Abstract
Background
Respiratory symptoms are associated with coronavirus disease 2019 (COVID-19) outcomes. However, the impacts of upper and lower respiratory symptoms on COVID-19 outcomes in the same population have not been compared. The objective of this study was to characterize upper and lower respiratory symptoms and compare their impacts on outcomes of hospitalized COVID-19 patients.
Methods
This was a multicenter, retrospective cohort study; the database from the Japan COVID-19 Task Force was used. A total of 3314 COVID-19 patients were included in the study, and the data on respiratory symptoms were collected. The participants were classified according to their respiratory symptoms (Group 1: no respiratory symptoms, Group 2: only upper respiratory symptoms, Group 3: only lower respiratory symptoms, and Group 4: both upper and lower respiratory symptoms). The impacts of upper and lower respiratory symptoms on the clinical outcomes were compared. The primary outcome was the percentage of patients with poor clinical outcomes, including the need for oxygen supplementation via high-flow oxygen therapy, mechanical ventilation, and extracorporeal membrane oxygenation or death.
Results
Of the 3314 COVID-19 patients, 605, 1331, 1229, and 1149 were classified as Group 1, Group 2, Group 3, and Group 4, respectively. In univariate analysis, patients in Group 2 had the best clinical outcomes among all groups (odds ratio [OR]: 0.21, 95% confidence interval [CI]: 0.11–0.39), while patients in Group 3 had the worst outcomes (OR: 3.27, 95% CI: 2.43–4.40). Group 3 patients had the highest incidence of pneumonia, other complications due to secondary infections, and thrombosis during the clinical course.
Conclusions
Upper and lower respiratory tract symptoms had vastly different impacts on the clinical outcomes of COVID-19.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-022-02222-3.
Keywords: SARS-CoV-2 infection, COVID-19, Upper respiratory tract symptoms, Lower respiratory tract symptoms, Primary care
Background
The most common symptoms of coronavirus disease 2019 (COVID-19) are cough, myalgia, and headache [1]. Additionally, various symptoms including gastrointestinal symptoms (diarrhea), dysgeusia, and dysosmia have been reported in COVID-19 patients [2, 3]. Of the 1.3 million patients reported by the Centers for Disease Control and Prevention (CDC) at the end of May 2020, 14% were hospitalized, 2% were treated in the intensive care unit (ICU), and 5% died [2, 4]. In recent years, several predictive tools have been proposed and used to identify patients prone to severe disease based on epidemiological, clinical, and laboratory characteristics [5, 6]. Primary physicians need to identify patients prone to severe outcomes based on limited clinical information and direct them to the appropriate higher-level medical facilities. Data on respiratory symptoms can be easily obtained during patient visits and could be crucial for primary care physicians.
Upper respiratory symptoms were reported to be present more frequently in COVID-19 than in the influenza virus infection [7, 8]. While sore throat and nasal discharge were reported in approximately 14.4% and 7.7% of the cases [9], respectively, dysgeusia or dysosmia were observed in 62% of the cases and were considered typical upper respiratory symptoms [8, 10]. Angiotensin-converting enzyme 2 (ACE2) receptors are highly expressed in the nasal epithelium, acting as entry and replication points for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [11], causing dysfunction of the olfactory neurons and taste buds and resulting in dysgeusia or dysosmia [12], although the exact mechanism is still unknown [13]. Additionally, dysosmia and dysgeusia were associated with the medical history of COVID-19 patients [12–15], with a higher incidence in younger adults and women with no comorbidities [12]. Dysosmia and dysgeusia occur in more than half of COVID-19 patients [8, 10]; however, previous studies have revealed an incidence of 4% in hospitalized patients [12, 16]. Thus, there could be an inverse association between dysosmia/dysgeusia and favorable clinical outcomes [13–15].
In the context of lower respiratory symptoms, a systematic review of 152 previous studies suggested cough as the most common symptom of COVID-19, occurring in approximately 50% of the cases [9]. Other lower respiratory symptoms such as sputum production and dyspnea were observed in approximately 25–30% of the cases [9]. Lower respiratory symptoms of cough and dyspnea indicate pneumonia and are associated with severe clinical outcomes [2, 6, 17, 18]. Additionally, some studies have suggested that cough and sputum production during the clinical course were caused by secondary bacterial infections [19, 20].
Hence, we hypothesized that these respiratory symptoms could be related to the clinical outcomes. However, no reports have compared the effect of both upper and lower respiratory symptoms on clinical outcomes. The aim of this present study was to investigate the impact of respiratory symptoms on the clinical outcomes of patients hospitalized with COVID-19.
Methods
Study design and settings
In this retrospective cohort study, data were collected from the Japan COVID-19 Task Force database from February 2020 to November 2021. The Japan COVID-19 Task Force collected clinical information on patients with COVID-19 aged > 18 years and diagnosed by polymerase chain reaction test or antigen test from 78 hospitals nationwide in Japan [21, 22]. Of the 3431 patients identified, 117 patients were excluded due to unknown respiratory symptoms, and thus, 3314 patients were included in the analysis (Additional file 1: Fig. S1). This study was approved by the Ethics Committee of Keio University School of Medicine (ID: 20200061), and written or oral informed consent was obtained. The study was conducted in accordance with the 1964 Declaration of Helsinki and its later amendments.
Definition of respiratory symptoms
Sore throat, nasal discharge, dysosmia, and dysgeusia were categorized as upper respiratory symptoms, while cough, sputum production, and dyspnea were categorized as lower respiratory symptoms. Based on the presence of upper or lower respiratory symptoms, the enrolled patients were classified into four groups as follows: Group 1: patients with no respiratory symptoms at all during the clinical course; Group 2: patients with only upper respiratory symptoms; Group 3: patients with only lower respiratory symptoms; and Group 4: patients with both upper and lower respiratory symptoms. The presence of all symptoms was reported subjectively by the patients, and the corresponding data were collected by the health care provider through medical interviews.
Data collection
The following patient data were obtained from the electronic case record form: age, sex, body mass index, number of days in the hospital, comorbidities, clinical symptoms and signs, laboratory and radiographic findings, complications after hospitalization, and medications administered during hospital stay (remdesivir, antibiotics, steroids, tocilizumab, baricitinib, and anti-coagulant drugs). In this study, poor clinical outcomes were defined as the need for oxygen supplementation via high-flow oxygen therapy, mechanical ventilation, and extracorporeal membrane oxygenation (ECMO) or death [22, 23]. All laboratory tests and radiography were performed within 48 h of the initial visit or admission based on the clinical care needs of the patients. The primary outcome was the percentage of patients with poor clinical outcomes.
Statistical analysis
For baseline variables, we reported categorial variables as frequencies and proportions and continuous variables as mean and standard error. Data were compared among the four groups using the chi-square test, ANOVA, and Dunnett’s test. In Dunnett’s tests, Group 1 was used as a control and was compared with the other groups. To assess the association between respiratory symptoms and poor clinical outcomes, we performed univariate analysis and calculated the odds ratio (OR). Data are presented as OR with 95% confidence interval (95% CI). Statistical significance was set at p < 0.05. To investigate the relationship between each group and poor prognosis, we performed a multivariable logistic regression analysis to adjust for previously reported factors [24–30]. Specifically, the models were adjusted for patient characteristics, such as age, sex, body mass index (BMI), smoking history, and comorbidities (hypertension, diabetes, cardiovascular disease and chronic kidney disease). We presented the adjusted odds ratio (aOR) with a 95% CI. Statistical significance was set at p < 0.05. All data were analyzed using the JMP 16 program (SAS Institute Japan Ltd., Tokyo, Japan).
Results
Comparison of baseline characteristics between the four groups stratified by respiratory symptoms
Table 1 shows the clinical characteristics of each group. Among the 3314 COVID-19 patients, 605 patients had no respiratory symptoms (Group 1). There were 2709 COVID-19 patients with respiratory symptoms, including 331 patients with only upper respiratory symptoms (Group 2), 1229 patients with only lower respiratory symptoms (Group 3), and 1149 patients with both upper and lower respiratory symptoms (Group 4). On comparing the clinical characteristics of patients in the four groups, parameters such as age and the incidence of hypertension, diabetes, cardiovascular disorders, and chronic kidney disease, generally associated with the severity of COVID-19 [24–28], were significantly lower in Groups 2 and 4 than in Group 1 (p < 0.05). The proportion of males and patients with a higher BMI, considered factors associated with severe outcomes of COVID-19 [29, 30], was significantly higher in Group 3.
Table 1.
Main clinical characteristics of each group
| All (n = 3314) | Group 1 (n = 605) | Group 2 (n = 331) | Group 3 (n = 1229) | Group 4 (n = 1149) | p value | |
|---|---|---|---|---|---|---|
| Age, years | 56.5 ± 17.5 | 62.0 ± 18.6 | 48.2 ± 19.2 | 60.2 ± 15.7 | 52.1 ± 16.3 | < 0.0001a = **/c = ** |
| Sex (Male), % | 67 | 65.5 | 59.8 | 71.3 | 65.4 | 0.0001 b= ** |
| BMI | 24.8 ± 4.8 | 23.9 ± 4.9 | 23.5 ± 4.1 | 25.2 ± 4.9 | 25.2 ± 4.8 | < 0.0001b = **/c = ** |
| Days of onset | 5.74 ± 4.0 | 4.35 ± 3.8 | 4.64 ± 3.3 | 6.29 ± 4.1 | 6.18 ± 4.0 | < 0.0001b = **/c = ** |
| Smoker, % | 14.8 | 12.2 | 16.2 | 13.3 | 17.5 | 0.0090c = ** |
| Hypertension, % | 33.5 | 41.3 | 21 | 40.4 | 25.8 | < 0.0001a = **/c = ** |
| Diabetes, % | 21 | 22.2 | 15.2 | 25.8 | 16.8 | < 0.0001 a = **/c = ** |
| Cardiovascular disorders, % | 10.2 | 13.7 | 4.9 | 13 | 6.9 | < 0.0001 a = **/c = ** |
| COPD, % | 4.1 | 3.9 | 3.1 | 5.7 | 2.8 | 0.0031 |
| Chronic kidney disease, % | 7 | 9.4 | 4.6 | 8.8 | 4.4 | < 0.0001a = */c = ** |
| Cancer, % | 6.6 | 9.4 | 5.2 | 5.8 | 6.4 | 0.0175a = */b = **/c = * |
| Hyperuricemia, % | 9.9 | 11.2 | 7.3 | 10.8 | 9.1 | 0.1456 |
| Chronic liver disease, % | 4.3 | 4.8 | 3.1 | 4.6 | 4.2 | 0.6131 |
| Asthma, % | 7.2 | 5.3 | 5.8 | 7.6 | 8.2 | 0.1056 |
| Fever, % | 80.7 | 72.3 | 71.5 | 81.7 | 86.7 | < 0.0001b = **/c = ** |
| WBC (/μL) | 5771.8 ± 2873.8 | 5560.0 ± 2495.1 | 5371.8 ± 2604.4 | 6266.3 ± 3406.3 | 5466.4 ± 2399.6 | < 0.0001b = ** |
| Neutrophil (/μL) | 4584.2 ± 10,509.2 | 3916.2 ± 2282.4 | 3648.2 ± 2204.4 | 5530.7 ± 1494.3 | 4190.8 ± 4190.8 | 0.0013b = ** |
| Lymphocytes (/μL) | 1145.2 ± 2342.1 | 1126.0 ± 556.0 | 1250.0 ± 595.8 | 1127.0 ± 3326.0 | 1145.3 ± 1920.1 | 0.8689 |
| Neutrophil lymphocyte ratio | 6.13 ± 17.0 | 4.85 ± 7.0 | 3.64 ± 3.6 | 7.27 ± 11.0 | 6.29 ± 25.9 | 0.0018 b = * |
| Eosinophil (/μL) | 42.3 ± 184.9 | 57.1 ± 153.5 | 58.2 ± 188.7 | 36.8 ± 258.9 | 36.1 ± 65.5 | 0.0429 |
| AST (IU/L) | 43.0 ± 58.5 | 38.9 ± 76.9 | 36.1 ± 98.5 | 47.1 ± 49.1 | 42.7 ± 37.3 | 0.0040b = * |
| ALT (IU/L) | 39.6 ± 71.5 | 32.9 ± 38.2 | 39.9 ± 190.5 | 42.1 ± 45.9 | 40.4 ± 38.5 | 0.0755b = * |
| T-B (mg/dL) | 0.7 ± 0.4 | 0.7 ± 0.4 | 0.7 ± 0.3 | 0.7 ± 0.5 | 0.6 ± 0.3 | 0.1603 |
| γ-GTP (IU/L) | 69.0 ± 87.5 | 55.7 ± 67.5 | 47.5 ± 63.1 | 75.7 ± 90.1 | 74.7 ± 97.5 | < 0.0001b = **/c = ** |
| Alb (mg/dL) | 3.7 ± 0.6 | 3.8 ± 0.6 | 4.1 ± 0.5 | 3.5 ± 0.6 | 3.8 ± 0.6 | < 0.0001a = **/b = ** |
| BUN (mg/dL) | 16.9 ± 11.8 | 18.1 ± 12.8 | 14.4 ± 8.8 | 18.9 ± 13.1 | 14.9 ± 9.9 | < 0.0001a = **/c = ** |
| Cr (mg/dL) | 1.1 ± 1.3 | 1.1 ± 1.6 | 1.0 ± 1.7 | 1.1 ± 1.4 | 1.0 ± 1.0 | 0.0220c = * |
| LDH (IU/L) | 292.2 ± 153.2 | 255.9 ± 131.1 | 222.6 ± 91.5 | 333.8 ± 176.0 | 286.3 ± 138.2 | < 0.0001a = **/b = **/c = ** |
| UA (mg/dL) | 4.9 ± 1.8 | 5.2 ± 1.9 | 4.8 ± 1.7 | 4.9 ± 1.9 | 4.7 ± 1.6 | 0.0002a = **/c = ** |
| HbA1c (%) | 6.4 ± 1.3 | 6.3 ± 1.4 | 6.0 ± 1.0 | 6.6 ± 1.4 | 6.2 ± 1.2 | < 0.0001a = */b = ** |
| CRP (mg/dL) | 5.7 ± 27.7 | 3.8 ± 5.1 | 2.7 ± 4.0 | 6.8 ± 6.9 | 6.4 ± 46.5 | 0.0286 |
| Procalcitonin (ng/mL) | 0.6 ± 17.2 | 0.2 ± 1.1 | 0.2 ± 0.8 | 1.4 ± 28.5 | 0.2 ± 0.6 | 0.3982 |
| D-dimer (μg/mL) | 2.2 ± 7.9 | 2.3 ± 8.4 | 1.2 ± 2.1 | 3.1 ± 11.0 | 1.4 ± 3.5 | < 0.0001 |
| Ferritin (ng/mL) | 628.0 ± 760.1 | 518.0 ± 880.4 | 390.9 ± 497.7 | 758.1 ± 768.4 | 611.2 ± 722.5 | < 0.0001b = ** |
| BNP (pg/mL) | 54.8 ± 287.4 | 55.5 ± 146.3 | 21.8 ± 51.3 | 89.1 ± 436.1 | 25.0 ± 91.4 | 0.0004 |
| KL-6 (IU/L) | 328.7 ± 326.3 | 300.9 ± 337.6 | 235.1 ± 116.2 | 393.5 ± 402.2 | 299.1 ± 249.1 | < 0.0001a = */b = ** |
Data are shown as mean ± standard Deviation (SD)
BMI body mass index, COPD chronic obstructive pulmonary disease, WBC white blood cell, AST aspartate aminotransferase, ALT alanine aminotransferase, T-B total bilirubin, Alb albumin, BUN blood urea nitrogen, Cr creatinine, LDH lactate dehydrogenase, UA uric acid, CRP C-reactive protein, BNP brain natriuretic peptide, KL-6 Krebs von den Lungen-6
aComparison of patients in group 1 versus group 2
bComparison of patients in group 1 versus group 3
cComparison of patients in group 1 versus group 4
*p < 0.05 ** p < 0.01
Laboratory results of the patients in the four groups
The clinical laboratory findings of the enrolled patients are presented in Table 1. Patients in Group 3 had higher levels of white blood cells, neutrophils, aspartate aminotransferase (AST), alanine aminotransferase (ALT), HbA1c, and ferritin; neutrophil lymphocyte ratio (NLR); and Krebs von den Lungen-6 values (all p < 0.05) than the patients in Group 1. Conversely, albumin (Alb), blood urea nitrogen, uric acid, HbA1c, and Krebs von den Lungen-6 levels (all p < 0.05) of Group 2 patients were significantly lower than those of Group 1 patients. The lactate dehydrogenase (LDH) levels in Group 2 patients were significantly lower than those of Group 1 patients, whereas LDH levels were significantly higher in Group 3 and 4 patients than in Group 1 patients.
Upper and lower respiratory symptoms of the patients in the four groups
In Group 2, most patients (58.9%) suffered from only one upper respiratory symptom. The frequency decreased as the number of symptoms increased, with only four patients (1.2%) developing all four upper respiratory symptoms (Fig. 1a). The most common upper respiratory symptom was sore throat (149 cases), followed by dysgeusia (145 cases) and dysosmia (134 cases). The incidence of nasal discharge was the lowest (74 cases) (Fig. 1b). The details of lower respiratory symptoms were as follows: 549 (44.6%) patients developed only one lower respiratory symptom, 464 (37.8%) developed two symptoms, and 216 (17.6%) patients developed all lower respiratory symptoms (Fig. 1c). Among these, cough was the most frequent symptom (988 cases), followed by dyspnea (695 cases) and sputum production (443 cases) (Fig. 1d). The most common symptoms in all groups, excluding respiratory presentations, were fever, fatigue, and diarrhea (Additional file 1: Table S1). In Group 4 patients, all systemic symptoms, except bloody stools, were significantly more frequently noted than in Group 1 patients. In contrast, only fever and fatigue were more prevalent in Group 3 patients.
Fig. 1.
Details of upper and lower respiratory symptoms. a Ratio of upper respiratory symptoms in Group 2. Of 331 patients in Group 2, 136 patients (41.1%) developed two or more upper respiratory symptoms at the same time. b Number of patients with each upper respiratory symptom in Group 2. Of 331 patients in Group 2, 149 patients developed sore throat, the most frequent upper respiratory symptom. Dysosmia and dysgeusia were also as common as sore throat. c Ratio of lower respiratory symptoms in Group 3. Of 1229 patients in Group 3, 549 patients (44.6%) developed a single lower respiratory symptom. d Number of patients with each lower respiratory symptom in Group 3. Of 1229 patients in Group 3, 988 patients presented with cough and 695 patients with dyspnea. The frequency of sputum production was the lowest
Radiographic findings of the patients in the four groups
In chest X-ray images, ground-glass opacities (GGO) and infiltrated shadows were significantly more frequent in Group 3 and 4 patients than in Group 1 patients (all p < 0.01), whereas these were less frequent in Group 2 patients than in Group 1 patients (p < 0.01). Additionally, GGO and infiltrated shadows in chest CT scans were more frequent in Group 3 and 4 patients than in Group 1 patients (Fig. 2).
Fig. 2.
Comparison of chest images among the four groups. a, b Proportions of patients with ground-glass opacities and infiltrative shadows on chest X-ray images among the four groups. c, d Proportions of patients with ground-glass opacities and infiltrative shadows on chest computed tomography scans among four the groups
Treatment of the patients in the four groups
The summary of the therapeutic agents (remdesivir, antibiotics, steroids, tocilizumab, baricitinib, and anti-coagulant drugs) used in each group during the hospital stay is presented in Table 2. The patients in Group 2 were administered drugs less frequently than those in Group 1, with the exception of tocilizumab and baricitinib, whereas the patients in Group 3 received all drugs more frequently than those in Group 1. The patients in Group 4 were administered therapeutic agents, except antibiotics and baricitinib, more frequently than those in Group 1.
Table 2.
Treatment of each respiratory symptoms group
| All (n = 3314) | Group 1 (n = 605) | Group 2 (n = 331) | Group 3 (n = 1229) | Group 4 (n = 1149) | p value | |
|---|---|---|---|---|---|---|
| Remdesivir, % | 35.8 | 24 | 16.4 | 46.9 | 35.9 | < 0.0001a = **/b = **/c = ** |
| Antibiotics, % | 23.2 | 20.3 | 12.7 | 30.6 | 20.1 | < 0.0001a = **/b = ** |
| Steroids, % | 50.4 | 38.1 | 24.6 | 64.7 | 49.2 | < 0.0001a = **/b = **/c = ** |
| Tocilizumab, % | 9.8 | 5 | 3.3 | 15.2 | 8.5 | < 0.0001b = **/c = ** |
| Baricitinib, % | 5.2 | 31.4 | 14.3 | 53.6 | 42.9 | 0.0014b = * |
| Anti-coagulant drugs, % | 29.3 | 21 | 14 | 41.3 | 25.4 | < 0.0001a = **/b = **/c = * |
Data are shown as mean ± standard deviation
aComparison of patients in group 1 versus group 2
bComparison of patients in group 1 versus group 3
cComparison of patients in group 1 versus group 4
*p < 0.05 **p < 0.01
Impact of respiratory symptoms on clinical outcomes
Poor clinical outcomes (using high-flow oxygen therapy, invasive mechanical ventilation (IMV), ECMO, or death) were observed in 321 cases (9.8%) in Group 1, 11 cases (3.3%) in Group 2, 321 cases (26.1%) in Group 3, and 161 cases (14.0%) in Group 4 (Fig. 3a). Compared to Group 1 in univariate analysis, Group 2 had a significantly lower severity rate, and Groups 3 and 4 had a significantly higher severity rate. While Group 2 was associated with a better prognosis [OR (95% CI) = 0.21 (0.11–0.39)], Group 3 and 4 patients had the highest risk for severe disease [OR (95% CI) = 3.27 (2.43–4.40) and 1.51 (1.10–2.07)] (Fig. 3b). However, in the multivariate logistic regression analysis, which adjusted for patient characteristics and comorbidities, the significant difference between the prognosis of Group 1 and Group 2 disappeared [aOR = 0.65 (0.31–1.34)]. In contrast, Groups 3 and 4 remained significantly associated with a poor prognosis in the multivariate analysis [aOR (95% CI) = 4.53 (3.12–6.59) and 2.62 (1.76–3.90)] (Fig. 3c). In univariate analysis of treatments among the four groups, percentage of high-flow oxygen therapy was significantly lower in Group 2 and significantly higher in Groups 3 and 4 as compared to that in Group 1. Group 2 patients had a significantly lower rate of IMV use, and Group 3 patients were associated with increased rates of IMV and ECMO use as compared to those in Group 1. In a similar analysis, no significant differences were found for mortality among the four groups (Additional file 1: Fig. S2). Analysis of complications showed that percentages of bacterial infections were lower in Group 2 and higher in Group 3, when compared to Group 1. Moreover, Group 2 patients had a significantly lower frequency of acute kidney injury, and Group 3 patients had a significantly higher incidence of thrombosis than Group 1 patients (Fig. 3d–g).
Fig. 3.
Relevance to clinical prognosis and percentage of complications in each group during in-patient treatment of COVID-19. a Univariate analysis of the proportion of poor prognosis cases in each group. b Comparison of odds ratios for poor clinical outcomes in each group. c Multivariate logistic regression analysis of the relationship between respiratory symptoms and critical outcomes in the whole cohort (adjusted for age, sex, BMI, smoking history and comorbidities (hypertension, cardiovascular disease, chronic kidney disease and chronic liver disease). d Univariate analysis of the proportion of patients with bacterial infection in each group. e Univariate analysis of the proportion of patients with heart failure in each group. f Univariate analysis of the proportion of patients with acute kidney injury in each group. g Univariate analysis of the proportion of patients with thrombosis in each group
Relevance of clinical outcomes and respiratory symptoms
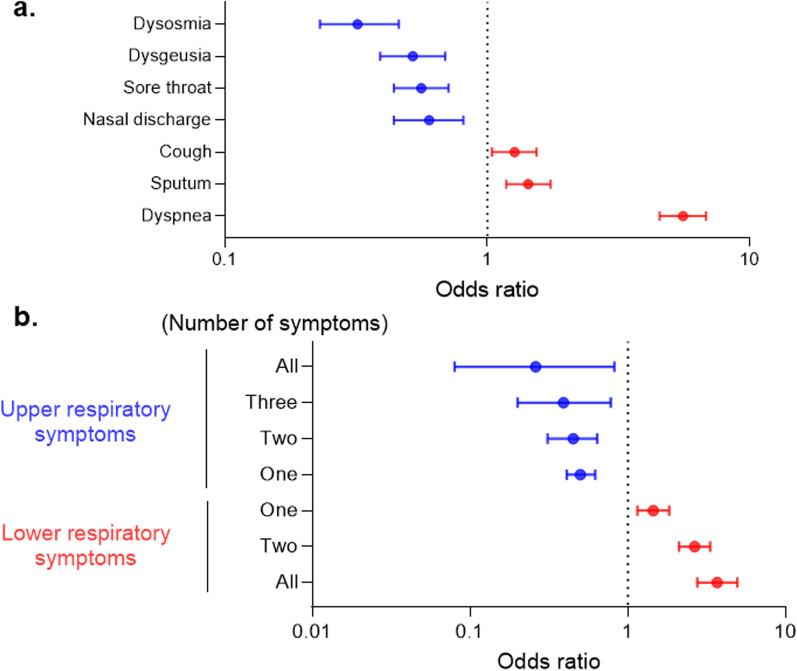
In the univariate analysis of the association of respiratory symptoms and clinical prognosis, all of the upper respiratory symptoms were good prognostic factors, whereas all of the lower respiratory symptoms were poor prognostic factors. Dysosmia (OR = 0.32 [0.23–0.46]) was the most favorable clinical factor, and dyspnea (OR = 5.56 [4.53–6.81]) was the worst risk factor associated with poor outcomes among respiratory symptoms. Interestingly, as the number of upper respiratory symptoms increased, the clinical course became better. Conversely, a higher incidence of lower respiratory symptoms was associated with a poorer prognosis (Fig. 4).
Fig. 4.
Relevance of symptoms in clinical prognosis. a Forest plot of odds ratios associated with poor clinical prognosis among respiratory symptoms. b Forest plot of odds ratios associated with poor clinical prognosis among numbers of respiratory symptoms
Discussion
This is a large-scale study of the association between the clinical outcomes of COVID-19 and respiratory symptoms. In this study, we characterized the clinical course of patients and classified them into four groups based on their respiratory symptoms. We report that upper respiratory symptoms are favorable prognostic factors, and lower respiratory symptoms are poor factors, similarly to previous studies [2, 13, 15, 17]. Additionally, our study provided two novel findings with clinical relevance. First, we identified that patients with only lower respiratory symptoms had a worse clinical course than those with both upper and lower respiratory symptoms. Second, this study is the first to show the association between the numbers and types of respiratory symptoms and clinical outcomes in COVID-19 patients. This study suggests better clinical outcomes with upper respiratory symptoms and worse clinical outcomes with lower respiratory symptoms. This study demonstrated the importance of conducting a respiratory symptoms interview in the primary care of COVID-19.
The appearance of symptoms in COVID-19 is known to be influenced by age and sex, with otorhinolaryngological symptoms being more common in younger patients; systemic symptoms such as fever, malaise, and anorexia being more common in older patients; and dysosmia, headache, nasal obstruction, and fatigue more common in women [31]. Moreover, several previous studies have revealed that upper respiratory symptoms are associated with favorable clinical outcomes [13, 15]. Similar to previous studies, our study suggested that all upper respiratory symptoms were favorable prognostic factors. Upper respiratory symptoms have been reported to be highly common in mild outpatient cases of COVID-19 [32, 33]. Additionally, in the Delta and Omicron variants, mild upper respiratory symptoms, for instance nasal discharge and sneezing, appear more frequently [34]. In our study, upper respiratory symptoms were less frequent than those in the previous studies [32–34]. This is because this study focused on hospitalized patients and excluded the period after November 2021, when infections with Omicron variants were common. Moreover, this study suggested that olfactory dysfunction was associated with the best prognosis among upper respiratory symptoms. In summary, conducting a medical interview of patients with respect to their respiratory symptoms can be useful for clinicians in primary care to predict the disease severity.
Among the lower respiratory symptoms of COVID-19 evaluated in this study, cough occurred most frequently, and all symptoms of the lower respiratory tract were associated with a severe clinical prognosis, similar to the results of previous studies [2, 4, 17, 18]. There are two reasons for this. First, patients in Groups 3 and 4 showed GGO and infiltrated shadows on chest X-ray images and CT scans more frequently. Several studies have shown a relationship between the extent of pneumonia on chest X-ray images or CT scans and the clinical prognosis [35–39]. Lower respiratory symptoms were considered to reflect the presence of pneumonia as a poor prognostic factor. It would be useful to predict pneumonia based on only medical interviews of the respiratory symptoms observed. Second, patients in Group 3 had bacterial infections and embolism more frequently than those in other groups. Both bacterial infections and embolism with COVID-19 were reported to worsen clinical outcomes [40–43], and these complications may affect the prognosis of patients with lower respiratory symptoms alone. Additionally, patients in Groups 2 and 4 had a significantly lower incidence of comorbidities associated with severe outcomes such as diabetes, hypertension, and cardiovascular disease. However, patients in Group 3, with the worst prognosis, had a significantly different incidence of comorbidities associated with severe outcomes, except sex and BMI, as compared to that of Group 1 patients. As Fig. 3c shows, lower respiratory symptoms were a poor prognostic factor, independent of comorbidities associated with poor clinical outcome, and lower respiratory symptoms were useful markers in predicting the severity of COVID-19. In contrast, our multivariate analysis showed that upper respiratory symptoms were not an independent risk factor for poor outcomes in patients with COVID-19, and the better prognosis in Group 2 may have resulted from the lower incidence of comorbidities associated with severe disease, as suggested by previous studies [12, 13].
In the laboratory data, NLR, AST, ALT, and ferritin levels associated with poor clinical outcomes were significantly elevated in patients in Group 2, consistent with previous reports [44–47]. In addition, Alb, LDH, and HbA1c values improved in Group 2 patients with good prognosis, whereas they worsened in Group 3 patients with poor prognosis. [47, 48]
Several studies have confirmed that SARS-CoV-2 uses human ACE2 as a receptor to enter host cells [49, 50]. These proteins were highly expressed in the nasal epithelium, and their levels were downregulated throughout the lower respiratory tract and type II alveolar cells in the lung [51]. Thus, SARS-CoV-2 may enter through the nasal epithelium followed by entry into the lungs by inhalation, triggering pneumonia. Additionally, elevated ACE2 expression is expected to increase the viral load. Some studies have shown that a high viral load is associated with death and disease severity [52, 53]; thus, increased expression of ACE2 could lead to poor prognosis. Moreover, ACE2 expression is higher in the lungs of males than of females as shown by single cell RNA-seq [54]. Thus, the significantly higher proportion of males in Group 3 with poor prognosis may have been due to a higher ACE2 expression. In addition, obesity and smoking significantly increase ACE2 expression in the lungs and bronchial epithelium [55, 56]; this could further explain the higher rates of obesity and smoking in Groups 3 and 4 with lower respiratory symptoms. The lower frequency of these comorbidities in Groups 2 and 4 may be because SARS-CoV-2 accumulated and proliferated in the upper respiratory tract, where ACE2 expression was the highest. Interestingly, patients in Group 3 had a worse prognosis than patients in Group 4. Thus, elevated ACE2 expression in the lower respiratory tract could prevent the restriction of SARS-CoV-2 to the upper respiratory tract, resulting in poor prognosis. However, the association of ACE2 expression and COVID-19 severity has not been reported [57], and various complex factors are assumed to be involved in the severity of COVID-19.
Our study has several limitations. First, this study included only hospitalized patients with COVID-19, which might have resulted in a biased sample due to the high severity of the disease. Patients with only upper respiratory symptoms were often treated as mild cases. Therefore, the population in Group 2 may not adequately reflect the clinical characteristics of COVID-19 patients with only upper respiratory symptoms. Second, several previous studies used objective scoring tools to assess olfactory and taste disorders [58, 59], but this study included information from only medical interviews, which may be less accurate for symptoms. Additionally, in COVID-19, there are some cases of rapid and severe respiratory failure without dyspnea characterized by silent hypoxia [60]. Although the prognosis of asymptomatic cases was relatively better, clinicians should not solely rely on interviews and use biomarkers such as those measured using pulse oximetry. Further studies are needed to address these limitations and develop optimal treatment strategies in the near future.
Conclusions
Based on the stratification of respiratory symptoms into upper and lower respiratory symptoms using medical interviews, clinicians may be able to predict the presence of pneumonia, clinical course, and complications of COVID-19. Especially in primary care, this easily obtained information is considered an important clinical tool.
Supplementary Information
Additional file 1. Supplemental Figure 1. Study flow chart of patient identification and selectionStudy flow chart of patient identification and selection. A total of 117 records were excluded from the 3431 cases registered in the coronavirus disease 2019 (COVID-19) taskforce database owing to lack of essential clinical information. Ultimately, 3314 patients met the eligibility criteria, of which 2709 had respiratory symptoms. Supplemental Figure 2. Frequency of assisted respiration therapy and death in all four groups (a) Univariate analysis of the proportion of high-flow oxygen therapy with COVID-19 in each group. (b) Univariate analysis of the proportion of use of invasive mechanical ventilation (IMV) with COVID-19 in each group. (c) Univariate analysis of the proportion of use of extracorporeal membrane oxygenation (ECMO) with COVID-19 in each group. (d) Univariate analysis of the proportion of death with COVID-19 in each group. Supplemental Table 1. Common non-respiratory symptoms in each group.
Acknowledgements
We would like to thank all the participants involved in this study and all members of the Japan COVID-19 Task Force engaged in clinical and research work on COVID-19 every day. All members contributed cases to this study.
Kazuhisa Takahashi6, Toshio Naito34, Makoto Hiki35,36, Yasushi Matsushita37, Haruhi Takagi6, Ryousuke Aoki38, Ai Nakamura6, Sonoko Harada6,39, Hitoshi Sasano6, Shinnosuke Ikemura1, Satoshi Okamori1, Hideki Terai1, Junichi Sasaki40, Hiroshi Morisaki41, Yoshifumi Uwamino42, Kosaku Nanki33, Yohei Mikami33, Sho Uchida2, Shunsuke Uno2, Rino Ishihara33, Yuta Matsubara33, Tomoyasu Nishimura2,43, Takunori Ogawa1, Toshiro Sato44, Masanori Azuma7, Ryuichi Saito7, Toshikatsu Sado7, Yoshimune Miyazaki7, Ryuichi Sato7, Yuki Haruta7, Tadao Nagasaki7, Yoshinori Yasui45, Yoshinori Hasegawa7, Ai Tada8, Masayoshi Miyawaki8, Masaomi Yamamoto8, Eriko Yoshida8, Reina Hayashi8, Tomoki Nagasaka8, Sawako Arai8, Yutaro Kaneko8, Kana Sasaki8, Taisuke Isono9, Shun Shibata9, Yuma Matsui9, Chiaki Hosoda9, Kenji Takano9, Takashi Nishida9, Yoichi Kobayashi9, Yotaro Takaku9, Noboru Takayanagi9, Etsuko Tagaya10, Masatoshi Kawana46, Yasushi Nakamori11, Kazuhisa Yoshiya11, Tomoyuki Yoshihara11, Daiki Wada11, Hiromu Iwamura11, Syuji Kanayama11, Shuhei Maruyama11, Takanori Hasegawa29, Kunihiko Takahashi29, Tatsuhiko Anzai29, Satoshi Ito29, Akifumi Endo47, Yuji Uchimura48, Yasunari Miyazaki49, Takayuki Honda49, Tomoya Tateishi49, Shuji Tohda50, Naoya Ichimura50, Kazunari Sonobe50, Chihiro Tani Sassa50, Jun Nakajima50, Masumi Ai51, Ken Ohta52, Hiroyuki Kokuto52, Hideo Ogata52, Yoshiaki Tanaka52, Kenichi Arakawa52, Masafumi Shimoda52, Takeshi Osawa52, Yukiko Nakajima13, Ryusuke Anan13, Ryosuke Arai13, Yuko Kurihara13, Yuko Harada13, Kazumi Nishio13, Tomonori Sato53, Reoto Takei53, Satoshi Hagimoto53, Yoichiro Noguchi53, Yasuhiko Yamano53, Hajime Sasano53, Sho Ota53, Sohei Nakayama4, Keita Masuzawa4, Tomomi Takano54, Kazuhiko Katayama55, Mitsuhiro Yamada16, Hisatoshi Sugiura16, Hirohito Sano16, Shuichiro Matsumoto16, Nozomu Kimura16, Yoshinao Ono16, Hiroaki Baba56, Rie Baba57, Daisuke Arai57, Takayuki Ogura57, Hidenori Takahashi57, Shigehiro Hagiwara57, Genta Nagao57, Shunichiro Konishi57, Ichiro Nakachi57, Hiroki Tateno58, Isano Hase58, Shuichi Yoshida58, Shoji Suzuki58, Miki Kawada59, Hirohisa Horinouchi60, Fumitake Saito61, Keiko Mitamura62, Masao Hagihara63, Junichi Ochi61, Tomoyuki Uchida63, Yuya Shirai15,18, Kyuto Sonehara18,19, Tatsuhiko Naito18, Kenichi Yamamoto18, Shinichi Namba18, Ken Suzuki18, Takayuki Shiroyama15, Yuichi Maeda15, Takuro Nii15, Yoshimi Noda15, Takayuki Niitsu15, Yuichi Adachi15, Takatoshi Enomoto15, Saori Amiya15, Reina Hara15, Toshihiro Kishikawa18,64,66, Shuhei Yamada65, Shuhei Kawabata65, Noriyuki Kijima65, Masatoshi Takagaki65,70, Noa Sasa18,64, Yuya Ueno64, Motoyuki Suzuki64, Norihiko Takemoto64, Hirotaka Eguchi64, Takahito Fukusumi64, Takao Imai64, Munehisa Fukushima64,69, Haruhiko Kishima65, Hidenori Inohara64, Kazunori Tomono67, Kazuto Kato68, Haruhiko Hirata15, Yoshito Takeda15, Atsushi Kumanogoh15,19,70,20, Naoki Miyazawa71, Yasuhiro Kimura71, Reiko Sado71, Hideyasu Sugimoto71, Akane Kamiya72, Naota Kuwahara73, Akiko Fujiwara73, Tomohiro Matsunaga73, Yoko Sato73, Takenori Okada73, Takashi Inoue74, Toshiyuki Hirano74, Keigo Kobayashi74, Hatsuyo Takaoka74, Koichi Nishi75, Masaru Nishitsuji75, Mayuko Tani75, Junya Suzuki75, Hiroki Nakatsumi75, Hidefumi Koh76, Tadashi Manabe76, Yohei Funatsu76, Fumimaro Ito76, Takahiro Fukui76, Keisuke Shinozuka76, Sumiko Kohashi76, Masatoshi Miyazaki76, Tomohisa Shoko77, Mitsuaki Kojima77, Tomohiro Adachi77, Motonao Ishikawa78, Kenichiro Takahashi79, Kazuyoshi Watanabe80, Yoshihiro Hirai81, Hidetoshi Kawashima81, Atsuya Narita81, Kazuki Niwa82, Yoshiyuki Sekikawa82, Hisako Sageshima83, Yoshihiko Nakamura84, Kota Hoshino,84 Junichi Maruyama84, Hiroyasu Ishikura84, Tohru Takata85, Takashi Ogura86, Hideya Kitamura86, Eri Hagiwara86, Kota Murohashi86, Hiroko Okabayashi86, Takao Mochimaru87,88, Shigenari Nukaga87, Ryosuke Satomi87, Yoshitaka Oyamada88, Nobuaki Mori89, Tomoya Baba90, Yasutaka Fukui90, Mitsuru Odate90, Shuko Mashimo90, Yasushi Makino90, Kazuma Yagi91, Mizuha Hashiguchi91, Junko Kagyo91, Tetsuya Shiomi91, Kodai Kawamura92, Kazuya Ichikado92, Kenta Nishiyama92, Hiroyuki Muranaka92, Kazunori Nakamura92, Satoshi Fuke93, Hiroshi Saito93, Tomoya Tsuchida94, Shigeki Fujitani95, Mumon Takita95, Daiki Morikawa95, Toru Yoshida95, Takehiro Izumo96, Minoru Inomata96, Naoyuki Kuse96, Nobuyasu Awano96, Mari Tone96, Akihiro Ito97, Toshio Odani98, Masaru Amishima99, Takeshi Hattori99, Yasuo Shichinohe100, Takashi Kagaya101, Toshiyuki Kita101, Kazuhide Ohta101, Satoru Sakagami101, Kiyoshi Koshida101, Morio Nakamura101, Koutaro Yokote102, Taka-Aki Nakada103, Ryuzo Abe103, Taku Oshima103, Tadanaga Shimada103, Kentaro Hayashi104, Tetsuo Shimizu104, Yutaka Kozu104, Hisato Hiranuma104, Yasuhiro Gon104, Namiki Izumi105, Kaoru Nagata105, Ken Ueda105, Reiko Taki105, Satoko Hanada105, Naozumi Hashimoto5, Keiko Wakahara5, Koji Sakamoto5, Norihito Omote5, Akira Ando5, Yu Kusaka106, Takehiko Ohba106, Susumu Isogai106, Aki Ogawa106, Takuya Inoue106, Nobuhiro Kodama107, Yasunari Kaneyama107, Shunsuke Maeda107, Takashige Kuraki108, Takemasa Matsumoto108, Masahiro Harada109, Takeshi Takahashi109, Hiroshi Ono109, Toshihiro Sakurai109, Takayuki Shibusawa109, Yusuke Kawamura110, Akiyoshi Nakayama110, Hirotaka Matsuo110, Yoshifumi Kimizuka111, Akihiko Kawana111, Tomoya Sano111, Chie Watanabe111, Ryohei Suematsu111, Makoto Masuda112, Aya Wakabayashi112, Hiroki Watanabe112, Suguru Ueda112, Masanori Nishikawa112, Ayumi Yoshifuji113, Kazuto Ito113, Saeko Takahashi114, Kota Ishioka114, Yusuke Chihara115, Mayumi Takeuchi115, Keisuke Onoi115, Jun Shinozuka115, Atsushi Sueyoshi115, Yoji Nagasaki116, Masaki Okamoto117,118, Sayoko Ishihara119, Masatoshi Shimo119, Yoshihisa Tokunaga117,118, Masafumi Watanabe120, Sumito Inoue120, Akira Igarashi120, Masamichi Sato120, Nobuyuki Hizawa121, Yoshiaki Inoue122, Shigeru Chiba123, Kunihiro Yamagata124, Yuji Hiramatsu125, Hirayasu Kai124, Satoru Fukuyama126, Yoshihiro Eriguchi127, Akiko Yonekawa127, Keiko Kan-o126, Koichiro Matsumoto126, Kensuke Kanaoka128, Shoichi Ihara128, Kiyoshi Komuta128, Koichiro Asano129, Tsuyoshi Oguma129, Yoko Ito129, Satoru Hashimoto130, Masaki Yamasaki130, Yu Kasamatsu131, Yuko Komase132, Naoya Hida132, Takahiro Tsuburai132, Baku Oyama132, Yuichiro Kitagawa133, Tetsuya Fukuta133, Takahito Miyake133, Shozo Yoshida133, Shinji Ogura133, Minoru Takada134, Hidenori Kanda134, Shinji Abe135, Yuta Kono135, Yuki Togashi135, Hiroyuki Takoi135, Ryota Kikuchi135, Shinichi Ogawa136, Tomouki Ogata136, Shoichiro Ishihara136, Arihiko Kanehiro137,138, Shinji Ozaki137, Yasuko Fuchimoto137, Sae Wada137, Nobukazu Fujimoto138, Kei Nishiyama139, Mariko Terashima140, Satoru Beppu140, Kosuke Yoshida140, Osamu Narumoto141, Hideaki Nagai141, Nobuharu Ooshima141, Mitsuru Motegi142, Akira Umeda143, Kazuya Miyagawa144, Hisato Shimada145, Mayu Endo146, Yoshiyuki Ohira147, Hironori Sagara147, Akihiko Tanaka147, Shin Ohta147, Tomoyuki Kimura147, Yoko Shibata148, Yoshinori Tanino148, Takefumi Nikaido148, Hiroyuki Minemura148, Yuki Sato148, Yuichiro Yamada149, Takuya Hashino149, Masato Shinoki149, Hajime Iwagoe150, Hiroshi Takahashi151, Kazuhiko Fujii151, Hiroto Kishi151, Tomoo Ishii152, Masayuki Kanai153, Tomonori Imamura153, Tatsuya Yamashita153, Masakiyo Yatomi154, Toshitaka Maeno154, Shinichi Hayashi155, Mai Takahashi155, Mizuki Kuramochi155, Isamu Kamimaki155, Yoshiteru Tominaga155, Mitsuyoshi Utsugi156, Akihiro Ono156, Toru Tanaka157, Takeru Kashiwada157, Kazue Fujita157, Yoshinobu Saito157, Masahiro Seike157, Masahiro Kanai158, Ryunosuke Saiki30, Takayoshi Hyugaji28, Eigo Shimizu28, Kotoe Katayama28, Satoru Miyawaki159, Meiko Takahashi160, Fumihiko Matsuda160, Yosuke Omae26, Yasuhito Nannya30, Takafumi Ueno161
34Department of General Medicine, Juntendo University Faculty of Medicine and Graduate School of Medicine, Tokyo, Japan. 35Department of Emergency and Disaster Medicine, Juntendo University Faculty of Medicine and Graduate School of Medicine, Tokyo, Japan. 36Department of Cardiovascular Biology and Medicine, Juntendo University Faculty of Medicine and Graduate School of Medicine, Tokyo, Japan. 37Department of Internal Medicine and Rheumatology, Juntendo University Faculty of Medicine and Graduate School of Medicine, Tokyo, Japan. 38Department of Nephrology, Juntendo University Faculty of Medicine and Graduate School of Medicine, Tokyo, Japan. 39Atopy (Allergy) Research Center, Juntendo University Graduate School of Medicine, Tokyo, Japan. 40Department of Emergency and Critical Care Medicine, Keio University School of Medicine, Tokyo, Japan. 41Department of Anesthesiology, Keio University School of Medicine, Tokyo, Japan. 42Department of Laboratory Medicine, Keio University School of Medicine, Tokyo, Japan. 43Keio University Health Center, Keio University School of Medicine, Tokyo, Japan. 44Department of Organoid Medicine, Keio University School of Medicine, Tokyo, Japan. 45Department of Infection Control, Osaka Saiseikai Nakatsu Hospital, Osaka, Japan. 46Department of General Medicine, Tokyo Women's Medical University, Tokyo, Japan. 47Clinical Research Center, Tokyo Medical and Dental University Hospital of Medicine, Tokyo, Japan. 48Department of Medical Informatics, Tokyo Medical and Dental University Hospital of Medicine, Tokyo, Japan. 49Respiratory Medicine, Tokyo Medical and Dental University, Tokyo, Japan. 50Clinical Laboratory, Tokyo Medical and Dental University Hospital of Medicine, Tokyo, Japan. 51Department of Insured Medical Care Management, Tokyo Medical and Dental University Hospital of Medicine, Tokyo, Japan. 52Fukujuji Hospital, Kiyose, Japan. 53Department of Respiratory Medicine and Allergy, Tosei General Hospital, Seto, Japan. 54School of Veterinary Medicine, Kitasato University, Towada, Japan. 55Laboratory of Viral Infection I, Department of Infection Control and Immunology, Ōmura Satoshi Memorial Institute & Graduate School of Infection Control Sciences, Kitasato University, Tokyo, Japan. 56Department of Infectious Diseases, Tohoku University Graduate School of Medicine, Sendai, Japan. 57Saiseikai Utsunomiya Hospital, Utsunomiya, Japan. 58Department of Pulmonary Medicine, Saitama City Hospital, Saitama, Japan. 59Department of Infectious Diseases, Saitama City Hospital, Saitama, Japan. 60Department of General Thoracic Surgery, Saitama City Hospital, Saitama, Japan. 61Department of Pulmonary Medicine, Eiju General Hospital, Tokyo, Japan. 62Division of Infection Control, Eiju General Hospital, Tokyo, Japan. 63Department of Hematology, Eiju General Hospital, Tokyo, Japan. 64Department of Otorhinolaryngology-Head and Neck Surgery, Osaka University Graduate School of Medicine, Suita, Japan. 65Department of Neurosurgery, Osaka University Graduate School of Medicine, Suita, Japan. 66Department of Head and Neck Surgery, Aichi Cancer Center Hospital, Nagoya, Japan. 67Division of Infection Control and Prevention, Osaka University Hospital, Suita, Japan. 68Department of Biomedical Ethics and Public Policy, Osaka University Graduate School of Medicine, Suita, Japan. 69Department of Otolaryngology and Head and Neck Surgery, Kansai Rosai Hospital, Hyogo, Japan. 70Department of Immunopathology, Immunology Frontier Research Center (WPI-IFReC), Osaka University, Suita, Japan. 71Department of Respirtory Medicine, Saiseikai Yokohamashi Nanbu Hospital, Yokohama, Japan. 72Department of Clinical Laboratory, Saiseikai Yokohamashi Nanbu Hospital, Yokohama, Japan. 73Internal Medicine, Internal Medicine Center, Showa University Koto Toyosu Hospital, Tokyo, Japan. 74Internal Medicine, Sano Kosei General Hospital, Sano, Japan. 75Ishikawa Prefectural Central Hospital, Kanazawa, Japan. 76Tachikawa Hospital, Tachikawa, Japan. 77Department of Emergency and Critical Care Medicine, Tokyo Women's Medical University Medical Center East, Tokyo, Japan. 78Department of Medicine, Tokyo Women's Medical University Medical Center East, Tokyo, Japan. 79Department of Pediatrics, Tokyo Women's Medical University Medical Center East, Tokyo, Japan. 80Japan Community Health care Organization Kanazawa Hospital, Kanazawa, Japan. 81Department of Respiratory Medicine, Japan Organization of Occupational Health and Safety, Kanto Rosai Hospital, Kawasaki, Japan. 82Department of Generai Internal Medicine, Japan Organization of Occupational Health and Safety, Kanto Rosai Hospital, Kawasaki, Japan. 83Sapporo City General Hospital, Sapporo, Japan. 84Department of Emergency and Critical Care Medicine, Faculty of Medicine, Fukuoka University, Fukuoka, Japan. 85Department of Infection Control, Fukuoka University Hospital, Fukuoka, Japan. 86Kanagawa Cardiovascular and Respiratory Center, Yokohama, Japan. 87Department of Respiratory Medicine, National Hospital Organization Tokyo Medical Center, Tokyo, Japan. 88Department of Allergy, National Hospital Organization Tokyo Medical Center, Tokyo, Japan. 89Department of General Internal Medicine and Infectious Diseases, National Hospital Organization Tokyo Medical Center, Tokyo, Japan. 90Department of Respiratory Medicine, Toyohashi Municipal Hospital, Toyohashi, Japan. 91Keiyu Hospital, Yokohama, Japan. 92Division of Respiratory Medicine, Social Welfare Organization Saiseikai Imperial Gift Foundation, Inc., Saiseikai Kumamoto Hospital, Kumamoto, Japan. 93KKR Sapporo Medical Center, Department of respiratory medicine, Sapporo, Japan. 94Division of General Internal Medicine, Department of Internal Medicine, StMarianna University School of Medicine, Kawasaki, Japan. 95Departmet of Emergency and Critical Care Medicine, StMarianna University School of Medicine, Kawasaki, Japan. 96Japanese Red Cross Medical Center, Tokyo, Japan. 97Matsumoto City Hostpital, Matsumoto, Japan. 98Department of Rheumatology, National Hospital Organization Hokkaido Medical Center, Sapporo, Japan. 99Department of Respiratory Medicine, National Hospital Organization Hokkaido Medical Center, Sapporo, Japan. 100Department of Emergency and Critical Care Medicine, National Hospital Organization Hokkaido Medical Center, Sapporo, Japan. 101NHO Kanazawa Medical Center, Kanazawa, Japan. 102Department of Endocrinology, Hematology and Gerontology, Chiba University Graduate School of Medicine, Chiba, Japan. 103Department of Emergency and Critical Care Medicine, Chiba University Graduate School of Medicine, Chiba, Japan. 104Nihon University School of Medicine, Department of Internal Medicine, Division of Respiratory Medicine, Tokyo, Japan. 105Musashino Red Cross Hospital, Musashino, Japan. 106Ome Municipal General Hospital, Ome, Japan. 107Fukuoka Tokushukai Hospital, Department of Internal Medicine, Kasuga, Japan. 108Fukuoka Tokushukai Hospital, Respiratory Medicine, Kasuga, Japan. 109National Hospital Organization Kumamoto Medical Center, Kumamoto, Japan. 110Department of Integrative Physiology and Bio-Nano Medicine, National Defense Medical College, Tokorozawa, Japan. 111Division of Infectious Diseases and Respiratory Medicine, Department of Internal Medicine, National Defense Medical College, Tokorozawa, Japan. 112Department of Respiratory Medicine, Fujisawa City Hospital, Fujisawa, Japan. 113Department of Internal Medicine, Tokyo Saiseikai Central Hospital, Tokyo, Japan. 114Department of Pulmonary Medicine, Tokyo Saiseikai Central Hospital, Tokyo, Japan. 115Uji-Tokushukai Medical Center, Uji, Japan. 116Department of Infectious Disease and Clinical Research Institute, National Hospital Organization Kyushu Medical Center, Fukuoka Japan. 117Department of Respirology, National Hospital Organization Kyushu Medical Center, Fukuoka, Japan. 118Division of Respirology, Rheumatology, and Neurology, Department of Internal Medicine, Kurume University School of Medicine, Kurume, Japan. 119Department of Infectious Disease, National Hospital Organization Kyushu Medical Center, Fukuoka Japan. 120Department of Cardiology, Pulmonology, and Nephrology, Yamagata University Faculty of Medicine, Yamagata, Japan. 121Department of Pulmonary Medicine, Faculty of Medicine, University of Tsukuba, Tsukuba, Japan. 122Department of Emergency and Critical Care Medicine, Facualty of Medicine, University of Tsukuba, Tsukuba, Japan. 123Departmentof Hematology, Facualty of Medicine, University of Tsukuba, Tsukuba, Japan. 124Department of Nephrology, Facualty of Medicine, University of Tsukuba, Tsukuba, Japan. 125Department of Cardiovascular Surgery, Facualty of Medicine, University of Tsukuba, Tsukuba, Japan. 126Research Institute for Diseases of the Chest, Graduate School of Medical Sciences, Kyushu University, Fukuoka, Japan. 127Department of Medicine and Biosystemic Science, Kyushu University Graduate School of Medical Sciences, Fukuoka, Japan. 128Daini Osaka Police Hospital, Osaka, Japan. 129Division of Pulmonary Medicine, Department of Medicine, Tokai University School of Medicine, Isehara, Japan. 130Department of Anesthesiology and Intensive Care Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan. 131Department of Infection Control and Laboratory Medicine, Kyoto Prefectural University of Medicine, Kyoto, Japan. 132Department of Respiratory Internal Medicine, StMarianna University School of Medicine, Yokohama-City Seibu Hospital, Yokohama, Japan. 133Gifu University School of Medicine Graduate School of Medicine, Emergency and Disaster Medicine, Gifu, Japan. 134KINSHUKAI Hanwa The Second Hospital, Osaka, Japan. 135Department of Respiratory Medicine, Tokyo Medical University Hospital, Tokyo, Japan. 136JA Toride medical hospital, Toride, Japan. 137Okayama Rosai Hospital, Okayama, Japan. 138Himeji StMary's Hospital, Himeji, Japan. 139Emergency & Critical Care, Niigata University, Niigata, Japan. 140Emergency & Critical Care Center, National Hospital Organization Kyoto Medical Center, Kyoto, Japan. 141National Hospital Organization Tokyo National Hospital, Kiyose, Japan. 142Fujioka General Hospital, Fujioka, Japan. 143Department of General Medicine, School of Medicine, International University of Health and Welfare Shioya Hospital, Ohtawara Japan. 144Department of Pharmacology, School of Pharmacy, International University of Health and Welfare Shioya Hospital, Ohtawara Japan. 145Department of Respiratory Medicine, International University of Health and Welfare Shioya Hospital, Ohtawara Japan. 146Department of Clinical Laboratory, International University of Health and Welfare Shioya Hospital, Ohtawara Japan. 147Department of General Medicine, School of Medicine, International University of Health and Welfare, Narita Japan. 148Department of Pulmonary Medicine, Fukushima Medical University, Fukushima, Japan. 149Kansai Electric Power Hospital, Osaka, Japan. 150Department of Infectious Diseases, Kumamoto City Hospital, Kumamoto, Japan. 151Department of Respiratory Medicine, Kumamoto City Hospital, Kumamoto, Japan. 152Tokyo Medical University Ibaraki Medical Center, Inashiki, Japan. 153Department of Emergency and Critical Care Medicine, Tokyo Metropolitan Police Hospital, Tokyo, Japan. 154Department of Respiratory Medicine, Gunma University Graduate School of Medicine, Maebashi, Japan. 155National hospital organization Saitama Hospital, Wako, Japan. 156Department of Internal Medicine, Kiryu Kosei General Hospital, Kiryu, Japan. 157Department of Pulmonary Medicine and Oncology, Graduate School of Medicine, Nippon Medical School, Tokyo, Japan. 158Department of Biomedical Informatics, Harvard Medical School, Boston, MA, USA. 159Department of Neurosurgery, Faculty of Medicine, the University of Tokyo, Tokyo, Japan. 160Center for Genomic Medicine, Kyoto University Graduate School of Medicine, Kyoto, Japan. 161Department of Biomolecular Engineering, Graduate School of Tokyo Institute of Technology, Tokyo, Japan.
Author contributions
Conceptualization: KN, SC, HN, TA, KM, HK, MI, NH, and KF. Data curation: KN, HT, HL, SO, TF, AM, MW, and TK. Formal analysis: KN, SC, and HN. Methodology: KN, SC, and HN. Supervision: KN, SC, NH, TA, KM, HK, MI, NH, NH, TU, SU, TI, KA, FS, TY, YN, YM, YS, RE, KM, YS, YO, RK, YK, KT, AK, SI, SM, SO, TK, and KF. Visualisation: KN, SC, and HN. Writing—original draft: KN, SC, and HN. Writing—review and editing: KN, SC, NH, TA, KM, HK, MI, NH, NH, TU, SU, TI, KA, FS, TY, YN, YM, YS, RE, KM, YS, YO, RK, YK, KT, AK, SI, SM, SO, TK, and KF. All authors read and approved the final manuscript.
Funding
This study was supported by AMED (JP20nk0101612, JP20fk0108415, JP21jk0210034, JP21km0405211, JP21km0405217, and JP21wm0325031), JST CREST (JPMJCR20H2), JST PRESTO (JPMJPR21R7), MHLW (20CA2054), Takeda Science Foundation, Mitsubishi Foundation, and Bioinformatics Initiative of Osaka University Graduate School of Medicine, Osaka University.
Availability of data and materials
The datasets generated during and/or analyzed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was approved by the ethics committee of Keio University School of Medicine (20200061) and affiliated institutes. Written informed consent was obtained from all patients. All aspects of the study conformed to the principles of the Declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shotaro Chubachi, Email: bachibachi472000@live.jp.
Ho Namkoong, Email: hounamugun@gmail.com.
References
- 1.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). https://stacks.cdc.gov/view/cdc/89980. Accessed 2 October 2022.
- 2.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan. China Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spinato G, Fabbris C, Polesel J, Cazzador D, Borsetto D, Hopkins C, et al. Alterations in smell or taste in mildly symptomatic outpatients with SARS-CoV-2 infection. JAMA. 2020;323:2089–2090. doi: 10.1001/jama.2020.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stokes EK, Zambrano LD, Anderson KN, Marder EP, Raz KM, El Burai FS, et al. Coronavirus disease 2019 case surveillance—United States, January 22–May 30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:759–765. doi: 10.15585/mmwr.mm6924e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, et al. Prediction models for diagnosis and prognosis of covid-19: systematic review and critical appraisal. BMJ. 2020;369:m1328. doi: 10.1136/bmj.m1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lombardi Y, Azoyan L, Szychowiak P, Bellamine A, Lemaitre G, Bernaux M, et al. External validation of prognostic scores for COVID-19: a multicenter cohort study of patients hospitalized in Greater Paris University Hospitals. Intensive Care Med. 2021;47:1426–1439. doi: 10.1007/s00134-021-06524-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zayet S, Kadiane-Oussou NJ, Lepiller Q, Zahra H, Royer PY, Toko L, et al. Clinical features of COVID-19 and influenza: a comparative study on Nord Franche-Comte cluster. Microbes Infect. 2020;22:481–488. doi: 10.1016/j.micinf.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Printza A, Constantinidis J. The role of self-reported smell and taste disorders in suspected COVID-19. Eur Arch Otorhinolaryngol. 2020;277:2625–2630. doi: 10.1007/s00405-020-06069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.da Rosa Mesquita R, Francelino Silva Junior LC, Santos Santana FM, Farias de Oliveira T, Campos Alcântara R, Monteiro Arnozo G, et al. Clinical manifestations of COVID-19 in the general population: systematic review. Wien Klin Wochenschr. 2021;133:377–382. doi: 10.1007/s00508-020-01760-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rocke J, Hopkins C, Philpott C, Kumar N. Is loss of sense of smell a diagnostic marker in COVID-19: a systematic review and meta-analysis. Clin Otolaryngol. 2020;45:914–922. doi: 10.1111/coa.13620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paderno A, Schreiber A, Grammatica A, Raffetti E, Tomasoni M, Gualtieri T, et al. Smell and taste alterations in COVID-19: a cross-sectional analysis of different cohorts. Int Forum Allergy Rhinol. 2020;10:955–962. doi: 10.1002/alr.22610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Husain Q, Kokinakos K, Kuo YH, Zaidi F, Houston S, Shargorodsky J. Characteristics of COVID-19 smell and taste dysfunction in hospitalized patients. Am J Otolaryngol. 2021;42:103068. doi: 10.1016/j.amjoto.2021.103068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA. 2020;323:2512–2514. doi: 10.1001/jama.2020.8391. [DOI] [PubMed] [Google Scholar]
- 15.Piu N, Isabella A, Airoldi C, Aleni C, Sarro A, Faggiano F. Taste and smell disorders in COVID-19 patients at a local healthcare trust in Northern Italy: a cross-sectional study. Ann Ig. 2022;34:122–127. doi: 10.7416/ai.2022.2474. [DOI] [PubMed] [Google Scholar]
- 16.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus Disease 2019 in Wuhan. China JAMA Neurol. 2020;77:683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Husain M, Valayer S, Poey N, Rondinaud E, d'Humières C, Visseaux B, et al. Pulmonary bacterial infections in adult patients hospitalized for COVID-19 in standard wards. Infect Dis Now. 2022;52:208–213. doi: 10.1016/j.idnow.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Song CL, Wang T, Ye YL, Du JR, Li SH, et al. Etiological and epidemiological characteristics of severe acute respiratory infection caused by multiple viruses and Mycoplasma pneumoniae in adult patients in Jinshan, Shanghai: a pilot hospital-based surveillance study. PLoS ONE. 2021;16:e0248750. doi: 10.1371/journal.pone.0248750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Namkoong H, Edahiro R, Fukunaga K, Shirai Y, Sonehara K, Tanaka H, et al. Japan COVID-19 Task Force: a nation-wide consortium to elucidate host genetics of COVID-19 pandemic in Japan. medRxiv. 2021:05.17.21256513.
- 22.Tanaka H, Lee H, Morita A, Namkoong H, Chubachi S, Kabata H, et al. Clinical characteristics of patients with coronavirus disease (COVID-19): preliminary baseline report of Japan COVID-19 Task Force, a nationwide consortium to investigate host genetics of COVID-19. Int J Infect Dis. 2021;113:74–81. doi: 10.1016/j.ijid.2021.09.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.COVID-19 therapeutic trial synopsis; 2022. https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis. Accessed 3 June 2022.
- 24.O’Driscoll M, Ribeiro dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 25.Geng L, He C, Kan H, Zhang K, Mao A, Zhang C, et al. The association between blood pressure levels and mortality in critically ill patients with COVID-19 in Wuhan, China: a case-series report. Hypertens Res. 2021;44:368–370. doi: 10.1038/s41440-020-00594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang I, Lim MA, Pranata R. Diabetes mellitus is associated with increased mortality and severity of disease in COVID-19 pneumonia—a systematic review, meta-analysis, and meta-regression. Diabetes Metab Syndr. 2020;14:395–403. doi: 10.1016/j.dsx.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santoso A, Pranata R, Wibowo A, Al-Farabi MJ, Huang I, Antariksa B. Cardiac injury is associated with mortality and critically ill pneumonia in COVID-19: a meta-analysis. Am J Emerg Med. 2021;44:352–357. doi: 10.1016/j.ajem.2020.04.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singh J, Malik P, Patel N, Pothuru S, Israni A, Chakinala RC, et al. Kidney disease and COVID-19 disease severity-systematic review and meta-analysis. Clin Exp Med. 2022;22:125–135. doi: 10.1007/s10238-021-00715-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jin JM, Bai P, He W, Wu F, Liu XF, Han DM, et al. Gender differences in patients with COVID-19: focus on severity and mortality. Front Public Health. 2020;8:152. doi: 10.3389/fpubh.2020.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized with COVID-19: results from the American Heart Association COVID-19 cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 31.Lechien JR, Chiesa-Estomba CM, Place S, Van Laethem Y, Cabaraux P, Mat Q, et al. Clinical and epidemiological characteristics of 1420 European patients with mild-to-moderate coronavirus disease 2019. J Intern Med. 2020;288:335–344. doi: 10.1111/joim.13089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tenforde MW, Billig Rose E, Lindsell CJ, Shapiro NI, Files DC, Gibbs KW, et al. Characteristics of adult outpatients and inpatients with COVID-19— 11 Academic Medical Centers, United States, March-May 2020. MMWR Morb Mortal Wkly Rep. 2020;69:841–846. doi: 10.15585/mmwr.mm6926e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Killerby ME, Link-Gelles R, Haight SC, Schrodt CA, England L, Gomes DJ, et al. Characteristics associated with hospitalization among patients with COVID-19—Metropolitan Atlanta, Georgia, March-April 2020. MMWR Morb Mortal Wkly Rep. 2020;69:790–794. doi: 10.15585/mmwr.mm6925e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menni C, Valdes AM, Polidori L, Antonelli M, Penamakuri S, Nogal A, et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shen B, Hoshmand-Kochi M, Abbasi A, Glass S, Jiang Z, Singer AJ, et al. Initial chest radiograph scores inform COVID-19 status, intensive care unit admission and need for mechanical ventilation. Clin Radiol. 2021;76:473.e1–473.e7. doi: 10.1016/j.crad.2021.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homayounieh F, Zhang EW, Babaei R, Karimi Mobin H, Sharifian M, Mohseni I, et al. Clinical and imaging features predict mortality in COVID-19 infection in Iran. PLoS ONE. 2020;15:e0239519. doi: 10.1371/journal.pone.0239519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng Y, Wang L, Ben S. Meta-analysis of chest CT features of patients with COVID-19 pneumonia. J Med Virol. 2021;93:241–249. doi: 10.1002/jmv.26218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55:327–331. doi: 10.1097/RLI.0000000000000672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colombi D, Bodini FC, Petrini M, Maffi G, Morelli N, Milanese G, et al. Well-aerated lung on admitting chest CT to predict adverse outcome in COVID-19 pneumonia. Radiology. 2020;296:E86–E96. doi: 10.1148/radiol.2020201433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Vidal C, Sanjuan G, Moreno-García E, Puerta-Alcalde P, Garcia-Pouton N, Chumbita M, et al. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin Microbiol Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musuuza JS, Watson L, Parmasad V, Putman-Buehler N, Christensen L, Safdar N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: a systematic review and meta-analysis. PLoS ONE. 2021;16:e0251170. doi: 10.1371/journal.pone.0251170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li JY, Wang HF, Yin P, Li D, Wang DL, Peng P, et al. Clinical characteristics and risk factors for symptomatic venous thromboembolism in hospitalized COVID-19 patients: a multicenter retrospective study. J Thromb Haemost. 2021;19:1038–1048. doi: 10.1111/jth.15261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meena RA, Sharifpour M, Gaddh M, Cui X, Xie Y, Di M, et al. COVID-19-associated venous thromboembolism portends worse survival. Semin Vasc Surg. 2021;34:117–124. doi: 10.1053/j.semvascsurg.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57:389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malik P, Patel U, Mehta D, Patel N, Kelkar R, Akrmah M, et al. Biomarkers and outcomes of COVID-19 hospitalisations: systematic review and meta-analysis. BMJ Evid Based Med. 2021;26:107–108. doi: 10.1136/bmjebm-2020-111536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng L, Li H, Li L, Liu C, Yan S, Chen H, et al. Ferritin in the coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. J Clin Lab Anal. 2020;34:e23618. doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu Z, Mao Y, Chen G. Predictive value of HbA1c for in-hospital adverse prognosis in COVID-19: a systematic review and meta-analysis. Prim Care Diabetes. 2021;15:910–917. doi: 10.1016/j.pcd.2021.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dai Z, Zeng D, Cui D, Wang D, Feng Y, Shi Y, et al. Prediction of COVID-19 patients at high risk of progression to severe disease. Front Public Health. 2020;8:574915. doi: 10.3389/fpubh.2020.574915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: identification of cell types and trends with age. ACS Chem Neurosci. 2020;11:1555–1562. doi: 10.1021/acschemneuro.0c00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH, et al. SARS-CoV-2 Reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–46.e14. doi: 10.1016/j.cell.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pujadas E, Chaudhry F, McBride R, Richter F, Zhao S, Wajnberg A, et al. SARS-CoV-2 viral load predicts COVID-19 mortality. Lancet Respir Med. 2020;8:e70. doi: 10.1016/S2213-2600(20)30354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aggarwal S, Aggarwal S, Aggarwal A, Jain K, Minhas S. High viral load and poor ventilation: cause of high mortality from COVID-19. Asia Pac J Public Health. 2020;32:377–378. doi: 10.1177/1010539520944725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhao Y, Zhao Z, Wang Y, Zhou Y, Ma Y, Zuo W. Single-Cell RNA expression profiling of ACE2, the receptor of SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:756–759. doi: 10.1164/rccm.202001-0179LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Higham A, Singh D. Increased ACE2 expression in bronchial epithelium of COPD patients who are overweight. Obesity (Silver Spring) 2020;28:1586–1589. doi: 10.1002/oby.22907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leung JM, Yang CX, Tam A, Shaipanich T, Hackett TL, Singhera GK, et al. ACE-2 expression in the small airway epithelia of smokers and COPD patients: implications for COVID-19. Eur Respir J. 2020 doi: 10.1183/13993003.00688-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hippisley-Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moein ST, Hashemian SM, Mansourafshar B, Khorram-Tousi A, Tabarsi P, Doty RL. Smell dysfunction: a biomarker for COVID-19. Int Forum Allergy Rhinol. 2020;10:944–950. doi: 10.1002/alr.22587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ninchritz-Becerra E, Soriano-Reixach MM, Mayo-Yánez M, Calvo-Henríquez C, Martínez-Ruiz de Apodaca P, Saga-Gutiérrez C, et al. Subjective evaluation of smell and taste dysfunction in patients with mild COVID-19 in Spain. Med Clin (Barc) 2021;156:61–64. doi: 10.1016/j.medcli.2020.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman A, Tabassum T, Araf Y, Al Nahid A, Ullah MA, Hosen MJ. Silent hypoxia in COVID-19: pathomechanism and possible management strategy. Mol Biol Rep. 2021;48:3863–3869. doi: 10.1007/s11033-021-06358-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental Figure 1. Study flow chart of patient identification and selectionStudy flow chart of patient identification and selection. A total of 117 records were excluded from the 3431 cases registered in the coronavirus disease 2019 (COVID-19) taskforce database owing to lack of essential clinical information. Ultimately, 3314 patients met the eligibility criteria, of which 2709 had respiratory symptoms. Supplemental Figure 2. Frequency of assisted respiration therapy and death in all four groups (a) Univariate analysis of the proportion of high-flow oxygen therapy with COVID-19 in each group. (b) Univariate analysis of the proportion of use of invasive mechanical ventilation (IMV) with COVID-19 in each group. (c) Univariate analysis of the proportion of use of extracorporeal membrane oxygenation (ECMO) with COVID-19 in each group. (d) Univariate analysis of the proportion of death with COVID-19 in each group. Supplemental Table 1. Common non-respiratory symptoms in each group.
Data Availability Statement
The datasets generated during and/or analyzed during the current study available from the corresponding author on reasonable request.