JA, Gold J, Bucuvalas M, & Kilpatrick D (2004, May). Consumption of cigarettes, alcohol, and marijuana among New York City residents six months after the September 11 terrorist attacks. Am J Drug Alcohol Abuse, 30(2), 385–407. https://www.ncbi.nlm.nih.gov/pubmed/15230082 [DOI] [PubMed] [Google Scholar]
- Walsh K, Elliott JC, Shmulewitz D, Aharonovich E, Strous R, Frisch A, Weizman A, Spivak B, Grant BF, & Hasin D (2014, Apr). Trauma exposure, posttraumatic stress disorder and risk for alcohol, nicotine, and marijuana dependence in Israel. Compr Psychiatry, 55(3), 621–630. 10.1016/j.comppsych.2013.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K, Resnick HS, Danielson CK, McCauley JL, Saunders BE, & Kilpatrick DG (2014, Mar). Patterns of drug and alcohol use associated with lifetime sexual revictimization and current posttraumatic stress disorder among three national samples of adolescent, college, and household-residing women. Addict Behav, 39(3), 684–689. 10.1016/j.addbeh.2013.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walukevich-Dienst K, Dylanne Twitty T, & Buckner JD (2019, May). Sexual minority women and Cannabis use: The serial impact of PTSD symptom severity and coping motives. Addict Behav, 92, 1–5. 10.1016/j.addbeh.2018.12.012 [DOI] [PubMed] [Google Scholar]
- Watson HJ, Swan A, & Nathan PR (2011, May-Jun). Psychiatric diagnosis and quality of life: the additional burden of psychiatric comorbidity. Compr Psychiatry, 52(3), 265–272. 10.1016/j.comppsych.2010.07.006 [DOI] [PubMed] [Google Scholar]
- Weathers FW, Bovin MJ, Lee DJ, Sloan DM, Schnurr PP, Kaloupek DG, Keane TM, & Marx BP (2018, Mar). The Clinician-Administered PTSD Scale for DSM-5 (CAPS-5): Development and initial psychometric evaluation in military veterans. Psychol Assess, 30(3), 383–395. 10.1037/pas0000486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weathers FW, Litz BT, Herman DS, Huska JA, & Keane TM (1993). The PTSD Checklist (PCL): Reliability, validity, and diagnostic utility. annual convention of the international society for traumatic stress studies, San Antonio, TX, [Google Scholar]
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP (2013). The PTSD checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at www.ptsd.va.gov.
- Weathers FW, Litz BT, Keane TM, Palmieri PA, Marx BP, & Schnurr PP (2013). The PTSD checklist for DSM-5 (PCL-5). Scale available from the National Center for PTSD at HYPERLINK “file:///Users/hicksta/Google Drive/VCU/Prelims/For Publication/Drug and Alcohol Dependence/R&R/www.ptsd.va.gov”www.ptsd.va.gov.
- Welsh JW, Knight JR, Hou SS, Malowney M, Schram P, Sherritt L, & Boyd JW (2017, Jun). Association Between Substance Use Diagnoses and Psychiatric Disorders in an Adolescent and Young Adult Clinic-Based Population. J Adolesc Health, 60(6), 648–652. 10.1016/j.jadohealth.2016.12.018 [DOI] [PubMed] [Google Scholar]
- Yoon J, & Chow A (2017, Aug 17). Comparing chronic condition rates using ICD-9 and ICD-10 in VA patients FY2014–2016. BMC Health Serv Res, 17(1), 572. 10.1186/s12913-017-2504-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurasek AM, Aston ER, & Metrik J (2017). Co-use of alcohol and cannabis: A review. Current Addiction Reports, 4(2), 184–193. [DOI] [PMC free article] [PubMed] [Google Scholar]


